Fast Kinetic Response and Efficient Removal of Methyl Blue and Methyl Green Dyes by Functionalized Multiwall Carbon Nanotubes Powered with Iron Oxide Nanoparticles and Citrus reticulata Peel Extract
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
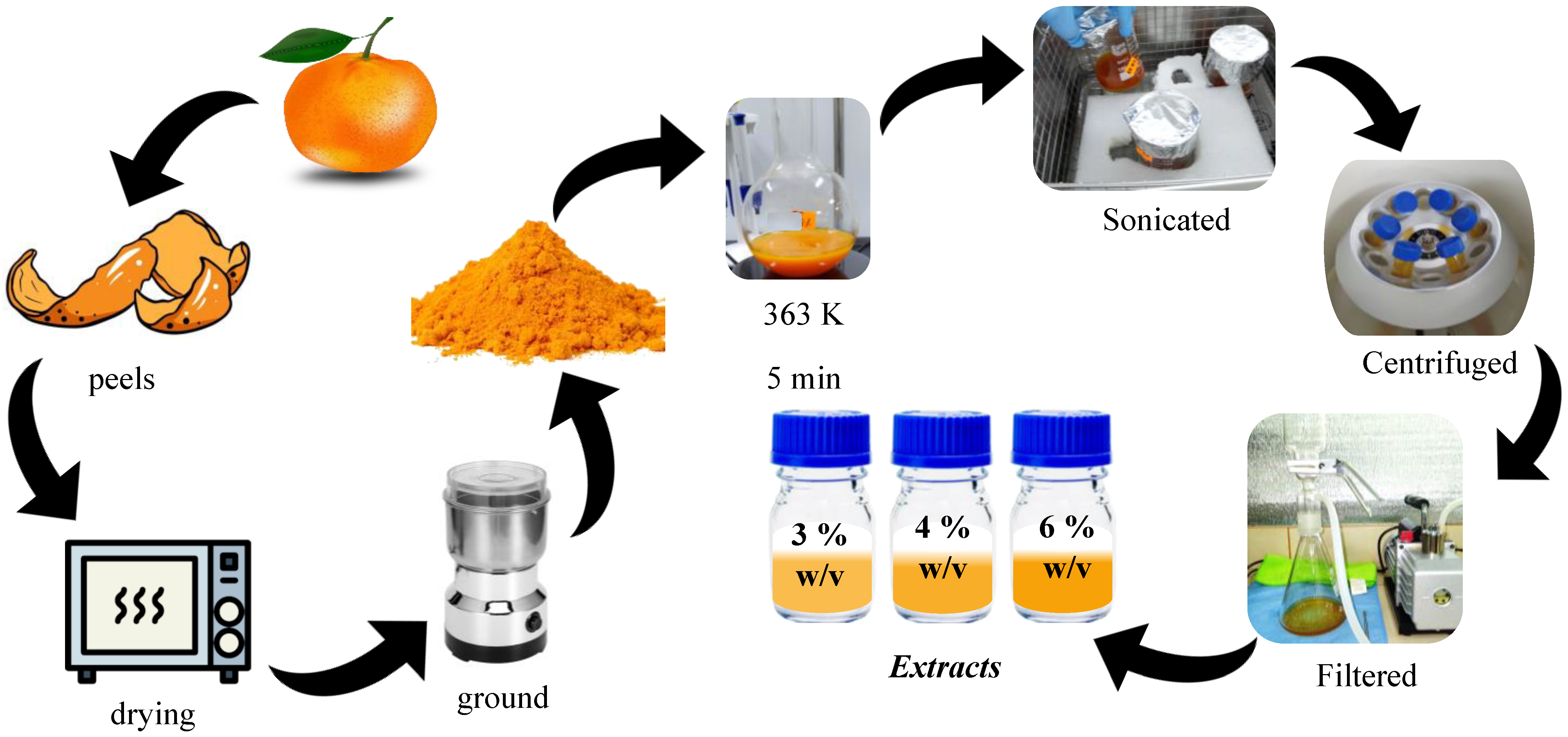
2.2. Extract Preparation
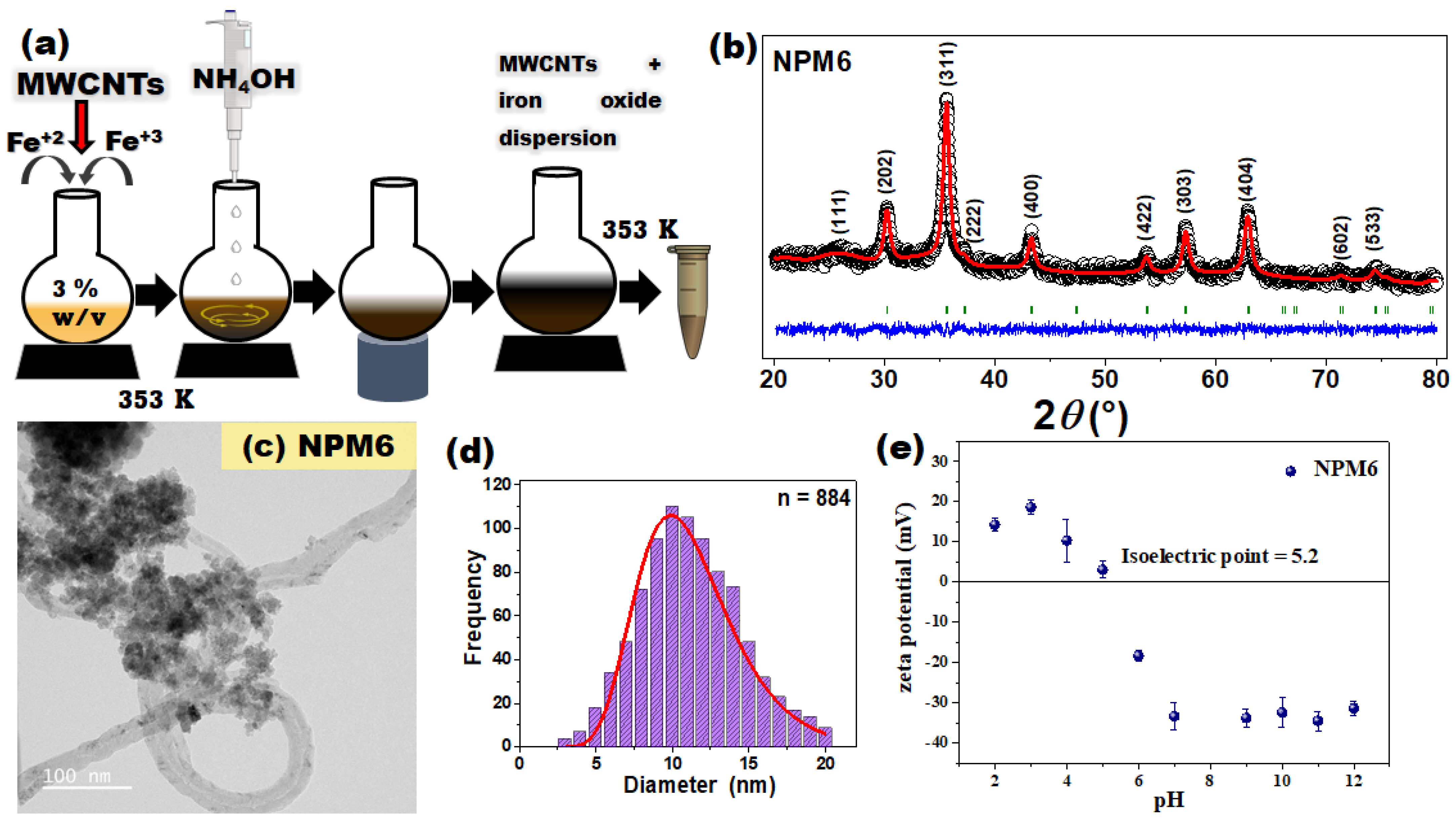
2.3. Biosynthesis
2.4. Characterization
2.4.1. Characterization of the Citrus reticulata Peel Extract
2.4.2. Characterization of the Adsorbent
2.5. Dye Adsorption Experiments
2.6. Adsorption Percentage and Adsorption Capacities for Dyes
3. Results and Discussion
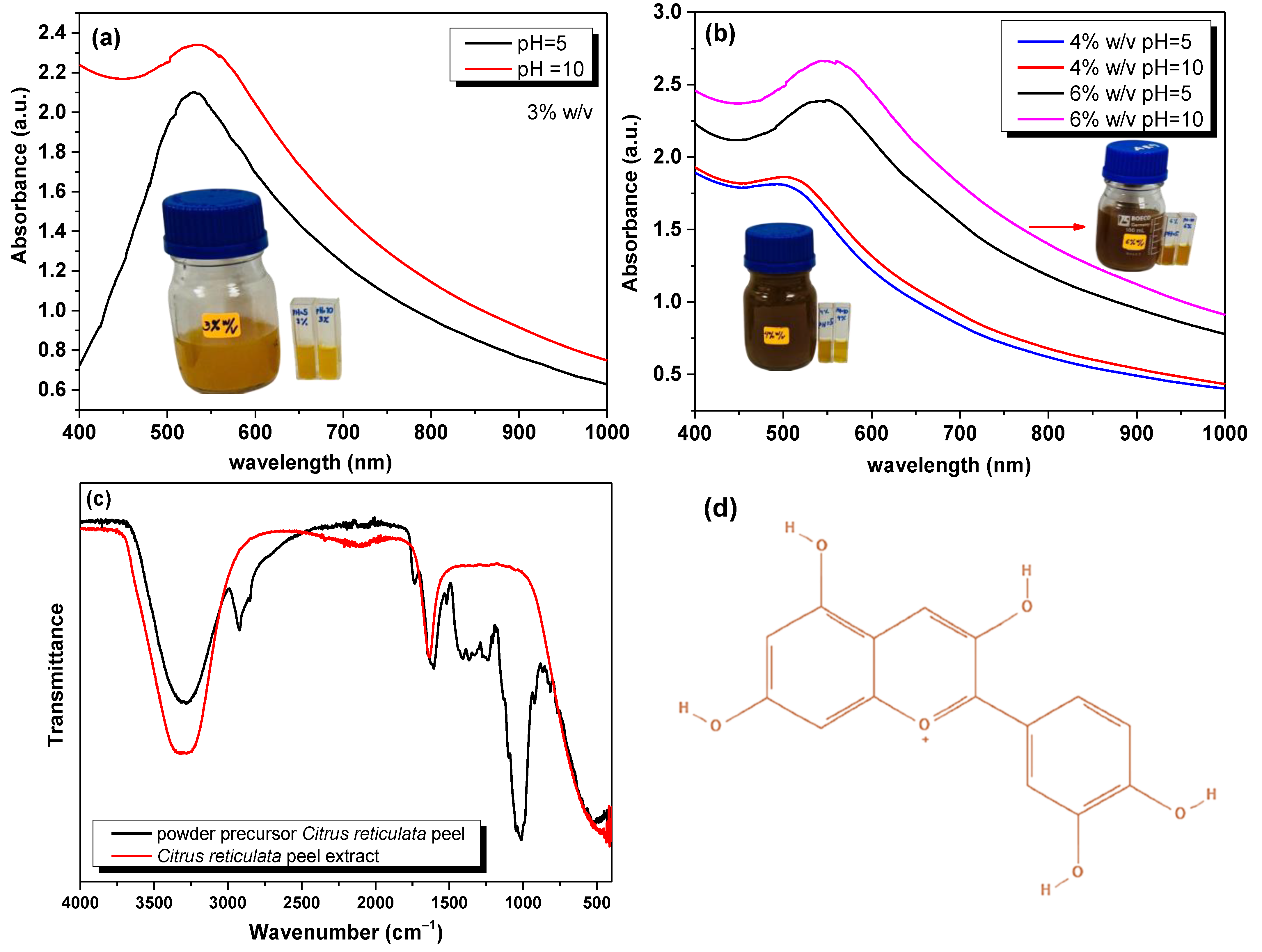
3.1. Citrus reticulata Peel Extract Characterization
3.2. Effect of Citrus reticulata Peel Extract in the Physicochemical Properties of the Adsorbents
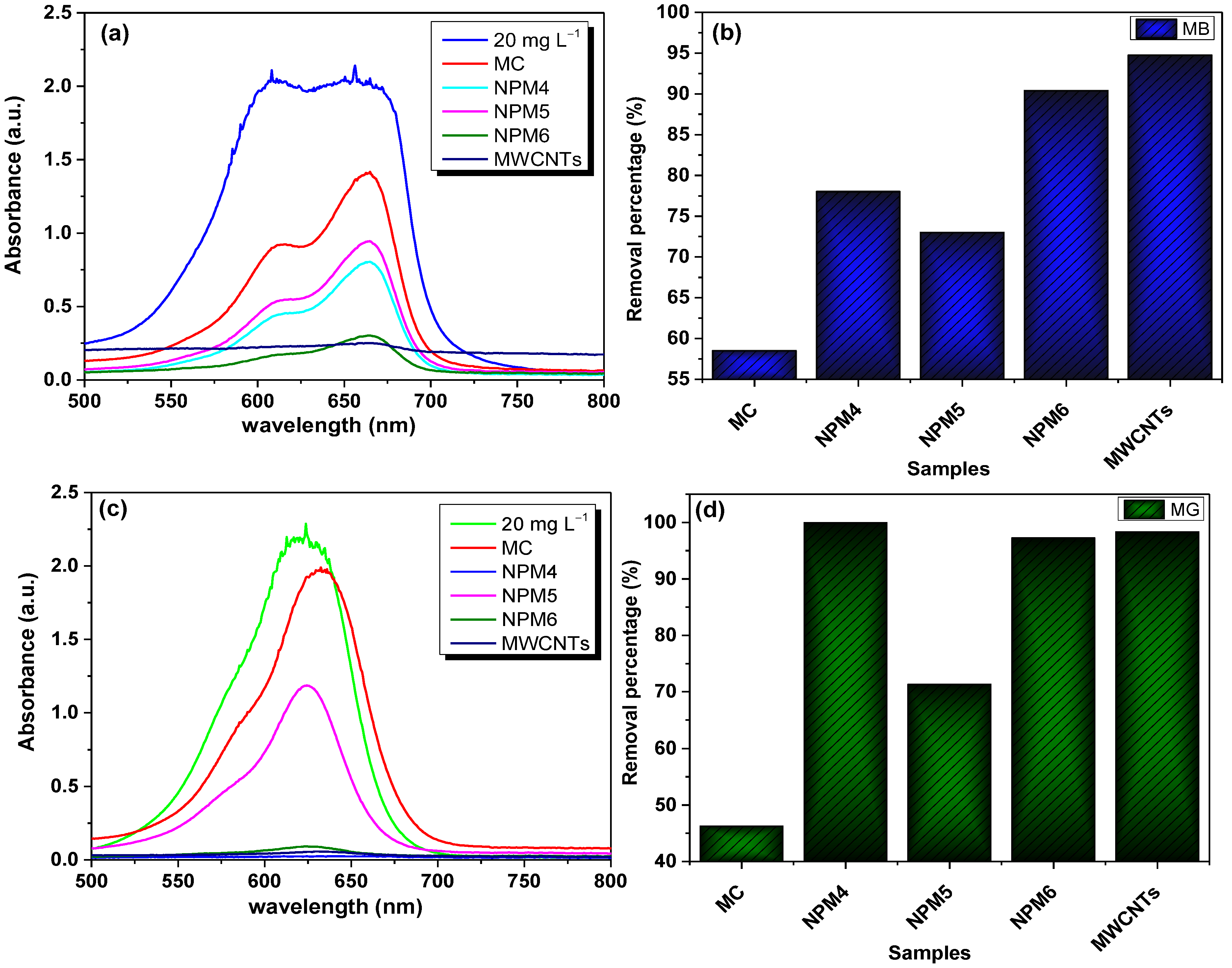
3.3. Preliminary Adsorption Test of the Adsorbents
3.4. Detailed Characterization of the Adsorbent with Best Adsorption Performance
