Abstract
The field of bio-nanotechnology has seen significant advancements in recent years, particularly in the synthesis and application of bio-nanoparticles (BNPs). This review focuses on the green synthesis of BNPs using biological entities such as plants, bacteria, fungi, and algae. The utilization of these organisms for nanoparticle synthesis offers an eco-friendly and sustainable alternative to conventional chemical and physical methods, which often involve toxic reagents and high energy consumption. Phytochemicals present in plant extracts, unique metabolic pathways, and biomolecules in bacteria and fungi, and the rich biochemical composition of algae facilitate the production of nanoparticles with diverse shapes and sizes. This review further explores the wide-ranging applications of BNPs in various fields like therapeutics, fuel cells, energy generation, and wastewater treatment. In therapeutics, BNPs have shown efficacy in antimicrobial, anti-inflammatory, antioxidant, and anticancer activities. In the energy sector, BNPs are being integrated into fuel cells and other energy generation systems like bio-diesel to improve efficiency and sustainability. Their catalytic properties and large surface area enhance the performance of these devices. Wastewater treatment is another critical area where BNPs are employed for the removal of heavy metals, organic pollutants, and microbial contaminants, offering a cost-effective and environmentally friendly solution to water purification. This comprehensive review highlights the potential of bio-nanoparticles synthesized through green methods. It highlights the need for further research to optimize synthesis processes, understand mechanisms of action, and expand the scope of their applications. BNPs can be utilized to address advantages and some of the pressing challenges in medicine, energy, and environmental sustainability, paving the way for innovative and sustainable technological advancements in future prospects.
1. Introduction
Nanotechnology research and studies have advanced rapidly worldwide, and the applications of nanoparticles (NPs) in various fields, including biomedical applications, cell labeling, drug delivery, plant tissue culture, biomarkers, the automobile industry, and the energy sector, have become significant subjects of study in recent years, [1,2,3]. Different synthesis methods can be used for the preparation of NPs with variations in size and morphology. Chemical and physical methods are widely used, while biological methods are currently emerging as an alternative [4]. The use of chemical agents such as sodium hydroxide, sodium borohydride, potassium hydroxide, and hydrazine for reduction purposes is common in chemical methods [5,6,7,8,9], and condensation, laser ablation, laser pyrolysis, evaporation lithography, and ball milling are widely used in physical methods [10,11,12,13,14,15,16,17] for NP synthesis. Bio nanoparticle synthesizing is a sustainable solution in the nanotechnology discipline since it uses renewable and biodegradable resources (Figure 1).
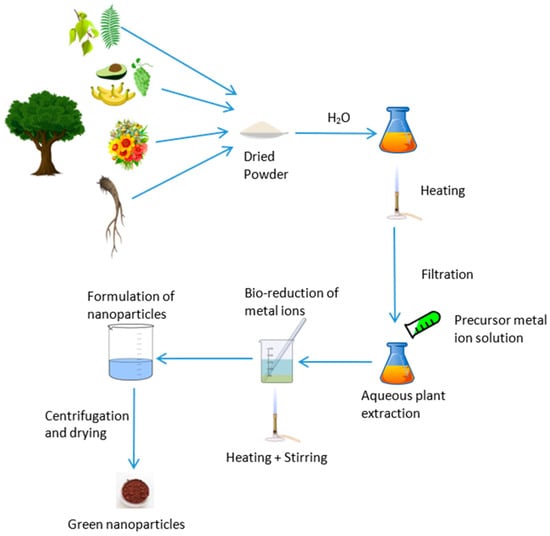
Figure 1.
Green synthesis of bio-nanoparticles from plants.
According to [18], no exact mechanism has been explained for the phytosynthesis of metallic nanoparticles. Similarly, [19] stated that identifying the precise biochemical reactions involved in the green synthesis of metallic nanoparticles remains a challenge. The general method for plant-based nanoparticle production is as follows: first, a plant and its specific part are selected and then crushed, and the plant extract is obtained. The plant extract is processed to remove any impurities. The precursor, typically a metallic solution, is then mixed with the plant extract, resulting in the production of nanoparticles. Maintaining appropriate pH, temperature, and continuous stirring (which ensures the production of uniformly sized nanoparticles) is crucial to facilitate the reaction effectively. A color change in the plant extract can be considered an indication of nanoparticle formation in some nanoparticles, such as Ag and Au, due to surface plasmon resonance (SPR) [20,21,22]. The color change observed during the reaction process serves as an indicator of nanoparticle formation. This change occurs due to SPR, where light interacts with the nanoparticles, causing them to display a different color compared to the bulk material. In addition to SPR, the quantum confinement effect also plays a role in the color variation observed during the synthesis of metallic nanoparticles [23,24].
Microorganisms produce various essential enzymes, while plants contain a range of secondary metabolites, such as phenols, terpenes, and alcohols. These enzymes and metabolites can act as reducing agents, facilitating the synthesis of nanoparticles. Additionally, plant extracts can function as stabilizers, eliminating the need for additional stabilizing agents in the solution [20,21,25]. Ref. [26] reported the presence of phytochemicals such as flavonoids, saponins, triterpenes, and steroids in Tithonia diversifolia. Similarly, [22] confirmed that the presence of functional groups of carbon (C) and oxygen (O) contributes to the stabilization and reduction processes involved in nanoparticle synthesis.
Plant extracts can also function as capping agents, stabilizing nanoparticles during synthesis. FTIR analysis has confirmed the involvement of various carbon (C), hydrogen (H), and oxygen (O) bonds in plant extracts, which contribute to the capping process [22,27,28]. Polyphenols, which contain multiple hydroxyl (-OH) groups attached to aromatic rings, are highly reactive in chemical reactions. For example, during the synthesis of gold nanoparticles, neighboring hydroxyl groups (typically in the ortho position) in polyphenols bind with gold ions, forming a stable five-membered chelate ring. The ortho-dihydroxyl groups (two -OH groups on adjacent carbons) are oxidized into quinones (C=O groups), while gold ions are reduced (gain electrons) to neutral gold atoms (Au0). This reduction occurs due to the high redox potential of gold [19,20]. Additionally, [19] reported that proteins act as stabilizing agents by providing carbonyl (-C=O) groups. These amino acid residues surround the nanoparticles, preventing aggregation and ensuring stability. FTIR analysis has provided supporting evidence for this stabilization mechanism [24].
The hydrogen radical donates its unpaired electron to silver ions (Ag+) in the solution, reducing them to neutral silver atoms (Ag). These silver atoms then cluster together, forming silver nanoparticles (Ag NPs). Following this reduction process, the leftover eugenol molecule, now containing a phenoxy radical on its oxygen atom, undergoes resonance stabilization. This stabilization occurs as the unpaired electron on the oxygen atom delocalizes across the benzene ring and its double bonds, making the radical more stable and less reactive. These stabilized radicals remain dissolved in the solution, aiding both nanoparticle formation and stabilization [19,29]. Ref. [30] reported that in polyphenolic compounds, neighboring hydroxyl groups form a five-membered chelate ring. Due to the extremely high oxidation-reduction potential of Au3+, the chelated ortho-dihydroxy groups are oxidized to quinones, while Au3+ is simultaneously reduced to Au. The formation of Au NPs occurs through the aggregation of nearby Au atoms, and quinones and polyphenolic compounds subsequently stabilize these nanoparticles. However, there exists several research areas for further development; for example, the efficiency of various natural resources for the green synthesis of nanomaterials has not been fully studied. Importantly, the negative impacts of those nanomaterials are also not sufficiently understood. Therefore, it is mandatory to focus on risk management throughout production, processing, preservation, and discharge [31,32]. Furthermore, the green synthesis of NPs using biological materials and their properties are summarized in Table 1.

Table 1.
Green synthesis of NPs using biological materials.
2. Applications of Bio-Nanoparticles
Bio-nanomaterials offer significant advantages such as biocompatibility, biodegradability, and enhanced biological functionality, making them ideal for several applications in energy storage, environmental remediation, and medicinal applications. However, several challenges still exist, such as synthesis complexity, stability issues, and scalability constraints that need to be addressed through advanced fabrication techniques, hybrid material development, and computational modeling to enhance their performance and applicability.
2.1. Applications of Bio-Nanoparticles in Fuel-Cells
The fuel cell was first introduced by Sir William Grove in the 1830s. Even though the fuel cell has a long history, nowadays, many research works are being carried out that are relevant to fuel cells compared to previous decades [108,109]. The fuel cell is an effective energy converter compared to other relevant energy sources, and it only emits water and heat, making it a more environmentally friendly solution. Due to their higher energy efficiency, fuel cells are currently used in several applications in electric vehicles, alternative power sources, energy-storing methods, and space programs [110,111].
Proton exchange membrane fuel cells (PEMFs), solid-oxide fuel cells (SOFs), alkaline fuel cells (AFCs), phosphoric acid fuel cells (PAFCs), direct methanol fuel cells (DMFC), and molten carbonate fuel cells (MCFCs) can be identified as the different fuel cells types that are currently at the development. These fuel cell types are used in different applications based on their power ratings and operating temperatures. Apart from conventional fuel cells, microbial fuel cells are also being developed by scientists and can also be used as fuel cells, which is an eco-friendly solution. Microbial fuel cells can generate electricity while purifying wastewater using the metabolism power of bacteria.
Apart from the anode, cathode, and electrolyte, electro-catalysts are used in fuel cells to increase the rate of reactions in the fuel cells [112]. Most of the catalysts are noble nanoparticles such as platinum (Pt) and platinum alloys. Currently, there is ongoing research to analyze the different extraction methods of Pt, Pt alloys, and non-precious materials. As an environmentally friendly solution, researchers are trying to develop bio-synthesized nanoparticles as nanocatalysts for fuel cells and microbial fuel cells [113,114,115]. Table 2 represents several recent studies that have been carried out regarding bio-synthesized nanoparticles as catalysts for conventional fuel and microbial fuel cells.

Table 2.
Bio-synthesized nanoparticle applications in fuel-cells.
2.2. Applications of Bio-Nanoparticles in Therapeutics
Bio-nanoparticles have garnered significant attention over the past decades owing to their excellent therapeutic capabilities. Their unique physicochemical properties, stability, solubility, and multi-functionality enhance their performance in various therapeutic applications, allowing for enhanced penetration and interaction with biological systems, targeted delivery, and efficacy. Moreover, their biocompatibility and ability to be functionalized for specific targeting further increase their effectiveness and safety in medical treatments [126]. In this section of the review, applications of bio nanoparticles in antioxidant, anticancer, anti-inflammatory, and antibacterial applications are discussed.
