Rational Design of Nanostructured Porous and Advanced Getter Materials for Vacuum Insulation Panels
Abstract
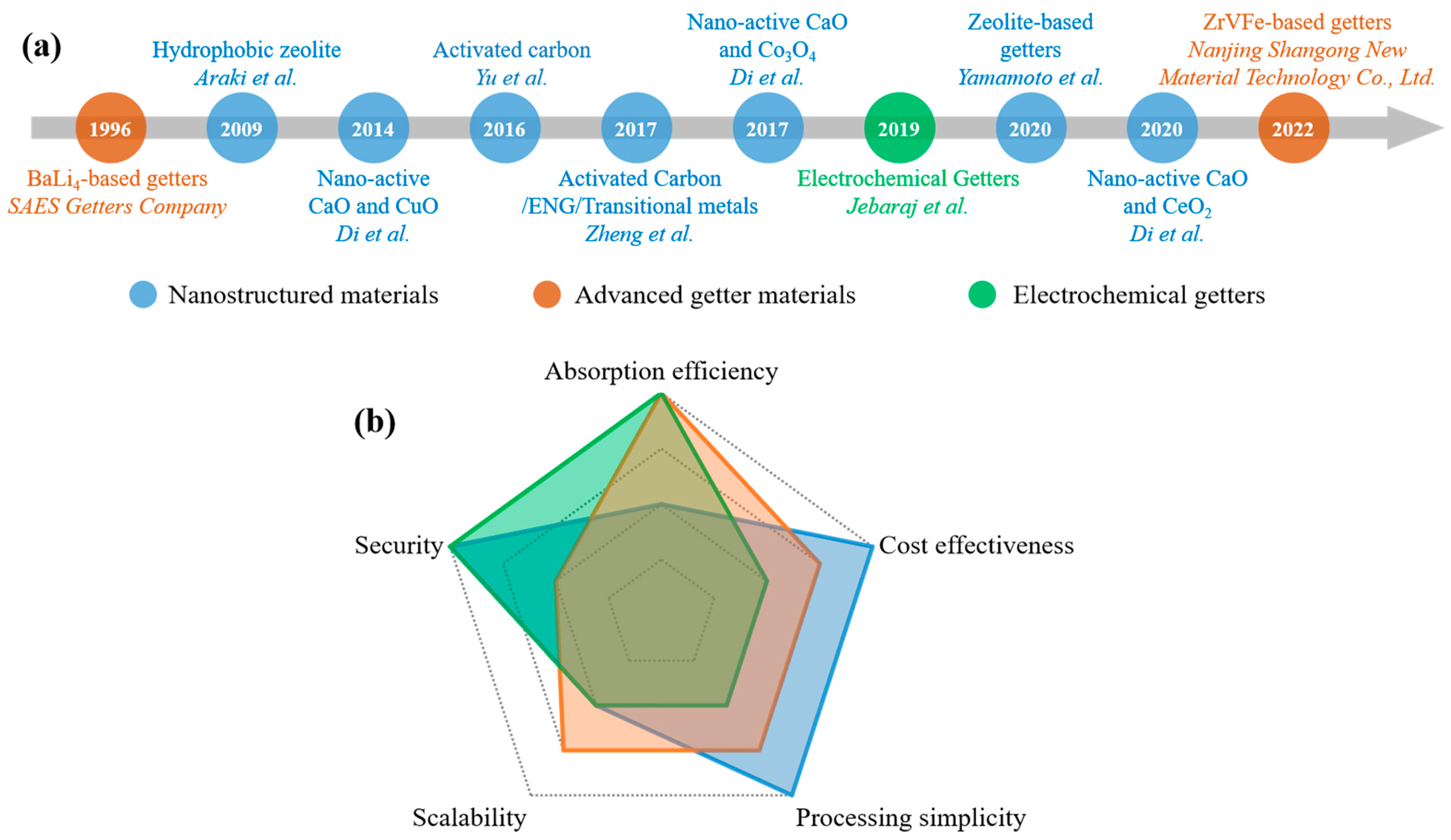
1. Introduction
2. Physical and Chemical Properties of Getter
3. Design Strategy for Getters in VIPs
3.1. Nanostructured Materials
3.2. Advanced Getter Materials
3.3. Electrochemical Getters
4. Challenges and Perspectives
Funding
Data Availability Statement
Conflicts of Interest
References
- Verma, S.; Singh, H. Vacuum insulation panels for refrigerators. Int. J. Refrig. 2020, 112, 215–228. [Google Scholar]
- Zhou, J.; Peng, Y.; Xu, J.; Wu, Y.; Huang, Z.; Xiao, X.; Cui, Y. Vacuum insulation arrays as damage-resilient thermal superinsulation materials for energy saving. Joule 2022, 6, 2358–2371. [Google Scholar]
- Jihwan, P.; Tae, K.K.; Kim, S.D.; Lee, M.Y.; Ari, H.; Kwanchul, K. Development of a vacuum insulation panel detection system for end-of-life refrigerators in Korea: A practical approach. J. Mater. Cycles Waste Manag. 2024, 26, 1713–1726. [Google Scholar]
- Kaushik, D.; Singh, H.; Tassou, S.A. Vacuum insulation panels for high-temperature applications-Design principles, challenges and pathways. Therm. Sci. Eng. Prog. 2024, 48, 102415. [Google Scholar]
- Aparicio-Fernández, C.; Torner, M.E.; Cañada-Soriano, M.; Vivancos, J. Analysis of the energy performance strategies in a historical building used as a music school. Dev. Built Environ. 2023, 15, 100195. [Google Scholar]
- Marcio, G.; Simões, N.; Serra, C.; Ines, F.C. A review of the challenges posed by the use of vacuum panels in external insulation finishing systems. Appl. Energy 2020, 257, 114028. [Google Scholar]
- Kan, A.; Zhang, X.; Chen, Z.; Cao, D. Effective thermal conductivity of vacuum insulation panels prepared with recyclable fibrous cotton core. Int. J. Therm. Sci. 2023, 187, 108176. [Google Scholar] [CrossRef]
- Verma, S.; Singh, H. Predicting the conductive heat transfer through evacuated perlite based vacuum insulation panels. Int. J. Therm. Sci. 2022, 171, 107245. [Google Scholar]
- Lee, J.; Song, T. Conduction/radiation combined heat transfer with contact resistance for application to vacuum insulation. Int. J. Heat Mass Transf. 2019, 129, 380. [Google Scholar]
- Di, X.; Gao, Y.; Bao, C.; Hu, Y.; Xie, Z. Optimization of glass fiber based core materials for vacuum insulation panels with laminated aluminum foils as envelopes. Vacuum 2013, 97, 55–59. [Google Scholar] [CrossRef]
- Kwon, J.-S.; Jang, C.H.; Jung, H.; Song, T.H. Vacuum maintenance in vacuum insulation panels exemplified with a staggered beam VIP. Energy Build. 2010, 42, 590–597. [Google Scholar]
