Ti-Doped, Mn-Based Polyanionic Compounds of Na4Fe1.2Mn1.8(PO4)2P2O7 for Sodium-Ion Battery Cathode
Abstract
1. Introduction
2. Experimental Section
2.1. Materials Synthesis
2.2. Material Characterization
2.3. Electrochemical Characterization
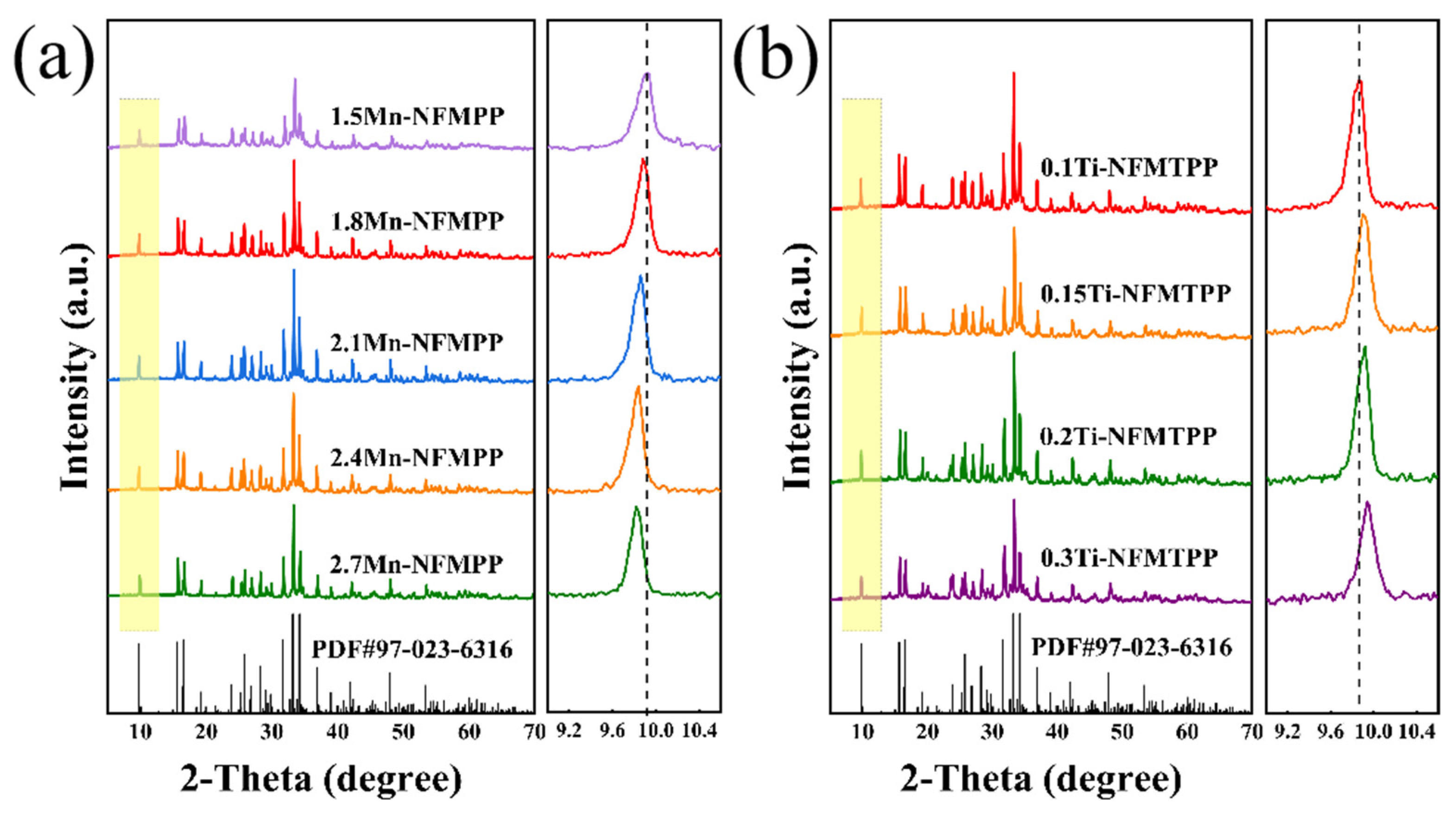
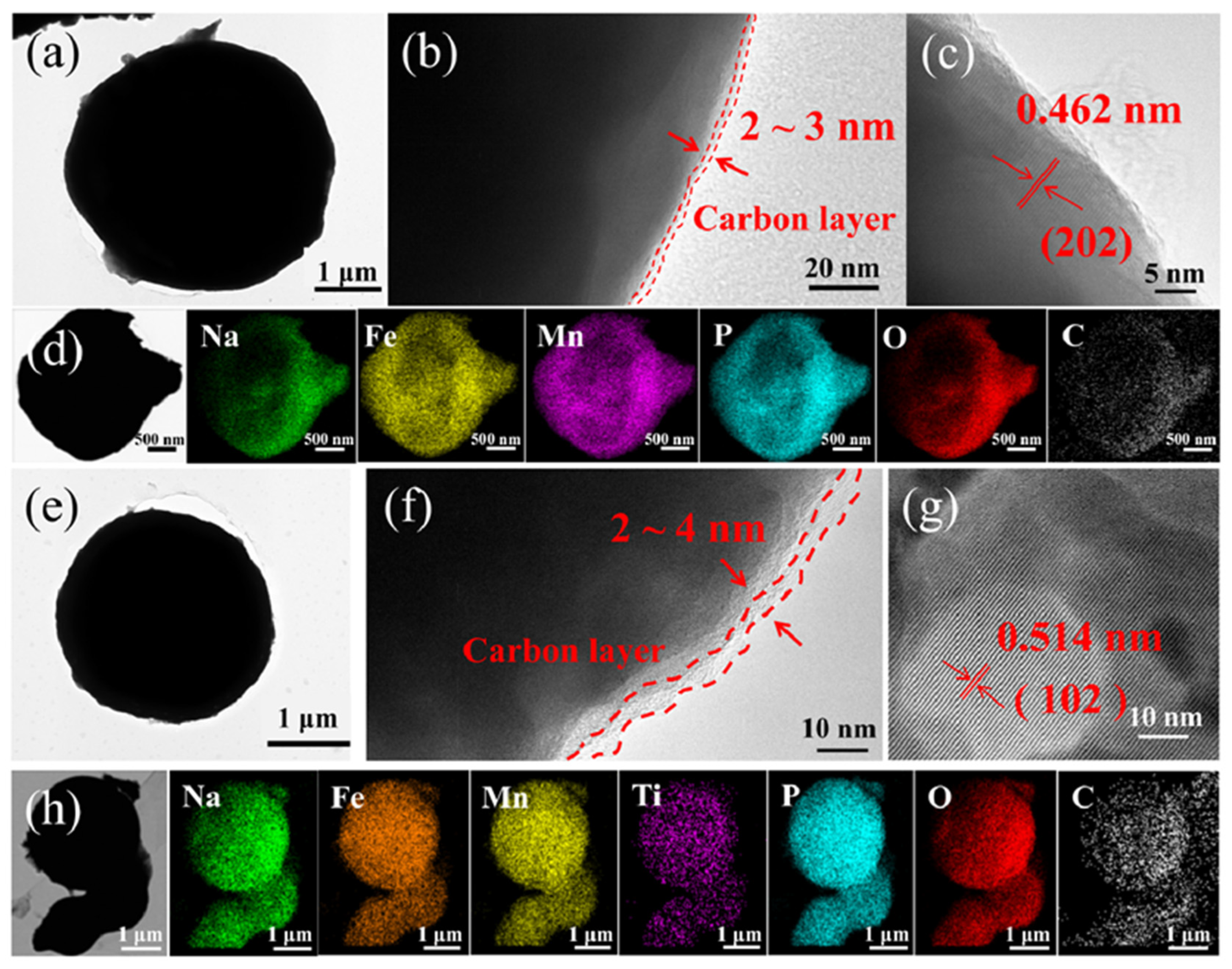
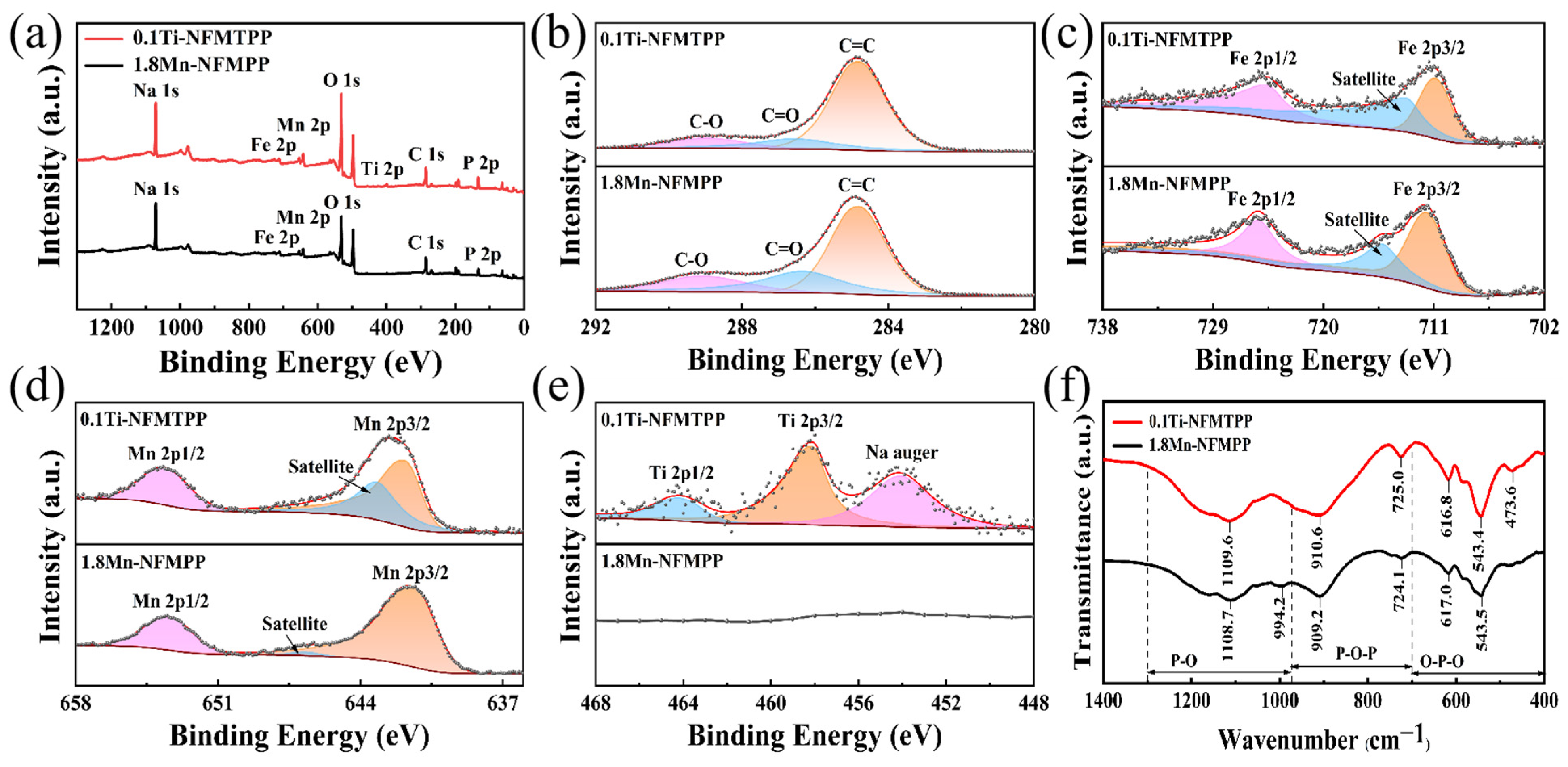
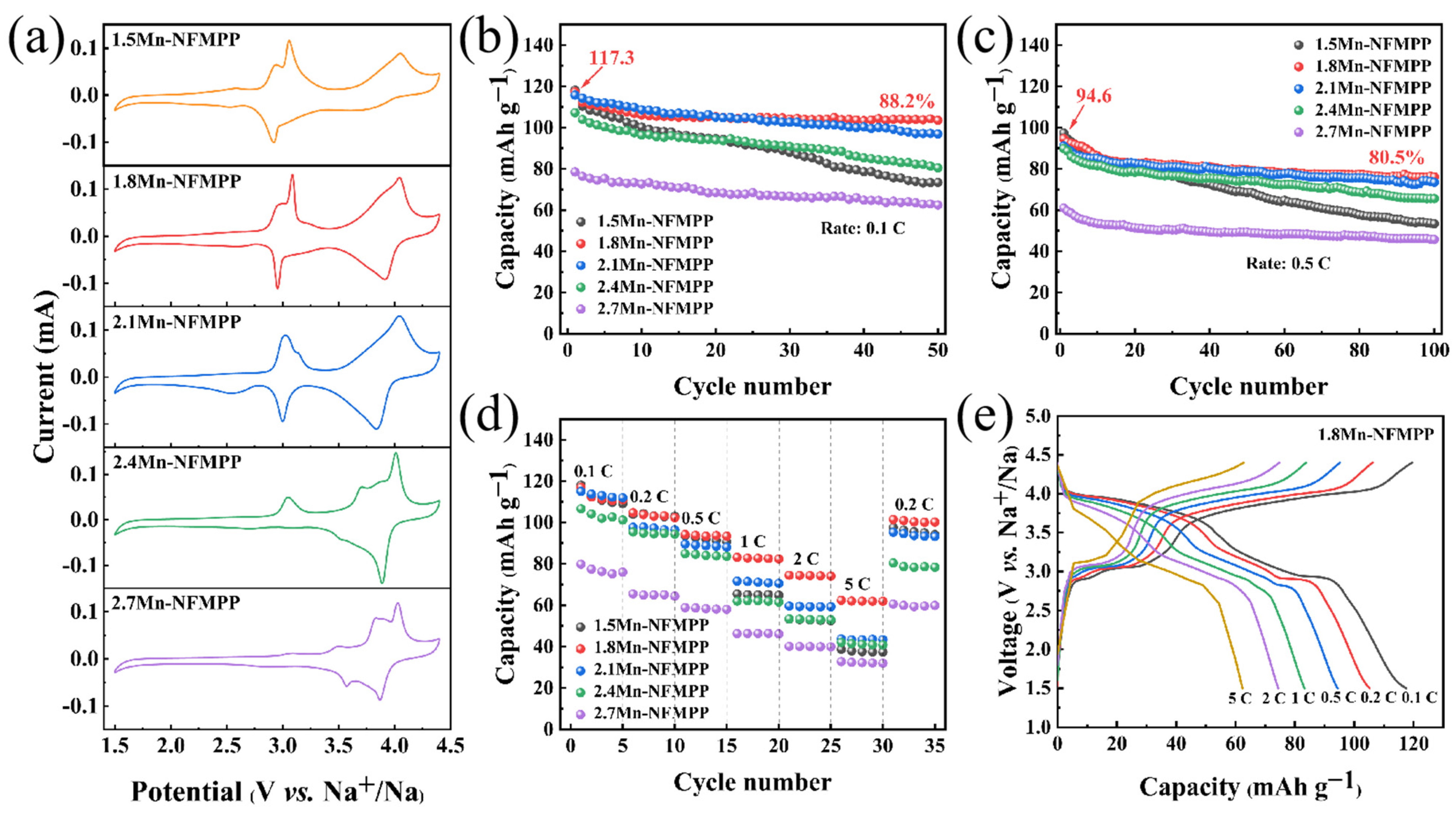
3. Result and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Armand, M.; Tarascon, J.M. Building better batteries. Nature 2008, 451, 652–657. [Google Scholar] [CrossRef]
- Li, Y.L.; Wei, Y.F.; Zhu, F.Q.; Du, J.Y.; Zhao, Z.M.; Ouyang, M.G. The path enabling storage of renewable energy toward carbon neutralization in China. ETransportation 2023, 16, 100226. [Google Scholar] [CrossRef]
- Zeng, X.Q.; Li, M.; EI-Hady, D.A.; Alshitari, W.; Abdullah, S.; Bogami, A.; Lu, J.; Amine, k. Commercialization of lithium battery technologies for electric vehicles. Adv. Energy Mater. 2019, 9, 1900161. [Google Scholar] [CrossRef]
- Shen, X.; Zhang, X.Q.; Ding, F.; Huang, J.Q.; Xu, R.; Chen, X.; Yan, C.; Su, F.Y.; Chen, C.M.; Liu, X.J.; et al. Advanced electrode materials in lithium batteries: Retrospect and prospect. Adv. Energy Mater. 2021, 2021, 1205324. [Google Scholar] [CrossRef]
- Tarascon, J.M. Na-ion versus Li-ion batteries: Complementarity rather than competitiveness. Joule 2020, 4, 1616–1620. [Google Scholar] [CrossRef]
- Roberts, S.; Kendrick, E. The re-emergence of sodium ion batteries: Testing, processing, and manufacturability. Nano. Technol. Sci. Appl. 2018, 11, 23–33. [Google Scholar] [CrossRef]
- Liu, T.F.; Zhang, Y.P.; Jiang, Z.G.; Zeng, X.Q.; Ji, J.P.; Li, Z.H.; Gao, X.H.; Sun, M.H.; Lin, Z.; Ling, M.; et al. Exploring competitive features of stationary sodium ion batteries for electrochemical energy storage. Energy Environ. Sci. 2019, 12, 1512–1533. [Google Scholar] [CrossRef]
- Abraham, K.M. How comparable are sodium-ion batteries to lithium-ion counterparts? ACS Energy Lett. 2020, 5, 3544–3547. [Google Scholar] [CrossRef]
