Catalytic Pyrolysis of Biomass: A Review of Zeolite, Carbonaceous, and Metal Oxide Catalysts
Abstract
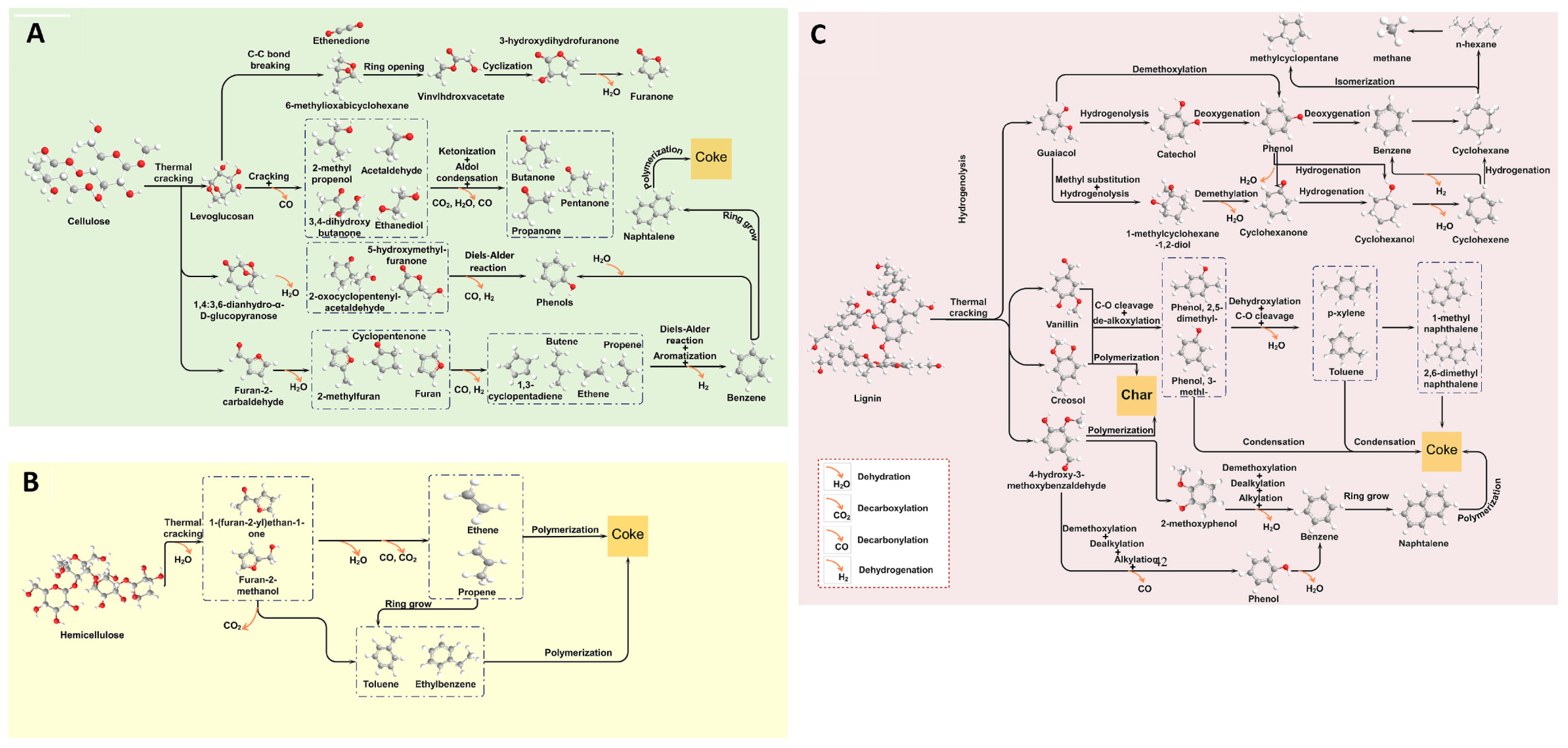
1. Introduction
2. Different Types of Pyrolysis
2.1. Slow Pyrolysis
2.2. Fast Pyrolysis
2.3. Flash Pyrolysis
3. Influential Parameters on Bio-Oil Yield
3.1. Biomass Characteristic
| Biomass | Biomass Component (%) | Biomass Yields (wt%) | Ref. | ||||
|---|---|---|---|---|---|---|---|
| Cellulose | Hemicellulose | Lignin | Liquid | Biochar | Biogas | ||
| Hardwood | 45–50 | 20–25 | 20–25 | 53.9 | 26.2 | 19.9 | [34] |
| Hardwood | 45–50 | 20–25 | 20–25 | 63.3 | 12.7 | 24 | [35] |
| Softwood | 35–40 | 20–25 | 27–30 | 45 | 27.6 | 27.4 | [34] |
| Softwood | 35–40 | 20–25 | 27–30 | 39.7 | 32.4 | 28.9 | [36] |
| Lignin free | 31–24 | 15–25 | / | 30 | 25 | 25 | [37] |
3.2. Effect of Pyrolysis Mode
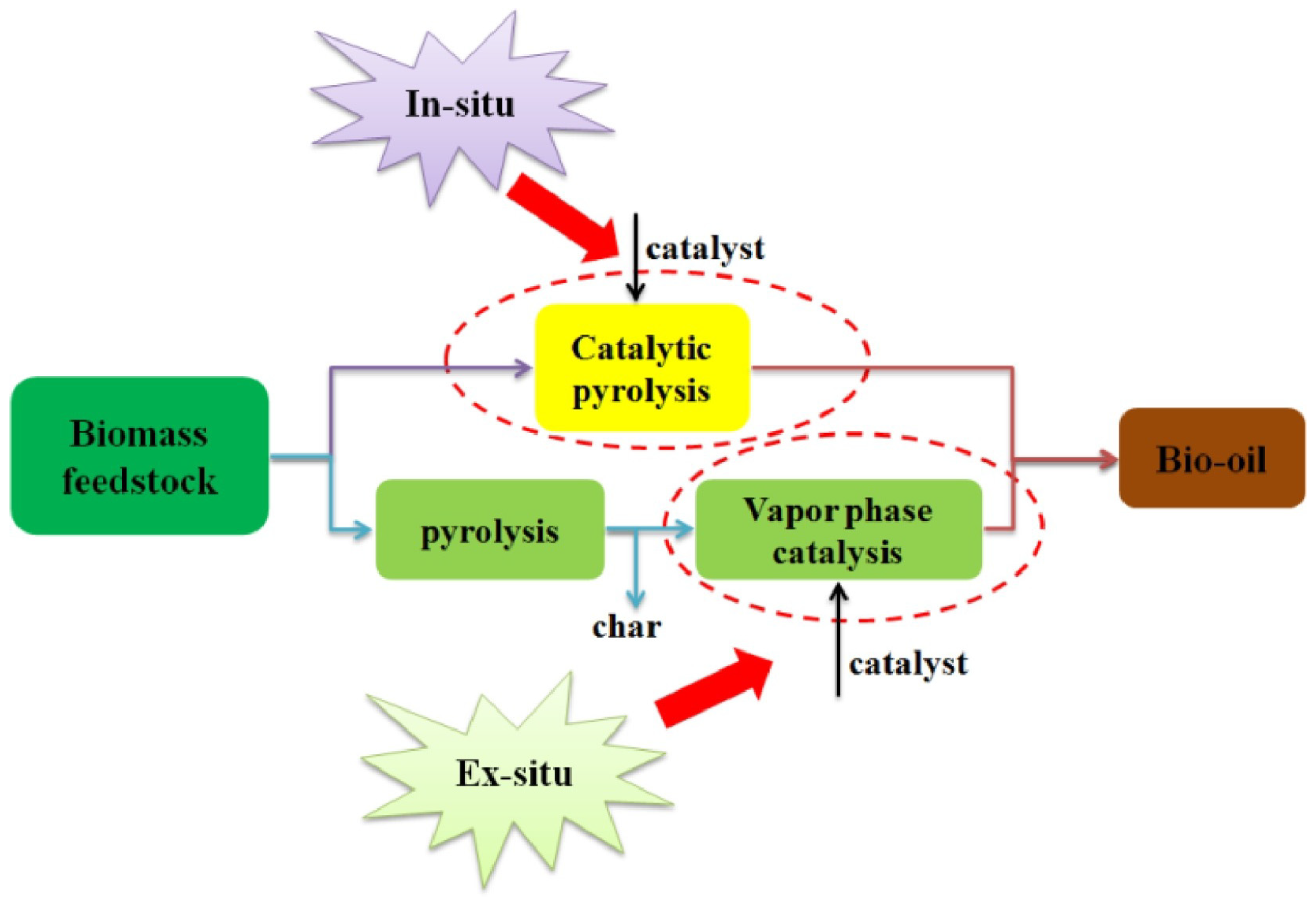
3.3. Effect of Catalysts
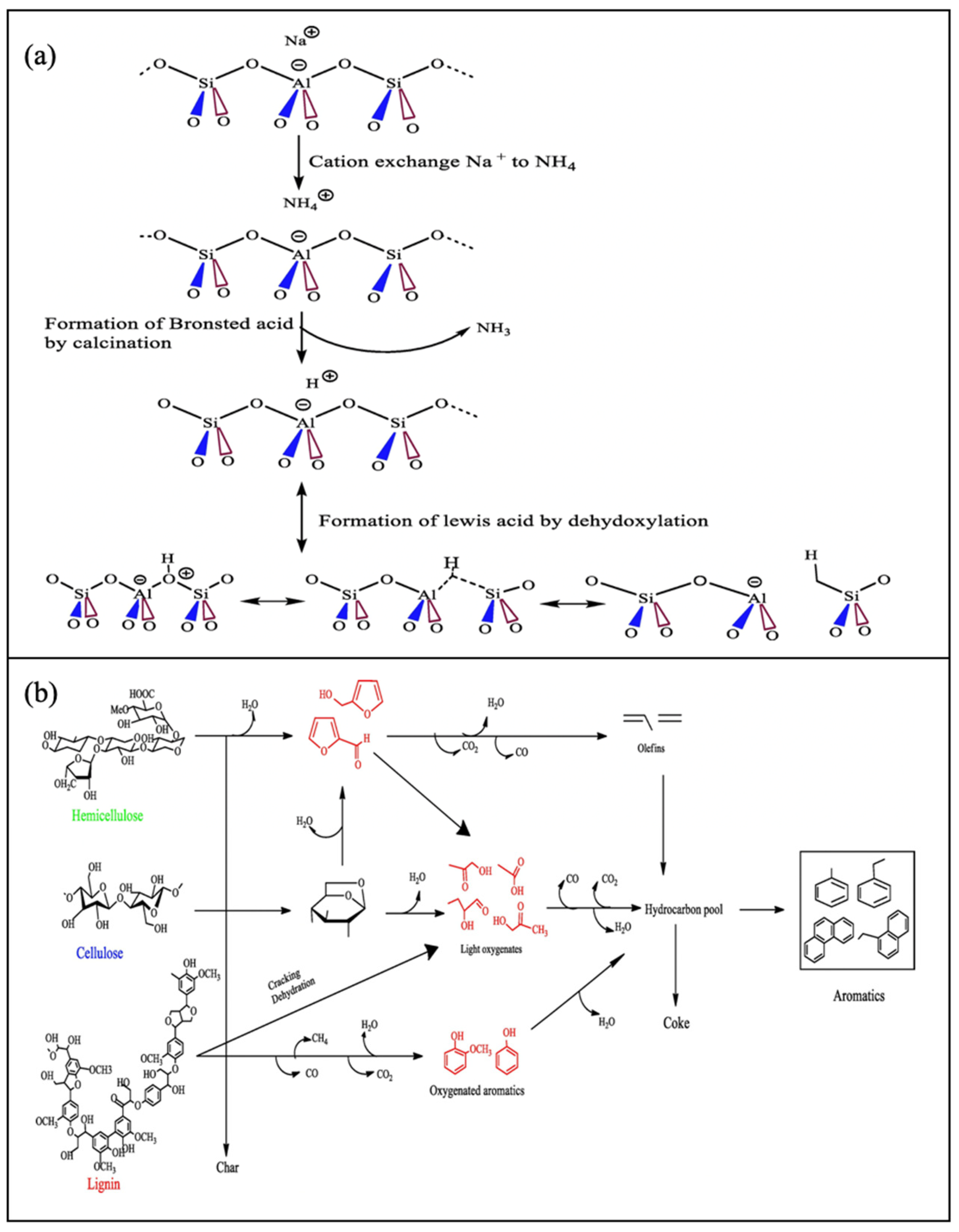
3.3.1. Zeolite Based Catalyst
3.3.2. Metal Oxide Catalysts
3.3.3. Carbonaceous Materials Catalysts
3.4. Impact of Nanostructured Catalysts on Bio-Oil Yield
4. Conclusions and Future Work
Author Contributions
Funding
Conflicts of Interest
References
- Antar, M.; Lyu, D.; Nazari, M.; Shah, A.; Zhou, X.; Smith, D.L. Biomass for a sustainable bioeconomy: An overview of world biomass production and utilization. Renew. Sustain. Energy Rev. 2021, 139, 110691. [Google Scholar] [CrossRef]
- Amenaghawon, A.N.; Anyalewechi, C.L.; Okieimen, C.O.; Kusuma, H.S. Biomass pyrolysis technologies for value-added products: A state-of-the-art review. Environ. Dev. Sustain. 2021, 23, 14324–14378. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, K.; Tao, Y.; Yang, Q.; Xu, L.; Liu, C.; Ma, L.; Xiao, R. Biomass directional pyrolysis based on element economy to produce high-quality fuels, chemicals, carbon materials—A review. Biotechnol. Adv. 2023, 69, 108262. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Chen, Y.; Lin, Y.; Chen, W.; Hu, J.; Yang, W.; Chang, C.; Pang, S. Research progress on the preparation of high-value carbon materials by biomass pyrolysis. Biomass Bioenergy 2025, 193, 107520. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, R.; Yin, R.; Mei, Y. Upgrading of bio-oil from biomass fast pyrolysis in China: A review. Renew. Sustain. Energy Rev. 2013, 24, 66–72. [Google Scholar] [CrossRef]
- Vuppaladadiyam, A.K.; Vuppaladadiyam, S.S.V.; Awasthi, A.; Sahoo, A.; Rehman, S.; Pant, K.K.; Murugavelh, S.; Huang, Q.; Anthony, E.; Fennel, P.; et al. Biomass pyrolysis: A review on recent advancements and green hydrogen production. Bioresour. Technol. 2022, 364, 128087. [Google Scholar] [CrossRef] [PubMed]
- Dada, T.K.; Sheehan, M.; Murugavelh, S.; Antunes, E. A review on catalytic pyrolysis for high-quality bio-oil production from biomass. Biomass Convers. Biorefin. 2023, 13, 2595–2614. [Google Scholar] [CrossRef]
