Cu0-Functionalized, ZIF-8-Derived, Nitrogen-Doped Carbon Composites for Efficient Iodine Elimination in Solution
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
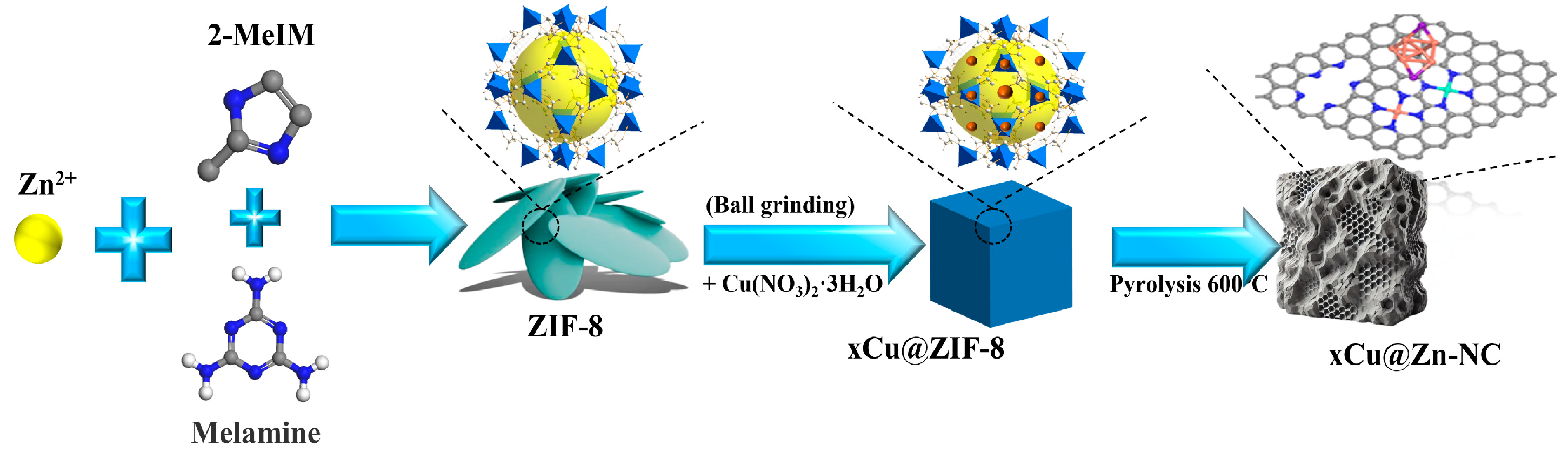
2.2. Synthesis of ZIF-8 and Cu@ZIF-8
2.3. Synthesis of Cu@Zn-NC
2.4. Characterization
2.5. Batch Adsorption Experiments
2.5.1. Adsorption Isotherm Experiment
2.5.2. Adsorption Kinetic Experiment
2.5.3. The Effect of the Solution Temperature
2.5.4. Regeneration and Reusability Evaluation
2.6. Theoretical Simulation Calculation
3. Results and Discussion
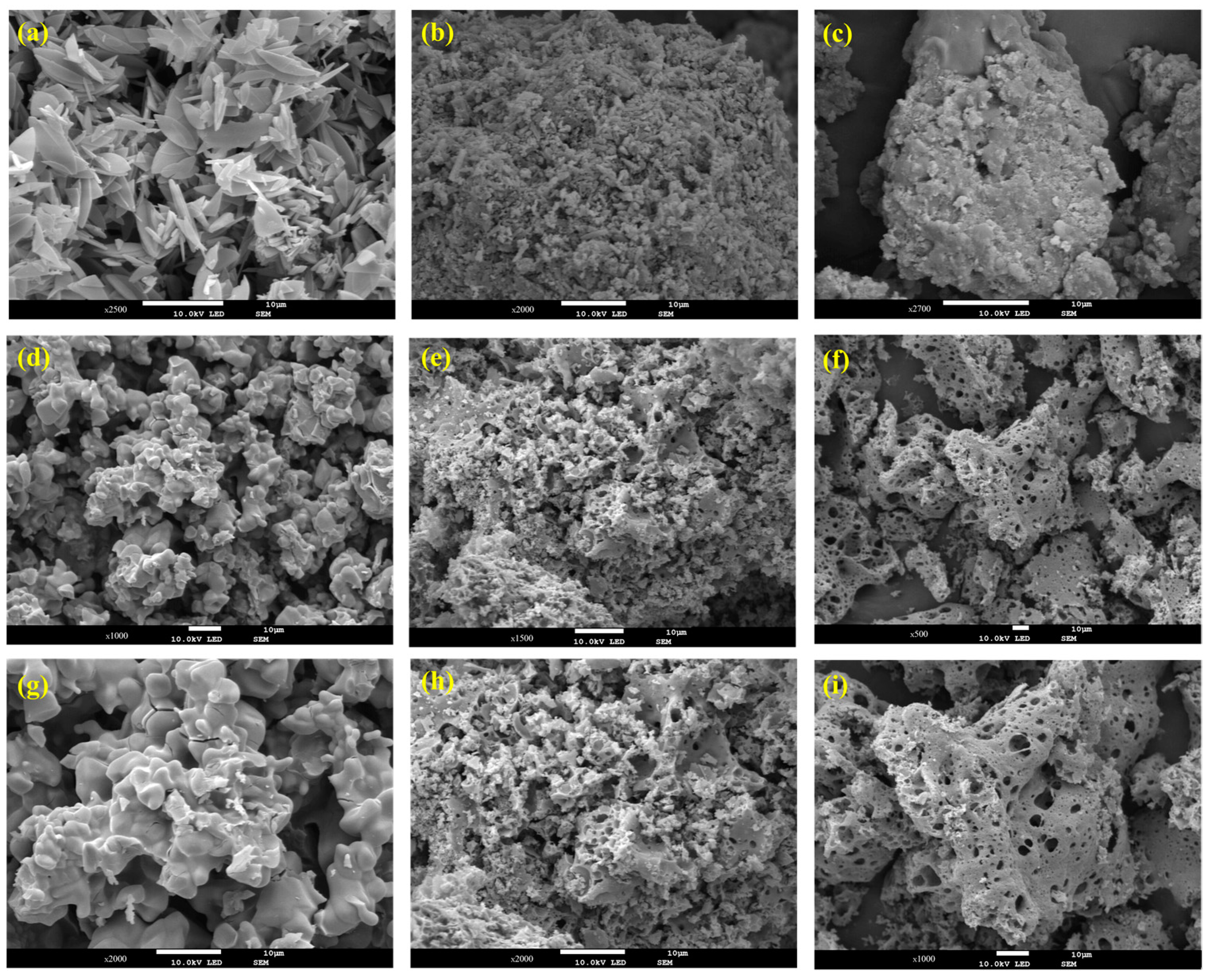
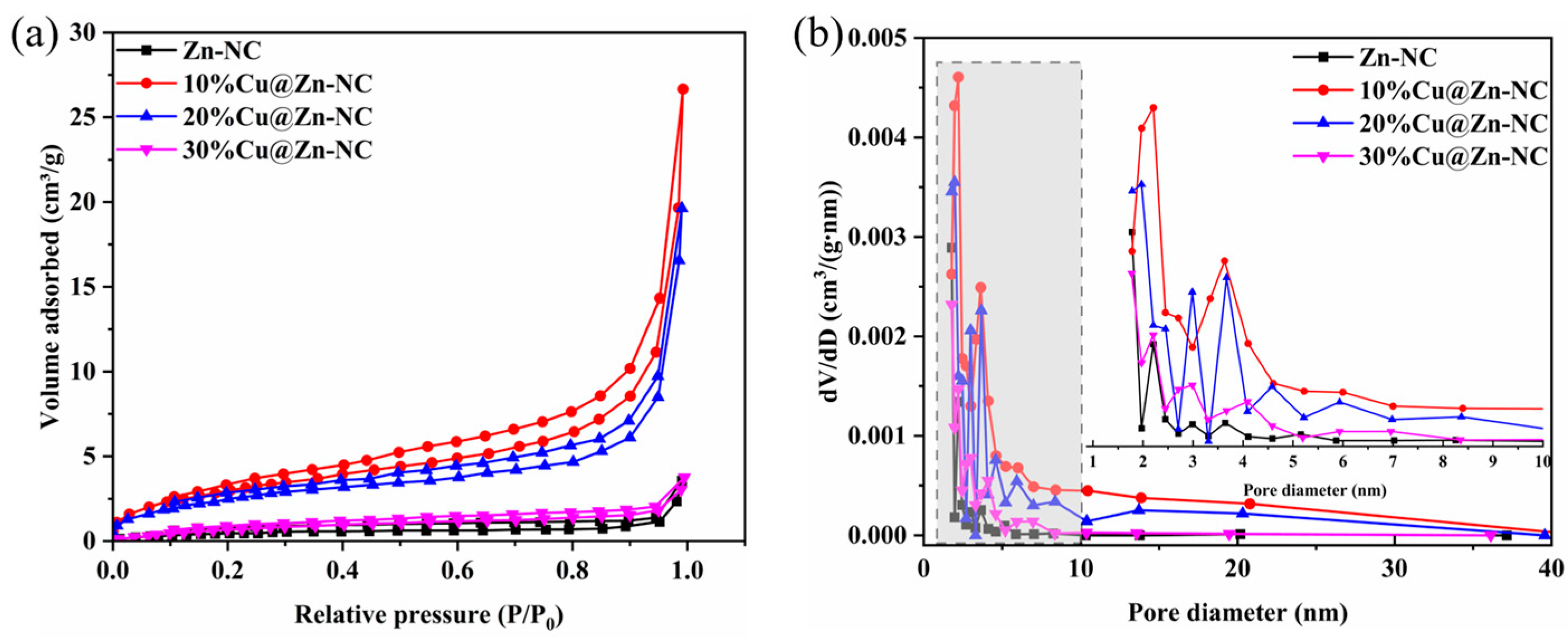
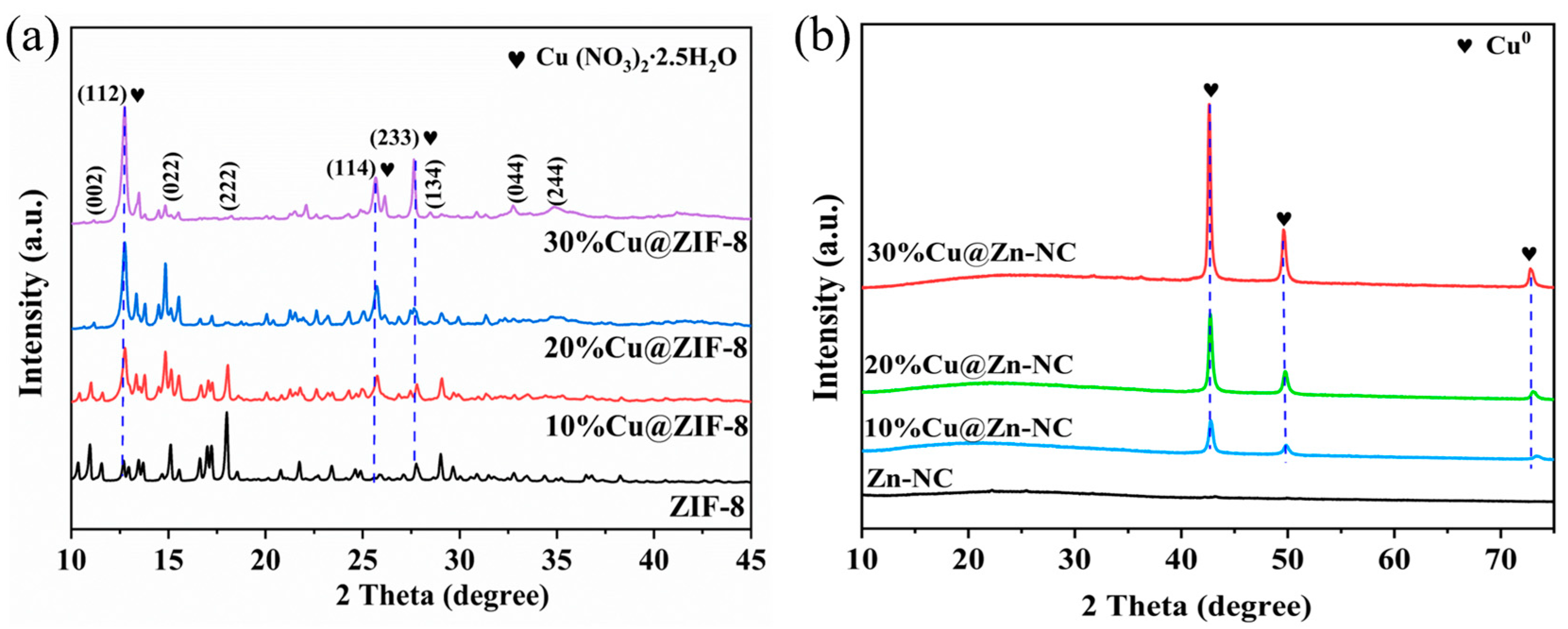
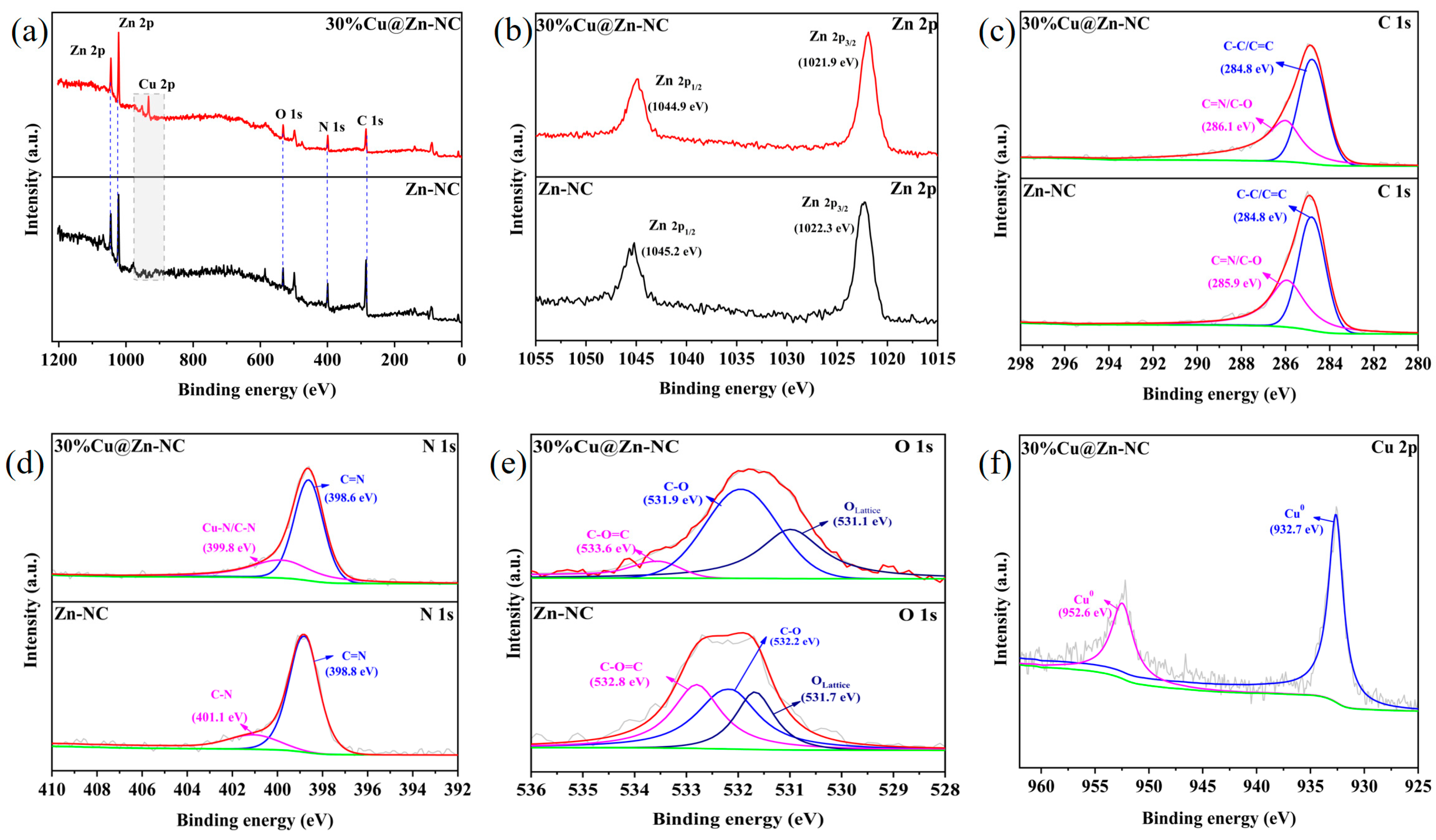
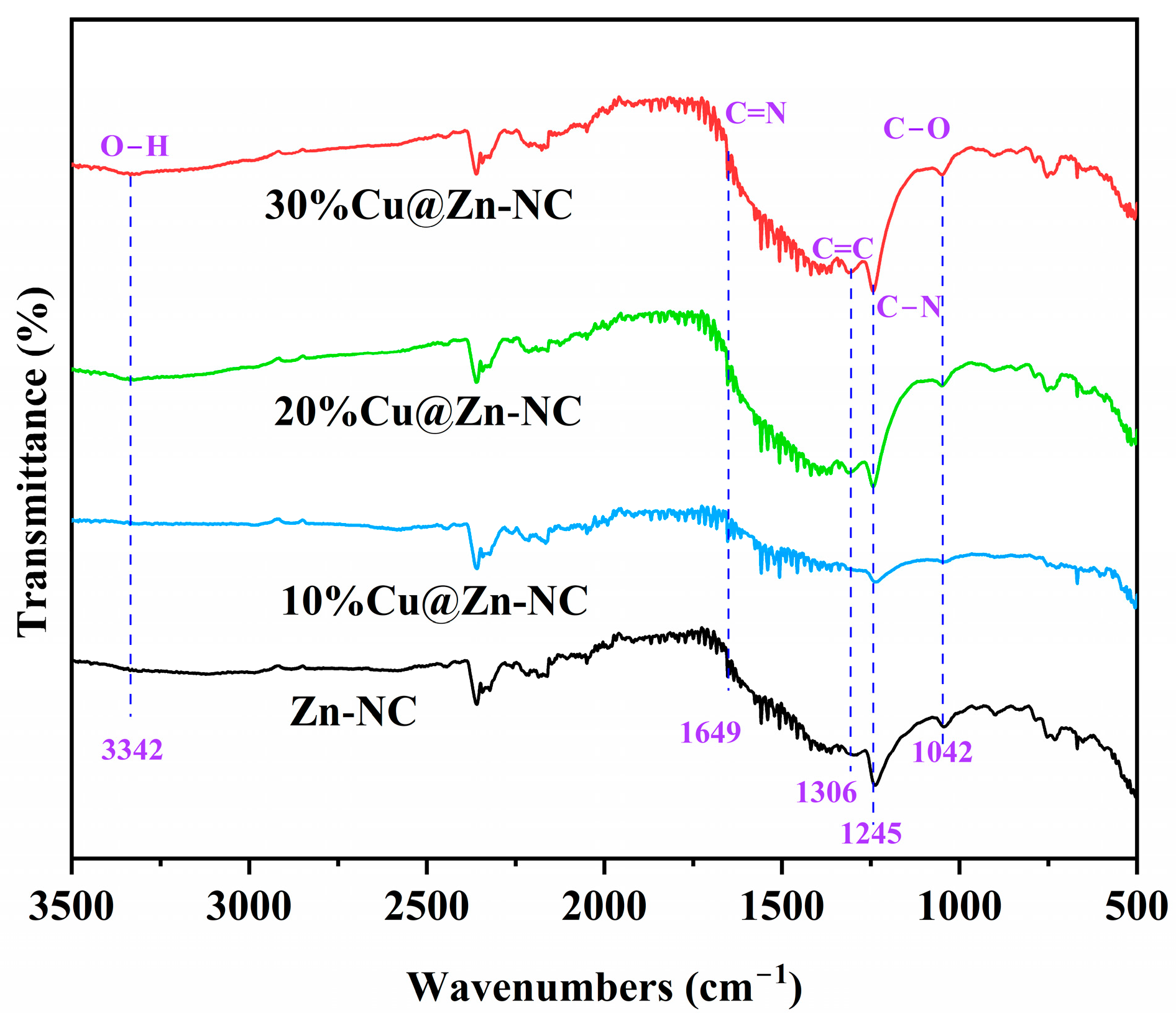
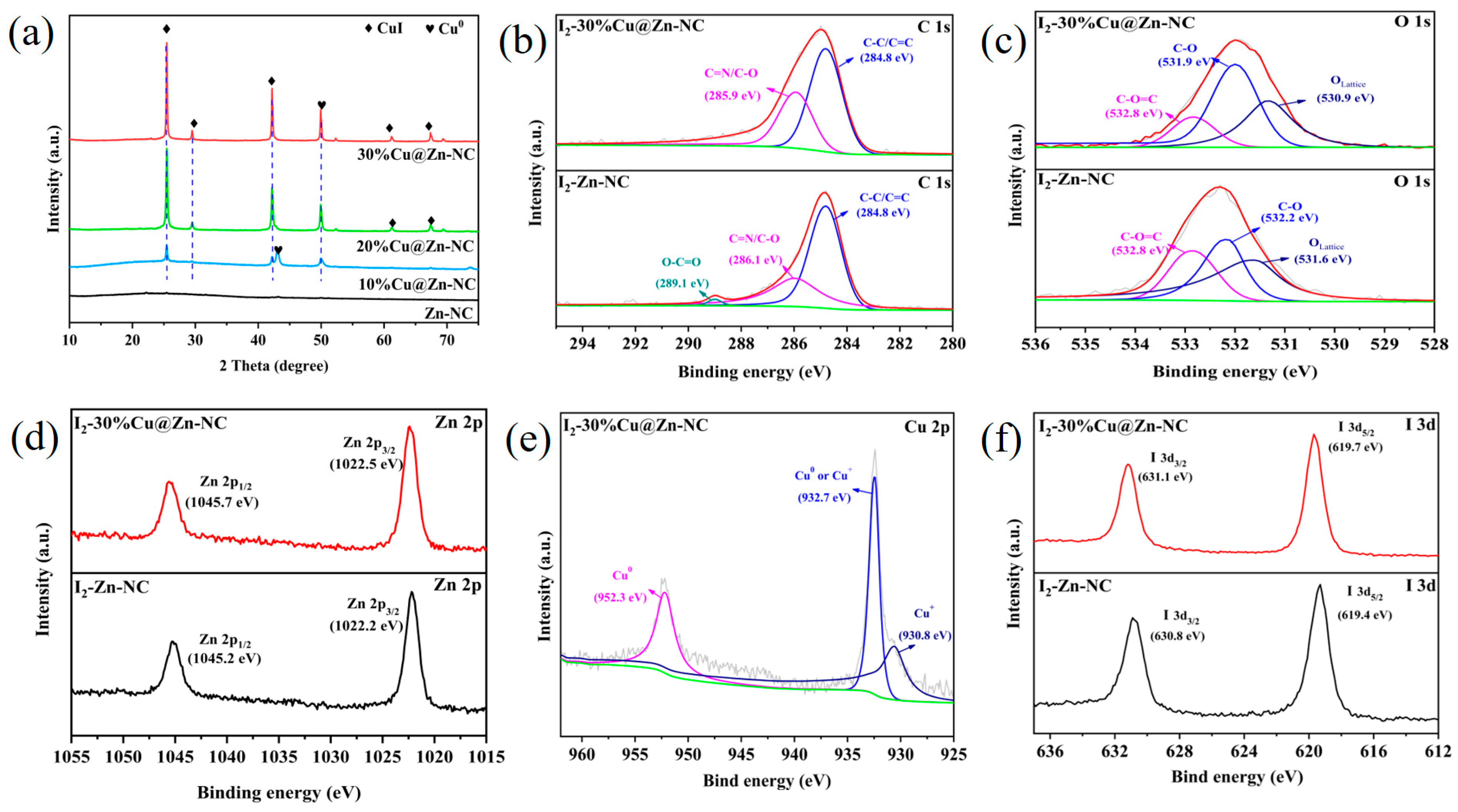
3.1. Materials Characterization
3.2. Batch Adsorption Performance
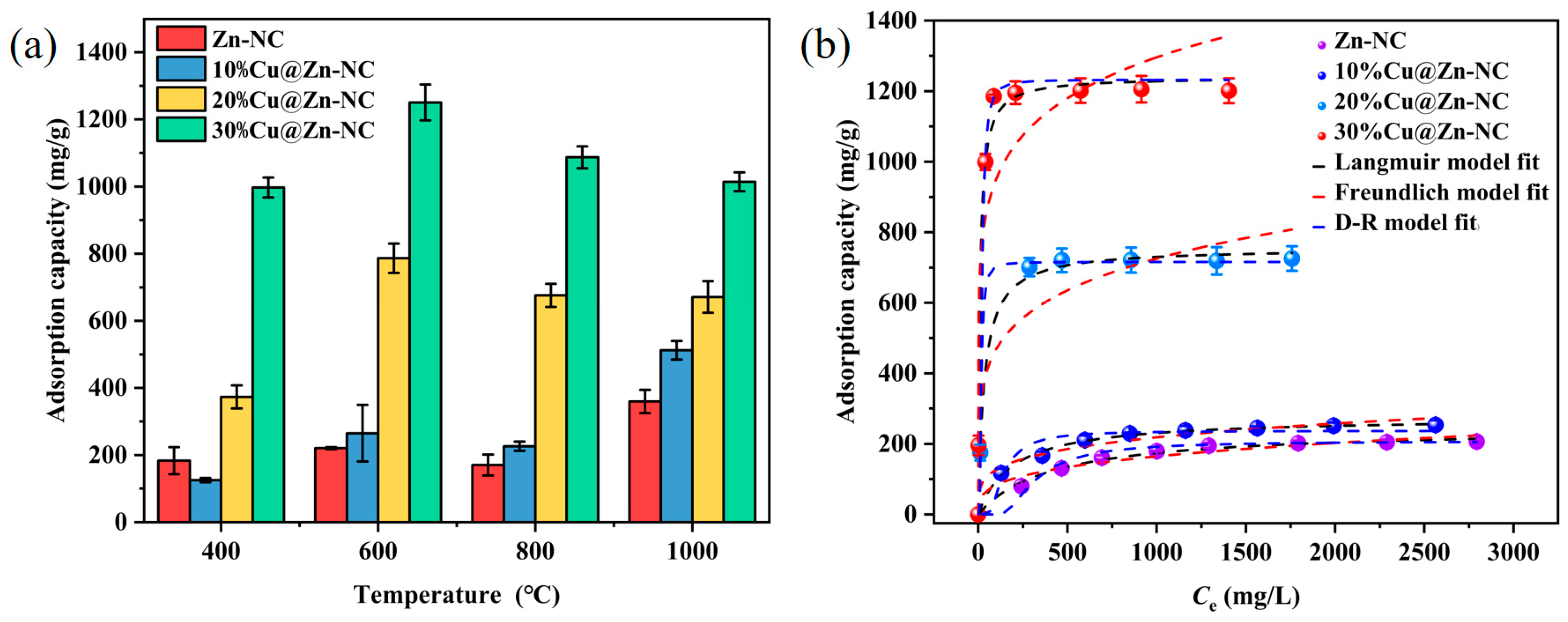
3.2.1. Adsorption Isothermal Analysis
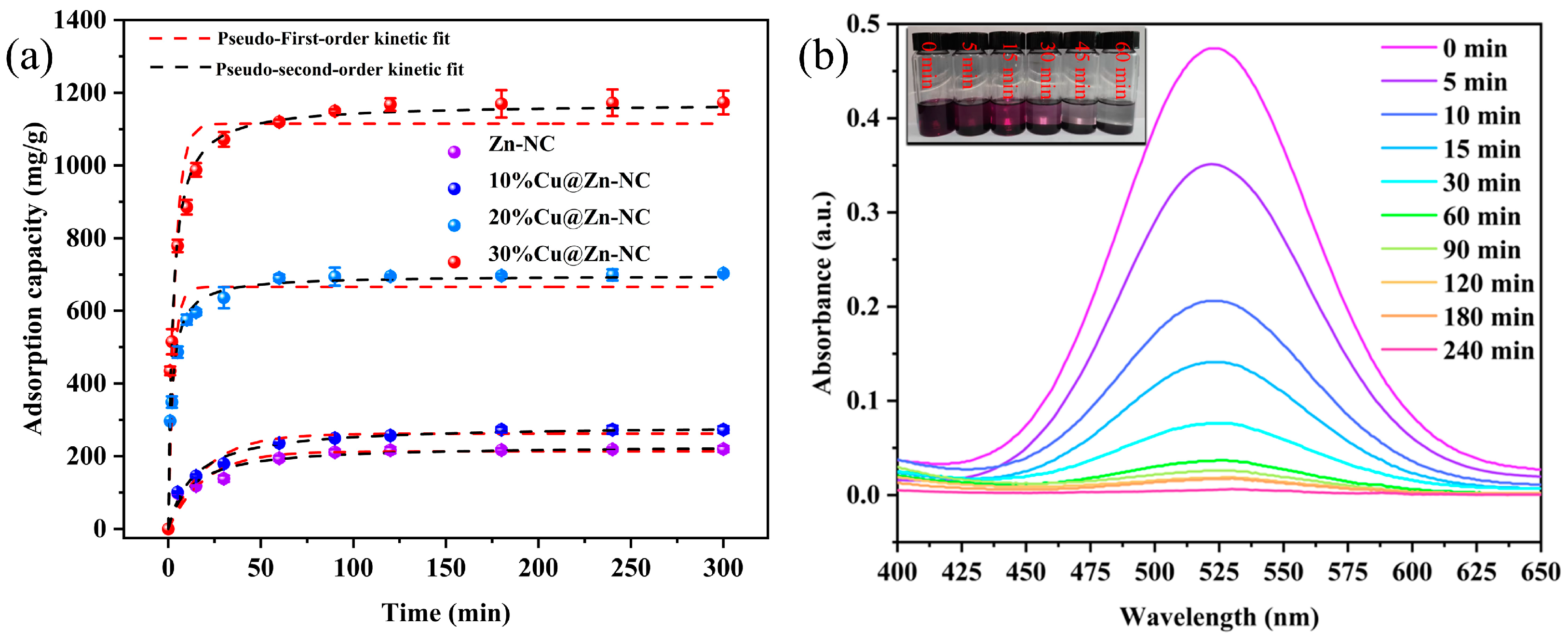
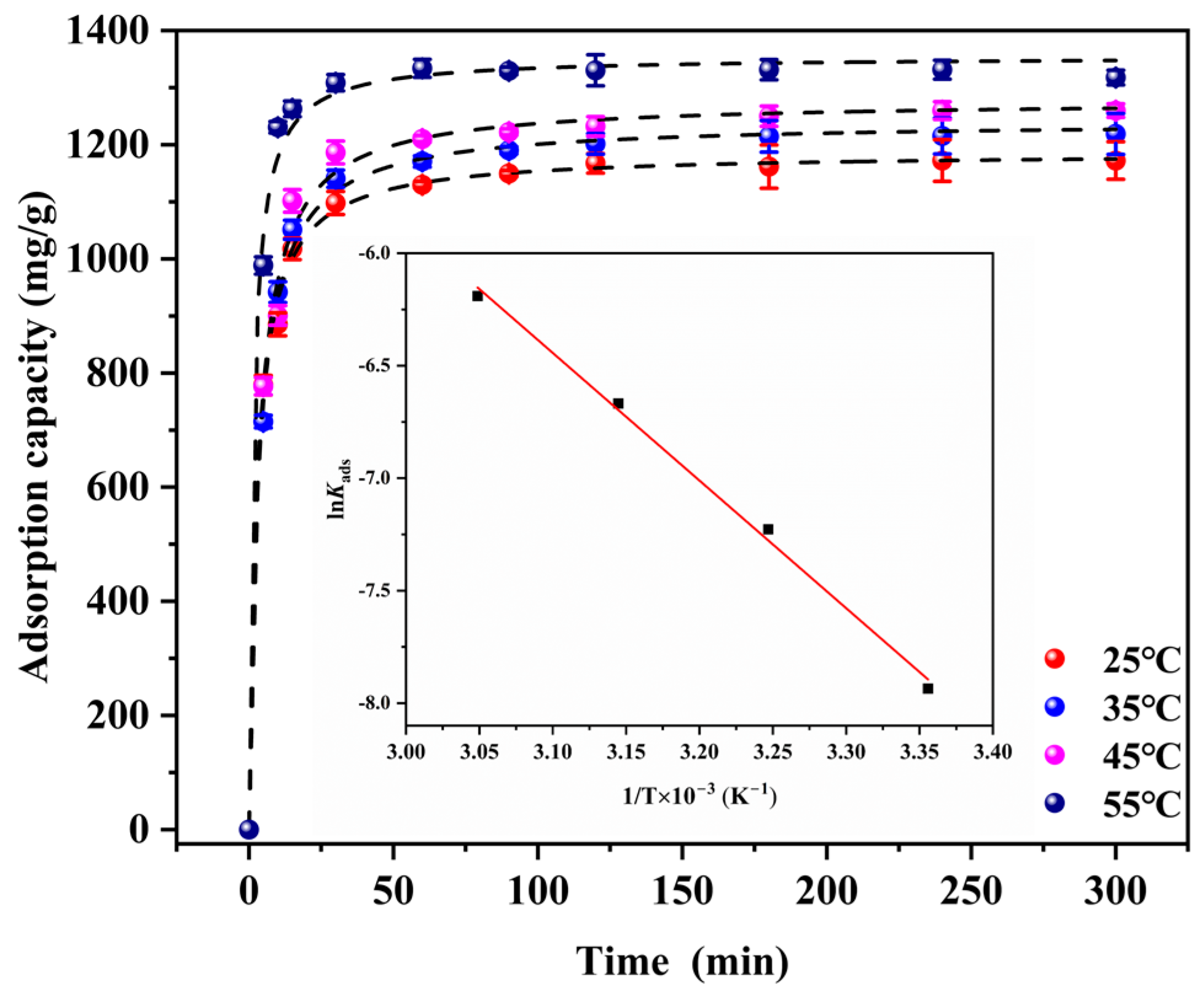
3.2.2. Adsorption Kinetic Analysis
3.2.3. The Activation Energy Analysis
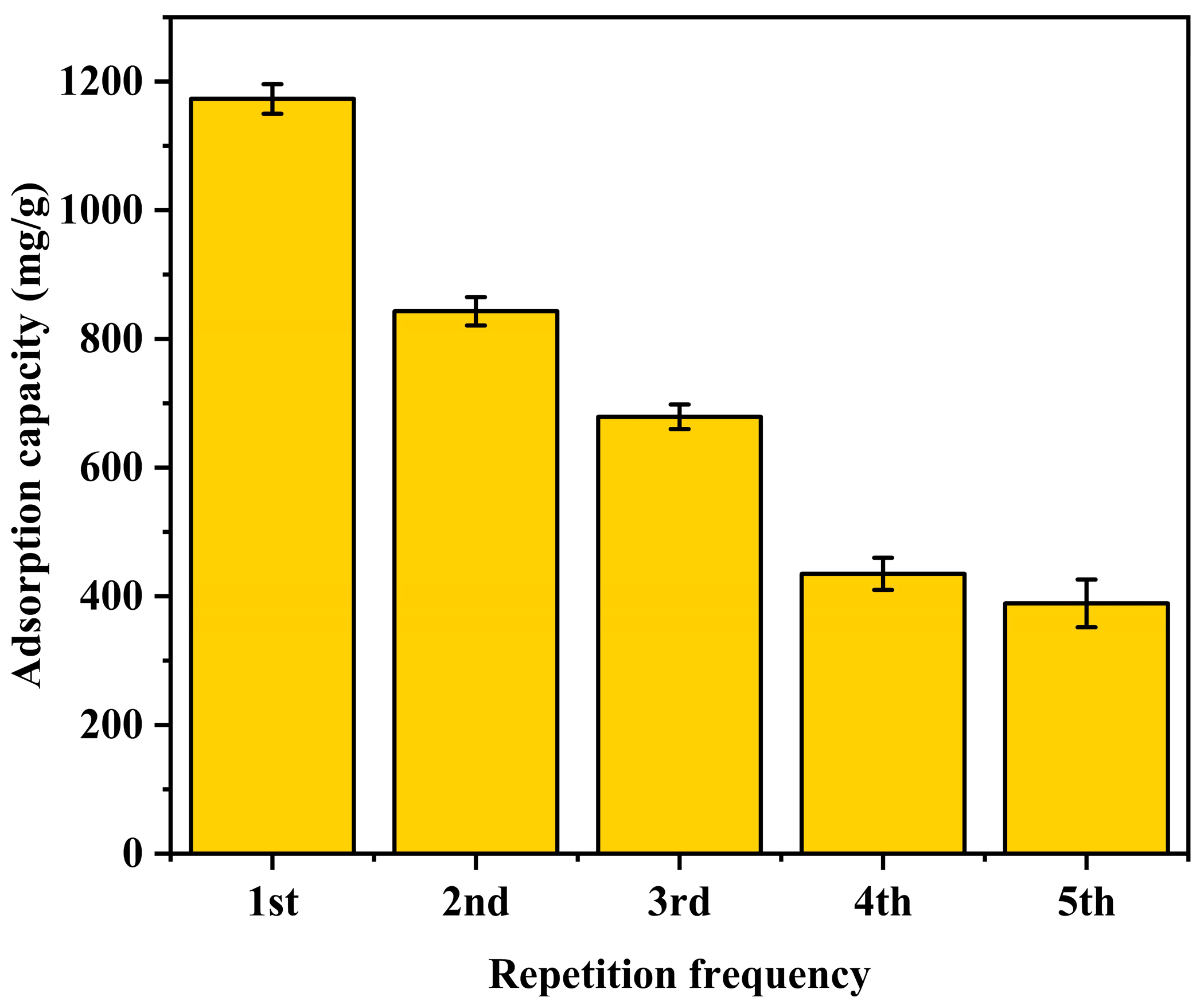
3.2.4. Regeneration and Reusability Performance
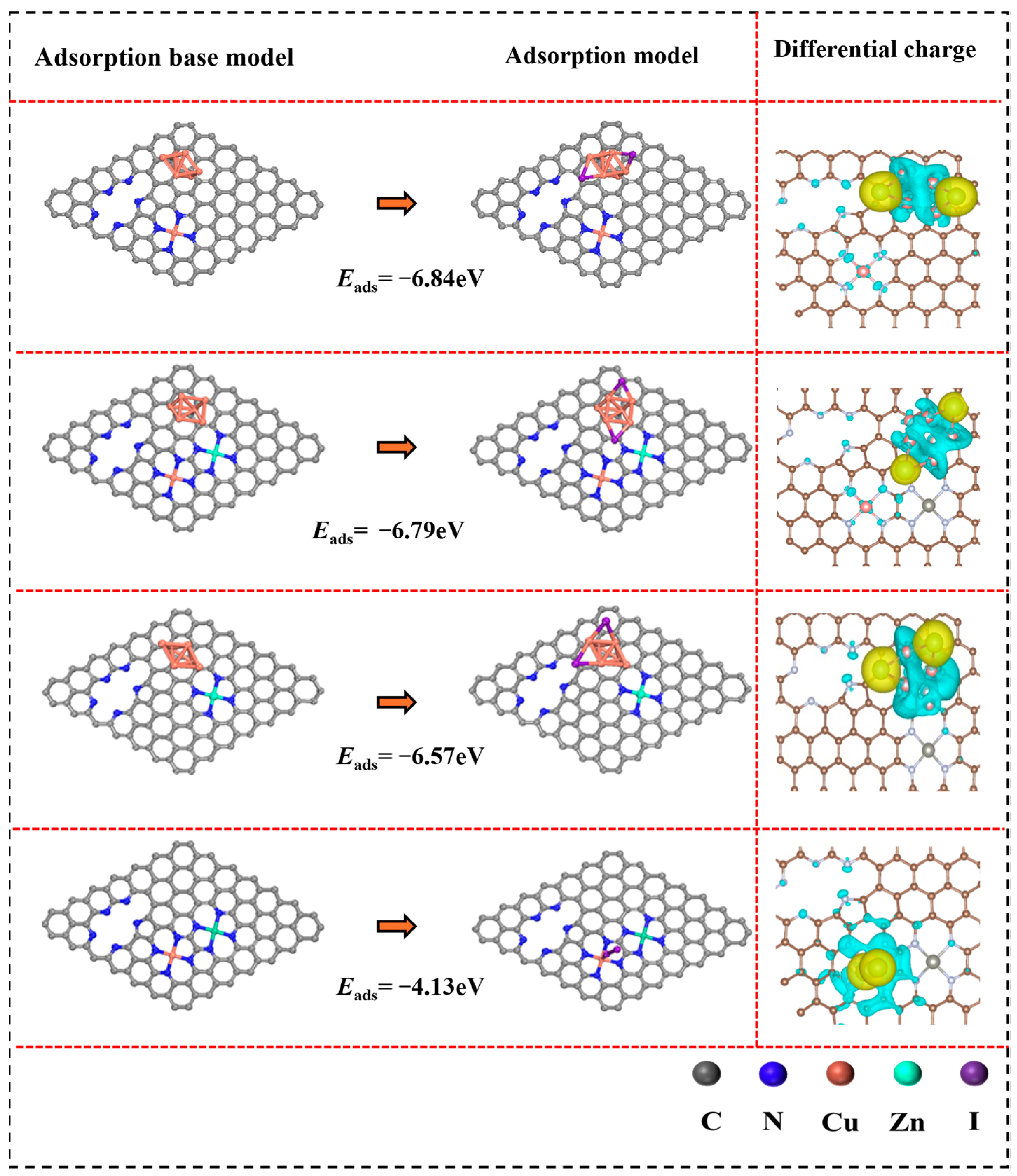
3.3. Adsorption Mechanism Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, M.; Samanta, J.; Atterberry, B.; Staples, R.; Rossini, A.; Ke, C. A crosslinked ionic organic framework for efficient iodine and iodide remediation in water. Angew. Chem. Int. Ed. Engl. 2022, 61, e202214189. [Google Scholar] [CrossRef]
- Changani, Z.; Razmjou, A.; Taheri-Kafrani, A.; Warkiani, M.; Asadnia, M. Surface modification of polypropylene membrane for the removal of iodine using polydopamine chemistry. Chemosphere 2020, 249, 126079. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Yin, H.; Zhang, Y.; Wei, F.; Hu, H.; Tang, Y.; Wang, S. Fe, Cu-coordinated ZIF-derived bimetal encapsulated N-doped carbon nanotube for efficient remediation of various aqueous pollutants. Chem. Eng. J. 2021, 426, 131801. [Google Scholar] [CrossRef]
