Nasal Spray Disinfectant for Respiratory Infections Based on Functionalized Silver Nanoparticles: A Physicochemical and Docking Approach
Abstract
1. Introduction
2. Materials and Methods
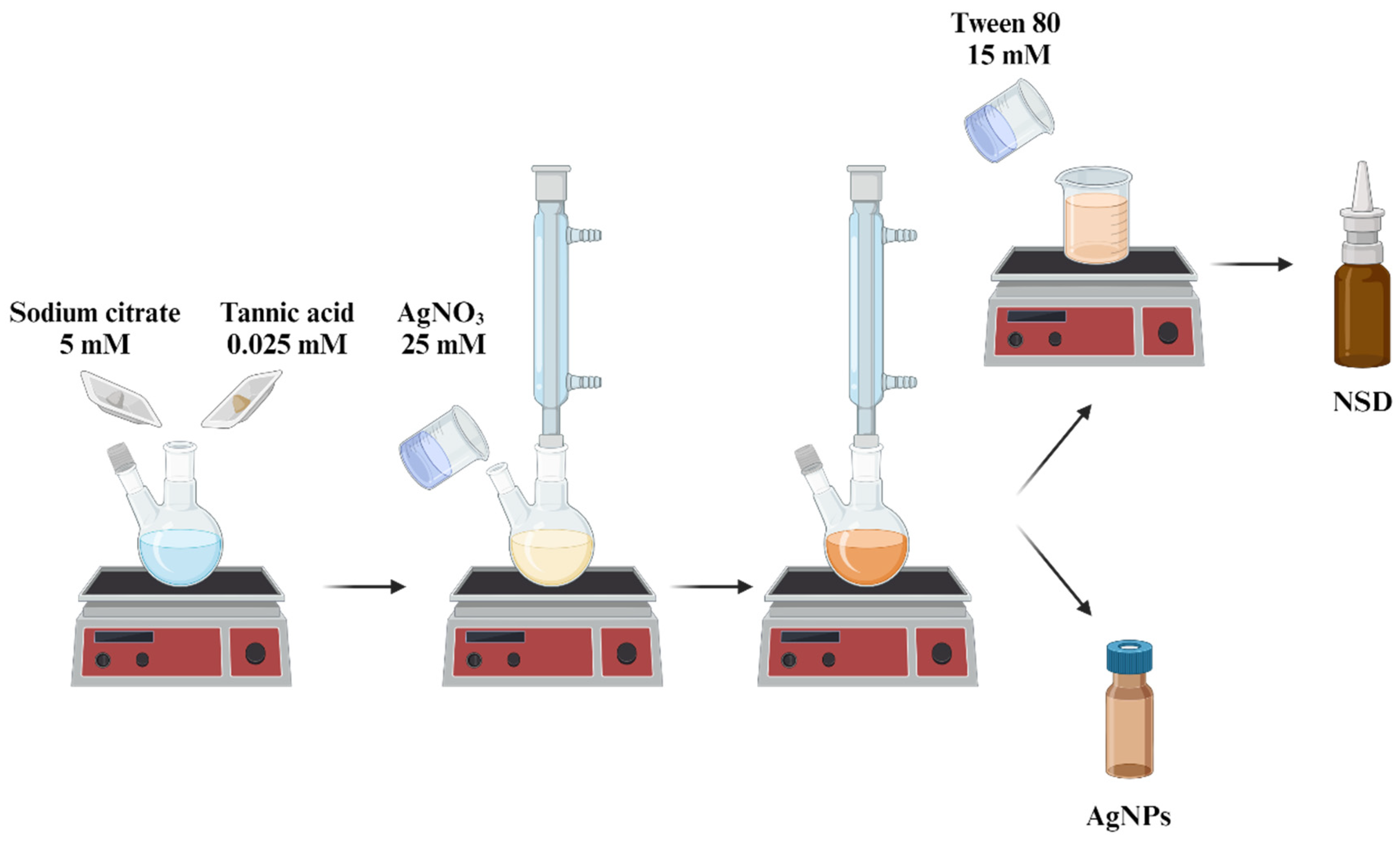
2.1. Synthesis of AgNPs
2.2. Preparation of Nasal Spray Disinfectant (NSD)
2.3. Physicochemical Characterization of AgNPs
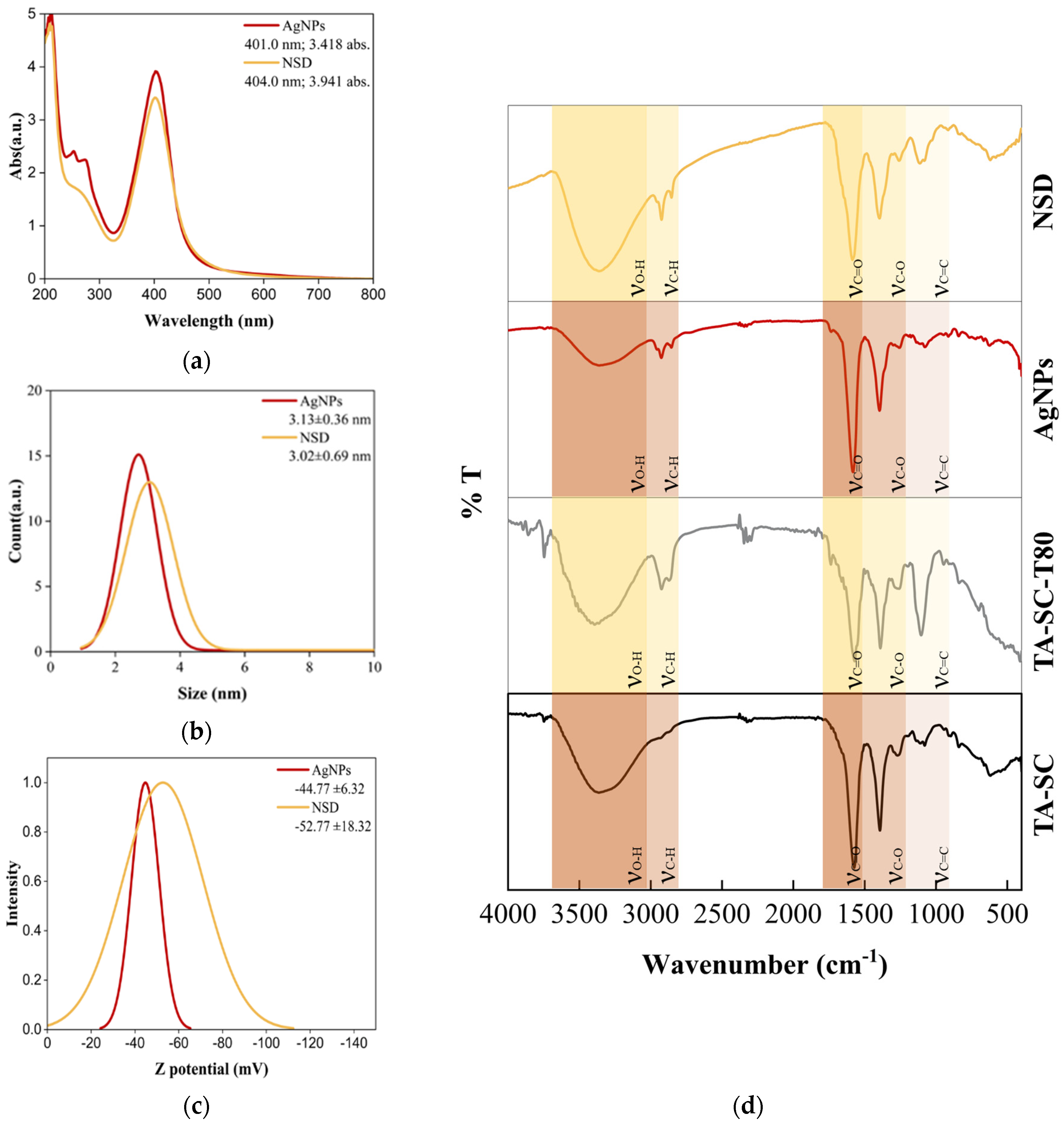
2.3.1. UV-Vis Spectroscopy
2.3.2. Dynamic Light Scattering (DLS)
2.3.3. Scanning Electron Microscopy (SEM) and Dispersive X-Ray Energy (EDX)
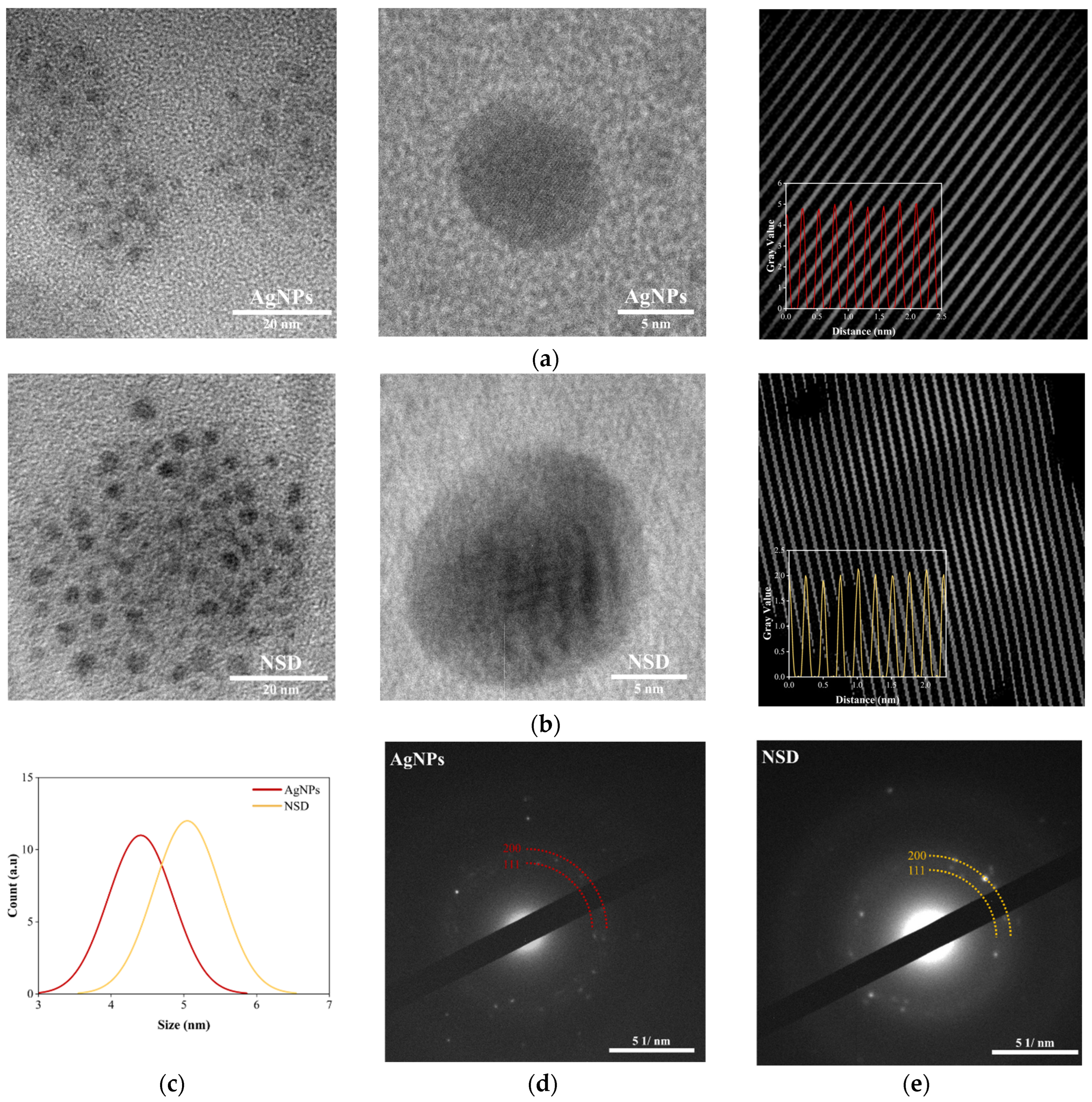
2.3.4. Transmission Electron Microscopy (TEM) and Selected-Area Electron Diffraction (SAED)
2.3.5. Fourier Transform Infrared Spectroscopy (FTIR)
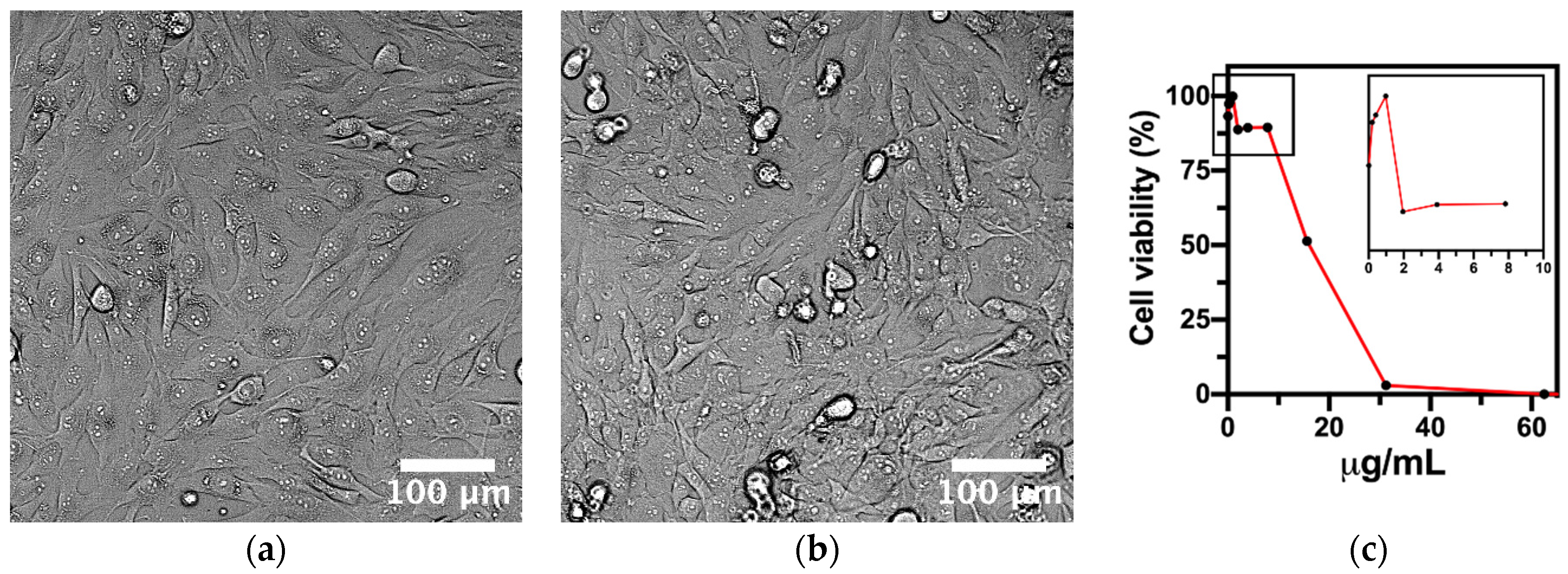
2.4. Cell Culture and Cytotoxicity Test
2.5. Nasal Disinfectant Spray Performance
2.5.1. Spray Coverage
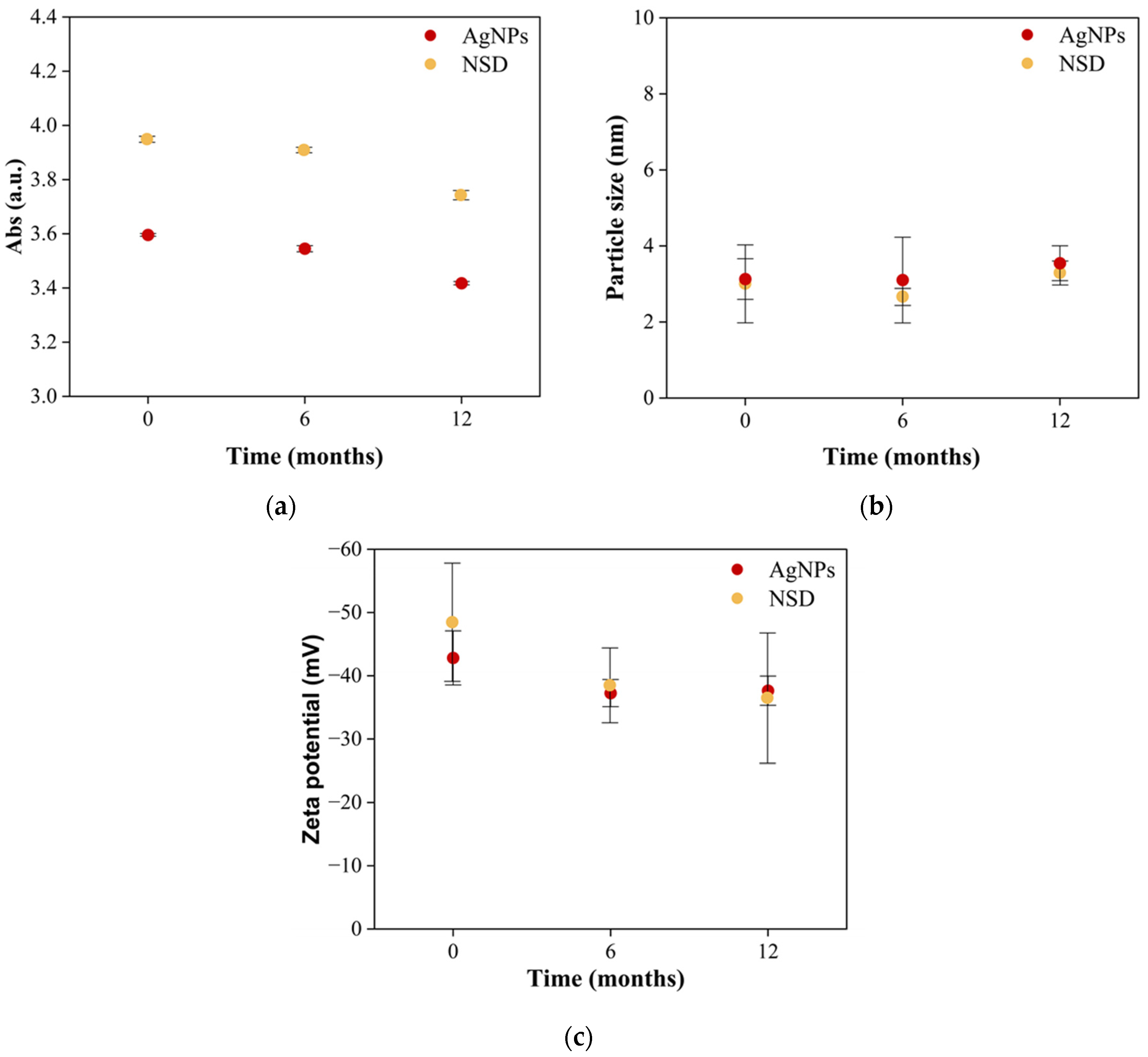
2.5.2. Storage Stability
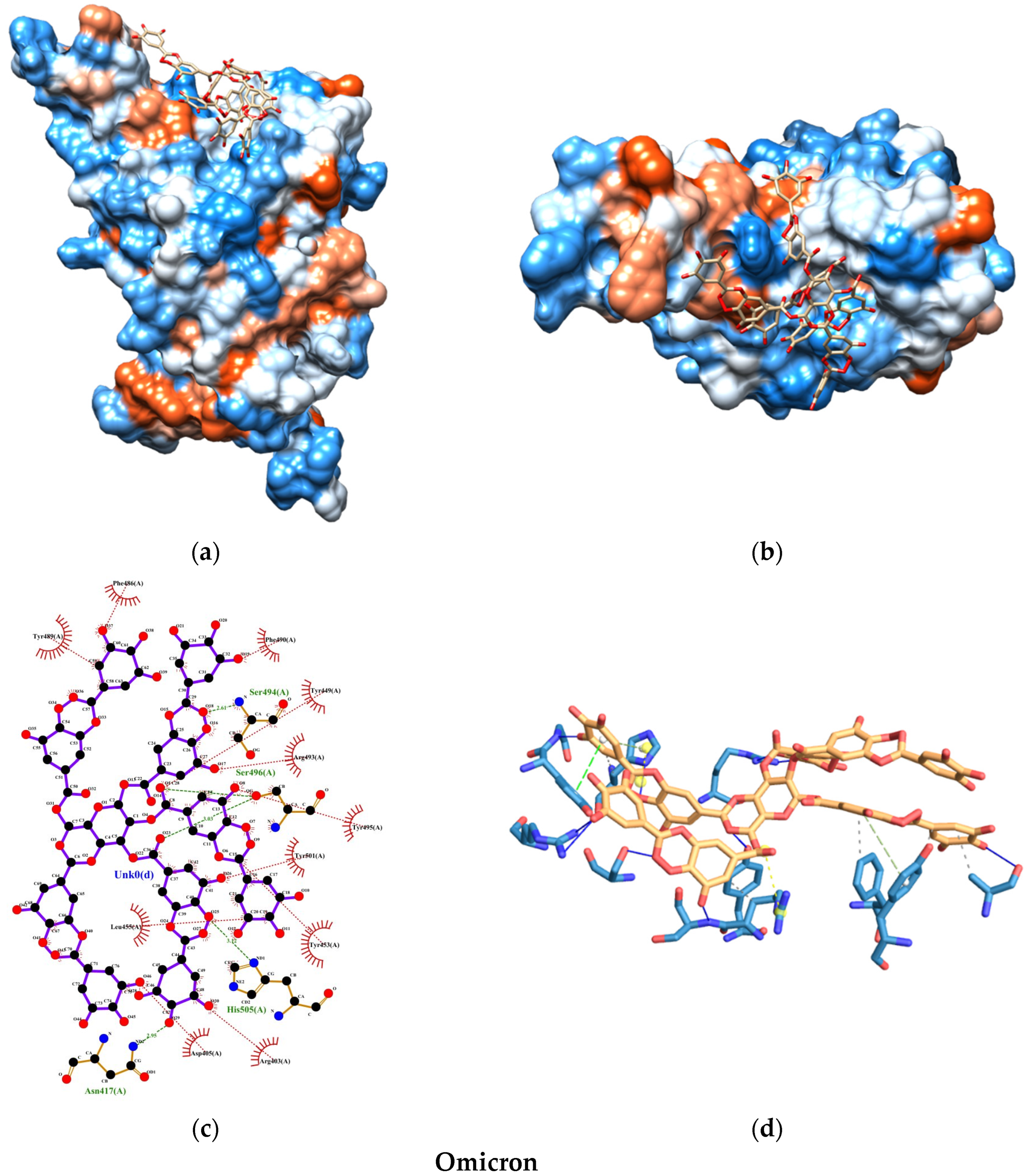
2.6. Molecular Docking Study
2.6.1. Ligand Preparation
2.6.2. Receptor Preparation
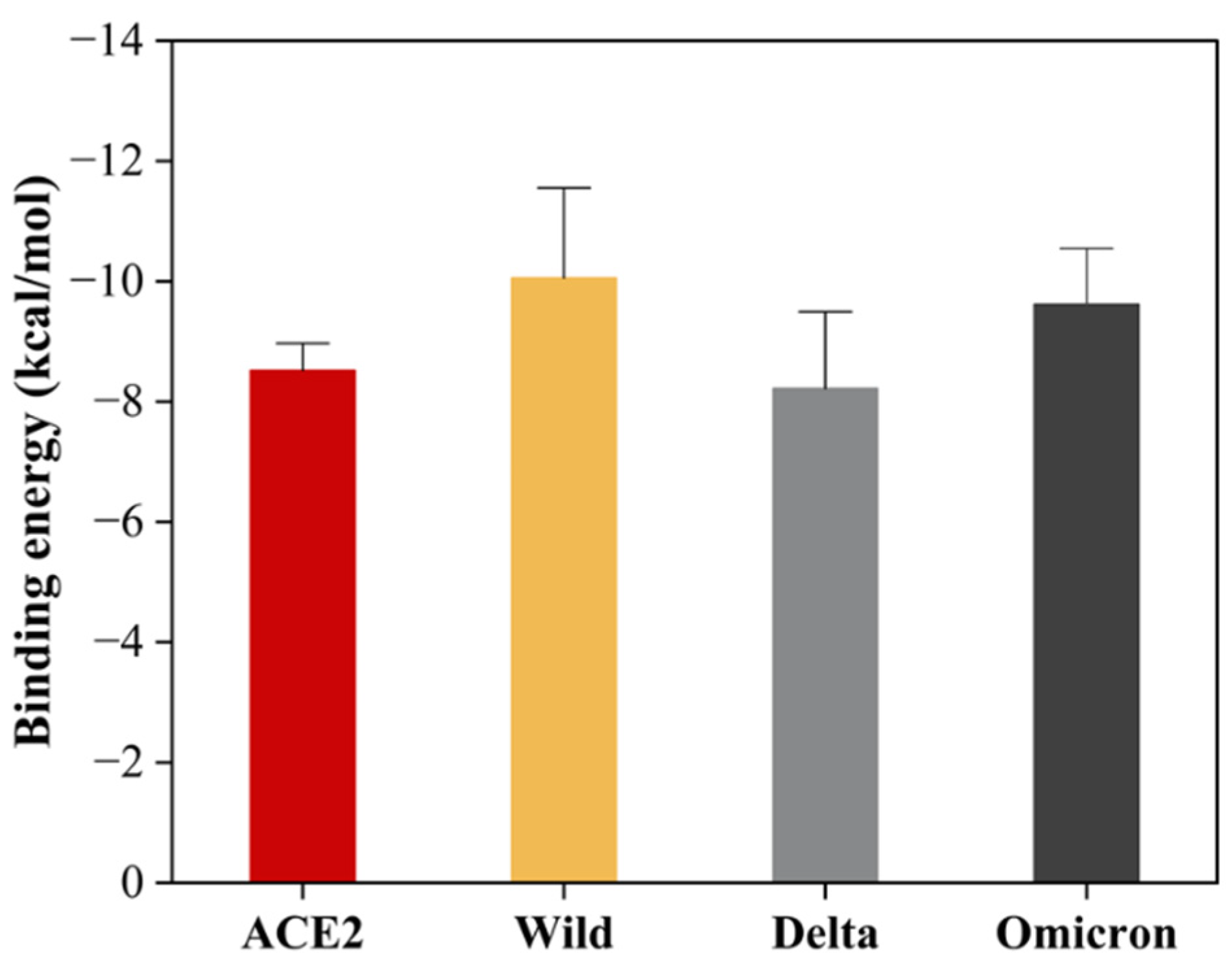
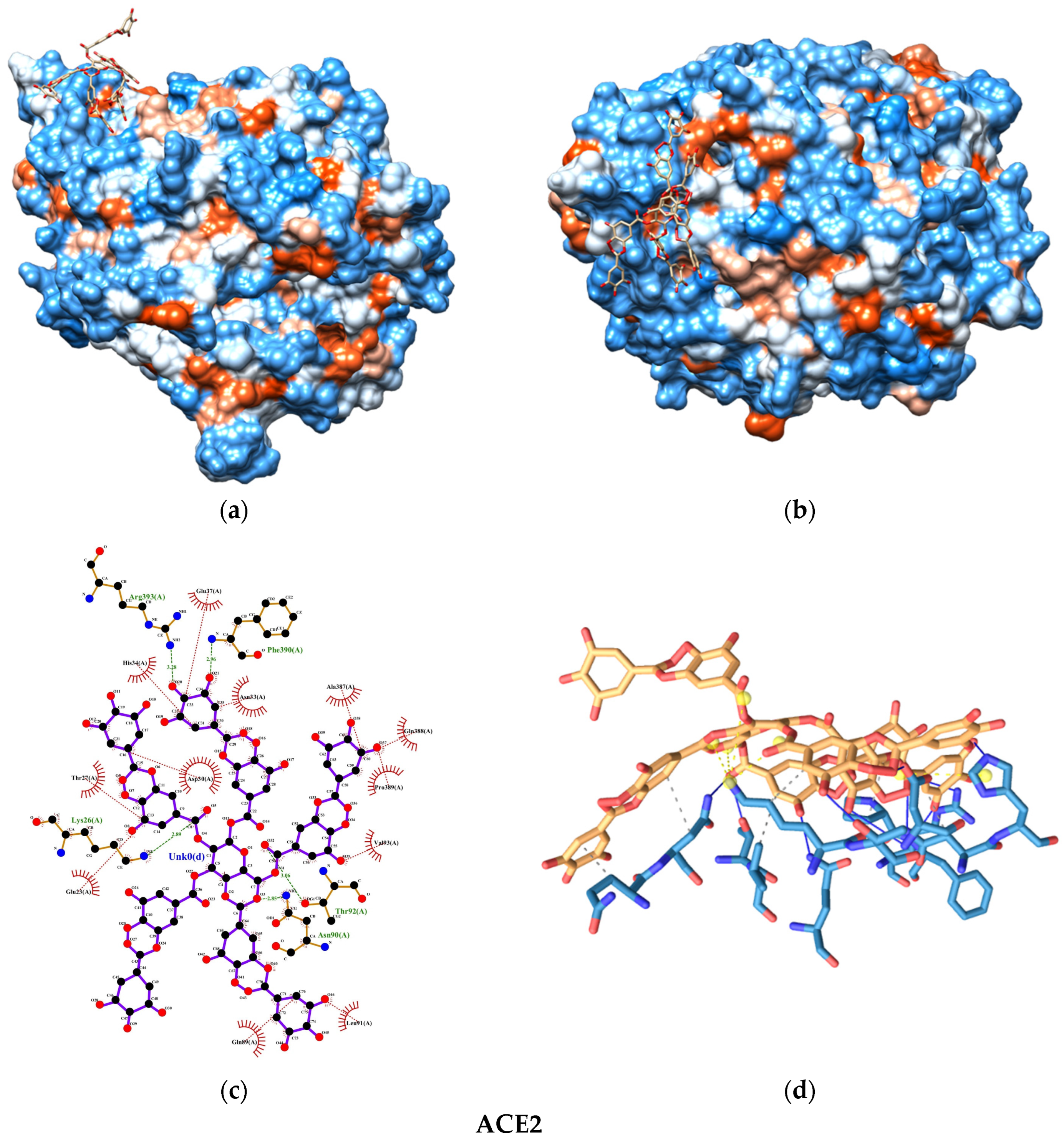
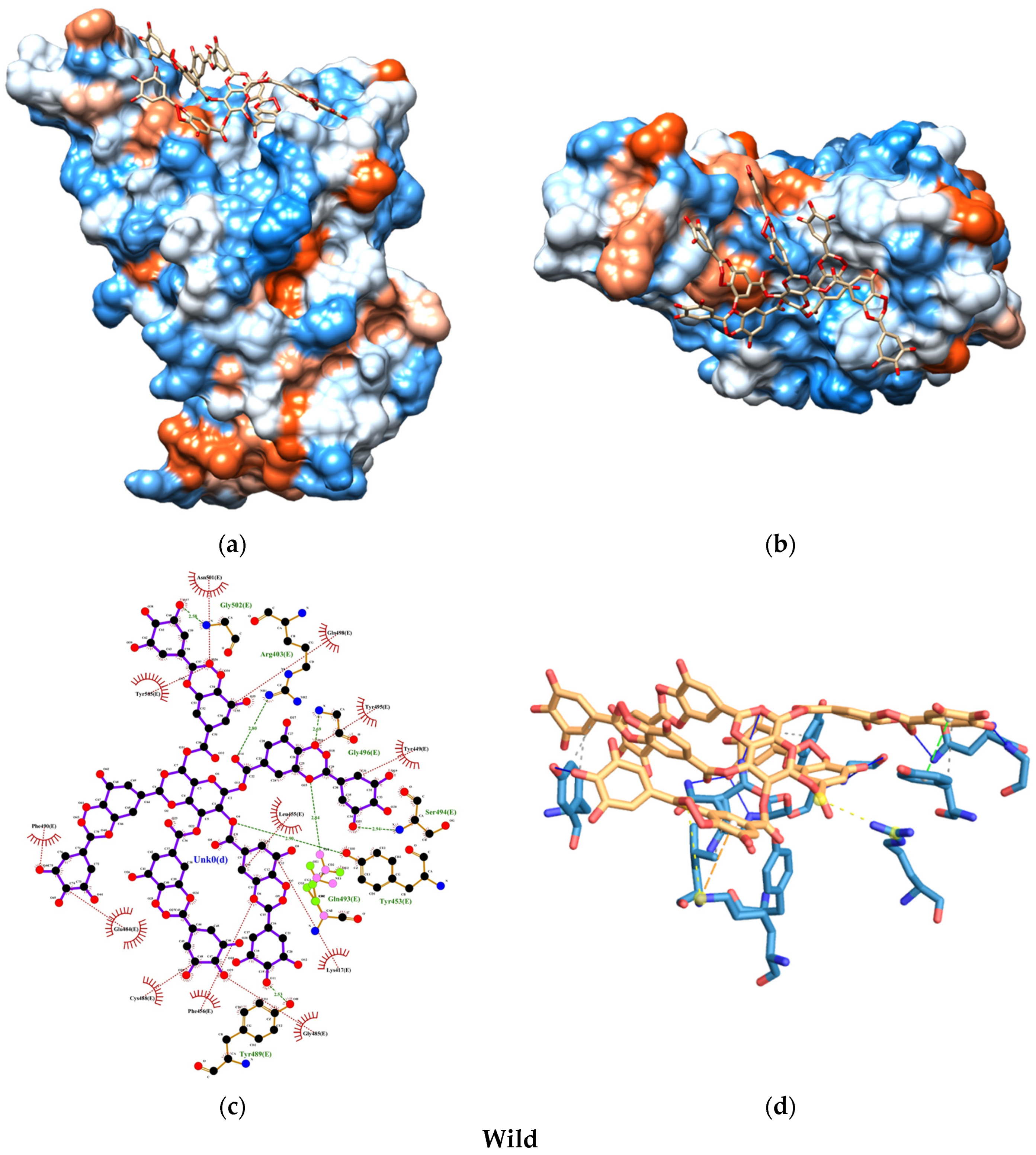
2.6.3. Interactions and Binding Energy
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nakayama, T.; Lee, I.T.; Jiang, S.; Matter, M.S.; Yan, C.H.; Overdevest, J.B.; Wu, C.-T.; Goltsev, Y.; Shih, L.-C.; Liao, C.-K.; et al. Determinants of SARS-CoV-2 entry and replication in airway mucosal tissue and susceptibility in smokers. Cell Rep. Med. 2021, 2, 100421. [Google Scholar] [CrossRef]
- Harrison, A.G.; Lin, T.; Wang, P. Mechanisms of SARS-CoV-2 Transmission and Pathogenesis. Trends Immunol. 2020, 41, 1100–1115. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.-D.; Wang, Z.-Y.; Zhang, S.-F.; Li, X.; Li, L.; Li, C.; Cui, Y.; Fu, R.-B.; Dong, Y.-Z.; Chi, X.-Y.; et al. Aerosol and Surface Distribution of Severe Acute Respiratory Syndrome Coronavirus 2 in Hospital Wards, Wuhan, China, 2020. Emerg. Infect. Dis. 2020, 26, 1583–1591. [Google Scholar] [CrossRef] [PubMed]