- Di, X.; Gao, Y.; Bao, C.; Ma, S. Thermal insulation property and service life of vacuum insulation panels with glass fiber chopped strand as core materials. Energy Build. 2014, 73, 176–183. [Google Scholar]
- Pei, Y.; Shen, Z.; Zhou, J.; Yang, B. Experimental and theoretical thermal performance analysis of additively manufactured polymer vacuum insulation panels. Appl. Therm. Eng. 2024, 256, 123957. [Google Scholar] [CrossRef]
- Di, X.; Xie, Z.; Chen, J.; Zheng, S. Residual gas analysis in vacuum insulation panel (VIP) with glass fiber core and investigation of getter for VIP. Build. Environ. 2020, 186, 107337. [Google Scholar]
- Porta, P.D. Gas problem and gettering in sealed-off vacuum devices. Vacuum 1996, 47, 771–777. [Google Scholar]
- Alam, M.; Singh, H.; Limbachiya, M.C. Vacuum Insulation Panels (VIPs) for building construction industry—A review of the contemporary developments and future directions. Appl. Energy 2011, 88, 3592–3602. [Google Scholar]
- Katsura, T.; Nagano, K. Double envelope vacuum insulation panel to contribute to the long-term thermal insulation performance. Vacuum 2024, 229, 113599. [Google Scholar]
- Ju, J.Z.; Zhao, J.Y.; Li, C.; Xue, Y. Vacuum Insulation Panel Production with Ultralow Thermal Conductivity-A Review. Int. J. Thermophys. 2024, 45, 163. [Google Scholar]
- Verma, S.; Sara, A.; Singh, H. Why and which opacifier for perlite based vacuum insulation panels (VIPs) in the average temperature range of 10–70 °C. Int. J. Therm. Sci. 2023, 186, 108136. [Google Scholar]
- Mao, S.; Kan, A.; Huang, Z.; Zhu, W. Prediction of thermal performance of vacuum insulation panels (VIPs) with micro-fiber core materials. Mater. Today Commun. 2020, 22, 100786. [Google Scholar] [CrossRef]
- Mao, S.; Kan, A.; Zhu, W.; Yuan, Y. The impact of vacuum degree and barrier envelope on thermal property and service life of vacuum insulation panels. Energy Build. 2020, 209, 109699. [Google Scholar] [CrossRef]
- Shi, M.; Yang, L.; Chen, Z.; Kan, A.; Chen, S.; He, T.; Zhang, J. Impacts investigation of gas barrier on effective thermal conductivity and service life of vacuum insulation panel. Sci. Rep. 2023, 13, 20055. [Google Scholar] [CrossRef] [PubMed]
- Katsura, T.; Miyata, T.; Memon, S.; Radwan, A.; Nagano, K. Experimental analysis of vacuum pressure and gas flow rate in structured-core transparent vacuum insulation panels. Energy Rep. 2023, 9, 1071–1078. [Google Scholar] [CrossRef]
