Photosensitizer and Charge Separator Roles of g-C₃N₄ Integrated into the CuO-Fe₂O₃ p-n Heterojunction Interface for Elevating PEC Water Splitting Potential
Abstract
1. Introduction
2. Experimental Work
2.1. Materials and Chemicals
2.2. Synthesis of CuO-Fe2O3@g-C3N4 Ternary Composite
2.3. Characterization and PEC Water-Splitting Analysis
3. Characterization Analysis
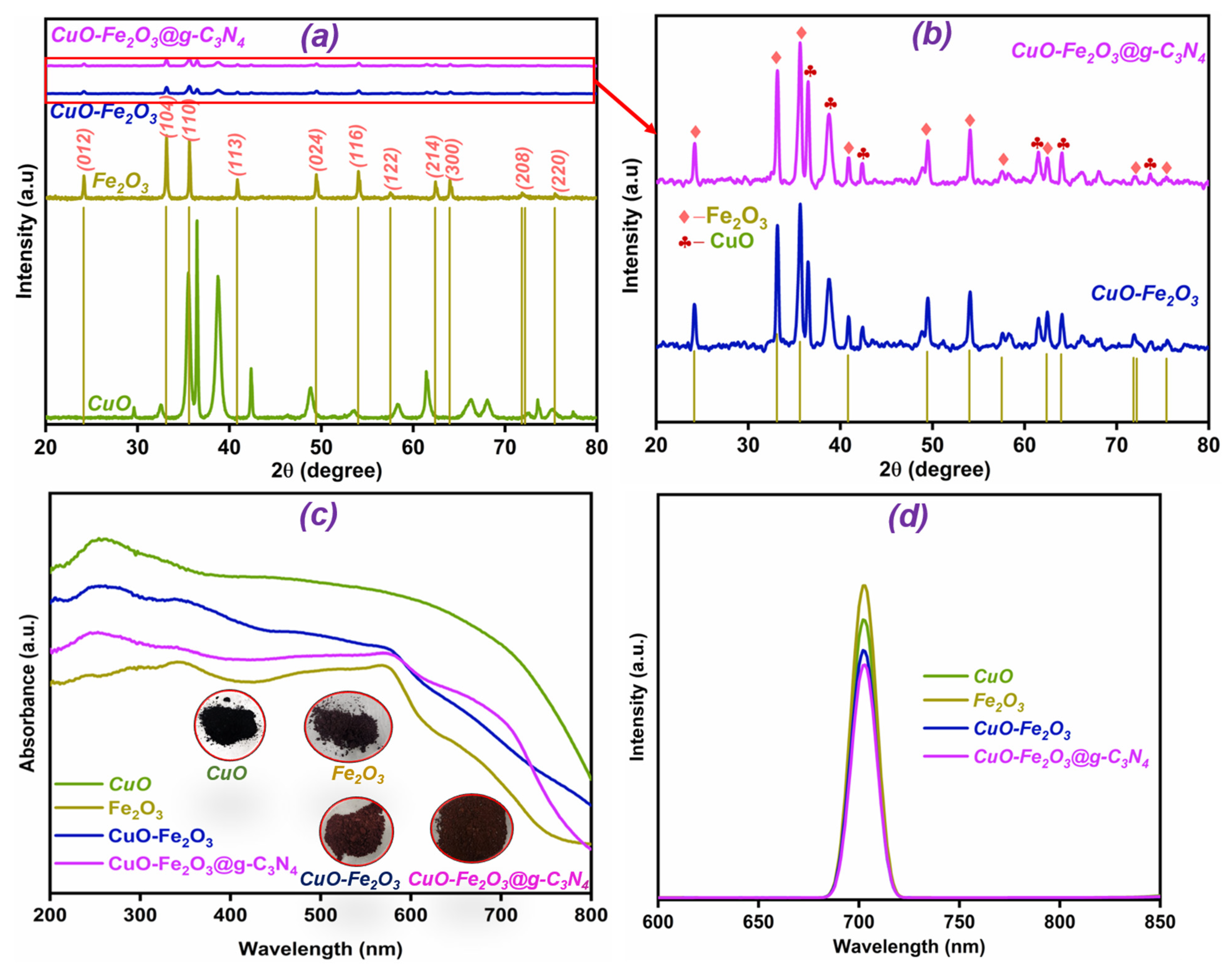
3.1. Crystallographic and Structural Features
3.2. Optical Characteristics
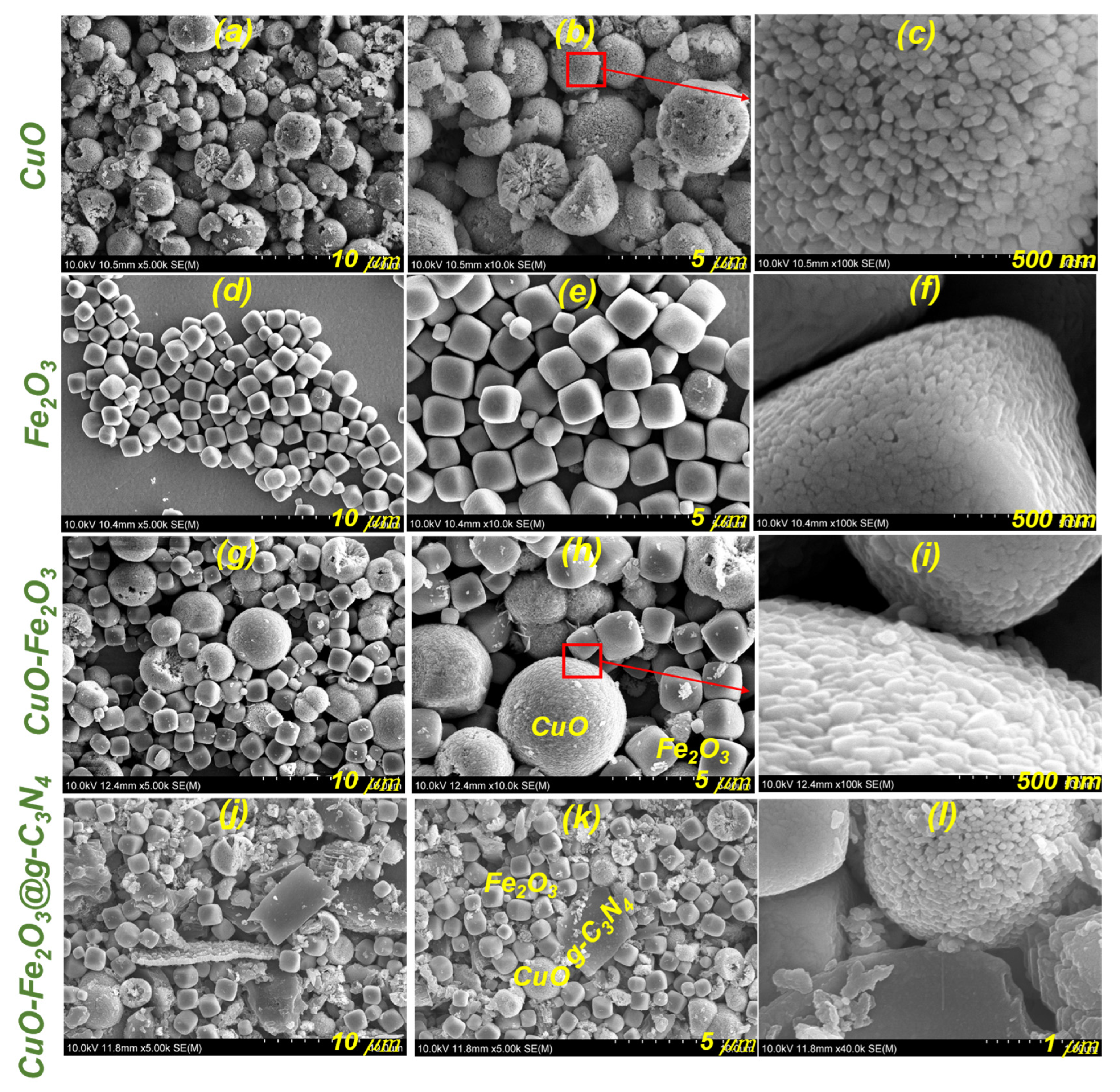
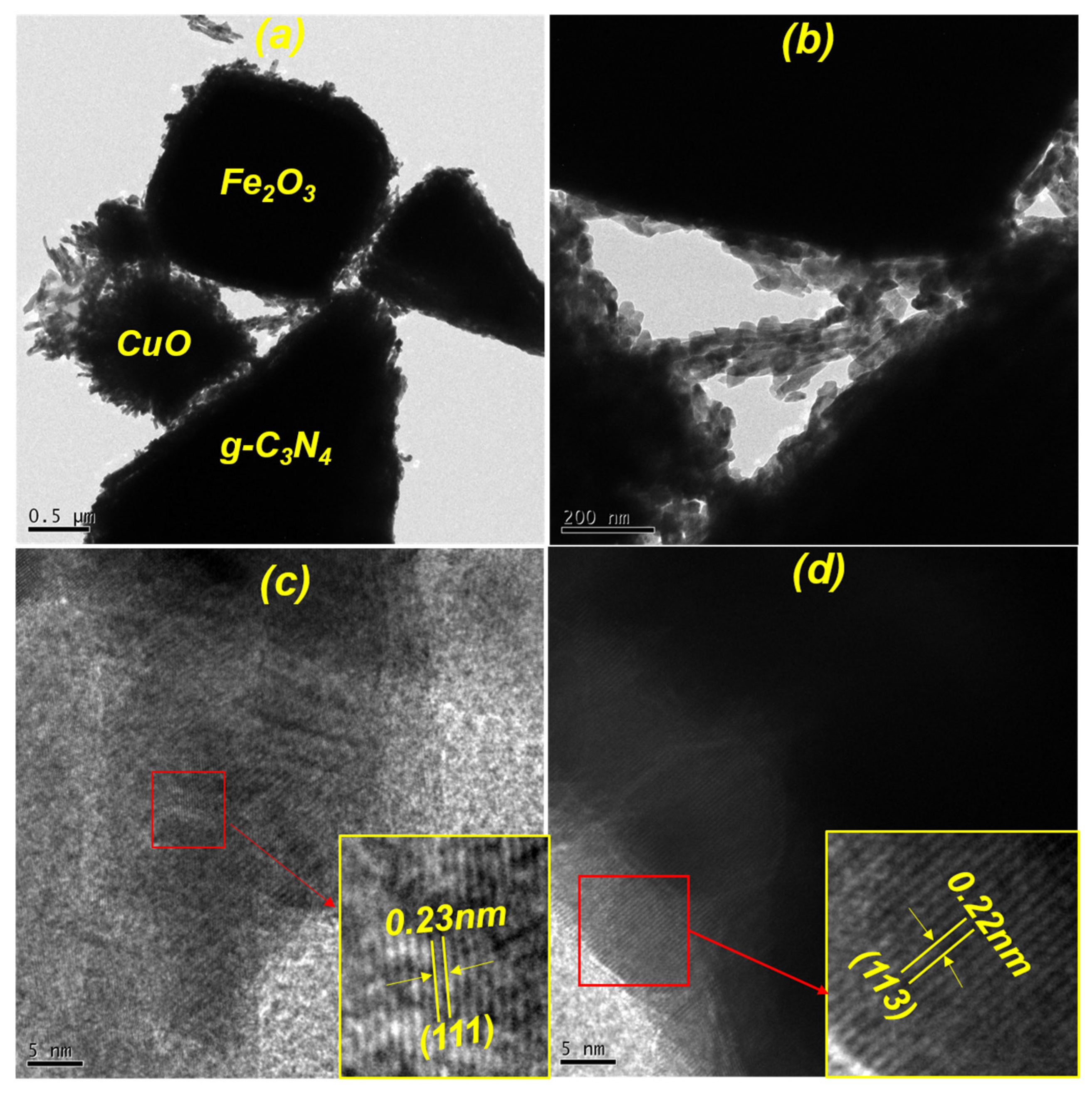
3.3. Morphological Properties
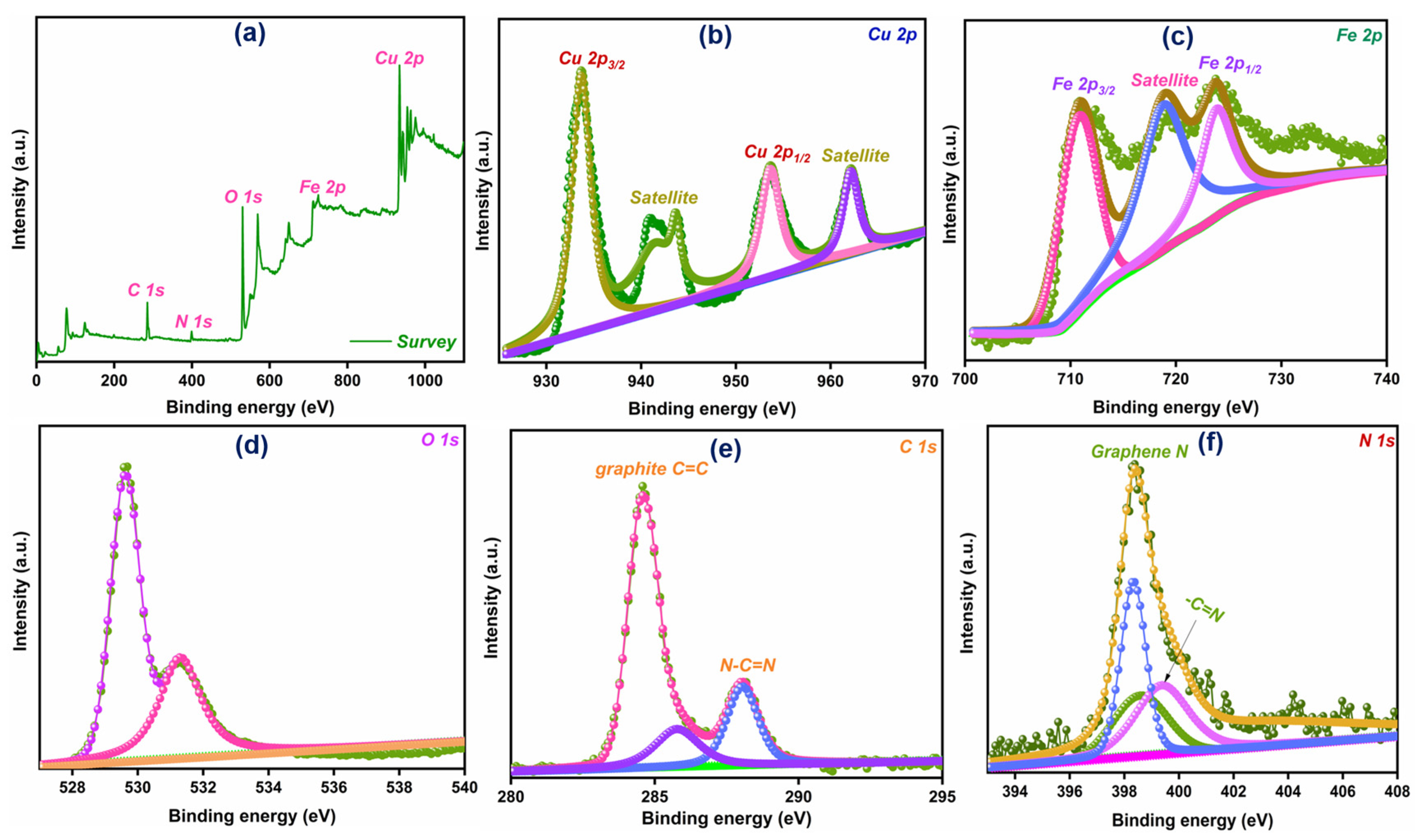
3.4. X-Ray Photoelectron Spectroscopy (XPS) Analysis
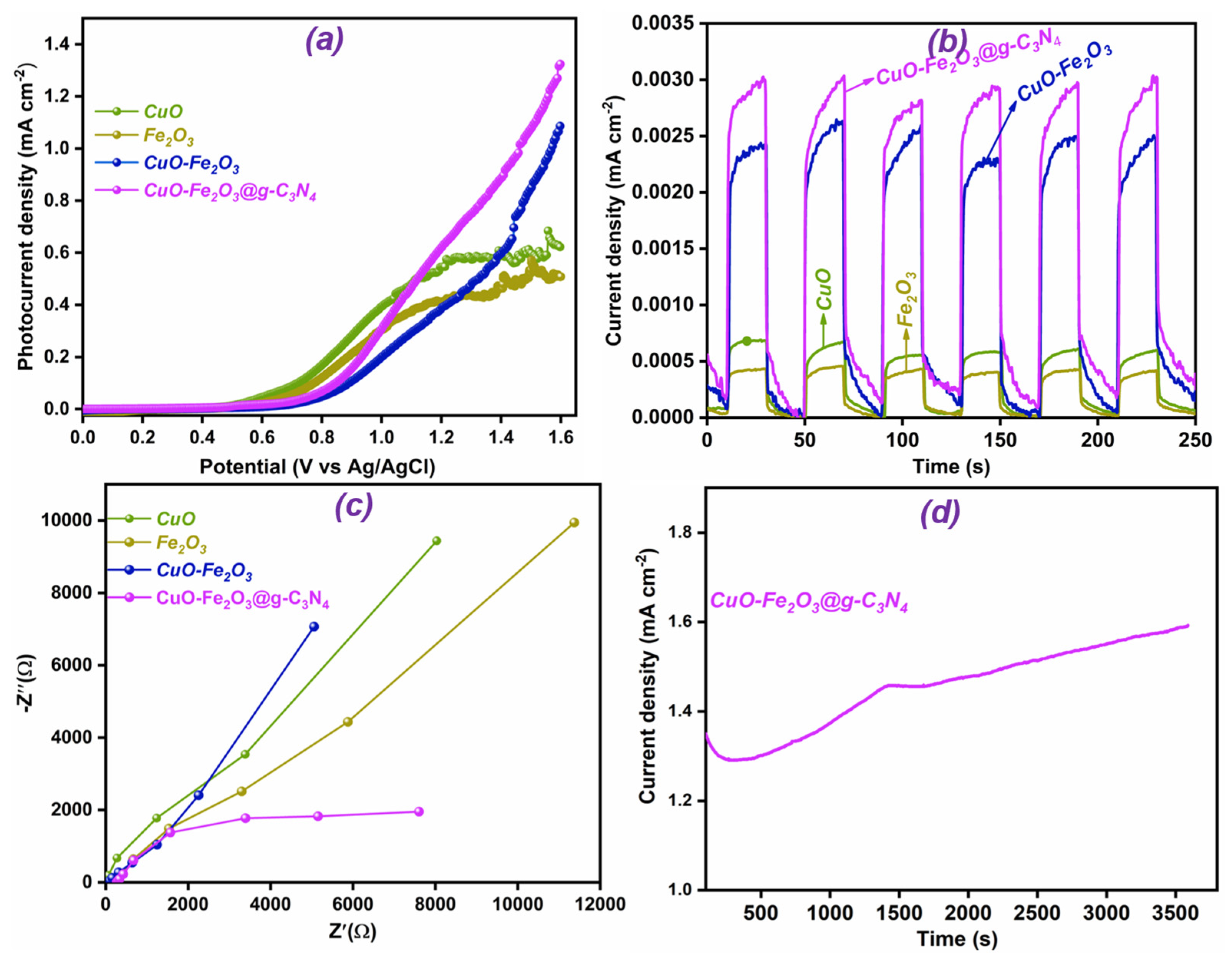
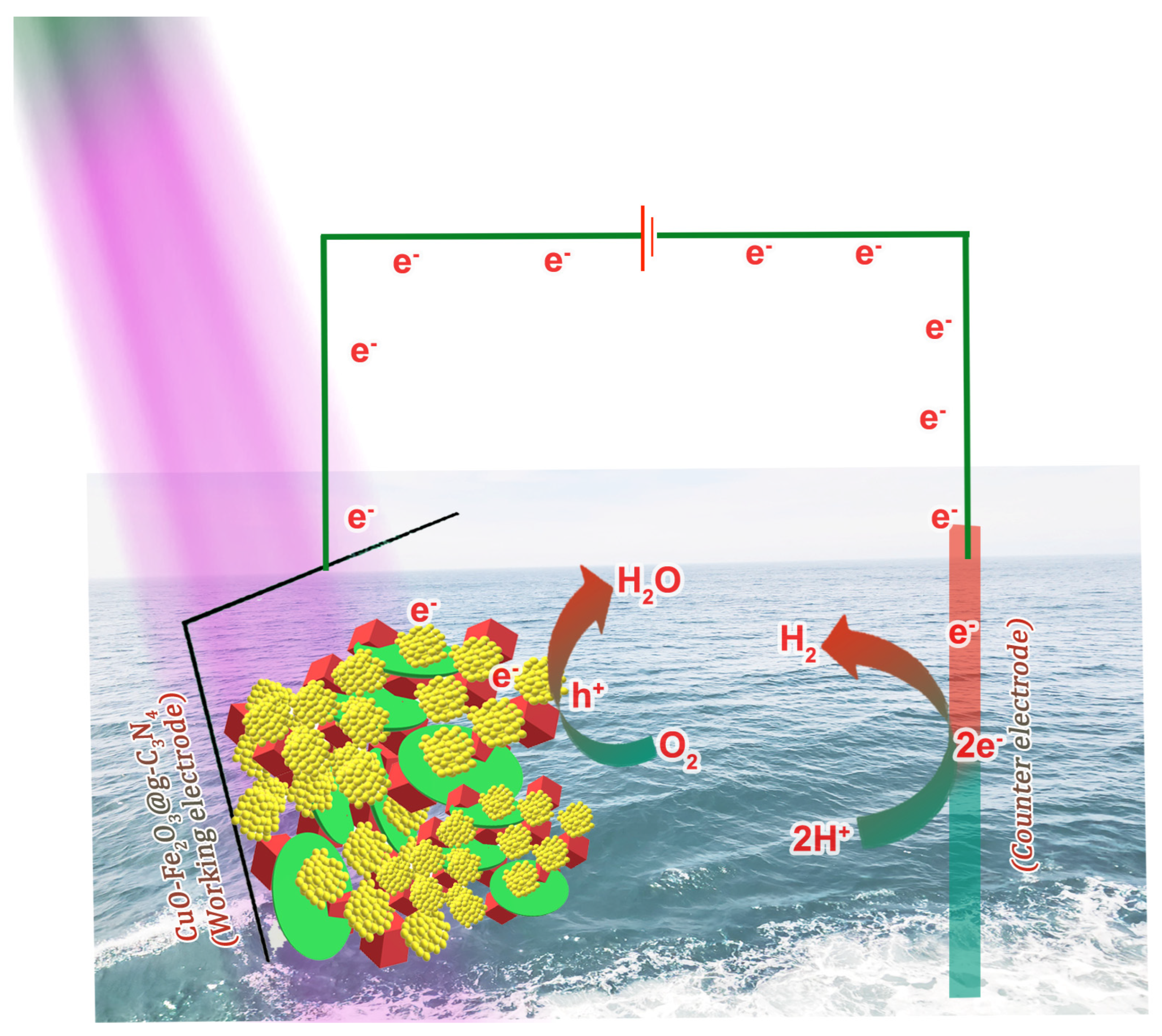
4. PEC Water-Splitting Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mohamed, N.A.; Safaei, J.; Ismail, A.F.; Khalid, M.N.; Mohd Jailani, M.F.A.; Noh, M.F.M.; Arzaee, N.A.; Zhou, D.; Sagu, J.S.; Teridi, M.A.M. Boosting Photocatalytic Activities of BiVO4 by Creation of g-C3N4/ZnO@BiVO4 Heterojunction. Mater. Res. Bull. 2020, 125, 110779. [Google Scholar] [CrossRef]
- Jiao, Z.; Guan, X.; Wang, M.; Wang, Q.; Xu, B.; Bi, Y.; Zhao, X.S. Undamaged Depositing Large-Area ZnO Quantum Dots/RGO Films on Photoelectrodes for the Construction of Pure Z-Scheme. Chem. Eng. J. 2019, 356, 781–790. [Google Scholar] [CrossRef]
- Yadav, J.; Singh, J.P. WO3/Ag2S Type-II Hierarchical Heterojunction for Improved Charge Carrier Separation and Photoelectrochemical Water Splitting Performance. J. Alloys Compd. 2022, 925, 166684. [Google Scholar] [CrossRef]
- Sharma, P.; Jang, J.W.; Lee, J.S. Key Strategies to Advance the Photoelectrochemical Water Splitting Performance of α-Fe2O3 Photoanode. ChemCatChem 2019, 11, 157–179. [Google Scholar] [CrossRef]
- Liao, J.; Feng, Y.; Zhang, X.; Huang, L.; Huang, S.; Liu, M.; Liu, Q.; Li, H. CuO-Co3O4 Composite Nanoplatelets for Hydrolyzing Ammonia Borane. ACS Appl. Nano Mater. 2021, 4, 7640–7649. [Google Scholar] [CrossRef]
- Yu, J.; Li, Z.; Liu, T.; Zhao, S.; Guan, D.; Chen, D.; Shao, Z.; Ni, M. Morphology Control and Electronic Tailoring of CoxAy (A = P, S, Se) Electrocatalysts for Water Splitting. Chem. Eng. J. 2023, 460, 141674. [Google Scholar]
- Guo, B.Y.; Zhang, X.Y.; Ma, X.; Chen, T.S.; Chen, Y.; Wen, M.L.; Qin, J.F.; Nan, J.; Chai, Y.M.; Dong, B. RuO2/Co3O4 Nanocubes Based on Ru Ions Impregnation into Prussian Blue Precursor for Oxygen Evolution. Int. J. Hydrogen Energy 2020, 45, 9575–9582. [Google Scholar]
