Synthesis and Characterization of a Novel Sol–Gel-Derived Ni-Doped TiO2 Photocatalyst for Rapid Visible Light-Driven Mineralization of Paracetamol
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Photocatalytic Nanoparticles
2.3. Characterization of the Photocatalysts
2.4. Experimental Setup for Photocatalytic Activity Tests
2.5. Evaluation of Possible Nickel Leaching
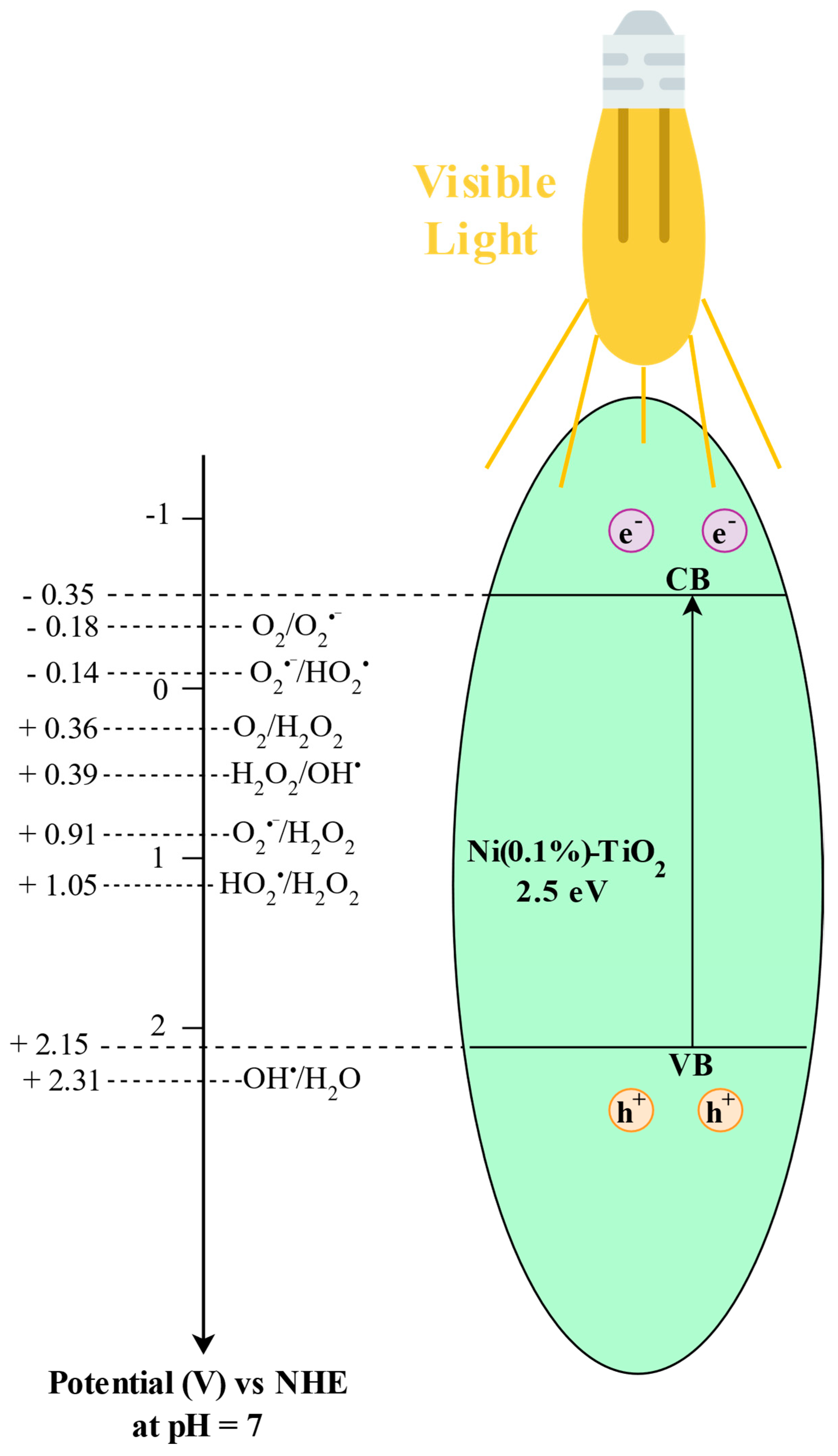
2.6. Band Structure Estimation
3. Results and Discussion
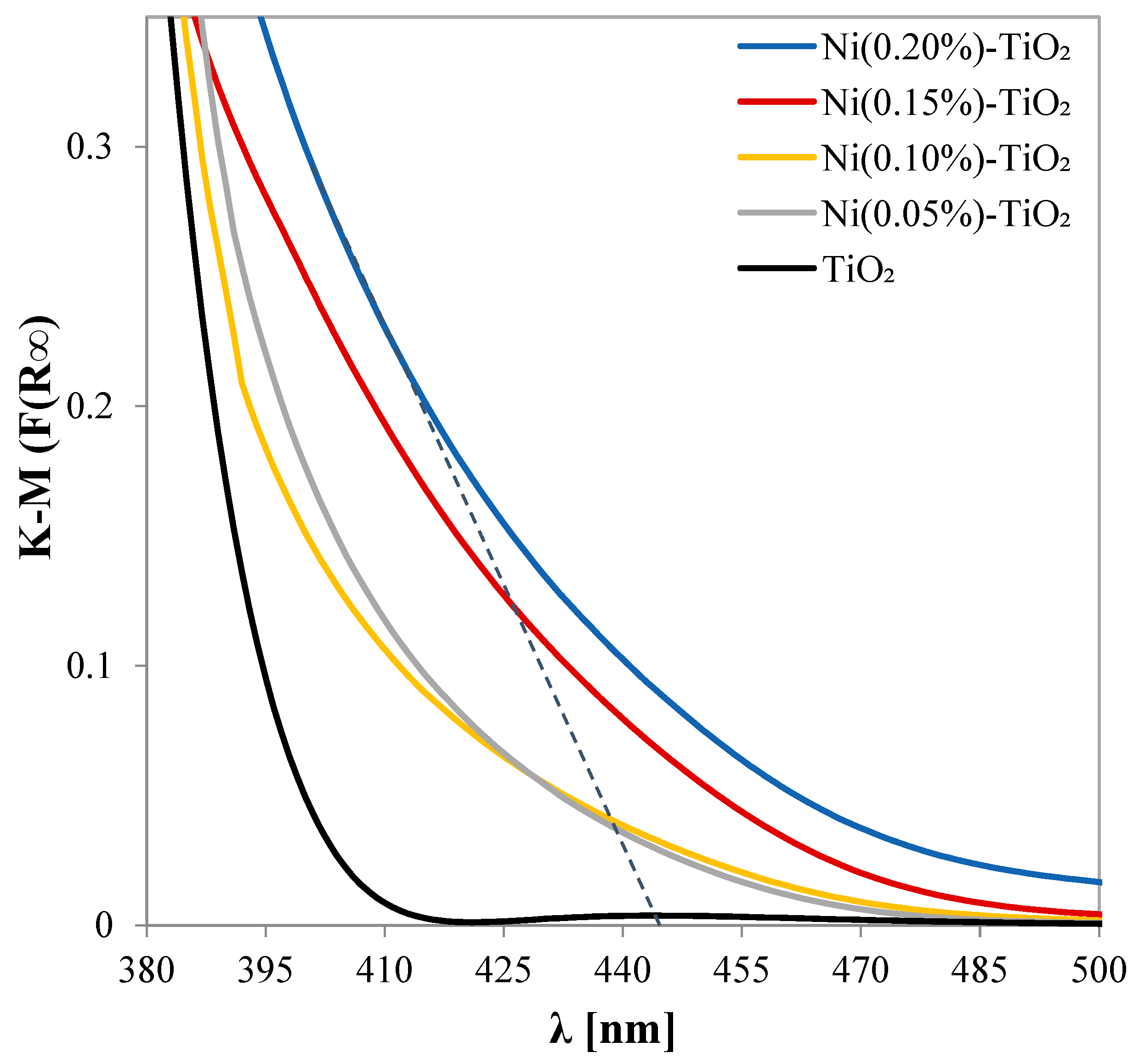
3.1. Photocatalysts Characterizations
3.2. Photocatalytic Activity Tests
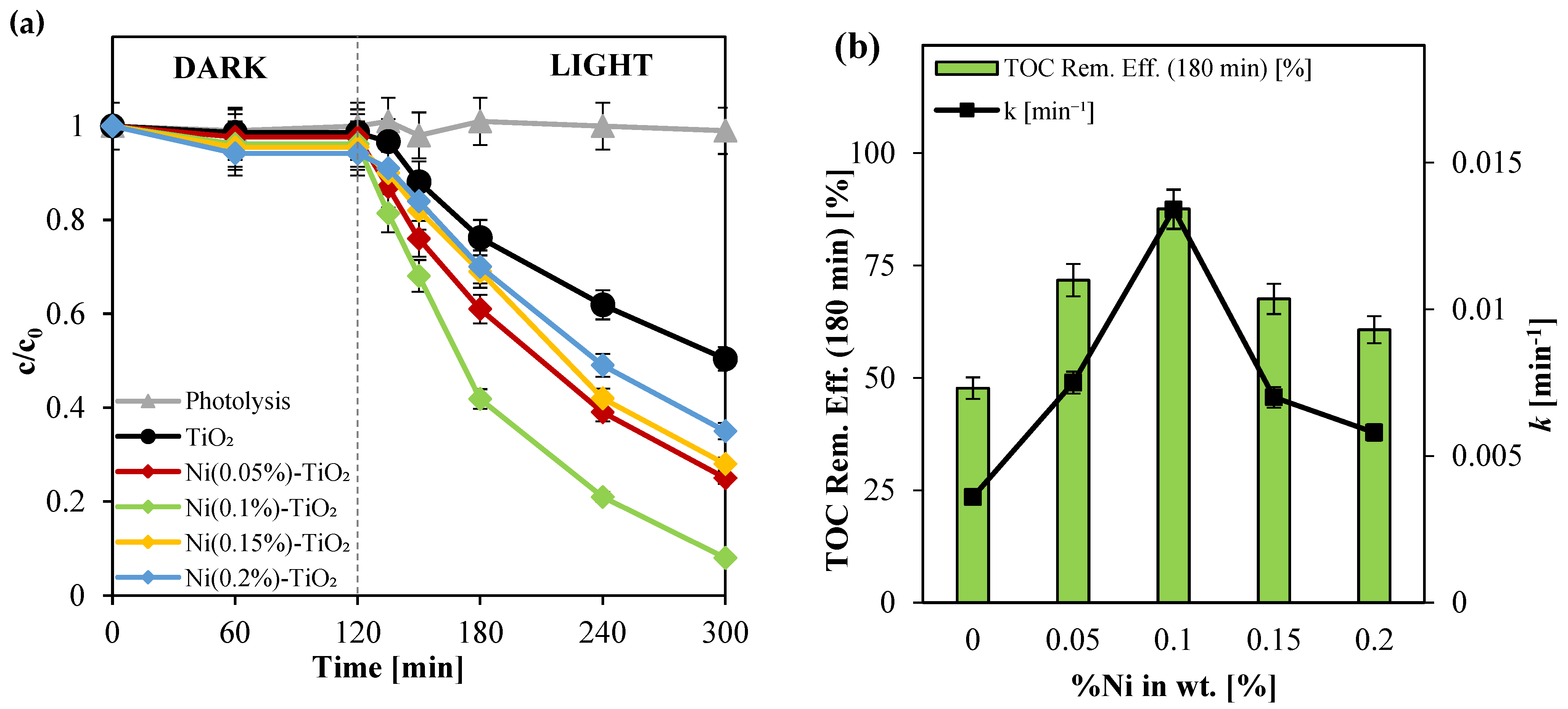
3.2.1. Screening Tests
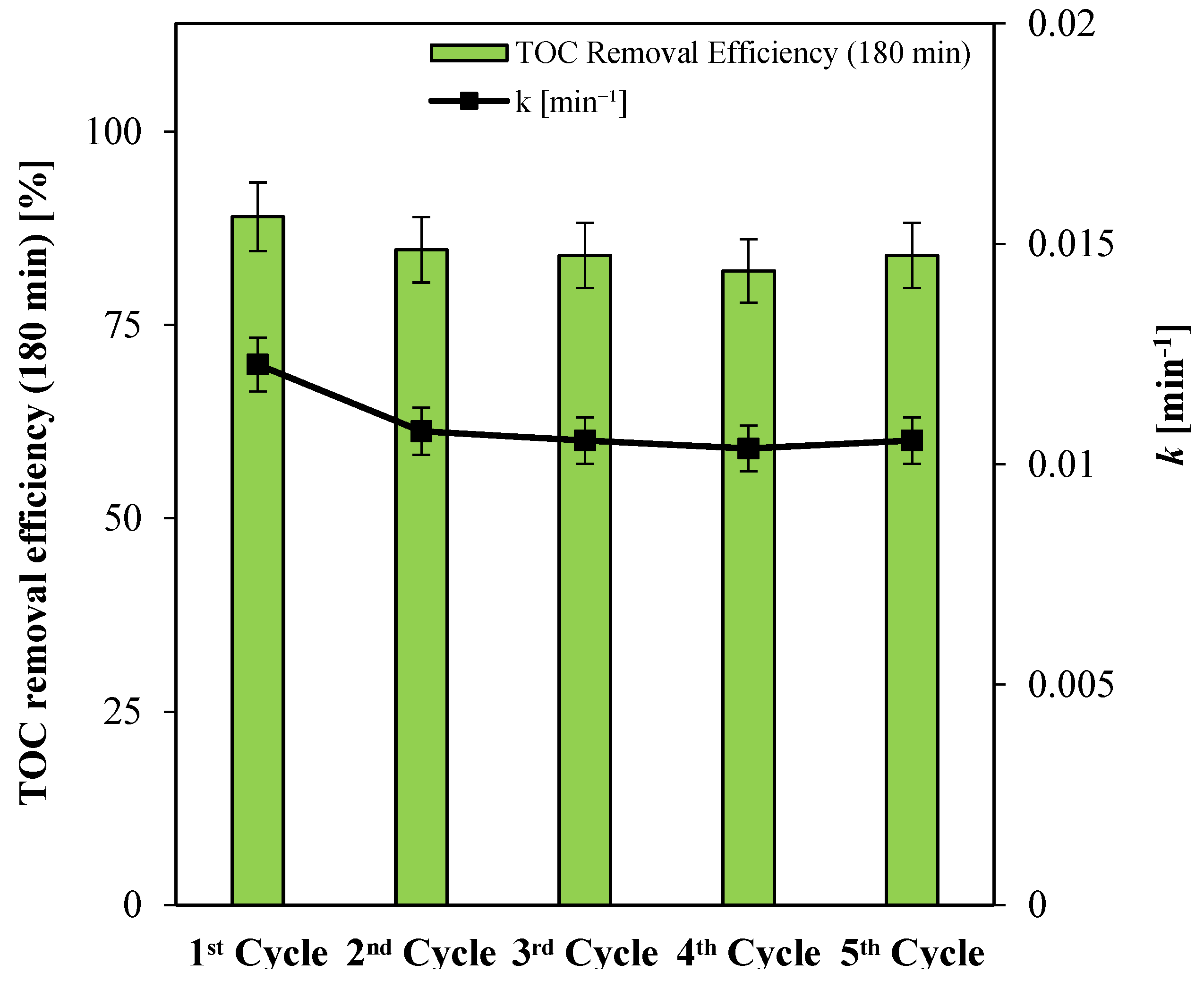
3.2.2. Stability Tests
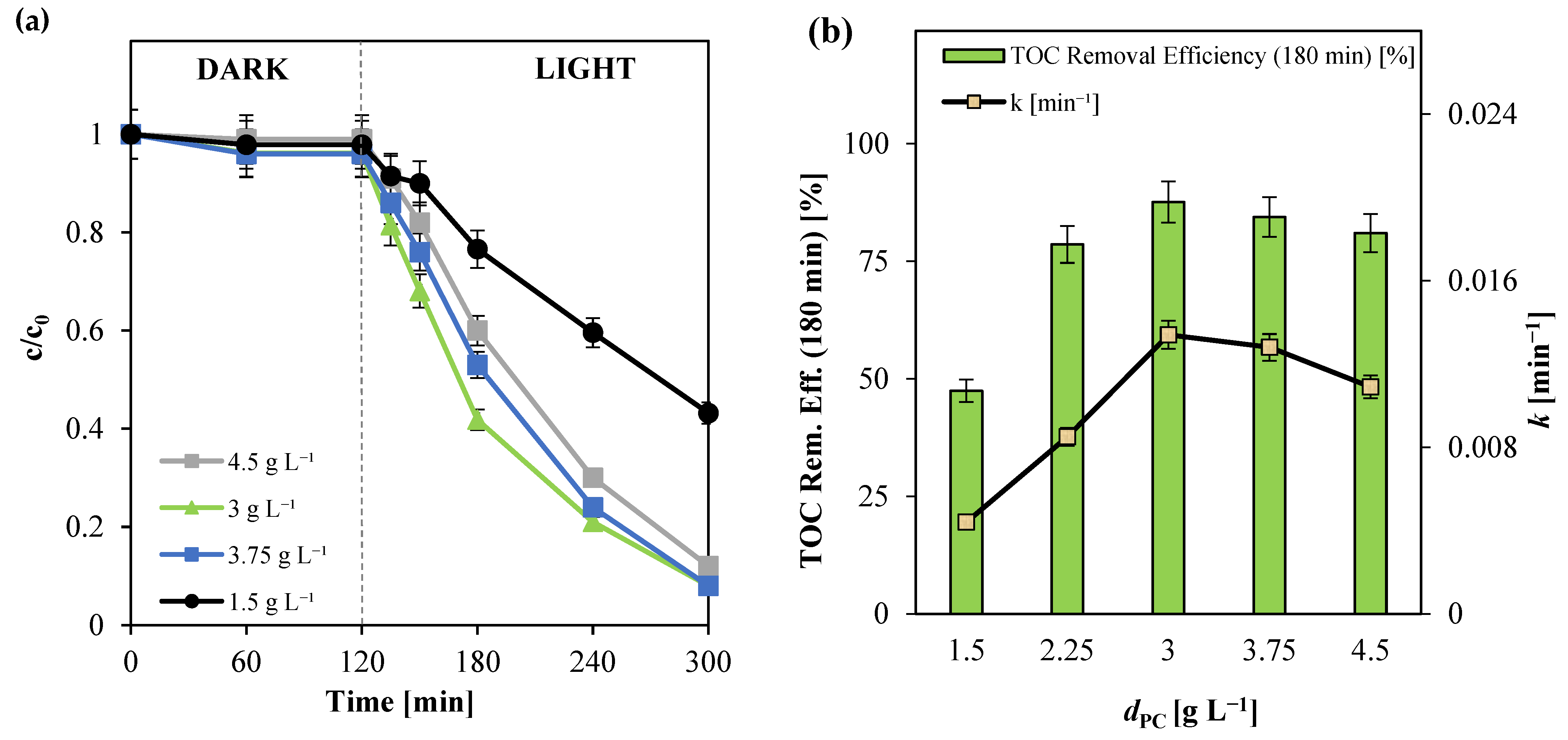
3.2.3. Influence of Photocatalyst Dosage
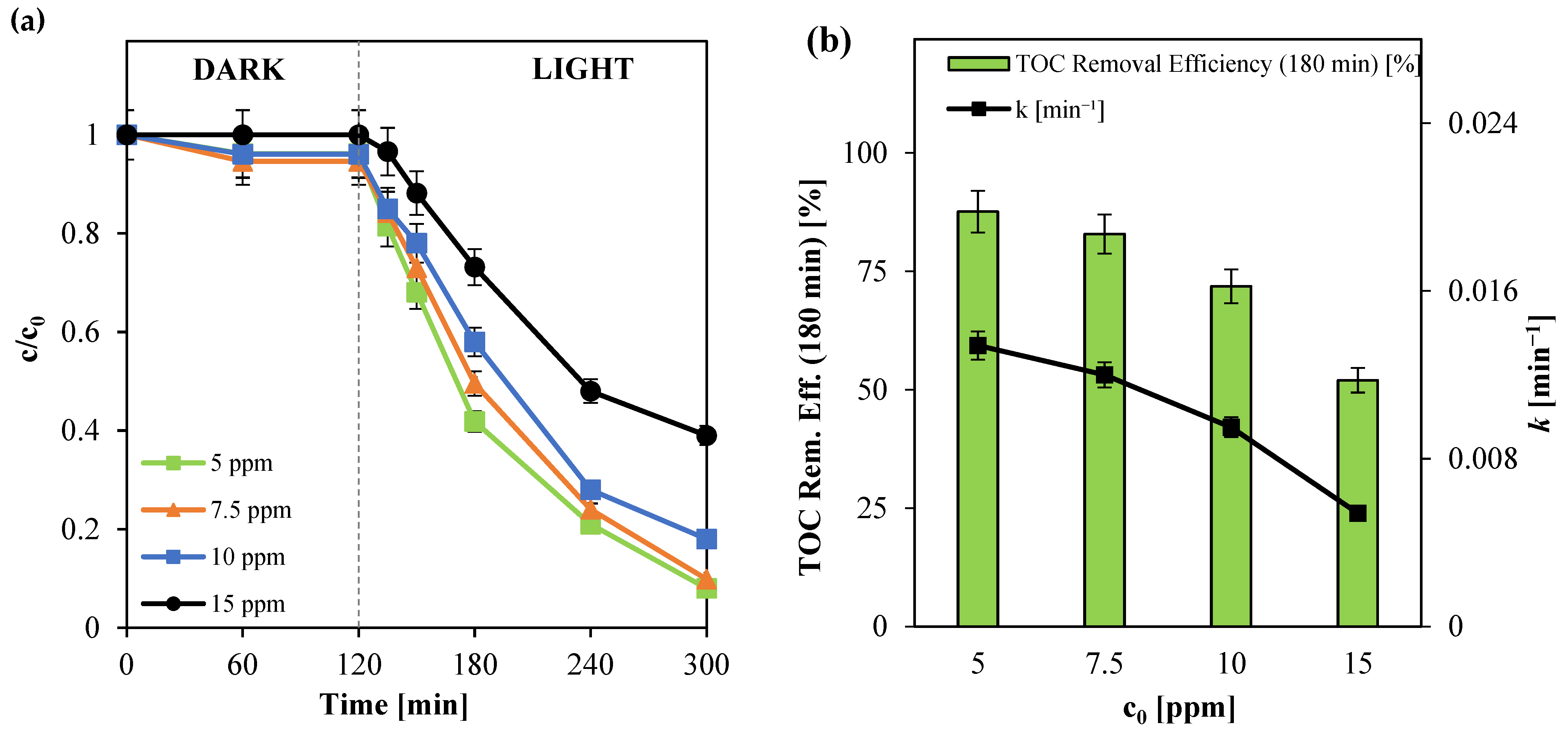
3.2.4. Influence of Initial Paracetamol Concentration (c0)
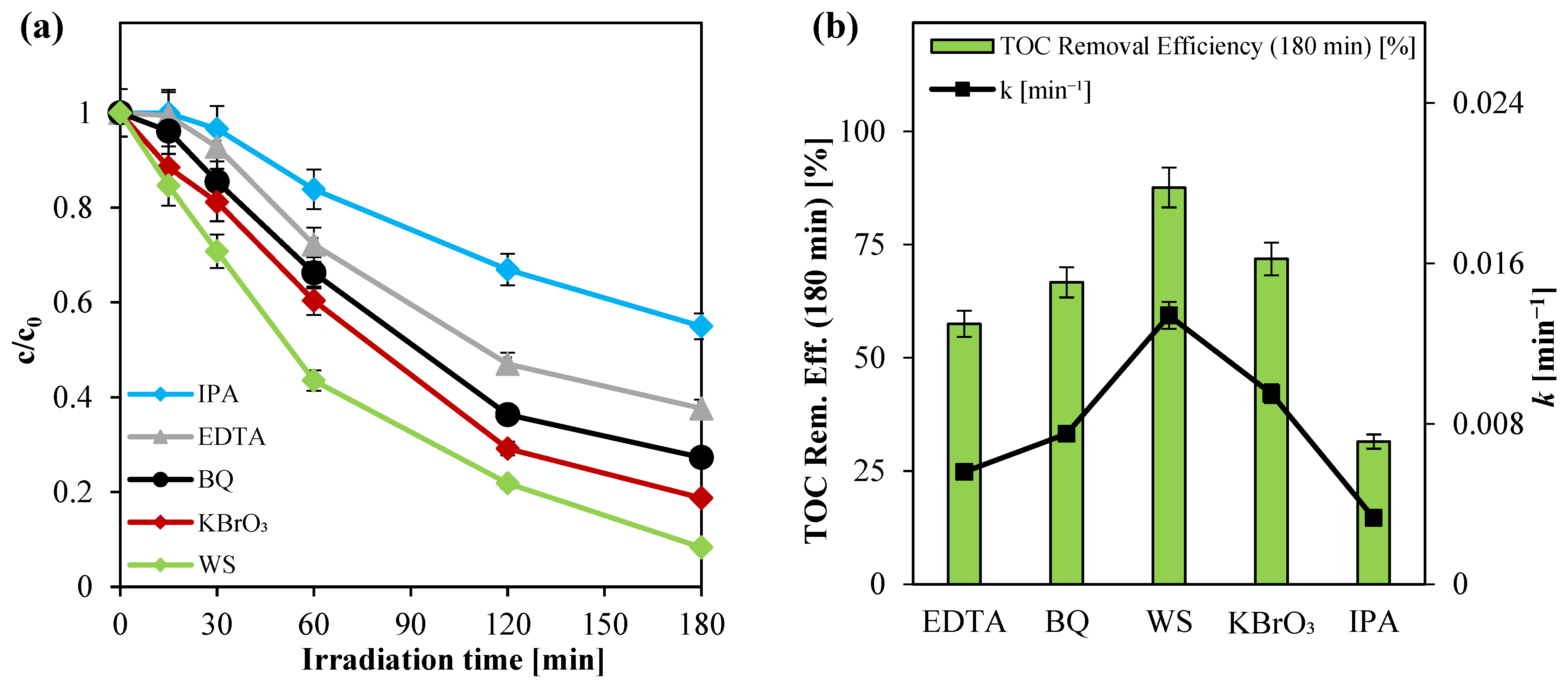
3.2.5. Investigation of Reactive Oxygen Species in the Photocatalytic Degradation of Paracetamol Using Ni(0.1%)-TiO2
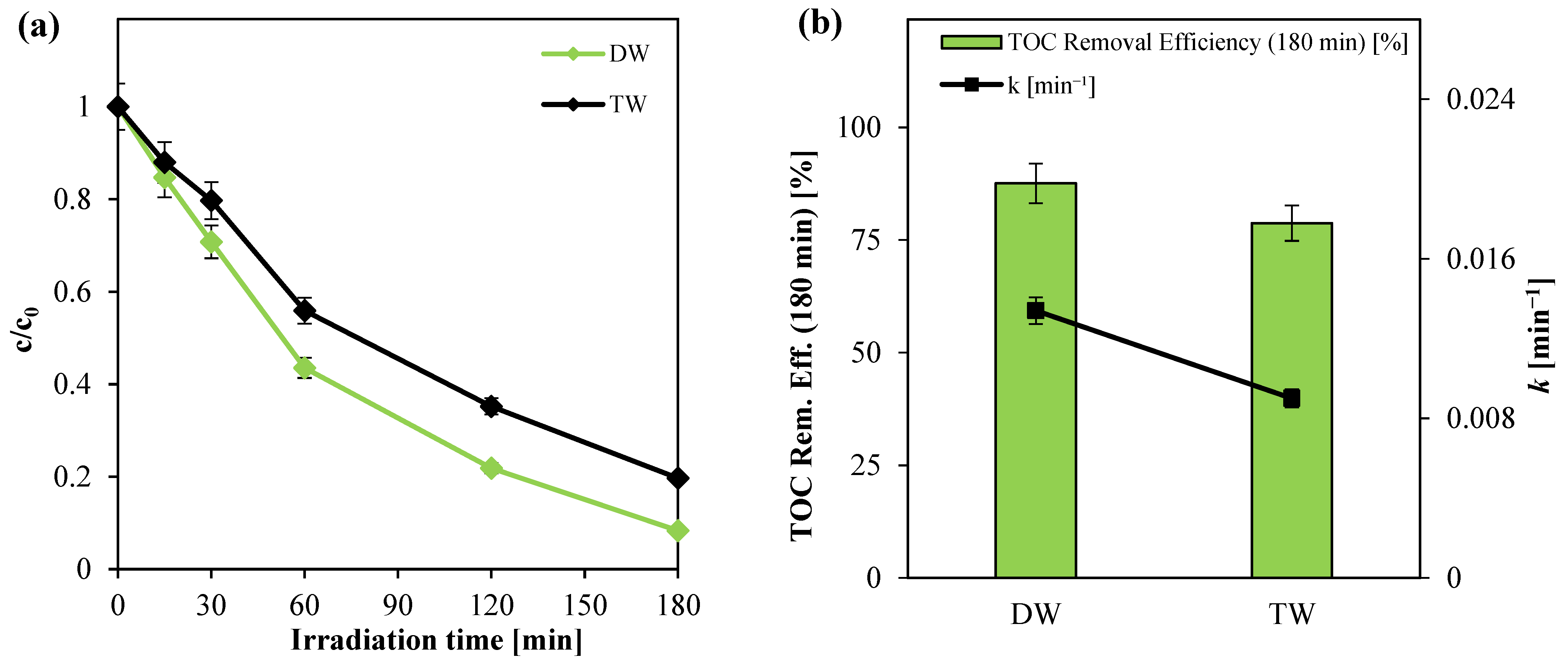
3.2.6. Effect of Water Matrix Nature on Photocatalytic Activity
3.2.7. Evaluation of Electrical Energy Consumption and Comparison of Photocatalytic Degradation of Paracetamol from Literature Studies
- W1: the photocatalytic activity of ZnO/GQDs/CdSe composite under visible light was investigated for paracetamol degradation [95]. A 50 mg portion of the synthesized photocatalyst was dispersed in 200 mL of a pollutant solution with a final concentration of 10 mg L−1. The mixture was stirred in complete darkness for 60 min to establish adsorption–desorption equilibrium. Subsequently, the system was exposed to visible light under an intensity of 75 W.
- W2: the photocatalytic performance of the ZnO/gC3N4 photocatalyst was assessed for the degradation of paracetamol under visible light irradiation in a photo-reactor equipped with a Xenon light source of 500 W power fitted with 420 nm UV cutoff filter [96]. The reaction involved a 100 mL paracetamol solution at an initial concentration of approximately 30 ppm.
- W3: The Ba0.95Bi0.05Fe0.95Cu0.05O3 photocatalyst was tested for paracetamol degradation under visible light (metal halide efficacy lamp, 244 W, HQI- T250/Daylight, OSRAM GmbH, Germany) [97]. The photocatalyst concentration was 0.75 g L−1, and the initial contaminant concentration was 20 ppm.
- W4: the use of ZnO/AgNPs composite in the degradation of paracetamol was examined in a slurry reactor under simulated solar light with an irradiance of ~1000 W m−2 (Sciencetech SS1.6 kW, Canada) [98]. The system treated a 3.5 mL solution of paracetamol with an initial contaminant concentration of 5 ppm.
- W5: the effects of 40% Pr/Bi4V2O11 on paracetamol degradation were studied using a 200 mL solution at an initial pollutant concentration of 10 ppm, irradiated by a visible light source (300 W) [99].
- W6: the photocatalytic efficiency of the chitosan-supported covalent organic framework (CSCF) photocatalyst was assessed for the degradation of paracetamol under visible light irradiation [100]. A 60 mL solution containing 3 ppm of paracetamol was treated with CSCF in a double-walled reactor maintained at room temperature. Prior to illumination, the solution was stirred in darkness for 30 min to ensure adsorption–desorption equilibrium. Visible light from a 300 W Xenon lamp equipped with a UV cutoff filter (λ ≥ 420 nm) was then applied, achieving a paracetamol degradation efficiency of 99.8% within 180 min.
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Peralta-Hernández, J.M.; Brillas, E. A Critical Review over the Removal of Paracetamol (Acetaminophen) from Synthetic Waters and Real Wastewaters by Direct, Hybrid Catalytic, and Sequential Ozonation Processes. Chemosphere 2023, 313, 137411. [Google Scholar] [PubMed]
- Brillas, E.; Manuel Peralta-Hernández, J. Removal of Paracetamol (Acetaminophen) by Photocatalysis and Photoelectrocatalysis. A Critical Review. Sep. Purif. Technol. 2023, 309, 122982. [Google Scholar]