Antioxidants are considered potent therapeutics for a variety of disease conditions. However, the use of these agents is doubtful in conventional therapy due to their instability, low permeability, and poor solubility [127]. Phytochemicals such as phenolic acids, terpenoids, and polyphenols from natural sources accompany substantial antioxidant potential. Bio-nanoparticles, functionalized with antioxidants derived from such bioactive compounds, have emerged as promising candidates for combating oxidative stress and are a heavily studied area in recent decades [128]. Cancer is considered to be an enormous challenge to human health. Bio-nanoparticle-based therapeutics have progressed significantly in the arena of cancer therapy, as conventional chemotherapy poses a multitude of limitations owing to the disadvantageous nature of the tumor microenvironment. Bio-nanoparticles offer a promising alternative to traditional chemotherapeutics with their enhanced capacities, including targeted delivery, selective anticancer effects, sustained release, and lower toxicity [129]. Various mechanisms have been proposed to explain the cytotoxicity mechanism of bio-nanoparticles, such as generation of reactive oxygen species (ROS), permeabilization of the mitochondrial outer membrane, activation of caspase-3, and specific DNA cleavage, all of which lead to apoptotic death of the cancer cell. There have been studies on bio-nanoparticles designed to treat cancer, including metallic nanoparticles from Ag, Au, Zn, and Cu, among the leading anticancer nanoparticles to date [130]. Inflammation is a localized physical response characterized by swelling, redness, pain, and other symptoms in the affected area in response to an infection or injury. Anti-inflammatory agents inhibit specific substances in the body that trigger inflammation [131]. Bio-nanoparticles are potent anti-inflammatory agents owing to their enhanced ability for selectivity and penetration and to restrict inflammatory messengers and enzymes compared to conventional therapy. Several bio-nanoparticles derived from metals and metal oxides, such as Ag, Au, Se, Cu, Ni, ZnO, FeO, and TiO2, are reported to be potent, with anti-inflammatory properties [132]. Multidrug-resistant bacterial pathogens are an escalating, highly debilitating threat worldwide, and conventional antibiotic therapeutics are rapidly becoming useless against the most resistant bacterial strains [133]. In pursuing alternative solutions, bio-nanoparticles have shown significant antibacterial activity, as they possess unique physical and chemical properties that enhance their interaction with microbial cells. The mechanisms through which bio-nanoparticles exhibit antibacterial effects include disruption of the bacterial cell membrane, generation of reactive oxygen species (ROS), and interference with cellular processes. The use of natural sources in the synthesis process imparts additional antibacterial properties due to the presence of bioactive compounds. Overall, the application of bio-nanoparticles in antibacterial treatments holds great promise for developing new, effective, and sustainable antimicrobial agents [134]. Table 3 provides examples of bio-nanoparticles synthesized from biological sources, including plants, fungi, bacteria, and algae, with their reported antioxidant, anticancer, anti-inflammatory, and antibacterial activities.

Table 3.
Therapeutic applications of green synthesized bio-nanoparticles.
2.3. Applications of Bio-Nanoparticles in Waste Water Treatment
Due to the unique properties such as high surface area, reactivity, and functionality of bio-nanoparticles, they have emerged as highly effective agents in the wastewater treatment industry. Their properties lead to the removal of a wide range of contaminants, including heavy metals, organic pollutants, and pathogenic microorganisms. The wastewater or effluent containing non-biodegradable dyes and organic pollutants into the water reservoirs is mainly discharged from various industries, factories, and laboratories without any treatment, and it leads to a global environmental and health hazard [153]. Large quantities of dyes are used in many industrial applications such as textiles, papers, leathers, laser materials, laser printing, foodstuffs, cosmetics, xerography, gasoline, etc. And byproducts discarded from industries contain heavy metal ions and dyes, or both in most cases [154]. Furthermore, according to the estimated data, the total worldwide production of dyes is lost in their synthesis and dyeing process, which is over 15% [155]. The studies proved that most of these dyes are toxic and carcinogenic and reduce the light penetration of the aqueous systems. As a result, it causes serious concern to society due to the complex structures and non-biodegradable nature. This leads to negative effects on photosynthesis, is toxic for living organisms, is harmful to human health, and contributes significantly to the overall imbalance of the ecosystem [156].
Due to the high surface area and affinity for metal ions, carbon-based and metal-oxide nanoparticles have shown exceptional adsorption capacity on heavy metals like lead, mercury, and cadmium from wastewater [157,158]. Nanoparticles such as titanium dioxide show photocatalytic activity, and they are employed to break down organic contaminants, including pesticides, dyes, and pharmaceutical residues, converting them into less harmful substances. TiO2 and other metal oxides demonstrate high photocatalytic activity, but their effectiveness depends on several conditions, such as pH level, light intensity, and the presence of additional catalysts. pH levels, can affect the surface charge and light intensity directly impacts the electron-hole pairs which is a critical factor for photocatalysis. At the same time the availability of a co-catalysts can improve the overall efficiency [159]. Furthermore, silver and gold nanoparticles exhibit potential antimicrobial effects against harmful viruses and bacteria [160]. Moreover, the efficiency and sustainability of the wastewater treatment process are enhanced by magnetically responsive nanoparticles due to their easy recovery and reusable properties. Table 4 demonstrates a summary of recent research works carried out by scientists on the applications of bio-based nanomaterials in wastewater treatment. These advanced bio-nanomaterials provide a versatile and robust solution for addressing the difficult challenges of wastewater treatment, improving the efficiency, effectiveness, and sustainability while contributing to the protection of public health and the environment. The studies summarized in Table 4 are conducted as laboratory based research activities. Even though the results from these studies demonstrate promising outcomes, it should be noted that these research are conducted under controlled laboratory conditions. In industrial applications there may be number of additional challenges such as variations in environmental conditions, cost effectiveness and scalability.

Table 4.
Bio-synthesized nanoparticle applications in wastewater treatment.
2.4. Applications of Bio-Nanoparticles in the Energy Industry
The increasing demand for energy due to rapid technological advancement and global population growth has caused a formidable challenge for human existence [176]. Global power generation is moving towards greener generation methods, discouraging conventional methods such as coal power, fossil fuel, natural gas, etc., to overcome environmental challenges such as global warming [177,178]. Throughout the last few decades, researchers have been working on finding a successful alternative to fossil fuels for power generation. As a result, many promising biofuels have emerged, such as bioethanol, biogas, biohydrogen, biodiesel, algal biofuels [179,180], bio-methanol, etc. However, biofuels still must achieve many milestones in order to challenge the fossil fuel industry. With the recent development of nanotechnology, a great deal of research has been conducted to improve the production efficiency of biofuels and the performance of biofuels using nanotechnology [181,182,183]. Nanoparticles can improve the efficiency of the manufacturing process of biofuels, as they have higher reactive surfaces [184]. Today, scientists have taken one step further by introducing bio-nanotechnology, a combination of biology and nanotechnology, to the energy sector, which results in more environmentally friendly outcomes. At the same time, the health-related concerns to the human body from the applications of nanotechnology are comparatively reduced with bio-nanotechnology [185].
There are a number of different applications of bio-nanotechnology in the energy industry. When considering the most recent research trends, the green synthesis of nanoparticles from plants is rapidly increasing in popularity due to environmental friendliness and health concerns due to the utility of toxic chemicals. The bio-nanoparticles that various plants synthesize are used in numerous types of research to observe their performance as catalysts for the biofuel production process. In Table 5, a summary of the recent research related to the enhancement of biofuel production using bio-nano catalysts is presented. All the nanoparticles used were synthesized using different plant components, such as orange peels [185], pomegranate peels [186], Euphorbia royleana leaves [187], rice husk [188], and also animal wastes such as chicken-egg shell [189], etc. All the research has shown very positive results in improving the production efficiency of biofuels, which have a promising number of industrial applications for nanotechnology in the future energy sector.

Table 5.
Recent studies on the utilization of bio nano-catalysts for biofuel production.
3. Conclusions
Green synthesis of BNPs using plants, bacteria, fungi, and algae presents a promising and eco-friendly alternative to conventional methods. The diverse biochemical properties of these biological entities enable the production of nanoparticles with varied shapes and sizes, enhancing their applicability across multiple fields. BNPs have shown significant potential in therapeutics as antimicrobial, anti-inflammatory, antioxidant, and anticancer agents. Additionally, they are being integrated into fuel cells and energy generation systems, providing green energy solutions. In wastewater treatment, BNPs offer an effective and environmentally friendly approach to removing heavy metals, organic pollutants, and microbial contaminants. However, further research is essential to optimize synthesis processes, fully elucidate their mechanisms of action, and expand the scope of their applications. BNPs can address some of the pressing challenges in medicine, energy, and environmental sustainability, paving the way for innovative and sustainable technological advancements. The continued exploration and development of bio-nanoparticles for advancements in material engineering, hybridization strategies, and computational design hold great promise for the future, offering sustainable solutions that align with the growing demand for environmentally conscious technologies.
Author Contributions
M.D.K.M.G., conceptualization, writing—original draft and writing—review and editing; G.D.C.P.G., conceptualization and writing—original draft; C.J.A., conceptualization and writing—original draft; D.K.A.I., conceptualization and writing—original draft; H.V.V.P., conceptualization and writing—original draft; S.S.M., writing—original draft; K.R.K., supervision; P.K.G.S.S.B., supervision. All authors have read and agreed to the published version of the manuscript.
Funding
This review was supported by the Science and Technology Human Resource Development Project, Ministry of Education, Sri Lanka, funded by the Asian Development Bank (Grant No CRG-R2-SB-1).