- Zarezadeh, S.; Habibi-Yangjeh, A.; Mousavi, M.; Ghosh, S. Synthesis of Novel P-n-p BiOBr/ZnO/BiOI Heterostructures and Their Efficient Photocatalytic Performances in Removals of Dye Pollutants under Visible Light. J. Photochem. Photobiol. A Chem. 2020, 389, 112247. [Google Scholar]
- Manh Hung, N.; Thi Bich, V.; Duc Quang, N.; Tien Hiep, N.; Nguyen, C.V.; Majumder, S.; Tien Hung, P.; Dinh Hoat, P.; Van Hoang, N.; Minh Hieu, N.; et al. CuS–CdS@TiO2 Multi-Heterostructure-Based Photoelectrode for Highly Efficient Photoelectrochemical Water Splitting. Ceram. Int. 2023, 49, 23796–23804. [Google Scholar] [CrossRef]
- Reddy, N.R.; Reddy, P.M.; Jyothi, N.; Kumar, A.S.; Jung, J.H.; Joo, S.W. Versatile TiO2 Bandgap Modification with Metal, Non-Metal, Noble Metal, Carbon Material, and Semiconductor for the Photoelectrochemical Water Splitting and Photocatalytic Dye Degradation Performance. J. Alloys Compd. 2023, 935, 167713. [Google Scholar] [CrossRef]
- Hernández, S.; Cauda, V.; Chiodoni, A.; Dallorto, S.; Sacco, A.; Hidalgo, D.; Celasco, E.; Pirri, C.F. Optimization of 1D ZnO@TiO2 Core-Shell Nanostructures for Enhanced Photoelectrochemical Water Splitting under Solar Light Illumination. ACS Appl. Mater. Interfaces 2014, 6, 12153–12167. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Ma, H.; Liu, Z. Highly Efficient Photocatalyst Based on All Oxides WO3/Cu2O Heterojunction for Photoelectrochemical Water Splitting. Appl. Catal. B Environ. 2017, 201, 84–91. [Google Scholar] [CrossRef]
- Ning, F.; Shao, M.; Xu, S.; Fu, Y.; Zhang, R.; Wei, M.; Evans, D.G.; Duan, X. TiO2/Graphene/NiFe-Layered Double Hydroxide Nanorod Array Photoanodes for Efficient Photoelectrochemical Water Splitting. Energy Environ. Sci. 2016, 9, 2633–2643. [Google Scholar] [CrossRef]
- Zhang, Z.; Sun, L.; Wu, Z.; Liu, Y.; Li, S. Facile Hydrothermal Synthesis of CuO-Cu2O/GO Nanocomposites for the Photocatalytic Degradation of Organic Dye and Tetracycline Pollutants. New J. Chem. 2020, 44, 6420–6427. [Google Scholar] [CrossRef]
- Fang, G.; Liu, Z.; Han, C. Enhancing the PEC Water Splitting Performance of BiVO4 Co-Modifying with NiFeOOH and Co-Pi Double Layer Cocatalysts. Appl. Surf. Sci. 2020, 515, 146095. [Google Scholar] [CrossRef]
- Zhang, J.; Zhu, Q.; Wang, L.; Nasir, M.; Cho, S.H.; Zhang, J. g-C3N4/CoAl-LDH 2D/2D Hybrid Heterojunction for Boosting Photocatalytic Hydrogen Evolution. Int. J. Hydrogen Energy 2020, 45, 21331–21340. [Google Scholar] [CrossRef]
- Kyesmen, P.I.; Nombona, N.; Diale, M. Heterojunction of Nanostructured α-Fe2O3/CuO for Enhancement of Photoelectrochemical Water Splitting. J. Alloys Compd. 2021, 863, 158724. [Google Scholar] [CrossRef]