- Zhou, W.; Li, A.; Zhou, M.; Xu, Y.; Zhang, Y.; He, Q. Nonporous amorphous superadsorbents for highly effective and selective adsorption of iodine in water. Nat. Commun. 2023, 14, 5388. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Ren, X.; Chen, L.; Ma, X.; Chen, Q.; Sun, Q.; Zhang, L.; Si, P.; Ci, L. High efficient adsorption and storage of iodine on S, N co-doped graphene aerogel. J. Hazard. Mater. 2019, 373, 705–715. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.; Yan, Q.; Wang, S.; Hu, H.; Xiao, S.; Su, X.; Xu, H.; Gao, Y. Conjugated microporous polymers based on octet and tetratopic linkers for efficient iodine capture. ACS Appl. Mater. Interfaces 2023, 15, 46408–46416. [Google Scholar] [CrossRef]
- Sen, A.; Sharma, S.; Dutta, S.; Shirolkar, M.M.; Dam, G.K.; Let, S.; Ghosh, S.K. Functionalized ionic porous organic polymers exhibiting high iodine uptake from both the vapor and aqueous medium. ACS Appl. Mater. Interfaces 2021, 13, 34188–34196. [Google Scholar] [CrossRef]
- Miensah, E.D.; Kokuloku, L.T.; Gu, A.; Chen, K.; Wang, P.; Gong, C.; Mao, P.; Chen, K.; Jiao, Y.; Yang, Y. In situ modification of JUC-160-derived carbon with Cu/ZnO nanoparticles for efficient capture and reversible storage of radioiodine. Surf. Interfaces 2022, 32, 102160. [Google Scholar] [CrossRef]
- Nandanwar, S.U.; Coldsnow, K.; Utgikar, V.; Sabharwall, P.; Aston, D.E. Capture of harmful radioactive contaminants from off-gas stream using porous solid sorbents for clean environment-A review. Chem. Eng. J. 2016, 306, 369–381. [Google Scholar] [CrossRef]
- Liu, S.; Zeng, Y.; Zhang, A.; Song, Y.; Ni, Y.; Li, J.; Chi, F.; Xiao, C. Efficient capture of radioactive iodine by ZIF-8 derived porous carbon. J. Environ. Radioact. 2022, 249, 106895. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Yuan, H.; Zheng, Q.; Huang, B.; Liao, F.; Gao, H.; Fu, H.; Zhang, J.; Liao, Y. Understanding the adsorption sites on nitrogen- and oxygen-doped carbon nanotubes for iodine uptake. Appl. Surf. Sci. 2023, 629, 157387. [Google Scholar] [CrossRef]
- Ma, J.; Xu, S.; Wang, X.; Zhang, M.; Qu, Y.; Cao, Q.; Jia, H.; Xu, J.; Wang, X. Biomass derived porous carbon for efficient iodine adsorption from vapor and solution. Sep. Purif. Technol. 2024, 347, 127613. [Google Scholar] [CrossRef]
- Kamran, U.; Bhatti, H.N.; Noreen, S.; Tahir, M.A.; Park, S.J. Chemically modified sugarcane bagasse-based biocomposites for efficient removal of acid red 1 dye: Kinetics, isotherms, thermodynamics, and desorption studies. Chemosphere 2022, 291, 132796. [Google Scholar] [CrossRef]
