Synthesis of High-Entropy Oxide Nanopowders with Different Crystal Structures by Electrical Explosion of Wires
Abstract
1. Introduction
2. Materials and Methods
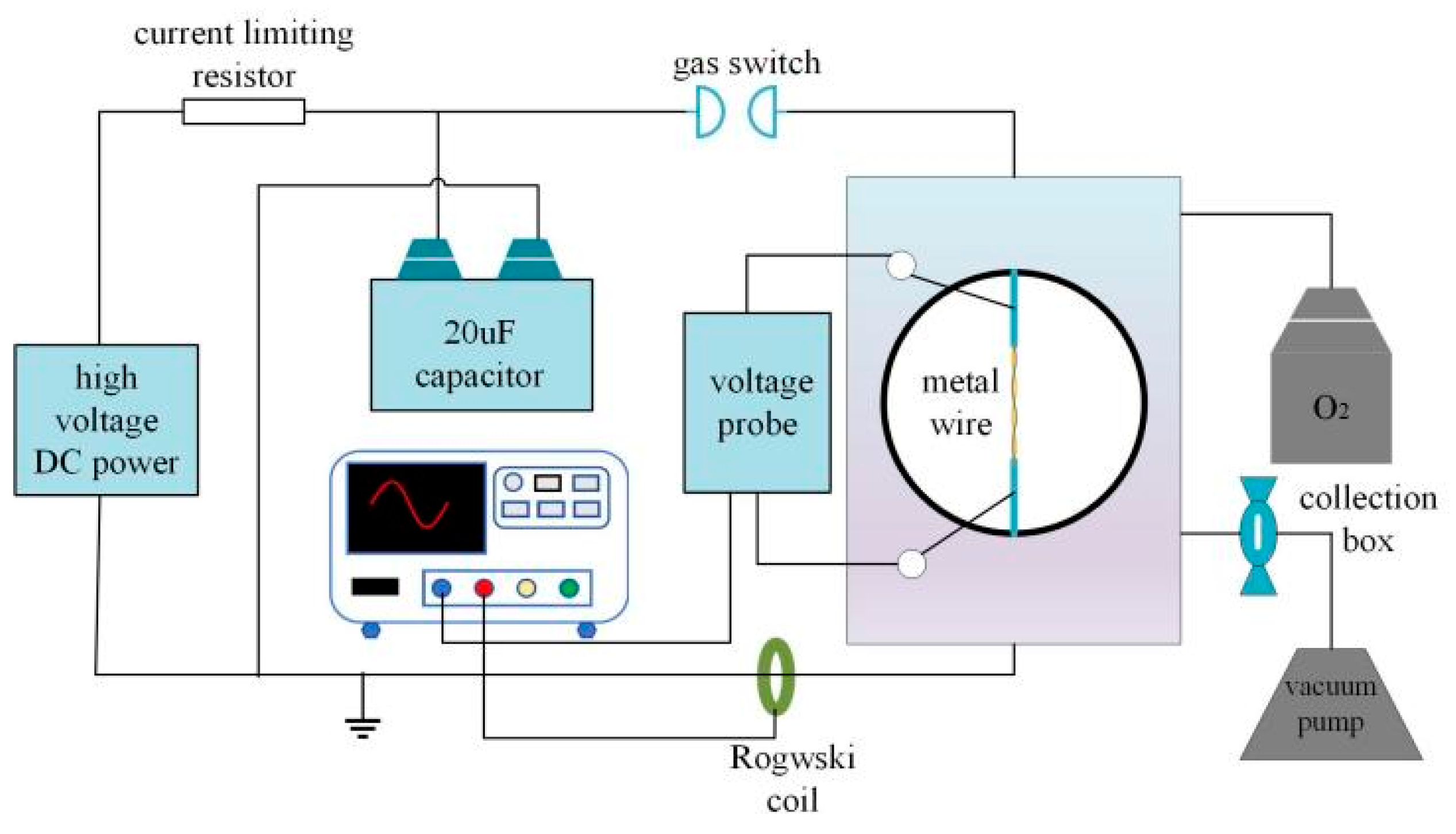
2.1. Experimental Setup
2.2. Materials
3. Results
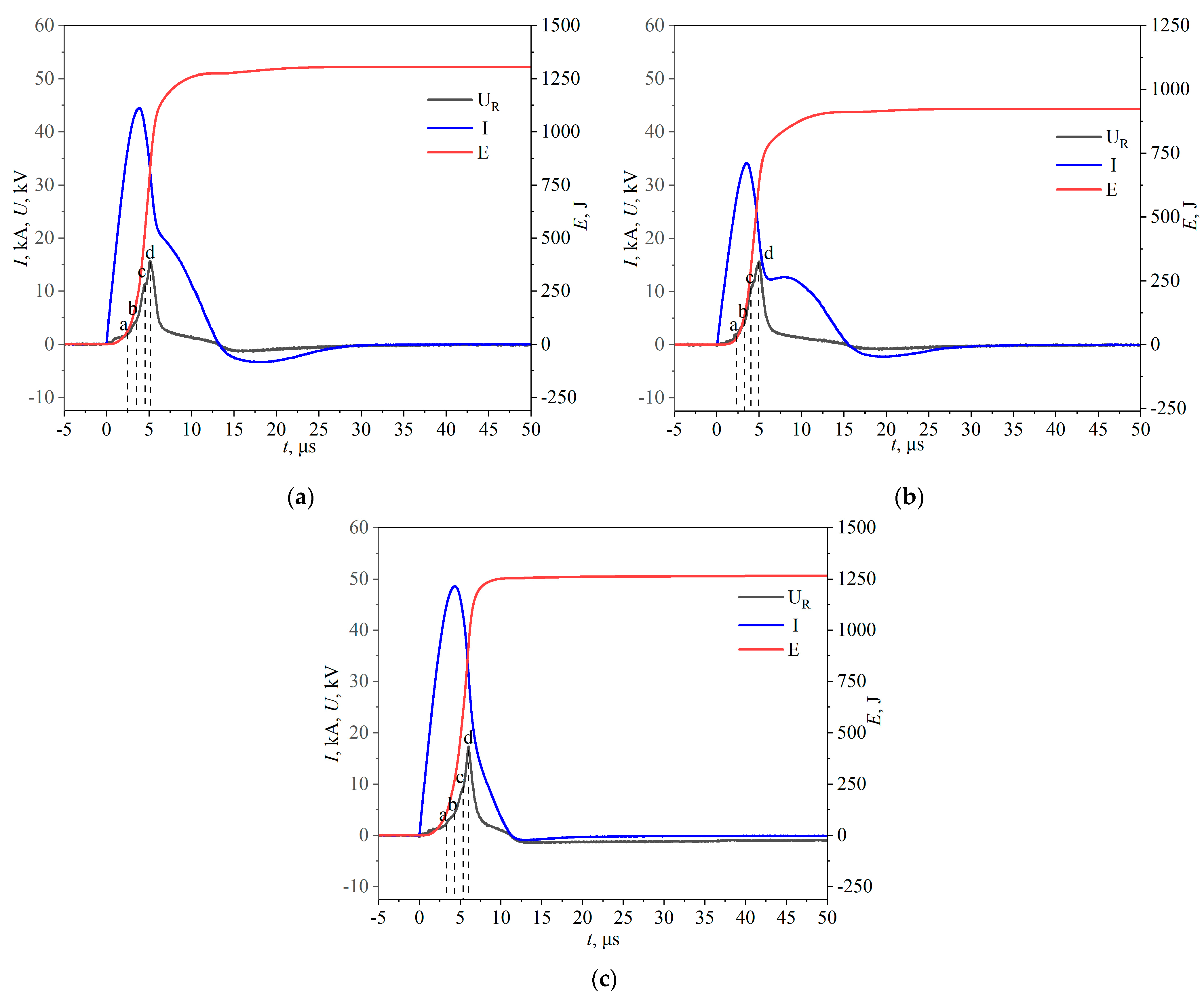
3.1. EEW Results
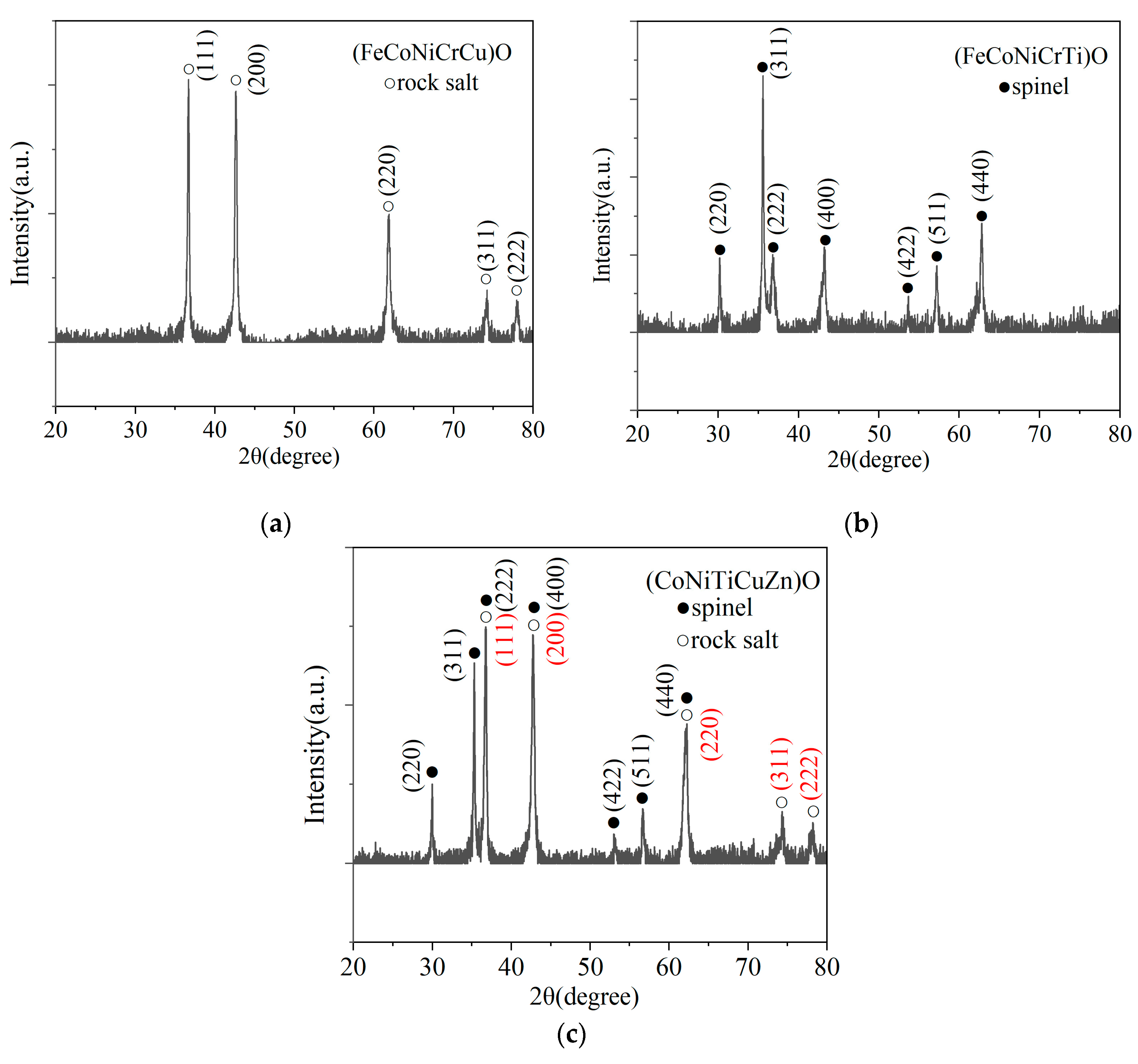
3.2. XRD Data
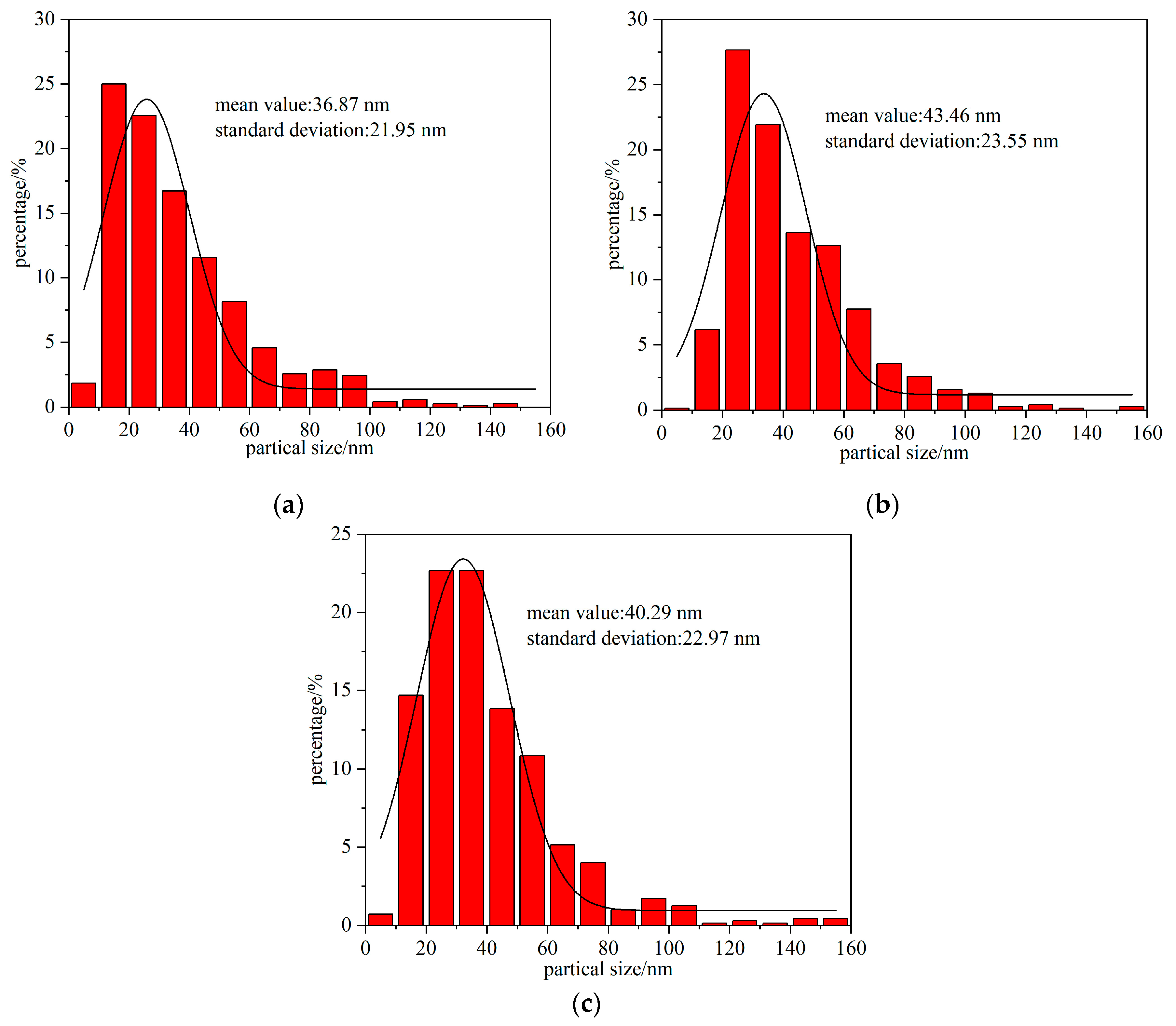
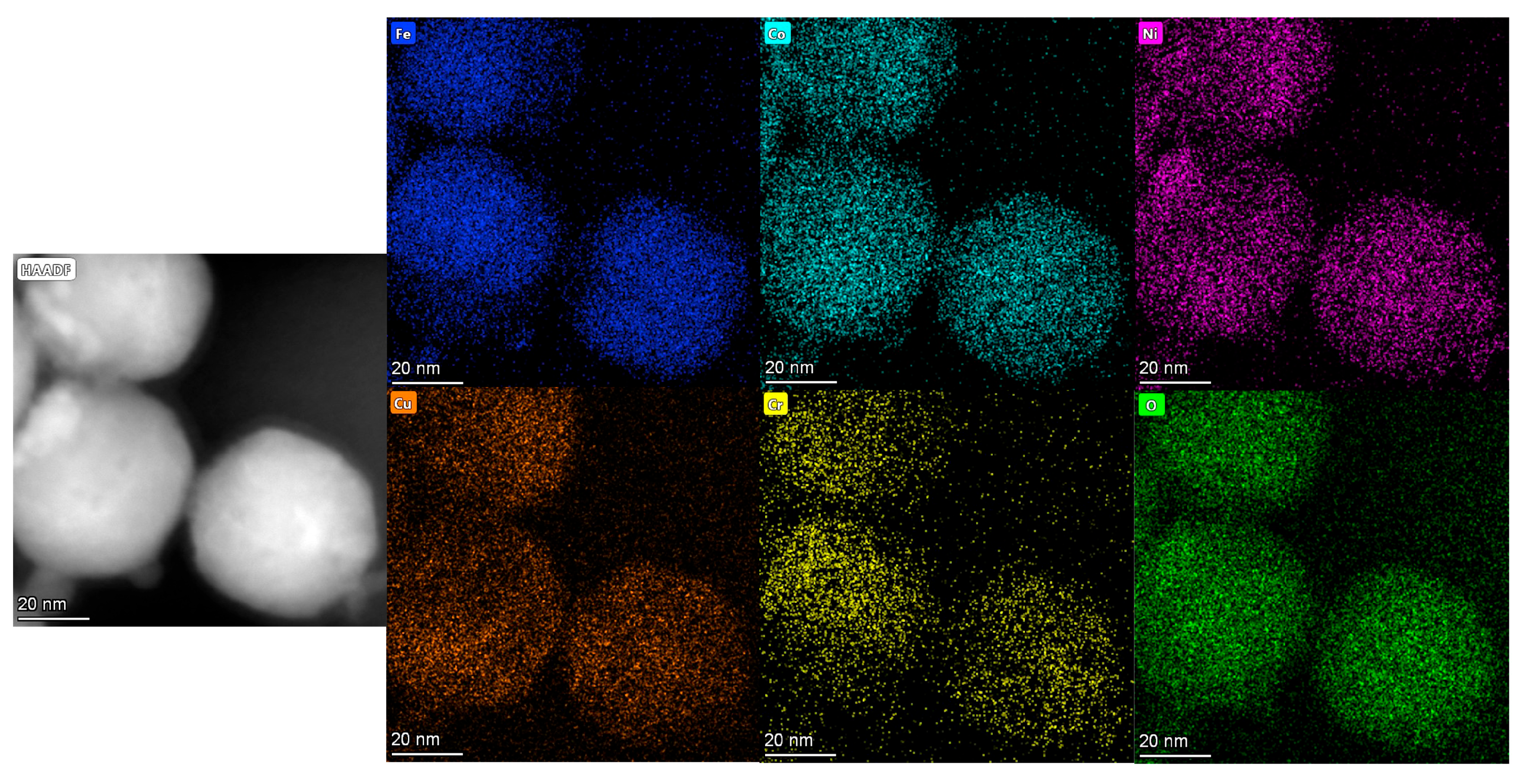
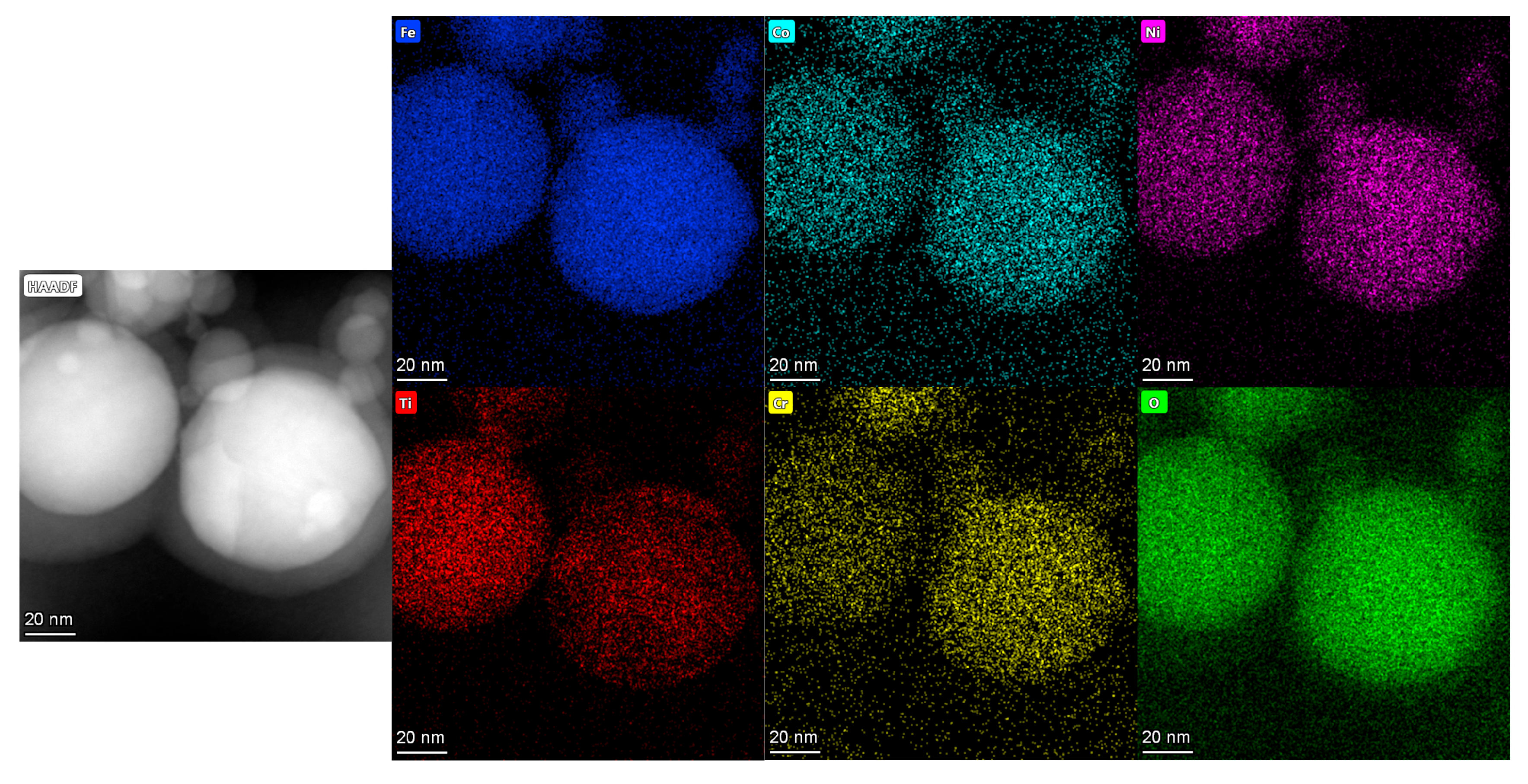
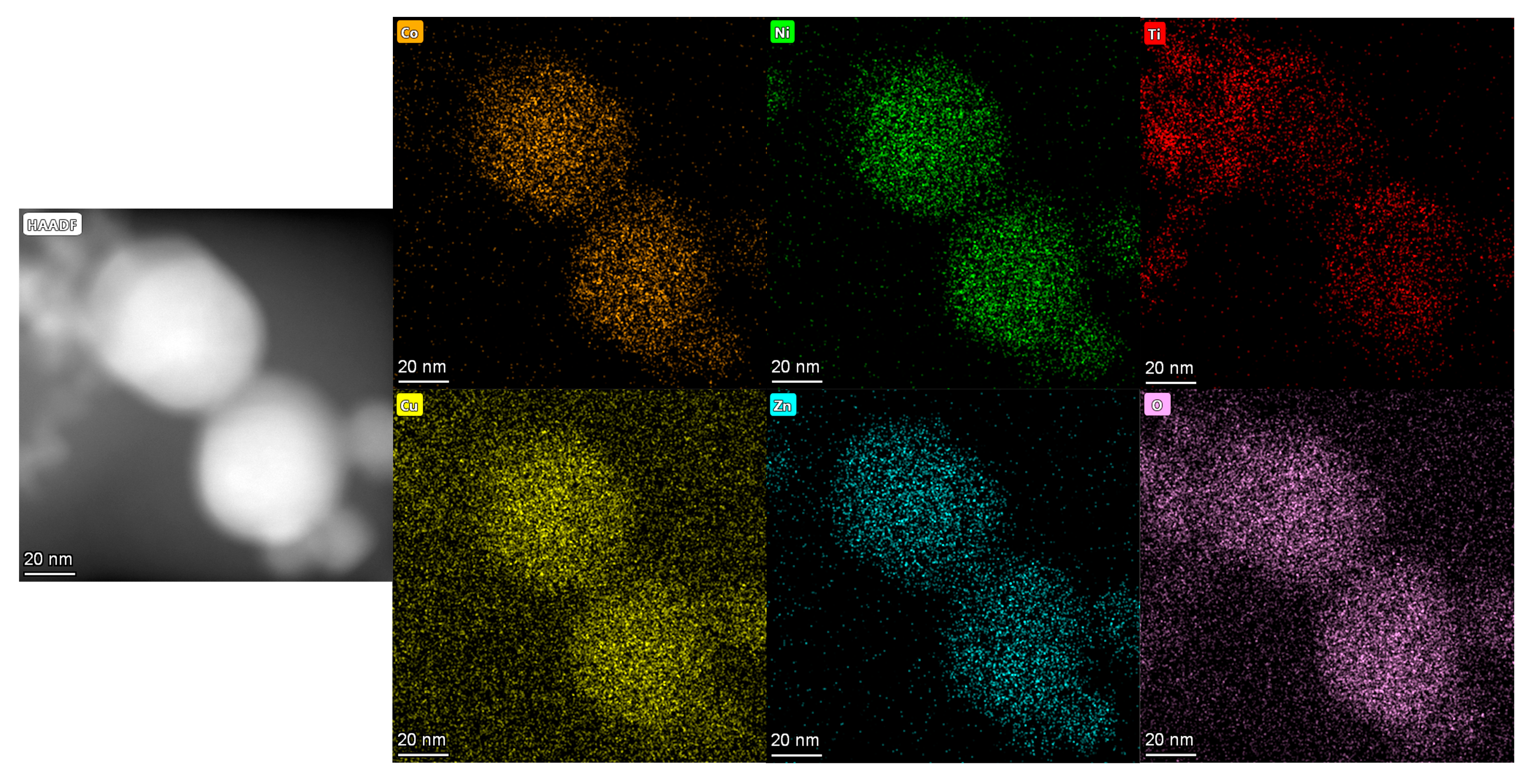
3.3. TEM Data
4. Discussion
4.1. Synthesis of Three Other HEOs with Different Crystal Structures by EEW
4.2. Influence of Component Elements on Crystal Structures
5. Conclusions
- The high-entropy oxide nanopowders with different crystal structures are successfully synthesized by EEW for the first time. (FeCoNiCrCu)O and (FeCoNiCuZn)O are rock salt structures, (FeCoNiCrTi)O and (FeCoNiTiZn)O are spinel structures, and (CoNiTiCuZn)O and (FeNiTiCuZn)O contain both rock salt and spinel phases;
- The waveforms of the EEW process demonstrate that the electrical explosions of the individual wires are approximately synchronized, and the deposition energy is sufficient to completely evaporate the wires;
- The TEM images demonstrate that the high-entropy oxides synthesized by the EEW method have a logarithmic distribution of particle sizes, with the vast majority of the particles below 100 nm and a small standard deviation. The distribution of each component element is uniform, and there is no enrichment;
- The crystal structure of high-entropy oxides is significantly influenced by the kind of metallic elements. In the EEW synthesis method, the element Cu tends to form rock salt structure, while the elements Ti tend to form spinel structure. HEOs have a dual-phase structure in the presence of both Cu and Ti elements.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhu, H.; Xie, H.; Zhao, Y.; Dai, S.; Li, M.; Wang, X. Structure and magnetic properties of a class of spinel high-entropy oxides. J. Magn. Magn. Mater. 2021, 535, 168063. [Google Scholar] [CrossRef]
- Yeh, J.W.; Chen, S.K.; Lin, S.J.; Gan, J.Y.; Chin, T.S.; Shun, T.T.; Tsau, C.H.; Chang, S.Y. Nanostructured High-Entropy Alloys with Multiple Principal Elements: Novel Alloy Design Concepts and Outcomes. Adv. Eng. Mater. 2004, 6, 299–303. [Google Scholar] [CrossRef]