- Klompas, M.; Baker, M.A.; Rhee, C. Airborne Transmission of SARS-CoV-2: Theoretical Considerations and Available Evidence. JAMA 2020, 324, 441–442. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Guan, X.; Wu, P.; Wang, X.; Zhou, L.; Tong, Y.; Ren, R.; Leung, K.S.M.; Lau, E.H.Y.; Wong, J.Y.; et al. Early Transmission Dynamics in Wuhan, China, of Novel Coronavirus–Infected Pneumonia. N. Engl. J. Med. 2020, 382, 1199–1207. [Google Scholar] [CrossRef]
- Zhou, L.; Ayeh, S.K.; Chidambaram, V.; Karakousis, P.C. Modes of transmission of SARS-CoV-2 and evidence for preventive behavioral interventions. BMC Infect. Dis. 2021, 21, 496. [Google Scholar] [CrossRef]
- Mason, R.J. Thoughts on the alveolar phase of COVID-19. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2020, 319, L115–L120. [Google Scholar] [CrossRef]
- Laveneziana, P.; Sesé, L.; Gille, T. Pathophysiology of pulmonary function anomalies in COVID-19 survivors. Breathe 2021, 17, 210065. [Google Scholar] [CrossRef]
- Machhi, J.; Herskovitz, J.; Senan, A.M.; Dutta, D.; Nath, B.; Oleynikov, M.D.; Blomberg, W.R.; Meigs, D.D.; Hasan, M.; Patel, M.; et al. The Natural History, Pathobiology, and Clinical Manifestations of SARS-CoV-2 Infections. J. Neuroimmune Pharmacol. 2020, 15, 359–386. [Google Scholar] [CrossRef]
- Baig, A.M. Computing the Effects of SARS-CoV-2 on Respiration Regulatory Mechanisms in COVID-19. ACS Chem. Neurosci. 2020, 11, 2416–2421. [Google Scholar] [CrossRef]
- Halawa, S.; Pullamsetti, S.S.; Bangham, C.R.M.; Stenmark, K.R.; Dorfmüller, P.; Frid, M.G.; Butrous, G.; Morrell, N.W.; De Jesus Perez, V.A.; Stuart, D.I.; et al. Potential long-term effects of SARS-CoV-2 infection on the pulmonary vasculature: A global perspective. Nat. Rev. Cardiol. 2022, 19, 314–331. [Google Scholar] [CrossRef] [PubMed]
- Spudich, S.; Nath, A. Nervous system consequences of COVID-19. Science 2022, 375, 267–269. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Geng, X.; Tan, Y.; Li, Q.; Xu, C.; Xu, J.; Hao, L.; Zeng, Z.; Luo, X.; Liu, F.; et al. New understanding of the damage of SARS-CoV-2 infection outside the respiratory system. Biomed. Pharmacother. 2020, 127, 110195. [Google Scholar] [CrossRef]
- Ivanova, N.; Sotirova, Y.; Gavrailov, G.; Nikolova, K.; Andonova, V. Advances in the Prophylaxis of Respiratory Infections by the Nasal and the Oromucosal Route: Relevance to the Fight with the SARS-CoV-2 Pandemic. Pharmaceutics 2022, 14, 530. [Google Scholar] [CrossRef]
