Pulsed Alternating Fields Magnetic Hyperthermia in Combination with Chemotherapy (5-Fluorouracil) as a Cancer Treatment for Glioblastoma Multiform: An In Vitro Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
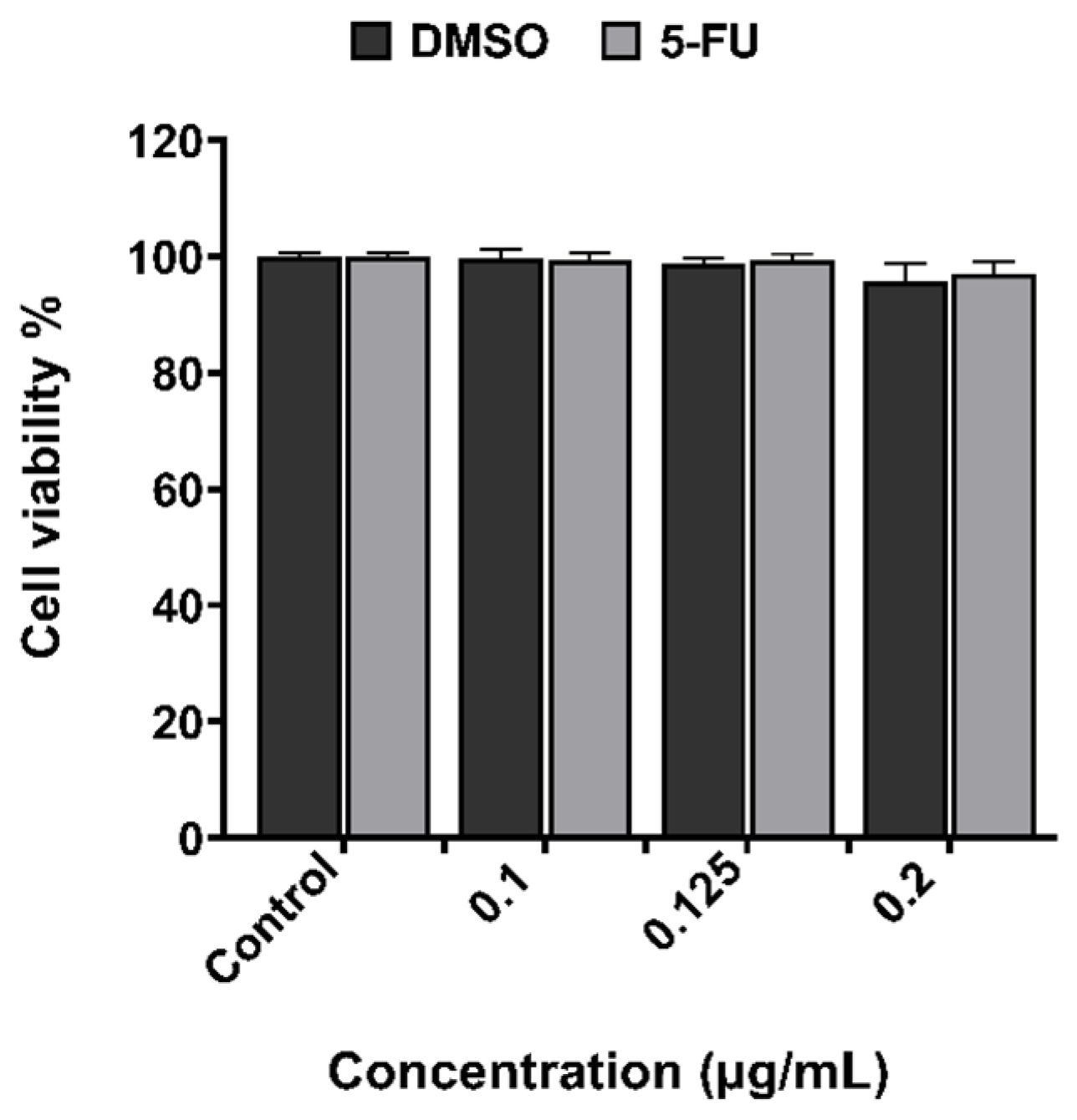
2.2. XTT Assay for Examination of Cytotoxicity Effect
2.2.1. 5-Fluorouracil
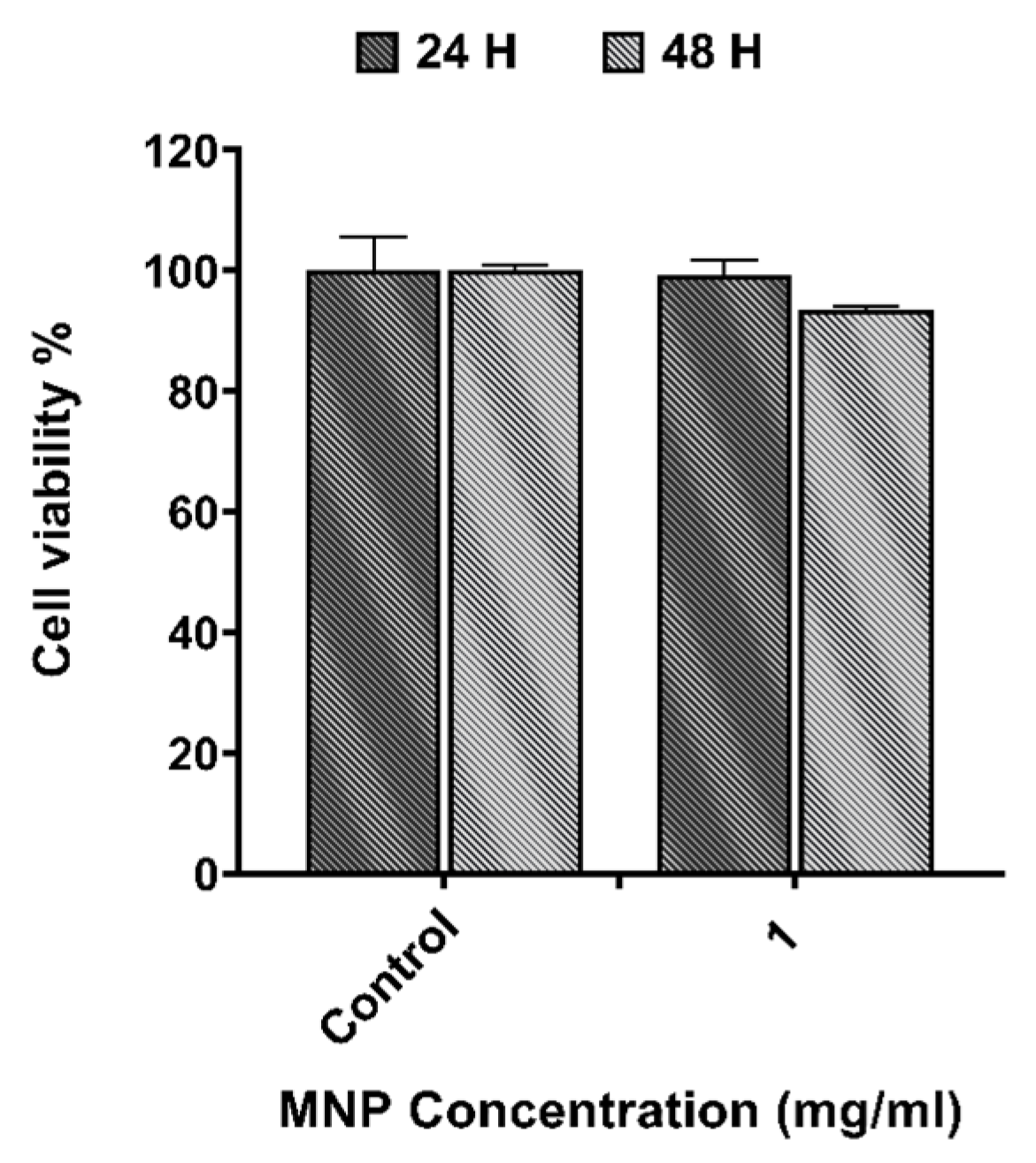
2.2.2. Magnetic Nanoparticles
2.3. Magnetic Hyperthermia Device
2.4. Evaluation of Chemo(5-FU)-Magnetic Hyperthermia Therapy
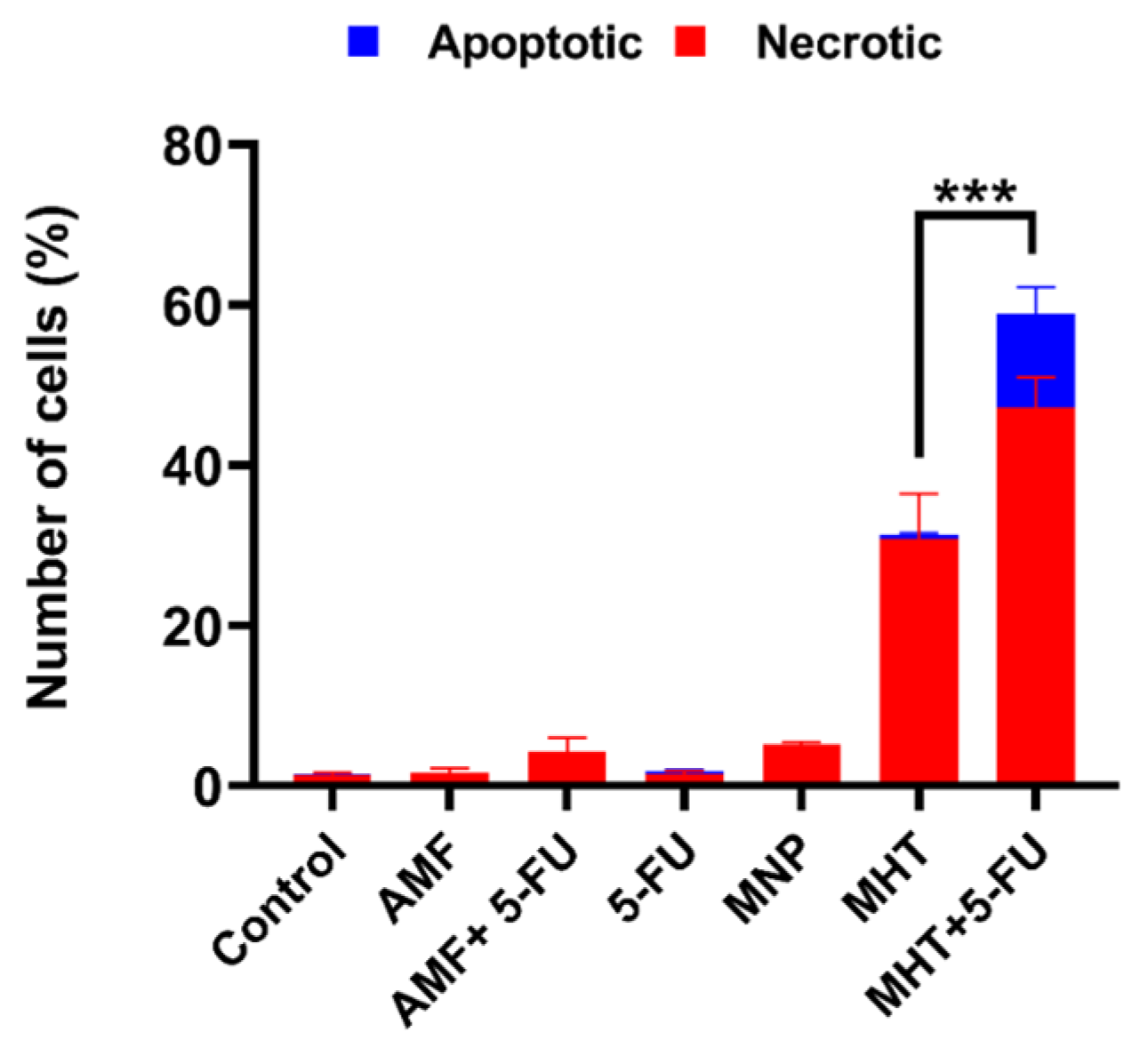
2.5. Annexin V/Propidium Iodide Assay (Apoptosis and Necrosis Quantification)
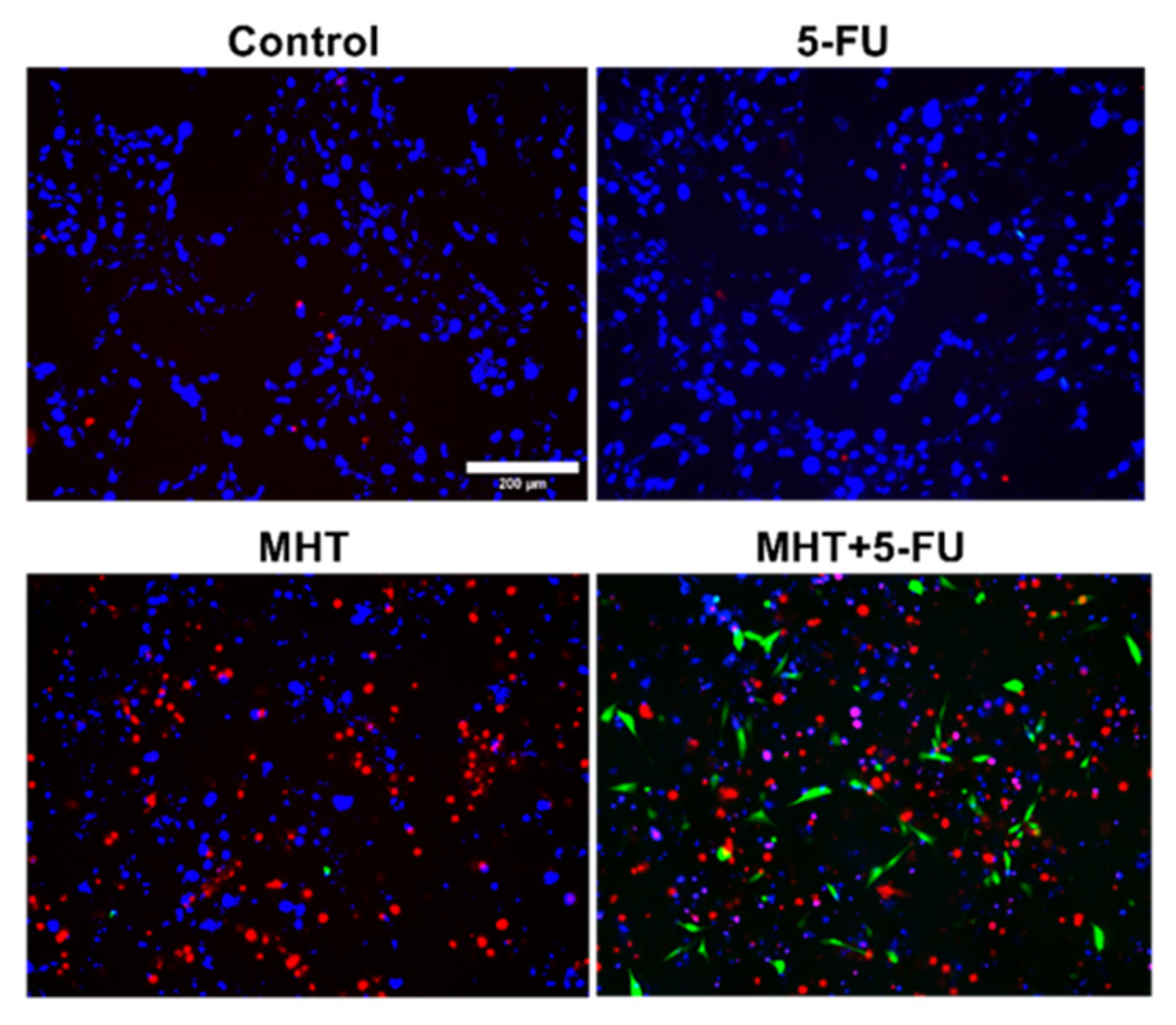
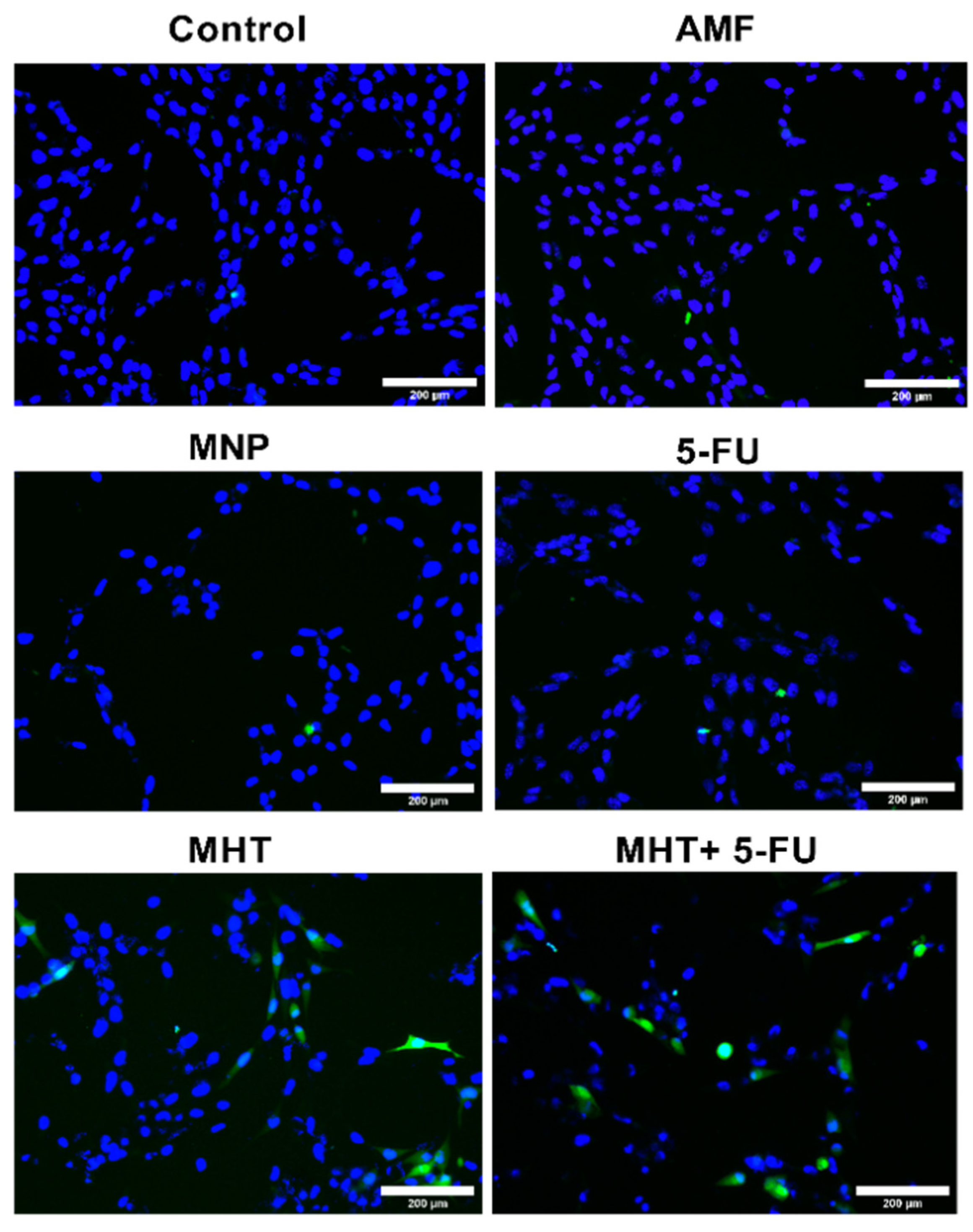
2.6. CRT Immunocytofluorescence Analysis
2.7. Statistical Analysis
3. Results
3.1. In Vitro Cytotoxicity Evaluation
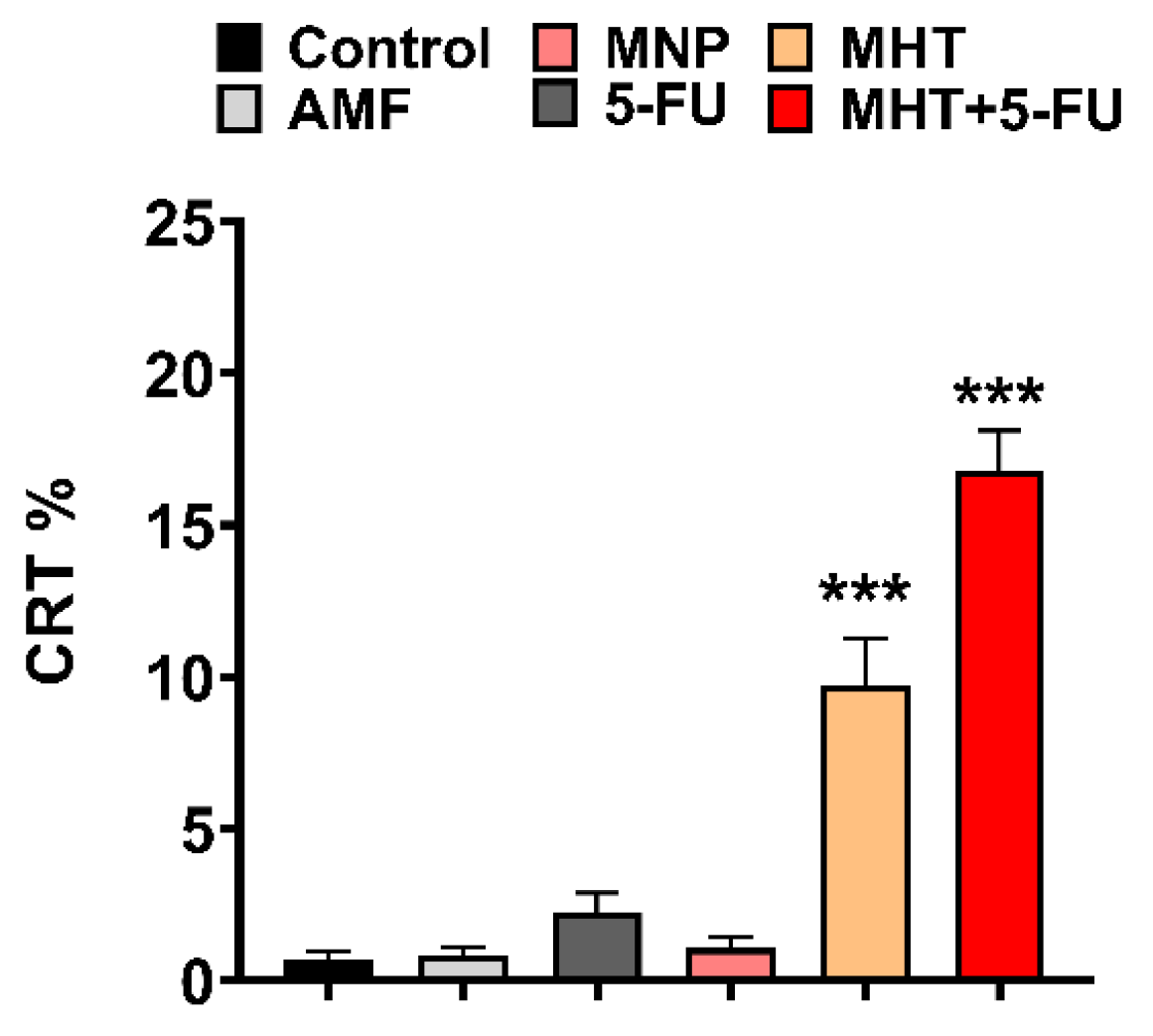
3.2. In Vitro Chemo(5-FU)-Magnetic Hyperthermia Therapy on Glioblastoma Cell Line
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MHT | Magnetic hyperthermia |
| MNPs | Magnetic nanoparticles |
| TP-PAMFs | Trapezoidal pulsed alternating magnetic fields |
| 5-FU | 5-Fluorouracil |
| ICD | Immunogenic cell death |
| GBM | Glioblastoma multiform |
| HT | Hyperthermia |
| SN | Sinusoidal |
| TS | Trapezoidal-square |
| TT | Trapezoidal-triangular |
| TR | Triangular |