- Dastanpour, E.; Huang, S.; Schönecker, S.; Mao, H.; Ström, V.; Eriksson, O.; Varga, L.K.; Vitos, L. On the structural and magnetic properties of Al-rich high entropy alloys: A joint experimental-theoretical study. J. Phys. D Appl. Phys. 2023, 56, 015003. [Google Scholar] [CrossRef]
- Vinnik, D.A.; Trofimov, E.A.; Zhivulin, V.E.; Gudkova, S.A.; Zaitseva, O.V.; Zherebtsov, D.A.; Starikov, A.Y.; Sherstyuk, D.P.; Amirov, A.A.; Kalgin, A.V.; et al. High Entropy Oxide Phases with Perovskite Structure. Nanomaterials 2020, 10, 268. [Google Scholar] [CrossRef]
- Zhivulin, V.E.; Trofimov, E.A.; Gudkova, S.A.; Pashkeev, I.Y.; Punda, A.Y.; Gavrilyak, M.; Zaitseva, O.V.; Taskaev, S.V.; Podgornov, F.V.; Darwish, M.A.; et al. Polysubstituted High-Entropy [LaNd](Cr0.2Mn0.2Fe0.2Co0.2Ni0.2)O3 Perovskites: Correlation of the Electrical and Magnetic Properties. Nanomaterials 2021, 11, 1014. [Google Scholar] [CrossRef]
- Surdu, V.-A.; Marinică, M.-A.; Pătru, R.-E.; Oprea, O.-C.; Nicoară, A.I.; Vasile, B.Ș.; Trușca, R.; Ianculescu, A.-C. High-Entropy Lead-Free Perovskite Bi0.2K0.2Ba0.2Sr0.2Ca0.2TiO3 Powders and Related Ceramics: Synthesis, Processing, and Electrical Properties. Nanomaterials 2023, 13, 2974. [Google Scholar] [CrossRef]
- Gild, J.; Samiee, M.; Braun, J.L.; Harrington, T.; Vega, H.; Hopkins, P.E.; Vecchio, K.; Luo, J. High-entropy fluorite oxides. J. Eur. Ceram. Soc 2018, 38, 3578–3584. [Google Scholar] [CrossRef]
- Mao, A.; Quan, F.; Xiang, H.-Z.; Zhang, Z.-G.; Kuramoto, K.; Xia, A.-L. Facile synthesis and ferrimagnetic property of spinel (CoCrFeMnNi)3O4 high-entropy oxide nanocrystalline powder. J. Mol. Struct. 2019, 1194, 11–18. [Google Scholar] [CrossRef]
- Ren, X.; Sun, W.; Sheng, Z.; Liu, M.; Hui, H.; Xiao, Y. Effects of Nano-CeO2 on Microstructure and Properties of WC/FeCoNiCrMo0.2 Composite High Entropy Alloy Coatings by Laser Cladding. Nanomaterials 2023, 13, 1104. [Google Scholar] [CrossRef]
- Sarkar, A.; Velasco, L.; Wang, D.; Wang, Q.; Talasila, G.; de Biasi, L.; Kübel, C.; Brezesinski, T.; Bhattacharya, S.S.; Hahn, H.; et al. High entropy oxides for reversible energy storage. Nat. Commun. 2018, 9, 3400. [Google Scholar] [CrossRef]