- Rudola, A.; Wright, C.J.; Barker, J. Reviewing the safe shipping of lithium-ion and sodium-ion cells: A materials chemistry perspective. Adv. Energy Mater. 2021, 2021, 9798460. [Google Scholar] [CrossRef]
- Rudola, A.; Sayers, R.; Christopher, J.; Wright, J.; Barker, J. Opportunities for moderate-range electric vehicles using sustainable sodium-ion batteries. Nat. Energy 2023, 8, 215–218. [Google Scholar] [CrossRef]
- Barpanda, P.; Lander, L.; Nishimura, S.I.; Yamada, A. Polyanionic insertion materials for sodium-ion batteries. Adv. Energy Mater. 2018, 8, 1703055. [Google Scholar] [CrossRef]
- Zheng, C.; Ji, D.L.; Yao, Q.; Bai, Z.C.; Zhu, Y.S.; Nie, C.H.; Liu, D.; Wang, N.N.; Yang, J.; Dou, S.X. Electrostatic Shielding Boosts Electrochemical Performance of Alloy-Type Anode Materials of Sodium-Ion Batteries. Angew. Chem. Int. Ed. 2023, 62, e202214258. [Google Scholar] [CrossRef]
- Yao, Q.; Zheng, C.; Ji, D.L.; Du, Y.Z.; Su, J.; Wang, N.N.; Yang, J.; Dou, S.X.; Qian, Y.T. Superior sodiophilicity and molecule crowding of crown ether boost the electrochemical performance of all- climate sodium- ion batteries. Proc. Natl. Acad. Sci. USA 2024, 121, e2312337121. [Google Scholar] [CrossRef] [PubMed]
- Hou, D.W.; Gabriel, E.; Graf, K.; Li, T.Y.; Ren, Y.; Wang, Z.B.; Liu, Y.Z.; Xiong, H. Thermal dynamics of P2-Na0.67Ni0.33Mn0.67O2 cathode materials for sodium ion batteries studied by in situ analysis. J. Mater. Sci. 2022, 37, 1156–1163. [Google Scholar] [CrossRef]
- Graf, K.; Hou, D.W.; Gabriel, E.; Park, J.; Koisch, A.; Schrock, R.; Conrado, A.; Schwartz, D.; Gutierrez, A.; Johnson, C.S.; et al. Tailoring P2/P3-Intergrowth in Manganese-Based Layered Transition Metal Oxide Positive Electrodes via Sodium Content for Na-Ion Batteries. Chem. Electro. Chem. 2025, 12, e202400662. [Google Scholar] [CrossRef]
- Li, W.J.; Han, C.; Cheng, G.; Chou, S.L.; Liu, H.K.; Dou, S.X. Chemical properties, structural properties, and energy storage applications of Prussian blue analogues. Small 2019, 15, 1900470. [Google Scholar] [CrossRef]