- Pons, E.; Yrieix, B.; Heymans, L.; Dubelley, F.; Planes, E. Permeation of water vapor through high performance laminates for VIPs and physical characterization of sorption and diffusion phenomena. Energy Build. 2014, 85, 604–616. [Google Scholar] [CrossRef]
- Liang, W.; Di, X.; Zheng, S.; Wu, L.; Zhang, J. A study on thermal bridge effect of vacuum insulation panels (VIPs). J. Build. Eng. 2023, 71, 106492. [Google Scholar] [CrossRef]
- Gonçalves, M.; Simões, N.; Serra, C.; Flores-Colen, I.; Rottenbacher, K.; Almeida, F. Study of the edge thermal bridging effect in vacuum insulation panels: Steady and unsteady-state approaches using numerical and experimental methods. Energ. Build. 2022, 258, 111821. [Google Scholar]
- Araki, K.; Kamoto, D.; Matsuoka, S.-i. Optimization about multilayer laminated film and getter device materials of vacuum insulation panel for using at high temperature. J. Mater. Process. Technol. 2009, 209, 271–282. [Google Scholar] [CrossRef]
- Van Malsen, J.; Tenpierik, M.J.; Looman, R.; Cauberg, J.J.M. Heat Seal Strength of Barrier Films Used in Vacuum Insulation Panels At Room Temperature and At −130 °C. J. Plast. Film Sheeting 2008, 24, 35–52. [Google Scholar] [CrossRef]
- Yamamoto, H.; Ogura, D. Study of long-term performance of vacuum insulation panels containing getter materials in building environment. Energy Build. 2022, 255, 111648. [Google Scholar] [CrossRef]
- Chuntonov, K.; Setina, J.; Douglass, G. The Newest Getter Technologies: Materials, Processes, Equipment. J. Mater. Sci. Chem. Eng. 2015, 3, 57–67. [Google Scholar]
- Yuan, B.; Ding, S.Q.; Wang, D.D.; Wang, G.; Li, H.X. Heat insulation properties of silica aerogel/glass fiber composites fabricated by press forming. Mater. Lett. 2012, 75, 204. [Google Scholar]
- Sun, Q.Q.; Xu, J.; Lu, C.B.; Zhu, S.Y.; Lin, G.Y.; Fan, M.Z.; Li, J.; Chen, K.F. Green and sustainable kapok fibre as novel core materials for vacuum insulations panels. Appl. Energy 2023, 347, 121394. [Google Scholar]
- Corker, J.; Marques, I.; Resalati, S.; Okoroafor, T.; Maalouf, A.; Fu, Z.Y.; Fan, M.Z. Al-rich industrial waste as new alternative of fumed silica for the manufacture of vacuum insulation panels for building energy conservation. J. Clean. Prod. 2023, 415, 137854. [Google Scholar]
- Yamamoto, H.; Ogura, D. Dependence of gas permeation and adsorption on temperature in vacuum insulation panels (VIPs) containing getter materials. J. Build. Phys. 2021, 45, 604–628. [Google Scholar]
- Zheng, Q.R.; Zhu, Z.W.; Chen, J.; Yu, W.S. Preparation of carbon based getter for glass fiber core vacuum insulation panels (VIPs) used on marine reefer containers. Vacuum 2017, 146, 111–119. [Google Scholar]
