Tunable Electrical Properties of Cobalt-Doped Maghemite Nanoparticles for Advanced Resistive and Thermistor Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of Pure and Cobalt-Doped Maghemite (γ-Fe2O3) Nanopowders
2.2. Characterization
3. Results and Discussion
3.1. Structural Behaviors
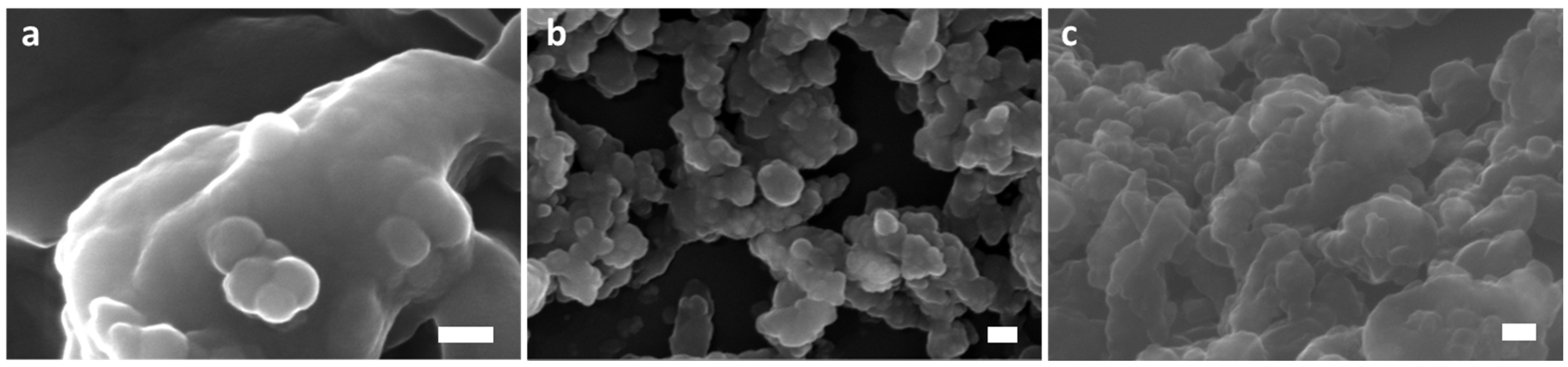
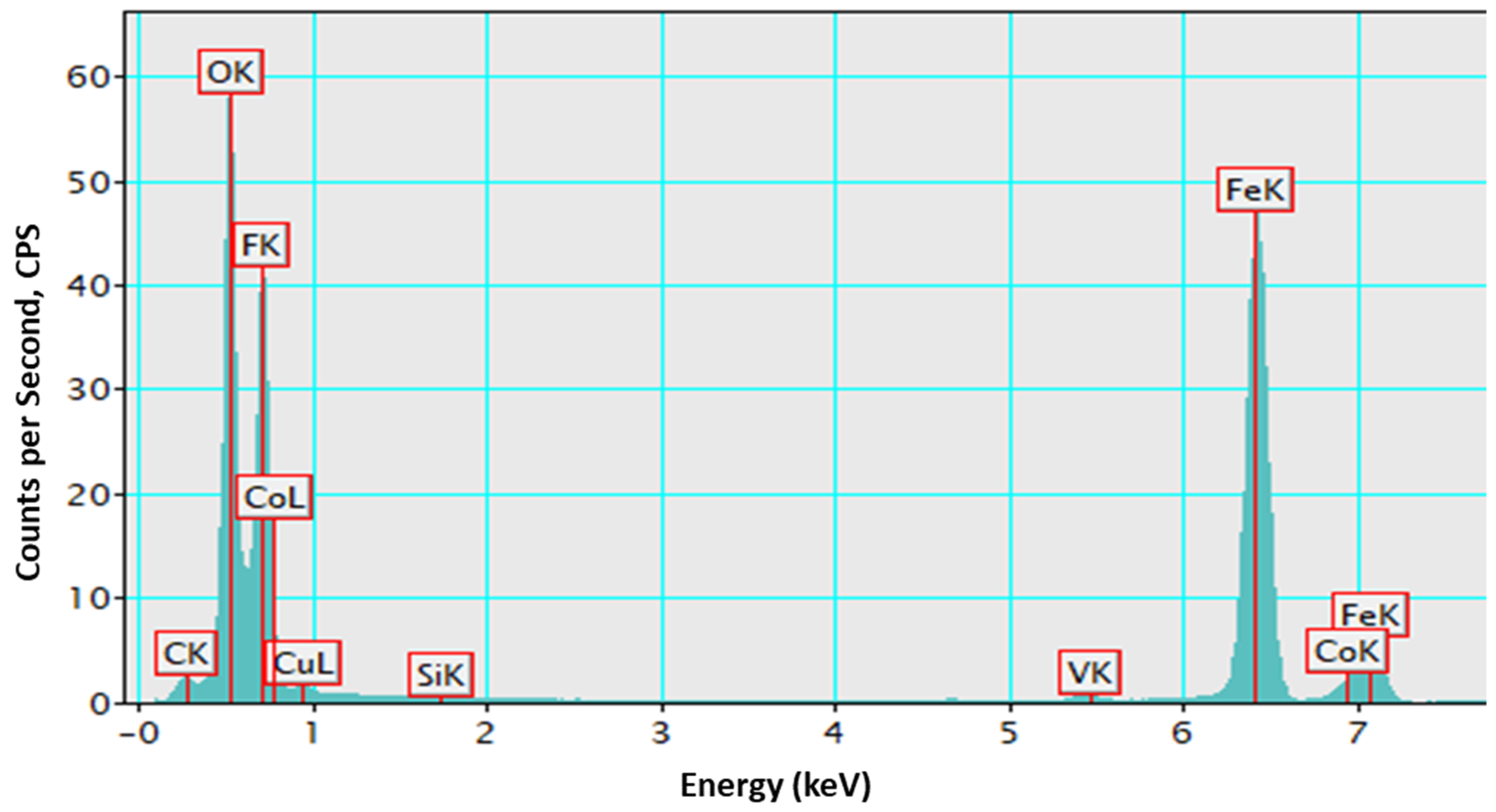
3.2. Morphological and Chemical Composition Properties
3.3. Electric Behaviors
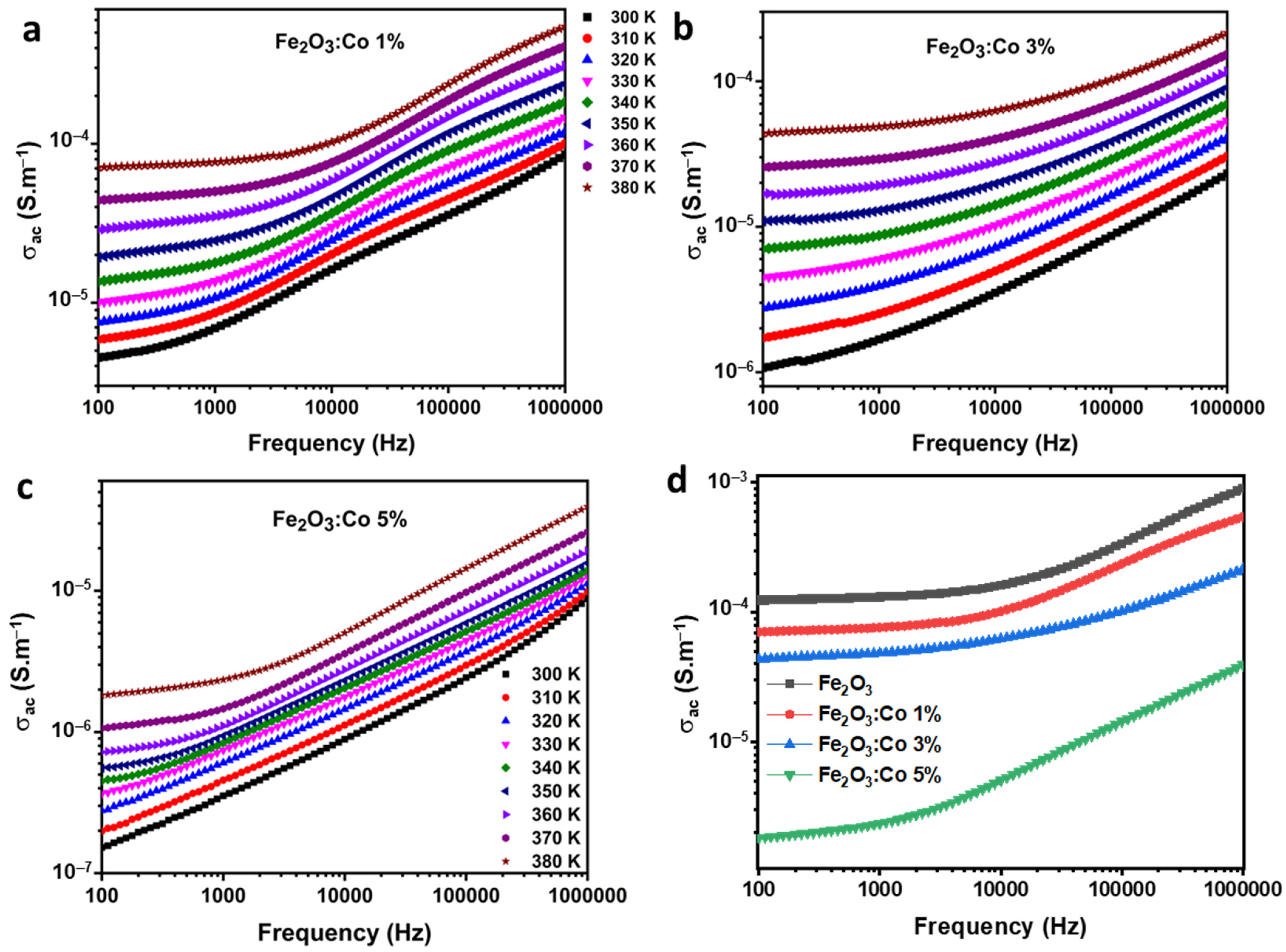
3.3.1. AC Conductivity
3.3.2. Activation Energy
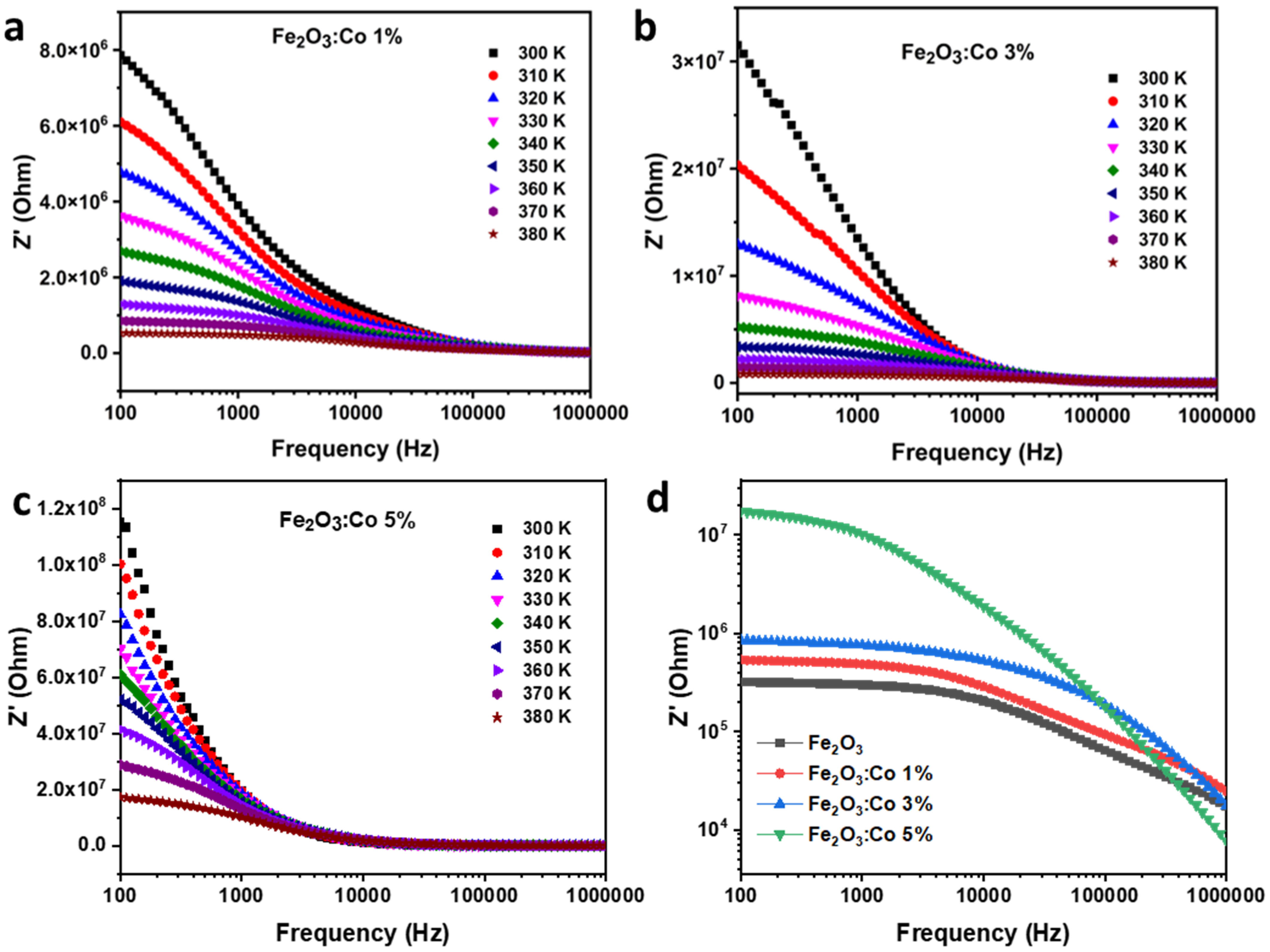
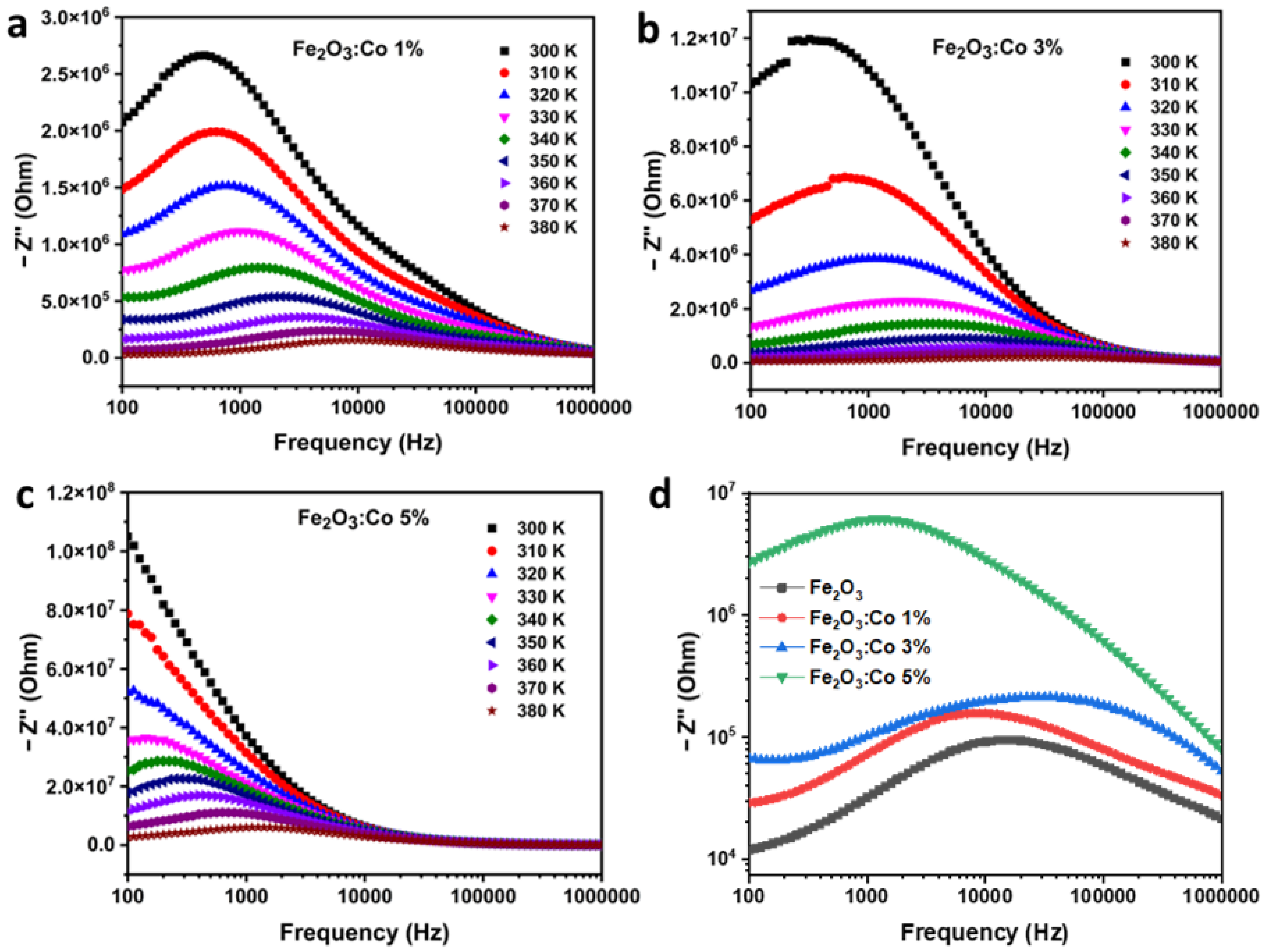
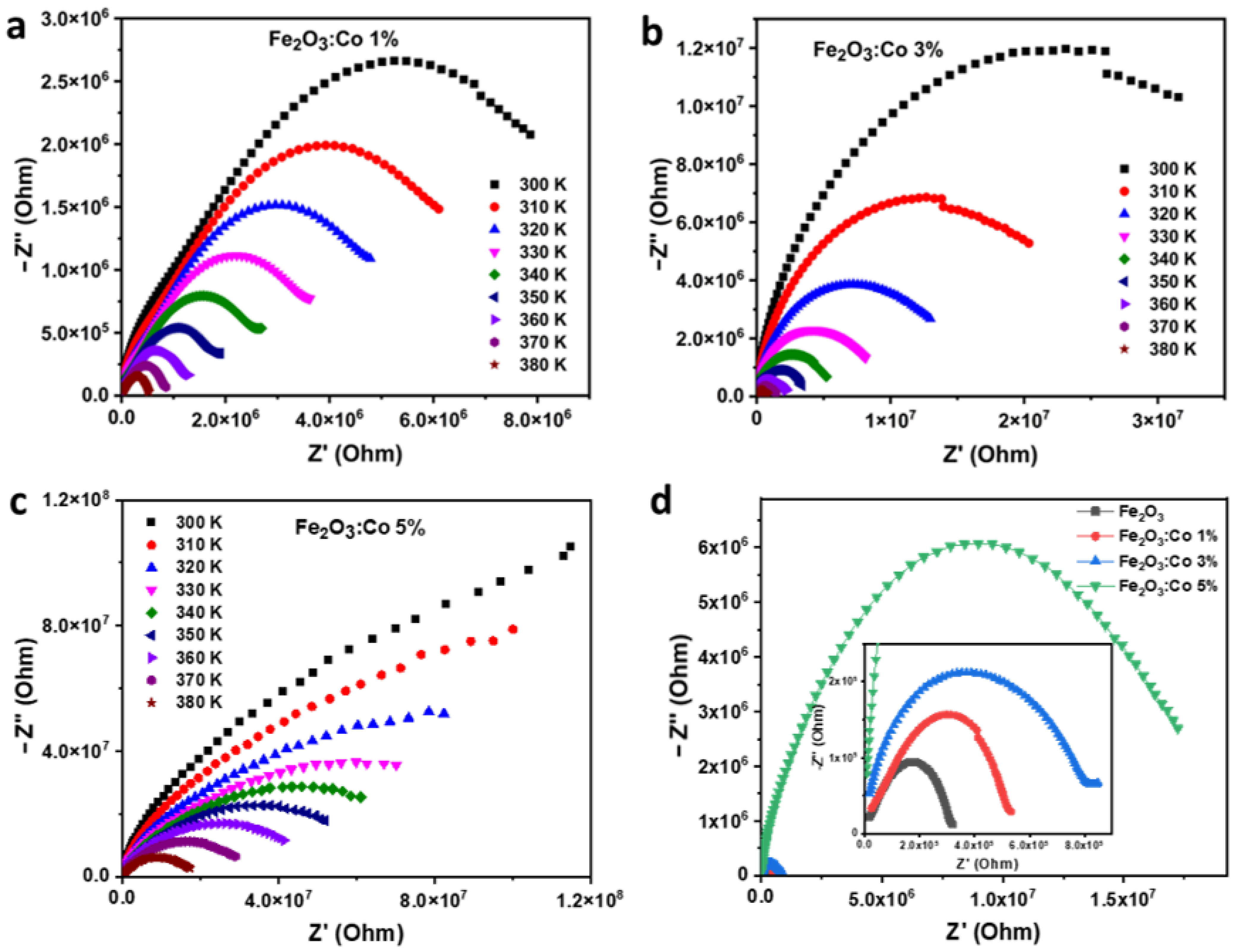
3.3.3. Electrical Impedance Studies
Real Part of Impedance (Z′)
Imaginary Part of Impedance (Z″)
Nyquist Diagram
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhou, H.; Guo, J.; Zhu, G.; Xu, H.; Tang, X.; Luo, X. Flotation Behavior and Mechanism of Smithsonite under the System of Bidentate Ligand Sulfide Sodium Thiocyanate. Sep. Purif. Technol. 2024, 334, 126086. [Google Scholar] [CrossRef]
- Li, N.; Hu, X.; Lu, Y.; Li, Y.; Ren, M.; Luo, X.; Sui, X. Wavelength-Selective Near-Infrared Organic Upconversion Detectors for Miniaturized Light Detection and Visualization. Adv. Funct. Mater. 2024, 34, 2411626. [Google Scholar]
- Yan, J.; Wei, D.; Zhao, Z.; Sun, K.; Sun, Y. Osteosarcoma-Targeting PtIV Prodrug Amphiphile for Enhanced Chemo-Immunotherapy via Ca2+ Trapping. Acta Biomater. 2025, 193, 474–483. [Google Scholar] [CrossRef]
- Zhuang, B.; Yang, Y.; Huang, K.; Yin, J. Plasmon-Enhanced NIR-II Photoluminescence of Rare-Earth Oxide Nanoprobes for High-Sensitivity Optical Temperature Sensing. Opt. Lett. 2024, 49, 6489–6492. [Google Scholar] [CrossRef]
- Li, X.; Hu, C.; Liu, H.; Shi, X.; Peng, J. Data-Driven Pressure Estimation and Optimal Sensor Selection for Noisy Turbine Flow with Blocked Clustering Strategy. Phys. Fluids 2024, 36, 125188. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, L.; Wang, J.; Pan, H.; Dou, M.; Teng, Y.; Wang, M. Bagasse-Based Porous Flower-Like MoS2/Carbon Composites for Efficient Microwave Absorption. Carbon Lett. 2024, 35, 145–160. [Google Scholar] [CrossRef]