- Qin, M.S.; Ren, W.H.; Jiang, R.X.; Li, Q.; Yao, X.H.; Wang, S.Q.; You, Y.; Mai, L.Q. Highly Crystallized Prussian Blue with Enhanced Kinetics for Highly Efficient Sodium Storage. ACS Appl. Mater. Interfaces 2021, 13, 3999–4007. [Google Scholar] [CrossRef]
- Gao, Y.; Zhang, H.; Liu, X.H.; Yang, Z.; He, X.X.; Li, L.; Qiao, Y.; Chou, S.L. Low-cost polyanion-type sulfate cathode for sodium-ion battery. Adv. Energy Mater. 2021, 11, 2101751. [Google Scholar] [CrossRef]
- Hao, Z.Q.; Shi, X.Y.; Yang, Z.; Zhou, X.Z.; Li, L.; Ma, C.Q.; Chou, S.L. The distance between phosphate-based polyanionic compounds and their practical application for sodium-ion batteries. Adv. Mater. 2024, 36, 2305135. [Google Scholar] [CrossRef]
- Hadouchi, M.; Hou, J.R.; Koketsu, T.; Lahmar, A.; Ma, J.W. Fluorophosphates and fluorosulfates cathode materials: Progress towards high energy density sodium-ion battery. Nano Res. 2023, 17, 1427–1440. [Google Scholar] [CrossRef]
- Zhang, H.; Cao, Y.J.; Liu, Z.L.; Cheng, X.S.; Li, X.L.; Xu, J.; Wang, N.; Yang, H.; Liu, Y.; Zhang, J.X. Structurally modulated Na4-xFe3-xVx(PO4)2P2O7 by vanadium doping for long-life sodium-ion batteries. ACS Sustain. Chem. Eng. 2024, 12, 5310–5318. [Google Scholar] [CrossRef]
- Yuan, T.C.; Wang, Y.X.; Zhang, J.X.; Pu, X.J.; Ai, X.P.; Chen, Z.X.; Yang, H.X.; Cao, Y.L. 3D graphene decorated Na4Fe3(PO4)2(P2O7) microspheres as low-cost and high-performance cathode materials for sodium-ion batteries. Nano Energy 2019, 56, 160–168. [Google Scholar] [CrossRef]
- Zhao, A.L.; Yuan, T.C.; Li, P.; Liu, C.Y.; Cong, H.J.; Pu, X.J.; Chen, Z.X.; Ai, X.P.; Yang, H.X.; Cao, Y.L. A novel Fe-defect induced pure-phase Na4Fe2.91(PO4)2P2O7 cathode material with high capacity and ultra-long lifetime for low-cost sodium-ion batteries. Nano Energy 2022, 91, 106680. [Google Scholar] [CrossRef]
- Chen, Y.; Su, Y.F.; Zhang, Y.X.; Lv, Z.K.; Xie, C.; Sun, W.B.; Zhao, Y.; Xie, M. Insights into iron-based polyanionic cathodes for scale-energy storage. Energy Storage Mater. 2024, 72, 103722. [Google Scholar] [CrossRef]