Data Availability Statement
No data were used for the research described in the article.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Abbreviations
The following abbreviations are used in this manuscript:
| ATR-FTIR | Attenuated total reflectance Fourier-transform infrared spectroscopy |
| BET | Brunauer–Emmett–Teller |
| DLS | Dynamic light scattering |
| EDAX | Energy-dispersive X-ray spectroscopy |
| EDS | Energy-dispersive X-ray spectroscopy |
| FESEM | Field emission scanning electron microscopy |
| FESEM-EDX | Field emission scanning electron microscopy with energy dispersive X-ray spectroscopy |
| FTIR | Fourier-transform infrared spectroscopy |
| HRSEM | High-resolution scanning electron microscopy |
| HRTEM | High-resolution transmission electron microscopy |
| SEM | Scanning electron microscopy |
| SEM-EDX | Scanning electron microscopy with energy dispersive X-ray spectroscopy |
| TEM | Transmission electron microscopy |
| TGA | Thermogravimetric analyzer |
| UV–vis | Ultraviolet–visible spectrophotometer |
References
- Galpaya, C.; Induranga, A.; Vithanage, V.; Mantilaka, P.; Koswattage, K.R. Comparative Study on the Thermal Properties of Engine Oils and Their Nanofluids Incorporating Fullerene-C60, TiO2 and Fe2O3 at Different Temperatures. Energies 2024, 17, 732. [Google Scholar] [CrossRef]
- Gunasena, M.D.K.M.; Alahakoon, A.M.P.D.; Polwaththa, K.P.G.D.M.; Galpaya, G.D.C.P.; Priyanjani, H.A.S.A.; Koswattage, K.R.; Senarath, W.T.P.S.K. Transforming Plant Tissue Culture with Nanoparticles: A Review of Current Applications. Plant Nano Biol. 2024, 10, 100102. [Google Scholar] [CrossRef]
- Induranga, A.; Galpaya, C.; Vithanage, V.; Koswattage, K.R. Thermal Properties of TiO2 Nanoparticle-Treated Transformer Oil and Coconut Oil. Energies 2024, 17, 49. [Google Scholar] [CrossRef]
- Shrivastava, V.; Chauhan, P.; Tomar, R.S. Bio-Fabrication of Metal Nanoparticles: A Review. Int. J. Curr. Res. Life Sci. 2019, 7, 1927–1932. [Google Scholar]
- Chen, W.; Cai, W.; Zhang, L.; Wang, G.; Zhang, L. Sonochemical Processes and Formation of Gold Nanoparticles within Pores of Mesoporous Silica. J. Colloid. Interface Sci. 2001, 238, 291–295. [Google Scholar] [CrossRef]
- Eustis, S.; Hsu, H.-Y.; El-Sayed, M.A. Gold Nanoparticle Formation from Photochemical Reduction of Au3+ by Continuous Excitation in Colloidal Solutions. A Proposed Molecular Mechanism. J. Phys. Chem. B 2005, 109, 4811–4815. [Google Scholar] [CrossRef]
- Frattini, A.; Pellegri, N.; Nicastro, D.; de Sanctis, O. Effect of Amine Groups in the Synthesis of Ag Nanoparticles Using Aminosilanes. Mater. Chem. Phys. 2005, 94, 148–152. [Google Scholar] [CrossRef]
- Rodríguez-Sánchez, L.; Blanco, M.C.; López-Quintela, M.A. Electrochemical Synthesis of Silver Nanoparticles. J. Phys. Chem. B 2000, 104, 9683–9688. [Google Scholar] [CrossRef]
- Starowicz, M.; Stypuła, B.; Banaś, J. Electrochemical Synthesis of Silver Nanoparticles. Electrochem. Commun. 2006, 8, 227–230. [Google Scholar] [CrossRef]
- Mafuné, F.; Kohno, J.; Takeda, Y.; Kondow, T. Dissociation and Aggregation of Gold Nanoparticles under Laser Irradiation. J. Phys. Chem. B 2001, 105, 9050–9056. [Google Scholar] [CrossRef]
- Mitrakos, D.; Jokiniemi, J.; Backman, U.; Housiadas, C. Aerosol Flow in a Tube Furnace Reactor of Gas-Phase Synthesised Silver Nanoparticles. J. Nanoparticle Res. 2008, 10, 153–161. [Google Scholar] [CrossRef]
- Zamiri, R.; Azmi, B.Z.; Ahangar, H.A.; Zamiri, G.; Husin, M.S.; Wahab, Z.A. Preparation and Characterization of Silver Nanoparticles in Natural Polymers Using Laser Ablation. Bull. Mater. Sci. 2012, 35, 727–731. [Google Scholar] [CrossRef]
- Zhang, G.; Wang, D. Fabrication of Heterogeneous Binary Arrays of Nanoparticles via Colloidal Lithography. J. Am. Chem. Soc. 2008, 130, 5616–5617. [Google Scholar] [CrossRef]
- Sarkar, T.; Kundu, S.; Ghorai, G.; Sahoo, P.; Reddy, V.; Bhattacharjee, A.C. Synthesis and Characterization of Zinc Ferrite Nanomaterials Vis-à-Vis Studies on Their Photocatalytic Application in Visible Light Dye Degradation. Appl. Phys. A 2025, 131, 266. [Google Scholar] [CrossRef]
- Mulay, M.; Patwardhan, S.; Martsinovich, N.D. Review of Bio-Inspired Green Synthesis of Titanium Dioxide for Photocatalytic Applications. Catalysts 2024, 14, 742. [Google Scholar] [CrossRef]
- Vijan, E.A.; Modan, E.M.; Moga, S.G.; Negrea, D.A.; Schiopu, A.-G.; Oproescu, M.; Istrate, D.E. Assisted Egg White Biogenic Synthesis for Elaboration of ZnO Nanoparticles. Crystals 2025, 15, 71. [Google Scholar] [CrossRef]
- Rajalakshmi, B.; Singh, N.; Madhavi, A.; Khan, I.; Hameed, A.A.; Singh, S.; Rao, A.V.L.F. Bio-Inspired Nanomaterial’s for Energy Harvesting and Storage: A Green Approach. E3S Web Conf. 2024, 552, 01122. [Google Scholar] [CrossRef]
- Akhtar, M.S.; Panwar, J.; Yun, Y.-S. Biogenic Synthesis of Metallic Nanoparticles by Plant Extracts. ACS Sustain. Chem. Eng. 2013, 1, 591–602. [Google Scholar] [CrossRef]
- Singh, H.; Desimone, M.F.; Pandya, S.; Jasani, S.; George, N.; Adnan, M.; Aldarhami, A.; Bazaid, A.S.; Alderhami, S.A. Revisiting the Green Synthesis of Nanoparticles: Uncovering Influences of Plant Extracts as Reducing Agents for Enhanced Synthesis Efficiency and Its Biomedical Applications. Int. J. Nanomed. 2023, 18, 4727–4750. [Google Scholar] [CrossRef]
- Dauthal, P.; Mukhopadhyay, M. Noble Metal Nanoparticles: Plant-Mediated Synthesis, Mechanistic Aspects of Synthesis, and Applications. Ind. Eng. Chem. Res. 2016, 55, 9557–9577. [Google Scholar] [CrossRef]
- Jadoun, S.; Arif, R.; Jangid, N.; Meena, R. Green Synthesis of Nanoparticles Using Plant Extracts: A Review. Environ. Chem. Lett. 2021, 19, 355–374. [Google Scholar] [CrossRef]
- Khatun, M.; Khatun, Z.; Karim, M.R.; Habib, M.R.; Rahman, M.H.; Aziz, M.A. Green Synthesis of Silver Nanoparticles Using Extracts of Mikania Cordata Leaves and Evaluation of Their Antioxidant, Antimicrobial and Cytotoxic Properties. Food Chem. Adv. 2023, 3, 100386. [Google Scholar] [CrossRef]
- Tesfaye, M.; Gonfa, Y.; Tadesse, G.; Temesgen, T.; Periyasamy, S. Green Synthesis of Silver Nanoparticles Using Vernonia Amygdalina Plant Extract and Its Antimicrobial Activities. Heliyon 2023, 9, e17356. [Google Scholar] [CrossRef]
- Tran, T.T.T.; Vu, T.T.H.; Nguyen, T.H. Biosynthesis of Silver Nanoparticles Using Tithonia Diversifolia Leaf Extract and Their Antimicrobial Activity. Mater. Lett. 2013, 105, 220–223. [Google Scholar] [CrossRef]
- Mohammadinejad, R.; Karimi, S.; Iravani, S.; Varma, R.S. Plant-Derived Nanostructures: Types and Applications. Green. Chem. 2015, 18, 20–52. [Google Scholar] [CrossRef]
- Dada, A.O.; Inyinbor, A.A.; Idu, E.I.; Bello, O.M.; Oluyori, A.P.; Adelani-Akande, T.A.; Okunola, A.A.; Dada, O. Effect of Operational Parameters, Characterization and Antibacterial Studies of Green Synthesis of Silver Nanoparticles Using Tithonia Diversifolia. PeerJ 2018, 6, e5865. [Google Scholar] [CrossRef] [PubMed]
- Kharissova, O.V.; Dias, H.V.R.; Kharisov, B.I.; Pérez, B.O.; Pérez, V.M.J. The Greener Synthesis of Nanoparticles. Trends Biotechnol. 2013, 31, 240–248. [Google Scholar] [CrossRef]
- Obayomi, K.; Oluwadiya, A.; Sie Yon, J.L.; Dada, A.O.; Akubuo, D.; Adelani-Akande, T.; Bari, A.S.M.F.; Temidayo, S.; Rahman, M.M. Biosynthesis of Tithonia Diversifolia Leaf Mediated Zinc Oxide Nanoparticles Loaded with Flamboyant Pods (Delonix Regia) for the Treatment of Methylene Blue Wastewater. Arab. J. Chem. 2021, 14, 103363. [Google Scholar] [CrossRef]
- Kaur, H.; Kaur, S.; Singh, M. Biosynthesis of Silver Nanoparticles by Natural Precursor from Clove and Their Antimicrobial Activity. Biologia 2013, 68, 1048–1053. [Google Scholar] [CrossRef]
- Dash, D.S.; Bag, B. Synthesis of Gold Nanoparticles Using Renewable Punica Granatum Juice and Study of Its Catalytic Activity. Appl. Nanosci. 2012, 4, 55–59. [Google Scholar] [CrossRef]