- Wu, H.; Zhang, H.; Yan, R.; Li, S.; Guo, X.; Qiu, L.; Yao, Y. Limosilactobacillus Regulating Microbial Communities to Overcome the Hydrolysis Bottleneck with Efficient One-Step Co-Production of H2 and CH4. Adv. Sci. 2024, 11, 2406119. [Google Scholar] [CrossRef]
- Jia, S.; Li, Y.; Gao, C.; Liu, G.; Ren, Y.; He, C.; An, X.T. Realization of P-Type MA-Based Perovskite Solar Cells Based on Exposure of the (002) Facet. Appl. Phys. Lett. 2025, 126, 023908. [Google Scholar] [CrossRef]
- Gao, C.; Jia, S.; Yin, X.; Li, Z.; Yang, G.; Chen, J.; An, X. Enhancing Open-Circuit Voltage in FAPbI3 Perovskite Solar Cells via Self-Formation of Coherent Buried Interface FAPbIxCl3−x. Chem. Commun. 2025, 61, 2758–2761. [Google Scholar] [CrossRef]
- Wu, H.; Li, A.; Zhang, H.; Li, S.; Yang, C.; Lv, H.; Yao, Y. Microbial Mechanisms for Higher Hydrogen Production in Anaerobic Digestion at Constant Temperature Versus Gradient Heating. Microbiome 2024, 12, 170. [Google Scholar] [CrossRef]
- Zhu, B.; Zhang, Z.; Ma, T.; Yang, X.; Li, Y.; Shung, K.K.; Zhou, Q. (100)-Textured KNN-Based Thick Film with Enhanced Piezoelectric Property for Intravascular Ultrasound Imaging. Appl. Phys. Lett. 2015, 106, 173504. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Chan, N.Y.; Dai, J.; Shung, K.K.; Takeuchi, S.; Zhou, Q. New Fabrication of High-Frequency (100-MHz) Ultrasound PZT Film Kerfless Linear Array [Correspondence]. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2013, 60, 854–857. [Google Scholar] [CrossRef]
- Zhu, B.P.; Wu, D.W.; Zhou, Q.F.; Shi, J.; Shung, K.K. Lead Zirconate Titanate Thick Film with Enhanced Electrical Properties for High Frequency Transducer Applications. Appl. Phys. Lett. 2008, 93, 012905. [Google Scholar] [CrossRef]
- Zhu, B.P.; Wu, D.W.; Zhang, Y.; Ou-Yang, J.; Chen, S.; Yang, X.F. Sol-Gel Derived PMN-PT Thick Films for High Frequency Ultrasound Linear Array Applications. Ceram. Int. 2013, 39, 8709–8714. [Google Scholar] [CrossRef]