- French, R.; Czernik, S. Catalytic pyrolysis of biomass for biofuels production. Fuel Process. Technol. 2010, 91, 25–32. [Google Scholar] [CrossRef]
- Hassan, N.S.; Jalil, A.A.; Hitam, C.N.C.; Vo, D.V.N.; Nabgan, W. Biofuels and renewable chemicals production by catalytic pyrolysis of cellulose: A review. Environ. Chem. Lett. 2020, 18, 1625–1648. [Google Scholar] [CrossRef]
- Luna-Murillo, B.; Pala, M.; Paioni, A.L.; Baldus, M.; Ronsse, F.; Prins, W.; Bruijnincx, P.C.A.; Weckhuysen, B.M. Catalytic Fast Pyrolysis of Biomass: Catalyst Characterization Reveals the Feed-Dependent Deactivation of a Technical ZSM-5-Based Catalyst. ACS Sustain. Chem. Eng. 2021, 9, 291–304. [Google Scholar] [CrossRef]
- Cai, R.; Pei, X.; Pan, H.; Wan, K.; Chen, H.; Zhang, Z.; Zhang, Y. Biomass Catalytic Pyrolysis over Zeolite Catalysts with an Emphasis on Porosity and Acidity: A State-of-the-Art Review. Energy Fuels 2020, 34, 11771–11790. [Google Scholar] [CrossRef]
- Kumar Mishra, R.; Singh, B.; Acharya, B. A comprehensive review on activated carbon from pyrolysis of lignocellulosic biomass: An application for energy and the environment. Carbon Resour. Convers. 2024, 7, 100228. [Google Scholar] [CrossRef]
- Villora-Picó, J.J.; González-Arias, J.; Baena-Moreno, F.M.; Reina, T.R. Renewable Carbonaceous Materials from Biomass in Catalytic Processes: A Review. Materials 2024, 17, 565. [Google Scholar] [CrossRef]
- Chong, Y.Y.; Thangalazhy-Gopakumar, S.; Ng, H.K.; Gan, S.; Lee, L.Y.; Ganesan, P.B. Catalytic pyrolysis of cellulose with oxides: Effects on physical properties and reaction pathways. Clean Technol. Environ. Policy 2019, 21, 1629–1643. [Google Scholar] [CrossRef]
- Ouedraogo, A.S.; Bhoi, P.R. Recent progress of metals supported catalysts for hydrodeoxygenation of biomass derived pyrolysis oil. J. Clean. Prod. 2020, 253, 119957. [Google Scholar] [CrossRef]
- Sekar, M.; Mathimani, T.; Alagumalai, A.; Chi, N.T.L.; Duc, P.A.; Bhatia, S.K.; Brindhadevi, K.; Pugazhendhi, A. A review on the pyrolysis of algal biomass for biochar and bio-oil—Bottlenecks and scope. Fuel 2021, 283, 119190. [Google Scholar] [CrossRef]
- Premchand, P.; Demichelis, F.; Chiaramonti, D.; Bensaid, S.; Fino, D. Biochar production from slow pyrolysis of biomass under CO2 atmosphere: A review on the effect of CO2 medium on biochar production, characterisation, and environmental applications. J. Environ. Chem. Eng. 2023, 11, 110009. [Google Scholar] [CrossRef]
- Al-Rumaihi, A.; Shahbaz, M.; McKay, G.; Mackey, H.; Al-Ansari, T. A review of pyrolysis technologies and feedstock: A blending approach for plastic and biomass towards optimum biochar yield. Renew. Sustain. Energy Rev. 2022, 167, 112715. [Google Scholar] [CrossRef]