- Leung, N.H.L. Transmissibility and transmission of respiratory viruses. Nat. Rev. Microbiol. 2021, 19, 528–545. [Google Scholar] [CrossRef] [PubMed]
- Grassin-Delyle, S.; Buenestado, A.; Naline, E.; Faisy, C.; Blouquit-Laye, S.; Couderc, L.-J.; Le Guen, M.; Fischler, M.; Devillier, P. Intranasal drug delivery: An efficient and non-invasive route for systemic administration. Pharmacol. Ther. 2012, 134, 366–379. [Google Scholar] [CrossRef]
- Xu, J.; Tao, J.; Wang, J. Design and Application in Delivery System of Intranasal Antidepressants. Front. Bioeng. Biotechnol. 2020, 8, 626882. [Google Scholar] [CrossRef]
- Clementino, A.R.; Pellegrini, G.; Banella, S.; Colombo, G.; Cantù, L.; Sonvico, F.; Del Favero, E. Structure and Fate of Nanoparticles Designed for the Nasal Delivery of Poorly Soluble Drugs. Mol. Pharm. 2021, 18, 3132–3146. [Google Scholar] [CrossRef]
- Ibarra-Sánchez, L.Á.; Gámez-Méndez, A.; Martínez-Ruiz, M.; Nájera-Martínez, E.F.; Morales-Flores, B.A.; Melchor-Martínez, E.M.; Sosa-Hernández, J.E.; Parra-Saldívar, R.; Iqbal, H.M.N. Nanostructures for drug delivery in respiratory diseases therapeutics: Revision of current trends and its comparative analysis. J. Drug Deliv. Sci. Technol. 2022, 70, 103219. [Google Scholar] [CrossRef]
- Bansal, S.; Jonsson, C.B.; Taylor, S.L.; Figueroa, J.M.; Dugour, A.V.; Palacios, C.; Vega, J.C. Iota-carrageenan and xylitol inhibit SARS-CoV-2 in Vero cell culture. PLoS ONE 2021, 16, e0259943. [Google Scholar] [CrossRef]
- Bentley, K.; Stanton, R.J. Hydroxypropyl Methylcellulose-Based Nasal Sprays Effectively Inhibit In Vitro SARS-CoV-2 Infection and Spread. Viruses 2021, 13, 2345. [Google Scholar] [CrossRef]
- Pyrć, K.; Milewska, A.; Duran, E.B.; Botwina, P.; Dabrowska, A.; Jedrysik, M.; Benedyk, M.; Lopes, R.; Arenas-Pinto, A.; Badr, M.; et al. SARS-CoV-2 inhibition using a mucoadhesive, amphiphilic chitosan that may serve as an anti-viral nasal spray. Sci. Rep. 2021, 11, 20012. [Google Scholar] [CrossRef]
- Varese, A.; Paletta, A.; Ceballos, A.; Palacios, C.A.; Figueroa, J.M.; Dugour, A.V. Iota-Carrageenan Prevents the Replication of SARS-CoV-2 in a Human Respiratory Epithelium Cell Line in vitro. Front. Virol. 2021, 1, 746824. [Google Scholar] [CrossRef]