- Al-howri, B.M.; Azha, S.F.; Shamsudin, M.S.; Hamid, N.A.; Alsobaai, A.M.; Ismail, S. Paracetamol in Diverse Water Sources: Health Hazards and Treatment Efficacy Emphasizing Adsorption Techniques—A Review. Int. J. Environ. Sci. Technol. 2024, 21, 9743–9762. [Google Scholar] [CrossRef]
- Ghanbari, F.; Hassani, A.; Wacławek, S.; Wang, Z.; Matyszczak, G.; Lin, K.Y.A.; Dolatabadi, M. Insights into Paracetamol Degradation in Aqueous Solutions by Ultrasound-Assisted Heterogeneous Electro-Fenton Process: Key Operating Parameters, Mineralization and Toxicity Assessment. Sep. Purif. Technol. 2021, 266, 118533. [Google Scholar] [CrossRef]
- Eniola, J.O.; Kumar, R.; Barakat, M.A.; Rashid, J. A Review on Conventional and Advanced Hybrid Technologies for Pharmaceutical Wastewater Treatment. J. Clean. Prod. 2022, 356, 131826. [Google Scholar]
- Moghaddam, A.; Khayatan, D.; Esmaeili Fard Barzegar, P.; Ranjbar, R.; Yazdanian, M.; Tahmasebi, E.; Alam, M.; Abbasi, K.; Esmaeili Gouvarchin Ghaleh, H.; Tebyaniyan, H. Biodegradation of Pharmaceutical Compounds in Industrial Wastewater Using Biological Treatment: A Comprehensive Overview. Int. J. Environ. Sci. Technol. 2023, 20, 5659–5696. [Google Scholar]
- Taoufik, N.; Boumya, W.; Achak, M.; Sillanpää, M.; Barka, N. Comparative Overview of Advanced Oxidation Processes and Biological Approaches for the Removal Pharmaceuticals. J. Environ. Manag. 2021, 288, 112404. [Google Scholar]
- Pandis, P.K.; Kalogirou, C.; Kanellou, E.; Vaitsis, C.; Savvidou, M.G.; Sourkouni, G.; Zorpas, A.A.; Argirusis, C. Key Points of Advanced Oxidation Processes (AOPs) for Wastewater, Organic Pollutants and Pharmaceutical Waste Treatment: A Mini Review. ChemEngineering 2022, 6, 8. [Google Scholar] [CrossRef]
- Fouad, K.; Bassyouni, M.; Alalm, M.G.; Saleh, M.Y. Recent Developments in Recalcitrant Organic Pollutants Degradation Using Immobilized Photocatalysts. Appl. Phys. A 2021, 127, 612. [Google Scholar] [CrossRef]
- Tufail, A.; Price, W.E.; Mohseni, M.; Pramanik, B.K.; Hai, F.I. A Critical Review of Advanced Oxidation Processes for Emerging Trace Organic Contaminant Degradation: Mechanisms, Factors, Degradation Products, and Effluent Toxicity. J. Water Process Eng. 2021, 40, 101778. [Google Scholar] [CrossRef]
- Titchou, F.E.; Zazou, H.; Afanga, H.; El Gaayda, J.; Ait Akbour, R.; Nidheesh, P.V.; Hamdani, M. Removal of Organic Pollutants from Wastewater by Advanced Oxidation Processes and Its Combination with Membrane Processes. Chem. Eng. Process. Process Intensif. 2021, 169, 108631. [Google Scholar] [CrossRef]
- Tufail, A.; Price, W.E.; Hai, F.I. A Critical Review on Advanced Oxidation Processes for the Removal of Trace Organic Contaminants: A Voyage from Individual to Integrated Processes. Chemosphere 2020, 260, 127460. [Google Scholar] [CrossRef] [PubMed]
- Saeed, M.; Muneer, M.; Ul Haq, A.; Akram, N. Photocatalysis: An Effective Tool for Photodegradation of Dyes—A Review. Environ. Sci. Pollut. Res. 2021, 29, 293–311. [Google Scholar] [CrossRef]
- AlMohamadi, H.; Awad, S.A.; Sharma, A.K.; Fayzullaev, N.; Távara-Aponte, A.; Chiguala-Contreras, L.; Amari, A.; Rodriguez-Benites, C.; Tahoon, M.A.; Esmaeili, H. Photocatalytic Activity of Metal- and Non-Metal-Anchored ZnO and TiO2 Nanocatalysts for Advanced Photocatalysis: Comparative Study. Catalysts 2024, 14, 420. [Google Scholar] [CrossRef]
- Morshedy, A.S.; El-Fawal, E.M.; Zaki, T.; El-Zahhar, A.A.; Alghamdi, M.M.; El Naggar, A.M.A. A Review on Heterogeneous Photocatalytic Materials: Mechanism, Perspectives, and Environmental and Energy Sustainability Applications. Inorg. Chem. Commun. 2024, 163, 112307. [Google Scholar] [CrossRef]
- Hsu, C.Y.; Mahmoud, Z.H.; Abdullaev, S.; Ali, F.K.; Ali Naeem, Y.; Mzahim Mizher, R.; Morad Karim, M.; Abdulwahid, A.S.; Ahmadi, Z.; Habibzadeh, S.; et al. Nano Titanium Oxide (Nano-TiO2): A Review of Synthesis Methods, Properties, and Applications. Case Stud. Chem. Environ. Eng. 2024, 9, 100626. [Google Scholar] [CrossRef]