3.4.1. Structural and Morphological Properties of NPM6 Adsorbent
3.4.2. Colloidal, Textural, and Surface Properties of NPM6 Adsorbent
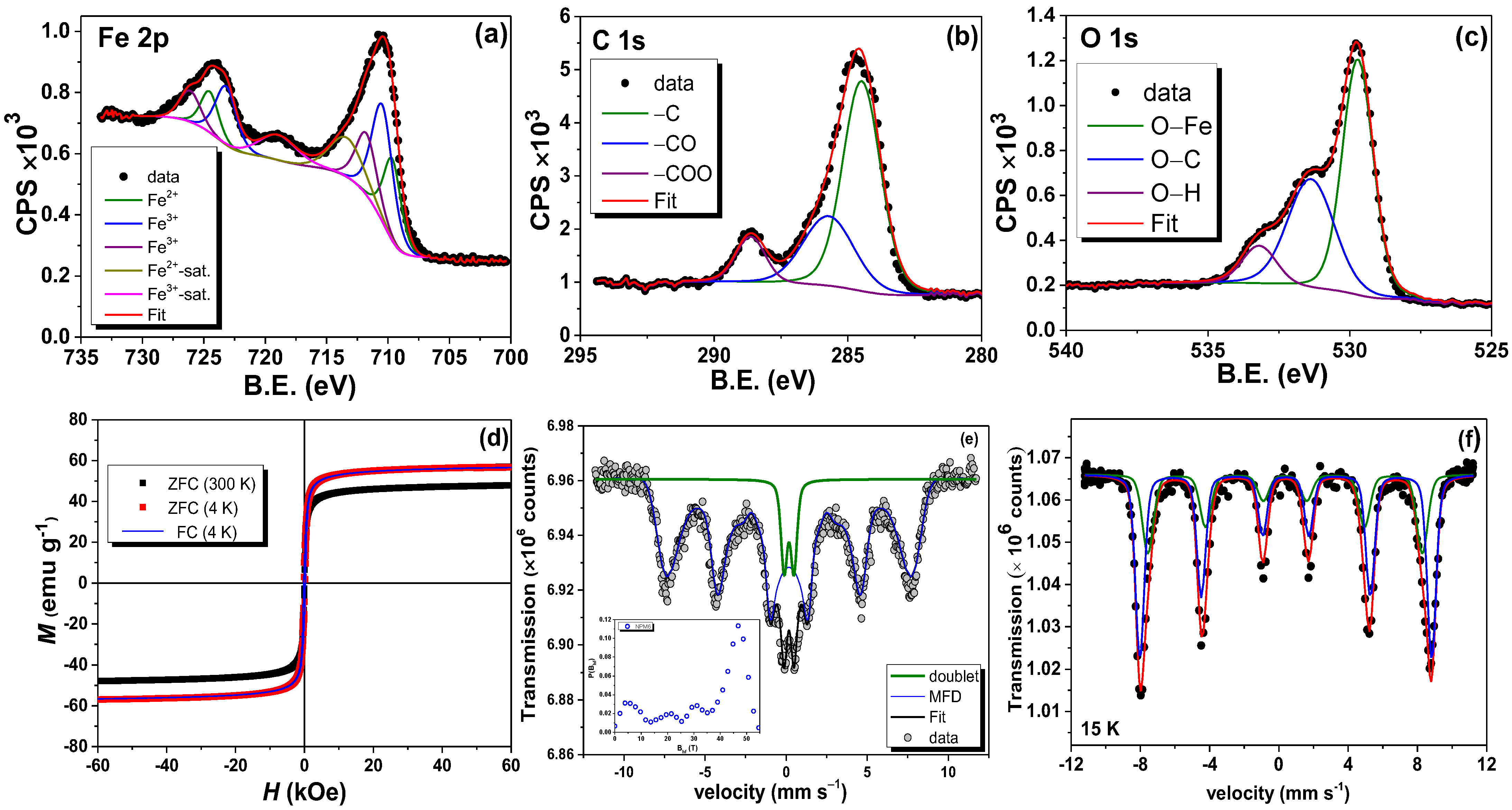
3.4.3. Magnetic and Hyperfine Properties
3.5. Batch Adsorption Analysis Using NPM6
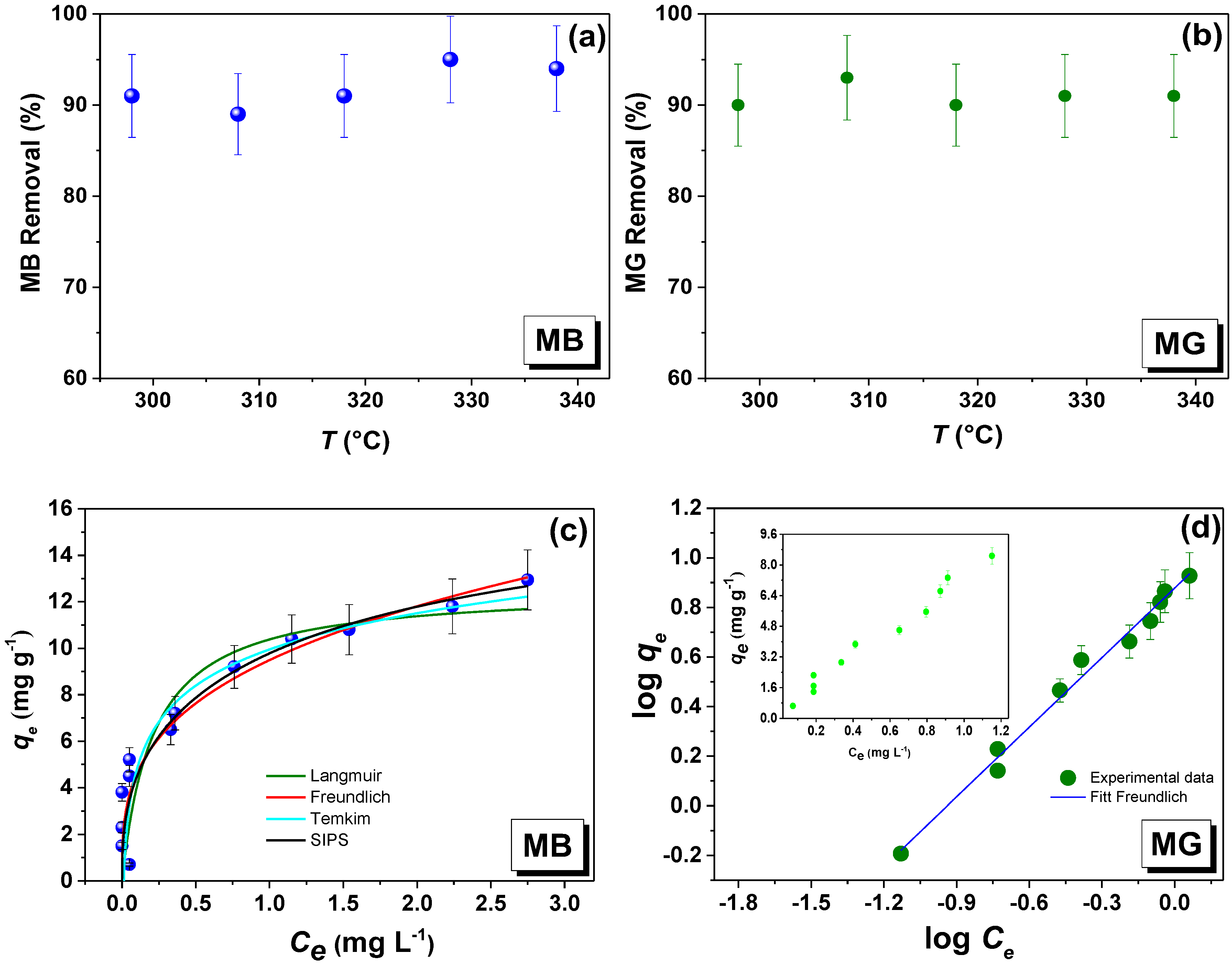
3.5.1. MB Removal Performance
| PFO | PSO | Elovich | IDM | |||||
|---|---|---|---|---|---|---|---|---|
| Parameters | qe | 4.78 (0.04) | qe | 4.31 (0.05) | α | 6.3 (0.8) × 1012 | kp | 0.29 (0.11) |
| k1 | 1.7 (0.2) | k2 | 1.1 (0.3) | β | 7 (2) | C1 | 3.1 (0.6) | |
| R2 | 0.987 | 0.981 | 0.970 | 0.297 | ||||
| RSS | 23.906 | 24.550 | 22.515 | 24.050 | ||||
| BIC | 12.769 | 12.396 | 11.011 | 9.790 | ||||
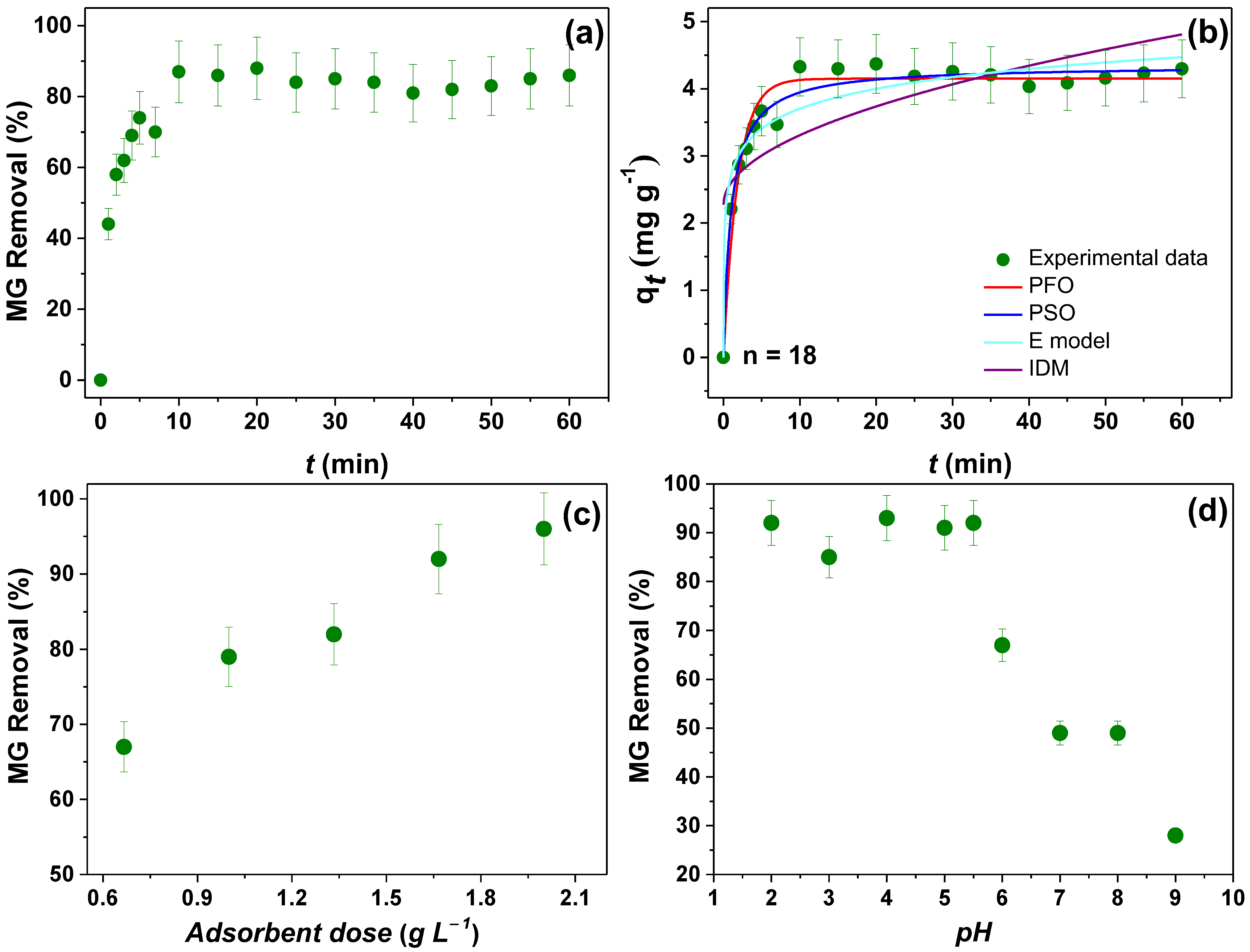
3.5.2. MG Removal Performance
3.5.3. Effect of Temperature and Isotherm Adsorption
3.5.4. Reuse Experiments
3.6. Physicochemical Properties of NPs After Adsorption
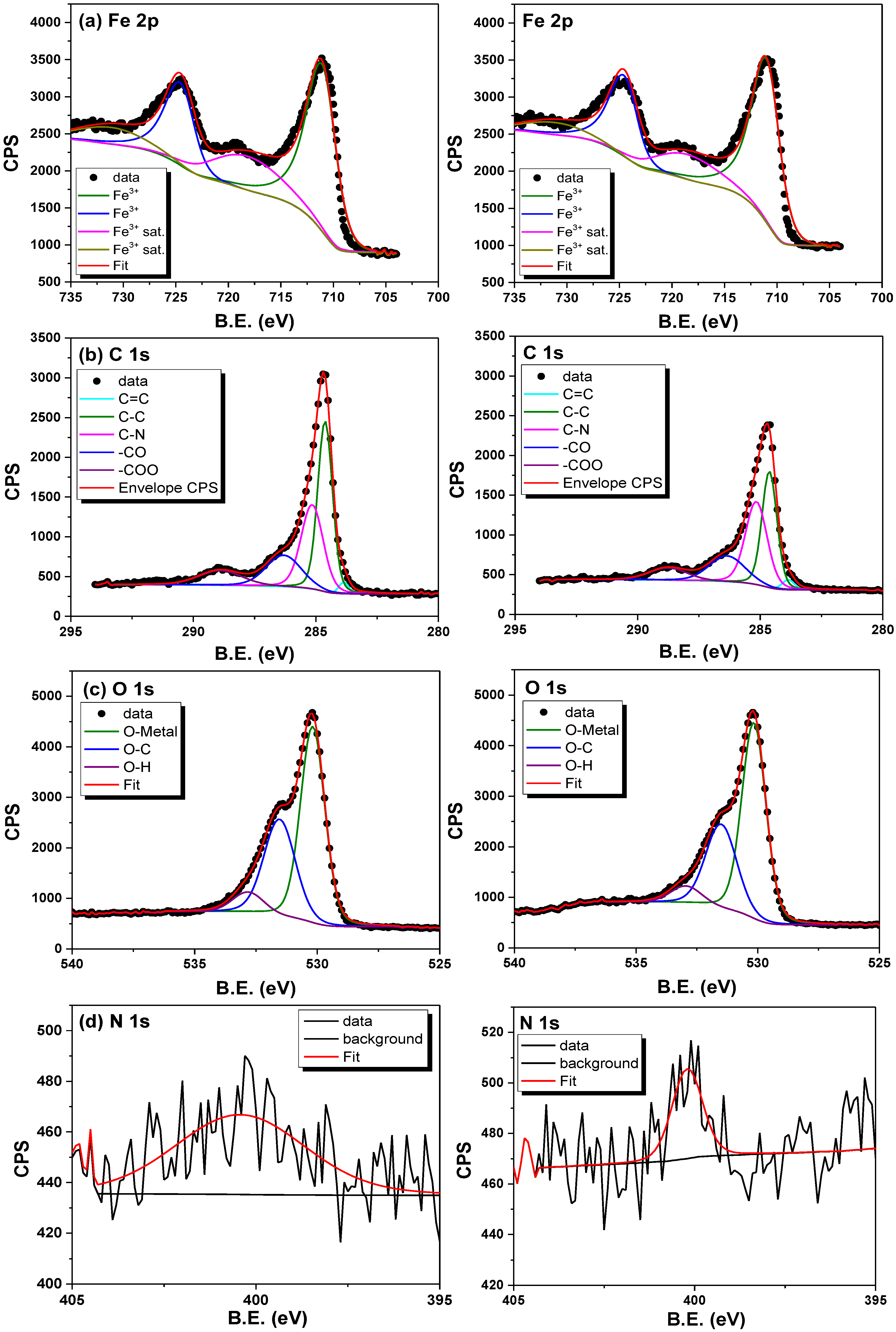
3.6.1. XPS After Adsorption Analysis
3.6.2. VSM After Adsorption Analysis
