Enhanced Photocatalytic Activity of CQDs-Modified Layered g-C3N4/Flower-like ZnO Heterojunction for Efficient Degradation of Ciprofloxacin
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
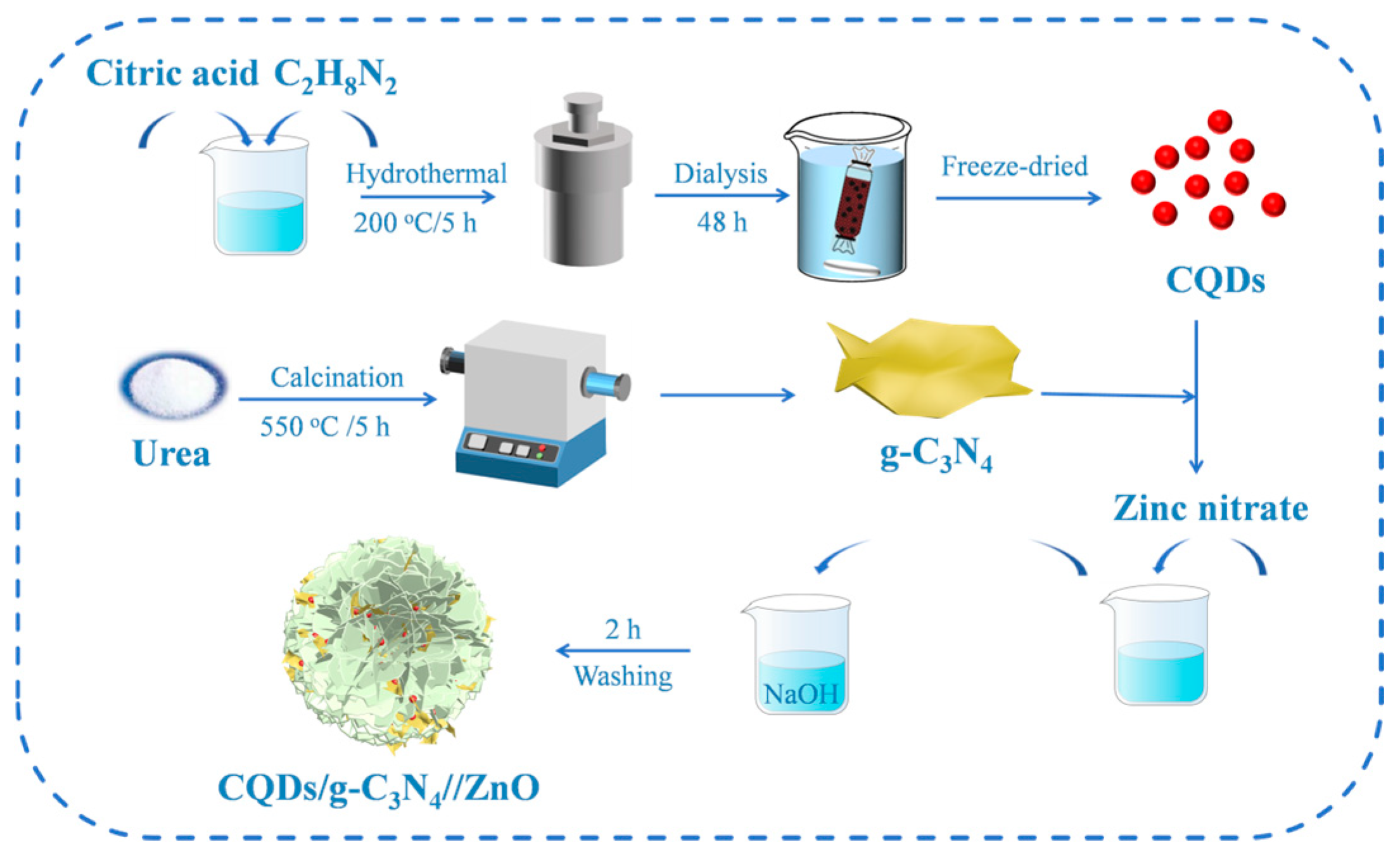
2.2. Catalyst Preparation
2.2.1. Preparation of CQDs
2.2.2. Preparation of g-C3N4
2.2.3. Preparation of CQDs Modified Layered g-C3N4/Flower-like ZnO
2.3. Characterization
2.4. Photocatalytic Degradation Experiments
2.5. Active Species Capturing and Recycling Experiments
2.6. Electrochemical Testing
3. Results
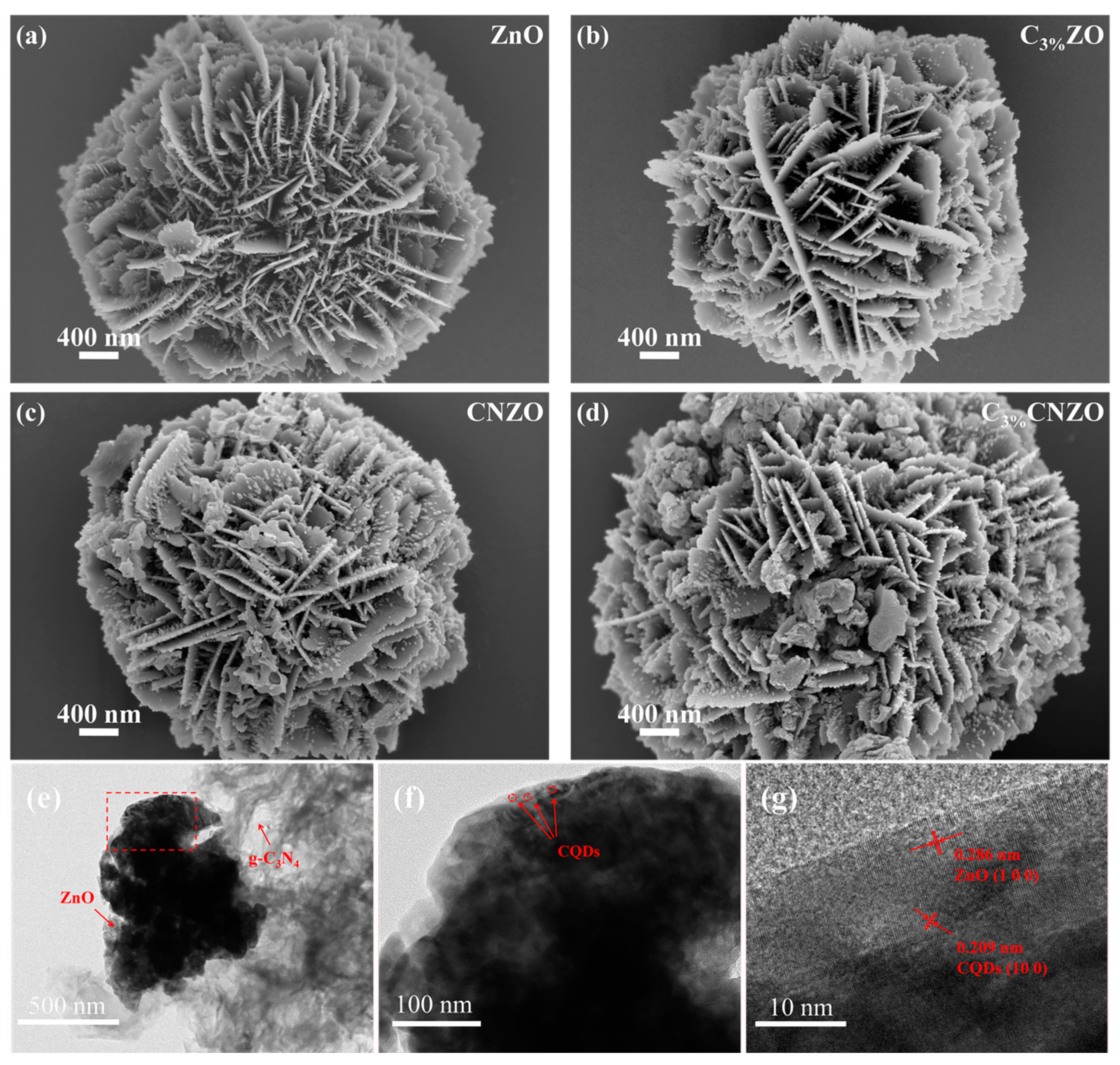
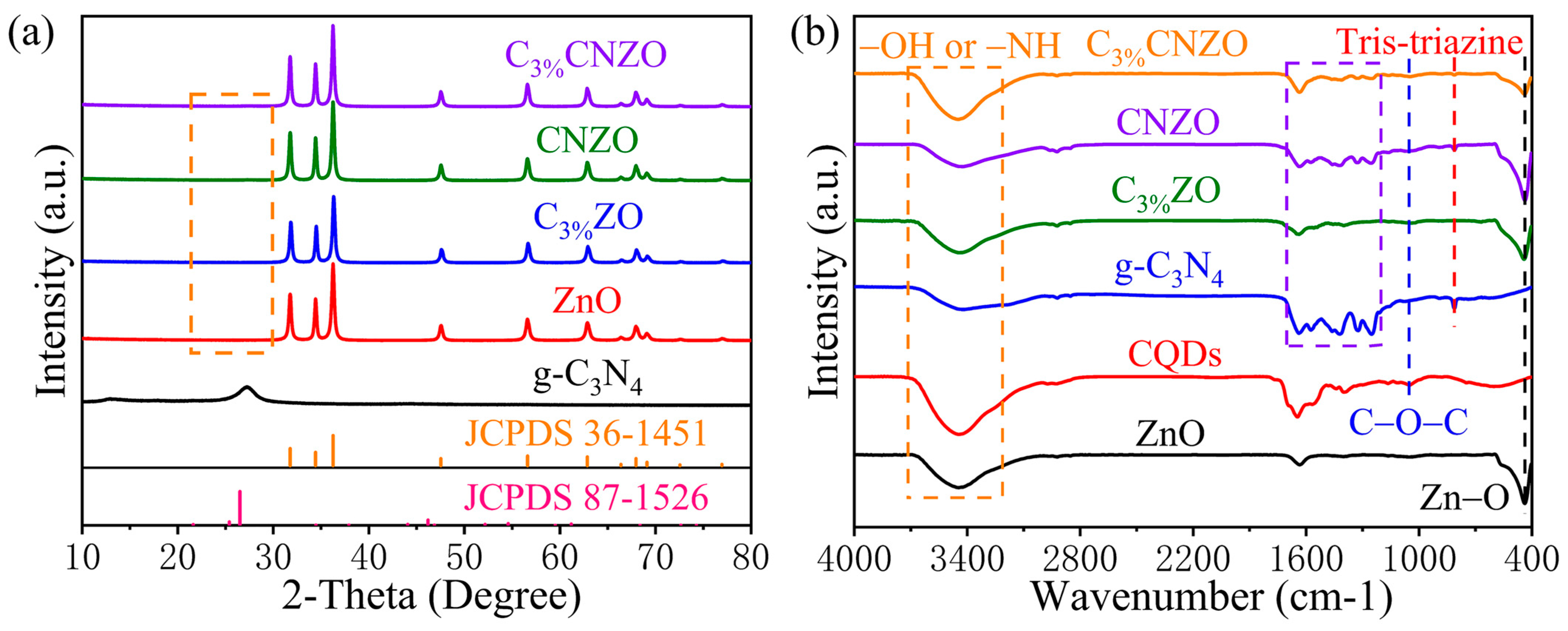
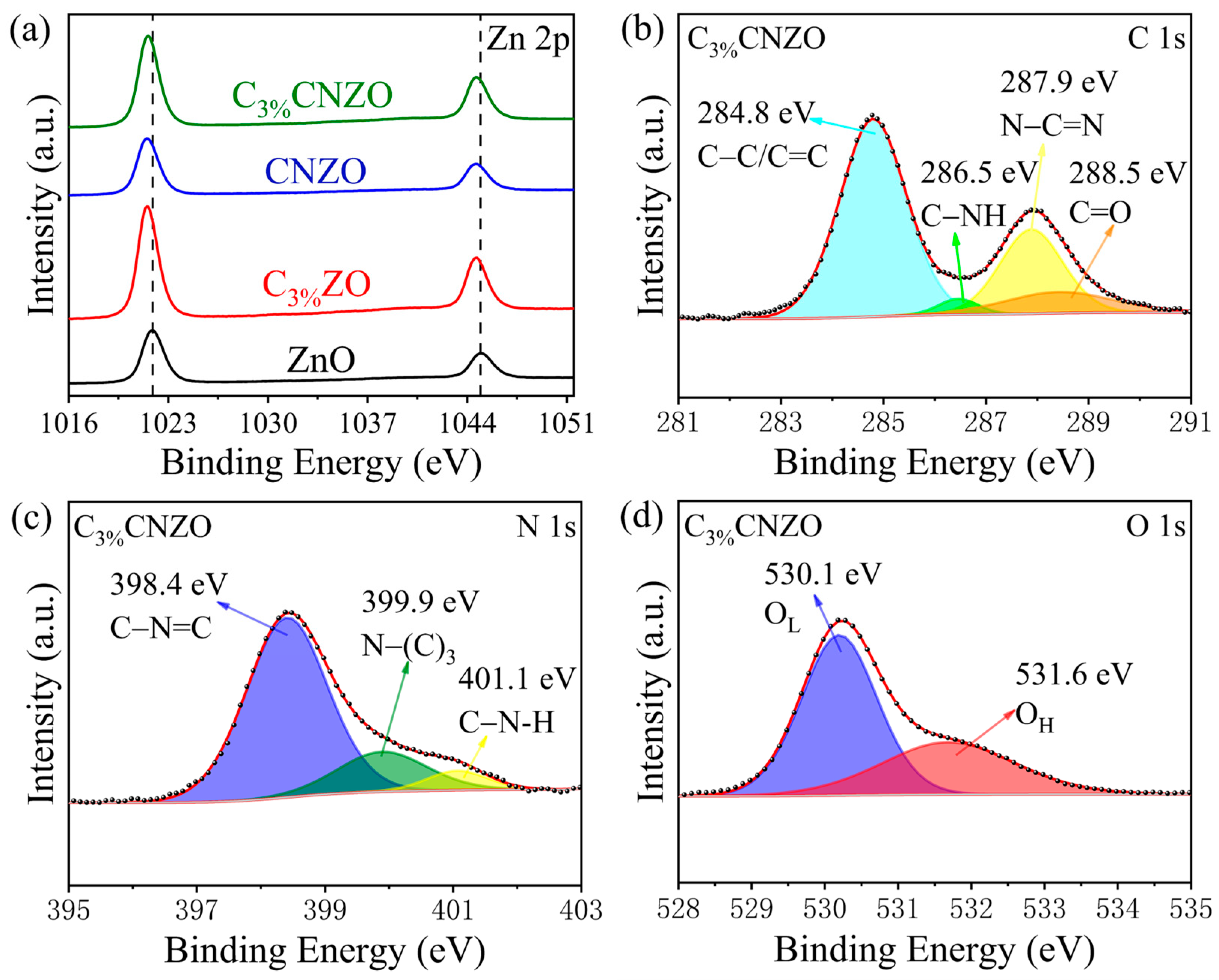
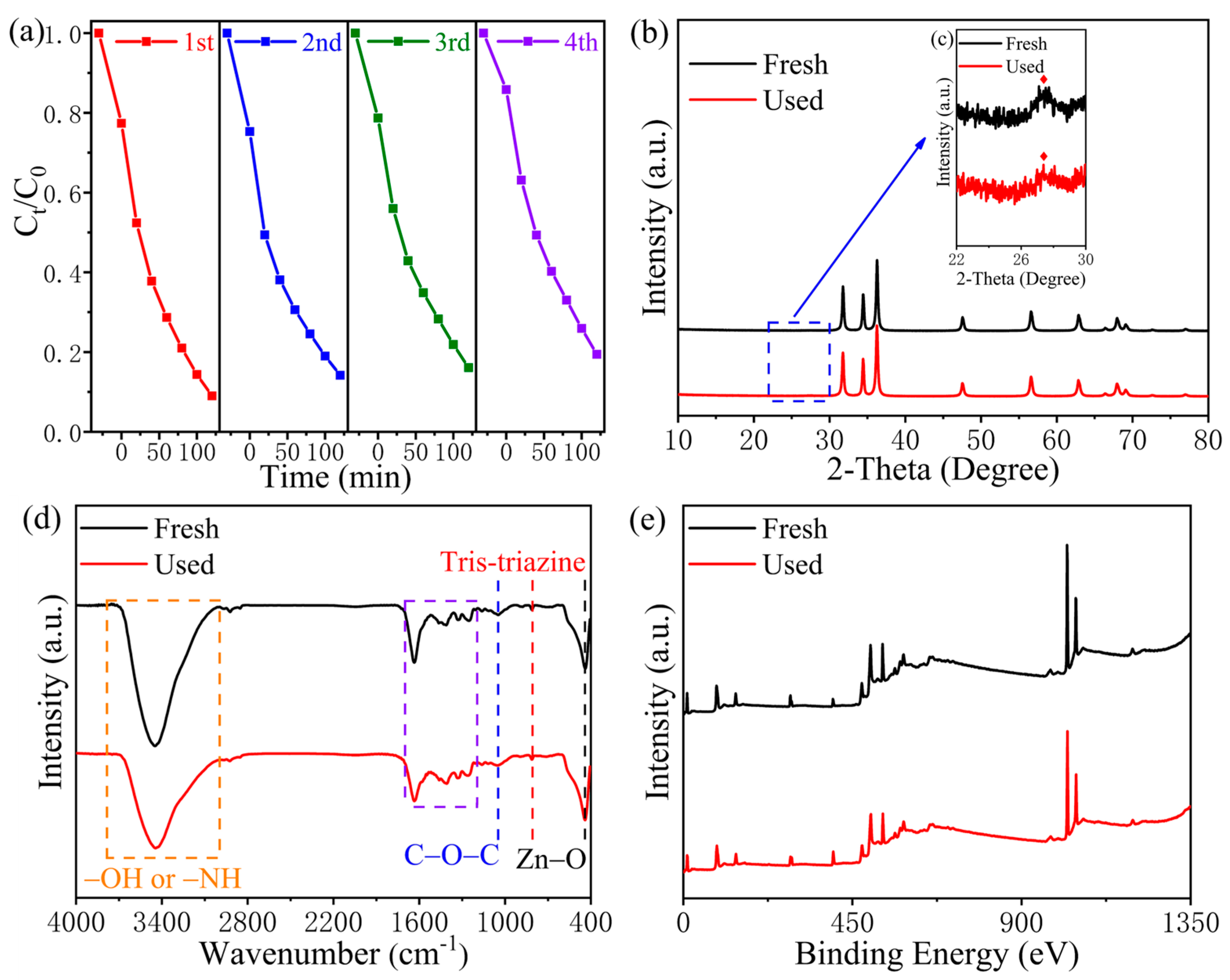
3.1. Characteristics of Materials
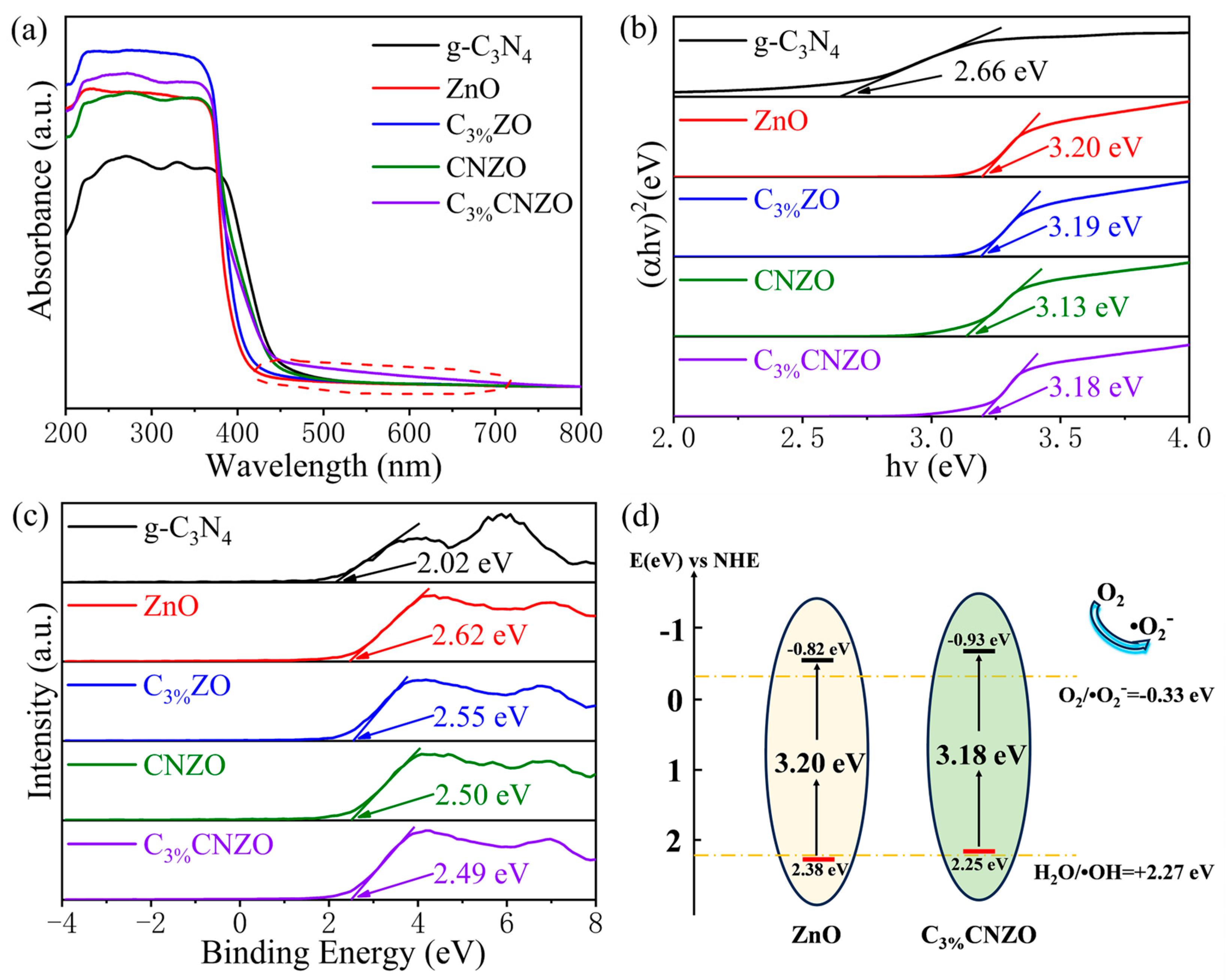
3.2. Photoelectric Chemical Performance Analysis
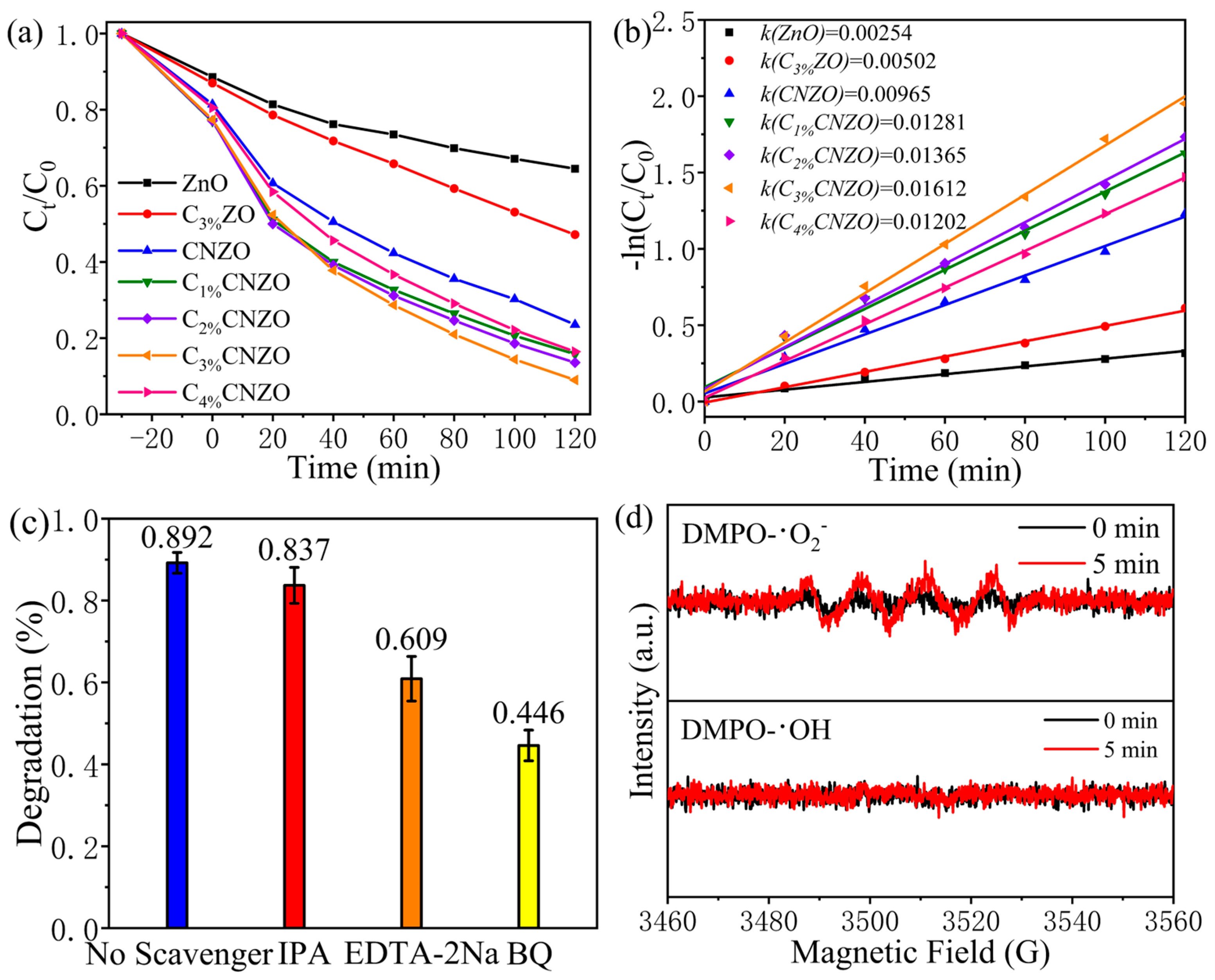
3.3. Photocatalytic Performance
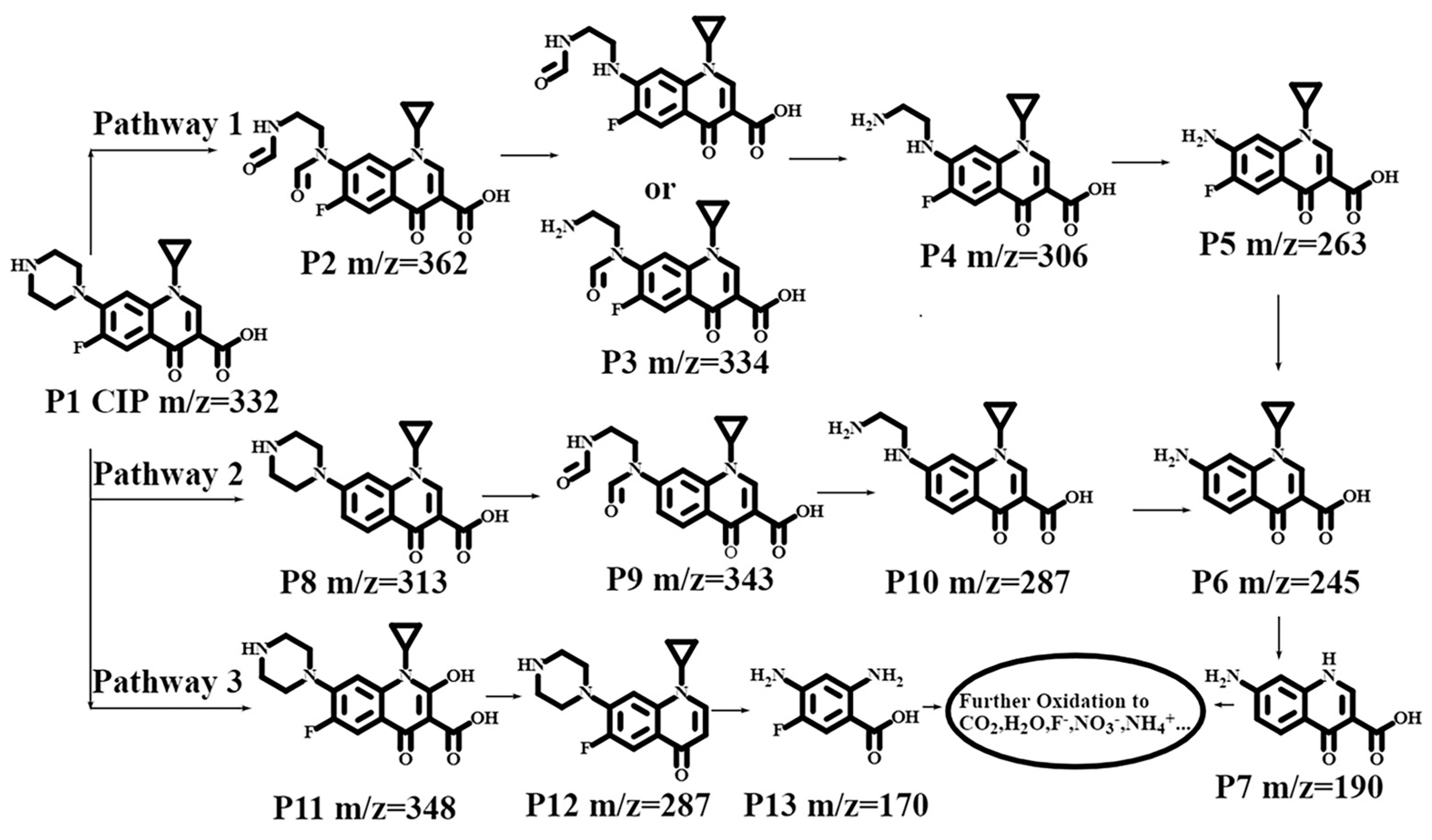
3.4. Ciprofloxacin Degradation Pathway
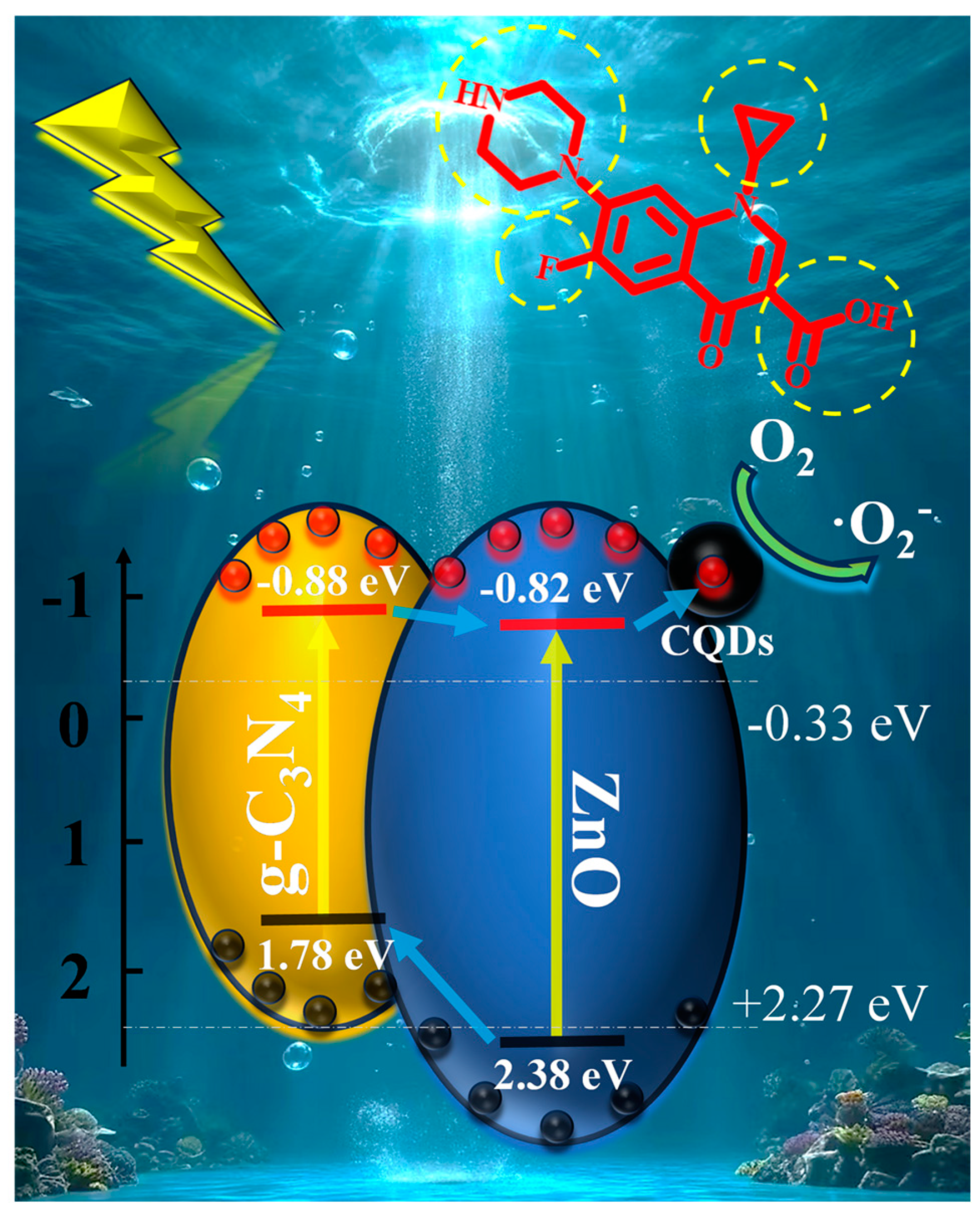
3.5. Photocatalytic Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Liu, D.; Jiang, L.; Chen, D.; Hao, Z.; Deng, B.; Sun, Y.; Liu, X.; Jia, B.; Chen, L.; Liu, H. Photocatalytic self-Fenton degradation of ciprofloxacin over S-scheme CuFe2O4/ZnIn2S4 heterojunction: Mechanism insight, degradation pathways and DFT calculations. Chem. Eng. J. 2024, 482, 149165. [Google Scholar] [CrossRef]
- Jiang, L.; Yuan, X.; Zeng, G.; Wu, Z.; Liang, J.; Chen, X.; Leng, L.; Wang, H.; Wang, H. Metal-free efficient photocatalyst for stable visible-light photocatalytic degradation of refractory pollutant. Appl. Catal. B Environ. 2018, 221, 715–725. [Google Scholar] [CrossRef]
- Rokesh, K.; Sakar, M.; Do, T.-O. Emerging Hybrid Nanocomposite Photocatalysts for the Degradation of Antibiotics: Insights into Their Designs and Mechanisms. Nanomaterials 2021, 11, 16. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Lin, L.; Fang, L.; Yang, Y.; Chen, E.; Yuan, K.; Zou, S.; Wang, X.; Luan, T. Complex pollution of antibiotic resistance genes due to beta-lactam and aminoglycoside use in aquaculture farming. Water Res. 2018, 134, 200–208. [Google Scholar] [CrossRef]
- Teye, G.K.; Huang, J.; Li, Y.; Li, K.; Chen, L.; Darkwah, W.K. Photocatalytic Degradation of Sulfamethoxazole, Nitenpyram and Tetracycline by Composites of Core Shell g-C3N4@ZnO, and ZnO Defects in Aqueous Phase. Nanomaterials 2021, 11, 2609. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, Y.; Ding, J.; Fang, J.; Yang, J.; Xie, Y.; Xu, X. A More Efficient Method for Preparing a MIP-CQDs/ZnO1–x Photodegradant with Highly Selective Adsorption and Photocatalytic Properties. ACS Appl. Mater. Interfaces 2024, 16, 2365–2377. [Google Scholar] [CrossRef]
