Factors Influencing Removal of Trichloroethylene in a Zero-Valent Iron Fenton System
Abstract
1. Introduction
2. Experimental
2.1. Chemicals and Reagents
2.2. Characterization of ZVI
2.3. Experimental Process
3. Results and Discussion
3.1. Characteristics of ZVI
3.1.1. Surface Morphological Characteristics of SEM
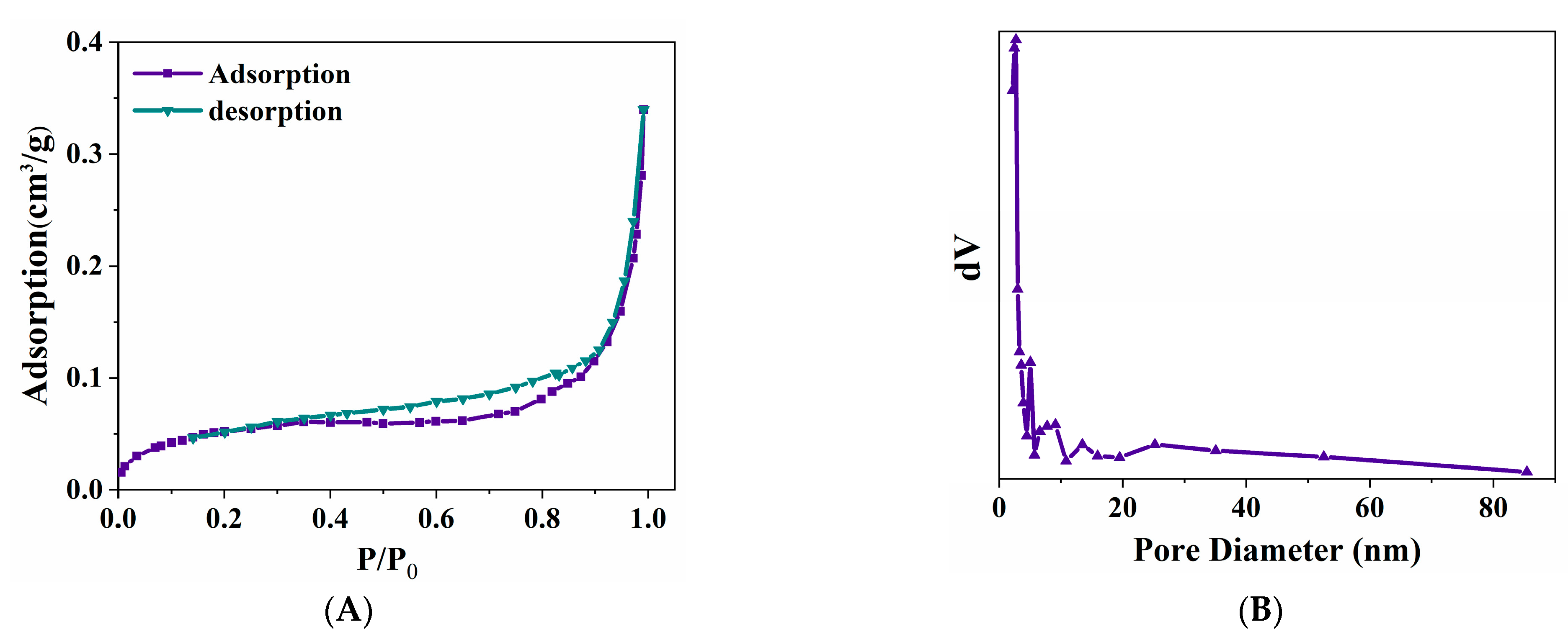
3.1.2. BET Analysis of ZVI
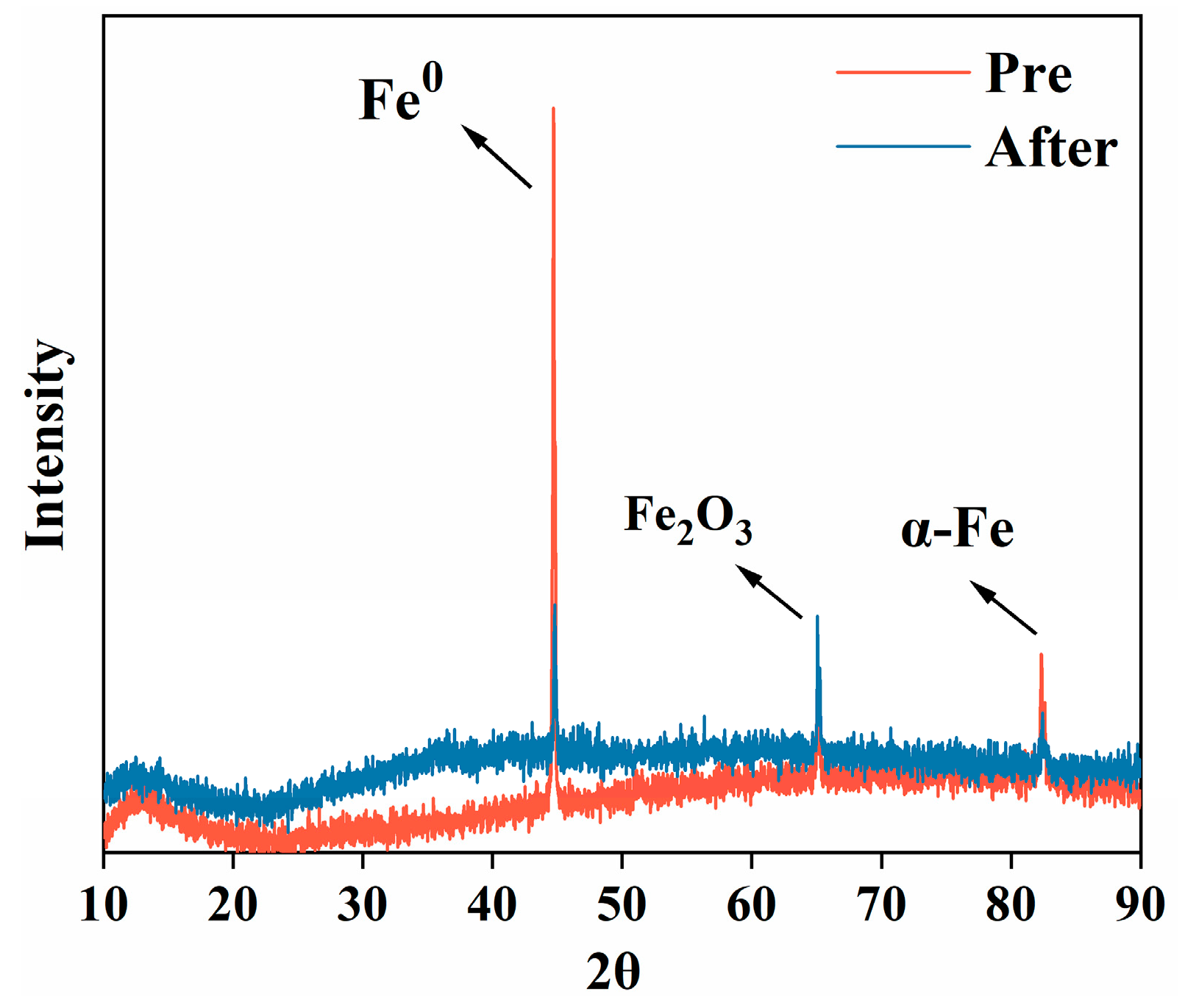
3.1.3. X-Ray Spectroscopy (XRD) Analysis of ZVI
3.2. Effects of TCE Concentration, Dosage of ZVI, H2O2 Concentration, Solution Temperature, and Initial pH on the Removal Efficiency of TCE by ZVI/H2O2
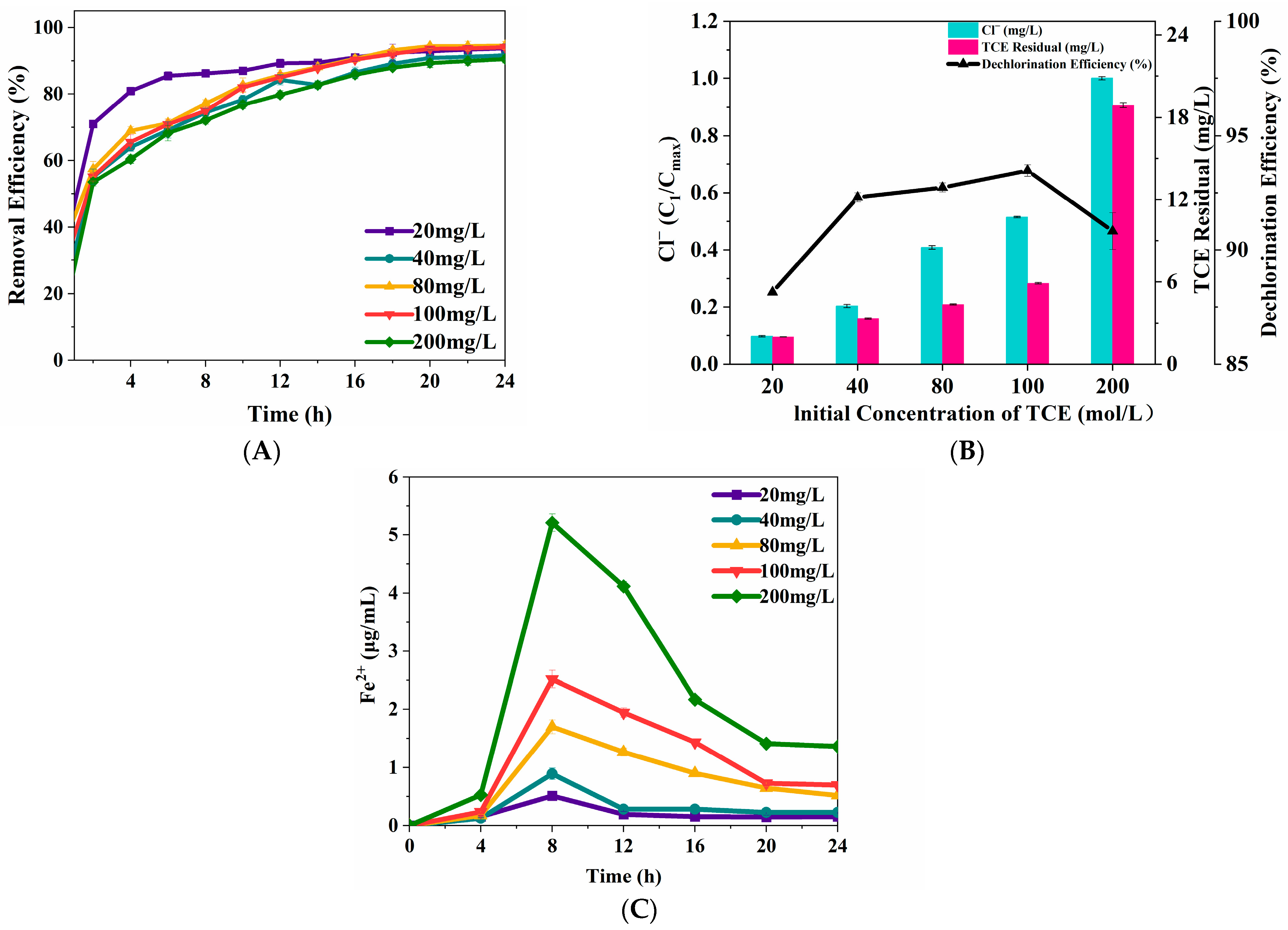
3.2.1. TCE Concentration
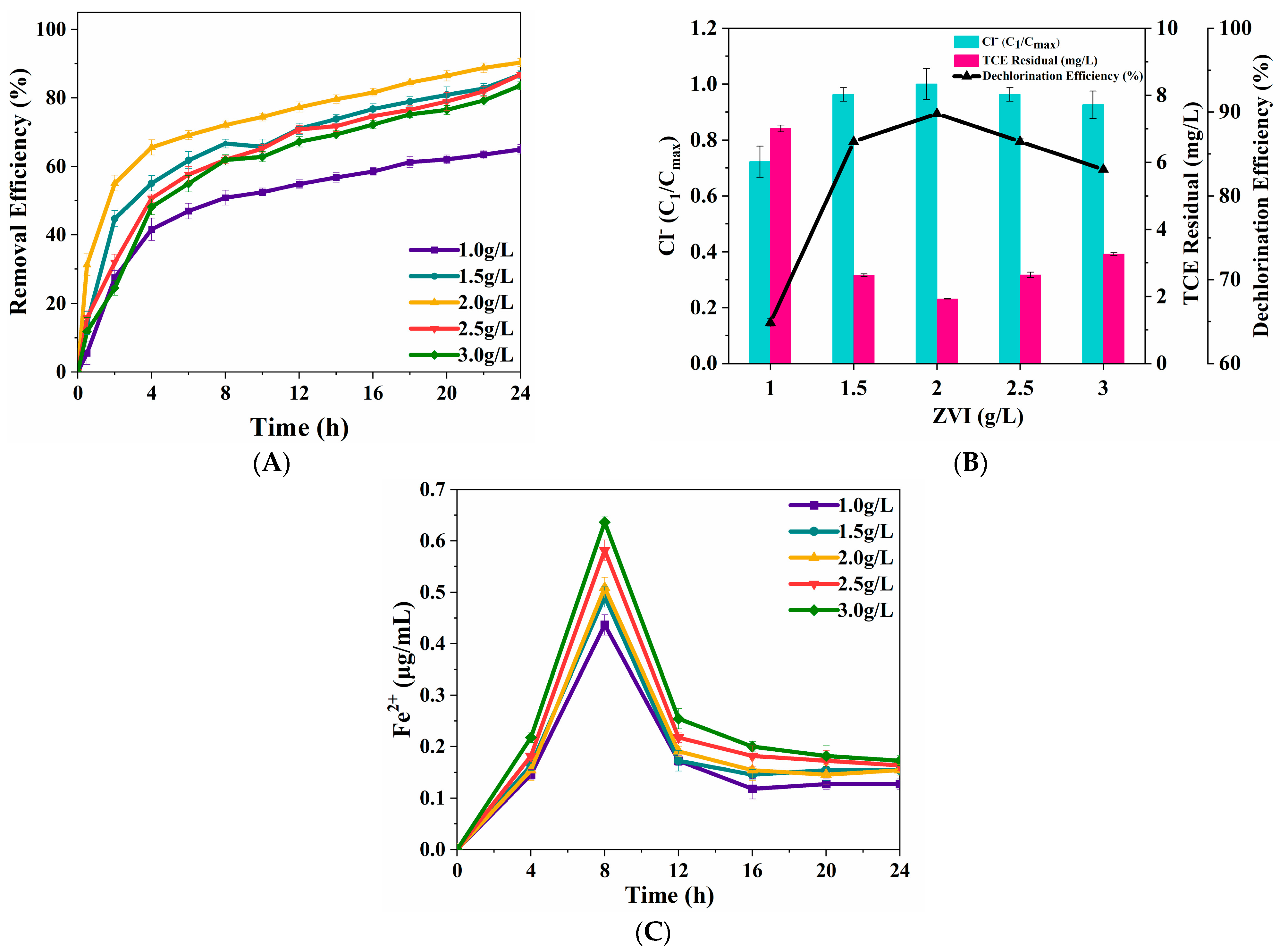
3.2.2. Dosage of ZVI
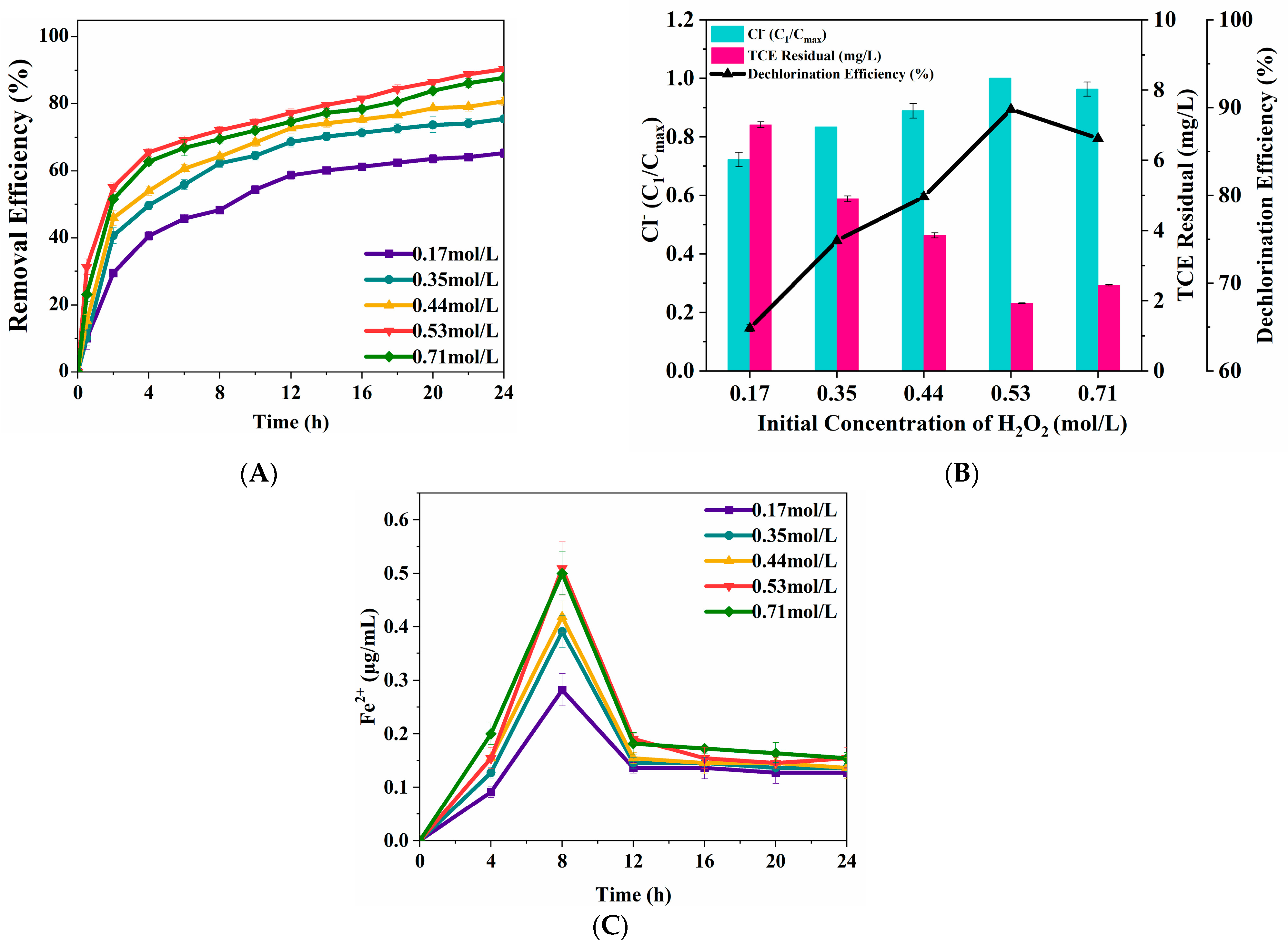
3.2.3. H2O2 Concentration
3.2.4. Solution Temperature
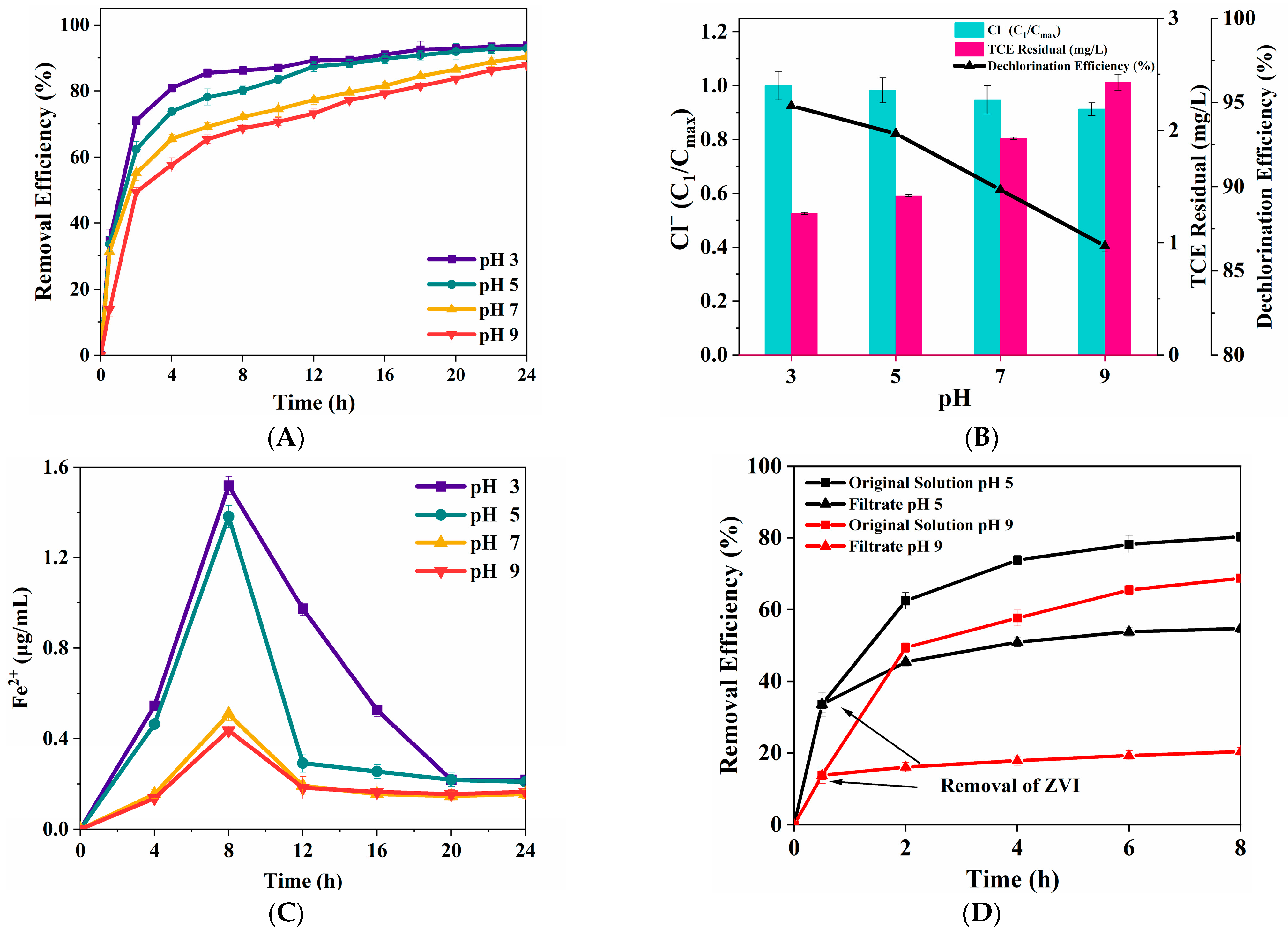
3.2.5. Initial pH
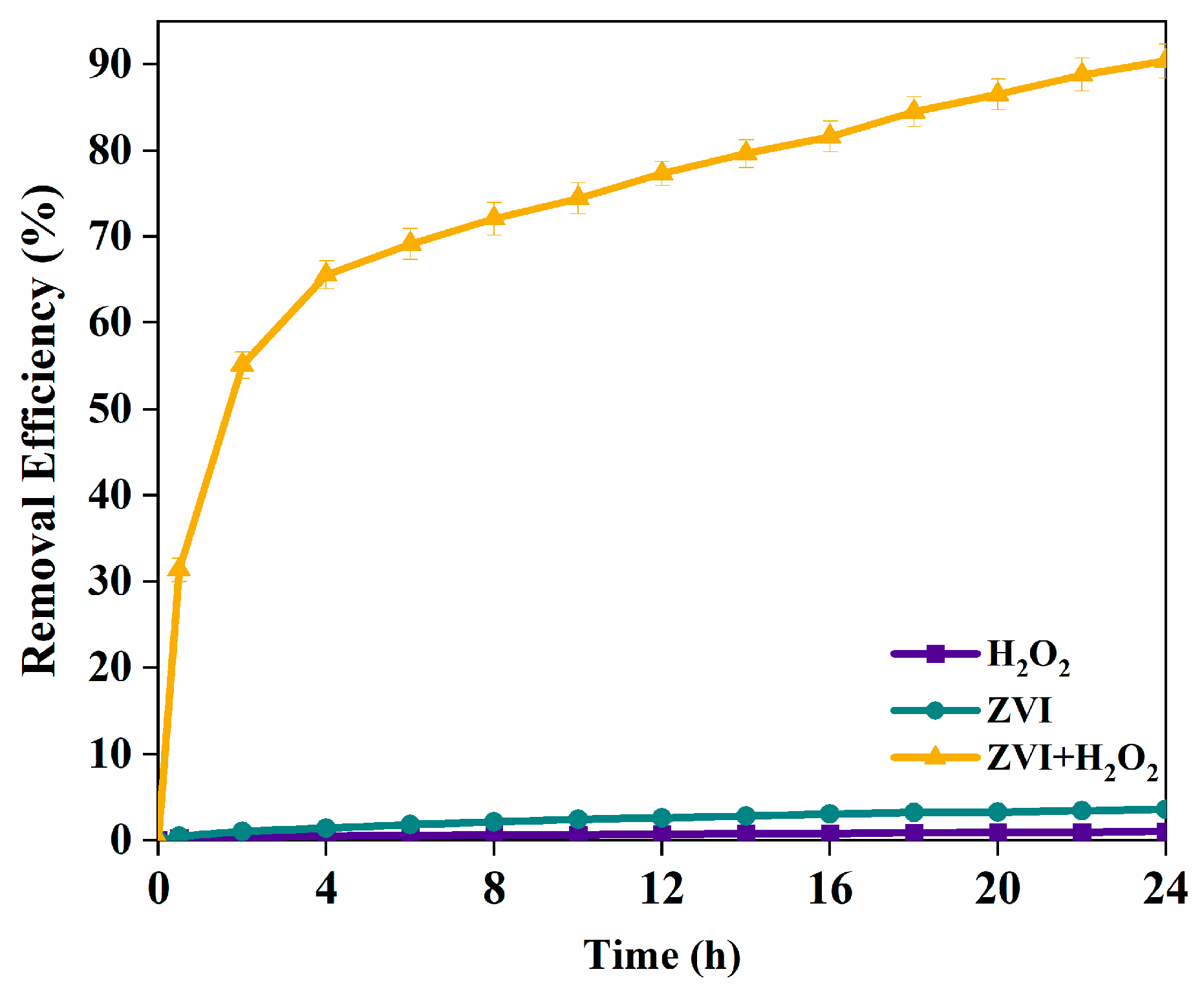
3.3. TCE Degradation Mechanisms in ZVI Fenton Process
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kube, M.; Beck, A.; Zinder, S.H.; Kuhl, H.; Reinhardt, R.; Adrian, L. Genome sequence of the chlorinated compound–respiring bacterium Dehalococcoides species strain CBDB1. Nat. Biotechnol. 2005, 23, 1269–1273. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Man, Q.; Niu, H.; Lyu, H.; Song, H.; Li, R.; Ren, G.; Zhu, F.; Peng, C.; Li, B.; et al. Recent advances and trends of trichloroethylene biodegradation: A critical review. Front. Microbiol. 2022, 13, 1053169. [Google Scholar] [CrossRef] [PubMed]
