Comparison of the Level and Mechanisms of Toxicity of Nanoparticles of Underwater Welding in Bioassay with Three Marine Microalgae
Abstract
1. Introduction
2. Materials and Methods
2.1. Seawater Samples for Underwater Welding
2.2. Microalgae Culture
2.3. Experimental Design and Sample Preparation
2.4. Flow Cytometry: Cell Count, Staining Protocols, and Post Processing
2.5. Statistical Analysis
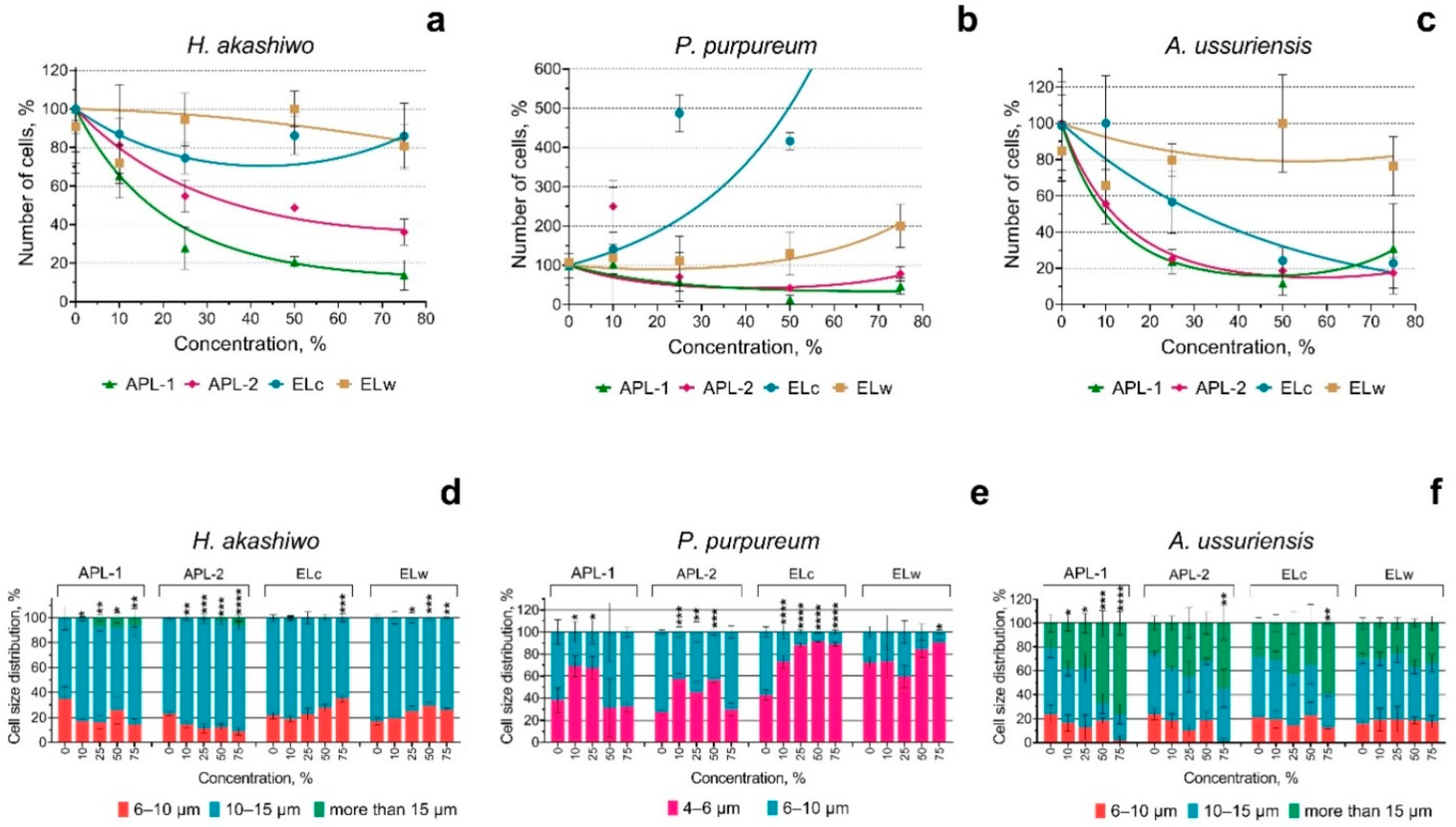
3. Results
4. Discussion
4.1. Toxicity of Welding Suspensions
4.2. Change in Membrane Potential
4.3. Environmental Impacts
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A
| H. akashiwo | ||||||||
|---|---|---|---|---|---|---|---|---|
| Concentration | APL-1 | APL-2 | ELc | ELw | ||||
| Summary | p Value | Summary | p Value | Summary | p Value | Summary | p Value | |
| 10 | * | 0.0147 | ns | 0.3119 | ns | 0.7163 | ns | 0.0550 |
| 25 | **** | <0.0001 | ** | 0.0051 | ns | 0.2108 | ns | 0.9582 |
| 50 | **** | <0.0001 | ** | 0.0019 | ns | 0.6740 | ns | 0.5088 |
| 75 | **** | <0.0001 | *** | 0.0003 | ns | 0.6650 | ns | 0.4341 |
| P. purpureum | ||||||||
| Concentration | APL-1 | APL-2 | ELc | ELw | ||||
| Summary | p Value | Summary | p Value | Summary | p Value | Summary | p Value | |
| 10 | ns | 0.9979 | ** | 0.0012 | ns | 0.9985 | ns | 0.9998 |
| 25 | ns | 0.1248 | ns | 0.7427 | ns | 0.1386 | ns | >0.9999 |
| 50 | *** | 0.0005 | ns | 0.2183 | ns | 0.3576 | ns | 0.9971 |
| 75 | * | 0.0258 | ns | 0.9029 | **** | <0.0001 | ns | 0.7750 |
| A. ussuriensis | ||||||||
| Concentration | APL-1 | APL-2 | ELc | ELw | ||||
| Summary | p Value | Summary | p Value | Summary | p Value | Summary | p Value | |
| 10 | * | 0.0225 | ** | 0.0039 | ns | 0.9999 | ns | 0.3472 |
| 25 | **** | <0.0001 | **** | <0.0001 | * | 0.0302 | ns | 0.9781 |
| 50 | **** | <0.0001 | **** | <0.0001 | *** | 0.0004 | ns | 0.5279 |
| 75 | **** | <0.0001 | **** | <0.0001 | ** | 0.0021 | ns | 0.8829 |
References
- Akhtyamova, D.R.; Khamitov, A.I.; Khamitova, A.R. Environmental damage during construction and operation of pipelines. In Professional communications in the scientific environment—A factor in ensuring the quality of research. In Proceedings of the XI All-Russian Scientific-Practical Conference, St. Petersburg, Russia, 12–16 September 2022; pp. 260–263. [Google Scholar]
- Kirichenko, K.; Elovskiy, E.; Golokhvast, K. Investigation of granulometric and chemical composition of samples of underwater welding. Reliab. Theory Appl. 2022, 17, 337–342. [Google Scholar] [CrossRef]
- Kirichenko, K.Y.; Drozd, V.A.; Chaika, V.V.; Gridasov, A.V.; Kholodov, A.S.; Golokhvast, K.S. Nano-and microparticles in welding aerosol: Granulometric analysis. Phys. Procedia 2017, 86, 50–53. [Google Scholar]