- Raza, M.; Inayat, A.; Ahmed, A.; Jamil, F.; Ghenai, C.; Naqvi, S.R.; Shanableh, A.; Ayoub, M.; Waris, A.; Park, Y.-K. Progress of the Pyrolyzer Reactors and Advanced Technologies for Biomass Pyrolysis Processing. Sustainability 2021, 13, 11061. [Google Scholar] [CrossRef]
- Yang, C.; Li, R.; Zhang, B.; Qiu, Q.; Wang, B.; Yang, H.; Ding, Y.; Wang, C. Pyrolysis of microalgae: A critical review. Fuel Process. Technol. 2019, 186, 53–72. [Google Scholar] [CrossRef]
- Vuppaladadiyam, A.K.; Vuppaladadiyam, S.S.V.; Sahoo, A.; Murugavelh, S.; Anthony, E.; Bhaskar, T.; Zheng, Y.; Zhao, M.; Duan, H.; Zhao, Y.; et al. Bio-oil and biochar from the pyrolytic conversion of biomass: A current and future perspective on the trade-off between economic, environmental, and technical indicators. Sci. Total Environ. 2023, 857, 159155. [Google Scholar] [CrossRef] [PubMed]
- Hoekstra, E.; van Swaaij, W.P.M.; Kersten, S.R.A.; Hogendoorn, K.J.A. Fast pyrolysis in a novel wire-mesh reactor: Design and initial results. Chem. Eng. J. 2012, 191, 45–58. [Google Scholar] [CrossRef]
- Mong, G.R.; Chong, C.T.; Chong, W.W.F.; Ng, J.-H.; Ong, H.C.; Ashokkumar, V.; Tran, M.-V.; Karmakar, S.; Goh, B.H.H.; Mohd Yasin, M.F. Progress and challenges in sustainable pyrolysis technology: Reactors, feedstocks and products. Fuel 2022, 324, 124777. [Google Scholar] [CrossRef]
- Nunez Manzano, M.; Gonzalez Quiroga, A.; Perreault, P.; Madanikashani, S.; Vandewalle, L.A.; Marin, G.B.; Heynderickx, G.J.; Van Geem, K.M. Biomass fast pyrolysis in an innovative gas-solid vortex reactor: Experimental proof of concept. J. Anal. Appl. Pyrolysis 2021, 156, 105165. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, Y.; Omairey, E.; Cai, J.; Gu, F.; Bridgwater, A.V. Intermediate pyrolysis of organic fraction of municipal solid waste and rheological study of the pyrolysis oil for potential use as bio-bitumen. J. Clean. Prod. 2018, 187, 390–399. [Google Scholar] [CrossRef]
- Adelawon, B.O.; Latinwo, G.K.; Eboibi, B.E.; Agbede, O.O.; Agarry, S.E. Comparison of the slow, fast, and flash pyrolysis of recycled maize-cob biomass waste, box-benhken process optimization and characterization studies for the thermal fast pyrolysis production of bio-energy. Chem. Eng. Commun. 2022, 209, 1246–1276. [Google Scholar] [CrossRef]
- Ighalo, J.O.; Iwuchukwu, F.U.; Eyankware, O.E.; Iwuozor, K.O.; Olotu, K.; Bright, O.C.; Igwegbe, C.A. Flash pyrolysis of biomass: A review of recent advances. Clean Technol. Environ. Policy 2022, 24, 2349–2363. [Google Scholar] [CrossRef]
- Kan, T.; Strezov, V.; Evans, T.J. Lignocellulosic biomass pyrolysis: A review of product properties and effects of pyrolysis parameters. Renew. Sustain. Energy Rev. 2016, 57, 1126–1140. [Google Scholar] [CrossRef]
- Zhang, C.; Chao, L.; Zhang, Z.; Zhang, L.; Li, Q.; Fan, H.; Zhang, S.; Liu, Q.; Qiao, Y.; Tian, Y.; et al. Pyrolysis of cellulose: Evolution of functionalities and structure of bio-char versus temperature. Renew. Sustain. Energy Rev. 2021, 135, 110416. [Google Scholar] [CrossRef]