- Wang, X.; Meng, R.; Zhao, S.; Jing, Z.; Jin, Y.; Zhang, J.; Pi, X.; Du, Q.; Chen, L.; Li, Y. Efficient adsorption of radioactive iodine by covalent organic framework/chitosan aerogel. Int. J. Biol. Macromol. 2024, 260, 129690. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Xu, Q.; Li, Z.P.; Jiang, W.M.; Jiang, Q.H.; Jiang, D.L. Exceptional iodine capture in 2D covalent organic frameworks. Adv. Mater. 2018, 30, 1801991. [Google Scholar] [CrossRef]
- Xu, G.; Lin, J.; Gao, X.; Yu, C.; Tang, C.; Huang, Y. Modification of boron nitride aerogels with APTES for enhanced iodine capture performance. Ceram. Int. 2024, 50, 27111–27119. [Google Scholar] [CrossRef]
- Zhou, X.; Jin, H.; Yun, S.; Huang, W.; Mao, P.; Chen, J.; Yang, Y. A novel Cu nanoporous aerogel for high-efficient immobilization of iodide in water. Chem. Eng. J. 2023, 454, 140217. [Google Scholar] [CrossRef]
- Zhou, X.; Jin, H.; Gu, A.; Chen, K.; Liu, Y.; Yun, S.; Mao, P.; Chen, J.; Yang, Y. Monolithic Cu/Al2O3-palygorskite composite aerogel for high-efficiency iodine elimination in a multimedia environment. Microporous Mesoporous Mater. 2024, 366, 112963. [Google Scholar] [CrossRef]
- Yu, R.; Li, Q.; Zhang, T.; Li, Z.; Xia, L. Zn, O Co-adsorption based on MOF-5 for efficient capture of radioactive iodine. Process. Saf. Environ. Prot. 2023, 174, 770–777. [Google Scholar] [CrossRef]
- Kamran, U.; Park, S.J. Hybrid biochar supported transition metal doped MnO2 composites: Efficient contenders for lithium adsorption and recovery from aqueous solutions. Desalination Water Treat. 2022, 522, 115387. [Google Scholar] [CrossRef]
- Wang, M.; Wang, C.; Liu, J.; Rong, F.; He, L.; Lou, Y.; Zhang, Z.; Du, M. Efficient Ag/Ag2O-doped cobalt metallo-covalent organic framework electrocatalysts for rechargeable Zinc-Air battery. ACS Sustain. Chem. Eng. 2021, 9, 5872–5883. [Google Scholar] [CrossRef]
- Wang, X.; Li, M.; Zhang, J.; He, X.; Crittenden, J.C.; Zhang, W. Silver ion-exchanged anionic metal–organic frameworks for iodine adsorption: Silver species evolution from ions to nanoparticles. ACS Appl. Nano Mater. 2023, 6, 7206–7217. [Google Scholar] [CrossRef]
- Qi, B.; Liu, Y.; Zheng, T.; Gao, Q.; Yan, X.; Jiao, Y.; Yang, Y. Highly efficient capture of iodine by Cu/MIL-101. J. Solid State Chem. 2018, 258, 49–55. [Google Scholar] [CrossRef]