- Xu, S.; Jia, C.; Maksymov, S.; Cai, Z.; Wu, C. Modeling the coupled bubble-arc-droplet evolution in underwater flux-cored arc welding. Int. J. Mech. Sci. 2024, 284, 109754. [Google Scholar] [CrossRef]
- Gong, N.; Shao, K.; Che, C.; Sun, Y. Stability of nickel oxide nanoparticles and its influence on toxicity to marine algae Chlorella vulgaris. Mar. Pollut. Bull. 2019, 149, 110532. [Google Scholar]
- Auffan, M.; Rose, J.; Wiesner, M.R.; Bottero, J.Y. Chemical stability of metallic nanoparticles: A parameter controlling their potential cellular toxicity in vitro. Environ. Pollut. 2009, 157, 1127–1133. [Google Scholar] [CrossRef]
- Morelli, E.; Cioni, P.; Posarelli, M.; Gabellieri, E. Chemical stability of CdSe quantum dots in seawater and their effects on a marine microalga. Aquat. Toxicol. 2012, 122–123, 153–162. [Google Scholar]
- Corsi, I.; Bergami, E.; Grassi, G. Behavior and Bio-Interactions of Anthropogenic Particles in Marine Environment for a More Realistic Ecological Risk Assessment REVIEW article. Front. Environ. Sci. 2020, 8, 60. [Google Scholar]
- Lekamge, S.; Ball, A.S.; Shukla, R.; Nugegoda, D. The Toxicity of Nanoparticles to Organisms in Freshwater. Rev. Environ. Contam Toxicol. Saf. 2018, 248, 1–80. [Google Scholar]
- Medici, S.; Peana, M.; Pelucelli, A.; Zoroddu, M.A. An updated overview on metal nanoparticles toxicity. Semin. Cancer Biol. 2021, 76, 17–26. [Google Scholar]
- Prajitha, N.; Athira, S.S.; Mohanan, P.V. Bio-interactions and risks of engineered nanoparticles. Environ. Res. 2019, 172, 98–108. [Google Scholar]
- Lee, J.; Hong, S.; An, S.A.; Khim, J.S. Methodological advances and future directions of microalgal bioassays for evaluation of potential toxicity in environmental samples: A review. Environ. Int. 2023, 173, 107869. [Google Scholar] [CrossRef] [PubMed]
- Pastorino, P.; Broccoli, A.; Anselmi, S.; Bagolin, E.; Prearo, M.; Barceló, D.; Renzi, M. The microalgae Chaetoceros tenuissimus exposed to contaminants of emerging concern: A potential alternative to standardized species for marine quality assessment. Ecol. Indic. 2022, 141, 109075. [Google Scholar] [CrossRef]
- Kirichenko, K.; Yelovsky, E.; Volkova, V.; Parshin, S.; Parshina, Y.; Pogodaev, A.; Kalinin, Y.; Gridasov, A.; Golokhvast, K. Mass spectrometric analysis of seawater during welding processes. Life Saf. Technol. 2024. [Google Scholar]
- Mudgal, V.; Madaan, N.; Mudgal, A.; Singh, R.B.; Mishra, S. Effect of Toxic Metals on Human Health. Open Nutraceuticals J. 2010, 3, 94–99. [Google Scholar] [CrossRef]