- Xu, Q.; Zhang, Z.; Song, X.; Yuan, S.; Qiu, Z.; Xu, H.; Cao, B. Improving the Triethylamine Sensing Performance Based on Debye Length: A Case Study on A-Fe2O3@NiO(CuO) Core-Shell Nanorods Sensor Working at near Room-Temperature. Sens. Actuators B Chem. 2017, 245, 375–385. [Google Scholar] [CrossRef]
- Murugan, C.; Karnan, M.; Sathish, M.; Pandikumar, A. Construction of Heterostructure Based on Hierarchical Bi2MoO6 and g-C3N4 with Ease for Impressive Performance in Photoelectrocatalytic Water Splitting and Supercapacitor. Catal. Sci. Technol. 2020, 10, 2427–2442. [Google Scholar] [CrossRef]
- Wen, P.; Sun, Y.; Li, H.; Liang, Z.; Wu, H.; Zhang, J.; Zeng, H.; Geyer, S.M.; Jiang, L. A Highly Active Three-Dimensional Z-Scheme ZnO/Au/g-C3N4 Photocathode for Efficient Photoelectrochemical Water Splitting. Appl. Catal. B Environ. 2020, 263, 118180. [Google Scholar] [CrossRef]
- Ghane, N.; Sadrnezhaad, S.K.; Hosseini H., S.M. Combustion Synthesis of g-C3N4/Fe2O3 Nanocomposite for Superior Photoelectrochemical Catalytic Performance. Appl. Surf. Sci. 2020, 534, 147563. [Google Scholar] [CrossRef]
- Li, D.; Liang, Z.; Zhang, W.; Dai, S.; Zhang, C. Preparation and Photocatalytic Performance of TiO2-RGO-CuO/Fe2O3 Ternary Composite Photocatalyst by Solvothermal Method. Mater. Res. Express 2021, 8, 015025. [Google Scholar] [CrossRef]
- Ma, J.; Wang, Q.; Li, L.; Zong, X.; Sun, H.; Tao, R.; Fan, X. Fe2O3 Nanorods/CuO Nanoparticles p-n Heterojunction Photoanode: Effective Charge Separation and Enhanced Photoelectrochemical Properties. J. Colloid Interface Sci. 2021, 602, 32–42. [Google Scholar] [CrossRef]
- Liu, Y.; Ye, Z.; Li, D.; Wang, M.; Zhang, Y.; Huang, W. Tuning CuOx -TiO2 Interaction and Photocatalytic Hydrogen Production of CuOx/TiO2 Photocatalysts via TiO2 Morphology Engineering. Appl. Surf. Sci. 2019, 473, 500–510. [Google Scholar] [CrossRef]
- Asen, P.; Shahrokhian, S. A High Performance Supercapacitor Based on Graphene/Polypyrrole/Cu2O-Cu(OH)2 Ternary Nanocomposite Coated on Nickel Foam. J. Phys. Chem. C 2017, 121, 6508–6519. [Google Scholar] [CrossRef]
- Djellabi, R.; Yang, B.; Adeel Sharif, H.M.; Zhang, J.; Ali, J.; Zhao, X. Sustainable and Easy Recoverable Magnetic TiO2-Lignocellulosic Biomass@Fe3O4 for Solar Photocatalytic Water Remediation. J. Clean. Prod. 2019, 233, 841–847. [Google Scholar] [CrossRef]
- Reddy, N.R.; Kumar, A.S.; Reddy, P.M.; Merum, D.; Kakarla, R.R.; Jung, J.H.; Joo, S.W.; Aminabhavi, T.M. Sharp-Edged Pencil Type ZnO Flowers and BiOI Flakes Combined with Carbon Nanofibers as Heterostructured Hybrid Photocatalysts for the Removal of Hazardous Pollutants from Contaminated Water. J. Environ. Manag. 2023, 332, 117397. [Google Scholar] [CrossRef]