- He, X.; Chen, L.; Xiao, X.; Gan, Y.; Yu, J.P.; Luo, J.; Dan, H.; Wang, Y.; Ding, Y.; Duan, T. Improved utilization of Cu0 for efficient adsorption of iodine in gas and solution by mesoporous Cu0-SBA-15 via solvothermal reduction method. Chem. Eng. J. 2023, 462, 142175. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, K.; Gu, A.; Zhou, X.; Wang, P.; Gong, C.; Mao, P.; Jiao, Y.; Chen, K.; Lu, J.; et al. Adsorption performance study of bismuth-doped ZIF-8 composites on radioactive iodine in the vapor and liquid phases. J. Solid State Chem. 2023, 325, 124186. [Google Scholar] [CrossRef]
- Jung, Y.E.; Yang, J.H.; Yim, M.S. Investigation of bismuth-based metal-organic frameworks for effective capture and immobilization of radioiodine gas. J. Hazard. Mater. 2024, 467, 133777. [Google Scholar] [CrossRef]
- Kamran, U.; Lee, S.; Rhee, K.Y.; Park, S.J. Rice husk valorization into sustainable Ni@TiO2/biochar nanocomposite for highly selective Pb (II) ions removal from an aqueous media. Chemosphere 2023, 323, 138210. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Chen, K.; Dai, X.; Qiao, T.; Liu, H.; Lu, J.; Wang, P.; Gong, C.; Deng, S.; Xia, M.; et al. Efficient elimination of trace iodide ions from medical wastewater by Cu/Cu2O@AC composites. J. Radioanal. Nucl. Chem. 2024, 333, 4955–4969. [Google Scholar] [CrossRef]
- Chen, J.; Wang, P.; Gong, C.; Sun, Y.; Zhu, B.; Yang, Y.; Liu, F. Bimetal ZIFs-derived Cu0 embedded in nitrogen-doped carbon framework activation of molecular oxygen for efficient iodide elimination. J. Environ. Chem. Eng. 2024, 12, 112235. [Google Scholar] [CrossRef]
- Li, M.; Wang, X.; Zhang, J.; Gao, Y.; Zhang, W. Cu-loaded MOF-303 for iodine adsorption: The roles of Cu species and pyrazole ligands. Appl. Surf. Sci. 2023, 619, 156819. [Google Scholar] [CrossRef]
- Luan, J.; Li, J.; Duan, W.; Zhang, X.; Liu, Y.; Guo, F.; Li, W. Fabrication of a family of Cu-based materials derived from a Cu-organic framework for enhanced iodine capture. Inorg. Chem. Commun. 2024, 167, 112783. [Google Scholar] [CrossRef]
- Liu, S.; Gao, X.; Li, P.; Zhang, X.; Wang, M.; Xiao, S.; Zhao, X. Copper nanoparticles embedded flexible graphene aerogel for effective capture of iodine vapor. Microporous Mesoporous Mater. 2024, 379, 113298. [Google Scholar] [CrossRef]