3.6.3. 57Fe Mössbauer Analysis
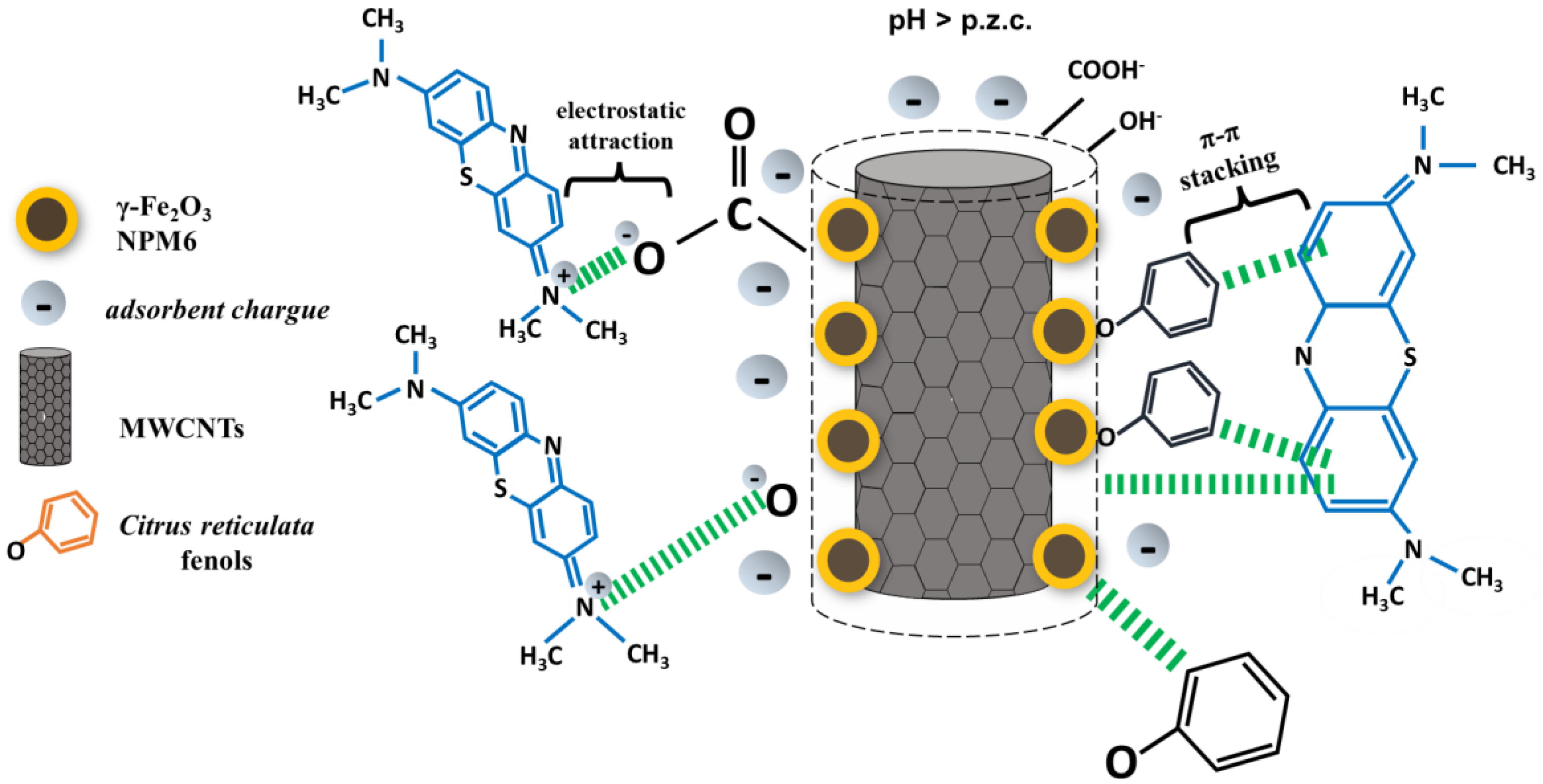
3.7. Surface Adsorption Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ukaogo, P.O.; Ewuzie, U.; Onwuka, C.V. Environmental pollution: Causes, effects, and the remedies. In Microorganisms for Sustainable Environment and Health; En Elsevier eBooks; Elsevier: Amsterdam, The Netherlands, 2020; pp. 419–429. [Google Scholar] [CrossRef]
- Akhtar, N.; Ishak, M.Z.; Bhawani, S.A.; Umar, K. Various natural and anthropogenic factors responsible for water quality degradation: A review. Water 2021, 13, 2660. [Google Scholar] [CrossRef]
- Tkaczyk, A.; Mitrowska, K.; Posyniak, A. Synthetic organic dyes as contaminants of the aquatic environment and their implications for ecosystems: A review. Sci. Total Environ. 2020, 717, 137222. [Google Scholar] [CrossRef] [PubMed]
- Al-Tohamy, R.; Ali, S.S.; Li, F.; Okasha, K.M.; Mahmoud, Y.A.