- Rost, C.M.; Sachet, E.; Borman, T.; Moballegh, A.; Dickey, E.C.; Hou, D.; Jones, J.L.; Curtarolo, S.; Maria, J.-P. Entropy-stabilized oxides. Nat. Commun. 2015, 6, 8485. [Google Scholar] [CrossRef] [PubMed]
- Rost, C.M.; Rak, Z.; Brenner, D.W.; Maria, J.-P. Local structure of the MgNiCoCuZnO(x=0.2) entropy-stabilized oxide: An EXAFS study. J. Am. Ceram. Soc. 2017, 100, 2732–2738. [Google Scholar] [CrossRef]
- Djenadic, R.; Sarkar, A.; Clemens, O.; Loho, C.; Botros, M.; Chakravadhanula, V.S.K.; Kübel, C.; Bhattacharya, S.S.; Gandhi, A.S.; Hahn, H. Multicomponent equiatomic rare earth oxides. Mater. Res. Lett. 2017, 5, 102–109. [Google Scholar] [CrossRef]
- Xu, Y.; Xu, X.; Bi, L. A high-entropy spinel ceramic oxide as the cathode for proton-conducting solid oxide fuel cells. J. Adv. Ceram. 2022, 11, 794–804. [Google Scholar] [CrossRef]
- Vinnik, D.A.; Zhivulin, V.E.; Trofimov, E.A.; Gudkova, S.A.; Punda, A.Y.; Valiulina, A.N.; Gavrilyak, M.; Zaitseva, O.V.; Taskaev, S.V.; Khandaker, M.U.; et al. A-Site Cation Size Effect on Structure and Magnetic Properties of Sm(Eu,Gd)Cr0.2Mn0.2Fe0.2Co0.2Ni0.2O3 High-Entropy Solid Solutions. Nanomaterials 2022, 12, 36. [Google Scholar] [CrossRef]
- Qiu, N.; Chen, H.; Yang, Z.; Sun, S.; Wang, Y.; Cui, Y. A high entropy oxide (Mg0.2Co0.2Ni0.2Cu0.2Zn0.2O) with superior lithium storage performance. J. Alloys Compd. 2019, 777, 767–774. [Google Scholar] [CrossRef]
- Dąbrowa, J.; Stygar, M.; Mikuła, A.; Knapik, A.; Mroczka, K.; Tejchman, W.; Danielewski, M.; Martin, M. Synthesis and microstructure of the (Co,Cr,Fe,Mn,Ni)3O4 high entropy oxide characterized by spinel structure. Mater. Lett. 2018, 216, 32–36. [Google Scholar] [CrossRef]
- Wang, Q.; Sarkar, A.; Wang, D.; Velasco, L.; Azmi, R.; Bhattacharya, S.S.; Bergfeldt, T.; Düvel, A.; Heitjans, P.; Brezesinski, T.; et al. Multi-anionic and -cationic compounds: New high entropy materials for advanced Li-ion batteries. Energy Environ. Sci. 2019, 12, 2433–2442. [Google Scholar] [CrossRef]
- Liu, C.; Li, S.; Zheng, Y.; Xu, M.; Su, H.; Miao, X.; Liu, Y.; Zhou, Z.; Qi, J.; Yang, B.; et al. Advances in high entropy oxides: Synthesis, structure, properties and beyond. Prog. Mater. Sci. 2025, 148, 101385. [Google Scholar] [CrossRef]
- Pei, Y.; Chen, Q.; Xiao, Y.-C.; Liu, L.; Xu, C.-Y.; Zhen, L.; Henkelman, G.; Cao, G. Understanding the phase transitions in spinel-layered-rock salt system: Criterion for the rational design of LLO/spinel nanocomposites. Nano Energy 2017, 40, 566–575. [Google Scholar] [CrossRef]