- Geng, R.; Wang, J.; Zhang, Z.; Dong, Q.; Wu, F.; Chen, S.; Su, T.; Qi, X. Adsorption of antibiotics by polydopamine-modified salecan hydrogel: Performance, kinetics and mechanism studies. Chem. Eng. J. 2023, 454, 140446. [Google Scholar] [CrossRef]
- Wang, J.; Fan, D.; Zhang, L.; Yang, D.; Qiu, X.; Lin, X. Lignin-derived hierarchical porous carbon with high surface area and interconnected pores for efficient antibiotics adsorption. Chem. Eng. J. 2023, 454, 139789. [Google Scholar] [CrossRef]
- Long, S.; Hamilton, P.B.; Wang, C.; Li, C.; Xue, X.; Zhao, Z.; Wu, P.; Gu, E.; Uddin, M.M.; Li, B.; et al. Bioadsorption, bioaccumulation and biodegradation of antibiotics by algae and their association with algal physiological state and antibiotic physicochemical properties. J. Hazard. Mater. 2024, 468, 133787. [Google Scholar] [CrossRef]
- Zhou, S.; Jia, Y.; Fang, H.; Jin, C.; Mo, Y.; Xiao, Z.; Zhang, N.; Sun, L.; Lu, H. A new understanding on the prerequisite of antibiotic biodegradation in wastewater treatment: Adhesive behavior between antibiotic-degrading bacteria and ciprofloxacin. Water Res. 2024, 252, 121226. [Google Scholar] [CrossRef]
- Núñez-de la Rosa, Y.; Durango, L.G.C.; Forim, M.R.; Nascimento, O.R.; Hammer, P.; Aquino, J.M. Unraveling the time evolution and post mortem changes of nanometric MnOOH during in situ oxidation of ciprofloxacin by activated peroxymonosulfate. Appl. Catal. B Environ. 2023, 327, 122439. [Google Scholar] [CrossRef]
- Lin, J.; Zhang, K.; Jiang, L.; Hou, J.; Yu, X.; Feng, M.; Ye, C. Removal of chloramphenicol antibiotics in natural and engineered water systems: Review of reaction mechanisms and product toxicity. Sci. Total Environ. 2022, 850, 158059. [Google Scholar] [CrossRef] [PubMed]
- Jia, Z.; Lv, R.; Guo, L.; Zhang, J.; Li, R.; Liu, J.; Fan, C. Rapid degradation of ciprofloxacin over BiOCl: Insight into the molecular structure transformation and antibacterial activity elimination. Sep. Purif. Technol. 2021, 257, 117872. [Google Scholar] [CrossRef]
- Calvete, M.J.F.; Piccirillo, G.; Vinagreiro, C.S.; Pereira, M.M. Hybrid materials for heterogeneous photocatalytic degradation of antibiotics. Coord. Chem. Rev. 2019, 395, 63–85. [Google Scholar] [CrossRef]