- Yu, W.S.; Zhu, Z.W.; Chen, J.; Zheng, Q.R. Synthesis of the Getter for Vacuum Insulation Panels (VIPs) used on Marine Reefer Containers. MATEC Web Conf. 2016, 67, 06105. [Google Scholar]
- Manini, P.; Belloni, F. Device for Maintaining a Vacuum in a Thermally Insulating Jacket and Method of Making Such Device. U.S. Patent 5544490, 13 August 1996. [Google Scholar]
- Jebaraj, A.; Xu, J.; Feng, Z.; Abramson, A.R.; Scherson, D.A. Electrochemical Getters: A Novel Approach toward Improved Thermal Insulation. J. Electrochem. Soc. 2019, 166, B1701. [Google Scholar]
- Kan, A.; Zheng, N.; Wu, Y.; Wang, W.; Zhang, X.; Cai, H.; Cao, D. Theoretical prediction and aging experimental verification of the service life of vacuum insulation panels. Clean. Eng. Technol. 2022, 8, 100484. [Google Scholar]
- Luft, G.; Recasens, F.; Velo, E. Chapter 3—Kinetic Properties at High Pressure. In Industrial Chemistry Library; Bertucco, A., Vetter, G., Eds.; Elsevier: Amsterdam, The Netherlands, 2001; Volume 9, pp. 65–140. [Google Scholar]
- Mulvihill, C.R.; Georgievskii, Y.; Klippenstein, S.J. Quantum and anharmonic effects in non-adiabatic transition state theory. J. Chem. Phys. 2023, 159, 174104. [Google Scholar]
- Rivlin, T.; Pollak, E. Nonadiabatic Couplings Can Speed Up Quantum Tunneling Transition Path Times. J. Phys. Chem. Lett. 2022, 13, 10558–10566. [Google Scholar]
- Elkatmis, A.; Kangi, R. Remarks concerning about the characteristics of the extractor vacuum gauge and the Quadrupole Mass Spectrometer. Measurement 2019, 131, 269–276. [Google Scholar] [CrossRef]
- Jousten, K.; Putzke, S.; Buthig, J. Partial pressure measurement standard for characterizing partial pressure analyzers and measuring outgassing rates. J. Vac. Sci. Technol. A 2015, 33, 061603. [Google Scholar] [CrossRef]
- Di, X.; Chen, Z. Investigation of composite getter for vacuum insulation panels. J. Nanjing Univ. Aeronaut. Astronaut. 2017, 49, 24–28. [Google Scholar]
- Jung, H.; Jang, C.H.; Seok, Y.I.; Song, T.H. Investigation of gas permeation through Al-metallized film for vacuum insulation panels. Int. J. Heat Mass Transf. 2013, 56, 436–446. [Google Scholar] [CrossRef]
- Torres, J.; Perry, C.C.; Wagner, A.J.; Fairbrother, D.H. Interaction of chlorine radicals with polyethylene and hydrocarbon thin films under vacuum conditions-a comparison with atomic oxygen reactivity. Surf. Sci. 2003, 543, 75–86. [Google Scholar] [CrossRef]