- Martins, G.; Caixeta, F.; de Souza, V.; Gonçalves, R.; Dias, A. Reddish-orange emitting Eu2-xGdxGe2O7 pyrogermanate ceramic phosphors: Synthesis and role of Eu3+/Gd3+ substitution on the optical features. J. Phys. D Appl. Phys. 2023, 57, 105304. [Google Scholar] [CrossRef]
- Yu, P.F.; Zhang, L.J.; Cheng, H.; Zhang, H.; Ma, M.Z.; Li, Y.C.; Li, G.; Liaw, P.K.; Liu, R.P. The high-entropy alloys with high hardness and soft magnetic property prepared by mechanical alloying and high-pressure sintering. Intermetallics 2016, 70, 82–87. [Google Scholar] [CrossRef]
- Lozhkomoev, A.S.; Pervikov, A.V.; Kazantsev, S.O.; Suliz, K.V.; Veselovskiy, R.V.; Miller, A.A.; Lerner, M.I. Controlled Oxidation of Cobalt Nanoparticles to Obtain Co/CoO/Co3O4 Composites with Different Co Content. Nanomaterials 2022, 12, 2523. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Han, R.; Geng, J.; Gao, M.; Li, Q. One-step preparation of pure and Cu-decorated graphite thin layers via electrical explosion in a confined environment: Physical process and product characterization. Ceram. Int. 2022, 48, 19874–19881. [Google Scholar] [CrossRef]
- Tanaka, S.; Inao, D.; Hasegawa, K.; Hokamoto, K.; Chen, P.; Gao, X. Graphene Formation through Pulsed Wire Discharge of Graphite Strips in Water: Exfoliation Mechanism. Nanomaterials 2021, 11, 1223. [Google Scholar] [CrossRef]
- Li, C.; Han, R.; Li, J.; Cao, Y.; Yuan, W.; Li, Q. Nanomaterial Production from Metallic Vapor Bubble Collapse in Liquid Nitrogen. Nanomaterials 2023, 13, 2021. [Google Scholar] [CrossRef]
- Kinemuchi, Y.; Murai, K.; Sangurai, C.; Cho, C.-H.; Suematsu, H.; Jiang, W.; Yatsui, K. Nanosize Powders of Aluminum Nitride Synthesized by Pulsed Wire Discharge. J. Am. Ceram. Soc. 2003, 86, 420–424. [Google Scholar] [CrossRef]
- Kinemuchi, Y.; Sato, K.; Watari, K.; Cho, C.; Murai, K.; Suematsu, H.; Jiang, W.; Yatsui, K. Particle Size Distribution of SnO2 Nano-Particles Synthesized by Pulsed Wire Discharge. J. Ceram. Soc. Jpn. 2004, 112, 355–362. [Google Scholar] [CrossRef]
- Tkachenko, S.I.; Barishpoltsev, D.V.; Ivanenkov, G.V.; Romanova, V.M.; Ter-Oganesyan, A.E.; Mingaleev, A.R.; Shelkovenko, T.A.; Pikuz, S.A. Analysis of the discharge channel structure upon nanosecond electrical explosion of wires. Phys. Plasmas 2007, 14, 046406–046477. [Google Scholar] [CrossRef]