- Doherty, R.E. A History of the Production and Use of Carbon Tetrachloride, Tetrachloroethylene, Trichloroethylene and 1,1,1-Trichloroethane in the United States: Part 2—Trichloroethylene and 1,1,1-Trichloroethane. Environ. Forensics 2000, 1, 83–93. [Google Scholar] [CrossRef]
- Wartenberg, D.; Reyner, D.; Scott, C.S. Trichloroethylene and cancer: Epidemiologic evidence. Environ. Health Perspect. 2000, 108, 161–176. [Google Scholar] [CrossRef]
- Milks, M.M.; Couri, D. Trichloroethylene effects on the formation of enzyme-altered foci in rat liver. Arch. Toxicol. 1984, 56, 69–73. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Zhang, Y.; Meng, Q.; Liu, Y.; Tuyiringire, D.; Chen, Z.; Liang, S. Effects of trichloroethylene stress on the microbiological characteristics of Mollisol. Ecotoxicol. Environ. Saf. 2019, 184, 109595. [Google Scholar] [CrossRef]
- Li, P.; Zhang, Y.; Meng, Q.; Liu, Y.; Liang, S. Trichloroethylene inhibits nitrogen transformation and microbial community structure in Mollisol. Ecotoxicology 2020, 29, 801–813. [Google Scholar] [CrossRef]
- An, J.; Li, G.; Si, W.; Chen, W.; He, J.; Feng, H.; Chen, J.; Fu, H. Highly efficient degradation of antibiotic residues with reagent-free photoelectro-Fenton via hollow fiber gas diffusion electrode. Chem. Eng. J. 2023, 477, 147259. [Google Scholar] [CrossRef]