- Yavary, M.; Ale Ebrahim, H.; Falamaki, C. Competitive Adsorption Equilibrium Isotherms of CO, CO2, CH4, and H2 on Activated Carbon and Zeolite 5A for Hydrogen Purification. J. Chem. Eng. Data 2016, 61, 3420–3427. [Google Scholar] [CrossRef]
- Mazza, F.; Boffito, C. Nonevaporable Getters: Properties and Applications. MRS Bull. 2013, 15, 50–52. [Google Scholar] [CrossRef]
- Pourhakkak, P.; Taghizadeh, A.; Taghizadeh, M.; Ghaedi, M.; Haghdoust, S. Chapter 1—Fundamentals of adsorption technology. In Interface Science and Technology; Ghaedi, M., Ed.; Elsevier: Amsterdam, The Netherlands, 2021; Volume 33, pp. 1–70. [Google Scholar]
- Liu, W.; Liu, J.; Ma, L.; Jing, D. Understanding the adsorption properties of CO2 and N2 by a typical MOF structure: Molecular dynamics and weak interaction visualization. Chem. Eng. Sci. 2024, 296, 120233. [Google Scholar] [CrossRef]
- Han, R.; Tao, Y.; Zhou, L. Growth Study of Hierarchical Pore SSZ-13 Molecular Sieves with Improved CO2 Adsorption Performance. Nanomaterials 2021, 11, 3171. [Google Scholar] [CrossRef]
- Kurnitski, J.; Yamamoto, H.; Ogura, D.; Kalamees, T. Study on the temperature dependence of gas permeation and adsorption behavior of the vacuum insulation panel with getter materials. E3S Web Conf. 2020, 172, 21001. [Google Scholar]
- Xu, H.; Wang, Q.Y.; Jiang, M.; Li, S.S. Application of valence-variable transition-metal-oxide-based nanomaterials in electrochemical analysis: A review. Anal. Chim. Acta 2024, 1295, 342270. [Google Scholar] [PubMed]
- Yamamoto, H.; Ogura, D. Prediction of water vapor permeation and desiccant saturation in vacuum insulation panels with a glass fiber core. J. Build. Eng. 2023, 76, 107347. [Google Scholar]
- Zhu, M.; Yu, Y.; Huang, Q.; Li, X.; Gu, C.; Zhang, B.; Yao, X.; Li, Z.; Hao, C. Experimental study on the sorption performance of New-Fashioned silver getters compared with the traditional PdO in High-Vacuum multi-layer insulation vessels. Vacuum 2023, 208, 111685. [Google Scholar] [CrossRef]
- Wang, J.; Zhan, Y.; Wei, W.; Chen, S.; Wang, R. A new cost effective composite getter for application in high-vacuum-multilayer-insulation tank. Vacuum 2016, 131, 44–50. [Google Scholar]
- Belousov, V.M.; Vasylyev, M.A.; Lyashenko, L.V.; Vilkova, N.Y.; Nieuwenhuys, B.E. The low-temperature reduction of Pd-doped transition metal oxide surfaces with hydrogen. Chem. Eng. J. 2003, 91, 143–150. [Google Scholar]
- Emmanuelle, P.; Yrieix, B.; Brunner, S. Evaluation of VIPs after mild artificial aging during 10 years: Focus on the core behavior. Energy Build. 2018, 162, 198–207. [Google Scholar]