- Sari, Y.; Gareso, P.L.; Armynah, B.; Tahir, D. A Review of TiO2 Photocatalyst for Organic Degradation and Sustainable Hydrogen Energy Production. Int. J. Hydrog. Energy 2024, 55, 984–996. [Google Scholar] [CrossRef]
- Ahmadpour, N.; Nowrouzi, M.; Madadi Avargani, V.; Sayadi, M.H.; Zendehboudi, S. Design and Optimization of TiO2-Based Photocatalysts for Efficient Removal of Pharmaceutical Pollutants in Water: Recent Developments and Challenges. J. Water Process Eng. 2024, 57, 104597. [Google Scholar] [CrossRef]
- TEE, S.Y.; Kong, J.; Koh, J.J.; Teng, C.P.; Wang, X.Z.; Wang, X.; Teo, S.L.; Thitsartarn, W.; Han, M.-Y.; Seh, Z.W. Structurally and Surficially Activated TiO2 Nanomaterials for Photochemical Reactions. Nanoscale 2024, 16, 18165–18212. [Google Scholar] [CrossRef]
- Baruah, M.; Kumar, S.; Ezung, S.L.; Jamir, L.; Bora Sinha, U.; Sinha, D. Preparation of Co-Doped TiO2 Activated Carbon Nanocomposite and Its Photocatalytic Degradation of Phenol Wastewater. Inorg. Chem. Commun. 2024, 166, 112644. [Google Scholar] [CrossRef]
- Rega, R.; Fioravanti, A.; Hejazi, S.M.H.; Shahrezaei, M.; Kment, Š.; Maddalena, P.; Naldoni, A.; Lettieri, S. Charge Carrier Recombination Processes, Intragap Defect States, and Photoluminescence Mechanisms in Stoichiometric and Reduced TiO2 Brookite Nanorods: An Interpretation Scheme through in Situ Photoluminescence Excitation Spectroscopy in Controlled Environment. Nanoscale 2024, 16, 11296–11309. [Google Scholar] [CrossRef] [PubMed]
- Fu, C.; Liu, L.; Wei, Y.; Huang, W.; Zhao, G. Linking the Doping-Induced Trap States to the Concentration of Surface-Reaching Photoexcited Holes in Transition-Metal-Doped TiO2 Nanoparticles. J. Phys. Chem. Lett. 2024, 15, 6504–6511. [Google Scholar] [CrossRef]
- Sultana, M.; Mondal, A.; Islam, S.; Khatun, M.A.; Rahaman, M.H.; Chakraborty, A.K.; Rahman, M.S.; Rahman, M.M.; Nur, A.S.M. Strategic Development of Metal Doped TiO2 Photocatalysts for Enhanced Dye Degradation Activity under UV–Vis Irradiation: A Review. Curr. Res. Green Sustain. Chem. 2023, 7, 100383. [Google Scholar]
- Khatun, M.M.; Islam, M.B.; Sumona, H.J.; Al Mahmood, A. Enhanced Photocatalytic Activity of Metal-Metal-Nonmetal Multidoped TiO2 Nanoparticles towards Visible Light. ChemistrySelect 2024, 9, e202304278. [Google Scholar] [CrossRef]
- Al-Madanat, O.; Alsalka, Y.; Ramadan, W.; Bahnemann, D.W. WTiO2 Photocatalysis for the Transformation of Aromatic Water Pollutants into Fuels. Catalysts 2021, 11, 317. [Google Scholar]
- Sangeeta; Onisha; Sandhu, N.; Kumar, C.; Mohajer, F.; Tomar, R. Critical Review on Titania-Based Nanoparticles: Synthesis, Characterization, and Application as a Photocatalyst. Chem. Afr. 2024, 7, 1749–1768. [Google Scholar] [CrossRef]
- Yang, X.; Zhao, R.; Zhan, H.; Zhao, H.; Duan, Y.; Shen, Z. Modified Titanium Dioxide-Based Photocatalysts for Water Treatment: Mini Review. Environ. Funct. Mater. 2024, 3, 1–12. [Google Scholar] [CrossRef]
- Kavaliunas, V.; Krugly, E.; Sriubas, M.; Mimura, H.; Laukaitis, G.; Hatanaka, Y. Influence of Mg, Cu, and Ni Dopants on Amorphous TiO2 Thin Films Photocatalytic Activity. Materials 2020, 13, 886. [Google Scholar] [CrossRef]
- Nguyen, T.L.; Pham, T.H.; Viet, N.M.; Thang, P.Q.; Rajagopal, R.; Sathya, R.; Jung, S.H.; Kim, T. Improved Photodegradation of Antibiotics Pollutants in Wastewaters by Advanced Oxidation Process Based on Ni-Doped TiO2. Chemosphere 2022, 302, 134837. [Google Scholar] [CrossRef]
- Ganesh, I.; Gupta, A.K.; Kumar, P.P.; Sekhar, P.S.C.; Radha, K.; Padmanabham, G.; Sundararajan, G. Preparation and Characterization of Ni-Doped TiO2 Materials for Photocurrent and Photocatalytic Applications. Sci. World J. 2012, 2012, 127326. [Google Scholar] [CrossRef]
- Yadav, H.M.; Otari, S.V.; Bohara, R.A.; Mali, S.S.; Pawar, S.H.; Delekar, S.D. Synthesis and Visible Light Photocatalytic Antibacterial Activity of Nickel-Doped TiO2 Nanoparticles against Gram-Positive and Gram-Negative Bacteria. J. Photochem. Photobiol. A Chem. 2014, 294, 130–136. [Google Scholar] [CrossRef]