- Gu, Y.; Meng, J.; Duo, J.; Khim, J.S.; Wang, T.; Su, G.; Li, Q.; Shi, B.; Sun, B.; Zhang, Y.; et al. Environmental fate and transformation mechanisms of chlorinated organic pollutants from the petrochemical industry: Insights for pollution control and remediation. J. Hazard. Mater. 2024, 480, 136329. [Google Scholar] [CrossRef]
- Zhao, X.; Yang, H.; Xu, J.; Cheng, T.; Li, Y. Bimetallic PdAu Nanoframes for Electrochemical H2O2 Production in Acids. ACS Mater. Lett. 2021, 3, 996–1002. [Google Scholar] [CrossRef]
- Chen, N.; Rioux, R.M.; Ribeiro, F.H. Investigation of Reaction Steps for the Hydrodechlorination of Chlorine-Containing Organic Compounds on Pd Catalysts. J. Catal. 2002, 211, 192–197. [Google Scholar] [CrossRef]
- Smith, B.A.; Teel, A.L.; Watts, R.J. Mechanism for the destruction of carbon tetrachloride and chloroform DNAPLs by modified Fenton’s reagent. J. Contam. Hydrol. 2006, 85, 229–246. [Google Scholar] [CrossRef]
- Walling, C.; Cheves, F. Fenton’s reagent revisited. Acc. Chem. Res. 1975, 8, 125–131. [Google Scholar] [CrossRef]
- Pignatello, J.; Oliveros, E.; MacKay, A. Advanced Oxidation Processes for Organic Contaminant Destruction Based on the Fenton Reaction and Related Chemistry. Crit. Rev. Environ. Sci. Technol. 2006, 36, 1–84. [Google Scholar] [CrossRef]
- Feng, W.; Li, J.; Gao, K.; An, H.; Wang, Y. A comparison of spent resin degradation by Fenton and O3-Fenton process. Prog. Nucl. Energy 2020, 130, 103566. [Google Scholar] [CrossRef]