- Magnano, E.; Vandré, S.; Kovac, J.; Narducci, E.; Caloi, R.; Manini, P.; Sancrotti, M. Surface and subsurface properties of a BaLi4 gettering alloy studied using X-ray photoemission spectroscopy. Surf. Sci. 1998, 402, 223–226. [Google Scholar] [CrossRef]
- Cuevas, F.; Latroche, M. Intermetallic alloys as hydrogen getters. J. Alloys Compd. 2022, 905, 164173. [Google Scholar]
- Zhang, R.; Shen, Z.; Park, B.; Feng, T.; Aldykiewicz, A.; Desjarlais, A.; Hun, D.; Shrestha, S. Natural fibers as promising core materials of vacuum insulation panels. Constr. Build. Mater. 2024, 453, 138890. [Google Scholar]
- Sun, Q.; Chen, J.; Xu, J.; Zhang, Z.; Zhu, S.; Li, J.; Chen, K.; Fan, M. Functionalising kapok fibre with lignin to enhance the structural and thermal performance of vacuum insulation panels. Ind. Crops Prod. 2024, 220, 119277. [Google Scholar]
- Sánchez-Calderón, I.; Bernardo, V.; Lizalde-Arroyo, F.; Martín-de-León, J.; Rodríguez-Pérez, M.Á. Development of new vacuum insulation core panels using micronized nanocellular poly(methyl-methacrylate) (PMMA). Appl. Mater. Today 2024, 41, 102483. [Google Scholar]
- Dong, X.; Zhang, Q.; Lan, Y.; Zeng, Q.; Fan, M.; Chen, L.; Zhao, W. Preparation and characterization of vacuum insulation panels with hybrid composite core materials of bamboo and glass fiber. Ind. Crop. Prod. 2022, 188, 115691. [Google Scholar]
- Pérez-Botella, E.; Valencia, S.; Rey, F. Zeolites in Adsorption Processes: State of the Art and Future Prospects. Chem. Rev. 2022, 122, 17647–17695. [Google Scholar]
- Rong, X.; Yang, Y.; Zhang, J. Factors affecting the thermal conductivity of vacuum-insulated panels: A review. Mater. Rep. 2025, 39, 24050221. [Google Scholar]
- Silva, J.A.C.; Rodrigues, A.E. Multisite Langmuir Model Applied to the Interpretation of Sorption of n -Paraffins in 5A Zeolite. Ind. Eng. Chem. Res. 1999, 38, 2434–2438. [Google Scholar]
- Son, K.N.; Cmarik, G.E.; Knox, J.C.; Weibel, J.A.; Garimella, S.V. Measurement and Prediction of the Heat of Adsorption and Equilibrium Concentration of CO2 on Zeolite 13X. J. Chem. Eng. Data 2018, 63, 1663–1674. [Google Scholar]
- Prasad, R.; Rattan, G. Preparation Methods and Applications of CuO-CeO2 Catalysts: A Short Review. ChemInform 2010, 41, 7–30. [Google Scholar]
- Xu, J.; Bian, Y.; Tian, W.; Pan, C.; Wu, C.; Xu, L.; Wu, M.; Chen, M. The Structures and Compositions Design of the Hollow Micro–Nano-Structured Metal Oxides for Environmental Catalysis. Nanomaterials 2024, 14, 1190. [Google Scholar] [CrossRef]