- Chung, S.; Peters, J.M.; Detyniecki, K.; Tatum, W.; Rabinowicz, A.L.; Carrazana, E. The nose has it: Opportunities and challenges for intranasal drug administration for neurologic conditions including seizure clusters. Epilepsy Behav. Rep. 2023, 21, 100581. [Google Scholar] [CrossRef]
- Illum, L. Nasal drug delivery—Possibilities, problems and solutions. J. Control. Release 2003, 87, 187–198. [Google Scholar] [CrossRef]
- Borghardt, J.M.; Kloft, C.; Sharma, A. Inhaled Therapy in Respiratory Disease: The Complex Interplay of Pulmonary Kinetic Processes. Can. Respir. J. 2018, 2018, 2732017. [Google Scholar] [CrossRef]
- Labiris, N.R.; Dolovich, M.B. Pulmonary drug delivery. Part I: Physiological factors affecting therapeutic effectiveness of aerosolized medications. Br. J. Clin. Pharmacol. 2003, 56, 588–599. [Google Scholar] [CrossRef] [PubMed]
- Mangal, S.; Gao, W.; Li, T.; Zhou, Q. Pulmonary delivery of nanoparticle chemotherapy for the treatment of lung cancers: Challenges and opportunities. Acta Pharmacol. Sin. 2017, 38, 782–797. [Google Scholar] [CrossRef] [PubMed]
- Nahhas, A.F.; Webster, T.J. Applications of peptide-functionalized or unfunctionalized selenium nanoparticles for the passivation of SARS-CoV-2 variants and the respiratory syncytial virus (RSV). Colloids Surf. B Biointerfaces 2024, 233, 113638. [Google Scholar] [CrossRef]
- Patil, J.; Sarasija, S. Pulmonary drug delivery strategies: A concise, systematic review. Lung India 2012, 29, 44–49. [Google Scholar] [CrossRef]
- Martín-Faivre, L.; Prince, L.; Cornu, C.; Villeret, B.; Sanchez-Guzman, D.; Rouzet, F.; Sallenave, J.-M.; Garcia-Verdugo, I. Pulmonary delivery of silver nanoparticles prevents influenza infection by recruiting and activating lymphoid cells. Biomaterials 2025, 312, 122721. [Google Scholar] [CrossRef]
- Wieler, L.; Vittos, O.; Mukherjee, N.; Sarkar, S. Reduction in the COVID-19 pneumonia case fatality rate by silver nanoparticles: A randomized case study. Heliyon 2023, 9, e14419. [Google Scholar] [CrossRef]