- Pradhan, A.C.; Uyar, T. Morphological Control of Mesoporosity and Nanoparticles within Co3O4-CuO Electrospun Nanofibers: Quantum Confinement and Visible Light Photocatalysis Performance. ACS Appl. Mater. Interfaces 2017, 9, 35757–35774. [Google Scholar] [CrossRef]
- Praveen Kumar, D.; Lakshmana Reddy, N.; Srinivas, B.; Durgakumari, V.; Roddatis, V.; Bondarchuk, O.; Karthik, M.; Ikuma, Y.; Shankar, M.V. Stable and Active CuxO/TiO2 nanostructured Catalyst for Proficient Hydrogen Production under Solar Light Irradiation. Sol. Energy Mater. Sol. Cells 2016, 146, 63–71. [Google Scholar] [CrossRef]
- Liu, Q.; Cao, J.; Ji, Y.; Liu, Y.; Liu, C.; Che, G.; Wang, D.; Cao, J.; Li, W.; Liu, X. The Direct Z-Scheme CdxZn1-XS Nanorods-Fe2O3 Quantum Dots Heterojunction/Reduced Graphene Oxide Nanocomposites for Photocatalytic Degradation and Photocatalytic Hydrogen Evolution. Appl. Surf. Sci. 2021, 570, 151085. [Google Scholar] [CrossRef]
- Habibi-Yangjeh, A.; Mousavi, M.; Nakata, K. Boosting Visible-Light Photocatalytic Performance of g-C3N4/Fe3O4 Anchored with CoMoO4 Nanoparticles: Novel Magnetically Recoverable Photocatalysts. J. Photochem. Photobiol. A Chem. 2019, 368, 120–136. [Google Scholar]
- Mousavi, M.; Habibi-Yangjeh, A. Magnetically Recoverable Highly Efficient Visible-Light-Active g-C3N4/Fe3O4/Ag2WO4/AgBr Nanocomposites for Photocatalytic Degradations of Environmental Pollutants. Adv. Powder Technol. 2018, 29, 94–105. [Google Scholar]
- Reddy, N.R.; Bhargav, U.; Kumari, M.M.; Cheralathan, K.K.; Shankar, M.V.; Reddy, K.R.; Saleh, T.A.; Aminabhavi, T.M. Highly Efficient Solar Light-Driven Photocatalytic Hydrogen Production over Cu/FCNTs-Titania Quantum Dots-Based Heterostructures. J. Environ. Manage. 2020, 254, 109747. [Google Scholar] [CrossRef] [PubMed]
- Reddy, N.R.; Kumar, A.S.; Reddy, P.M.; Reddy, R.; Woo, S.; Aminabhavi, T.M. Novel Rhombus Co3O4 -Nanocapsule CuO Heterohybrids for Efficient Photocatalytic Water Splitting and Electrochemical Energy Storage Applications. J. Environ. Manage. 2023, 325, 116650. [Google Scholar] [CrossRef]
- Fang, Y.; Wang, Y.; Wang, F.; Shu, C.; Zhu, J.; Wu, W. Fe-Mn Bimetallic Oxides-Catalyzed Oxygen Reduction Reaction in Alkaline Direct Methanol Fuel Cells. RSC Adv. 2018, 8, 8678–8687. [Google Scholar]
- May, Y.A.; Wei, S.; Yu, W.Z.; Wang, W.W.; Jia, C.J. Highly Efficient CuO/α-MnO2 Catalyst for Low-Temperature CO Oxidation. Langmuir 2020, 36, 11196–11206. [Google Scholar]
- Wang, Y.; Zhou, M.; He, Y.; Zhou, Z.; Sun, Z. In Situ Loading CuO Quantum Dots on TiO2 Nanosheets as Cocatalyst for Improved Photocatalytic Water Splitting. J. Alloys Compd. 2020, 813, 152184. [Google Scholar]