- Dimitriou, C.; Psathas, P.; Solakidou, M.; Deligiannakis, Y. Advanced Flame Spray Pyrolysis (FSP) Technologies for Engineering Multifunctional Nanostructures and Nanodevices. Nanomaterials 2023, 13, 3006. [Google Scholar] [CrossRef] [PubMed]
- Orizu, G.E.; Ugwuoke, P.E.; Asogwa, P.U.; Offiah, S.U. A Review on the Inference of Doping TiO2 with Metals/Non-Metals to Improve Its Photocatalytic Activities. In Proceedings of the IOP Conference Series: Earth and Environmental Science, 5th International Conference/Training Workshop on Energy for Sustainable Development in Africa (ICTWESDA-2022), Nsukka, Nigeria, 16–19 May 2022; Institute of Physics: London, UK, 2023; Volume 1178. [Google Scholar]
- Ahmad, M.M.; Mushtaq, S.; Al Qahtani, H.S.; Sedky, A.; Alam, M.W. Investigation of TiO2 Nanoparticles Synthesized by Sol-Gel Method for Effectual Photodegradation, Oxidation and Reduction Reaction. Crystals 2021, 11, 1456. [Google Scholar] [CrossRef]
- Shabaninia, M.; Khorasani, M.; Baniyaghoob, S. Synthesis of Silver-Doped Titanium Dioxide Nanoparticles by Sol-Gel and Coprecipitation Techniques: Evaluation of Antimicrobial Activity and Cytotoxic Effects. ChemistrySelect 2024, 9, e202303358. [Google Scholar] [CrossRef]
- Anju, K.R.; Thankapan, R.; Rajabathar, J.R.; Al-Lohedan, H.A. Hydrothermal Synthesis of Nanosized (Fe, Co, Ni)-TiO2 for Enhanced Visible Light Photosensitive Applications. Optik 2018, 165, 408–415. [Google Scholar] [CrossRef]
- Ndlwana, L.; Raleie, N.; Dimpe, K.M.; Ogutu, H.F.; Oseghe, E.O.; Motsa, M.M.; Msagati, T.A.M.; Mamba, B.B. Sustainable Hydrothermal and Solvothermal Synthesis of Advanced Carbon Materials in Multidimensional Applications: A Review. Materials 2021, 14, 5094. [Google Scholar] [CrossRef] [PubMed]
- Sobhani, A.; Alinavaz, S. Co-Precipitation Synthesis of CuMn2O4/CuMnO Nanocomposites without Capping Agent and Investigation of Their Applications for Removing Pollutants from Wastewater. Biomass Convers. Biorefin 2023, 14, 28901–28911. [Google Scholar] [CrossRef]
- Armaković, S.J.; Savanović, M.M.; Armaković, S. Titanium Dioxide as the Most Used Photocatalyst for Water Purification: An Overview. Catalysts 2023, 13, 26. [Google Scholar] [CrossRef]
- Thong, H.C.; Payne, A.; Li, J.W.; Cheng, Y.Y.S.; Jones, J.L.; Wang, K. The Origin of Chemical Inhomogeneity in Lead-Free Potassium Sodium Niobate Ceramic: Competitive Chemical Reaction during Solid-State Synthesis. Acta Mater. 2021, 211, 116833. [Google Scholar] [CrossRef]
- Akpan, U.G.; Hameed, B.H. The Advancements in Sol-Gel Method of Doped-TiO2 Photocatalysts. Appl. Catal. A Gen. 2010, 375, 1–11. [Google Scholar] [CrossRef]
- Kuspanov, Z.; Bakbolat, B.; Baimenov, A.; Issadykov, A.; Yeleuov, M.; Daulbayev, C. Photocatalysts for a Sustainable Future: Innovations in Large-Scale Environmental and Energy Applications. Sci. Total Environ. 2023, 885, 163914. [Google Scholar]
- Waghchaure, R.H.; Koli, P.B.; Adole, V.A.; Jagdale, B.S. Exploration of Photocatalytic Performance of TiO2, 5% Ni/TiO2, and 5% Fe/TiO2 for Degradation of Eosine Blue Dye: Comparative Study. Results Chem. 2022, 4, 100488. [Google Scholar] [CrossRef]
- Solis-Cortazar, J.C.; Zamudio-Torres, I.; Rojas-Blanco, L.; Perez-Hernandez, G.; Arellano-Cortaza, M.; Castillo-Palomera, R.; de los Monteros, A.E.; Ramirez-Morales, E. Photoresponse Enhancement in TiO2 Thin Films by Incorporation Ni and Cr Nanoparticles Using Sol–Gel Method. J. Mater. Sci. Mater. Electron. 2022, 33, 7668–7678. [Google Scholar] [CrossRef]
- Kumar, A.; Pandey, G. Photocatalytic Degradation of Eriochrome Black-T by the Ni:TiO2 Nanocomposites. Desalination Water Treat. 2017, 71, 406–419. [Google Scholar] [CrossRef]
- Park, J.; Lam, S.S.; Park, Y.K.; Kim, B.J.; An, K.H.; Jung, S.C. Fabrication of Ni/TiO2 Visible Light Responsive Photocatalyst for Decomposition of Oxytetracycline. Environ. Res. 2023, 216, 114657. [Google Scholar] [CrossRef]
- Lee, W.J.; Goh, P.S.; Lau, W.J.; Ismail, A.F. Removal of Pharmaceutical Contaminants from Aqueous Medium: A State-of-the-Art Review Based on Paracetamol. Arab. J. Sci. Eng. 2020, 45, 7109–7135. [Google Scholar] [CrossRef]