- He, Q.; Lu, J.; Liu, N.; Lu, W.; Li, Y.; Shang, C.; Li, X.; Hu, L.; Jiang, G. Antiviral Properties of Silver Nanoparticles against SARS-CoV-2: Effects of Surface Coating and Particle Size. Nanomaterials 2022, 12, 990. [Google Scholar] [CrossRef]
- Lara, H.H.; Garza-Treviño, E.N.; Ixtepan-Turrent, L.; Singh, D.K. Silver nanoparticles are broad-spectrum bactericidal and virucidal compounds. J. Nanobiotechnol. 2011, 9, 30. [Google Scholar] [CrossRef] [PubMed]
- Naumenko, K.; Zahorodnia, S.; Pop, C.V.; Rizun, N. Antiviral activity of silver nanoparticles against the influenza A virus. J. Virus Erad. 2023, 9, 100330. [Google Scholar] [CrossRef] [PubMed]
- Pilaquinga, F.; Morey, J.; Torres, M.; Seqqat, R.; Piña, M.D.L.N. Silver nanoparticles as a potential treatment against SARS-CoV -2: A review. WIREs Nanomed. Nanobiotechnol. 2021, 13, e1707. [Google Scholar] [CrossRef]
- Rivera-Martinez, T.; Valdez-Salas, B.; Salvador-Carlos, J.; Stoytcheva, M.; Zlatev, R.; Beltrán-Partida, E. Improvement of the antibacterial and skin-protective performance of alcohol-based sanitizers using hydroglycolic phytocompounds. Biotechnol. Biotechnol. Equip. 2023, 37, 2253927. [Google Scholar] [CrossRef]
- Valdez-Salas, B.; Beltrán-Partida, E.; Zlatev, R.; Stoytcheva, M.; Gonzalez-Mendoza, D.; Salvador-Carlos, J.; Moreno-Ulloa, A.; Cheng, N. Structure-activity relationship of diameter controlled Ag@Cu nanoparticles in broad-spectrum antibacterial mechanism. Mater. Sci. Eng. C 2021, 119, 111501. [Google Scholar] [CrossRef] [PubMed]
- Moakes, R.J.A.; Davies, S.P.; Stamataki, Z.; Grover, L.M. Formulation of a Composite Nasal Spray Enabling Enhanced Surface Coverage and Prophylaxis of SARS-COV-2. Adv. Mater. 2021, 33, 2008304. [Google Scholar] [CrossRef]
- Ranoszek-Soliwoda, K.; Tomaszewska, E.; Socha, E.; Krzyczmonik, P.; Ignaczak, A.; Orlowski, P.; Krzyzowska, M.; Celichowski, G.; Grobelny, J. The role of tannic acid and sodium citrate in the synthesis of silver nanoparticles. J. Nanopart. Res. 2017, 19, 273. [Google Scholar] [CrossRef]
- Rizvi, S.M.D.; Shakil, S.; Haneef, M. A simple click by click protocol to perform docking: AutoDock 4.2 made easy for non-bioinformaticians. EXCLI J. 2013, 12, 831–857. [Google Scholar] [PubMed]