- Wang, L.; Chen, H.L.; Zhang, Y.X.; Liu, J.Y.; Peng, L. Research Progress in Strategies for Enhancing the Conductivity and Conductive Mechanism of LiFePO4 Cathode Materials. Molecules 2024, 29, 5250. [Google Scholar] [CrossRef]
- Akhmetova, K.; Sultanov, F.; Mentbayeva, A.; Umirov, N.; Bakenov, Z.; Tatykayev, B. Advances in multi-element doping of LiFePO4 cathode material for capacity enhancement in Li-ion batteries. J. Power Sources 2024, 624, 235531. [Google Scholar] [CrossRef]
- Yi, T.F.; Peng, P.P.; Fang, Z.K.; Zhu, Y.R.; Xie, Y.; Luo, S.H. Carbon-coated LiMn1-xFexPO4 (0 ≤ x ≤ 0.5) nanocomposites as high-performance cathode materials for Li-ion battery. Compos. Part B 2019, 175, 107067. [Google Scholar] [CrossRef]
- Fang, K.B.; Zhu, J.H.; Xie, Q.; Men, Y.F.; Yang, W.; Li, J.P.; Yu, X.W. Synthesis of Fe2+ Substituted High-Performance LiMn1-xFexPO4/C (x = 0, 0.1, 0.2, 0.3, 0.4) Cathode Materials for Lithium-Ion Batteries via Sol-Gel Processes. Molecules 2021, 26, 7641. [Google Scholar] [CrossRef]
- Sun, K.; Luo, S.H.; Du, N.Y.; Wei, Y.; Yan, S.X. Research progress of lithium manganese iron phosphate cathode materials: From preparation to modification. Electroanalysis 2024, 36, 00120. [Google Scholar] [CrossRef]
- Li, G.D.; Zhang, L.J.; Xu, Z.M.; Liu, Y.; Zhang, K.; Zhao, S.Y.; Cao, Y.J.; Xia, Y.Y. Constructing Internal Coupling Reactions Through Iron Substitution to Facilitate Manganese-Based Mixed-Phosphate Cathode for Sodium-Ion Storage. Adv. Funct. Mater. 2025, 2425598. [Google Scholar] [CrossRef]
- Fei, W.B.; Wang, Y.; Zhang, X.P.; Zhang, J.X.; Lu, S.X.; Rao, K.X.; Sun, K.Y.; Deng, M.T.; Liu, Y.X.; Li, Q.Q.; et al. A novel bimetallic-polyanion Na4Fe2.82Ni0.18(PO4)2P2O7 cathode with superior rate performance and long cycle-life for sodium-ion batteries. Chem. Eng. J. 2024, 493, 152523. [Google Scholar] [CrossRef]