- Li, J.; Wang, M.; Zhao, X.; Li, Z.; Niu, Y.; Wang, S.; Sun, Q. Efficient iodine removal by porous biochar-Confined nano-Cu2O/Cu0: Rapid and selective adsorption of iodide and iodate ions. Nanomaterials 2023, 13, 576. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, X.; Hou, Y.; Liu, C.; Xie, G.; Chen, X. Current research status of MOF materials for catalysis applications. Mol. Catal. 2024, 555, 113851. [Google Scholar] [CrossRef]
- Li, Y.; Guo, Q.; Ding, Z.; Jiang, H.; Yang, H.; Du, W.; Zheng, Y.; Huo, K.; Shaw, L.L. MOFs-based materials for solid-state hydrogen storage: Strategies and perspectives. Chem. Eng. J. 2024, 485, 149665. [Google Scholar] [CrossRef]
- Wu, Y.; Guo, Y.; Su, R.; Ma, X.; Wu, Q.; Zeng, Z.; Li, L.; Yao, X.; Wang, S. Hierarchical porous carbon with an ultrahigh surface area for high-efficient iodine capture: Insights into adsorption mechanisms through experiments, simulations and modeling. Sep. Purif. Technol. 2022, 303, 122237. [Google Scholar] [CrossRef]
- He, L.; Chen, L.; Dong, X.; Zhang, S.; Zhang, M.; Dai, X.; Liu, X.J.; Lin, P.; Li, K.F.; Chen, C.; et al. A nitrogen-rich covalent organic framework for simultaneous dynamic capture of iodine and methyl iodide. Chem 2021, 7, 699–714. [Google Scholar] [CrossRef]
- Chen, J.; Gu, A.; Miensah, E.D.; Liu, Y.; Wang, P.; Mao, P.; Gong, C.; Jiao, Y.; Chen, K.; Yang, Y. Cu-Zn bimetal ZIFs derived nanowhisker zero-valent copper decorated ZnO nanocomposites induced oxygen activation for high-efficiency iodide elimination. J. Hazard. Mater. 2021, 416, 126097. [Google Scholar] [CrossRef]
- Yang, H.; Xie, A.; Tang, Y.; Wang, Z.; Zhang, J.; Kong, L.; Song, P.; Sun, Y.; Yang, X.; Wan, P. Fe-ZIF8 coating Cu foil derived Carbon as a pH-universal electrocatalyst for efficient oxygen reduction reaction. Chem. Eur. J. 2021, 28, 21506010. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; He, Y.; Zhu, L.; Zhang, L.; Liu, B.; Zhang, Y.K.; Duan, T. Natural porous wood decorated with ZIF-8 for high efficient iodine capture. Mater. Chem. Phys. 2021, 258, 123964. [Google Scholar] [CrossRef]
- Jing, Y.; Cheng, Y.; Wang, L.; Liu, Y.; Yu, B.; Yang, C. MOF-derived Co, Fe, and Ni co-doped N-enriched hollow carbon as e fficient electrocatalyst for oxygen reduction reaction. Chem. Eng. J. 2020, 397, 125539. [Google Scholar] [CrossRef]
- Schejn, A.; Balan, L.; Falk, V.; Aranda, L.; Medjahdi, G.; Schneider, R. Controlling ZIF-8 nano- and microcrystal formation and reactivity through zinc salt variations. Cryst. Eng. Comm. 2014, 16, 4493–4500. [Google Scholar] [CrossRef]