- Holt, P.A.; Chaires, J.B.; Trent, J.O. Molecular docking of intercalators and groove-binders to nucleic acids using Autodock and Surflex. J. Chem. Inf. Model. 2008, 48, 1602–1615. [Google Scholar] [CrossRef]
- Amirjani, A.; Haghshenas, D.F. Ag nanostructures as the surface plasmon resonance (SPR)˗based sensors: A mechanistic study with an emphasis on heavy metallic ions detection. Sens. Actuators B Chem. 2018, 273, 1768–1779. [Google Scholar] [CrossRef]
- La Spina, R.; Mehn, D.; Fumagalli, F.; Holland, M.; Reniero, F.; Rossi, F.; Gilliland, D. Synthesis of Citrate-Stabilized Silver Nanoparticles Modified by Thermal and pH Preconditioned Tannic Acid. Nanomaterials 2020, 10, 2031. [Google Scholar] [CrossRef] [PubMed]
- Gangwar, C.; Yaseen, B.; Kumar, I.; Singh, N.K.; Naik, R.M. Growth Kinetic Study of Tannic Acid Mediated Monodispersed Silver Nanoparticles Synthesized by Chemical Reduction Method and Its Characterization. ACS Omega 2021, 6, 22344–22356. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, T. Reviewing the Tannic Acid Mediated Synthesis of Metal Nanoparticles. J. Nanotechnol. 2014, 2014, 954206. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, N.; Goebl, J.; Lu, Z.; Yin, Y. A Systematic Study of the Synthesis of Silver Nanoplates: Is Citrate a “Magic” Reagent? J. Am. Chem. Soc. 2011, 133, 18931–18939. [Google Scholar] [CrossRef] [PubMed]
- Franco-Ulloa, S.; Tatulli, G.; Bore, S.L.; Moglianetti, M.; Pompa, P.P.; Cascella, M.; De Vivo, M. Dispersion state phase diagram of citrate-coated metallic nanoparticles in saline solutions. Nat. Commun. 2020, 11, 5422. [Google Scholar] [CrossRef]
- Ricci, A.; Olejar, K.J.; Parpinello, G.P.; Kilmartin, P.A.; Versari, A. Application of Fourier Transform Infrared (FTIR) Spectroscopy in the Characterization of Tannins. Appl. Spectrosc. Rev. 2015, 50, 407–442. [Google Scholar] [CrossRef]
- Husanu, E.; Chiappe, C.; Bernardini, A.; Cappello, V.; Gemmi, M. Synthesis of colloidal Ag nanoparticles with citrate based ionic liquids as reducing and capping agents. Colloids Surf. A Physicochem. Eng. Asp. 2018, 538, 506–512. [Google Scholar] [CrossRef]
- FDA. Nasal Spray and Inhalation Solution, Suspension, and Spray Drug Products—Chemistry, Manufacturing, and Controls Documentation; FDA: Silver Spring, MD, USA, 2002.