- Li, D.; Shi, W. Recent developments in visible-light photocatalytic degradation of antibiotics. Chin. J. Catal. 2016, 37, 792–799. [Google Scholar] [CrossRef]
- Gusain, R.; Gupta, K.; Joshi, P.; Khatri, O.P. Adsorptive removal and photocatalytic degradation of organic pollutants using metal oxides and their composites: A comprehensive review. Adv. Colloid Interface Sci. 2019, 272, 102009. [Google Scholar] [CrossRef]
- Shandilya, P.; Sambyal, S.; Sharma, R.; Mandyal, P.; Fang, B. Properties, optimized morphologies, and advanced strategies for photocatalytic applications of WO3 based photocatalysts. J. Hazard. Mater. 2022, 428, 128218. [Google Scholar] [CrossRef]
- Kshirsagar, S.D.; Shelake, S.P.; Biswas, B.; Ramesh, K.; Gaur, R.; Abraham, B.M.; Sainath, A.V.S.; Pal, U. Emerging ZnO Semiconductors for Photocatalytic CO2 Reduction to Methanol. Small 2024, 20, 2407318. [Google Scholar] [CrossRef]
- Verma, S.; Younis, S.A.; Kim, K.-H.; Dong, F. Anisotropic ZnO nanostructures and their nanocomposites as an advanced platform for photocatalytic remediation. J. Hazard. Mater. 2021, 415, 125651. [Google Scholar] [CrossRef]
- Ruan, X.; Li, S.; Huang, C.; Zheng, W.; Cui, X.; Ravi, S.K. Catalyzing Artificial Photosynthesis with TiO2 Heterostructures and Hybrids: Emerging Trends in a Classical yet Contemporary Photocatalyst. Adv. Mater. 2023, 36, 2305285. [Google Scholar] [CrossRef]
- Sanchez Tobon, C.; Panžić, I.; Bafti, A.; Matijašić, G.; Ljubas, D.; Ćurković, L. Rapid Microwave-Assisted Synthesis of N/TiO2/rGO Nanoparticles for the Photocatalytic Degradation of Pharmaceuticals. Nanomaterials 2022, 12, 3975. [Google Scholar] [CrossRef] [PubMed]
- Vinoth, S.; Ong, W.-J.; Pandikumar, A. Defect engineering of BiOX (X = Cl, Br, I) based photocatalysts for energy and environmental applications: Current progress and future perspectives. Coord. Chem. Rev. 2022, 464, 214541. [Google Scholar] [CrossRef]
- Song, D.; Li, M.; Liao, L.; Guo, L.; Liu, H.; Wang, B.; Li, Z. High-Crystallinity BiOCl Nanosheets as Efficient Photocatalysts for Norfloxacin Antibiotic Degradation. Nanomaterials 2023, 13, 1841. [Google Scholar] [CrossRef]
- Ali Khan, A.; Tahir, M.; Khan, N. LDH-based nanomaterials for photocatalytic applications: A comprehensive review on the role of bi/trivalent cations, anions, morphology, defect engineering, memory effect, and heterojunction formation. J. Energy Chem. 2023, 84, 242–276. [Google Scholar] [CrossRef]