- Mohammadi, A.; Nakhaei Pour, A. Triethylenetetramine-impregnated ZIF-8 nanoparticles for CO2 adsorption. J. CO2 Util. 2023, 69, 102424. [Google Scholar] [CrossRef]
- Jiang, X.; Wu, K.; Shao, L.; Shui, M.; Lin, X.; Lao, M.; Long, N.; Ren, Y.; Shu, J. Lithium storage mechanism in superior high capacity copper nitrate hydrate anode material. J. Power Sources 2014, 260, 218–224. [Google Scholar] [CrossRef]
- Zhu, H.; Dai, Y.; Di, S.; Tian, L.; Wang, F.; Wang, Z.; Lu, Y. Bimetallic ZIF-based PtCuCo/NC electrocatalyst Pt supported with an N-doped porous carbon for oxygen reduction reaction in PEM fuel cells. ACS Appl. Energy Mater. 2023, 6, 1575–1584. [Google Scholar] [CrossRef]
- Hu, C.; Hu, X.; Li, R.; Xing, Y. MOF derived ZnO/C nanocomposite with enhanced adsorption capacity and photocatalytic performance under sunlight. J. Hazard. Mater. 2020, 385, 121599. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Chen, L.; Thummavichai, K.; Ye, Z.; Wang, Y.; Fujita, T.; Wang, X. Amino-functionalized MOF-on-MOF architectural nanocomplexes composed for radioactive-iodine efficient adsorption. Chem. Eng. J. 2023, 474, 145858. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, J.; Wang, Z.; Zhang, J. Bimetallic Fe-Cu-based metal-organic frameworks as efficient adsorbents for gaseous elemental mercury removal. Ind. Eng. Chem. Res. 2020, 60, 781–789. [Google Scholar] [CrossRef]
- Jin, Y.; Wu, J.; Wang, J.; Fan, Y.; Zhang, S.; Ma, N.; Dai, W. Highly efficient capture of benzothiophene with a novel water-resistant-bimetallic Cu-ZIF-8 material. Inorg. Chim. Acta 2020, 503, 119412. [Google Scholar] [CrossRef]
- Wei, Y.; Liu, Y.; Wang, T.; Zhang, G.; Yang, L.; He, C.; Xiong, Z.; Pan, Z.; Lai, B. N, S co-doped porous carbon to accelerate Fe3+/Fe2+ redox cycle for peroxymonosulfate activation. Sep. Purif. Technol. 2024, 328, 125080. [Google Scholar] [CrossRef]
- Luo, G.; Deng, Y.; Zhang, X.P.; Zou, R.; Sun, W.; Li, B.; Sun, B.; Wang, Y.; Li, G. A ZIF-8 derived nitrogen-doped porous carbon and nitrogen-doped graphene nanocomposite modified electrode for simultaneous determination of ascorbic acid, dopamine and uric acid. New J. Chem. 2019, 43, 16819–16828. [Google Scholar] [CrossRef]