- Zhu, B.P.; Li, D.D.; Zhou, Q.F.; Shi, J.; Shung, K.K. Piezoelectric PZT Thick Films on LaNiO3 Buffered Stainless Steel Foils for Flexible Device Applications. J. Phys. D Appl. Phys. 2008, 42, 025504. [Google Scholar] [CrossRef]
- Zhang, T.; Ou-Yang, J.; Yang, X.; Zhu, B. Transferred PMN-PT Thick Film on Conductive Silver Epoxy. Materials 2018, 11, 1621. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.Q.; Zhu, H.L. Study on the Effect of Carbon Nanotubes on the Microstructure and Anti-Carbonation Properties of Cement-Based Materials. J. Funct. Mater. 2023, 54, 8217–8227. [Google Scholar]
- Yang, Z.Q.; Hou, K.P.; Guo, T.T. Study on the Effects of Different Water-Cement Ratios on the Flow Pattern Properties of Cement Grouts. Appl. Mech. Mater. 2011, 71, 1264–1267. [Google Scholar] [CrossRef]
- Feng, L.; Yang, Y.; Zhao, Y.; Ma, K.; Cui, J. Corrosion behaviors and mechanism of AlxCrFeMnCu high-entropy alloys in a 3.5 wt% NaCl solution. Corros. Sci. 2024, 233, 112087. [Google Scholar] [CrossRef]
- Zhao, L.C.; Tang, M.; Zhang, Q.H.; Hu, Z.Y.; Gao, H.W.; Liao, X.Y.; Leng, J. Fabrication of a Porous Metal-Organic Framework with Polar Channels for 5-Fu Delivery and Inhibiting Human Osteosarcoma Cells. J. Chem. 2018, 2018, 1523154. [Google Scholar] [CrossRef]
- Wu, C.W.; Ren, X.; Xie, G.; Zhou, W.X.; Zhang, G.; Chen, K.Q. Enhanced High-Temperature Thermoelectric Performance by Strain Engineering in BiOCl. Phys. Rev. Appl. 2022, 18, 014053. [Google Scholar] [CrossRef]
- Chen, X.K.; Zhang, E.M.; Wu, D.; Chen, K.Q. Strain-Induced Medium-Temperature Thermoelectric Performance of Cu4TiSe4: The Role of Four-Phonon Scattering. Phys. Rev. Appl. 2023, 19, 044052. [Google Scholar] [CrossRef]
- Zhou, W.X.; Wu, C.W.; Cao, H.R.; Zeng, Y.J.; Xie, G.; Zhang, G. Abnormal Thermal Conductivity Increase in β-Ga2O3 by an Unconventional Bonding Mechanism Using Machine-Learning Potential. Mater. Today Phys. 2025, 52, 101677. [Google Scholar]
- Chen, X.K.; Zhang, Y.; Luo, Q.Q.; Jia, P.Z.; Zhou, W.X. Strain-Driven Anisotropic Enhancement in the Thermal Conductivity of KCaBi: The Role of Optical Phonons. Int. J. Heat Mass Transf. 2025, 236, 126364. [Google Scholar]