- Pervikov, A.V.; Pustovalov, A.V.; Afonnikova, S.D.; Bauman, Y.I.; Mishakov, I.V.; Vedyagin, A.A. Synthesis and structure of NiCu and NiAl electroexplosive nanoparticles for production of carbon nanofibers. Powder Technol. 2023, 415, 118164. [Google Scholar] [CrossRef]
- Pervikov, A.V.; Kazantsev, S.O.; Lozhkomoev, A.S.; Lerner, M.I. Bimetallic AlAg, AlCu and AlZn nanoparticles with controllable phase compositions prepared by the electrical explosion of two wires. Powder Technol. 2020, 372, 136–147. [Google Scholar] [CrossRef]
- Suliz, K.; Miller, A.; Ivanov, K.; Pervikov, A. Synthesizing multicomponent AlCrFeCuNi nanoparticles by joint electrical explosion of wires. Powder Technol. 2022, 404, 117491. [Google Scholar] [CrossRef]
- Liang, L.; Wu, J.; Yin, Z.; Kong, C.; Pervikov, A.; Shi, H.; Li, X.; Qiu, A. Synthesis of FCC structure Fe10Co25Ni34Cu23Al8 high-entropy-alloy nanoparticles by electrical wire explosion: For electromagnetic wave absorption. Appl. Phys. Lett. 2024, 124, 053502. [Google Scholar] [CrossRef]
- Weihua, J.; Yatsui, K. Pulsed wire discharge for nanosize powder synthesis. IEEE Trans. Plasma Sci. 1998, 26, 1498–1501. [Google Scholar] [CrossRef]
- Égerházi, L.; Kovács, B.; Szörényi, T. Spectroscopic quantification of the nanoparticle production efficiency of copper wire explosion. J. Appl. Phys. 2021, 129, 195902. [Google Scholar] [CrossRef]
- An, V.; Potgieter, H.; Usoltseva, N.; Valiev, D.; Stepanov, S.; Pustovalov, A.; Baryshnikov, A.; Titov, M.; Dolinina, A. MoS2@ZnO Nanoheterostructures Prepared by Electrospark Erosion for Photocatalytic Applications. Nanomaterials 2021, 11, 157. [Google Scholar] [CrossRef]
- Wang, Q.; Yang, H.; Shi, J.; Zou, G. One-step synthesis of the nanometer particles of γ-Fe2O3 by wire electrical explosion method. Mater. Res. Bull. 2001, 36, 503–509. [Google Scholar] [CrossRef]
- Bakina, O.; Glazkova, E.; Rodkevich, N.; Mosunov, A.; Chzhou, V.; Lerner, M. Electroexplosive synthesis of composite ZnO/ZnFe2O4/Zn nanoparticles with photocatalytic and antibacterial activity. Mater. Sci. Semicond. Process. 2022, 152, 107076. [Google Scholar] [CrossRef]