- Li, X.Q.; Zhang, J.H.; Zhang, Y.; Zhang, B.L.; Liu, H.M.; Xu, Q.J.; Xia, Y.Y. A facile Ball-Milling preparation strategy of Nitrogen-Doped carbon coated Na4Fe3(PO4)2P2O7 Nano-Flakes with superior sodium ion storage performance. Chem. Eng. J. 2022, 260, 117951. [Google Scholar] [CrossRef]
- Qi, X.R.; Dong, Q.Y.; Dong, H.H.; Hou, B.X.; Liu, H.Y.; Shang, N.Z.; Zhang, S.H.; Wang, L.G.; Shao, H.; Shen, Y.B.; et al. Copper-induced lattice distortion in Na4Fe3(PO4)2(P2O7) cathode enabling high power density Na-ion batteries with good cycling stability. Energy Storage Mater. 2024, 73, 103861. [Google Scholar] [CrossRef]
- Li, X.Q.; Zhang, Y.; Zhang, B.L.; Qin, K.; Liu, H.M.; Ma, Z.F. Mn-doped Na4Fe3(PO4)2(P2O7) facilitating Na+ migration at low temperature as a high performance cathode material of sodium ion batteries. J. Power Sources 2022, 521, 230922. [Google Scholar] [CrossRef]
- Tao, Q.D.; Ding, H.Y.; Tang, X.; Zhang, K.B.; Teng, J.H.; Zhao, H.M.; Li, J. Mn-Doped Na4Fe3(PO4)2P2O7 as a Low-Cost and High-Performance Cathode Material for Sodium-Ion Batteries. Energy Fuels 2023, 37, 6230–6239. [Google Scholar] [CrossRef]
- Wu, H.L.; Wen, T.Z.; Chen, L.; Ding, Y.; Pu, X.J.; Cao, Y.L.; Chen, Z.X. Understanding the Role of Mn Substitution for Boosting High-Voltage Na4Fe3-xMnx(PO4)2P2O7 Cathode in Sodium-Ion Batteries. Small Methods 2024, 9, e2400642. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.Y.; Li, H.M.; Wang, Z.H.; Liu, M.Z.; Lu, Y.Y.; Zhang, Y.; Li, J.J.; Ding, K.; Liu, H.M.; Ma, Z.F.; et al. High Entropy Helps Na4Fe3(PO4)2P2O7 Improve Its Sodium Storage Performance. Adv. Funct. Mater. 2024, 35, 2. [Google Scholar] [CrossRef]
- Sun, C.; Ni, Q.; Li, M.; Sun, Z.; Yuan, X.Y.; Li, L.; Wang, K.Y.; Jin, H.B.; Zhao, Y.J. Improving Rate Performance by Inhibiting Jahn-Teller Effect in Mn-Based Phosphate Cathode for Na-Ion Batteries. Adv. Funct. Mater. 2024, 34, 7. [Google Scholar] [CrossRef]
- Wang, Y.S.; Liu, J.; Lee, B.; Qiao, R.B.; Yang, Z.Z.; Xu, S.Y.; Yu, X.Q.; Gu, L.; Hu, Y.S.; Yang, W.L.; et al. Ti-substituted tunnel-type Na0.44MnO2 oxide as a negative electrode for aqueous sodium-ion batteries. Nat. Commun. 2015, 6, 6401. [Google Scholar] [CrossRef]