- Nallapureddy, R.R.; Pallavolu, M.R.; Nallapureddy, J.; Yedluri, A.K.; Joo, S.W. Z-Scheme Photocatalysis and Photoelectrochemical Platform with a Co3O4-CuO Heterogeneous Catalyst for the Removal of Water Pollutants and Generation of Energy. J. Clean. Prod. 2023, 382, 135302. [Google Scholar] [CrossRef]
- Suresh, R.; Giribabu, K.; Manigandan, R.; Stephen, A.; Narayanan, V. Fabrication of Ni-Fe2O3 Magnetic Nanorods and Application to the Detection of Uric Acid. RSC Adv. 2014, 4, 17146–17155. [Google Scholar]
- Zou, D.; Yi, Y.; Song, Y.; Guan, D.; Xu, M.; Ran, R.; Wang, W.; Zhou, W.; Shao, Z. The BaCe0.16Y0.04Fe0.8O3-d Nanocomposite: A New High-Performance Cobalt-Free Triple-Conducting Cathode for Protonic Ceramic Fuel Cells Operating at Reduced Temperatures. J. Mater. Chem. A 2022, 10, 5381–5390. [Google Scholar]
- Li, J.; Li, F.; Qian, M.; Han, M.; Liu, H.; Zhang, D.; Ma, J.; Zhao, C. Characteristics and Regulatory Pathway of the PrupeSEP1 SEPALLATA Gene during Ripening and Softening in Peach Fruits. Plant Sci. 2017, 257, 63–73. [Google Scholar]
- Lyu, J.; Ge, M.; Hu, Z.; Guo, C. One-Pot Synthesis of Magnetic CuO/Fe2O3/CuFe2O4 Nanocomposite to Activate Persulfate for Levofloxacin Removal: Investigation of Efficiency, Mechanism and Degradation Route. Chem. Eng. J. 2020, 389, 124456. [Google Scholar]
- Tan, L.; Xu, J.; Zhang, X.; Hang, Z.; Jia, Y.; Wang, S. Synthesis of g-C3N4/CeO2 Nanocomposites with Improved Catalytic Activity on the Thermal Decomposition of Ammonium Perchlorate. Appl. Surf. Sci. 2015, 356, 447–453. [Google Scholar]
- Huang, Z.; Sun, Q.; Lv, K.; Zhang, Z.; Li, M.; Li, B. Effect of Contact Interface between TiO2 and g-C3N4 on the Photoreactivity of g-C3N4/TiO2 Photocatalyst: (001) vs (101) Facets of TiO2. Appl. Catal. B Environ. 2015, 164, 420–427. [Google Scholar]
- Cao, S.W.; Yuan, Y.P.; Barber, J.; Loo, S.C.J.; Xue, C. Noble-Metal-Free g-C3N4 /Ni(DmgH)2 Composite for Efficientphotocatalytic Hydrogen Evolution under Visible Light Irradiation. Appl. Surf. Sci. 2014, 319, 344–349. [Google Scholar]
- Kakinuma, K.; Suda, K.; Kobayashi, R.; Tano, T.; Arata, C.; Amemiya, I.; Watanabe, S.; Matsumoto, M.; Imai, H.; Iiyama, A.; et al. Electronic States and Transport Phenomena of Pt Nanoparticle Catalysts Supported on Nb-Doped SnO2 for Polymer Electrolyte Fuel Cells. ACS Appl. Mater. Interfaces 2019, 11, 34957–34963. [Google Scholar] [PubMed]
- Arunachalam, M.; Lee, D.G.; Das, P.K.; Subhash, K.R.; Ahn, K.S.; Kang, S.H. Surface Engineering of Ba-Doped TiO2 Nanorods by Bi2O3 Passivation and (NiFe)OOH Co-Catalyst Layers for Efficient and Stable Solar Water Oxidation. Int. J. Hydrogen Energy 2022, 47, 40920–40931. [Google Scholar] [CrossRef]