- Tato, T.; Beiras, R. The use of the marine microalga Tisochrysis lutea (T-iso) in standard toxicity tests; comparative sensitivity with other test species. Front. Mar. Sci. 2019, 6, 488. [Google Scholar]
- Zheng, G.; Lu, L.; Yang, Y.; Wei, J.; Han, B.; Zhang, Q.; Wang, Y. Development of microfluidic dilution network-based system for lab-on-a-chip microalgal bioassays. Anal. Chem. 2018, 90, 13280–13289. [Google Scholar] [CrossRef]
- Falkowski, P.G. The role of phytoplankton photosynthesis in global biogeochemical cycles. Photosynth. Res. 1994, 39, 235–258. [Google Scholar] [CrossRef]
- Orlova, T.Y.; Stonik, I.; Shevchenko, O. Flora of planktonic microalgae of Amursky Bay, Sea of Japan. Russ. J. Mar. Biol. 2009, 35, 60–78. [Google Scholar] [CrossRef]
- Pikula, K.S.; Chernyshev, V.V.; Zakharenko, A.M.; Chaika, V.V.; Waissi, G.; Hai, L.H.; Hien, T.T.; Tsatsakis, A.M.; Golokhvast, K.S. Toxicity assessment of particulate matter emitted from different types of vehicles on marine microalgae. Environ. Res. 2019, 179, 108785. [Google Scholar] [CrossRef]
- Özhan, K.; Bargu, S. Responses of sympatric Karenia brevis, Prorocentrum minimum, and Heterosigma akashiwo to the exposure of crude oil. Ecotoxicology 2014, 23, 1387–1398. [Google Scholar]
- OECD. Test No. 201: Freshwater Alga and Cyanobacteria, Growth Inhibition Test; OECD: Paris, France, 2011. [Google Scholar]
- Guillard, R.R.; Ryther, J.H. Studies of marine planktonic diatoms. I. Cyclotella nana Hustedt, and Detonula confervacea (cleve) Gran. Can. J. Microbiol. 1962, 8, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Crowley, L.C.; Scott, A.P.; Marfell, B.J.; Boughaba, J.A.; Chojnowski, G.; Waterhouse, N.J. Measuring cell death by propidium iodide uptake and flow cytometry. Cold Spring Harb. Protoc. 2016, 2016, 27371595. [Google Scholar]
- Suzuki, T.; Fujikura, K.; Higashiyama, T.; Takata, K. DNA staining for fluorescence and laser confocal microscopy. J. Histochem. Cytochem. 1997, 45, 49–53. [Google Scholar] [PubMed]
- Sabnis, R.W.; Deligeorgiev, T.G.; Jachak, M.N.; Dalvi, T.S. DiOC6(3): A useful dye for staining the endoplasmic reticulum. Biotech. Histochem. 1997, 72, 253–258. [Google Scholar]
- Grégori, G.; Denis, M.; Lefèvre, D.; Beker, B. A flow cytometric approach to assess phytoplankton respiration. In Advanced Flow Cytometry: Applications in Biological Research; Springer: Dordrecht, The Netherlands, 2003; pp. 99–106. [Google Scholar]
- Pinto, E.; Sigaud-Kutner, C.S.T.; Zajac, M.A.; Okamoto, O.; Morse, D.; Colepicolo, P. Heavy metal-induced oxidative stress in algae. J. Phycol. 2003, 39, 1008–1018. [Google Scholar]