- Zhu, B.; Wang, Z.; Zhang, Y.; Yu, Z.; Shi, J.; Xiong, R. Low Temperature Fabrication of the Giant Dielectric Material CaCu3Ti4O12 by Oxalate Coprecipitation Method. Mater. Chem. Phys. 2009, 113, 746–748. [Google Scholar]
- Zhu, B.P.; Tang, Z.; Zhao, L.H.; Wang, L.L.; Li, C.Z.; Yin, D.; Ruan, X.F. Synthesis of Mg[Ti2]O4 by Spark Plasma Sintering. Mater. Lett. 2007, 61, 578–581. [Google Scholar] [CrossRef]
- Wang, D.; Ou-Yang, J.; Guo, W.; Yang, X.; Zhu, B. Novel Fabrication of PZT Thick Films by an Oil-Bath Based Hydrothermal Method. Ceram. Int. 2017, 43, 9573–9576. [Google Scholar]
- Zhang, T.; Shi, X.L.; Hu, Q.; Gong, H.; Shi, K.; Li, Z. Ultrahigh-Performance Fiber-Supported Iron-Based Ionic Liquid for Synthesizing 3, 4-Dihydropyrimidin-2-(1H)-ones in a Cleaner Manner. Langmuir 2024, 40, 9579–9591. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, L.; Xiao, C.; Huang, Z.; Zhao, F.; Yin, J. Highly Efficient Upconversion Photodynamic Performance of Rare-Earth-Coupled Dual-Photosensitizers: Ultrafast Experiments and Excited-State Calculations. Nanophotonics 2024, 13, 443–455. [Google Scholar]
- Meyer, J.; Hamwi, S.; Kröger, M.; Kowalsky, W.; Riedl, T.; Kahn, A. Transition Metal Oxides for Organic Electronics: Energetics, Device Physics and Applications. Adv. Mater. 2012, 24, 5408–5427. [Google Scholar]
- Fernandez-Garcia, M.; Martinez-Arias, A.; Hanson, J.C.; Rodriguez, J.A. Nanostructured Oxides in Chemistry: Characterization and Properties. Chem. Rev. 2004, 104, 4063–4104. [Google Scholar] [PubMed]
- Rastogi, A.; Zivcak, M.; Sytar, O.; Kalaji, H.M.; He, X.; Mbarki, S.; Brestic, M. Impact of Metal and Metal Oxide Nanoparticles on Plants: A Critical Review. Front. Chem. 2017, 5, 78. [Google Scholar]
- Falcaro, P.; Ricco, R.; Yazdi, A.; Imaz, I.; Furukawa, S.; Maspoch, D.; Doonan, C.J. Application of Metal and Metal Oxide Nanoparticles@MOFs. Coord. Chem. Rev. 2016, 307, 237–254. [Google Scholar]
- Zhu, B.; Fei, C.; Wang, C.; Zhu, Y.; Yang, X.; Zheng, H.; Shung, K.K. Self-Focused AlScN Film Ultrasound Transducer for Individual Cell Manipulation. ACS Sens. 2017, 2, 172–177. [Google Scholar] [CrossRef] [PubMed]