- Glazkova, E.; Bakina, O.; Rodkevich, N.; Mosunov, A.; Evstigneev, M.; Evstigneev, V.; Klimenko, V.; Lerner, M. Antibacterial Properties of PMMA Functionalized with CuFe2O4/Cu2O/CuO Nanoparticles. Coatings 2022, 12, 957. [Google Scholar] [CrossRef]
- Pervikov, A.; Glazkova, E.; Lerner, M. Energy characteristics of the electrical explosion of two intertwined wires made of dissimilar metals. Phys. Plasmas 2018, 25, 070701. [Google Scholar] [CrossRef]
- Liu, G.; Lu, Z.; Zhang, X. Nano-Structure Evolution and Mechanical Properties of AlxCoCrFeNi2.1 (x = 0, 0.3, 0.7, 1.0, 1.3) High-Entropy Alloy Prepared by Mechanical Alloying and Spark Plasma Sintering. Nanomaterials 2024, 14, 641. [Google Scholar] [CrossRef]
- Suzuki, T.; Keawchai, K.; Jiang, W.; Yatsui, K. Nanosize Al2O3 Powder Production by Pulsed Wire Discharge. Jpn. J. Appl. Phys. 2001, 40, 1073. [Google Scholar] [CrossRef]
- Sarkar, A.; Wang, Q.; Schiele, A.; Chellali, M.R.; Bhattacharya, S.S.; Wang, D.; Brezesinski, T.; Hahn, H.; Velasco, L.; Breitung, B. High-Entropy Oxides: Fundamental Aspects and Electrochemical Properties. Adv. Mater. 2019, 31, 1806236. [Google Scholar] [CrossRef] [PubMed]
- Ganesh, U.L.; Raghavendra, H. Review on the transition from conventional to multi-component-based nano-high-entropy alloys—NHEAs. J. Therm. Anal. Calorim. 2020, 139, 207–216. [Google Scholar] [CrossRef]
- Yeh, J.-W. Alloy Design Strategies and Future Trends in High-Entropy Alloys. JOM 2013, 65, 1759–1771. [Google Scholar] [CrossRef]
- Voskanyan, A.A.; Lilova, K.; McCormack, S.J.; Kriven, W.M.; Navrotsky, A. A new class of entropy stabilized oxides: Commensurately modulated A6B2O17 (A = Zr, Hf; B = Nb, Ta) structures. Scr. Mater. 2021, 204, 114139. [Google Scholar] [CrossRef]
- Pervikov, A.; Khrustalyov, A.; Filippov, A.; Mironov, Y.; Lozhkomoev, A.; Lerner, M.; Tarasov, S. Structural, Mechanical, and Tribological Characterization of Magnetic Pulse Compacted Fe–Cu Bimetallic Particles Produced by Electric Explosion of Dissimilar Metal Wires. Metals 2019, 9, 1287. [Google Scholar] [CrossRef]
- Giacovazzo, C.; Monaco, H.L.; Artioli, G.; Viterbo, D.; Milanesio, M.; Gilli, G.; Gilli, P.; Zanotti, G.; Ferraris, G.; Catti, M. Fundamentals of Crystallography, 3rd ed.; Oxford University Press: New York, NY, USA, 2011. [Google Scholar] [CrossRef]