| ER | Endoplasmic reticulum |
| CRT | Calreticulin |
| DMEM | Dulbecco’s modified eagle’s medium |
| FBS | Fetal bovine serum |
| APS | 3-aminopropyl-trietoxysilane |
| ROS | Reactive oxygen species |
| PFA | Paraformaldehyde |
| SEM | Standard error of the mean |
| HSP | Heat shock protein |
| DAMPs | Damage associated molecular patterns |
| APCs | Antigen presenting cells |
| ATP | Adenosine triphosphate |
| HMGB1 | High-mobility group box-1 |
| PI | Propidium Iodide |
References
- Czarnywojtek, A.; Borowska, M.; Dyrka, K.; Van Gool, S.; Sawicka-Gutaj, N.; Moskal, J.; Kościński, J.; Graczyk, P.; Hałas, T.; Lewandowska, A.M.; et al. Glioblastoma Multiforme: The Latest Diagnostics and Treatment Techniques. Pharmacology 2023, 108, 423–431. [Google Scholar] [CrossRef] [PubMed]
- Szwed, M.; Marczak, A. Application of Nanoparticles for Magnetic Hyperthermia for Cancer Treatment—The Current State of Knowledge. Cancers 2024, 16, 1156. [Google Scholar] [CrossRef] [PubMed]
- Oskoui, P.R.; Rezvani, M. Revolution in cancer treatment methods: Perspective review of factors affecting the final results of nanoparticles used in magnetic fluid hyperthermia. Health Sci. Rev. 2025, 14, 100212. [Google Scholar] [CrossRef]
- Fatima, H.; Charinpanitkul, T.; Kim, K.-S. Fundamentals to Apply Magnetic Nanoparticles for Hyperthermia Therapy. Nanomaterials 2021, 11, 1203. [Google Scholar] [CrossRef]