- Xu, S.Y.; Wang, Y.S.; Ben, L.B.; Lyu, Y.C.; Song, N.N.; Yang, Z.Z.; Li, Y.M.; Mu, L.Q.; Yang, H.T.; Gu, L.; et al. Fe-Based Tunnel-Type Na0.61[Mn0.27Fe0.34Ti0.39]O2 Designed by a New Strategy as a Cathode Material for Sodium-Ion Batteries. Adv. Energy Mater. 2015, 5, 22. [Google Scholar] [CrossRef]
- Darbar, D.; Muralidharan, N.; Hermann, R.P.; Nanda, J.; Bhattacharya, I. Hermann Evaluation of electrochemical performance and redox activity of Fe in Ti doped layered P2-Na0.67Mn0.5Fe0.5O2 cathode for sodium ion batteries. Electrochim. Acta 2021, 380, 138156. [Google Scholar] [CrossRef]
- Zhao, C.L.; Wang, Q.D.; Yao, Z.P.; Wang, J.L.; Ding, F.X.; Qi, X.G.; Lu, Y.X.; Bai, X.D.; Li, B.H.; Li, H.; et al. Rational design of layered oxide materials for sodium-ion batteries. Science 2020, 370, 708–711. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.Y.; Wu, Z.; Su, J.; Long, Y.F.; Lv, X.Y.; Wen, Y.X. Synthesis and electrochemical performance of Ti-Fe co-doped LiMnPO4/C as cathode material for lithium-ion batteries. Ceram. Int. 2016, 42, 11348–11354. [Google Scholar] [CrossRef]
- Zhao, L.N.; Zhao, H.L.; Long, X.Y.; Li, Z.L.; Du, Z.H. Superior High-Rate and Ultra long-Life span Na3V2(PO4)3@C Cathode by Enhancing the Conductivity Both in Bulk and on Surface. ACS Appl. Mater. Interfaces 2018, 10, 42. [Google Scholar]
- Zhang, J.S.; Lin, C.F.; Xia, Q.B.; Wang, C.; Zhao, X.S. Improved Performance of Na3TiMn(PO4)3 Using a Non-stoichiometric Synthesis Strategy. ACS Energy Lett. 2021, 6, 6. [Google Scholar] [CrossRef]
- Yin, Q.M.; Gu, Z.Y.; Liu, Y.; Lv, H.Y.; Liu, Y.T.; Liu, Y.N.; Su, M.Y.; Guo, J.Z.; Wu, X.L. Mn-Rich Phosphate Cathode for Sodium-Ion Batteries: Anion-Regulated Solid Solution Behavior and Long-Term Cycle Life. Adv. Funct. Mater. 2023, 33, 2304046. [Google Scholar] [CrossRef]