- Chen, Y.C.; Yeh, H.Y.; Popescu, R.; Gerthsen, D.; Hsu, Y.K. Solution–Processed Cu2O/ZnO/TiO2/Pt Nanowire Photocathode for Efficient Photoelectrochemical Water Splitting. J. Alloys Compd. 2022, 899, 163348. [Google Scholar]
- Zheng, Y.; Ruan, Q.; Ren, J.X.; Guo, X.; Zhou, Y.; Zhou, B.; Xu, Q.; Fu, Q.; Wang, S.; Huang, Y. Plasma- Assisted Liquid-Based Growth of g-C3N4/Mn2O3 p-n Heterojunction with Tunable Valence Band for Photoelectrochemical Application. Appl. Catal. B Environ. 2023, 323, 122170. [Google Scholar]
- Zhang, S.; Yan, J.; Yang, S.; Xu, Y.; Cai, X.; Li, X.; Zhang, X.; Peng, F.; Fang, Y. Electrodeposition of Cu2O/g-C3N4 Heterojunction Film on an FTO Substrate for Enhancing Visible Light Photoelectrochemical Water Splitting. Cuihua Xuebao/Chinese J. Catal. 2017, 38, 365–371. [Google Scholar]
- Moakhar, R.S.; Soleimani, F.; Sadrnezhaad, S.K.; Masudy-Panah, S.; Katal, R.; Seza, A.; Ghane, N.; Ramakrishna, S. One-Pot Microwave Synthesis of Hierarchical C-Doped CuO Dandelions/g-C3N4 Nanocomposite with Enhanced Photostability for Photoelectrochemical Water Splitting. Appl. Surf. Sci. 2020, 530, 147271. [Google Scholar]
- Parvari, R.; Ghorbani-Shahna, F.; Bahrami, A.; Azizian, S.; Assari, M.J.; Farhadian, M. A Novel Core-Shell Structured α-Fe2O3/Cu/g-C3N4 Nanocomposite for Continuous Photocatalytic Removal of Air Ethylbenzene under Visible Light Irradiation. J. Photochem. Photobiol. A Chem. 2020, 399, 112643. [Google Scholar] [CrossRef]
- Reddy, I.N.; Sreedhar, A.; Shim, J.; Gwag, J.S. Multifunctional Monoclinic VO2 Nanorod Thin Films for Enhanced Energy Applications: Photoelectrochemical Water Splitting and Supercapacitor. J. Electroanal. Chem. 2019, 835, 40–47. [Google Scholar] [CrossRef]
- Chen, M.; Chang, X.; Li, C.; Wang, H.; Jia, L. Ni-Doped BiVO4 Photoanode for Efficient Photoelectrochemical Water Splitting. J. Colloid Interface Sci. 2023, 640, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chen, R.; Xiang, L.; Komarneni, S. Synthesis, Properties and Applications of ZnO Nanomaterials with Oxygen Vacancies: A Review. Ceram. Int. 2018, 44, 7357–7377. [Google Scholar] [CrossRef]
- Li, S.; Wang, L.; Li, Y.D.; Zhang, L.; Wang, A.; Xiao, N.; Gao, Y.; Li, N.; Song, W.; Ge, L.; et al. Novel Photocatalyst Incorporating Ni-Co Layered Double Hydroxides with P-Doped CdS for Enhancing Photocatalytic Activity towards Hydrogen Evolution. Appl. Catal. B Environ. 2019, 254, 145–155. [Google Scholar] [CrossRef]
- Hao, C.; Wang, W.; Zhang, R.; Zou, B.; Shi, H. Enhanced Photoelectrochemical Water Splitting with TiO2@Ag2O Nanowire Arrays via P-n Heterojunction Formation. Sol. Energy Mater. Sol. Cells 2018, 174, 132–139. [Google Scholar] [CrossRef]