- Dai, G.; Wang, G.; Wang, K.; Zhou, Z.; Wang, S. Mechanism study of hemicellulose pyrolysis by combining in-situ DRIFT, TGA-PIMS and theoretical calculation. Proc. Combust. Inst. 2021, 38, 4241–4249. [Google Scholar] [CrossRef]
- Chen, D.; Cen, K.; Zhuang, X.; Gan, Z.; Zhou, J.; Zhang, Y.; Zhang, H. Insight into biomass pyrolysis mechanism based on cellulose, hemicellulose, and lignin: Evolution of volatiles and kinetics, elucidation of reaction pathways, and characterization of gas, biochar and bio-oil. Combust. Flame 2022, 242, 112142. [Google Scholar] [CrossRef]
- Leng, E.; Guo, Y.; Chen, J.; Liu, S.; Xue, Y. A comprehensive review on lignin pyrolysis: Mechanism, modeling and the effects of inherent metals in biomass. Fuel 2022, 309, 122102. [Google Scholar] [CrossRef]
- Shafizadeh, A.; Rastegari, H.; Shahbeik, H.; Mobli, H.; Pan, J.; Peng, W.; Li, G.; Tabatabaei, M.; Aghbashlo, M. A critical review of the use of nanomaterials in the biomass pyrolysis process. J. Clean. Prod. 2023, 400, 136705. [Google Scholar] [CrossRef]
- Garcìa-Pérez, M.; Chaala, A.; Pakdel, H.; Kretschmer, D.; Roy, C. Vacuum pyrolysis of softwood and hardwood biomass: Comparison between product yields and bio-oil properties. J. Anal. Appl. Pyrolysis 2007, 78, 104–116. [Google Scholar] [CrossRef]
- Agblevor, F.A.; Beis, S.; Kim, S.S.; Tarrant, R.; Mante, N.O. Biocrude oils from the fast pyrolysis of poultry litter and hardwood. Waste Manag. 2010, 30, 298–307. [Google Scholar] [CrossRef] [PubMed]
- Demirbas, M.F. Characterization of Bio-oils from Spruce Wood (Picea orientalis L.) via Pyrolysis. Energy Sources Part A Recovery Util. Environ. Eff. 2010, 32, 909–916. [Google Scholar] [CrossRef]
- Umeki, K.; Moilanen, A.; Gómez-Barea, A.; Konttinen, J. A model of biomass char gasification describing the change in catalytic activity of ash. Chem. Eng. J. 2012, 207-208, 616–624. [Google Scholar] [CrossRef]
- Wang, S.; Ru, B.; Lin, H.; Sun, W.; Yu, C.; Luo, Z. Pyrolysis mechanism of hemicellulose monosaccharides in different catalytic processes. Chem. Res. Chin. Univ. 2014, 30, 848–854. [Google Scholar] [CrossRef]
- Yildiz, G.; Pronk, M.; Djokic, M.; van Geem, K.M.; Ronsse, F.; van Duren, R.; Prins, W. Validation of a new set-up for continuous catalytic fast pyrolysis of biomass coupled with vapour phase upgrading. J. Anal. Appl. Pyrolysis 2013, 103, 343–351. [Google Scholar] [CrossRef]
- Wang, K.; Johnston, P.A.; Brown, R.C. Comparison of in-situ and ex-situ catalytic pyrolysis in a micro-reactor system. Bioresour. Technol. 2014, 173, 124–131. [Google Scholar] [CrossRef]
- Wang, S.; Dai, G.; Yang, H.; Luo, Z. Lignocellulosic biomass pyrolysis mechanism: A state-of-the-art review. Prog. Energy Combust. Sci. 2017, 62, 33–86. [Google Scholar] [CrossRef]