- Ivask, A.; Kurvet, I.; Kasemets, K.; Blinova, I.; Aruoja, V.; Suppi, S.; Vija, H.; Käkinen, A.; Titma, T.; Heinlaan, M.; et al. Size-Dependent Toxicity of Silver Nanoparticles to Bacteria, Yeast, Algae, Crustaceans and Mammalian Cells In Vitro. PLoS ONE 2014, 9, e102108. [Google Scholar] [CrossRef] [PubMed]
- Osonga, F.J.; Akgul, A.; Yazgan, I.; Akgul, A.; Eshun, G.B.; Sakhaee, L.; Sadik, O.A. Size and Shape-Dependent Antimicrobial Activities of Silver and Gold Nanoparticles: A Model Study as Potential Fungicides. Molecules 2020, 25, 2682. [Google Scholar] [CrossRef]
- Wang, L.; Hu, C.; Shao, L. The antimicrobial activity of nanoparticles: Present situation and prospects for the future. Int. J. Nanomed. 2017, 12, 1227–1249. [Google Scholar] [CrossRef]
- Das, C.; Paul, S.S.; Saha, A.; Singh, T.; Saha, A.; Im, J.; Biswas, G. Silver-Based Nanomaterials as Therapeutic Agents Against Coronaviruses: A Review. Int. J. Nanomed. 2020, 15, 9301–9315. [Google Scholar] [CrossRef]
- Khoshnevisan, K.; Maleki, H.; Baharifar, H. Nanobiocide Based-Silver Nanomaterials Upon Coronaviruses: Approaches for Preventing Viral Infections. Nanoscale Res. Lett. 2021, 16, 100. [Google Scholar] [CrossRef]
- Allawadhi, P.; Singh, V.; Khurana, A.; Khurana, I.; Allwadhi, S.; Kumar, P.; Banothu, A.K.; Thalugula, S.; Barani, P.J.; Naik, R.R.; et al. Silver nanoparticle based multifunctional approach for combating COVID-19. Sens. Int. 2021, 2, 100101. [Google Scholar] [CrossRef] [PubMed]
- Jeremiah, S.S.; Miyakawa, K.; Morita, T.; Yamaoka, Y.; Ryo, A. Potent antiviral effect of silver nanoparticles on SARS-CoV-2. Biochem. Biophys. Res. Commun. 2020, 533, 195–200. [Google Scholar] [CrossRef]
- Valdez-Salas, B.; Beltran-Partida, E.; Cheng, N.; Salvador-Carlos, J.; Valdez-Salas, E.A.; Curiel-Alvarez, M.; Ibarra-Wiley, R. Promotion of Surgical Masks Antimicrobial Activity by Disinfection and Impregnation with Disinfectant Silver Nanoparticles. Int. J. Nanomed. 2021, 16, 2689–2702. [Google Scholar] [CrossRef]
- Abbaszadegan, A.; Ghahramani, Y.; Gholami, A.; Hemmateenejad, B.; Dorostkar, S.; Nabavizadeh, M.; Sharghi, H. The Effect of Charge at the Surface of Silver Nanoparticles on Antimicrobial Activity against Gram-Positive and Gram-Negative Bacteria: A Preliminary Study. J. Nanomater. 2015, 2015, 720654. [Google Scholar] [CrossRef]
- Anees Ahmad, S.; Sachi Das, S.; Khatoon, A.; Tahir Ansari, M.; Afzal, M.; Saquib Hasnain, M.; Kumar Nayak, A. Bactericidal activity of silver nanoparticles: A mechanistic review. Mater. Sci. Energy Technol. 2020, 3, 756–769. [Google Scholar] [CrossRef]
- Ferreyra Maillard, A.P.V.; Dalmasso, P.R.; López De Mishima, B.A.; Hollmann, A. Interaction of green silver nanoparticles with model membranes: Possible role in the antibacterial activity. Colloids Surf. B Biointerfaces 2018, 171, 320–326. [Google Scholar] [CrossRef] [PubMed]
- Salvioni, L.; Galbiati, E.; Collico, V.; Alessio, G.; Avvakumova, S.; Corsi, F.; Tortora, P.; Prosperi, D.; Colombo, M. Negatively charged silver nanoparticles with potent antibacterial activity and reduced toxicity for pharmaceutical preparations. Int. J. Nanomed. 2017, 12, 2517–2530. [Google Scholar] [CrossRef] [PubMed]
- Acharya, D.; Singha, K.M.; Pandey, P.; Mohanta, B.; Rajkumari, J.; Singha, L.P. Shape dependent physical mutilation and lethal effects of silver nanoparticles on bacteria. Sci. Rep. 2018, 8, 201. [Google Scholar] [CrossRef]
- Cheon, J.Y.; Kim, S.J.; Rhee, Y.H.; Kwon, O.H.; Park, W.H. Shape-dependent antimicrobial activities of silver nanoparticles. Int. J. Nanomedicine 2019, 14, 2773–2780. [Google Scholar] [CrossRef]
- Helmlinger, J.; Sengstock, C.; Groß-Heitfeld, C.; Mayer, C.; Schildhauer, T.A.; Köller, M.; Epple, M. Silver nanoparticles with different size and shape: Equal cytotoxicity, but different antibacterial effects. RSC Adv. 2016, 6, 18490–18501. [Google Scholar] [CrossRef]
- Liu, Y.; Tan, J.; Thomas, A.; Ou-Yang, D.; Muzykantov, V.R. The Shape of Things to Come: Importance of Design in Nanotechnology for Drug Delivery. Ther. Deliv. 2012, 3, 181–194. [Google Scholar] [CrossRef]
- Jawad, B.; Adhikari, P.; Podgornik, R.; Ching, W.-Y. Key Interacting Residues between RBD of SARS-CoV-2 and ACE2 Receptor: Combination of Molecular Dynamics Simulation and Density Functional Calculation. J. Chem. Inf. Model. 2021, 61, 4425–4441. [Google Scholar] [CrossRef]
- Lan, J.; Ge, J.; Yu, J.; Shan, S.; Zhou, H.; Fan, S.; Zhang, Q.; Shi, X.; Wang, Q.; Zhang, L.; et al. Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor. Nature 2020, 581, 215–220. [Google Scholar] [CrossRef]
- Verma, J.; Subbarao, N. Insilico study on the effect of SARS-CoV-2 RBD hotspot mutants’ interaction with ACE2 to understand the binding affinity and stability. Virology 2021, 561, 107–116. [Google Scholar] [CrossRef]
- Yi, C.; Sun, X.; Ye, J.; Ding, L.; Liu, M.; Yang, Z.; Lu, X.; Zhang, Y.; Ma, L.; Gu, W.; et al. Key residues of the receptor binding motif in the spike protein of SARS-CoV-2 that interact with ACE2 and neutralizing antibodies. Cell. Mol. Immunol. 2020, 17, 621–630. [Google Scholar] [CrossRef]
- Das, N.C.; Chakraborty, P.; Bayry, J.; Mukherjee, S. In Silico Analyses on the Comparative Potential of Therapeutic Human Monoclonal Antibodies Against Newly Emerged SARS-CoV-2 Variants Bearing Mutant Spike Protein. Front. Immunol. 2022, 12, 782506. [Google Scholar] [CrossRef]
- Stadler, E.; Burgess, M.T.; Schlub, T.E.; Khan, S.R.; Chai, K.L.; McQuilten, Z.K.; Wood, E.M.; Polizzotto, M.N.; Kent, S.J.; Cromer, D.; et al. Monoclonal antibody levels and protection from COVID-19. Nat. Commun. 2023, 14, 4545. [Google Scholar] [CrossRef]