- Sun, T.; Levin, B.D.A.; Guzman, J.J.L.; Enders, A.; Muller, D.A.; Angenent, L.T.; Lehmann, J. Rapid electron transfer by the carbon matrix in natural pyrogenic carbon. Nat. Commun. 2017, 8, 14873. [Google Scholar] [CrossRef] [PubMed]
- Demiral, H.; Güngör, C. Adsorption of copper(II) from aqueous solutions on activated carbon prepared from grape bagasse. J. Clean. Prod. 2016, 124, 103–113. [Google Scholar] [CrossRef]
- Karthik, R.; Meenakshi, S. Removal of Pb(II) and Cd(II) ions from aqueous solution using polyaniline grafted chitosan. Chem. Eng. J. 2015, 263, 168–177. [Google Scholar] [CrossRef]
- Pezoti, O.; Cazetta, A.L.; Bedin, K.C.; Souza, L.S.; Martins, A.C.; Silva, T.L.; Santos Júnior, O.O.; Visentainer, J.V.; Almeida, V.C. NaOH-activated carbon of high surface area produced from guava seeds as a high-efficiency adsorbent for amoxicillin removal: Kinetic, isotherm and thermodynamic studies. Chem. Eng. J. 2016, 288, 778–788. [Google Scholar] [CrossRef]
- Yun, S.; Zhang, Y.; Zhang, L.; Liu, Z.; Deng, Y. Ni and Fe nanoparticles, alloy and Ni/Fe-Nx coordination co-boost the catalytic activity of the carbon-based catalyst for triiodide reduction and hydrogen evolution reaction. J. Colloid Interface Sci. 2022, 615, 501–516. [Google Scholar] [CrossRef] [PubMed]
- Han, T.; Wang, L.; Potgieter, J.H. ZIF-11 derived nanoporous carbons with ultrahigh uptakes for capture and reversible storage of volatile iodine. J. Solid State Chem. 2020, 282, 121108. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, X. Convenient synthesis of novel Cu2O/Cu@C/SiO2 composite from rice-husk for efficient iodine gas capture. J. Radioanal. Nucl. Chem. 2024, 333, 4377–4386. [Google Scholar] [CrossRef]
- Yue, T.C.; Yin, L.; Huang, J.B.; Wang, L.L.; Wang, D.Z. Assembly of a three-dimensional Cu-MOF for efficient Fenton-like degradation of dyes and iodine capture. Appl. Organomet. Chem. 2024, 38, e7629. [Google Scholar] [CrossRef]
- Yu, Q.; Jiang, X.; Cheng, Z.; Liao, Y.; Duan, M. Porous ZIF-8@polyacrylonitrile composite beads for iodine capture. RSC Adv. 2021, 11, 30259–30269. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Chai, L.; Yang, Z.; Wang, Q. Kinetics and thermodynamics of Pb(II) adsorption onto modified spent grain from aqueous solutions. Appl. Surf. Sci. 2009, 255, 4298–4303. [Google Scholar] [CrossRef]