- Chavali, M.S.; Nikolova, M.P. Metal Oxide Nanoparticles and Their Applications in Nanotechnology. SN Appl. Sci. 2019, 1, 607. [Google Scholar]
- Ali, A.; Zafar, H.; Zia, M.; ul Haq, I.; Phull, A.R.; Ali, J.S.; Hussain, A. Synthesis, Characterization, Applications, and Challenges of Iron Oxide Nanoparticles. Nanotechnol. Sci. Appl. 2016, 9, 49–67. [Google Scholar]
- Rahman, M.M.; Khan, S.B.; Jamal, A.; Faisal, M.; Aisiri, A.M. Iron Oxide Nanoparticles. Nanomaterials 2011, 3, 43–67. [Google Scholar]
- Riaz, S.; Bashir, M.; Naseem, S. Iron Oxide Nanoparticles Prepared by Modified Co-Precipitation Method. IEEE Trans. Magn. 2013, 50, 1–4. [Google Scholar]
- Takami, S.; Sato, T.; Mousavand, T.; Ohara, S.; Umetsu, M.; Adschiri, T. Hydrothermal Synthesis of Surface-Modified Iron Oxide Nanoparticles. Mater. Lett. 2007, 61, 4769–4772. [Google Scholar]
- Mishra, D.; Arora, R.; Lahiri, S.; Amritphale, S.S.; Chandra, N. Synthesis and Characterization of Iron Oxide Nanoparticles by Solvothermal Method. Prot. Met. Phys. Chem. Surf. 2014, 50, 628–631. [Google Scholar]
- Hufschmid, R.; Arami, H.; Ferguson, R.M.; Gonzales, M.; Teeman, E.; Brush, L.N.; Krishnan, K.M. Synthesis of Phase-Pure and Monodisperse Iron Oxide Nanoparticles by Thermal Decomposition. Nanoscale 2015, 7, 11142–11154. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.P.; Guo, W.K.; Shen, G.Z.; Zhou, Q.; Shung, K.K. Structure and Electrical Properties of (111)-Oriented Pb(Mg1/3Nb2/3)O3-PbZrO3-PbTiO3 Thin Film for Ultra-High-Frequency Transducer Applications. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2011, 58, 1962–1967. [Google Scholar] [CrossRef]
- Piñeiro, Y.; González Gómez, M.; de Castro Alves, L.; Arnosa Prieto, A.; García Acevedo, P.; Seco Gudiña, R.; Rivas, J. Hybrid nanostructured magnetite nanoparticles: From bio-detection and theragnostics to regenerative medicine. Magnetochem. 2020, 6, 4. [Google Scholar] [CrossRef]