- Attia, G.H.; Moemen, Y.S.; Youns, M.; Ibrahim, A.M.; Abdou, R.; El Raey, M.A. Antiviral zinc oxide nanoparticles mediated by hesperidin and in silico comparison study between antiviral phenolics as anti-SARS-CoV-2. Colloids Surf. B Biointerfaces 2021, 203, 111724. [Google Scholar] [CrossRef]
- Coelho, C.; Gallo, G.; Campos, C.B.; Hardy, L.; Würtele, M. Biochemical screening for SARS-CoV-2 main protease inhibitors. PLoS ONE 2020, 15, e0240079. [Google Scholar] [CrossRef]
- Elfiky, A.A. Natural products may interfere with SARS-CoV-2 attachment to the host cell. J. Biomol. Struct. Dyn. 2020, 39, 3194–3203. [Google Scholar] [CrossRef]
- Goc, A.; Sumera, W.; Rath, M.; Niedzwiecki, A. Phenolic compounds disrupt spike-mediated receptor-binding and entry of SARS-CoV-2 pseudo-virions. PLoS ONE 2021, 16, e0253489. [Google Scholar] [CrossRef]
- Wang, S.-C.; Chen, Y.; Wang, Y.-C.; Wang, W.-J.; Yang, C.-S.; Tsai, C.-L.; Hou, M.-H.; Chen, H.-F.; Shen, Y.-C.; Hung, M.-C. Tannic acid suppresses SARS-CoV-2 as a dual inhibitor of the viral main protease and the cellular TMPRSS2 protease. Am. J. Cancer Res. 2020, 10, 4538–4546. [Google Scholar]
- Gupta, G.; Hamawandi, B.; Sheward, D.J.; Murrell, B.; Hanke, L.; McInerney, G.; Blosi, M.; Costa, A.L.; Toprak, M.S.; Fadeel, B. Silver nanoparticles with excellent biocompatibility block pseudotyped SARS-CoV-2 in the presence of lung surfactant. Front. Bioeng. Biotechnol. 2022, 10, 1083232. [Google Scholar] [CrossRef]
- Gao, M.; Shen, X.; Mao, S. Factors influencing drug deposition in thenasal cavity upon delivery via nasal sprays. J. Pharm. Investig. 2020, 50, 251–259. [Google Scholar] [CrossRef]
- HCA Lung Biological Network; Sungnak, W.; Huang, N.; Bécavin, C.; Berg, M.; Queen, R.; Litvinukova, M.; Talavera-López, C.; Maatz, H.; Reichart, D.; et al. SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes. Nat. Med. 2020, 26, 681–687. [Google Scholar] [CrossRef]
- Chavda, V.P.; Vora, L.K.; Pandya, A.K.; Patravale, V.B. Intranasal vaccines for SARS-CoV-2: From challenges to potential in COVID-19 management. Drug Discov. Today 2021, 26, 2619–2636. [Google Scholar] [CrossRef] [PubMed]
- Kooij, S.; Sijs, R.; Denn, M.M.; Villermaux, E.; Bonn, D. What Determines the Drop Size in Sprays? Phys. Rev. X 2018, 8, 031019. [Google Scholar] [CrossRef]
- Li, Y.; Li, J.; Zhang, X.; Ding, J.; Mao, S. Non-ionic surfactants as novel intranasal absorption enhancers: In vitro and in vivo characterization. Drug Deliv. 2016, 23, 2272–2279. [Google Scholar] [CrossRef]
- Sijs, R.; Kooij, S.; Bonn, D. How surfactants influence the drop size in sprays from flat fan and hollow cone nozzles. Phys. Fluids 2021, 33, 113608. [Google Scholar] [CrossRef]
- Robinson, T.E.; Moakes, R.J.A.; Grover, L.M. Low Acyl Gellan as an Excipient to Improve the Sprayability and Mucoadhesion of Iota Carrageenan in a Nasal Spray to Prevent Infection With SARS-CoV-2. Front. Med. Technol. 2021, 3, 687681. [Google Scholar] [CrossRef]
- Mannar, D.; Saville, J.W.; Zhu, X.; Srivastava, S.S.; Berezuk, A.M.; Tuttle, K.S.; Marquez, A.C.; Sekirov, I.; Subramaniam, S. SARS-CoV-2 Omicron Variant: Antibody Evasion and Cryo-EM Structure of Spike Protein–ACE2 Complex. Science 2022, 375, 760–764. [Google Scholar] [CrossRef]
- Adasme, M.F.; Linnemann, K.L.; Bolz, S.N.; Kaiser, F.; Salentin, S.; Haupt, V.J.; Schroeder, M. PLIP 2021: Expanding the Scope of the Protein–Ligand Interaction Profiler to DNA and RNA. Nucleic Acids Res. 2021, 49, W530–W534. [Google Scholar] [CrossRef]



| Crystal | Description | Method | Resolution (Å) |
|---|---|---|---|
| 6MOJ | ACE2 | X-ray diffraction | 2.43 |
| 6MOJ | Wild | X-ray diffraction | 2.43 |
| 7V8B | Delta | Cryo-electron microscopy | 3.20 |
| 7T9L | Omicron | Cryo-electron microscopy | 2.66 |
| TA-SC | TA-SA-T80 | AgNPs | NSD | Description |
|---|---|---|---|---|
| 3364 | 3364 | 3364 | 3364 | ν(O-H) |
| 2924 | 2924 | 2924 | 2924 | ν(C-H) |
| 2854 | 2854 | 2854 | 2854 | ν(C=O) |
| - | 1736 | 1734 | - | ν(C=O) |
| 1576 | 1572 | 1586 | 1586 | ν(C=O) |
| 1392 | 1390 | 1398 | 1398 | ν(C-OH) |
| 1264 | 1262 | 1260 | 1260 | ν(C-H) |
| - | 1104 | - | 1114 | ν(C-O-C) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valdez-Salas, B.; Salvador-Carlos, J.; Valdez-Salas, E.; Beltrán-Partida, E.; Castillo-Saenz, J.; Curiel-Álvarez, M.; Gonzalez-Mendoza, D.; Cheng, N. Nasal Spray Disinfectant for Respiratory Infections Based on Functionalized Silver Nanoparticles: A Physicochemical and Docking Approach. Nanomaterials 2025, 15, 533. https://doi.org/10.3390/nano15070533
Valdez-Salas B, Salvador-Carlos J, Valdez-Salas E, Beltrán-Partida E, Castillo-Saenz J, Curiel-Álvarez M, Gonzalez-Mendoza D, Cheng N. Nasal Spray Disinfectant for Respiratory Infections Based on Functionalized Silver Nanoparticles: A Physicochemical and Docking Approach. Nanomaterials. 2025; 15(7):533. https://doi.org/10.3390/nano15070533
Chicago/Turabian StyleValdez-Salas, Benjamín, Jorge Salvador-Carlos, Ernesto Valdez-Salas, Ernesto Beltrán-Partida, Jhonathan Castillo-Saenz, Mario Curiel-Álvarez, Daniel Gonzalez-Mendoza, and Nelson Cheng. 2025. "Nasal Spray Disinfectant for Respiratory Infections Based on Functionalized Silver Nanoparticles: A Physicochemical and Docking Approach" Nanomaterials 15, no. 7: 533. https://doi.org/10.3390/nano15070533
APA StyleValdez-Salas, B., Salvador-Carlos, J., Valdez-Salas, E., Beltrán-Partida, E., Castillo-Saenz, J., Curiel-Álvarez, M., Gonzalez-Mendoza, D., & Cheng, N. (2025). Nasal Spray Disinfectant for Respiratory Infections Based on Functionalized Silver Nanoparticles: A Physicochemical and Docking Approach. Nanomaterials, 15(7), 533. https://doi.org/10.3390/nano15070533