- Alsaiari, N.; Alzahrani, F.; Amari, A.; Osman, H.; Harharah, H.; Elboughdiri, N.; Tahoon, M.A. Plant and Microbial Approaches as Green Methods for the Synthesis of Nanomaterials: Synthesis, Applications, and Future Perspectives. Molecules 2023, 28, 463. [Google Scholar] [CrossRef] [PubMed]
- Arif, M.; Hoque, M.E.; Rahman, M.Z.; Shafoyat, M.U.B. Emerging Directions in Green Nanomaterials: Synthesis, Physicochemical Properties and Applications. Mater. Commun. 2024, 40, 109335. [Google Scholar] [CrossRef]
- Faheem, I.; Shahid, S.; Khan, S.; Ahmad, W.; Zaman, S. Green Synthesis of Copper Oxide Nanoparticles Using Abutilon Indicum Leaf Extract: Antimicrobial, Antioxidant and Photocatalytic Dye Degradation Activitie. Trop. J. Pharm. Res. 2017, 16, 743–753. [Google Scholar] [CrossRef]
- Ahmad, B.; Chang, L.; Satti, U.Q.; Rehman, S.u.; Arshad, H.; Mustafa, G.; Shaukat, U.; Wang, F.; Tong, C. Phyto-Synthesis, Characterization, and In Vitro Antibacterial Activity of Silver Nanoparticles Using Various Plant Extracts. Bioengineering 2022, 9, 779. [Google Scholar] [CrossRef] [PubMed]
- Dulta, K.; Koşarsoy Ağçeli, G.; Chauhan, P.; Jasrotia, R.; Chauhan, P.K.; Ighalo, J.O.I. Multifunctional CuO Nanoparticles with Enhanced Photocatalytic Dye Degradation and Antibacterial Activity. Sustain. Environ. Res. 2022, 32, 2. [Google Scholar] [CrossRef]
- Abou-Zeid, H.; Ismail, G. The Role of Priming with Biosynthesized Silver Nanoparticles in the Response of Triticum Aestivum L to Salt Stress. Egypt. J. Bot. 2018, 58, 73–85. [Google Scholar] [CrossRef]
- Mishra, D.; Chitara, M.K.; Negi, S.; Pal singh, J.; Kumar, R.; Chaturvedi, P. Biosynthesis of Zinc Oxide Nanoparticles via Leaf Extracts of Catharanthus Roseus (L.) G. Don and Their Application in Improving Seed Germination Potential and Seedling Vigor of Eleusine Coracana (L.) Gaertn. Adv. Agric. 2023, 2023, 7412714. [Google Scholar]
- Gonçalves, J.P.Z.; Seraglio, J.; Macuvele, D.L.P.; Padoin, N.; Soares, C.; Riella, H.G. Green Synthesis of Manganese Based Nanoparticles Mediated by Eucalyptus Robusta and Corymbia Citriodora for Agricultural Applications. Colloids Surf. A Physicochem. Eng. Asp. 2022, 636, 128180. [Google Scholar]
- Mathew, S.S.; Sunny, N.E.; Shanmugam, V. Green Synthesis of Anatase Titanium Dioxide Nanoparticles Using Cuminum cyminum Seed Extract; Effect on Mung Bean (Vigna radiata) Seed Germination. Inorg. Chem. Commun. 2021, 126, 108485. [Google Scholar]
- Golzarnezhad, F.; Allahdou, M.; Mehravaran, L.; Naderi, S. Green Synthesis of ZnO Nanoparticles from the Extract of Cymbopogon Olivieri and Investigation of Their Antimicrobial and Anticancer Effects. Discov. Appl. Sci. 2025, 7, 196. [Google Scholar] [CrossRef]
- Batool, S.U.; Javed, B.; Sohail; Zehra, S.S.; Mashwani, Z.-R.; Raja, N.I.; Khan, T.; ALHaithloul, H.A.S.; Alghanem, S.M.; Al-Mushhin, A.A. Exogenous Applications of Bio-Fabricated Silver Nanoparticles to Improve Biochemical, Antioxidant, Fatty Acid and Secondary Metabolite Contents of Sunflower. Nanomaterials 2021, 11, 1750. [Google Scholar] [CrossRef] [PubMed]
- Sackey, J.; Nwanya, A.; Bashir, A.K.H.; Matinise, N.; Ngilirabanga, J.B.; Ameh, A.E.; Coetsee, E.; Maaza, M. Electrochemical Properties of Euphorbia Pulcherrima Mediated Copper Oxide Nanoparticles. Mater. Chem. Phys. 2020, 244, 122714. [Google Scholar]
- Hemmati, S.; Ahmeda, A.; Salehabadi, Y.; Zangeneh, A.; Zangeneh, M.M. Synthesis, Characterization, and Evaluation of Cytotoxicity, Antioxidant, Antifungal, Antibacterial, and Cutaneous Wound Healing Effects of Copper Nanoparticles Using the Aqueous Extract of Strawberry Fruit and L-Ascorbic Acid. Polyhedron 2020, 180, 114425. [Google Scholar]
- Jafarirad, S.; Kosari-Nasab, M.; Tavana, R.M.; Mahjouri, S.; Ebadollahi, R. Impacts of Manganese Bio-Based Nanocomposites on Phytochemical Classification, Growth and Physiological Responses of Hypericum perforatum L. Shoot Cultures. Ecotoxicol. Environ. Saf. 2021, 209, 111841. [Google Scholar] [PubMed]
- Del Buono, D.; Di Michele, A.; Costantino, F.; Trevisan, M.; Lucini, L. Biogenic ZnO Nanoparticles Synthesized Using a Novel Plant Extract: Application to Enhance Physiological and Biochemical Traits in Maize. Nanomaterials 2021, 11, 1270. [Google Scholar] [CrossRef]
- Rafique, M.; Jahangir, J.; Amin, B.A.Z.; Bilal Tahir, M.; Nabi, G.; Isa Khan, M.; Khalid, N.R.; Gillani, S.S.A.; Sadaf, I. Investigation of Photocatalytic and Seed Germination Effects of TiO2 Nanoparticles Synthesized by Melia azedarach L. Leaf Extract. J. Inorg. Organomet. Polym. Mater. 2019, 29, 2133–2144. [Google Scholar]
- Raja, K.; Sowmya, R.; Sudhagar, R.; Moorthy, P.S.; Govindaraju, K.; Subramanian, K.S. Biogenic ZnO and Cu Nanoparticles to Improve Seed Germination Quality in Blackgram (Vigna Mungo). Mater. Lett. 2019, 235, 164–167. [Google Scholar]
- Ambedkar, A.K.; Gautam, D.; Vikal, S.; Singh, M.; Kumar, A.; Sanger, A.; Sharma, K.; Singh, B.P.; Gautam, Y.K. Ocimum sanctum Leaf Extract-Assisted Green Synthesis of Pd-Doped CuO Nanoparticles for Highly Sensitive and Selective NO2 Gas Sensors. ACS Omega 2023, 8, 29663–29673. [Google Scholar]
- Lima, A.K.O.; Vieira, Í.R.S.; Souza, L.M.d.S.; Florêncio, I.; da Silva, I.G.M.; Tavares Junior, A.G.; Machado, Y.A.A.; dos Santos, L.C.; Taube, P.S.; Nakazato, G.; et al. Green Synthesis of Silver Nanoparticles Using Paullinia Cupana Kunth Leaf Extract Collected in Different Seasons: Biological Studies and Catalytic Properties. Pharmaceutics 2025, 17, 356. [Google Scholar] [CrossRef]
- Abd El Aty, A.A. Hafr Al Batin Phoenix dactylifera L. Leaves Extract as Efficient Catalyst for Green Synthesis of New Silver Nanoparticles with Broad Spectrum Antimicrobial Activity: Characterization and Evaluation Compared to Fungi. Arab. J. Sci. Eng. 2025. [Google Scholar] [CrossRef]
- Fatima, E.; Arooj, I.; Javeed, M.; Yin, J.V. Green Synthesis, Characterization and Applications of Phyllanthus Emblica Fruit Extract Mediated Chromium Oxide Nanoparticles. Discov. Nano 2024, 19, 68. [Google Scholar] [CrossRef] [PubMed]
- Mary, A.A.; Ansari, A.T.; Subramanian, R. Sugarcane Juice Mediated Synthesis of Copper Oxide Nanoparticles, Characterization and Their Antibacterial Activity. J. King Saud. Univ. Sci. 2019, 31, 1103–1114. [Google Scholar]
- Buazar, F.; Sweidi, S.; Badri, M.; Kroushawi, F. Biofabrication of Highly Pure Copper Oxide Nanoparticles Using Wheat Seed Extract and Their Catalytic Activity: A Mechanistic Approach. Green. Process. Synth. 2019, 8, 691–702. [Google Scholar]
- Mann, S.; Frankel, R.B.; Blakemore, R.P. Structure, Morphology and Crystal Growth of Bacterial Magnetite. Nature 1984, 310, 405–407. [Google Scholar]
- Shivaji, S.; Madhu, S.; Singh, S. Extracellular Synthesis of Antibacterial Silver Nanoparticles Using Psychrophilic Bacteria. Process Biochem. 2011, 46, 1800–1807. [Google Scholar]
- Sunkar, S.; Nachiyar, C.V. Biogenesis of Antibacterial Silver Nanoparticles Using the Endophytic Bacterium Bacillus Cereus Isolated from Garcinia Xanthochymus. Asian Pac. J. Trop. Biomed. 2012, 2, 953–959. [Google Scholar]
- Wen, L.; Lin, Z.; Gu, P.; Zhou, J.; Yao, B.; Chen, G.; Fu, J. Extracellular Biosynthesis of Monodispersed Gold Nanoparticles by a SAM Capping Route. J. Nanoparticle Res. 2009, 11, 279–288. [Google Scholar]
- Southam, G.; Beveridge, T.J. The in Vitro Formation of Placer Gold by Bacteria. Geochim. Et Cosmochim. Acta 1994, 58, 4527–4530. [Google Scholar]
- Chen, Y.-L.; Tuan, H.-Y.; Tien, C.-W.; Lo, W.-H.; Liang, H.-C.; Hu, Y.-C. Augmented Biosynthesis of Cadmium Sulfide Nanoparticles by Genetically Engineered Escherichia Coli. Biotechnol. Prog. 2009, 25, 1260–1266. [Google Scholar]
- Du, L.; Jiang, H.; Liu, X.; Wang, E. Biosynthesis of Gold Nanoparticles Assisted by Escherichia Coli DH5α and Its Application on Direct Electrochemistry of Hemoglobin. Electrochem. Commun. 2007, 9, 1165–1170. [Google Scholar]