- Kaur, M.; Noonia, A.; Dogra, A.; Singh Thind, P. Optimising the Parameters Affecting Degradation of Cypermethrin in an Aqueous Solution Using TiO2/H2O2 Mediated UV Photocatalysis: RSM-BBD, Kinetics, Isotherms and Reusability. Int. J. Environ. Anal. Chem. 2023, 103, 1153–1167. [Google Scholar] [CrossRef]
- Thind, P.S.; Kumari, D.; John, S. TiO2/H2O2 Mediated UV Photocatalysis of Chlorpyrifos: Optimization of Process Parameters Using Response Surface Methodology. J. Environ. Chem. Eng. 2018, 6, 3602–3609. [Google Scholar] [CrossRef]
- Merdoud, R.; Aoudjit, F.; Mouni, L.; Ranade, V.V. Degradation of Methyl Orange Using Hydrodynamic Cavitation, H2O2, and Photo-Catalysis with TiO2-Coated Glass Fibers: Key Operating Parameters and Synergistic Effects. Ultrason. Sonochemistry 2024, 103, 106772. [Google Scholar] [CrossRef]
- Morante, N.; Viscusi, G.; Gorrasi, G.; Monzillo, K.; Sannino, D. Immobilization of Solar Light-Active Photocatalyst Cr-TiO2 into the Polyurethane/Polycaprolactone Electrospun Membrane for the Photocatalytic Oxidation of Acid Orange 7. J. Water Process Eng. 2025, 69, 106529. [Google Scholar] [CrossRef]
- Morante, N.; Tammaro, O.; Albarano, L.; De Guglielmo, L.; Oliva, N.; Sacco, O.; Mancuso, A.; Castellino, M.; Sannino, D.; Femia, N.; et al. Influence of Visible Light LEDs Modulation Techniques on Photocatalytic Degradation of Ceftriaxone in a Flat Plate Reactor. Chem. Eng. J. 2024, 482, 149175. [Google Scholar] [CrossRef]
- Hernández-Laverde, M.; Morante, N.; Gutiérrez, B.L.; Murcia, J.J.; Monzillo, K.; Sannino, D.; Vaiano, V. Solar Light Elimination of Bacteria, Yeast and Organic Pollutants by Effective Photocatalysts Based on Ag/Cr-TiO2 and Pd/Cr-TiO2. Nanomaterials 2024, 14, 1730. [Google Scholar] [CrossRef] [PubMed]
- Balakrishnan, M.; John, R. Impact of Ni Metal Ion Concentration in TiO2 Nanoparticles for Enhanced Photovoltaic Performance of Dye Sensitized Solar Cell. J. Mater. Sci. Mater. Electron. 2021, 32, 5295–5308. [Google Scholar] [CrossRef]
- Noh, J.S.; Schwarz, J.A. Estimation of the Point of Zero Charge of Simple Oxides by Mass Titration. J. Colloid Interface Sci. 1989, 130, 157–164. [Google Scholar] [CrossRef]
- Alomar, M.S.; Bakather, O.Y.; Zouli, N.; Eldoma, M.A.; Mahmoud, M.A.; Hegazi, S.E.F.; Ahmed, Z.M.; Hassan, D.A.; Abouatiaa, A.F.F.; Al Askar, A.; et al. Solar-Driven Purification: Removing Pharmaceutical Contaminants from Water Using Carbon-Doped NASICON Photocatalyst. Mater. Sci. Semicond. Process. 2025, 185, 108901. [Google Scholar] [CrossRef]
- Morante, N.; Folliero, V.; Dell’Annunziata, F.; Capuano, N.; Mancuso, A.; Monzillo, K.; Galdiero, M.; Sannino, D.; Franci, G. Characterization and Photocatalytic and Antibacterial Properties of Ag- and TiOx-Based (x = 2, 3) Composite Nanomaterials under UV Irradiation. Materials 2024, 17, 2178. [Google Scholar] [CrossRef]
- Carpani, I.; Scavetta, E.; Tonelli, D. Spectrophotometric Determination of Aluminium and Nickel. Ann. Chim. 2004, 94, 365–372. [Google Scholar] [CrossRef]
- Ter Haar, K.; Bazen, J. The Titration of Nickel with Edta at Ph 2.8. Anal. Chim. Acta 1956, 14, 409–413. [Google Scholar] [CrossRef]
- Yu, X.; Yang, J.; Ren, X.; Sui, Z. Influences of Ph and Edta Additive on the Structure of Ni Films Electrodeposited by Using Bubble Templates as Electrocatalysts for Hydrogen Evolution Reaction. Membranes 2021, 11, 165. [Google Scholar] [CrossRef]
- Lanigan, K.C.; Jeong, W.; Gerges, M.A.; Ahmed, M. Photoinduced Oxidation of Nickel in Ni(II)EDTA with TiO2 Nanoparticles. J. Photochem. Photobiol. A Chem. 2022, 433, 114117. [Google Scholar] [CrossRef]
- Kong, L.; Jiang, Z.; Lai, H.H.C.; Xiao, T.; Edwards, P.P. Does Noble Metal Modification Improve the Photocatalytic Activity of BiOCl? Prog. Nat. Sci. Mater. Int. 2013, 23, 286–293. [Google Scholar] [CrossRef]
- Morante, N.; Tammaro, O.; Monzillo, K.; Sannino, D.; Battiato, A.; Vittone, E.; Castellino, M.; Esposito, S.; Vaiano, V. Unraveling the Role of CuO in CuxO/TiO2 Photocatalyst for the Direct Propylene Epoxidation With O2 in a Fluidized Bed Reactor. ChemSusChem 2024. [Google Scholar] [CrossRef]