- Gupta, S.K.; Singh, J. Evaluation of mollusc as sensitive indicator of heavy metal pollution in aquatic system: A review. IIOAB J. 2011, 2, 49–57. [Google Scholar]
- Khan, M.J.; Rai, A.; Ahirwar, A.; Sirotiya, V.; Mourya, M.; Mishra, S.; Schoefs, B.; Marchand, J.; Bhatia, S.K.; Varjani, S. Diatom microalgae as smart nanocontainers for biosensing wastewater pollutants: Recent trends and innovations. Bioengineered 2021, 12, 9531–9549. [Google Scholar] [CrossRef]
- Wang, J.; Zhu, X.; Tan, L.; Zhao, T.; Ni, Z.; Zhang, N.; Wang, J. Single and combined nanotoxicity of ZnO nanoparticles and graphene quantum dots against the microalga Heterosigma akashiwo. Environ. Sci. Nano 2022, 9, 3094–3109. [Google Scholar]
- Nguyen, M.K.; Moon, J.-Y.; Lee, Y.-C. Microalgal ecotoxicity of nanoparticles: An updated review. Ecotoxicol. Environ. Saf. 2020, 201, 110781. [Google Scholar]
- Zhang, C.; Chen, X.; Wang, J.; Tan, L. Toxicity of Zinc Oxide Nanoparticles on Marine Microalgae Possessing Different Shapes and Surface Structures. Environ. Eng. Sci. 2018, 35, 785–790. [Google Scholar] [CrossRef]

| Sample Code | Welding Process | Mode Description | Dissolved Oxygen (O2), mg/L |
|---|---|---|---|
| APL-1 | Flux-cored wire. Wet cutting | Automatic wet arc cutting with a 2 mm diameter PPR-APL1 flux-cored wire on DC polarity, at a current of 280–300 A, voltage of 37 V, with the torch position at an angle of 90 ± 15°, cutting speed of 180–200 mm/min. Two samples were taken 60 s after the start of cutting. | 7.39 ± 0.11 * |
| APL-2 | Flux-cored wire. Wet welding | Automatic wet arc welding with 1.6 mm diameter PPS-APL2 flux-cored wire (technical specifications 1274-001-83763787-2014) on reverse polarity current, at a current of 180 A, an arc voltage of 32 V, with the torch position at an angle of 90 ± 15° and a speed of 20 mm/min. A sample was taken after 60 s of welding. | 8.59 ± 0.12 * |
| ELc | Coated electrode. Wet cutting | Manual wet arc cutting using Arcair size 5/16 × 14 (8.0 × 356 mm) electrodes, P/N: 42-059-007. A sample was taken 60 s after the start of cutting. | 5.46 ± 0.11 |
| ELw | Coated electrode. Wet welding | Manual wet arc welding using Arcair size 5/32 × 14 (3.97 × 356 mm) coated electrodes P/N: 42-984-004 on reverse current. A sample was taken after 60 s of welding. | 6.15 ± 0.04 * |
| Element | ELw, ppb | ELc, ppb | APL-2, ppb | APL-1, ppb |
|---|---|---|---|---|
| Li | 153 | 122 | 137 | 498 |
| Mg | 873,000 | 828,000 | 896,000 | 903,000 |
| Al | 309 | 280 | 8080 | 474 |
| Ti | 622 | 108 | 34.3 | 610 |
| V | 6.04 | 6.56 | 3.67 | 3.54 |
| Cr | 10.6 | 12.5 | 197 | 22.4 |
| Mn | 887 | 612 | 2480 | 1500 |
| Fe | 8440 | 20,700 | 43,100 | 12,300 |