- Shahzad, H.; Goutam, D.; Aktar, M.S.; Hasan, M.K.; Zakaria, A.K.M.; Datta, T.K.; Kamal, I.; Yunus, S.M.; Ahsan, M.H.; Azad, A.K. Line profiles of neutron powder-diffraction peaks for structure refinement. Acta Crystallogr. 1967, 22, 151–152. [Google Scholar]
- Xu, C.L.; Zhao, J.M.; Yang, C.; Hu, Y.S. Polyanionic Cathode Materials for Practical Na-Ion Batteries toward High Energy Density and Long Cycle Life. ACS Central Sci. 2023, 9, 1721–1736. [Google Scholar] [CrossRef]
- Jin, T.; Li, H.X.; Zhu, K.J.; Wang, P.H.; Liu, P.; Jiao, L.F. Polyanion-type cathode materials for sodium-ion batteries. Chem. Soc. Rev. 2020, 49, 2342–2377. [Google Scholar] [CrossRef]
- Yang, T.T.; Huang, Y.L.; Zhang, J.; Zhu, H.; Ren, J.C.; Li, T.Y.; Gallington, L.C.; Lan, S.; Yang, L.G.; Liu, Q. Insights into Ti doping for stabilizing the Na2/3Fe1/3Mn2/3O2 cathode in sodium ion battery. J. Energy Chem. 2022, 73, 542–548. [Google Scholar] [CrossRef]
- Lin, Z.Y.; Waller, G.; Liu, Y.; Liu, M.L.; Wong, C.P. Facile Synthesis of Nitrogen-Doped Graphene via Pyrolysis of Graphene Oxide and Urea, and its Electrocatalytic Activity toward the OxygenReduction Reaction. Adv. Energy Mater. 2012, 2, 884–888. [Google Scholar] [CrossRef]
- Shen, B.L.; Xu, M.W.; Niu, Y.B.; Han, J.; Lu, S.Y.; Jiang, J.; Li, Y.; Dai, C.L.; Hu, L.Y.; Li, C.M. Sodium-Rich ferric pyrophosphate cathode for stationary room-temperature sodium-ion batteries. ACS Appl. Mater. Interfaces 2018, 10, 502–508. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.X.; Ma, J.X.; He, X.X.; Sun, J.; Yang, L.H.; Jiang, R.B.; Lei, Z.B.; Liu, Z.H.; Li, Q. Single-crystalline Mg-substituted Na4Mn3(PO4)2P2O7 nanoparticles as a high capacity and superior cycling cathode for sodium-ion batteries. Nanoscale 2023, 15, 4830–4838. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Wang, R.Q.; Jiang, M.Q.; Zhang, J.X. Electrochemical properties of mixed-phosphates Nax+2Fex+1(PO4)x(P2O7) with different ratios of PO43−/P2O74−. J. Alloys Compd. 2021, 870, 159382. [Google Scholar] [CrossRef]