- Xie, J.; Yan, C.; Yan, Y.; Chen, L.; Song, L.; Zang, F.; An, Y.; Teng, G.; Gu, N.; Zhang, Y. Multi-modal Mn-Zn ferrite nanocrystals for magnetically-induced cancer targeted hyperthermia: A comparison of passive and active targeting effects. Nanoscale 2016, 8, 16902–16915. [Google Scholar] [CrossRef]
- Ganguly, S.; Margel, S. Design of magnetic hydrogels for hyperthermia and drug delivery. Polymers 2021, 13, 4259. [Google Scholar] [CrossRef]
- Vicentini, M.; Ferrero, R.; Manzin, A. Influence of coil geometry, supply conditions and nanoparticle heating properties on magnetic hyperthermia in mouse models. Int. J. Therm. Sci. 2024, 203, 109151. [Google Scholar] [CrossRef]
- Yoshida, T.; Enpuku, K. Optimization of excitation field depending on magnetic nanoparticle parameters for magnetic hyperthermia under safety constraint. AIP Adv. 2024, 14, 075105. [Google Scholar] [CrossRef]
- Allia, P.; Barrera, G.; Tiberto, P. Nonharmonic Driving Fields for Enhancement of Nanoparticle Heating Efficiency in Magnetic Hyperthermia. Phys. Rev. Appl. 2019, 12, 034041. [Google Scholar] [CrossRef]
- Zeinoun, M.; Serrano, D.; Medina, P.T.; Garcia, O.; Vasic, M.; Serrano-Olmedo, J.J. Configurable High-Frequency Alternating Magnetic Field Generator for Nanomedical Magnetic Hyperthermia Applications. IEEE Access 2021, 9, 105805–105816. [Google Scholar] [CrossRef]