- Krýsa, J.; Waldner, G.; Měšt’ánková, H.; Jirkovský, J.; Grabner, G. Photocatalytic Degradation of Model Organic Pollutants on an Immobilized Particulate TiO2 Layer. Roles of Adsorption Processes and Mechanistic Complexity. Appl. Catal. B 2006, 64, 290–301. [Google Scholar] [CrossRef]
- Chenab, K.K.; Sohrabi, B.; Jafari, A.; Ramakrishna, S. Water Treatment: Functional Nanomaterials and Applications from Adsorption to Photodegradation. Mater. Today Chem. 2020, 16, 100262. [Google Scholar]
- Ohsaka, T.; Fujiki, Y. Raman Spectrum of Anatase, TiO2. J. Raman Spectrosc. 1978, 7, 321–324. [Google Scholar]
- Birla, P.N.; Arbuj, S.; Shinde, M.D.; Joseph, S.; Rane, S.; Kulkarni, S.; Kale, B. Electroless Ni Plated Nanostructured TiO2 as a Photocatalyst for Solar Hydrogen Production. RSC Adv. 2023, 13, 20068–20080. [Google Scholar] [CrossRef] [PubMed]
- Ahmetović, S.; Vasiljević, Z.; Krstić, J.B.; Finšgar, M.; Solonenko, D.; Bartolić, D.; Tadić, N.B.; Miskovic, G.; Cvijetićanin, N.; Nikolic, M.V. Looking into How Nickel Doping Affects the Structure, Morphology, and Optical Properties of TiO2 Nanofibers. Surf. Interfaces 2024, 49, 104434. [Google Scholar] [CrossRef]
- Shaban, M.; Ahmed, A.M.; Shehata, N.; Betiha, M.A.; Rabie, A.M. Ni-Doped and Ni/Cr Co-Doped TiO2 Nanotubes for Enhancement of Photocatalytic Degradation of Methylene Blue. J. Colloid. Interface Sci. 2019, 555, 31–41. [Google Scholar] [CrossRef]
- Maleki, F.; Di Liberto, G.; Pacchioni, G. PH- and Facet-Dependent Surface Chemistry of TiO2 in Aqueous Environment from First Principles. ACS Appl. Mater. Interfaces 2023, 15, 11216–11224. [Google Scholar] [CrossRef]
- Liu, Y.; Wan, K.; Deng, N.; Wu, F. Photodegradation of Paracetamol in Montmorillonite KSF Suspension. React. Kinet. Mech. Catal. 2010, 99, 493–502. [Google Scholar] [CrossRef]
- Sathishkumar, K.; Harishsenthil, P.; Naidu, S.M.; Srividhya, B.; Guganathan, L.; Batoo, K.M.; Ijaz, M.F.; Ramamoorthy, M.; Ragupathy, S. Impact of Metal Doping and Non Metal Loading on the Photocatalytic Degradation of Organic Pollutants Using Tin Dioxide Catalyst under Sunlight Irradiation. J. Mol. Struct. 2025, 1321, 139841. [Google Scholar] [CrossRef]
- Zakir, O.; Ait-Karra, A.; Idouhli, R.; Khadiri, M.; Dikici, B.; Zegzouti, A.; Abouelfida, A.; Outzourhit, A. A Study on the Influence of Metal Ag, Cu, and Fe Doping on the Morphological, Structural, and Photocatalytic Activity of TiO2 Nanostructures. J. Alloys Compd. 2025, 1010, 177141. [Google Scholar] [CrossRef]
- Tian, B.; Li, C.; Gu, F.; Jiang, H. Synergetic Effects of Nitrogen Doping and Au Loading on Enhancing the Visible-Light Photocatalytic Activity of Nano-TiO2. Catal. Commun. 2009, 10, 925–929. [Google Scholar] [CrossRef]
- Tan, Y.N.; Wong, C.L.; Mohamed, A.R. An Overview on the Photocatalytic Activity of Nano-Doped-TiO2 in the Degradation of Organic Pollutants. ISRN Mater. Sci. 2011, 2011. [Google Scholar] [CrossRef]
- Mancuso, A.; Sacco, O.; Vaiano, V.; Sannino, D.; Pragliola, S.; Venditto, V.; Morante, N. Visible Light Active Fe-Pr Co-Doped TiO2 for Water Pollutants Degradation. Catal. Today 2021, 380, 93–104. [Google Scholar] [CrossRef]
- Yusuff, A.S.; Obende, B.A.; Egbosiuba, T.C. Photocatalytic Decolorization of Textile Effluent over ZnO Nanoparticles Immobilized on Eucalyptus Bark Biochar: Parametric Optimization, Kinetic and Economic Analyses. Water Resour. Ind. 2024, 31, 100245. [Google Scholar] [CrossRef]
- Binjhade, R.; Mondal, R.; Mondal, S. Continuous Photocatalytic Reactor: Critical Review on the Design and Performance. J. Environ. Chem. Eng. 2022, 10, 107746. [Google Scholar]
- Zeinali Heris, S.; Bagheri Khaniani, P.; Mousavi, S.B. Photocatalytic Degradation of Co-Amoxiclav Using Hybrid TiO2/ZnO Nanoparticles: Experimental and Optimization. J. Water Process Eng. 2025, 7, 107040. [Google Scholar] [CrossRef]
- Naeimi, A.; Sharifi, A.; Montazerghaem, L.; Abhari, A.R.; Mahmoodi, Z.; Bakr, Z.H.; Soldatov, A.V.; Ali, G.A.M. Transition Metals Doped WO3 Photocatalyst towards High Efficiency Decolourization of Azo Dye. J. Mol. Struct. 2022, 1250, 131800. [Google Scholar] [CrossRef]