- Xiong, Y.H.; Wei, X.Y.; Qin, G.R.; Yuan, P.; Mao, C.H.; Du, J. Preparation and hydrogen sorption performance of a modified Zr-C getter. Vacuum 2008, 82, 737–741. [Google Scholar]
- Heidary Moghadam, A.; Dashtizad, V.; Kaflou, A.; Yoozbashizadeh, H. Effect of rare earth elements on sorption characteristics of nanostructured Zr-Co sintered porous getters. Vacuum 2015, 111, 9–14. [Google Scholar]
- Li, L.; Zeng, F.; Li, W.; Ziwei, W.; Liu, H.; Peng, Y.; Yi, G.; Song, J.; Wensheng, L. Nitrogen absorption behavior and mechanism of TiZrMnFe getter alloy. Vacuum 2021, 183, 109814. [Google Scholar]
- Barra, G.; Guadagno, L.; Raimondo, M.; Santonicola, M.G.; Toto, E.; Vecchio Ciprioti, S. A Comprehensive Review on the Thermal Stability Assessment of Polymers and Composites for Aeronautics and Space Applications. Polymers 2023, 15, 3786. [Google Scholar] [CrossRef] [PubMed]
- Kan, A.; Zheng, N.; Zhu, W.; Cao, D.; Wang, W. Innovation and development of vacuum insulation panels in China: A state-of-the-art review. J. Build. Eng. 2022, 48, 103937. [Google Scholar]
- Wegger, E.; Jelle, B.P.; Sveipe, E.; Grynning, S.; Gustavsen, A.; Baetens, R.; Thue, J.V. Aging effects on thermal properties and service life of vacuum insulation panels. J. Build. Phys. 2011, 35, 128–167. [Google Scholar]
- Liu, M.; Liu, H.; Chen, K.; Jiangyong, S.; Hui, W.; Liu, J.; Liuzhang, O. An Al-Li alloy/water system for superior and low-temperature hydrogen production. Inorg. Chem. Front. 2021, 8, 3473–3481. [Google Scholar]
- Jones, J.R.; Gaedtke, M.; Sebastian, S.; Matthias, R.; Hermann, N.; Krause, M.J. Conjugate heat transfer through nano scale porous media to optimize vacuum insulation panels with lattice Boltzmann methods. Comput. Math. Appl. 2019, 77, 209–221. [Google Scholar]
- Jiao, L.; Seow, J.Y.R.; Skinner, W.S.; Wang, Z.U.; Jiang, H.L. Metal-organic frameworks: Structures and functional applications. Mater. Today 2019, 27, 43–68. [Google Scholar] [CrossRef]
- Nath, K.; Wright, K.R.; Ahmed, A.; Siegel, D.J.; Matzger, A.J. Adsorption of Natural Gas in Metal-Organic Frameworks: Selectivity, Cyclability, and Comparison to Methane Adsorption. J. Am. Chem. Soc. 2024, 146, 10517–10523. [Google Scholar]
- Kim, S.C.; Choi, S.Q.; Park, J. Asymmetric Supercapacitors Using Porous Carbons and Iron Oxide Electrodes Derived from a Single Fe Metal-Organic Framework (MIL-100 (Fe)). Nanomaterials 2023, 13, 1824. [Google Scholar] [CrossRef]
- Qu, J.; Sui, M.; Li, R. Recent advances in in-situ transmission electron microscopy techniques for heterogeneous catalysis. iScience 2023, 26, 107072. [Google Scholar]