- Barrera, G.; Allia, P.; Tiberto, P. Magnetic nanoparticles in square-wave fields for breakthrough performance in hyperthermia and magnetic particle imaging. Sci. Rep. 2024, 14, 10704. [Google Scholar] [CrossRef]
- Souiade, L.; Domingo-Diez, J.; Alcaide, C.; Gámez, B.; Gámez, L.; Ramos, M.; Serrano Olmedo, J.J. Improving the Efficacy of Magnetic Nanoparticle-Mediated Hyperthermia Using Trapezoidal Pulsed Electromagnetic Fields as an In Vitro Anticancer Treatment in Melanoma and Glioblastoma Multiforme Cell Lines. Int. J. Mol. Sci. 2023, 24, 15933. [Google Scholar] [CrossRef]
- Zhang, Y.F.; Lu, M. Advances in magnetic induction hyperthermia. Front. Bioeng. Biotechnol. 2024, 12, 1432189. [Google Scholar] [CrossRef] [PubMed]
- Troitskaya, O.S.; Novak, D.D.; Richter, V.A.; Koval, O.A. Immunogenic Cell Death in Cancer Therapy. Acta Naturae 2022, 14, 40–53. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Yang, M.; Zhang, J.; Yin, Y.; Fan, X.; Zhang, Y.; Qin, S.; Zhang, H.; Yu, F. Immunogenic Cell Death Induction by Ionizing Radiation. Front. Immunol. 2021, 12, 705361. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Lai, X.; Fu, S.; Ren, L.; Cai, H.; Zhang, H.; Gu, Z.; Ma, X.; Luo, K. Immunogenic Cell Death Activates the Tumor Immune Microenvironment to Boost the Immunotherapy Efficiency. Adv. Sci. 2022, 9, 2201734. [Google Scholar] [CrossRef]