- Gil, M.A.; Murcia, J.J.; Hernández-Laverde, M.; Morante, N.; Sannino, D.; Vaiano, V. Ag/Cr-TiO2 and Pd/Cr-TiO2 for Organic Dyes Elimination and Treatment of Polluted River Water in Presence of Visible Light. Nanomaterials 2023, 13, 2341. [Google Scholar] [CrossRef]
- Morante, N.; De Guglielmo, L.; Oliva, N.; Monzillo, K.; Femia, N.; Di Capua, G.; Vaiano, V.; Sannino, D. Influence of UV-A Light Modulation on Phenol Mineralization by TiO2 Photocatalytic Process Coadjuvated with H2O2. Catalysts 2024, 14, 544. [Google Scholar] [CrossRef]
- Enesca, A. The Influence of Photocatalytic Reactors Design and Operating Parameters on the Wastewater Organic Pollutants Removal—A Mini-review. Catalysts 2021, 11, 556. [Google Scholar] [CrossRef]
- Rabeie, B.; Mahmoodi, N.M. Green and Environmentally Friendly Architecture of Starch-Based Ternary Magnetic Biocomposite (Starch/MIL100/CoFe2O4): Synthesis and Photocatalytic Degradation of Tetracycline and Dye. Int. J. Biol. Macromol. 2024, 274, 133318. [Google Scholar] [CrossRef]
- Zhang, J.; Ying, Z.; Li, H.; Liu, X.; Ma, D.; Yu, H. Preparation of Soybean Dreg-Based Biochar@TiO2 Composites and the Photocatalytic Degradation of Aflatoxin B1 Exposed to Simulated Sunlight Irradiation. Toxins 2024, 16, 429. [Google Scholar] [CrossRef] [PubMed]
- Boudechiche, N.; Morante, N.; Sannino, D.; Monzillo, K.; Trari, M.; Sadaoui, Z. Enhanced Visible-Light Photocatalysis Activity of TiO2/Ag Nanocomposites Prepared by the Ultrasound-Assisted Sol–Gel Method: Characterization and Degradation–Mineralization of Cationic and Anionic Dyes. Catalysts 2024, 14, 883. [Google Scholar] [CrossRef]
- Koppenol, W.H.; Stanbury, D.M.; Bounds, P.L. Electrode Potentials of Partially Reduced Oxygen Species, from Dioxygen to Water. Free Radic. Biol. Med. 2010, 49, 317–322. [Google Scholar] [PubMed]
- Petri, B.G.; Watts, R.J.; Teel, A.L.; Huling, S.G.; Brown, R.A. Fundamentals of ISCO Using Hydrogen Peroxide. In Situ Chemical Oxidation for Groundwater Remediation; Springer: New York, NY, USA, 2011; pp. 33–88. [Google Scholar]
- Wang, Z.; Chen, H.; Rong, C.; Li, A.; Hua, X.; Dong, D.; Liang, D.; Liu, H. Photocatalytic Degradation of Acetaminophen in Aqueous Environments: A Mini Review. Toxics 2023, 11, 604. [Google Scholar] [CrossRef]
- Yang, L.; Yu, L.E.; Ray, M.B. Photocatalytic Oxidation of Paracetamol: Dominant Reactants, Intermediates, and Reaction Mechanisms. Environ. Sci. Technol. 2009, 43, 460–465. [Google Scholar] [CrossRef]
- Feng, J.; Xu, P.; Qin, B.; Hu, M.; Ye, H.; Lv, L.; Luo, M.; Zhou, W.; Zhang, D.; Du, H.; et al. Boosting Photocatalytic Hydrogen Peroxide Production by Plasmonic Ag Decorated Perovskite-Type BaSnO3. Inorg. Chem. Commun. 2025, 174, 113975. [Google Scholar] [CrossRef]
- Ahmad, S.; Almehmadi, M.; Janjuhah, H.T.; Kontakiotis, G.; Abdulaziz, O.; Saeed, K.; Ahmad, H.; Allahyani, M.; Aljuaid, A.; Alsaiari, A.A.; et al. The Effect of Mineral Ions Present in Tap Water on Photodegradation of Organic Pollutants: Future Perspectives. Water 2023, 15, 175. [Google Scholar] [CrossRef]
- Jiménez-Salcedo, M.; Monge, M.; Tena, M.T. The Photocatalytic Degradation of Sodium Diclofenac in Different Water Matrices Using G-C3N4 Nanosheets: A Study of the Intermediate by-Products and Mechanism. J. Environ. Chem. Eng. 2021, 9, 105827. [Google Scholar] [CrossRef]
- Bolton, J.R.; Bircher, K.G.; Tumas, W.; Tolman, C.A. Figures-of-Merit for the Technical Development and Application of Advanced Oxidation Technologies for Both Electric-and Solar-Driven Systems (IUPAC Technical Report). Pure Appl. Chem. 2001, 73, 627–637. [Google Scholar] [CrossRef]
- Nugroho, D.; Kabir, L.; Joo, Y.J.; Cho, K.Y.; Nanan, S.; Chanthai, S.; Benchawattananon, R.; Oh, W.C. Photocatalytic Performance with Electrochemical Effects for ZnO/Graphene Quantum Dots/CdSe Nanocomposite toward Ciprofloxacin Antibiotics and Paracetamol Degradation under Visible Light. Mater. Today Chem. 2024, 42, 102393. [Google Scholar] [CrossRef]