- Sankaranarayanan, S.; Won, W. Catalytic pyrolysis of biomass to produce bio-oil using layered double hydroxides (LDH)-derived materials. GCB Bioenergy 2024, 16, e13124. [Google Scholar] [CrossRef]
- Reza, M.S.; Iskakova, Z.B.; Afroze, S.; Kuterbekov, K.; Kabyshev, A.; Bekmyrza, K.Z.; Kubenova, M.M.; Bakar, M.S.; Azad, A.K.; Roy, H.; et al. Influence of Catalyst on the Yield and Quality of Bio-Oil for the Catalytic Pyrolysis of Biomass: A Comprehensive Review. Energies 2023, 16, 5547. [Google Scholar] [CrossRef]
- Stošić, D.; Auroux, A. Characterization of Acid–Base Sites in Zeolites. In Calorimetry and Thermal Methods in Catalysis; Auroux, A., Ed.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 353–384. [Google Scholar]
- Rahman, M.M.; Liu, R.; Cai, J. Catalytic fast pyrolysis of biomass over zeolites for high quality bio-oil—A review. Fuel Process. Technol. 2018, 180, 32–46. [Google Scholar] [CrossRef]
- Yu, Y.; Li, X.; Su, L.; Zhang, Y.; Wang, Y.; Zhang, H. The role of shape selectivity in catalytic fast pyrolysis of lignin with zeolite catalysts. Appl. Catal. A Gen. 2012, 447–448, 115–123. [Google Scholar] [CrossRef]
- Mihalcik, D.J.; Mullen, C.A.; Boateng, A.A. Screening acidic zeolites for catalytic fast pyrolysis of biomass and its components. J. Anal. Appl. Pyrolysis 2011, 92, 224–232. [Google Scholar] [CrossRef]
- Rahman, M.M.; Chai, M.; Sarker, M.; Nishu; Liu, R. Catalytic pyrolysis of pinewood over ZSM-5 and CaO for aromatic hydrocarbon: Analytical Py-GC/MS study. J. Energy Inst. 2020, 93, 425–435. [Google Scholar] [CrossRef]
- Hu, C.; Xiao, R.; Zhang, H. Ex-situ catalytic fast pyrolysis of biomass over HZSM-5 in a two-stage fluidized-bed/fixed-bed combination reactor. Bioresour. Technol. 2017, 243, 1133–1140. [Google Scholar] [CrossRef]
- Dai, G.; Wang, S.; Zou, Q.; Huang, S. Improvement of aromatics production from catalytic pyrolysis of cellulose over metal-modified hierarchical HZSM-5. Fuel Process. Technol. 2018, 179, 319–323. [Google Scholar] [CrossRef]
- Xue, X.; Liu, Y.; Wu, L.; Pan, X.; Liang, J.; Sun, Y. Catalytic fast pyrolysis of maize straw with a core–shell ZSM-5@SBA-15 catalyst for producing phenols and hydrocarbons. Bioresour. Technol. 2019, 289, 121691. [Google Scholar] [CrossRef]
- Lin, X.; Zhang, Z.; Wang, Q. Evaluation of zeolite catalysts on product distribution and synergy during wood-plastic composite catalytic pyrolysis. Energy 2019, 189, 116174. [Google Scholar] [CrossRef]
- Lazaridis, P.A.; Fotopoulos, A.P.; Karakoulia, S.A.; Triantafyllidis, K.S. Catalytic Fast Pyrolysis of Kraft Lignin With Conventional, Mesoporous and Nanosized ZSM-5 Zeolite for the Production of Alkyl-Phenols and Aromatics. Front. Chem. 2018, 6, 295. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-M.; Jeong, J.; Ryu, S.; Lee, H.W.; Jung, J.S.; Siddiqui, M.Z.; Jung, S.-C.; Jeon, J.-K.; Jae, J.; Park, Y.-K. Catalytic pyrolysis of wood polymer composites over hierarchical mesoporous zeolites. Energy Convers. Manag. 2019, 195, 727–737. [Google Scholar] [CrossRef]