- Persano, S.; Vicini, F.; Poggi, A.; Fernandez, J.L.C.; Rizzo, G.M.R.; Gavilán, H.; Silvestri, N.; Pellegrino, T. Elucidating the innate immunological effects of mild magnetic hyperthermia on U87 human glioblastoma cells: An in vitro study. Pharmaceutics 2021, 13, 1668. [Google Scholar] [CrossRef]
- Heshmati Aghda, N.; Abdulsahib, S.M.; Severson, C.; Lara, E.J.; Torres Hurtado, S.; Yildiz, T.; Castillo, J.A.; Tunnell, J.W.; Betancourt, T. Induction of immunogenic cell death of cancer cells through nanoparticle-mediated dual chemotherapy and photothermal therapy. Int. J. Pharm. 2020, 589, 119787. [Google Scholar] [CrossRef]
- Clares, B.; Biedma-Ortiz, R.A.; Sáez-Fernández, E.; Prados, J.C.; Melguizo, C.; Cabeza, L.; Ortiz, R.; Arias, J.L. Nano-engineering of 5-fluorouracil-loaded magnetoliposomes for combined hyperthermia and chemotherapy against colon cancer. Eur. J. Pharm. Biopharm. 2013, 85, 329–338. [Google Scholar] [CrossRef]
- Li, T.J.; Huang, C.C.; Ruan, P.W.; Chuang, K.Y.; Huang, K.J.; Shieh DBin Yeh, C.S. In vivo anti-cancer efficacy of magnetite nanocrystal-based system using locoregional hyperthermia combined with 5-fluorouracil chemotherapy. Biomaterials 2013, 34, 7873–7883. [Google Scholar] [CrossRef]