- Hassan, F.; Backer, S.N.; Almanassra, I.W.; Ali Atieh, M.; Elbahri, M.; Shanableh, A. Solar-Matched S-Scheme ZnO/g-C3N4 for Visible Light-Driven Paracetamol Degradation. Sci. Rep. 2024, 14, 12220. [Google Scholar] [CrossRef]
- Abbas, H.A.; Nasr, R.A.; Vannier, R.N.; Jamil, T.S. Improving of Photocatalytic Activity of Barium Ferrate via Bismuth and Copper Co-Doping for Degradation of Paracetamol under Visible Light Irradiation. J. Environ. Sci. 2022, 112, 331–342. [Google Scholar] [CrossRef]
- Al-Gharibi, M.A.; Kyaw, H.H.; Al-Sabahi, J.N.; Zar Myint, M.T.; Al-Sharji, Z.A.; Al-Abri, M.Z. Silver Nanoparticles Decorated Zinc Oxide Nanorods Supported Catalyst for Photocatalytic Degradation of Paracetamol. Mater. Sci. Semicond. Process 2021, 134, 105994. [Google Scholar] [CrossRef]
- Anwar, K.; Naqvi, F.K.; Beg, S.; Haneef, S. Photocatalytic Degradation of MB Dye and Paracetamol Drug, via Hydrothermally Synthesised Praseodymium Doped Bi4V2O11 Nanoparticles. J. Mol. Struct. 2023, 1272, 134183. [Google Scholar] [CrossRef]
- Zhang, B.; Liu, F.; Nie, C.; Hou, Y.; Tong, M. Photocatalytic Degradation of Paracetamol and Bisphenol A by Chitosan Supported Covalent Organic Framework Thin Film with Visible Light Irradiation. J. Hazard. Mater. 2022, 435, 128966. [Google Scholar] [CrossRef]



| Photocatalyst | mNi(OCOCH3)2·4H2O [g] | mTTIP [g] | nNi/nTiO2 |
|---|---|---|---|
| TiO2 | 0 | 12 | |
| Ni(0.05%)-TiO2 | 0.00725 | 12 | 0.00069 |
| Ni(0.10%)-TiO2 | 0.0145 | 12 | 0.00138 |
| Ni(0.15%)-TiO2 | 0.02175 | 12 | 0.00207 |
| Ni(0.20%)-TiO2 | 0.029 | 12 | 0.00276 |
| Photocatalyst | SSA [m2 g−1] | Ebg [eV] | PZC |
|---|---|---|---|
| TiO2 | 101 | 3.11 | 5.95 |
| Ni(0.05%)-TiO2 | 102 | 2.53 | 5.75 |
| Ni(0.10%)-TiO2 | 103 | 2.5 | 5.47 |
| Ni(0.15%)-TiO2 | 103.5 | 2.44 | 5.18 |
| Ni(0.20%)-TiO2 | 105 | 2.49 | 4.95 |
| Parameter | Value |
|---|---|
| Conductivity (µS cm−1) | 370 |
| Sodium (ppm) | 3.16 |
| Potassium (ppm) | 1.08 |
| Calcium (ppm) | 59.8 |
| Magnesium (ppm) | 12.9 |
| Chlorides, Cl− (ppm) | 6 |
| Sulfates, SO42− (ppm) | 3.4 |
| Bicarbonates, HCO3− (ppm) | 249 |
| Nitrates, NO3− (ppm) | 7.1 |
| Photocatalyst | Type of Light | P [kW] | ksp [min−1 kW−1] | V [L] | EE/O [kWh m−3] | Reference |
|---|---|---|---|---|---|---|
| ZnO/GQDs/CdSe | Visible Light | 0.075 | 0.533 | 0.2 | 156.3 | [95] |
| ZnO/gC3N4 | Visible Light | 0.5 | 0.100 | 0.1 | 1666.7 | [96] |
| Ba0.95Bi0.05Fe0.95Cu0.05O3 | Visible Light | 0.244 | 0.135 | 0.1 | 1232.3 | [97] |
| ZnO/AgNPs | Simulated Solar Light | 1.6 | 0.005 | 0.0035 | 9.52 × 105 | [98] |
| 40% Pr/Bi4V2O11 | Visible Light | 0.3 | 0.025 | 0.2 | 3289.5 | [99] |
| CSCF | Visible Light | 0.3 | 0.113 | 0.06 | 2451.0 | [100] |
| Ni(0.1%)-TiO2 | Visible Light | 0.012 | 1.125 | 0.1 | 148.1 | Our reaction system |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morante, N.; Monzillo, K.; Vaiano, V.; Kadirova, Z.C.; Sannino, D. Synthesis and Characterization of a Novel Sol–Gel-Derived Ni-Doped TiO2 Photocatalyst for Rapid Visible Light-Driven Mineralization of Paracetamol. Nanomaterials 2025, 15, 530. https://doi.org/10.3390/nano15070530
Morante N, Monzillo K, Vaiano V, Kadirova ZC, Sannino D. Synthesis and Characterization of a Novel Sol–Gel-Derived Ni-Doped TiO2 Photocatalyst for Rapid Visible Light-Driven Mineralization of Paracetamol. Nanomaterials. 2025; 15(7):530. https://doi.org/10.3390/nano15070530
Chicago/Turabian StyleMorante, Nicola, Katia Monzillo, Vincenzo Vaiano, Zukhra C. Kadirova, and Diana Sannino. 2025. "Synthesis and Characterization of a Novel Sol–Gel-Derived Ni-Doped TiO2 Photocatalyst for Rapid Visible Light-Driven Mineralization of Paracetamol" Nanomaterials 15, no. 7: 530. https://doi.org/10.3390/nano15070530
APA StyleMorante, N., Monzillo, K., Vaiano, V., Kadirova, Z. C., & Sannino, D. (2025). Synthesis and Characterization of a Novel Sol–Gel-Derived Ni-Doped TiO2 Photocatalyst for Rapid Visible Light-Driven Mineralization of Paracetamol. Nanomaterials, 15(7), 530. https://doi.org/10.3390/nano15070530