- Holmes, J.D.; Smith, P.R.; Evans-Gowing, R.; Richardson, D.J.; Russell, D.A.; Sodeau, J.R. Energy-Dispersive X-Ray Analysis of the Extracellular Cadmium Sulfide Crystallites of Klebsiella Aerogenes. Arch. Microbiol. 1995, 163, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Korbekandi, H.; Iravani, S.; Abbasi, S. Optimization of Biological Synthesis of Silver Nanoparticles Using Lactobacillus Casei Subsp. Casei. J. Chem. Technol. Biotechnol. 2012, 87, 932–937. [Google Scholar] [CrossRef]
- Philipse, A.P.; Maas, D. Magnetic Colloids from Magnetotactic Bacteria: Chain Formation and Colloidal Stability. Langmuir 2002, 18, 9977–9984. [Google Scholar] [CrossRef]
- Lengke, M.F.; Fleet, M.E.; Southam, G. Morphology of Gold Nanoparticles Synthesized by Filamentous Cyanobacteria from Gold (I)−Thiosulfate and Gold (III)−Chloride Complexes. Langmuir 2006, 22, 2780–2787. [Google Scholar] [CrossRef]
- He, S.; Guo, Z.; Zhang, Y.; Zhang, S.; Wang, J.; Gu, N. Biosynthesis of Gold Nanoparticles Using the Bacteria Rhodopseudomonas Capsulata. Mater. Lett. 2007, 61, 3984–3987. [Google Scholar] [CrossRef]
- Singh, D.; Jain, D.; Rajpurohit, D.; Jat, G.; Kushwaha, H.S.; Singh, A.; Mohanty, S.R.; Al-Sadoon, M.K.; Zaman, W.; Upadhyay, S.K. Bacteria Assisted Green Synthesis of Copper Oxide Nanoparticles and Their Potential Applications as Antimicrobial Agents and Plant Growth Stimulants. Front. Chem. 2023, 11, 1154128. [Google Scholar] [CrossRef]
- Marshall, M.J.; Beliaev, A.S.; Dohnalkova, A.C.; Kennedy, D.W.; Shi, L.; Wang, Z.; Boyanov, M.I.; Lai, B.; Kemner, K.M.; McLean, J.S. C-Type Cytochrome-Dependent Formation of U (IV) Nanoparticles by Shewanella Oneidensis. PLoS Biol. 2006, 4, e268. [Google Scholar] [CrossRef]
- Konishi, Y.; Tsukiyama, T.; Tachimi, T.; Saitoh, N.; Nomura, T.; Nagamine, S. Microbial Deposition of Gold Nanoparticles by the Metal-Reducing Bacterium Shewanella Algae. Electrochim. Acta 2007, 53, 186–192. [Google Scholar] [CrossRef]
- Gajbhiye, M.; Kesharwani, J.; Ingle, A.; Gade, A.; Rai, M. Fungus-Mediated Synthesis of Silver Nanoparticles and Their Activity against Pathogenic Fungi in Combination with Fluconazole. Nanomed. Nanotechnol. Biol. Med. 2009, 5, 382–386. [Google Scholar] [CrossRef]
- Vigneshwaran, N.; Kathe, A.A.; Varadarajan, P.V.; Nachane, R.P.; Balasubramanya, R.H. Biomimetics of Silver Nanoparticles by White Rot Fungus, Phaenerochaete Chrysosporium. Colloids Surf. B Biointerfaces 2006, 53, 55–59. [Google Scholar] [CrossRef]
- Raliya, R.; Biswas, P.; Tarafdar, J.C. TiO2 Nanoparticle Biosynthesis and Its Physiological Effect on Mung Bean (Vigna radiata L.). Biotechnol. Rep. 2015, 5, 22–26. [Google Scholar] [CrossRef] [PubMed]
- Bhainsa, K.C.; D’souza, S.F. Extracellular Biosynthesis of Silver Nanoparticles Using the Fungus Aspergillus Fumigatus. Colloids Surf. B Biointerfaces 2006, 47, 160–164. [Google Scholar] [PubMed]
- Gade, A.K.; Bonde, P.; Ingle, A.P.; Marcato, P.D.; Duran, N.; Rai, M.K. Exploitation of Aspergillus Niger for Synthesis of Silver Nanoparticles. J. Biobased Mater. Bioenergy 2008, 2, 243–247. [Google Scholar]
- Raliya, R.; Tarafdar, J.C. Biosynthesis and Characterization of Zinc, Magnesium and Titanium Nanoparticles: An Eco-Friendly Approach. Int. Nano Lett. 2014, 4, 93. [Google Scholar] [CrossRef]
- Sanghi, R.; Verma, P. Biomimetic Synthesis and Characterisation of Protein Capped Silver Nanoparticles. Bioresour. Technol. 2009, 100, 501–504. [Google Scholar] [CrossRef]
- Balaji, D.S.; Basavaraja, S.; Deshpande, R.; Mahesh, D.B.; Prabhakar, B.K.; Venkataraman, A. Extracellular Biosynthesis of Functionalized Silver Nanoparticles by Strains of Cladosporium Cladosporioides Fungus. Colloids Surf. B Biointerfaces 2009, 68, 88–92. [Google Scholar] [CrossRef]
- Senapati, S.; Ahmad, A.; Khan, M.I.; Sastry, M.; Kumar, R. Extracellular Biosynthesis of Bimetallic Au–Ag Alloy Nanoparticles. Small 2005, 1, 517–520. [Google Scholar] [CrossRef]
- Basavaraja, S.; Balaji, S.D.; Lagashetty, A.; Rajasab, A.H.; Venkataraman, A. Extracellular Biosynthesis of Silver Nanoparticles Using the Fungus Fusarium Semitectum. Mater. Res. Bull. 2008, 43, 1164–1170. [Google Scholar]
- Ingle, A.; Rai, M.; Gade, A.; Bawaskar, M. Fusarium Solani: A Novel Biological Agent for the Extracellular Synthesis of Silver Nanoparticles. J. Nanoparticle Res. 2009, 11, 2079–2085. [Google Scholar] [CrossRef]
- Shaligram, N.S.; Bule, M.; Bhambure, R.; Singhal, R.S.; Singh, S.K.; Szakacs, G.; Pandey, A. Biosynthesis of Silver Nanoparticles Using Aqueous Extract from the Compactin Producing Fungal Strain. Process Biochem. 2009, 44, 939–943. [Google Scholar]
- Kathiresan, K.; Manivannan, S.; Nabeel, M.A.; Dhivya, B. Studies on Silver Nanoparticles Synthesized by a Marine Fungus, Penicillium Fellutanum Isolated from Coastal Mangrove Sediment. Colloids Surf. B Biointerfaces 2009, 71, 133–137. [Google Scholar]
- Vigneshwaran, N.; Ashtaputre, N.M.; Varadarajan, P.V.; Nachane, R.P.; Paralikar, K.M.; Balasubramanya, R.H. Biological Synthesis of Silver Nanoparticles Using the Fungus Aspergillus Flavus. Mater. Lett. 2007, 61, 1413–1418. [Google Scholar]
- Birla, S.S.; Tiwari, V.V.; Gade, A.K.; Ingle, A.P.; Yadav, A.P.; Rai, M.K. Fabrication of Silver Nanoparticles by Phoma Glomerata and Its Combined Effect against Escherichia Coli, Pseudomonas Aeruginosa and Staphylococcus Aureus. Lett. Appl. Microbiol. 2009, 48, 173–179. [Google Scholar]
- Ravindra, B.K.; Rajasab, A.H. A Comparative Study on Biosynthesis of Silver Nanoparticles Using Four Different Fungal Species. Int. J. Pharm. Pharm. Sci. 2014, 6, 372–376. [Google Scholar]
- Binupriya, A.R.; Sathishkumar, M.; Yun, S.-I. Biocrystallization of Silver and Gold Ions by Inactive Cell Filtrate of Rhizopus Stolonifer. Colloids Surf. B Biointerfaces 2010, 79, 531–534. [Google Scholar] [PubMed]
- Mourato, A.; Gadanho, M.; Lino, A.R.; Tenreiro, R. Biosynthesis of Crystalline Silver and Gold Nanoparticles by Extremophilic Yeasts. Bioinorg. Chem. Appl. 2011, 2011, 546074. [Google Scholar]
- Fayaz, A.M.; Balaji, K.; Girilal, M.; Yadav, R.; Kalaichelvan, P.T.; Venketesan, R. Biogenic Synthesis of Silver Nanoparticles and Their Synergistic Effect with Antibiotics: A Study against Gram-Positive and Gram-Negative Bacteria. Nanomed. Nanotechnol. Biol. Med. 2010, 6, 103–109. [Google Scholar]
- Ahmad, A.; Senapati, S.; Khan, M.I.; Kumar, R.; Sastry, M. Extra-/Intracellular Biosynthesis of Gold Nanoparticles by an Alkalotolerant Fungus, Trichothecium sp. J. Biomed. Nanotechnol. 2005, 1, 47–53. [Google Scholar]
- Mukherjee, P.; Ahmad, A.; Mandal, D.; Senapati, S.; Sainkar, S.R.; Khan, M.I.; Parishcha, R.; Ajaykumar, P.V.; Alam, M.; Kumar, R. Fungus-Mediated Synthesis of Silver Nanoparticles and Their Immobilization in the Mycelial Matrix: A Novel Biological Approach to Nanoparticle Synthesis. Nano Lett. 2001, 1, 515–519. [Google Scholar]
- Gericke, M.; Pinches, A. Microbial Production of Gold Nanoparticles. Gold. Bull. 2006, 39, 22–28. [Google Scholar]
- Abboud, Y.; Saffaj, T.; Chagraoui, A.; El Bouari, A.; Brouzi, K.; Tanane, O.; Ihssane, B. Biosynthesis, Characterization and Antimicrobial Activity of Copper Oxide Nanoparticles (CONPs) Produced Using Brown Alga Extract (Bifurcaria Bifurcata). Appl. Nanosci. 2014, 4, 571–576. [Google Scholar] [CrossRef]
- Kathiraven, T.; Sundaramanickam, A.; Shanmugam, N.; Balasubramanian, T. Green Synthesis of Silver Nanoparticles Using Marine Algae Caulerpa Racemosa and Their Antibacterial Activity against Some Human Pathogens. Appl. Nanosci. 2015, 5, 499–504. [Google Scholar] [CrossRef]
- Kannan, R.R.R.; Arumugam, R.; Ramya, D.; Manivannan, K.; Anantharaman, P. Green Synthesis of Silver Nanoparticles Using Marine Macroalga Chaetomorpha Linum. Appl. Nanosci. 2013, 3, 229–233. [Google Scholar] [CrossRef]
- Barwal, I.; Ranjan, P.; Kateriya, S.; Yadav, S.C. Cellular Oxido-Reductive Proteins of Chlamydomonas reinhardtii Control the Biosynthesis of Silver Nanoparticles. J. Nanobiotechnology 2011, 9, 56. [Google Scholar] [CrossRef] [PubMed]
- Annamalai, J.; Nallamuthu, T. Characterization of Biosynthesized Gold Nanoparticles from Aqueous Extract of Chlorella Vulgaris and Their Anti-Pathogenic Properties. Appl. Nanosci. 2015, 5, 603–607. [Google Scholar] [CrossRef]