- Resende Leite, R.; Colombo, R.; Eduardo Bimbi Júnior, F.; Roberto de Vasconcelos Lanza, M.; da Silva Barud, H.; Ramos Moreira Afonso, C.; Inês Basso Bernardi, M. Precursor effect on the hydrothermal synthesis of pure ZnO nanostructures and enhanced photocatalytic performance for norfloxacin degradation. Chem. Eng. J. 2024, 496, 154374. [Google Scholar] [CrossRef]
- Foophow, T.; Lertkowit, P.; Kitthawee, U.; Phoohinkong, W. Preparation and characterization of dextran-modified ZnO and Cu-doped ZnO nanohybrid material for enhanced antimicrobial delivery and activity. Carbohydr. Polym. 2025, 349, 122947. [Google Scholar] [CrossRef] [PubMed]
- Vittal, R.; Ho, K.-C. Zinc oxide based dye-sensitized solar cells: A review. Renew. Sustain. Energy Rev. 2017, 70, 920–935. [Google Scholar] [CrossRef]
- Lee, K.M.; Lai, C.W.; Ngai, K.S.; Juan, J.C. Recent developments of zinc oxide based photocatalyst in water treatment technology: A review. Water Res. 2016, 88, 428–448. [Google Scholar] [CrossRef]
- Cheng, J.; Shen, Y.; Chen, K.; Wang, X.; Guo, Y.; Zhou, X.; Bai, R. Flower-like Bi2WO6/ZnO composite with excellent photocatalytic capability under visible light irradiation. Chin. J. Catal. 2018, 39, 810–820. [Google Scholar] [CrossRef]
- Cheng, Q.; Benipal, M.K.; Liu, Q.; Wang, X.; Crozier, P.A.; Chan, C.K.; Nemanich, R.J. Al2O3 and SiO2 Atomic Layer Deposition Layers on ZnO Photoanodes and Degradation Mechanisms. ACS Appl. Mater. Interfaces 2017, 9, 16138–16147. [Google Scholar] [CrossRef]
- Hailili, R.; Ji, H.; Wang, K.; Dong, X.A.; Chen, C.; Sheng, H.; Bahnemann, D.W.; Zhao, J. ZnO with Controllable Oxygen Vacancies for Photocatalytic Nitrogen Oxide Removal. ACS Catal. 2022, 12, 10004–10017. [Google Scholar] [CrossRef]
- Li, X.; Hu, Z.; Liu, J.; Li, D.; Zhang, X.; Chen, J.; Fang, J. Ga doped ZnO photonic crystals with enhanced photocatalytic activity and its reaction mechanism. Appl. Catal. B Environ. 2016, 195, 29–38. [Google Scholar] [CrossRef]
- Chen, L.; Tsai, M.-L.; Chuang, Y.; Chen, C.-W.; Dong, C.-D. Construction of carbon nanotubes bridged MoS2/ZnO Z-scheme nanohybrid towards enhanced visible light driven photocatalytic water disinfection and antibacterial activity. Carbon 2022, 196, 877–889. [Google Scholar] [CrossRef]
- Luo, C.; Liu, Y.; Yu, J.; Zhou, L.; Zhang, R.; Wang, X. ZnO@In2O3 Core–Shell Heterojunctions Constructed With ZIF-8 and MIL-68 (In) for Improving Photogenerated Carrier Transfer Process. Small 2024, 20, 2404303. [Google Scholar] [CrossRef] [PubMed]