- Dabaghi, M.; Quaas, R.; Hilger, I. The treatment of heterotopic human colon xenograft tumors in mice with 5-fluorouracil attached to magnetic nanoparticles in combination with magnetic hyperthermia is more efficient than either therapy alone. Cancers 2020, 12, 2562. [Google Scholar] [CrossRef] [PubMed]
- Mirzaghavami, P.S.; Khoei, S.; Khoee, S.; Shirvalilou, S.; Mahdavi, S.R.; Pirhajati Mahabadi, V. Radio-sensitivity enhancement in HT29 cells through magnetic hyperthermia in combination with targeted nano-carrier of 5-Flourouracil. Mater. Sci. Eng. C 2021, 124, 112043. [Google Scholar] [CrossRef]
- Shirvalilou, S.; Khoee, S.; Khoei, S.; Karimi, M.R.; Sadri, E.; Shirvaliloo, M. Targeted magnetochemotherapy modified by 5-Fu-loaded thermally on/off switching nanoheaters for the eradication of CT26 murine colon cancer by inducing apoptotic and autophagic cell death. Cancer Nanotechnol. 2023, 14, 11. [Google Scholar] [CrossRef]
- Osial, M.; Ha, G.N.; Vu, V.H.; Nguyen, P.T.; Nieciecka, D.; Pietrzyk-Thel, P.; Urbanek, O.; Olusegun, S.J.; Wilczewski, S.; Giersig, M.; et al. One-pot synthesis of magnetic hydroxyapatite (SPION/HAp) for 5-fluorouracil delivery and magnetic hyperthermia. J. Nanopart. Res. 2024, 26, 7. [Google Scholar] [CrossRef]