- Park, S.H.; Lee, K.W.; Otto, E.; Fissan, H. The log-normal size distribution theory of brownian aerosol coagulation for the entire particle size range: Part I—Analytical solution using the harmonic mean coagulation kernel. J. Aerosol Sci. 1999, 30, 3–16. [Google Scholar] [CrossRef]
- Li, X.; Wu, J.; Li, X.; Chen, L.; Shi, H.; Qiu, A. Influence of Partial Reheating on Aluminum Nanoparticles From Electrical Exploding Wires. IEEE Trans. Nanotechnol. 2019, 18, 1103–1109. [Google Scholar] [CrossRef]
- Zhang, R.-Z.; Reece, M.J. Review of high entropy ceramics: Design, synthesis, structure and properties. J. Mater. Chem. A 2019, 7, 22148–22162. [Google Scholar] [CrossRef]
- Wu, H.; Lu, Q.; Li, Y.; Zhao, M.; Wang, J.; Li, Y.; Zhang, J.; Zheng, X.; Han, X.; Zhao, N.; et al. Structural Framework-Guided Universal Design of High-Entropy Compounds for Efficient Energy Catalysis. J. Am. Chem. Soc. 2023, 145, 1924–1935. [Google Scholar] [CrossRef] [PubMed]
- Rafiq, M.A.; Javed, A.; Rasul, M.N.; Khan, M.A.; Hussain, A. Understanding the structural, electronic, magnetic and optical properties of spinel MFe2O4 (M = Mn, Co, Ni) ferrites. Ceram. Int. 2020, 46, 4976–4983. [Google Scholar] [CrossRef]
- Bularzik, J.; Davies, P.K.; Navrotsky, A. Thermodynamics of Solid-Solution Formation in NiO-CuO. J. Am. Ceram. Soc. 1986, 69, 453–457. [Google Scholar] [CrossRef]
- Mebrek, A.; Alleg, S.; Benayache, S.; Benabdeslem, M. Preparation and characterization of spinel type Zn2TiO4 nanocomposite. Ceram. Int. 2018, 44, 10921–10928. [Google Scholar] [CrossRef]
- Mohanty, G.C.; Das, S.; Verma, A. Fabrication of aqueous asymmetric supercapacitor device by using spinel type (FeCoNiCuZn)3O4 high entropy oxide and green carbon derived from plastic wastes. Ceram. Int. 2024, 50, 48938–48947. [Google Scholar] [CrossRef]
- Hong, D.; Choi, Y.-H. High-entropy oxide (Mg0.2Fe0.2Co0.2Cu0.2Zn0.2)O with rocksalt-to-spinel transformation and its electrocatalytic activity for the oxygen evolution reaction. J. Alloys Compd. 2024, 985, 174029. [Google Scholar] [CrossRef]


| Sample | Wire Material | d, mm | l, mm | c, %mol |
|---|---|---|---|---|
| 1 | Fe | 0.3 | 44 | 24.45 |
| Co | 0.3 | 44 | 26.2 | |
| Ni70Cr30 | 0.3 | 44 | Ni-17.4 Cr-7.45 | |
| Cu | 0.3 | 44 | 24.5 | |
| 2 | Fe | 0.3 | 44 | 26.63 |
| Co | 0.3 | 44 | 28.57 | |
| Ni70Cr30 | 0.3 | 44 | Ni-18.9 Cr-8.1 | |