-G.; Elsamahy, T.; Jiao, H.; Fu, Y.; Sun, J. A critical review on the treatment of dye-containing wastewater: Ecotoxicological and health concerns of textile dyes and possible remediation approaches for environmental safety. Ecotoxicol. Environ. Saf. 2022, 231, 113160. [Google Scholar] [CrossRef] [PubMed]
- Ali, Q.; Zia, M.A.; Kamran, M.; Shabaan, M.; Zulfiqar, U.; Ahmad, M.; Iqbal, R.; Maqsood, M.F. Nanoremediation for heavy metal contamination: A review. Hybrid Adv. 2023, 4, 100091. [Google Scholar] [CrossRef]
- Ahmed, T.; Noman, M.; Ijaz, M.; Ali, S.; Rizwan, M.; Ijaz, U.; Hameed, A.; Ahmad, U.; Wang, Y.; Sun, G.; et al. Current trends and future prospective in nanoremediation of heavy metals contaminated soils: A way forward towards sustainable agriculture. Ecotoxicol. Environ. Saf. 2021, 227, 112888. [Google Scholar] [CrossRef]
- Malik, S.; Muhammad, K.; Waheed, Y. Nanotechnology: A revolution in modern industry. Molecules 2023, 28, 661. [Google Scholar] [CrossRef]
- Suppiah, D.D.; Julkapli, N.M.; Sagadevan, S.; Johan, M.R. Eco-friendly green synthesis approach and evaluation of environmental and biological applications of Iron oxide nanoparticles. Inorg. Chem. Commun. 2023, 152, 110700. [Google Scholar] [CrossRef]
- Huston, M.; DeBella, M.; DiBella, M.; Gupta, A. Green synthesis of nanomaterials. Nanomaterials 2021, 11, 2130. [Google Scholar] [CrossRef]
- Kumar, B.; Smita, K.; Galeas, S.; Guerrero, V.H.; Debut, A.; Cumbal, L. One-Pot biosynthesis of maghemite (γ-Fe2O3) nanoparticles in aqueous extract of Ficus carica fruit and their application for antioxidant and 4-Nitrophenol reduction. Waste Biomass Valori. 2020, 12, 3575–3587. [Google Scholar] [CrossRef]
- Abdullah, J.A.A.; Jiménez-Rosado, M.; Perez-Puyana, V.; Guerrero, A.; Romero, A. Green synthesis of FeXOY nanoparticles with potential antioxidant properties. Nanomaterials 2022, 12, 2449. [Google Scholar] [CrossRef]
- Match!—Phase Identification from Powder Diffraction—Version 3, Crystal Impact—Dr. H. Put, Dr. K. Brandenburg GbR, Kreuzherrenstr. 102, 53227 Bonn, Germany. Available online: https://www.crystalimpact.de/match (accessed on 1 April 2025).