- Bai, S.; Yang, X.; Liu, C.; Xiang, X.; Luo, R.; He, J.; Chen, A. An Integrating Photoanode of WO3/Fe2O3 Heterojunction Decorated with NiFe-LDH to Improve PEC Water Splitting Efficiency. ACS Sustain. Chem. Eng. 2018, 6, 12906–12913. [Google Scholar] [CrossRef]
- Chen, L.; Zuo, X.; Yang, S.; Cai, T.; Ding, D. Rational Design and Synthesis of Hollow Co3O4@Fe2O3 Core-Shell Nanostructure for the Catalytic Degradation of Norfloxacin by Coupling with Peroxymonosulfate. Chem. Eng. J. 2019, 359, 373–384. [Google Scholar] [CrossRef]
- Sitara, E.; Nasir, H.; Mumtaz, A.; Ehsan, M.F.; Sohail, M.; Iram, S.; Bukhari, S.A.B.; Ullah, S.; Akhtar, T.; Iqbal, A. Enhanced Photoelectrochemical Water Splitting Using Zinc Selenide/Graphitic Carbon Nitride Type-II Heterojunction Interface. Int. J. Hydrogen Energy 2021, 46, 25424–25435. [Google Scholar] [CrossRef]
- Mustafa, E.; Dawi, E.A.; Ibupoto, Z.H.; Ibrahim, A.M.M.; Elsukova, A.; Liu, X.; Tahira, A.; Adam, R.E.; Willander, M.; Nur, O. Efficient CuO/Ag2WO4 Photoelectrodes for Photoelectrochemical Water Splitting Using Solar Visible Radiation. RSC Adv. 2023, 13, 11297–11310. [Google Scholar] [CrossRef] [PubMed]
- Yun, X.; Lei, Y.; Wang, Z.; Bo, X.; Ma, Y. Highly Enhanced Photoelectrocatalytic Activity of NiFe/Ni/BiVO4 Photoanode by a Facile Photoelectron-Activation Process in Neutral Solution. J. Photochem. Photobiol. A Chem. 2025, 458, 2–11. [Google Scholar] [CrossRef]
- Ding, Y.; Huang, L.; Barakat, T.; Su, B.L. A Novel 3DOM TiO2 Based Multifunctional Photocatalytic and Catalytic Platform for Energy Regeneration and Pollutants Degradation. Adv. Mater. Interfaces 2021, 8, 1–12. [Google Scholar]
- John, S.; Roy, S.C. CuO/Cu2O nanoflake/nanowire heterostructure photocathode with enhanced surface area for photoelectrochemical solar energy conversion. Appl. Surf. Sci. 2020, 509, 144703. [Google Scholar] [CrossRef]
- Tian, J.; Li, H.; Xing, Z.; Wang, L.; Luo, Y.; Asiri, A.M.; Al-Youbi, A.O.; Sun, X. One-pot green hydrothermal synthesis of CuO–Cu2O–Cu nanorod-decorated reduced graphene oxide composites and their application in photocurrent generation. Catal. Sci. Technol. 2012, 2, 2227–2230. [Google Scholar] [CrossRef]
- Mahmood, A.; Tezcan, F.; Kardaş, G. Molybdenum disulfide as the interfacial layer in the CuO–TiO2 photocathode for photoelectrochemical cells. J. Mater. Sci. Mater. Electron. 2017, 28, 12937–12943. [Google Scholar] [CrossRef]
- Borkar, R.; Dahake, R.; Rayalu, S.; Bansiwal, A. Copper Oxide Nanograss for Efficient and Stable Photoelectrochemical Hydrogen Production by Water Splitting. J. Electron. Mater. 2017, 47, 1824–1831. [Google Scholar] [CrossRef]