- Xiao, J.; Wang, C.; Lyu, S.; Liu, H.; Jiang, C.; Lei, Y. Enhancement of Fenton degradation by catechol in a wide initial pH range. Sep. Purif. Technol. 2016, 169, 202–209. [Google Scholar] [CrossRef]
- Subramanian, G.; Madras, G. Remarkable enhancement of Fenton degradation at wide pH range promoted by thioglycolic acid. Chem. Commun. 2016, 3, 1136–1139. [Google Scholar] [CrossRef]
- Aline, M.M.; Iara, R.G.; Guilherme, M.M.d.C.; Mateus, A.G.; Teodorico, C.R.; Mário, C.G. Boron as a promoter in the goethite (α-FeOOH) phase: Organic compound degradation by Fenton reaction. Appl. Catal. B Environ. Energy 2016, 192, 286–295. [Google Scholar] [CrossRef]
- Anaëlle, G.; Charlotte, G.; Arezou, F.; Hélène, M.; Christine de, B.; Marcello, B.; Gilles, M. The ability of recycled magnetite nanoparticles to degrade carbamazepine in water through photo-Fenton oxidation at neutral pH. Sep. Purif. Technol. 2023, 317, 123877. [Google Scholar] [CrossRef]
- Zhang, Y.; Dong, K.; Liu, Z.; Wang, H.; Ma, S.; Zhang, A.; Li, M.; Yu, L.; Li, Y. Sulfurized hematite for photo-Fenton catalysis. Prog. Nat. Sci. Mater. Int. 2017, 27, 4. [Google Scholar] [CrossRef]
- Zhu, K.; Sun, C.; Chen, H.; Baig, S.A.; Sheng, T.; Xu, X. Enhanced catalytic hydrodechlorination of 2,4-dichlorophenoxyacetic acid by nanoscale zero-valent iron with electrochemical technique using a palladium/nickel foam electrode. Chem. Eng. J. 2013, 223, 192–199. [Google Scholar] [CrossRef]