- Zheng, Y.; Wang, F.; Yang, X.; Huang, Y.; Liu, C.; Zheng, Z.; Gu, J. Study on aromatics production via the catalytic pyrolysis vapor upgrading of biomass using metal-loaded modified H-ZSM-5. J. Anal. Appl. Pyrolysis 2017, 126, 169–179. [Google Scholar] [CrossRef]
- Mullen, C.A.; Tarves, P.C.; Boateng, A.A. Role of Potassium Exchange in Catalytic Pyrolysis of Biomass over ZSM-5: Formation of Alkyl Phenols and Furans. ACS Sustain. Chem. Eng. 2017, 5, 2154–2162. [Google Scholar] [CrossRef]
- Che, Q.; Yang, M.; Wang, X.; Yang, Q.; Rose Williams, L.; Yang, H.; Zou, J.; Zeng, K.; Zhu, Y.; Chen, Y.; et al. Influence of physicochemical properties of metal modified ZSM-5 catalyst on benzene, toluene and xylene production from biomass catalytic pyrolysis. Bioresour. Technol. 2019, 278, 248–254. [Google Scholar] [CrossRef]
- Zhang, Z.; Cheng, H.; Chen, H.; Chen, K.; Lu, X.; Ouyang, P.; Fu, J. Enhancement in the aromatic yield from the catalytic fast pyrolysis of rice straw over hexadecyl trimethyl ammonium bromide modified hierarchical HZSM-5. Bioresour. Technol. 2018, 256, 241–246. [Google Scholar] [CrossRef]
- Li, X.; Zhang, X.; Shao, S.; Dong, L.; Zhang, J.; Hu, C.; Cai, Y. Catalytic upgrading of pyrolysis vapor from rape straw in a vacuum pyrolysis system over La/HZSM-5 with hierarchical structure. Bioresour. Technol. 2018, 259, 191–197. [Google Scholar] [CrossRef]
- Jiang, L.; Wang, Y.; Dai, L.; Yu, Z.; Yang, Q.; Yang, S.; Jiang, D.; Ma, Z.; Wu, Q.; Zhang, B.; et al. Co-pyrolysis of biomass and soapstock in a downdraft reactor using a novel ZSM-5/SiC composite catalyst. Bioresour. Technol. 2019, 279, 202–208. [Google Scholar] [CrossRef]
- Ding, K.; He, A.; Zhong, D.; Fan, L.; Liu, S.; Wang, Y.; Liu, Y.; Chen, P.; Lei, H.; Ruan, R. Improving hydrocarbon yield via catalytic fast co-pyrolysis of biomass and plastic over ceria and HZSM-5: An analytical pyrolyzer analysis. Bioresour. Technol. 2018, 268, 1–8. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, H.; Liu, X.; Zhu, S.; Hu, L.; Zhang, Q. Upgrading of bio-oil from catalytic pyrolysis of pretreated rice husk over Fe-modified ZSM-5 zeolite catalyst. Fuel Process. Technol. 2018, 175, 17–25. [Google Scholar] [CrossRef]
- Gu, B.; Cao, J.-P.; Wei, F.; Zhao, X.-Y.; Ren, X.-Y.; Zhu, C.; Guo, Z.-X.; Bai, J.; Shen, W.-Z.; Wei, X.-Y. Nitrogen migration mechanism and formation of aromatics during catalytic fast pyrolysis of sewage sludge over metal-loaded HZSM-5. Fuel 2019, 244, 151–158. [Google Scholar] [CrossRef]
- Wang, S.; Cao, B.; Liu, X.; Xu, L.; Hu, Y.; Afonaa-Mensah, S.; Abomohra, A.E.-F.; He, Z.; Wang, Q.; Xu, S. A comparative study on the quality of bio-oil derived from green macroalga Enteromorpha clathrata over metal modified ZSM-5 catalysts. Bioresour. Technol. 2018, 256, 446–455. [Google Scholar] [CrossRef]