- El-Rafie, H.M.; El-Rafie, M.; Zahran, M.K. Green Synthesis of Silver Nanoparticles Using Polysaccharides Extracted from Marine Macro Algae. Carbohydr. Polym. 2013, 96, 403–410. [Google Scholar] [CrossRef]
- Prasad, T.N.; Kambala, V.S.R.; Naidu, R. Phyconanotechnology: Synthesis of Silver Nanoparticles Using Brown Marine Algae Cystophora Moniliformis and Their Characterisation. J. Appl. Phycol. 2013, 25, 177–182. [Google Scholar] [CrossRef]
- Venkatesan, J.; Manivasagan, P.; Kim, S.-K.; Kirthi, A.V.; Marimuthu, S.; Rahuman, A.A. Marine Algae-Mediated Synthesis of Gold Nanoparticles Using a Novel Ecklonia Cava. Bioprocess. Biosyst. Eng. 2014, 37, 1591–1597. [Google Scholar] [CrossRef] [PubMed]
- Yousefzadi, M.; Rahimi, Z.; Ghafori, V. The Green Synthesis, Characterization and Antimicrobial Activities of Silver Nanoparticles Synthesized from Green Alga Enteromorpha flexuosa (wulfen) J. Agardh. Mater. Lett. 2014, 137, 1–4. [Google Scholar] [CrossRef]
- Francavilla, M.; Pineda, A.; Romero, A.A.; Colmenares, J.C.; Vargas, C.; Monteleone, M.; Luque, R. Efficient and Simple Reactive Milling Preparation of Photocatalytically Active Porous ZnO Nanostructures Using Biomass Derived Polysaccharides. Green Chem. 2014, 16, 2876–2885. [Google Scholar] [CrossRef]
- Sharma, B.; Purkayastha, D.D.; Hazra, S.; Thajamanbi, M.; Bhattacharjee, C.R.; Ghosh, N.N.; Rout, J. Biosynthesis of Fluorescent Gold Nanoparticles Using an Edible Freshwater Red Alga, Lemanea fluviatilis (L.) C. Ag. and Antioxidant Activity of Biomatrix Loaded Nanoparticles. Bioprocess. Biosyst. Eng. 2014, 37, 2559–2565. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Kalaivani, R.; Manikandan, S.; Sangeetha, N.; Kumaraguru, A.K. Facile Green Synthesis of Variable Metallic Gold Nanoparticle Using Padina Gymnospora, a Brown Marine Macroalga. Appl. Nanosci. 2013, 3, 145–151. [Google Scholar]
- Sharma, B.; Purkayastha, D.D.; Hazra, S.; Gogoi, L.; Bhattacharjee, C.R.; Ghosh, N.N.; Rout, J. Biosynthesis of Gold Nanoparticles Using a Freshwater Green Alga, Prasiola Crispa. Mater. Lett. 2014, 116, 94–97. [Google Scholar]
- Mahdavi, M.; Namvar, F.; Ahmad, M.B.; Mohamad, R. Green Biosynthesis and Characterization of Magnetic Iron Oxide (Fe3O4) Nanoparticles Using Seaweed (Sargassum Muticum) Aqueous Extract. Molecules 2013, 18, 5954–5964. [Google Scholar] [CrossRef] [PubMed]
- Azizi, S.; Ahmad, M.B.; Namvar, F.; Mohamad, R. Green Biosynthesis and Characterization of Zinc Oxide Nanoparticles Using Brown Marine Macroalga Sargassum Muticum Aqueous Extract. Mater. Lett. 2014, 116, 275–277. [Google Scholar]
- Namvar, F.; Azizi, S.; Ahmad, M.B.; Shameli, K.; Mohamad, R.; Mahdavi, M.; Tahir, P.M. Green Synthesis and Characterization of Gold Nanoparticles Using the Marine Macroalgae Sargassum Muticum. Res. Chem. Intermed. 2015, 41, 5723–5730. [Google Scholar]
- Senapati, S.; Syed, A.; Moeez, S.; Kumar, A.; Ahmad, A. Intracellular Synthesis of Gold Nanoparticles Using Alga Tetraselmis Kochinensis. Mater. Lett. 2012, 79, 116–118. [Google Scholar]
- Fan, L.; Tu, Z.; Chan, S.H. Recent Development of Hydrogen and Fuel Cell Technologies: A Review. Energy Rep. 2021, 7, 8421–8446. [Google Scholar] [CrossRef]
- Sazali, N.; Wan Salleh, W.N.; Jamaludin, A.S.; Mhd Razali, M.N. New Perspectives on Fuel Cell Technology: A Brief Review. Membranes 2020, 10, 99. [Google Scholar] [CrossRef]
- Baroutaji, A.; Wilberforce, T.; Ramadan, M.; Olabi, A.G. Comprehensive Investigation on Hydrogen and Fuel Cell Technology in the Aviation and Aerospace Sectors. Renew. Sustain. Energy Rev. 2019, 106, 31–40. [Google Scholar] [CrossRef]
- Manoharan, Y.; Hosseini, S.E.; Butler, B.; Alzhahrani, H.; Senior, B.T.F.; Ashuri, T.; Krohn, J. Hydrogen Fuel Cell Vehicles; Current Status and Future Prospect. Appl. Sci. 2019, 9, 2296. [Google Scholar] [CrossRef]
- Chaturvedi, S.; Dave, P.N.; Shah, N.K. Applications of Nano-Catalyst in New Era. J. Saudi Chem. Soc. 2012, 16, 307–325. [Google Scholar] [CrossRef]
- Kamali, M.; Aminabhavi, T.M.; Abbassi, R.; Dewil, R.; Appels, L. Engineered Nanomaterials in Microbial Fuel Cells—Recent Developments, Sustainability Aspects, and Future Outlook. Fuel 2022, 310, 122347. [Google Scholar] [CrossRef]
- Macaskie, L.E.; Mikheenko, I.P.; Omajai, J.B.; Stephen, A.J.; Wood, J. Metallic Bionanocatalysts: Potential Applications as Green Catalysts and Energy Materials. Microb. Biotechnol. 2017, 10, 1171–1180. [Google Scholar] [CrossRef]
- Obileke, K.; Onyeaka, H.; Meyer, E.L.; Nwokolo, N. Microbial Fuel Cells, a Renewable Energy Technology for Bio-Electricity Generation: A Mini-Review. Electrochem. Commun. 2021, 125, 107003. [Google Scholar] [CrossRef]
- Stephen, A.J.; Rees, N.V.; Mikheenko, I.; Macaskie, L.E. Platinum and Palladium Bio-Synthesized Nanoparticles as Sustainable Fuel Cell Catalysts. Front. Energy Res. 2019, 7, 66. [Google Scholar]
- Yong, P.; Mikheenko, I.P.; Macaskie, L.E. A Novel Fuel Cell Catalyst for Clean Energy Production Based on a Bionanocatalyst. Adv. Mater. Res. 2007, 20, 655–658. [Google Scholar]
- Sekar, A.D.; Jayabalan, T.; Muthukumar, H.; Chandrasekaran, N.I.; Mohamed, S.N.; Matheswaran, M. Enhancing Power Generation and Treatment of Dairy Waste Water in Microbial Fuel Cell Using Cu-Doped Iron Oxide Nanoparticles Decorated Anode. Energy 2019, 172, 173–180. [Google Scholar]
- Matsena, M.T.; Tichapondwa, S.M.; Chirwa, E.M.N. Synthesis of Biogenic Palladium Nanoparticles Using Citrobacter Sp. for Application as Anode Electrocatalyst in a Microbial Fuel Cell. Catalysts 2020, 10, 838. [Google Scholar] [CrossRef]
- Lee, D.W.; Jang, J.-H.; Jang, I.; Kang, Y.S.; Jang, S.; Lee, K.Y.; Jang, J.H.; Kim, H.-J.; Yoo, S.J. Bio-derived Co2P Nanoparticles Supported on Nitrogen-doped Carbon as Promising Oxygen Reduction Reaction Electrocatalyst for Anion Exchange Membrane Fuel Cells. Small 2019, 15, 1902090. [Google Scholar]
- Fuku, X.; Modibedi, M.; Matinise, N.; Mokoena, P.; Xaba, N.; Mathe, M. Single Step Synthesis of Bio-Inspired NiO/C as Pd Support Catalyst for Dual Application: Alkaline Direct Ethanol Fuel Cell and CO2 Electro-Reduction. J. Colloid Interface Sci. 2019, 545, 138–152. [Google Scholar] [PubMed]
- Cui, Y.; Chen, X.; Pan, Z.; Wang, Y.; Xu, Q.; Bai, J.; Jia, H.; Zhou, J.; Yong, X.; Wu, X. Biosynthesized Iron Sulfide Nanoparticles by Mixed Consortia for Enhanced Extracellular Electron Transfer in a Microbial Fuel Cell. Bioresour. Technol. 2020, 318, 124095. [Google Scholar]
- Ishak, N.; Kamarudin, S.K.; Timmiati, S.N.; Karim, N.A.; Basri, S. Biogenic Platinum from Agricultural Wastes Extract for Improved Methanol Oxidation Reaction in Direct Methanol Fuel Cell. J. Adv. Res. 2021, 28, 63–75. [Google Scholar] [PubMed]
- Kasturi, P.R.; Arunchander, A.; Kalpana, D.; Selvan, R.K. Bio-Derived Carbon as an Efficient Supporting Electrocatalyst for the Oxygen Reduction Reaction. J. Phys. Chem. Solids 2019, 124, 305–311. [Google Scholar]
- Qian, C.; Guo, X.; Zhang, W.; Yang, H.; Qian, Y.; Xu, F.; Qian, S.; Lin, S.; Fan, T. Co3O4 Nanoparticles on Porous Bio-Carbon Substrate as Catalyst for Oxygen Reduction Reaction. Microporous Mesoporous Mater. 2019, 277, 45–51. [Google Scholar]
- Wang, A.Z.; Gu, F.; Zhang, L.; Chan, J.M.; Radovic-Moreno, A.; Shaikh, M.R.; Farokhzad, O.C. Biofunctionalized Targeted Nanoparticles for Therapeutic Applications. Expert. Opin. Biol. Ther. 2008, 8, 1063–1070. [Google Scholar] [CrossRef]
- Hu, B.; Liu, X.; Zhang, C.; Zeng, X. Food Macromolecule Based Nanodelivery Systems for Enhancing the Bioavailability of Polyphenols. J. Food Drug Anal. 2017, 25, 3–15. [Google Scholar] [CrossRef]
- Kumar, H.; Bhardwaj, K.; Nepovimova, E.; Kuča, K.; Singh Dhanjal, D.; Bhardwaj, S.; Bhatia, S.K.; Verma, R.; Kumar, D. Antioxidant Functionalized Nanoparticles: A Combat against Oxidative Stress. Nanomaterials 2020, 10, 1334. [Google Scholar] [CrossRef]