- Li, T.; Zhang, X.; Zhou, Y.; Du, X.; Fang, D.; Liang, J.; Li, J.; Zhou, L. Schwertmannite-based heterogeneous Fenton for enhancing sludge dewaterability over a wide pH range. J. Hazard. Mater. 2023, 459, 132240. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, Y.; Chen, L.; Zhang, H. Goethite as an efficient heterogeneous Fenton catalyst for the degradation of methyl orange. Catal. Today 2015, 252, 107–112. [Google Scholar] [CrossRef]
- Tiar, K.; Soualah, A.; Bisio, C.; Guidotti, M. Effect of initial solution pH on 4-nitrophenol oxidation through homogeneous/heterogeneous photo-Fenton process using goethite/H2O2 system. J. Photochem. Photobiol. A Chem. 2024, 447, 115184. [Google Scholar] [CrossRef]
- Zhang, G.S.; Wang, Q.; Zhang, W.; Li, T.; Yuan, Y.X.; Wang, P. Effects of organic acids and initial solution pH on photocatalytic degradation of bisphenol A (BPA) in a photo-Fenton-like process using goethite (α-FeOOH). Photoch. Photobio. Sci. 2024, 15, 1046–1053. [Google Scholar] [CrossRef]
- Choi, K.; Lee, W. Enhanced degradation of trichloroethylene in nano-scale zero-valent iron Fenton process with Cu(II). J. Hazard. Mater. 2011, 211–212, 146–153. [Google Scholar] [CrossRef]
- Gao, J.; Zhang, L.; Xu, Z.; Sheng, X.; Li, D.; Chen, Y.; Habib, M.; Lyu, S. Degradation of 1,2,3-trichloropropane in aqueous solution by ZVI-enhanced Fenton process: Performances and mechanisms. J. Environ. Chem. Eng. 2023, 11, 110432. [Google Scholar] [CrossRef]
- Lin, C.-C.; Cheng, Y.-J. Effectiveness of using nanoscale zero-valent iron and hydrogen peroxide in degrading sulfamethazine in water. J. Taiwan Inst. Chem. Eng. 2021, 118, 179–186. [Google Scholar] [CrossRef]