| Ti | 0.3 | 44 | 17.8 | |
| 3 | Co | 0.3 | 44 | 23.34 |
| Ni | 0.3 | 44 | 23.44 | |
| Ti | 0.3 | 44 | 14.55 | |
| Cu | 0.3 | 44 | 21.79 | |
| Zn | 0.3 | 44 | 16.88 |
| Elements | (FeCoNiCrCu)O | Elements | (FeCoNiCrTi)O | Elements | (CoNiTiCuZn)O |
|---|---|---|---|---|---|
| O (At, %) | 42.86 | O (At, %) | 62.56 | O (At, %) | 48.63 |
| Fe (At, %) | 13.29 | Fe (At, %) | 10.3 | Co (At, %) | 11.72 |
| Co (At, %) | 15.26 | Co (At, %) | 10.78 | Ni (At, %) | 12.1 |
| Ni (At, %) | 8.86 | Ni (At, %) | 7.08 | Ti (At, %) | 7.08 |
| Cr (At, %) | 3.48 | Cr (At, %) | 3.03 | Cu (At, %) | 12.42 |
| Cu (At, %) | 16.25 | Ti (At, %) | 6.25 | Zn (At, %) | 8.05 |
| Sample | Wire Material | d, mm | l, mm | c, %mol |
|---|---|---|---|---|
| 1 | Fe | 0.3 | 44 | 21.74 |
| Co | 0.3 | 44 | 23.33 | |
| Ni | 0.3 | 44 | 16.27 | |
| Cu | 0.3 | 44 | 21.78 | |
| Zn | 0.3 | 44 | 16.87 | |
| 2 | Fe | 0.3 | 44 | 23.45 |
| Co | 0.3 | 44 | 25.17 | |
| Ni | 0.3 | 44 | 17.55 | |
| Ti | 0.3 | 44 | 15.67 | |
| Zn | 0.3 | 44 | 18.2 | |
| 3 | Fe | 0.3 | 44 | 22.1 |
| Ni | 0.3 | 44 | 23.8 | |
| Ti | 0.3 | 44 | 14.8 | |
| Cu | 0.3 | 44 | 22.15 | |
| Zn | 0.3 | 44 | 17.15 |
| Crystal Structure | High-Entropy Oxide Nanoproducts | |
|---|---|---|
| Rock-salt | (FeCoNiCrCu)O | (FeCoNiCuZn)O |
| Spinel | (FeCoNiCrTi)O | (FeCoNiTiZn)O |
| Dual-phase | (CoNiTiCuZn)O | (FeNiTiCuZn)O |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, S.; Liu, K.; Qi, L.; Yang, L. Synthesis of High-Entropy Oxide Nanopowders with Different Crystal Structures by Electrical Explosion of Wires. Nanomaterials 2025, 15, 571. https://doi.org/10.3390/nano15080571
Liu S, Liu K, Qi L, Yang L. Synthesis of High-Entropy Oxide Nanopowders with Different Crystal Structures by Electrical Explosion of Wires. Nanomaterials. 2025; 15(8):571. https://doi.org/10.3390/nano15080571
Chicago/Turabian StyleLiu, Shuai, Kangqi Liu, Liangwen Qi, and Lanjun Yang. 2025. "Synthesis of High-Entropy Oxide Nanopowders with Different Crystal Structures by Electrical Explosion of Wires" Nanomaterials 15, no. 8: 571. https://doi.org/10.3390/nano15080571
APA StyleLiu, S., Liu, K., Qi, L., & Yang, L. (2025). Synthesis of High-Entropy Oxide Nanopowders with Different Crystal Structures by Electrical Explosion of Wires. Nanomaterials, 15(8), 571. https://doi.org/10.3390/nano15080571