- Chen, Y.H.; Fan, Q.H.; Li, J.J.; Chen, M.; Jin, H.L.; Wen, N.; Dong, Y.Z.; Kuang, Q.; Zhao, Y.M. Preparation and electrochemical performance of nanowire-shaped Na3Mn2-xFex(P2O7)(PO4) for sodium-ion and lithium-ion batteries. Daltont 2022, 51, 4173–4181. [Google Scholar] [CrossRef]
- Fei, W.B.; Sui, Y.L.; Wang, Y.; Sun, K.Y.; Zhang, X.P.; Deng, M.T.; Tao, C.D.; Liu, L.Z.; Wang, R.H.; Wu, L. Regulating Na/Mn Antisite Defects and Reactivating Anomalous Jahn-Teller Behavior for Na4Fe1.5Mn1.5(PO4)2(P2O7) Cathode Material with Superior Performance. ACS Nano 2025, 19, 8. [Google Scholar] [CrossRef]
- Kagesawa, K.; Hosono, E.; Okubo, M.; Hamane, D.N.; Kudo, T.; Zhou, H.S. Electrochemical properties of LiMnxFe1-xPO4 (x = 0, 0.2, 0.4, 0.6, 0.8 and 1.0)/vapor grown carbon fiber coreesheath composite nanowire synthesized by electrospinning method. J. Power Sources 2014, 248, 615–620. [Google Scholar] [CrossRef]
- Chen, Y.; Peng, P.; Sun, K.Y.; Wu, L.; Zheng, J.W. Stabilizing NASICON-type Na4MnCr(PO4)3 by Ti-substitution toward a high-voltage cathode material for sodium ion batteries. J. Colloid Interface Sci. 2024, 671, 385–393. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Pang, G.; Zhang, W.; Zhang, Q.; Hou, L.; Yuan, C. Ti-Doped, Mn-Based Polyanionic Compounds of Na4Fe1.2Mn1.8(PO4)2P2O7 for Sodium-Ion Battery Cathode. Nanomaterials 2025, 15, 581. https://doi.org/10.3390/nano15080581
Li H, Pang G, Zhang W, Zhang Q, Hou L, Yuan C. Ti-Doped, Mn-Based Polyanionic Compounds of Na4Fe1.2Mn1.8(PO4)2P2O7 for Sodium-Ion Battery Cathode. Nanomaterials. 2025; 15(8):581. https://doi.org/10.3390/nano15080581
Chicago/Turabian StyleLi, Hualin, Gang Pang, Weilong Zhang, Qingan Zhang, Linrui Hou, and Changzhou Yuan. 2025. "Ti-Doped, Mn-Based Polyanionic Compounds of Na4Fe1.2Mn1.8(PO4)2P2O7 for Sodium-Ion Battery Cathode" Nanomaterials 15, no. 8: 581. https://doi.org/10.3390/nano15080581
APA StyleLi, H., Pang, G., Zhang, W., Zhang, Q., Hou, L., & Yuan, C. (2025). Ti-Doped, Mn-Based Polyanionic Compounds of Na4Fe1.2Mn1.8(PO4)2P2O7 for Sodium-Ion Battery Cathode. Nanomaterials, 15(8), 581. https://doi.org/10.3390/nano15080581