- Luengo, Y.; Nardecchia, S.; Morales, M.P.; Serrano, M.C. Different cell responses induced by exposure to maghemite nanoparticles. Nanoscale 2013, 5, 11428–11437. [Google Scholar] [CrossRef]
- Ahmed, A.; Tait, S.W.G. Targeting immunogenic cell death in cancer. Mol. Oncol. 2020, 14, 2994–3006. [Google Scholar] [CrossRef]
- Beola, L.; Iturrioz-Rodríguez, N.; Pucci, C.; Bertorelli, R.; Ciofani, G. Drug-Loaded Lipid Magnetic Nanoparticles for Combined Local Hyperthermia and Chemotherapy against Glioblastoma Multiforme. ACS Nano 2023, 17, 18441–18455. [Google Scholar] [CrossRef]
- Adachi, Y.; Kuwahata, A.; Nakamura, E.; Yabukami, S. Enhancing heating efficiency of magnetic hyperthermia using pulsed magnetic fields. AIP Adv. 2024, 14, 015144. [Google Scholar] [CrossRef]
- Yin, H.; Dong, B.; Zhao, Y.; Ma, Y.; Zhao, S.; Sun, Z.; Du, Z.; Peng, J.; Yang, T. Hyperthermia inhibits cellular function and induces immunogenic cell death in renal cell carcinoma. BMC Cancer 2023, 23, 972. [Google Scholar] [CrossRef]
- Ribeiro, T.P.; Moreira, J.A.; Monterio, F.J.; Laranjeira, M.S. Nanomaterials in cancer: Reviewing the combination of hyperthermia and triggered chemotherapy. J. Control. Release 2022, 347, 89–103. [Google Scholar] [CrossRef]