- Muhmood, T.; Ahmad, I.; Haider, Z.; Haider, S.K.; Shahzadi, N.; Aftab, A.; Ahmed, S.; Ahmad, F. Graphene-like graphitic carbon nitride (g-C3N4) as a semiconductor photocatalyst: Properties, classification, and defects engineering approaches. Mater. Today Sustain. 2024, 25, 100633. [Google Scholar] [CrossRef]
- Pan, Y.; Qiao, K.; Ning, C.; Wang, X.; Liu, Z.; Chen, Z. Electrostatic Self-Assembled Synthesis of Amorphous/Crystalline g-C3N4 Homo-Junction for Efficient Photocatalytic H2 Production with Simultaneous Antibiotic Degradation. Nanomaterials 2023, 13, 2964. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yang, Z.; Gao, X.; Yao, W.; Wei, W.; Chen, X.; Zong, R.; Zhu, Y. Core-shell g-C3N4@ZnO composites as photoanodes with double synergistic effects for enhanced visible-light photoelectrocatalytic activities. Appl. Catal. B Environ. 2017, 217, 169–180. [Google Scholar] [CrossRef]
- Gao, X.; Yang, B.; Yao, W.; Wang, Y.; Zong, R.; Wang, J.; Li, X.; Jin, W.; Tao, D. Enhanced photocatalytic activity of ZnO/g-C3N4 composites by regulating stacked thickness of g-C3N4 nanosheets. Environ. Pollut. 2020, 257, 113577. [Google Scholar] [CrossRef]
- Jo, W.-K.; Lee, J.Y.; Selvam, N.C.S. Synthesis of MoS2 nanosheets loaded ZnO–g-C3N4 nanocomposites for enhanced photocatalytic applications. Chem. Eng. J. 2016, 289, 306–318. [Google Scholar] [CrossRef]
- Lv, Y.; Zheng, Y.; Zhu, H.; Wu, Y. Designing a dual-functional material with barrier anti-corrosion and photocatalytic antifouling properties using g-C3N4 nanosheet with ZnO nanoring. J. Mater. Sci. Technol. 2022, 106, 56–69. [Google Scholar] [CrossRef]
- Meena, P.L.; Poswal, K.; Surela, A.K.; Saini, J.K. Synthesis of graphitic carbon nitride/zinc oxide (g-C3N4/ZnO) hybrid nanostructures and investigation of the effect of ZnO on the photodegradation activity of g-C3N4 against the brilliant cresyl blue (BCB) dye under visible light irradiation. Adv. Compos. Hybrid Mater. 2022, 6, 16. [Google Scholar] [CrossRef]
- Mirzaei, A.; Yerushalmi, L.; Chen, Z.; Haghighat, F. Photocatalytic degradation of sulfamethoxazole by hierarchical magnetic ZnO@g-C3N4: RSM optimization, kinetic study, reaction pathway and toxicity evaluation. J. Hazard. Mater. 2018, 359, 516–526. [Google Scholar] [CrossRef] [PubMed]
- Jo, W.-K.; Clament Sagaya Selvam, N. Enhanced visible light-driven photocatalytic performance of ZnO–g-C3N4 coupled with graphene oxide as a novel ternary nanocomposite. J. Hazard. Mater. 2015, 299, 462–470. [Google Scholar] [CrossRef]