- Yang, M.; Shao, J.; Yang, H.; Zeng, K.; Wu, Z.; Chen, Y.; Bai, X.; Chen, H. Enhancing the production of light olefins and aromatics from catalytic fast pyrolysis of cellulose in a dual-catalyst fixed bed reactor. Bioresour. Technol. 2019, 273, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Chen, P.; Zhou, N.; Liu, S.; Zhang, Y.; Liu, Y.; Wang, Y.; Omar, M.M.; Peng, P.; Addy, M.; et al. In-situ and ex-situ catalytic upgrading of vapors from microwave-assisted pyrolysis of lignin. Bioresour. Technol. 2018, 247, 851–858. [Google Scholar] [CrossRef] [PubMed]
- Paysepar, H.; Rao, K.T.V.; Yuan, Z.; Nazari, L.; Shui, H.; Xu, C. Zeolite catalysts screening for production of phenolic bio-oils with high contents of monomeric aromatics/phenolics from hydrolysis lignin via catalytic fast pyrolysis. Fuel Process. Technol. 2018, 178, 362–370. [Google Scholar] [CrossRef]
- Paysepar, H.; Rao, K.T.V.; Yuan, Z.; Shui, H.; Xu, C. Improving activity of ZSM-5 zeolite catalyst for the production of monomeric aromatics/phenolics from hydrolysis lignin via catalytic fast pyrolysis. Appl. Catal. A Gen. 2018, 563, 154–162. [Google Scholar] [CrossRef]
- Zhao, T.; Li, F.; Yu, H.; Ding, S.; Li, Z.; Huang, X.; Li, X.; Wei, X.; Wang, Z.; Lin, H. Synthesis of mesoporous ZSM-5 zeolites and catalytic cracking of ethanol and oleic acid into light olefins. Appl. Catal. A Gen. 2019, 575, 101–110. [Google Scholar] [CrossRef]
- Casoni, A.I.; Nievas, M.L.; Moyano, E.L.; Álvarez, M.; Diez, A.; Dennehy, M.; Volpe, M.A. Catalytic pyrolysis of cellulose using MCM-41 type catalysts. Appl. Catal. A Gen. 2016, 514, 235–240. [Google Scholar] [CrossRef]
- Qiu, B.; Tao, X.; Wang, J.; Liu, Y.; Li, S.; Chu, H. Research progress in the preparation of high-quality liquid fuels and chemicals by catalytic pyrolysis of biomass: A review. Energy Convers. Manag. 2022, 261, 115647. [Google Scholar] [CrossRef]
- Zhang, J.; Gu, J.; Yuan, H.; Chen, Y. Catalytic fast pyrolysis of waste mixed cloth for the production of value-added chemicals. Waste Manag. 2021, 127, 141–146. [Google Scholar] [CrossRef]
- Chong, Y.Y.; Thangalazhy-Gopakumar, S.; Ng, H.K.; Lee, L.Y.; Gan, S. Effect of oxide catalysts on the properties of bio-oil from in-situ catalytic pyrolysis of palm empty fruit bunch fiber. J. Environ. Manag. 2019, 247, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Chireshe, F.; Collard, F.-X.; Görgens, J.F. Production of an upgraded bio-oil with minimal water content by catalytic pyrolysis: Optimisation and comparison of CaO and MgO performances. J. Anal. Appl. Pyrolysis 2020, 146, 104751. [Google Scholar] [CrossRef]
- Gupta, J.; Papadikis, K.; Konysheva, E.Y.; Lin, Y.; Kozhevnikov, I.V.; Li, J. CaO catalyst for multi-route conversion of oakwood biomass to value-added chemicals and fuel precursors in fast pyrolysis. Appl. Catal. B Environ. 2021, 285, 119858. [Google Scholar] [CrossRef]