- Klencsár, Z. Mosswinn 4.0i, Revision. Available online: http://www.mosswinn.hu/ (accessed on 18 March 2025).
- Lowell, S.; Shields, J.E.; Thomas, M.A.; Thommes, M. Characterization of Porous Solids and Powders: Surface Area, Pore Size and Density; Science + Business Media: New York, NY, USA, 2004; ISBN 978-90-481-6633-6. [Google Scholar]
- Harborne, J. Spectral methods of characterizing anthocyanins. Biochem. J. 1958, 70, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Maylinda, E.V.; Rinadi, A.; Putri, E.A.; Fadillah, G.; Wayuningsih, S. Color stability of anthocyanins copigmentation from red rice (Oryza sativa L.) bran by spectrophotometry UV-Vis. IOP Conf. Ser. Mater. Sci. Eng. 2019, 578, 012001. [Google Scholar] [CrossRef]
- Skaar, I.; Adaku, C.; Jordheim, M.; Byamukama, R.; Kiremire, B.; Andersen, Ø.M. Purple anthocyanin colouration on lower (abaxial) leaf surface of Hemigraphis colorata (Acanthaceae). Phytochemistry 2014, 105, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Kit, I.; Kahyaoglu, L.N. Colorimetric sensing films of visible-light curable furfuryl gelatin for visual monitoring of chicken freshness. Food Bioprocess Technol. 2024, 17, 528–543. [Google Scholar] [CrossRef]
- Marković, J.M.D.; Baranac, J.M.; Brdarić, T.P. Electronic and infrared vibrational analysis of cyanidin–quercetin copigment complex. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2025, 62, 673–680. [Google Scholar] [CrossRef]
- Arias-Contreras, M.A.; Checca-Huaman, N.R.; Arévalo-Gardini, E.; Arévalo-Hernández, C.O.; Passamani, E.C.; Ramos-Guivar, J.A. Medium scale-up synthesis of nanomaghemite as an inhibitor of cadmium uptake in seedlings of Theobroma cacao L. J. Agric. Food Res. 2024, 18, 101295. [Google Scholar] [CrossRef]
- Ma-Hock, L.; Treumann, S.; Strauss, V.; Brill, S.; Luizi, F.; Mertler, M.; Wiench, K.; Gamer, A.O.; van Ravenzwaay, B.; Landsiedel, R. Inhalation toxicity of multiwall carbon nanotubes in rats exposed for 3 months. Toxicol. Sci. 2009, 112, 468–481. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Yamashita, T.; Hayes, P.C. Analysis of XPS Spectra of Fe2+ and Fe3+ Ions in Oxide Materials. Appl. Surf. Sci. 2008, 254, 2441–2449. [Google Scholar] [CrossRef]
- Ramos-Guivar, J.A.; Flores-Cano, D.A.; Caetano Passamani, E. Differentiating nanomaghemite and nanomagnetite and discussing their importance in arsenic and lead removal from contaminated effluents: A critical review. Nanomaterials 2021, 11, 2310. [Google Scholar] [CrossRef]
- Lapuente, R.; Quijada, C.; Huerta, F.; Cases, F.; Vázquez, J.L. X-Ray Photoelectron Spectroscopy Study of the Composition of Polyphenol Films Formed on PT by Electropolymerisation of Phenol in the Presence of Sulphide in Carbonate Medium. Polym. J. 2003, 35, 911–919. [Google Scholar] [CrossRef]
- McCafferty, E.W.J.P.; Wightman, J.P. Determination of the concentration of surface hydroxyl groups on metal oxide films by a quantitative XPS method. Surf. Interface Anal. 1998, 26, 549–564. [Google Scholar] [CrossRef]
- Ramos-Guivar, J.A.; Gonzalez-Gonzalez, J.C.; Litterst, F.J.; Passamani, E.C. Rietveld Refinement, µ-Raman, X-Ray Photoelectron, and Mössbauer Studies of Metal Oxide-Nanoparticles Growth on Multiwall Carbon Nanotubes and Graphene Oxide. Cryst. Growth Des. 2021, 21, 2128–2141. [Google Scholar] [CrossRef]
- Cornell, R.M.; Schwertmann, U. The Iron Oxides: Structure, Properties, Reactions, Occurrences, and Uses; Wiley-vch: Weinheim, Germany, 2003; Volume 664. [Google Scholar]
- Stoner, E.C.; Wohlfarth, E.P. A Mechanism of Magnetic Hysteresis in Heterogeneous Alloys. Philos. Trans. R. Soc. 1948, 240, 599–642. [Google Scholar] [CrossRef]
- Chaudhari, H.N.; Dhruv, P.N.; Singh, C.; Meena, S.S.; Kavita, S.; Jotania, R.B. Effect of heating temperature on structural, magnetic, and dielectric properties of Magnesium ferrites prepared in the presence of Solanum Lycopersicum fruit extract. J. Mater. Sci. Mater. Electron. 2020, 31, 18445–18463. [Google Scholar] [CrossRef]
- Prado, Y.; Daffé, N.; Michel, A.; Georgelin, T.; Yaacoub, N.; Greneche, J.M.; Choueikani, F.; Otero, E.; Ohresser, P.; Arrio, M.-A.; et al. Enhancing the magnetic anisotropy of maghemite nanoparticles via the surface coordination of molecular complexes. Nat. Commun. 2015, 6, 10139. [Google Scholar] [CrossRef]
- Demirezen, D.A.; Yılmaz, D.D.; Yildiz, Y.Ş. Magnetic Chitosan/Calcium Alginate Double-Network Hydrogel Beads: Preparation, Adsorption of Anionic and Cationic Surfactants, and Reuse in the Removal of Methylene Blue. Int. J. Biol. Macromol. 2023, 239, 124311. [Google Scholar] [CrossRef]
- Obayomi, K.S.; Lau, S.Y.; Zahir, A.; Meunier, L.; Zhang, J.; Dada, A.O.; Rahman, M.M. Removing Methylene Blue from Water: A Study of Sorption Effectiveness onto Nanoparticles-Doped Activated Carbon. Chemosphere 2023, 313, 137533. [Google Scholar] [CrossRef]
- Munagapati, V.S.; Wen, H.-Y.; Gollakota, A.R.K.; Wen, J.; Lin, K.A.; Shu, C.; Vijaya, Y.; Basivi, P.K.; Reddy, G.M.; Zyryanov, G.V. Magnetic Fe3O4 Nanoparticles Loaded Guava Leaves Powder Impregnated into Calcium Alginate Hydrogel Beads (Fe3O4-GLP@CAB) for Efficient Removal of Methylene Blue Dye from Aqueous Environment: Synthesis, Characterization, and Its Adsorption Performance. Int. J. Biol. Macromol. 2023, 246, 125675. [Google Scholar] [CrossRef]
- Bhakta, A.K.; Kumari, S.; Hussain, S.; Martis, P.; Mascarenhas, R.J.; Delhalle, J.; Mekhalif, Z. Synthesis and Characterization of Maghemite Nanocrystals Decorated Multi-Wall Carbon Nanotubes for Methylene Blue Dye Removal. J. Mater. Sci. 2018, 54, 200–216. [Google Scholar] [CrossRef]