- Feitz, A.J.; Joo, S.H.; Guan, J.; Sun, Q.; Sedlak, D.L.; Waite, T.D. Oxidative transformation of contaminants using colloidal zero-valent iron. Colloids Surf. A Physicochem. Eng. Asp. 2005, 265, 88–94. [Google Scholar] [CrossRef]
- Dong, H.; Zhang, C.; Deng, J.; Jiang, Z.; Zhang, L.; Cheng, Y.; Hou, K.; Tang, L.; Zeng, G. Factors influencing degradation of trichloroethylene by sulfide-modified nanoscale zero-valent iron in aqueous solution. Water Res. 2018, 15, 1–10. [Google Scholar] [CrossRef]
- Peng, X.; Xi, B.; Zhao, Y.; Shi, Q.; Meng, X.; Mao, X.; Jiang, Y.; Ma, Z.; Tan, W.; Liu, H.; et al. Effect of Arsenic on the Formation and Adsorption Property of Ferric Hydroxide Precipitates in ZVI Treatment. Environ. Sci. Technol. 2017, 51, 10100–10108. [Google Scholar] [CrossRef]
- Li, J.; Dou, X.; Qin, H.; Sun, Y.; Yin, D.; Guan, X. Characterization methods of zerovalent iron for water treatment and remediation. Water Res. 2018, 148, 70–85. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.-J.; Kim, J.-H.; Azad, A.-M.; Chang, Y.-S. Facile Synthesis and Characterization of Fe/FeS Nanoparticles for Environmental Applications. ACS Appl. Mater. Interfaces 2011, 3, 1457–1462. [Google Scholar] [CrossRef] [PubMed]
- Velimirovic, M.; Auffan, M.; Carniato, L.; Micić Batka, V.; Schmid, D.; Wagner, S.; Borschneck, D.; Proux, O.; von der Kammer, F.; Hofmann, T. Effect of field site hydrogeochemical conditions on the corrosion of milled zerovalent iron particles and their dechlorination efficiency. Sci. Total Environ. 2017, 618, 1619–1627. [Google Scholar] [CrossRef] [PubMed]