- Li, Y.; Zheng, X.; Chu, Q. Bio-Based Nanomaterials for Cancer Therapy. Nano Today 2021, 38, 101134. [Google Scholar] [CrossRef]
- Andleeb, A.; Andleeb, A.; Asghar, S.; Zaman, G.; Tariq, M.; Mehmood, A.; Nadeem, M.; Hano, C.; Lorenzo, J.M.; Abbasi, B.H. A Systematic Review of Biosynthesized Metallic Nanoparticles as a Promising Anti-Cancer-Strategy. Cancers 2021, 13, 2818. [Google Scholar] [CrossRef]
- Calhelha, R.C.; Haddad, H.; Ribeiro, L.; Heleno, S.A.; Carocho, M.; Barros, L. Inflammation: What’s There and What’s New? Appl. Sci. 2023, 13, 2312. [Google Scholar] [CrossRef]
- Agarwal, H.; Nakara, A.; Shanmugam, V.K. Anti-Inflammatory Mechanism of Various Metal and Metal Oxide Nanoparticles Synthesized Using Plant Extracts: A Review. Biomed. Pharmacother. 2019, 109, 2561–2572. [Google Scholar] [CrossRef] [PubMed]
- Saha, M.; Sarkar, A. Review on Multiple Facets of Drug Resistance: A Rising Challenge in the 21st Century. J. Xenobiotics 2021, 11, 197–214. [Google Scholar] [CrossRef]
- Madubuonu, N.; Aisida, S.O.; Ahmad, I.; Botha, S.; Zhao, T.; Maaza, M.; Ezema, F.I. Bio-Inspired Iron Oxide Nanoparticles Using Psidium Guajava Aqueous Extract for Antibacterial Activity. Appl. Phys. A 2020, 126, 72. [Google Scholar] [CrossRef]
- Xu, C.; Qiao, L.; Guo, Y.; Ma, L.; Cheng, Y. Preparation, Characteristics and Antioxidant Activity of Polysaccharides and Proteins-Capped Selenium Nanoparticles Synthesized by Lactobacillus Casei ATCC 393. Carbohydr. Polym. 2018, 195, 576–585. [Google Scholar] [CrossRef]
- Oladipo, I.C.; Lateef, A.; Elegbede, J.A.; Azeez, M.A.; Asafa, T.B.; Yekeen, T.A.; Akinboro, A.; Gueguim-Kana, E.B.; Beukes, L.S.; Oluyide, T.O. Enterococcus Species for the One-Pot Biofabrication of Gold Nanoparticles: Characterization and Nanobiotechnological Applications. J. Photochem. Photobiol. B Biol. 2017, 173, 250–257. [Google Scholar] [CrossRef]
- Netala, V.R.; Kotakadi, V.S.; Bobbu, P.; Gaddam, S.A.; Tartte, V. Endophytic Fungal Isolate Mediated Biosynthesis of Silver Nanoparticles and Their Free Radical Scavenging Activity and Anti Microbial Studies. 3 Biotech. 2016, 6, 132. [Google Scholar] [CrossRef]
- Joshi, C.G.; Danagoudar, A.; Poyya, J.; Kudva, A.K.; Dhananjaya, B.L. Biogenic Synthesis of Gold Nanoparticles by Marine Endophytic Fungus-Cladosporium Cladosporioides Isolated from Seaweed and Evaluation of Their Antioxidant and Antimicrobial Properties. Process Biochem. 2017, 63, 137–144. [Google Scholar]
- Firdhouse, J.; Lalitha, P. Apoptotic Efficacy of Biogenic Silver Nanoparticles on Human Breast Cancer MCF-7 Cell Lines. Progress. Biomater. 2015, 4, 113. [Google Scholar]
- Prabhu, D.; Arulvasu, C.; Babu, G.; Manikandan, R.; Srinivasan, P. Biologically Synthesized Green Silver Nanoparticles from Leaf Extract of Vitex Negundo L. Induce Growth-Inhibitory Effect on Human Colon Cancer Cell Line HCT15. Process Biochem. 2013, 48, 317–324. [Google Scholar] [CrossRef]
- KS, U.S.; Govindaraju, K.; Prabhu, D.; Arulvasu, C.; Karthick, V.; Changmai, N. Anti-Proliferative Effect of Biogenic Gold Nanoparticles against Breast Cancer Cell Lines (MDA-MB-231 & MCF-7). Appl. Surf. Sci. 2016, 371, 415–424. [Google Scholar]
- Sulaiman, G.M.; Tawfeeq, A.T.; Jaaffer, M.D. Biogenic Synthesis of Copper Oxide Nanoparticles Using Olea Europaea Leaf Extract and Evaluation of Their Toxicity Activities: An in Vivo and in Vitro Study. Biotechnol. Prog. 2018, 34, 218–230. [Google Scholar] [CrossRef]
- Noor, S.; Shah, Z.; Javed, A.; Ali, A.; Hussain, S.B.; Zafar, S.; Ali, H.; Muhammad, S.A. A Fungal Based Synthesis Method for Copper Nanoparticles with the Determination of Anticancer, Antidiabetic and Antibacterial Activities. J. Microbiol. Methods 2020, 174, 105966. [Google Scholar]
- David, L.; Moldovan, B.; Vulcu, A.; Olenic, L.; Perde-Schrepler, M.; Fischer-Fodor, E.; Florea, A.; Crisan, M.; Chiorean, I.; Clichici, S. Green Synthesis, Characterization and Anti-Inflammatory Activity of Silver Nanoparticles Using European Black Elderberry Fruits Extract. Colloids Surf. B Biointerfaces 2014, 122, 767–777. [Google Scholar]
- Moldovan, B.; David, L.; Vulcu, A.; Olenic, L.; Perde-Schrepler, M.; Fischer-Fodor, E.; Baldea, I.; Clichici, S.; Filip, G.A. In Vitro and in Vivo Anti-Inflammatory Properties of Green Synthesized Silver Nanoparticles Using Viburnum opulus L. Fruits Extract. Mater. Sci. Eng. C 2017, 79, 720–727. [Google Scholar]
- Muniyappan, N.; Nagarajan, N.S. Green Synthesis of Silver Nanoparticles with Dalbergia Spinosa Leaves and Their Applications in Biological and Catalytic Activities. Process Biochem. 2014, 49, 1054–1061. [Google Scholar] [CrossRef]
- Islam, N.U.; Amin, R.; Shahid, M.; Amin, M.; Zaib, S.; Iqbal, J. A Multi-Target Therapeutic Potential of Prunus Domestica Gum Stabilized Nanoparticles Exhibited Prospective Anticancer, Antibacterial, Urease-Inhibition, Anti-Inflammatory and Analgesic Properties. BMC Complement. Altern. Med. 2017, 17, 276. [Google Scholar] [CrossRef]
- Krithika, S.; Niraimathi, K.L.; Arun, K.P.; Narendran, R.; Balaji, K.; Brindha, P. In Vitro Anti-Inflammatory Studies on Silver Nanoparticles Synthesized from Centratherum Punctatum Cass. Int. J. Res. Ayurveda Pharm. 2016, 7, 61–66. [Google Scholar] [CrossRef]
- Aref, M.S.; Salem, S.S. Bio-Callus Synthesis of Silver Nanoparticles, Characterization, and Antibacterial Activities via Cinnamomum Camphora Callus Culture. Biocatal. Agric. Biotechnol. 2020, 27, 101689. [Google Scholar]
- Alsamhary, K.I. Eco-Friendly Synthesis of Silver Nanoparticles by Bacillus Subtilis and Their Antibacterial Activity. Saudi J. Biol. Sci. 2020, 27, 2185–2191. [Google Scholar]
- Coelho, N.; Jacinto, J.P.; Silva, R.; Soares, J.C.; Pereira, A.S.; Tavares, P. Green Synthesis and Antibacterial Activity of Silver Nanoparticles Obtained from Moringa Oleifera Seed Cake. Coatings 2023, 13, 1439. [Google Scholar] [CrossRef]
- Al Moudani, N.; Ouahidi, I.; Laaraj, S.; Aarab, L. Silver Bio-Nanoparticles Synthesis from Tetraclinis Articulata Leaves Extract and Their Anti-Inflammatory, Antioxidant, and Cytotoxicity Activities. ChemistrySelect 2025, 10, e202404860. [Google Scholar] [CrossRef]
- Kumar, B.; Smita, K.; Kumar, B. Phytochemical Functionalized Metal Nanocatalyst (Ag, Au, Fe, Zn and Pd) for Remediation of Organic Dyes. In Advances in Chemistry Research; Nova Science Publishers: Hauppauge, NY, USA, 2017; pp. 87–120. ISBN 978-1-5361-1054-8. [Google Scholar]
- Sharma, M.; Jain, T.; Singh, S.; Pandey, O.P. Photocatalytic Degradation of Organic Dyes under UV–Visible Light Using Capped ZnS Nanoparticles. Sol. Energy 2012, 86, 626–633. [Google Scholar] [CrossRef]
- Safavi, A.; Momeni, S. Highly Efficient Degradation of Azo Dyes by Palladium/Hydroxyapatite/Fe3O4 Nanocatalyst. J. Hazard. Mater. 2012, 201–202, 125–131. [Google Scholar] [CrossRef]
- Kumar, B. Green Synthesis of Gold, Silver, and Iron Nanoparticles for the Degradation of Organic Pollutants in Wastewater. J. Compos. Sci. 2021, 5, 219. [Google Scholar] [CrossRef]
- Ghosh, N.; Das, S.; Biswas, G.; Haldar, P.K. Review on Some Metal Oxide Nanoparticles as Effective Adsorbent in Wastewater Treatment. Water Sci. Technol. 2022, 85, 3370–3395. [Google Scholar] [CrossRef]
- Gupta, K.; Joshi, P.; Gusain, R.; Khatri, O.P. Recent Advances in Adsorptive Removal of Heavy Metal and Metalloid Ions by Metal Oxide-Based Nanomaterials. Coord. Chem. Rev. 2021, 445, 214100. [Google Scholar] [CrossRef]
- Eldoma, M.A.; Alaswad, S.O.; Mahmoud, M.A.; Qudsieh, I.Y.; Hassan, M.; Bakather, O.Y.; Elawadi, G.A.; Abouatiaa, A.F.F.; Alomar, M.S.; Elhassan, M.S.; et al. Enhancing Photocatalytic Performance of Co-TiO2 and Mo-TiO2-Based Catalysts through Defect Engineering and Doping: A Study on the Degradation of Organic Pollutants under UV Light. J. Photochem. Photobiol. A Chem. 2024, 446, 115164. [Google Scholar] [CrossRef]
- Balestri, A.; Cardellini, J.; Berti, D. Gold and Silver Nanoparticles as Tools to Combat Multidrug-Resistant Pathogens. Curr. Opin. Colloid. Interface Sci. 2023, 66, 101710. [Google Scholar] [CrossRef]