| Co | 1.14 | 1.43 | 2.68 | 1.25 |
| Ni | 25.3 | 19.7 | 261 | 152 |
| Cu | 190 | 270 | 8710 | 2790 |
| Zn | 326 | 4100 | 1910 | 188 |
| Ga | 1.14 | 2.52 | 8.28 | 1.69 |
| Ge | 0.893 | 0.828 | 2.96 | 0.87 |
| Se | 2.02 | 1.21 | 1.38 | 2.16 |
| Y | 0.14 | 0.108 | 0.222 | 0.148 |
| Zr | 9.04 | 0.605 | 0.584 | 2.69 |
| Nb | 2.15 | 0.149 | 0.195 | 2.05 |
| Ag | 0.0779 | 0.0657 | 1.69 | 0.232 |
| Cd | 4.36 | ≤0.012 | 0.283 | 0.0872 |
| Sn | 11 | 50.8 | 41.4 | 4.97 |
| Pb | 7.41 | 4.95 | 160 | 10.9 |
| Endpoint | Fluorescent Dye or Registered Parameter | Emission Channel/Band Width, nm | Dye Concentration for H. akashiwo | Dye Concentration for P. purpureum | Dye Concentration for A. ussuriensis |
|---|---|---|---|---|---|
| Growth rate inhibition | PI | 610/20 | 15 µM | 20 µM | 15 µM |
| Cell size change | Forward scatter intensity (size calibration kit F13838 by Molecular Probes, USA) | FSC | – | – | – |
| Membrane potential change | DiOC6 | 525/40 | 2.5 µM | 5 µM | 1 µM |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kirichenko, K.Y.; Pikula, K.S.; Chayka, V.V.; Gridasov, A.V.; Vakhniuk, I.A.; Volkova, V.N.; Pogodaev, A.V.; Parshin, S.G.; Parshina, Y.S.; Kalinin, Y.E.; et al. Comparison of the Level and Mechanisms of Toxicity of Nanoparticles of Underwater Welding in Bioassay with Three Marine Microalgae. Nanomaterials 2025, 15, 518. https://doi.org/10.3390/nano15070518
Kirichenko KY, Pikula KS, Chayka VV, Gridasov AV, Vakhniuk IA, Volkova VN, Pogodaev AV, Parshin SG, Parshina YS, Kalinin YE, et al. Comparison of the Level and Mechanisms of Toxicity of Nanoparticles of Underwater Welding in Bioassay with Three Marine Microalgae. Nanomaterials. 2025; 15(7):518. https://doi.org/10.3390/nano15070518
Chicago/Turabian StyleKirichenko, Konstantin Yu., Konstantin S. Pikula, Vladimir V. Chayka, Alexander V. Gridasov, Igor A. Vakhniuk, Vladislava N. Volkova, Anton V. Pogodaev, Sergei G. Parshin, Yulia S. Parshina, Yuri E. Kalinin, and et al. 2025. "Comparison of the Level and Mechanisms of Toxicity of Nanoparticles of Underwater Welding in Bioassay with Three Marine Microalgae" Nanomaterials 15, no. 7: 518. https://doi.org/10.3390/nano15070518
APA StyleKirichenko, K. Y., Pikula, K. S., Chayka, V. V., Gridasov, A. V., Vakhniuk, I. A., Volkova, V. N., Pogodaev, A. V., Parshin, S. G., Parshina, Y. S., Kalinin, Y. E., Kholodov, A. S., Ugay, S. M., Orlova, T. Y., & Golokhvast, K. S. (2025). Comparison of the Level and Mechanisms of Toxicity of Nanoparticles of Underwater Welding in Bioassay with Three Marine Microalgae. Nanomaterials, 15(7), 518. https://doi.org/10.3390/nano15070518