- Samaraweera, H.; Andrena, D.; Carter, K.A.; Felder, T.; Nawalage, S.; Chui, I.; Pérez, F.; Khan, A.; Mlsna, T. Green Iron Oxide-Modified Biochar for Methylene Blue Removal from Aqueous Solutions. Groundw. Sustain. Dev. 2023, 21, 100945. [Google Scholar] [CrossRef]
- El-Katori, E.E.; Ahmed, M.A.; El-Bindary, A.A.; Oraby, A.M. Impact of CDS/SNO2 Heterostructured Nanoparticle as Visible Light Active Photocatalyst for the Removal Methylene Blue Dye. J. Photochem. Photobiol. Chem. 2020, 392, 112403. [Google Scholar] [CrossRef]
- Zhang, P.; O’Connor, D.; Wang, Y.; Jiang, L.; Xia, T.; Wang, L.; Tsang, D.C.W.; Ok, Y.S.; Hou, D. A Green Biochar/Iron Oxide Composite for Methylene Blue Removal. J. Hazard. Mater. 2020, 384, 121286. [Google Scholar] [CrossRef]
- Mbachu, C.A.; Babayemi, A.K.; Egbosiuba, T.C.; Ike, J.I.; Ani, J.I.; Mustapha, S. Green Synthesis of Iron Oxide Nanoparticles by Taguchi Design of Experiment Method for Effective Adsorption of Methylene Blue and Methyl Orange from Textile Wastewater. Results Eng. 2023, 19, 101198. [Google Scholar] [CrossRef]
- Ashraf, I.; Singh, N.B.; Agarwal, A. Green Synthesis of Iron Oxide Nanoparticles Using AMLA Seed for Methylene Blue Dye Removal from Water. Mater. Today Proc. 2023, 72, 311–316. [Google Scholar] [CrossRef]
- Eniola, J.O.; Sızırıcı, B.; Khaleel, A.; Yildiz, İ. Fabrication of Engineered Biochar-Iron Oxide from Date Palm Frond for the Effective Removal of Cationic Dye from Wastewater. J. Water Process Eng. 2023, 54, 104046. [Google Scholar] [CrossRef]
- Mohammadpour, A.; Karami, N.; Zabihi, R.; Fazeliyan, E.; Abbasi, A.; Karimi, S.; De Farias, M.B.; Vieira, M.G.A.; Shahsavani, E.; Khaneghah, A.M. Green Synthesis, Characterization, and Application of Fe3O4 Nanoparticles for Methylene Blue Removal: RSM Optimization, Kinetic, Isothermal Studies, and Molecular Simulation. Environ. Res. 2023, 225, 115507. [Google Scholar] [CrossRef]
- Ai, L.; Jiang, J. Removal of Methylene Blue from Aqueous Solution with Self-Assembled Cylindrical Graphene–Carbon Nanotube Hybrid. Chem. Eng. J. 2012, 192, 156–163. [Google Scholar] [CrossRef]
- Bayram, O.; Moral, E.; Köksal, E.; Göde, F.; Pehlivan, E. Removal of Methyl Blue and Malachite Green from Water Using Biodegradable Magnetic Tamarindus Indica Fruit Seed Biochar: Characterization, Equilibrium Study, Modelling and Thermodynamics. Sustain. Chem. Environ. 2023, 3, 100023. [Google Scholar] [CrossRef]
- Ansari, M.J.; Jasim, S.A.; Bokov, D.O.; Thangavelu, L.; Yasin, G.; Khalaji, A.D. Preparation of New Bio-Based Chitosan/Fe2O3/NiFe2O4 as an Efficient Removal of Methyl Green from Aqueous Solution. Int. J. Biol. Macromol. 2022, 198, 128–134. [Google Scholar] [CrossRef]
- Iqbal, M.; Bhatti, H.N.; Younis, S.; Rehmat, S.; Alwadai, N.; Almuqrin, A.H.; Iqbal, M. Graphene Oxide Nanocomposite with CUSE and Photocatalytic Removal of Methyl Green Dye under Visible Light Irradiation. Diam. Relat. Mater. 2021, 113, 108254. [Google Scholar] [CrossRef]
- Alardhi, S.M.; Albayati, T.M.; Alrubaye, J.M. Adsorption of the Methyl Green Dye Pollutant from Aqueous Solution Using Mesoporous Materials MCM-41 in a Fixed-Bed Column. Heliyon 2020, 6, e03253. [Google Scholar] [CrossRef] [PubMed]
- Alalwan, H.A.; Mohammed, M.M.; Sultan, A.J.; Abbas, M.N.; Ibrahim, T.A.; Aljaafari, H.A.S.; Alminshid, A.A. Adsorption of Methyl Green Stain from Aqueous Solutions Using Non-Conventional Adsorbent Media: Isothermal Kinetic and Thermodynamic Studies. Bioresour. Technol. Rep. 2021, 14, 100680. [Google Scholar] [CrossRef]
- Yang, X.; Xiong, L.; Hu, X.; He, B.; Chu, G. Photocatalytic Reaction and Degradation of Methylene Blue on TiO2 Films in Vacuum: An X-Ray Photoelectron Spectroscopy Study. Res. Chem. Intermed. 2011, 38, 67–75. [Google Scholar] [CrossRef]
- Zhang, F.; Lan, J.; Yang, Y.; Wei, T.; Tan, R.; Song, W. Adsorption behavior and mechanism of methyl blue on zinc oxide nanoparticles. J. Nanoparticle Res. 2013, 15, 2034. [Google Scholar] [CrossRef]
- Zhang, F.; Yin, X.; Lan, J.; Zhang, W. Application of Ba3(PO4)2/Fe3O4 as a Novel Magnetic Adsorbent to Remove Methyl Blue from Aqueous Solution. J. Mater. Sci. 2015, 51, 3525–3535. [Google Scholar] [CrossRef]
- John, S.-M.; Mathew, J.M. Determination of Ferrimagnetic and Superparamagnetic Components of Magnetization and the Effect of Particle Size on Structural, Magnetic and Hyperfine Properties of Mg0.5Zn0.5Fe2O4 Nanoparticles. J. Alloys Compd. 2021, 869, 159242. [Google Scholar] [CrossRef]
- Lü, K.; Wang, T.; Zhai, L.; Wu, W.; Dong, S.; Gao, S.; Mao, L. Adsorption Behavior and Mechanism of FE-MN Binary Oxide Nanoparticles: Adsorption of Methylene Blue. J. Colloid Interface Sci. 2019, 539, 553–562. [Google Scholar] [CrossRef]
- Ai, L.; Zhang, C.; Liao, F.; Wang, Y.; Li, M.; Meng, L.; Jiang, J. Removal of Methylene Blue from Aqueous Solution with Magnetite Loaded Multi-Wall Carbon Nanotube: Kinetic, Isotherm and Mechanism Analysis. J. Hazard. Mater. 2011, 198, 282–290. [Google Scholar] [CrossRef]






| Sample | Adsorption Capacity (mg g−1) | % | pH | Time (min) | Dose | Reference |
|---|---|---|---|---|---|---|
| SDS-CSMAB Chitosan/Calcium Alginate Double Network Magnetic Hydrogel Beads | 1.9 | 61 | 5.5 | 60 | 0.1 g L−1 | [32] |
| CSAC@AgNPs@TiO2 NP | 184 | 97 | 10 | 60 | 30 mg | [33] |