- Rafique, M.; Tahir, M.B.; Irshad, M.; Nabi, G.; Gillani, S.S.A.; Iqbal, T.; Mubeen, M. Novel Citrus Aurantifolia Leaves Based Biosynthesis of Copper Oxide Nanoparticles for Environmental and Wastewater Purification as an Efficient Photocatalyst and Antibacterial Agent. Optik 2020, 219, 165138. [Google Scholar]
- Arumugam, V.; Sriram, P.; Yen, T.-J.; Redhi, G.G.; Gengan, R.M. Nano-Material as an Excellent Catalyst for Reducing a Series of Nitroanilines and Dyes: Triphosphonated Ionic Liquid-CuFe2O4-Modified Boron Nitride. Appl. Catal. B Environ. 2018, 222, 99–114. [Google Scholar]
- Sebeia, N.; Jabli, M.; Ghith, A. Biological Synthesis of Copper Nanoparticles, Using Nerium Oleander Leaves Extract: Characterization and Study of Their Interaction with Organic Dyes. Inorg. Chem. Commun. 2019, 105, 36–46. [Google Scholar]
- Varshney, S.; Gupta, A. Forest Industrial Biomass Residue-Mediated Green Synthesized Multifunctional Copper Oxide Nanoparticles for Efficient Wastewater Treatment and Biomedical Applications. J. Clean. Prod. 2024, 434, 140109. [Google Scholar] [CrossRef]
- Eid, A.M.; Fouda, A.; Hassan, S.E.-D.; Hamza, M.F.; Alharbi, N.K.; Elkelish, A.; Alharthi, A.; Salem, W.M. Plant-Based Copper Oxide Nanoparticles; Biosynthesis, Characterization, Antibacterial Activity, Tanning Wastewater Treatment, and Heavy Metals Sorption. Catalysts 2023, 13, 348. [Google Scholar] [CrossRef]
- Fatima, B.; Siddiqui, S.; Ahmed, R.; Chaudhry, S.A. Preparation of Functionalized CuO Nanoparticles Using Brassica Rapa Leave Extract for Water Purification. Desalin. Water Treat. 2019, 164, 192–205. [Google Scholar]
- Vasantharaj, S.; Sathiyavimal, S.; Senthilkumar, P.; Kalpana, V.N.; Rajalakshmi, G.; Alsehli, M.; Elfasakhany, A.; Pugazhendhi, A. Enhanced Photocatalytic Degradation of Water Pollutants Using Bio-Green Synthesis of Zinc Oxide Nanoparticles (ZnO NPs). J. Environ. Chem. Eng. 2021, 9, 105772. [Google Scholar]
- Rambabu, K.; Bharath, G.; Banat, F.; Show, P.L. Green Synthesis of Zinc Oxide Nanoparticles Using Phoenix dactylifera Waste as Bioreductant for Effective Dye Degradation and Antibacterial Performance in Wastewater Treatment. J. Hazard. Mater. 2021, 402, 123560. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, A.K.; Kataria, N.; Garg, V.K. Green Fabrication of ZnO Nanoparticles Using Eucalyptus Spp. Leaves Extract and Their Application in Wastewater Remediation. Chemosphere 2020, 247, 125803. [Google Scholar] [CrossRef] [PubMed]
- Kumar, B.; Smita, K.; Debut, A.; Cumbal, L. Utilization of Persea Americana (Avocado) Oil for the Synthesis of Gold Nanoparticles in Sunlight and Evaluation of Antioxidant and Photocatalytic Activities. Environ. Nanotechnol. Monit. Manag. 2018, 10, 231–237. [Google Scholar]
- Baruah, D.; Goswami, M.; Yadav, R.N.S.; Yadav, A.; Das, A.M. Biogenic Synthesis of Gold Nanoparticles and Their Application in Photocatalytic Degradation of Toxic Dyes. J. Photochem. Photobiol. B Biol. 2018, 186, 51–58. [Google Scholar]
- Sharma, P.; Pant, S.; Rai, S.; Yadav, R.B.; Dave, V. Green Synthesis of Silver Nanoparticle Capped with Allium Cepa and Their Catalytic Reduction of Textile Dyes: An Ecofriendly Approach. J. Polym. Environ. 2018, 26, 1795–1803. [Google Scholar]
- Rather, M.Y.; Sundarapandian, S. Magnetic Iron Oxide Nanorod Synthesis by Wedelia Urticifolia (Blume) DC. Leaf Extract for Methylene Blue Dye Degradation. Appl. Nanosci. 2020, 10, 2219–2227. [Google Scholar]
- Lohrasbi, S.; Kouhbanani, M.A.J.; Beheshtkhoo, N.; Ghasemi, Y.; Amani, A.M.; Taghizadeh, S. Green Synthesis of Iron Nanoparticles Using Plantago Major Leaf Extract and Their Application as a Catalyst for the Decolorization of Azo Dye. BioNanoScience 2019, 9, 317–322. [Google Scholar]
- El Golli, A.; Contreras, S.; Dridi, C. Bio-Synthesized ZnO Nanoparticles and Sunlight-Driven Photocatalysis for Environmentally-Friendly and Sustainable Route of Synthetic Petroleum Refinery Wastewater Treatment. Sci. Rep. 2023, 13, 20809. [Google Scholar] [CrossRef]
- Yusuf, M.; Abdullah, B. Fossil Fuels, Rising Population, and Global Warming: The Interlinked Phenomena. Orient. J. Phys. Sci. 2020, 5, 49–52. [Google Scholar] [CrossRef]
- Fang, H. Analysis of the Causes and Crisis of Global Warming. MATEC Web Conf. 2023, 386, 03018. [Google Scholar] [CrossRef]
- Ghosh, S.; Ghosh, B. 160-Fossil Fuel Consumption Trend and Global Warming Scenario: Energy Overview. Glob. J. Eng. Sci. 2020, 5, 1–5. [Google Scholar] [CrossRef]
- Akram, F.; Saleem, B.; Irfan, M.; Shakir, H.A.; Khan, M.; Ali, S.; Saeed, S.; Mehmood, T.; Franco, M. Recent Trends for Production of Biofuels Using Algal Biomass. In Basic Research Advancement for Algal Biofuels Production; Srivastava, N., Mishra, P.K., Eds.; Springer: Singapore, 2023; pp. 27–58. ISBN 978-981-19-6810-5. [Google Scholar]
- Sanju, S.; Thakur, A.; Misra, P.; Shukla, P.K. Algal Biomass and Biofuel Production. In Bioprospecting of Microorganism-Based Industrial Molecules; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2021; pp. 357–376. ISBN 978-1-119-71731-7. [Google Scholar]
- Dabirian, E.; Hajipour, A.; Mehrizi, A.A.; Karaman, C.; Karimi, F.; Loke-Show, P.; Karaman, O. Nanoparticles Application on Fuel Production from Biological Resources: A Review. Fuel 2023, 331, 125682. [Google Scholar] [CrossRef]
- Jayabal, R.; Soundararajan, G.; Kumar, R.A.; Choubey, G.; Devarajan, Y.; Raja, T.; Kaliappan, N. Study of the Effects of Bio-Silica Nanoparticle Additives on the Performance, Combustion, and Emission Characteristics of Biodiesel Produced from Waste Fat. Sci. Rep. 2023, 13, 18907. [Google Scholar] [CrossRef]
- Karpagam, R.; Rani, K.; Ashokkumar, B.; Ganesh Moorthy, I.; Dhakshinamoorthy, A.; Varalakshmi, P. Green Energy from Coelastrella sp. M-60: Bio-Nanoparticles Mediated Whole Biomass Transesterification for Biodiesel Production. Fuel 2020, 279, 118490. [Google Scholar] [CrossRef]
- Lahiri, D.; Nag, M.; Ghosh, S.; Ray, R.R. Chapter 8—Green Synthesis of Nanoparticles and Their Applications in the Area of Bioenergy and Biofuel Production. In Nanomaterials; Kumar, R.P., Bharathiraja, B., Eds.; Academic Press: Cambridge, MA, USA, 2021; pp. 195–219. ISBN 978-0-12-822401-4. [Google Scholar]
- Ettefaghi, E.; Ghobadian, B.; Rashidi, A.; Najafi, G.; Khoshtaghaza, M.H.; Rashtchi, M.; Sadeghian, S. A Novel Bio-Nano Emulsion Fuel Based on Biodegradable Nanoparticles to Improve Diesel Engines Performance and Reduce Exhaust Emissions. Renew. Energy 2018, 125, 64–72. [Google Scholar] [CrossRef]
- Duman, F.; Sahin, U.; Atabani, A.E. Harvesting of Blooming Microalgae Using Green Synthetized Magnetic Maghemite (γ-Fe2O3) Nanoparticles for Biofuel Production. Fuel 2019, 256, 115935. [Google Scholar] [CrossRef]
- Sawaira; Alsaiari, M.; Ahmad, M.; Munir, M.; Zafar, M.; Sultana, S.; Dawood, S.; Almohana, A.I.; Hassan, M.H.A.-M.; Alharbi, A.F.; et al. Efficient Application of Newly Synthesized Green Bi2O3 Nanoparticles for Sustainable Biodiesel Production via Membrane Reactor. Chemosphere 2023, 310, 136838. [Google Scholar] [CrossRef]
- Hazmi, B.; Rashid, U.; Taufiq-Yap, Y.H.; Ibrahim, M.L.; Nehdi, I.A. Supermagnetic Nano-Bifunctional Catalyst from Rice Husk: Synthesis, Characterization and Application for Conversion of Used Cooking Oil to Biodiesel. Catalysts 2020, 10, 225. [Google Scholar] [CrossRef]
- Pandit, P.R.; Fulekar, M.H. Egg Shell Waste as Heterogeneous Nanocatalyst for Biodiesel Production: Optimized by Response Surface Methodology. J. Environ. Manag. 2017, 198, 319–329. [Google Scholar] [CrossRef]
- Umeagukwu, O.E.; Onukwuli, D.O.; Ude, C.N.; Esonye, C.; Ekwueme, B.N.; Asadu, C.O.; Okey-Onyesolu, F.C.; Ikenna, M.U.; Chukwudi, E.I.; Makhkamov, T.; et al. Transesterification of Persea Americana Seed Oil to Methyl Ester Using Bio-Based Heterogeneous Catalyst: Optimization and Techno-Economic Analysis. Green. Technol. Sustain. 2024, 2, 100086. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).