- Harada, T.; Yatagai, T.; Kawase, Y. Hydroxyl radical generation linked with iron dissolution and dissolved oxygen consumption in zero-valent iron wastewater treatment process. Chem. Eng. J. 2016, 303, 611–620. [Google Scholar] [CrossRef]
- Duesterberg, C.K.; Waite, T.D. Process Optimization of Fenton Oxidation Using Kinetic Modeling. Environ. Sci. Technol. 2006, 40, 4189–4195. [Google Scholar] [CrossRef]
- Fontmorin, J.M.; Burgos Castillo, R.C.; Tang, W.Z.; Sillanpää, M. Stability of 5,5-dimethyl-1-pyrroline-N-oxide as a spin-trap for quantification of hydroxyl radicals in processes based on Fenton reaction. Water Res. 2016, 99, 24–32. [Google Scholar] [CrossRef]
- Metzgen, A.D.; Dahmke, A.; Ebert, M. Significance of temperature as a key driver in ZVI PRB applications for PCE degradation. J. Contam. Hydrol. 2023, 258, 104236. [Google Scholar] [CrossRef]
- Fan, C.; Zheng, N.; Zheng, J.; Xiao, L.; Liu, Y. Kinetic-molecular theory optimization algorithm using opposition-based learning and varying accelerated motion. Soft Comput. 2020, 24, 17. [Google Scholar] [CrossRef]
- Matheson, L.J.; Tratnyek, P.G. Reductive dehalogenation of chlorinated methanes by iron metal. Environ. Sci. Technol. 1994, 28, 2045–2053. [Google Scholar] [CrossRef]