- Fritea, L.; Banica, F.; Costea, T.O.; Moldovan, L.; Dobjanschi, L.; Muresan, M.; Cavalu, S. Metal nanoparticles and carbon-based nanomaterials for improved performances of electrochemical (Bio) sensors with biomedical applications. Mater. 2021, 14, 6319. [Google Scholar] [CrossRef] [PubMed]
- Parashar, M.; Shukla, V.K.; Singh, R. Metal oxides nanoparticles via sol–gel method: A review on synthesis, characterization and applications. J. Mater. Sci. Mater. Electron. 2020, 31, 3729–3749. [Google Scholar] [CrossRef]
- Dehghanghadikolaei, A.; Ansary, J.; Ghoreishi, R. Sol-gel process applications: A mini-review. Proc. Nat. Res. Soc. 2018, 2, 2008–2029. [Google Scholar] [CrossRef]
- Gao, J.; Wang, K.; Cao, J.; Zhang, M.; Lin, F.; Ling, M.; Chen, J. Recent Progress of Self-Supported Metal Oxide Nano-Porous Arrays in Energy Storage Applications. Small 2023, 19, 2302786. [Google Scholar] [CrossRef]
- Zheng, B.; Fan, J.; Chen, B.; Qin, X.; Wang, J.; Wang, F.; Liu, X. Rare-earth doping in nanostructured inorganic materials. Chem. Rev. 2022, 122, 5519–5603. [Google Scholar] [CrossRef]
- Baranovskaya, V.B.; Karpov, Y.A.; Petrova, K.V.; Korotkova, N.A. Actual trends in the application of rare-earth metals and their compounds in the production of magnetic and luminescent materials: A review. Russ. J. Non-Ferr. Met. 2021, 62, 10–31. [Google Scholar] [CrossRef]
- Zhu, B.P.; Zhou, Q.F.; Shi, J.; Shung, K.K.; Irisawa, S.; Takeuchi, S. Self-Separated Hydrothermal Lead Zirconate Titanate Thick Films for High Frequency Transducer Applications. Appl. Phys. Lett. 2009, 94, 102901. [Google Scholar] [CrossRef]
- Wei, J.; Fan, P.; Huang, Y.; Zeng, H.; Jiang, R.; Wu, Z.; Hu, Z. (±)-Hypandrone A, a Pair of Polycyclic Polyprenylated Acylphloroglucinol Enantiomers with a Caged 7/6/5/6/6 Pentacyclic Skeleton from Hypericum androsaemum. Org. Chem. Front. 2024, 11, 3459–3464. [Google Scholar]
- Popov, N.; Bošković, M.; Perović, M.; Németh, Z.; Wang, J.; Kuang, Z.; Krehula, S. Influence of low-spin Co3+ for high-spin Fe3+ substitution on the structural, magnetic, optical and catalytic properties of hematite (α-Fe2O3) nanorods. J. Phys. Chem. Solids 2021, 152, 109929. [Google Scholar]