- Wen, J.; Zhou, L.; Tang, Q.; Xiao, X.; Sun, S. Photocatalytic degradation of organic pollutants by carbon quantum dots functionalized g-C3N4: A review. Ecotoxicol. Environ. Saf. 2023, 262, 115133. [Google Scholar] [CrossRef]
- Yao, Y.; Zhang, H.; Hu, K.; Nie, G.; Yang, Y.; Wang, Y.; Duan, X.; Wang, S. Carbon dots based photocatalysis for environmental applications. J. Environ. Chem. Eng. 2022, 10, 107336. [Google Scholar] [CrossRef]
- Ren, H.; Qi, F.; Labidi, A.; Zhao, J.; Wang, H.; Xin, Y.; Luo, J.; Wang, C. Chemically bonded carbon quantum dots/Bi2WO6 S-scheme heterojunction for boosted photocatalytic antibiotic degradation: Interfacial engineering and mechanism insight. Appl. Catal. B Environ. 2023, 330, 122587. [Google Scholar] [CrossRef]
- Huang, K.; Lin, L.; Zhang, L.; Zhao, M.; Dai, X.; Jiang, Y.; Yang, R.; Liao, J.; Zhang, Y.; Wang, Y. Rapid synthesis of ultra-bright blue and cyan CQDs fluorescent powders based on chemical dispersion and concentration effects. Ceram. Int. 2024, 50, 4046–4052. [Google Scholar] [CrossRef]
- Xu, N.; Gao, S.; Xu, C.; Fang, Y.; Xu, L.; Zhang, W. Carbon quantum dots derived from waste acorn cups and its application as an ultraviolet absorbent for polyvinyl alcohol film. Appl. Surf. Sci. 2021, 556, 149774. [Google Scholar] [CrossRef]
- Rathi, V.; Panneerselvam, A.; Sathiyapriya, R. Graphitic carbon nitride (g-C3N4) decorated ZnWO4 heterojunctions architecture synthesis, characterization and photocatalytic activity evaluation. Diam. Relat. Mater. 2020, 108, 107981. [Google Scholar] [CrossRef]
- Wang, F.; Feng, Y.; Chen, P.; Wang, Y.; Su, Y.; Zhang, Q.; Zeng, Y.; Xie, Z.; Liu, H.; Liu, Y.; et al. Photocatalytic degradation of fluoroquinolone antibiotics using ordered mesoporous g-C3N4 under simulated sunlight irradiation: Kinetics, mechanism, and antibacterial activity elimination. Appl. Catal. B Environ. 2018, 227, 114–122. [Google Scholar] [CrossRef]
- Xu, H.-Y.; Zhang, S.-Q.; Wang, Y.-F.; Xu, Y.; Dong, L.-M.; Komarneni, S. New insights into the photocatalytic mechanism of pristine ZnO nanocrystals: From experiments to DFT calculations. Appl. Surf. Sci. 2023, 614, 156225. [Google Scholar] [CrossRef]
- Lu, W.; Qi, L.; Dong, D.; Shen, X.; Xu, L.; Zhang, Y.; Mei, X.; Qiao, W.; Guo, X.; Pan, Y. A comparison study of photocatalytic performance of g-C3N4 prepared from different precursors for the activation of different peroxides. Sep. Purif. Technol. 2023, 327, 124904. [Google Scholar] [CrossRef]