- Arzaee, N.A.; Noh, M.F.M.; Ita, N.S.H.M.; Mohamed, N.A.; Nasir, S.N.F.M.; Mumthas, I.N.N.; Ismail, A.F.; Teridi, M.A.M. Nanostructure-assisted charge transfer in α-Fe2O3/g-C3N4 heterojunctions for efficient and highly stable photoelectrochemical water splitting. Dalt. Trans. 2020, 49, 11317–11328. [Google Scholar]
- Liu, Y.; Su, F.-Y.; Yu, Y.-X.; Zhang, W.-D. Nano g-C3N4 modified Ti-Fe2O3 vertically arrays for efficient photoelectrochemical generation of hydrogen under visible light. Int. J. Hydrogen Energy 2016, 41, 7270–7279. [Google Scholar] [CrossRef]
- Lei, N.; Li, J.; Song, Q.; Liang, Z. Construction of g-C3N4/BCN two-dimensional heterojunction photoanode for enhanced photoelectrochemical water splitting. Int. J. Hydrogen Energy 2019, 44, 10498–10507. [Google Scholar] [CrossRef]
- Ragupathi, V.; Raja, M.A.; Panigrahi, P.; Subramaniam, N.G. CuO/g-C3N4 nanocomposite as promising photocatalyst for photoelectrochemical water splitting. Optik 2020, 208, 164569. [Google Scholar] [CrossRef]
- Li, X.; Wang, Z.; Zhang, Z.; Chen, L.; Cheng, J.; Ni, W.; Wang, B.; Xie, E. Light Illuminated α-Fe2O3/Pt Nanoparticles as Water Activation Agent for photoelectrochemical water splitting. Sci. Rep. 2015, 5, 9130. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nallapureddy, R.R.; Arla, S.K.; Ibáñez, A.; Pabba, D.P.; Jung, J.H.; Joo, S.W. Photosensitizer and Charge Separator Roles of g-C₃N₄ Integrated into the CuO-Fe₂O₃ p-n Heterojunction Interface for Elevating PEC Water Splitting Potential. Nanomaterials 2025, 15, 551. https://doi.org/10.3390/nano15070551
Nallapureddy RR, Arla SK, Ibáñez A, Pabba DP, Jung JH, Joo SW. Photosensitizer and Charge Separator Roles of g-C₃N₄ Integrated into the CuO-Fe₂O₃ p-n Heterojunction Interface for Elevating PEC Water Splitting Potential. Nanomaterials. 2025; 15(7):551. https://doi.org/10.3390/nano15070551
Chicago/Turabian StyleNallapureddy, Ramesh Reddy, Sai Kumar Arla, Andrés Ibáñez, Durga Prasad Pabba, Jae Hak Jung, and Sang Woo Joo. 2025. "Photosensitizer and Charge Separator Roles of g-C₃N₄ Integrated into the CuO-Fe₂O₃ p-n Heterojunction Interface for Elevating PEC Water Splitting Potential" Nanomaterials 15, no. 7: 551. https://doi.org/10.3390/nano15070551
APA StyleNallapureddy, R. R., Arla, S. K., Ibáñez, A., Pabba, D. P., Jung, J. H., & Joo, S. W. (2025). Photosensitizer and Charge Separator Roles of g-C₃N₄ Integrated into the CuO-Fe₂O₃ p-n Heterojunction Interface for Elevating PEC Water Splitting Potential. Nanomaterials, 15(7), 551. https://doi.org/10.3390/nano15070551