- Zhao, J.; Tobler, D.J.; Yin, W.; Hansen, H.C.B. ZVI-biochar granules for reactive chlorinated solvent filters generated by high temperature pyrolysis of iron(III) amended biomass. Sep. Purif. Technol. 2025, 357, 129979. [Google Scholar] [CrossRef]
- Guo, J.; Gao, F.; Zhang, C.; Ahmad, S.; Tang, J. Sulfidation of zero-valent iron for enhanced reduction of chlorinated contaminants: A review on the reactivity, selectivity, and interference resistance. Chem. Eng. J. 2023, 477, 147049. [Google Scholar] [CrossRef]

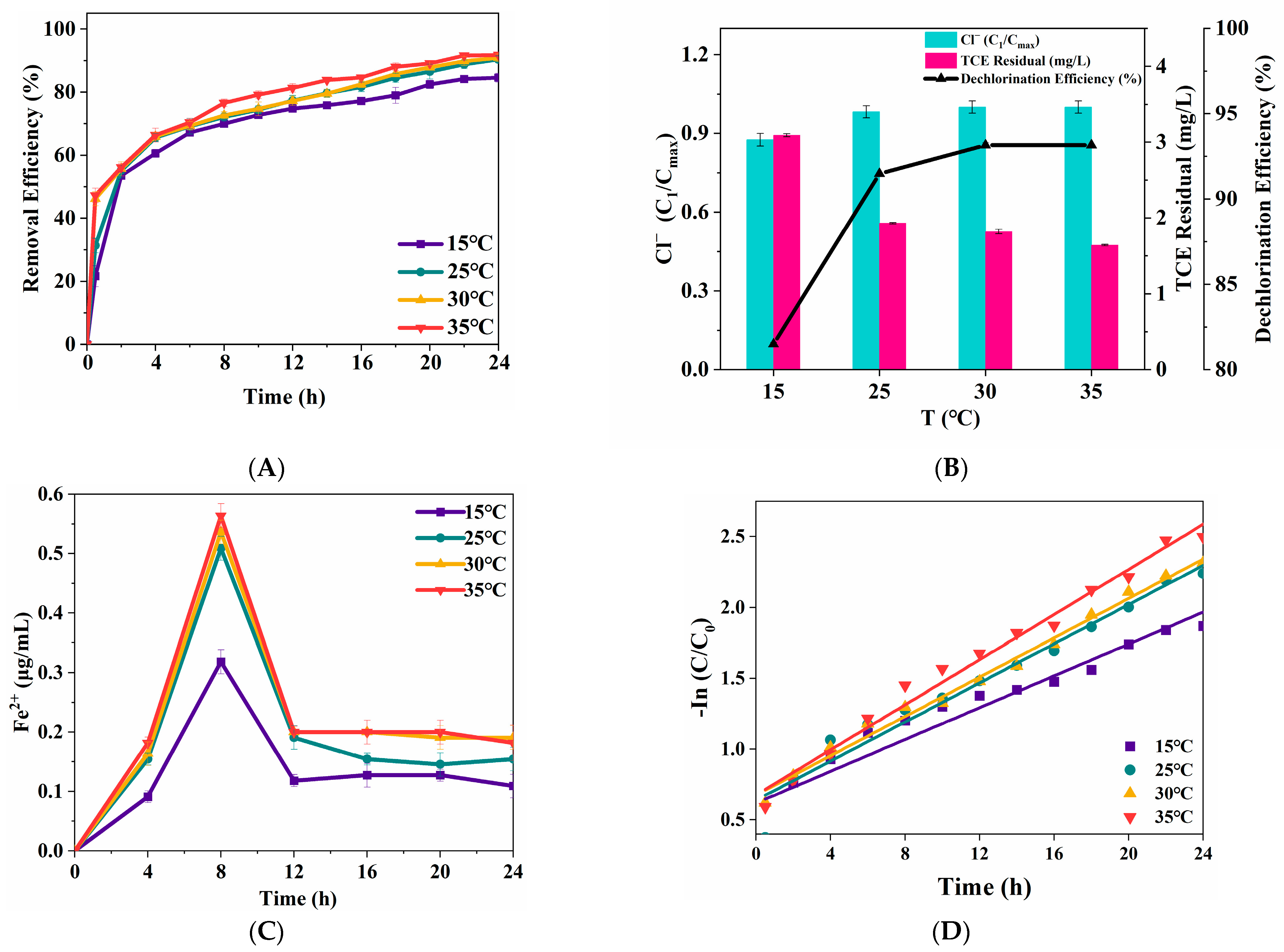
| T (°C) | Reaction Kinetics Constant (/h) | R2 |
|---|---|---|
| 15 | 0.0947 | 0.9760 |
| 25 | 0.1250 | 0.9902 |
| 30 | 0.1347 | 0.9907 |
| 35 | 0.1537 | 0.9846 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Y.; Liang, S.; Li, P. Factors Influencing Removal of Trichloroethylene in a Zero-Valent Iron Fenton System. Nanomaterials 2025, 15, 558. https://doi.org/10.3390/nano15070558
Sun Y, Liang S, Li P. Factors Influencing Removal of Trichloroethylene in a Zero-Valent Iron Fenton System. Nanomaterials. 2025; 15(7):558. https://doi.org/10.3390/nano15070558
Chicago/Turabian StyleSun, Yangyang, Shichao Liang, and Pengfei Li. 2025. "Factors Influencing Removal of Trichloroethylene in a Zero-Valent Iron Fenton System" Nanomaterials 15, no. 7: 558. https://doi.org/10.3390/nano15070558
APA StyleSun, Y., Liang, S., & Li, P. (2025). Factors Influencing Removal of Trichloroethylene in a Zero-Valent Iron Fenton System. Nanomaterials, 15(7), 558. https://doi.org/10.3390/nano15070558