- Meng, X.; Dang, T.; Chai, J. From Apoptosis to Necroptosis: The Death Wishes to Cancer. Cancer Control 2021, 28, 10732748211066311. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Souiade, L.; Rodriguez-Garcia, M.-R.; Serrano-Olmedo, J.-J.; Ramos-Gómez, M. Pulsed Alternating Fields Magnetic Hyperthermia in Combination with Chemotherapy (5-Fluorouracil) as a Cancer Treatment for Glioblastoma Multiform: An In Vitro Study. Nanomaterials 2025, 15, 556. https://doi.org/10.3390/nano15070556
Souiade L, Rodriguez-Garcia M-R, Serrano-Olmedo J-J, Ramos-Gómez M. Pulsed Alternating Fields Magnetic Hyperthermia in Combination with Chemotherapy (5-Fluorouracil) as a Cancer Treatment for Glioblastoma Multiform: An In Vitro Study. Nanomaterials. 2025; 15(7):556. https://doi.org/10.3390/nano15070556
Chicago/Turabian StyleSouiade, Lilia, Miguel-Ramon Rodriguez-Garcia, José-Javier Serrano-Olmedo, and Milagros Ramos-Gómez. 2025. "Pulsed Alternating Fields Magnetic Hyperthermia in Combination with Chemotherapy (5-Fluorouracil) as a Cancer Treatment for Glioblastoma Multiform: An In Vitro Study" Nanomaterials 15, no. 7: 556. https://doi.org/10.3390/nano15070556
APA StyleSouiade, L., Rodriguez-Garcia, M.-R., Serrano-Olmedo, J.-J., & Ramos-Gómez, M. (2025). Pulsed Alternating Fields Magnetic Hyperthermia in Combination with Chemotherapy (5-Fluorouracil) as a Cancer Treatment for Glioblastoma Multiform: An In Vitro Study. Nanomaterials, 15(7), 556. https://doi.org/10.3390/nano15070556