- Chu, M.N.; Nguyen, L.T.H.; Truong, M.X.; Do, T.H.; Duong, T.T.A.; Nguyen, L.T.T.; Pham, M.A.; Tran, T.K.N.; Ngo, T.C.Q.; Pham, V.H. Ce3+/Ce4+-Doped ZrO2/CuO Nanocomposite for Enhanced Photocatalytic Degradation of Methylene Blue under Visible Light. Toxics 2022, 10, 463. [Google Scholar] [CrossRef] [PubMed]
- Grabchenko, M.V.; Mikheeva, N.N.; Mamontov, G.V.; Cortés Corberán, V.; Litvintseva, K.A.; Svetlichnyi, V.A.; Vodyankina, O.V.; Salaev, M.A. Unraveling the Structural and Compositional Peculiarities in CTAB-Templated CeO2-ZrO2-MnOx Catalysts for Soot and CO Oxidation. Nanomaterials 2023, 13, 3108. [Google Scholar] [CrossRef] [PubMed]
- Lorger, S.; Fischer, D.; Usiskin, R.; Maier, J. Sputter deposition and thermal evaporation of Li2O, Li2S, and Li2Se films. J. Vac. Sci. Technol. A 2019, 37, 061515. [Google Scholar]
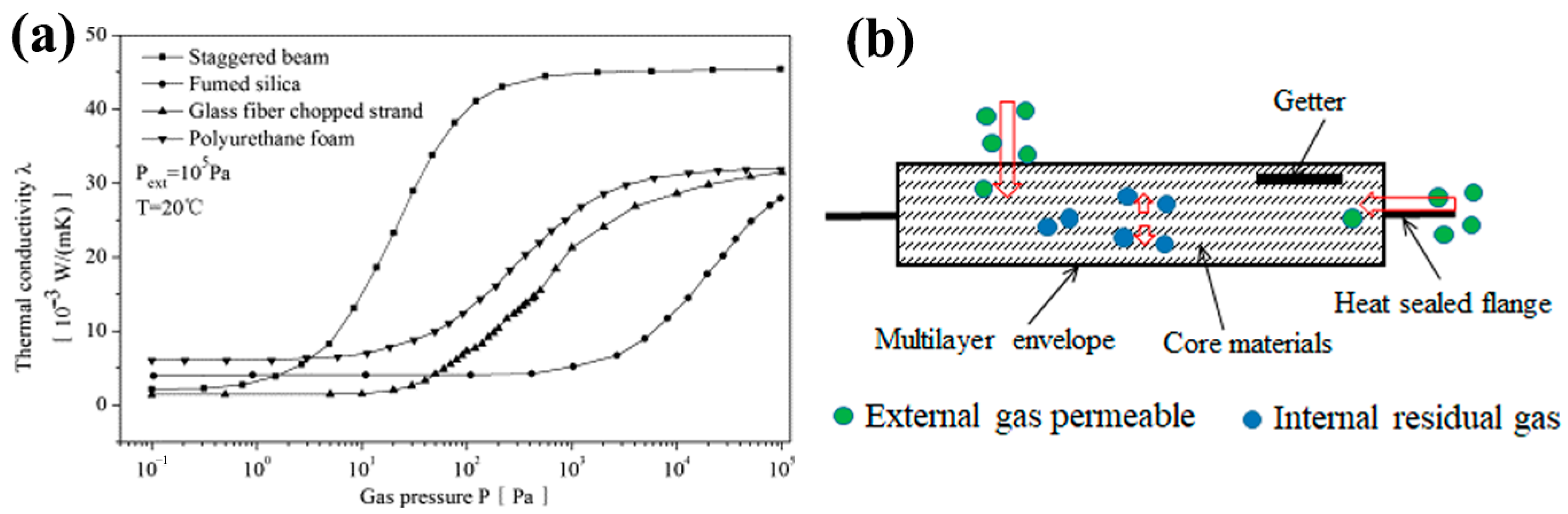

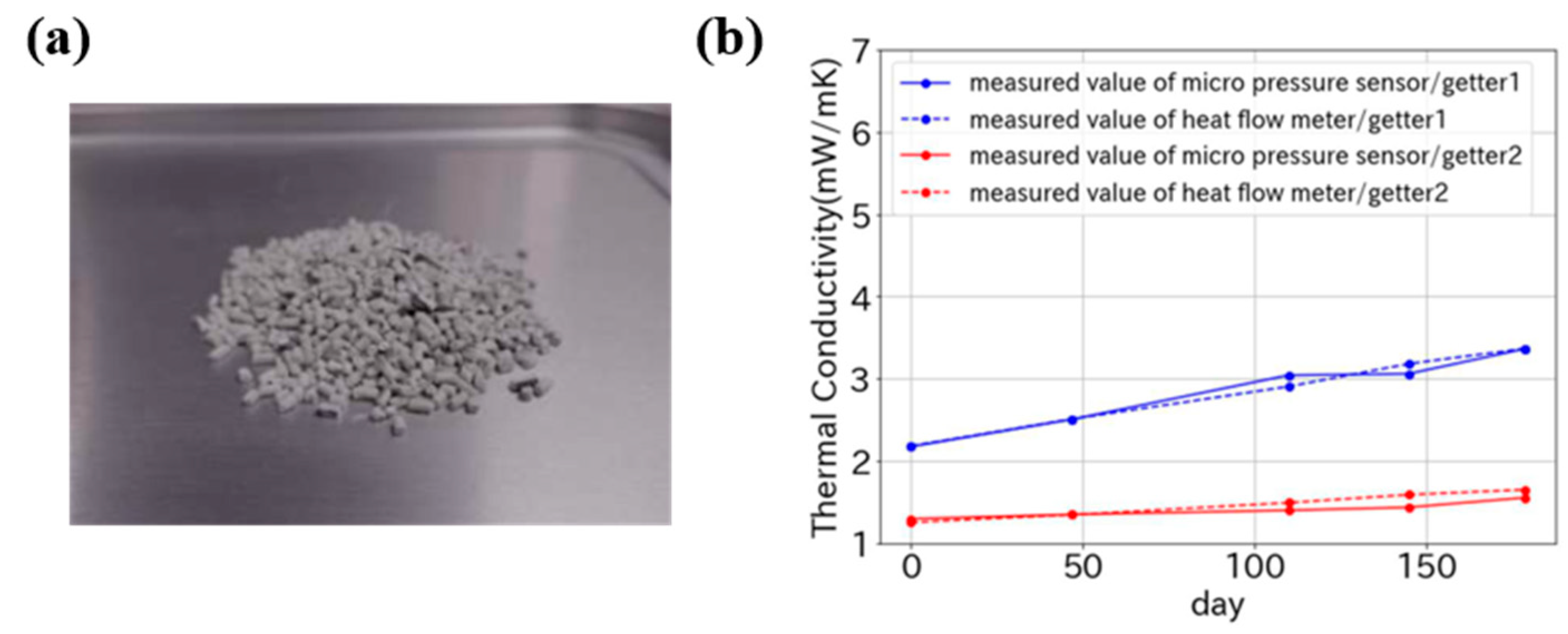
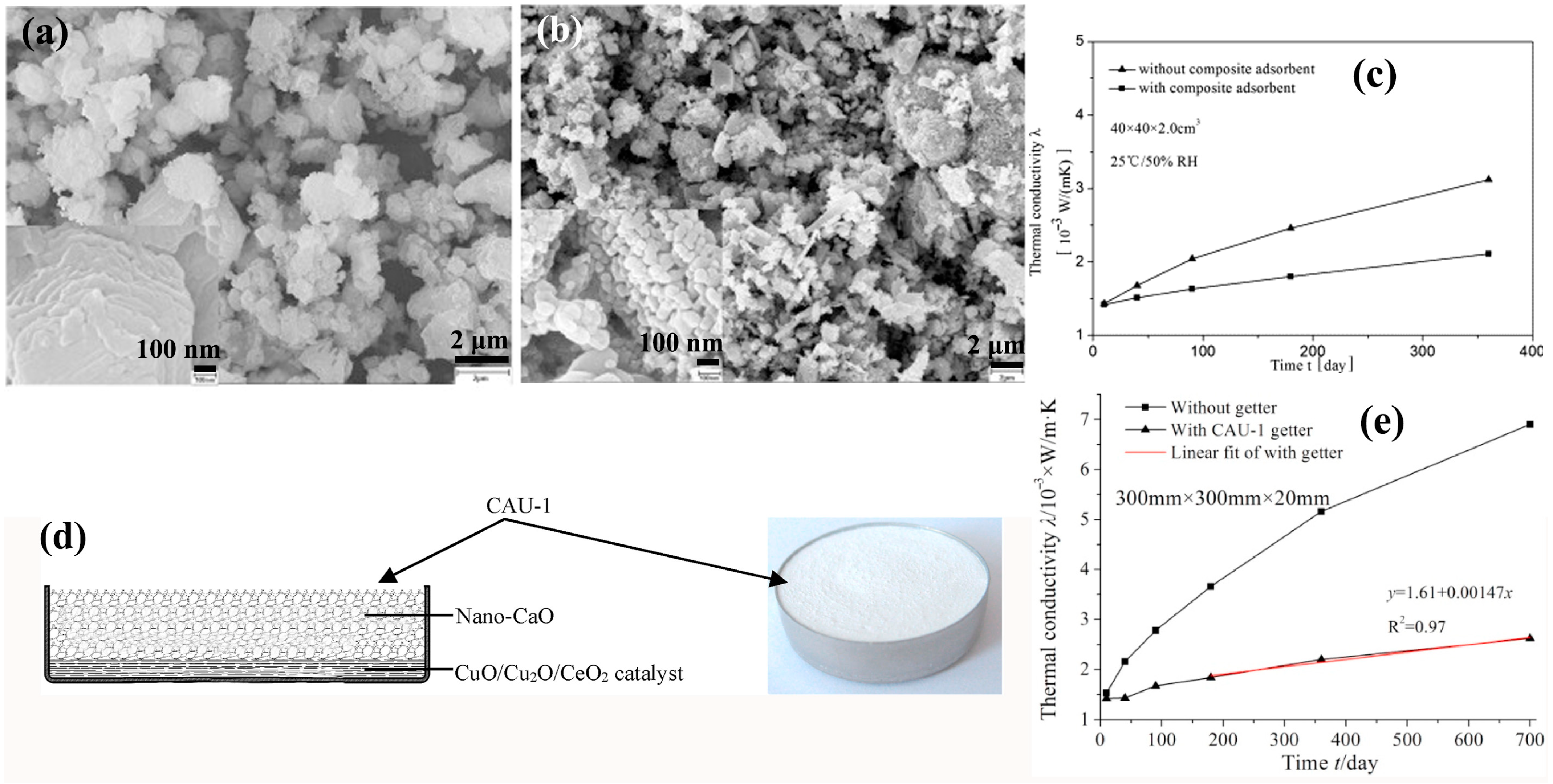
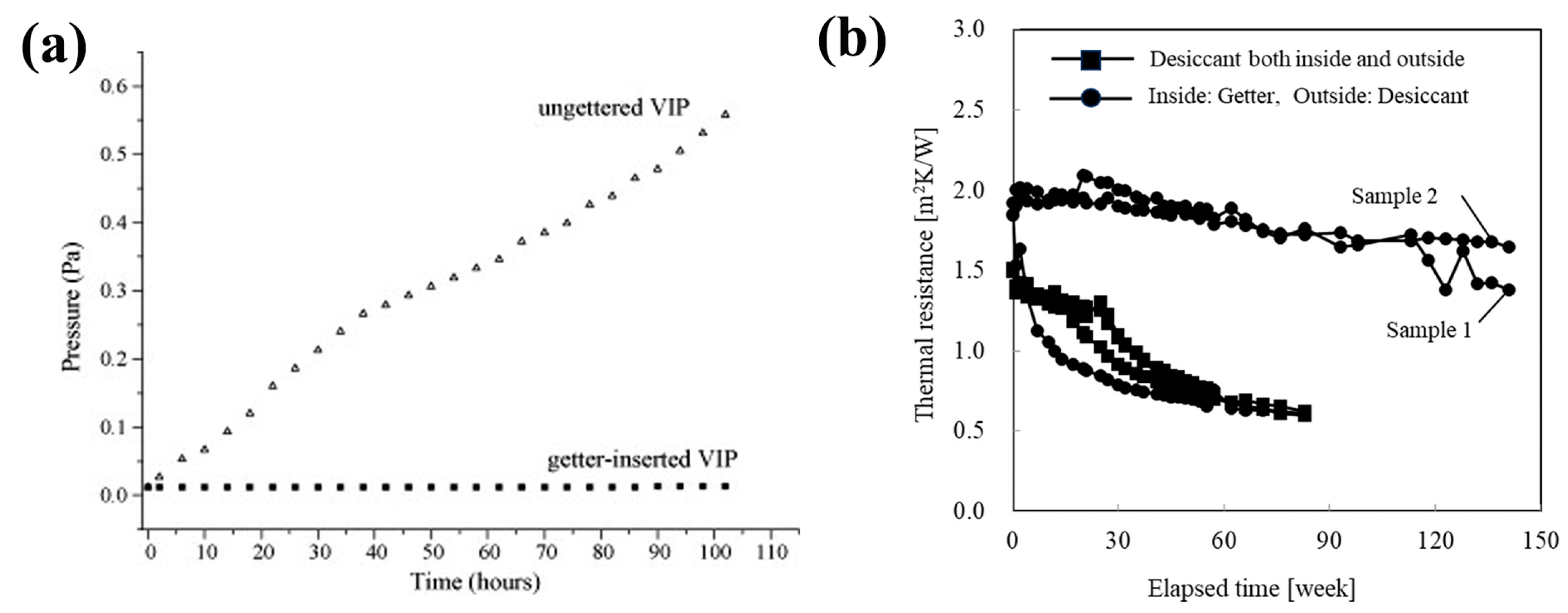
| Core Material | Initial Gas Composition | Gas Composition After Aging (Without Getter) | Getter Material | Initial Thermal Conductivity mW/(m·K) | Thermal Conductivity After Aging mW/(m·K) @720 day | Ref. |
|---|---|---|---|---|---|---|
| Glass fiber | N2/O2/Ar/H2O | N2/O2/Ar/H2O/H2/CO/CO2/CxHy | CaO/CuO/Cu2O/CeO2 | 1.52 | 6.8 | [14] |
| Glass fiber | N2/O2/H2/Ar/H2O/CO2 | / | Zeolite | 2.0 | 2.2 | [34] |
| Glass fiber | Air/H2/ethylene and propylene | / | Activated Carbon /ENG/Transitional metals | 2.45 | / | [35] |
| Glass fiber | N2/O2/H2O/H2/Ar | H2/H2O/N2/O2/CO/CO2/Ar | Nano-CaO | 2.2 | 5.8 | [45] |
| Nano-CaO/Co3O4 | 2.2 | 3.9 | ||||
| / | 2.4 | 7.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Pei, Z.; Zhou, N. Rational Design of Nanostructured Porous and Advanced Getter Materials for Vacuum Insulation Panels. Nanomaterials 2025, 15, 532. https://doi.org/10.3390/nano15070532
Wang J, Pei Z, Zhou N. Rational Design of Nanostructured Porous and Advanced Getter Materials for Vacuum Insulation Panels. Nanomaterials. 2025; 15(7):532. https://doi.org/10.3390/nano15070532
Chicago/Turabian StyleWang, Juan, Zhibin Pei, and Ningning Zhou. 2025. "Rational Design of Nanostructured Porous and Advanced Getter Materials for Vacuum Insulation Panels" Nanomaterials 15, no. 7: 532. https://doi.org/10.3390/nano15070532
APA StyleWang, J., Pei, Z., & Zhou, N. (2025). Rational Design of Nanostructured Porous and Advanced Getter Materials for Vacuum Insulation Panels. Nanomaterials, 15(7), 532. https://doi.org/10.3390/nano15070532