- Hu, X.; Hu, X.; Peng, Q.; Zhou, L.; Tan, X.; Jiang, L.; Tang, C.; Wang, H.; Liu, S.; Wang, Y.; et al. Mechanisms underlying the photocatalytic degradation pathway of ciprofloxacin with heterogeneous TiO2. Chem. Eng. J. 2020, 380, 122366. [Google Scholar] [CrossRef]
- Yu, H.; Huang, J.; Jiang, L.; Yuan, X.; Yi, K.; Zhang, W.; Zhang, J.; Chen, H. Steering photo-excitons towards active sites: Intensified substrates affinity and spatial charge separation for photocatalytic molecular oxygen activation and pollutant removal. Chem. Eng. J. 2021, 408, 127334. [Google Scholar] [CrossRef]
- Hui, J.; Wu, R.; Zhu, Y.; Zhang, Z.; Wei, S.; Ouyang, F. Citric acid-assisted in situ preparation of MoIn2S4/CQDs with few-layer promotes charge transfer and enhances photocatalytic activity. Appl. Surf. Sci. 2024, 667, 160420. [Google Scholar] [CrossRef]
- Hidayat, R.N.; Widiyandari, H.; Parasdila, H.; Prilita, O.; Astuti, Y.; Mufti, N.; Ogi, T. Green synthesis of ZnO photocatalyst composited carbon quantum dots (CQDs) from lime (Citrus aurantifolia). Catal. Commun. 2024, 187, 106888. [Google Scholar] [CrossRef]
- Li, Z.; Wen, C.; Li, D.; Fang, Z.; Lin, Z.; Liu, D.; Wang, Y.; Zhang, X.; Chen, P.; Lv, W.; et al. Insights into nitrogen-doped BiOBr with oxygen vacancy and carbon quantum dots photocatalysts for the degradation of sulfonamide antibiotics: Actions to promote exciton dissociation and carrier migration. Chem. Eng. J. 2024, 492, 152449. [Google Scholar] [CrossRef]
- Li, W.; Zuo, Y.; Jiang, L.; Yao, D.; Chen, Z.; He, G.; Chen, H. Bi2Ti2O7/TiO2/RGO composite for the simulated sunlight-driven photocatalytic degradation of ciprofloxacin. Mater. Chem. Phys. 2020, 256, 123650. [Google Scholar] [CrossRef]
- Wen, X.-J.; Niu, C.-G.; Zhang, L.; Liang, C.; Guo, H.; Zeng, G.-M. Photocatalytic degradation of ciprofloxacin by a novel Z-scheme CeO2–Ag/AgBr photocatalyst: Influencing factors, possible degradation pathways, and mechanism insight. J. Catal. 2018, 358, 141–154. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, J.; Wu, D. Enhanced photocatalytic degradation of ciprofloxacin over Bi2MoO6/g-C3N4/BiFeO3 heterojunction photocatalyst under visible light irradiation. Mater. Sci. Semicond. Process. 2022, 151, 107011. [Google Scholar] [CrossRef]