- Kiymaz, K.; Uysal, S. The synthesis and characterization of pyridine cored dentrimeric s-Triazine Schiff bases: Investigation of their [MSalen/Salophen] (M = Cr3+, Fe3+, Co3+ and Zr4+) capped complexes. J. Mol. Struct. 2023, 1271, 134029. [Google Scholar]
- Guan, D.; Zhong, J.; Xu, H.; Huang, Y.-C.; Hu, Z.; Chen, B.; Zhang, Y.; Ni, M.; Xu, X.; Zhou, W.; et al. A Universal Chemical-Induced Tensile Strain Tuning Strategy to Boost Oxygen-Evolving Electrocatalysis on Perovskite Oxides. Appl. Phys. Rev. 2022, 9, 011422. [Google Scholar]
- Liu, Z.; Bai, Y.; Sun, H.; Guan, D.; Li, W.; Huang, W.-H.; Pao, C.-W.; Hu, Z.; Yang, G.; Zhu, Y.; et al. Synergistic Dual-Phase Air Electrode Enables High and Durable Performance of Reversible Proton Ceramic Electrochemical Cells. Nat. Commun. 2024, 15, 472. [Google Scholar]
- Gao, Y.; Nolan, A.M.; Du, P.; Wu, Y.; Yang, C.; Chen, Q.; Bo, S.H. Classical and emerging characterization techniques for investigation of ion transport mechanisms in crystalline fast ionic conductors. Chem. Rev. 2020, 120, 5954–6008. [Google Scholar]
- Chung, C.Y.; Chen, W.M.; Chen, Y.R.; Chen, L.Y.; Su, Y.H.; Chi, P.W.; Wu, M.K. Enhanced Fast Charging Capabilities in Natural Graphite/Iron Cross-Linked Pectin Electrodes for Lithium-Ion Batteries. Mater. Adv. 2024, 5, 6820–6829. [Google Scholar]
- Hota, S.S.; Panda, D.; Choudhary, R.N.P. Studies of structural, dielectric, conductivity, leakage current mechanism, and efficiency of complex electroceramic. Ceram. Int. 2024, 50, 4031–4045. [Google Scholar]
- Ahmad, N.; Khan, S.; Ansari, M.M.N. Exploration of Raman spectroscopy, dielectric and magnetic properties of (Mn, Co) co-doped SnO2 nanoparticles. Physica B Condens. Matter 2019, 558, 131–141. [Google Scholar]
- Abdel-Karim, A.M.; Salama, A.H.; El-Samahy, F.A. Dependence of Electrical Properties on Frequency and Temperature for Novel Cyclopentenone Derivatives. Nanoarchitectonics 2020, 13–25. Available online: https://www.researchgate.net/publication/349157892_Dependence_of_Electrical_Properties_on_Frequency_and_Temperature_for_Novel_Cyclopentenone_Derivatives (accessed on 25 February 2025).