| Fe3O4-GLP@CAB Magnetic Fe3O4 NPs loaded guava leaves powder impregnated into calcium alginate hydrogel beads | 137 | 95 | 10 | 90 | 1 g | [34] |
| MWCNTs + γ-Fe2O3 NPs | 59.4 | - | ND | 1 | 0.3 g L−1 | [35] |
| Fe3O4-modified Douglas fir biochar (FDBC) | 741 | 90 | 6.8 | 5 | 50 mg | [36] |
| CdS/SnO2 heterostructured NP | - | 90 | photocatalytic degradation | 180 | 0.1 g | [37] |
| Biochar/FexOy composite | 862 | - | 6.1 | 720 | 0.01 g | [38] |
| NP CN-Fe2O3 Green synthesis | 530.4 | 88 | 5 | 60 | 25 mg L−1 | [39] |
| IONPs Green synthesis | - | 80 | ND | 30 | 2 mg | [40] |
| Biochar-FexOy date palm frond | 85.1 | - | 8 | 198 | 0.02 g | [41] |
| Fe3O4 P. factra extract | 26.8 | 91 | 9 | 50 | 0.7 g L−1 | [42] |
| NPM6 | 9.5 | 93 | 5.5 | 10 | 1.3 g L−1 | This work |
| Sample | Adsorption Capacity (mg g−1) | % | pH | Time (min) | Dose | Reference |
|---|---|---|---|---|---|---|
| BC | 5.6 | 95 | 2–9 | 50 | 0.9 g | [44] |
| Tamarindus indica fruit seed nanomagnetic/biocarbon composite (nM-BC) | 20.4 | 99 | 2–9 | 50 | 0.9 g | [44] |
| adsorbent chitosan/Fe2O3/NiFe2O4 | 77.2 | 97 | 8 | 120 | 0.02 g | [45] |
| graphene oxide (CuSe/GO) | - | 81 | 5 | 45 | 0.025 g | [46] |
| Fixed bed adsorption MCM-41 | 21 | 50 | 6 | - | 0.8 mL min−1 | [47] |
| NPM6 | 8 | 84 | 5.5 | 25 | 1.7 g L−1 | This work |
| PFO | PSO | Elovich | IDM | |||||
|---|---|---|---|---|---|---|---|---|
| Parameters | qe | 4.15 (0.07) | qe | 4.35 (0.06) | α | 244.2 (2) | kp | 0.33 (0.08) |
| k1 | 0.54 (0.05) | k2 | 0.23 (0.03) | β | 2.3 (0.3) | C1 | 2.3 (0.4) | |
| R2 | 0.95 | 0.98 | 0.93 | 0.51 | ||||
| RSS | 25.07 | 29.52 | 24.08 | 21.24 | ||||
| BIC | 11.74 | 14.69 | 11.02 | 5.87 | ||||
| Langmuir | Freundlich | Temkim | Sips | ||||
|---|---|---|---|---|---|---|---|
| qmax | 13 (1) | KF | 9.5 (0.6) | bT | 2.25 (0.37) | qms | 2 (1) × 10 |
| KL | 5 (2) | n | 0.32 (0.06) | KT | 8 (5) × 10 | Ks | 0.3 (0.9) |
| ms | 0.4 (0.2) | ||||||
| R2 | 0.775 | R2 | 0.81 | R2 | 0.80 | R2 | 0.795 |
| BIC | 46.041 | BIC | 56.049 | BIC | 118.04 | BIC | 55.79 |
| Langmuir | Freundlich | Temkim | Sips | ||||
|---|---|---|---|---|---|---|---|
| b | 0.17 (0.02) | 1/n | 0.88 (0.03) | KT | 2.9 (0.4) | 1/n | 0.8 (0.2) |
| qmax | 8.72 (0.01) | KF | 7.96 (0.05) | bT | 10.8 (0.3) | bs | 7.9 (0.2) |
| R2 | 0.992 | R2 | 0.987 | R2 | 0.888 | R2 | 0.926 |
| BIC | 36.19 | BIC | 34.46 | BIC | 43.41 | BIC | 34.21 |
| Systems | Number of Reuses | % | Desorbing Reagent | Reference |
|---|---|---|---|---|
| SDS-CSMAB/MB | 2 | 61 | surfactant loaded CSMAB. | [32] |
| CSAC@AgNPs@TiO2NPs/MB | 6 | 99.33–86.97 | ethanol mixed with acetic acid, and ultrapure water | [33] |
| Biochar-FexOy/MB | 4 | 85 to 39.1 mg g−1 | ethanol and filtered | [41] |
| nM- BC/MB | 4 | 88.36 to 86.74 | 0.1 M NaOH | [44] |
| nM-BC/MG | 4 | 91.25 to 88.43 | 0.1 M NaOH | [44] |
| chitosan/Fe2O3/NiFe2O4/MG | 5 | 97 to 91 | - | [45] |
| MCM-41 fixed-bed regeneration/MG | 4 | 44.38 to 32 | 0.1 M NaOH | [47] |
| NPM6/MB | 7 | 93 to 88 | 0.1 M NaOH | This work |
| NPM6/MG | 7 | 97 to 94 | 0.1 M NaOH | This work |
| Sample | T (K) | Mr (emu g−1) ± 0.1 | Hc (Oe) ± 3 | Ms (emu g−1) ± 1 | Mr/Ms | χ 10−4 (emu/g kOe) ± 0.1 | Keff × 105 (J m−3) ± 0.1 |
|---|---|---|---|---|---|---|---|
| MB2 | 5 | 9.0 | 300 | 23 | 0.39 | 1.3 | 0.6 |
| 300 | 1.4 | 40 | 16 | 0.09 | 1.5 | 0.5 | |
| MB7 | 5 | 16.1 | 340 | 40 | 0.41 | 2.4 | 1.2 |
| 300 | 1.6 | 20 | 29 | 0.05 | 2.4 | 0.9 | |
| MG2 | 5 | 14.5 | 310 | 38 | 0.38 | 2.1 | 1.1 |
| 300 | 2.3 | 40 | 27 | 0.08 | 2.2 | 0.8 | |
| MG7 | 5 | 1.7 | 340 | 4 | 0.40 | 0.3 | 0.1 |
| 300 | 0.2 | 30 | 3 | 0.05 | 0.3 | 0.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manrique-Castillo, E.V.; Marcos-Carrillo, M.d.P.; Checca-Huaman, N.-R.; Santos, B.L.D.; Macedo, W.A.A.; Barrero Meneses, C.A.; Passamani, E.C.; Greneche, J.-M.; Ramos-Guivar, J.A. Fast Kinetic Response and Efficient Removal of Methyl Blue and Methyl Green Dyes by Functionalized Multiwall Carbon Nanotubes Powered with Iron Oxide Nanoparticles and Citrus reticulata Peel Extract. Nanomaterials 2025, 15, 603. https://doi.org/10.3390/nano15080603
Manrique-Castillo EV, Marcos-Carrillo MdP, Checca-Huaman N-R, Santos BLD, Macedo WAA, Barrero Meneses CA, Passamani EC, Greneche J-M, Ramos-Guivar JA. Fast Kinetic Response and Efficient Removal of Methyl Blue and Methyl Green Dyes by Functionalized Multiwall Carbon Nanotubes Powered with Iron Oxide Nanoparticles and Citrus reticulata Peel Extract. Nanomaterials. 2025; 15(8):603. https://doi.org/10.3390/nano15080603
Chicago/Turabian StyleManrique-Castillo, Erich V., Mercedes del Pilar Marcos-Carrillo, Noemi-Raquel Checca-Huaman, Bruno L. D. Santos, Waldemar A. A. Macedo, César A. Barrero Meneses, Edson C. Passamani, Jean-Marc Greneche, and Juan A. Ramos-Guivar. 2025. "Fast Kinetic Response and Efficient Removal of Methyl Blue and Methyl Green Dyes by Functionalized Multiwall Carbon Nanotubes Powered with Iron Oxide Nanoparticles and Citrus reticulata Peel Extract" Nanomaterials 15, no. 8: 603. https://doi.org/10.3390/nano15080603
APA StyleManrique-Castillo, E. V., Marcos-Carrillo, M. d. P., Checca-Huaman, N.-R., Santos, B. L. D., Macedo, W. A. A., Barrero Meneses, C. A., Passamani, E. C., Greneche, J.-M., & Ramos-Guivar, J. A. (2025). Fast Kinetic Response and Efficient Removal of Methyl Blue and Methyl Green Dyes by Functionalized Multiwall Carbon Nanotubes Powered with Iron Oxide Nanoparticles and Citrus reticulata Peel Extract. Nanomaterials, 15(8), 603. https://doi.org/10.3390/nano15080603