- Fu, H.; Tang, Y.; Yuan, Q.; Chang, J.; Liao, F.; Zhang, J.; Gao, H.; Yang, Y.; Liao, Y. Understanding iodine adsorption sites on monolithic N/O co-doped carbon fibers with scaffolding structure. Fuel 2024, 371, 132035. [Google Scholar] [CrossRef]
- Zhao, Q.; Zhu, L.; Lin, G.H.; Chen, G.Y.; Liu, B.; Zhang, L.; Duan, T.; Lei, J.H. Controllable Synthesis of Porous Cu-BTC@polymer Composite Beads for Iodine Capture. ACS Appl. Mater Interfaces 2019, 11, 42635–42645. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Xie, D.; Li, S.; Yang, X. Novel hydrophobic hierarchical porous carbon from sewage sludge for the efficient capture of gaseous iodine. Sep. Purif. Technol. 2024, 335, 126214. [Google Scholar] [CrossRef]
| Adsorbent | Iodine State | T (°C) | Adsorption Capacity (mg/g) | Equilibration Time (h) | Reference |
|---|---|---|---|---|---|
| Cu/MIL-101 | Gas/I2 | 75 | 432.0 | 24 | [23] |
| Cu0-SBA-15 | Cyclohexane/I2 | RT | 842.0 | 8 | [24] |
| Cu/ZnO@C | Cyclohexane/I2 | 25 | 1280.1 | 15 | [8] |
| Cu-MOF | Cyclohexane/I2 | RT | 971.0 | 10 | [31] |
| Cu2O/Cu@C/SiO2 | Gas/I2 | 150 | 820.0 | 2 | [58] |
| XJU-1 | Cyclohexane/I2 | RT | 368.0 | 48 | [59] |
| ZIF-8@polyacrylonitrile | Cyclohexane/I2 | RT | 643.0 | 24 | [60] |
| ZIF-8@wood | Gas/I2 | 75 | 1091.0 | 4.1 | [40] |
| CZIF-1000 | Cyclohexane/I2 | RT | 790.8 | 8 | [10] |
| 30%Cu@Zn-NC | Cyclohexane/I2 | RT | 1204.9 | 0.5 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.; Gao, C.; Chen, J.; Liu, F.; Liu, Z. Cu0-Functionalized, ZIF-8-Derived, Nitrogen-Doped Carbon Composites for Efficient Iodine Elimination in Solution. Nanomaterials 2025, 15, 105. https://doi.org/10.3390/nano15020105
Chen J, Gao C, Chen J, Liu F, Liu Z. Cu0-Functionalized, ZIF-8-Derived, Nitrogen-Doped Carbon Composites for Efficient Iodine Elimination in Solution. Nanomaterials. 2025; 15(2):105. https://doi.org/10.3390/nano15020105
Chicago/Turabian StyleChen, Jiuyu, Chensheng Gao, Jingwen Chen, Fei Liu, and Zhiwen Liu. 2025. "Cu0-Functionalized, ZIF-8-Derived, Nitrogen-Doped Carbon Composites for Efficient Iodine Elimination in Solution" Nanomaterials 15, no. 2: 105. https://doi.org/10.3390/nano15020105
APA StyleChen, J., Gao, C., Chen, J., Liu, F., & Liu, Z. (2025). Cu0-Functionalized, ZIF-8-Derived, Nitrogen-Doped Carbon Composites for Efficient Iodine Elimination in Solution. Nanomaterials, 15(2), 105. https://doi.org/10.3390/nano15020105