- Farag, A.A.M.; Mansour, A.M.; Ammar, A.H.; Rafea, M.A.; Farid, A.M. Electrical conductivity, dielectric properties and optical absorption of organic based nanocrystalline sodium copper chlorophyllin for photodiode application. J. Alloys Compd. 2012, 513, 404–413. [Google Scholar]
- Shukla, N.; Kumar, V.; Dwivedi, D.K. Dependence of dielectric parameters and AC conductivity on frequency and temperature in bulk Se90Cd8In2 glassy alloy. J. Non-Oxide Glasses 2016, 8, 47–57. [Google Scholar]
- Halder, S.; Sheikh, M.S.; Ghosh, B.; Sinha, T.P. Electronic structure and electrical conduction by polaron hopping mechanism in A2LuTaO6 (A= Ba, Sr, Ca) double perovskite oxides. Ceram. Int. 2017, 43, 11097–11108. [Google Scholar]
- Zhu, B.; Zhu, Y.; Yang, J.; Ou-Yang, J.; Yang, X.; Li, Y.; Wei, W. New Potassium Sodium Niobate Single Crystal with Thickness-Independent High-Performance for Photoacoustic Angiography of Atherosclerotic Lesion. Sci. Rep. 2016, 6, 39679. [Google Scholar]
- Badapanda, T.; Harichandan, R.K.; Nayak, S.S.; Mishra, A.; Anwar, S. Frequency and temperature dependence behaviour of impedance, modulus and conductivity of BaBi4Ti4O15 Aurivillius ceramic. Process. Appl. Ceram. 2014, 8, 145–153. [Google Scholar] [CrossRef]
- Panda, R.K.; Behera, D. Investigation of electric transport behavior of bulk CoFe2O4 by complex impedance spectroscopy. J. Alloys Compd. 2014, 587, 481–486. [Google Scholar]
- Solanki, P.S.; Khachar, U.; Vagadia, M.; Ravalia, A.; Katba, S.; Kuberkar, D.G. Electroresistance and field effect studies on manganite based heterostructure. J. Appl. Phys. 2015, 117, 145306. [Google Scholar]
- Dussan, S.; Kumar, A.; Scott, J.F.; Katiyar, R.S. Effect of electrode resistance on dielectric and transport properties of multiferroic superlattice: A Impedance spectroscopy study. AIP Adv. 2012, 2, 032136. [Google Scholar]
- Fan, Q.; Li, Z.; Wu, C.; Yin, Y. Magnetically induced anisotropic interaction in colloidal assembly. Precis. Chem. 2023, 1, 272–298. [Google Scholar] [CrossRef]
- Chen, J.; Lou, Y.H.; Wang, Z.K. Characterizing spatial and energetic distributions of trap states toward highly efficient perovskite photovoltaics. Small 2023, 19, 2305064. [Google Scholar]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hjiri, M.; Soltani, S.; Jbeli, A.; Mustapha, N.; Ahmed Althumairi, N.; Benamara, M.; Valente, M.A. Tunable Electrical Properties of Cobalt-Doped Maghemite Nanoparticles for Advanced Resistive and Thermistor Applications. Nanomaterials 2025, 15, 534. https://doi.org/10.3390/nano15070534
Hjiri M, Soltani S, Jbeli A, Mustapha N, Ahmed Althumairi N, Benamara M, Valente MA. Tunable Electrical Properties of Cobalt-Doped Maghemite Nanoparticles for Advanced Resistive and Thermistor Applications. Nanomaterials. 2025; 15(7):534. https://doi.org/10.3390/nano15070534
Chicago/Turabian StyleHjiri, Mokhtar, Sonia Soltani, Anouar Jbeli, Nazir Mustapha, Nouf Ahmed Althumairi, Majdi Benamara, and Manuel Almeida Valente. 2025. "Tunable Electrical Properties of Cobalt-Doped Maghemite Nanoparticles for Advanced Resistive and Thermistor Applications" Nanomaterials 15, no. 7: 534. https://doi.org/10.3390/nano15070534
APA StyleHjiri, M., Soltani, S., Jbeli, A., Mustapha, N., Ahmed Althumairi, N., Benamara, M., & Valente, M. A. (2025). Tunable Electrical Properties of Cobalt-Doped Maghemite Nanoparticles for Advanced Resistive and Thermistor Applications. Nanomaterials, 15(7), 534. https://doi.org/10.3390/nano15070534