- Zhang, H.; Xu, J.; Yuan, Y.; Guo, Y.; Tan, X.; Wang, H.; Hu, X.; Tang, C. Highly conductive Ti3C2 MXene reinforced g-C3N4 heterojunction photocatalytic for the degradation of ciprofloxacin: Mechanism insight. Sep. Purif. Technol. 2024, 330, 125520. [Google Scholar] [CrossRef]
- Bai, J.; Zhang, B.; Xiong, T.; Jiang, D.; Ren, X.; Lu, P.; Fu, M. Enhanced visible light driven photocatalytic performance of Bi2WO6 nano-catalysts by introducing oxygen vacancy. J. Alloys Compd. 2021, 887, 161297. [Google Scholar] [CrossRef]
- Yin, Y.; Yao, Y.; Qian, X.; Sun, M.; Huang, B.; He, G.; Chen, H. Fabrication of Fe/BiOCl/RGO with enhanced photocatalytic degradation of ciprofloxacin under visible light irradiation. Mater. Sci. Semicond. Process. 2022, 140, 106384. [Google Scholar] [CrossRef]
- Lu, Z.; Zhu, Z.; Wang, D.; Ma, Z.; Shi, W.; Yan, Y.; Zhao, X.; Dong, H.; Yang, L.; Hua, Z. Specific oriented recognition of a new stable ICTX@Mfa with retrievability for selective photocatalytic degrading of ciprofloxacin. Catal. Sci. Technol. 2016, 6, 1367–1377. [Google Scholar] [CrossRef]
- Hu, K.; Li, R.; Ye, C.; Wang, A.; Wei, W.; Hu, D.; Qiu, R.; Yan, K. Facile synthesis of Z-scheme composite of TiO2 nanorod/g-C3N4 nanosheet efficient for photocatalytic degradation of ciprofloxacin. J. Clean. Prod. 2020, 253, 120055. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Q.; Deng, W.; Zhang, H.; Fang, J.; Xie, Y.; Liu, C.; Han, X.; Xu, X.; Zhou, Z. Enhanced Photocatalytic Activity of CQDs-Modified Layered g-C3N4/Flower-like ZnO Heterojunction for Efficient Degradation of Ciprofloxacin. Nanomaterials 2025, 15, 550. https://doi.org/10.3390/nano15070550
Liu Q, Deng W, Zhang H, Fang J, Xie Y, Liu C, Han X, Xu X, Zhou Z. Enhanced Photocatalytic Activity of CQDs-Modified Layered g-C3N4/Flower-like ZnO Heterojunction for Efficient Degradation of Ciprofloxacin. Nanomaterials. 2025; 15(7):550. https://doi.org/10.3390/nano15070550
Chicago/Turabian StyleLiu, Qing, Wei Deng, Hai Zhang, Jiajun Fang, Yushi Xie, Congwen Liu, Xiaochen Han, Xiaoling Xu, and Zuowan Zhou. 2025. "Enhanced Photocatalytic Activity of CQDs-Modified Layered g-C3N4/Flower-like ZnO Heterojunction for Efficient Degradation of Ciprofloxacin" Nanomaterials 15, no. 7: 550. https://doi.org/10.3390/nano15070550
APA StyleLiu, Q., Deng, W., Zhang, H., Fang, J., Xie, Y., Liu, C., Han, X., Xu, X., & Zhou, Z. (2025). Enhanced Photocatalytic Activity of CQDs-Modified Layered g-C3N4/Flower-like ZnO Heterojunction for Efficient Degradation of Ciprofloxacin. Nanomaterials, 15(7), 550. https://doi.org/10.3390/nano15070550