- Raymundo, L.M.; Mullen, C.A.; Boateng, A.A.; DeSisto, W.J.; Trierweiler, J.O. Production of Partially Deoxygenated Pyrolysis Oil from Switchgrass via Ca(OH)2, CaO, and Ca(COOH)2 Cofeeding. Energy Fuels 2020, 34, 12616–12625. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, L.; Li, Q.; Wang, Y.; Liu, Q.; Wei, T.; Dong, D.; Salavati, S.; Gholizadeh, M.; Hu, X. Catalytic pyrolysis of poplar wood over transition metal oxides: Correlation of catalytic behaviors with physiochemical properties of the oxides. Biomass Bioenergy 2019, 124, 125–141. [Google Scholar] [CrossRef]
- Veses, A.; Aznar, M.; Martínez, I.; Martínez, J.D.; López, J.M.; Navarro, M.V.; Callén, M.S.; Murillo, R.; García, T. Catalytic pyrolysis of wood biomass in an auger reactor using calcium-based catalysts. Bioresour. Technol. 2014, 162, 250–258. [Google Scholar] [CrossRef]
- Wang, W.; Lemaire, R.; Bensakhria, A.; Luart, D. Review on the catalytic effects of alkali and alkaline earth metals (AAEMs) including sodium, potassium, calcium and magnesium on the pyrolysis of lignocellulosic biomass and on the co-pyrolysis of coal with biomass. J. Anal. Appl. Pyrolysis 2022, 163, 105479. [Google Scholar] [CrossRef]
- Chen, X.; Chen, Y.; Yang, H.; Chen, W.; Wang, X.; Chen, H. Fast pyrolysis of cotton stalk biomass using calcium oxide. Bioresour. Technol. 2017, 233, 15–20. [Google Scholar] [CrossRef]
- Vichaphund, S.; Sricharoenchaikul, V.; Atong, D. Industrial waste derived CaO-based catalysts for upgrading volatiles during pyrolysis of Jatropha residues. J. Anal. Appl. Pyrolysis 2017, 124, 568–575. [Google Scholar] [CrossRef]
- Liu, C.; Wang, H.; Karim, A.M.; Sun, J.; Wang, Y. Catalytic fast pyrolysis of lignocellulosic biomass. Chem. Soc. Rev. 2014, 43, 7594–7623. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Zhang, Z.-x.; Xie, W.-l.; Liu, J.; Li, Y.; Zhang, W.-m.; Fu, H.; Lu, Q. Advances on the fast pyrolysis of biomass for the selective preparation of phenolic compounds. Fuel Process. Technol. 2022, 237, 107465. [Google Scholar] [CrossRef]
- Sharifzadeh, M.; Sadeqzadeh, M.; Guo, M.; Borhani, T.N.; Murthy Konda, N.V.S.N.; Garcia, M.C.; Wang, L.; Hallett, J.; Shah, N. The multi-scale challenges of biomass fast pyrolysis and bio-oil upgrading: Review of the state of art and future research directions. Prog. Energy Combust. Sci. 2019, 71, 1–80. [Google Scholar] [CrossRef]
- Lin, X.; Zhang, Z.; Zhang, Z.; Sun, J.; Wang, Q.; Pittman, C.U. Catalytic fast pyrolysis of a wood-plastic composite with metal oxides as catalysts. Waste Manag. 2018, 79, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Hu, B.; Fu, H.; Zhang, Z.-x.; Guo, Z.-t.; Zhou, G.-z.; Zhu, L.-j.; Liu, J.; Lu, Q. Fast pyrolysis of bagasse catalyzed by mixed alkaline-earth metal oxides for the selective production of 4-vinylphenol. J. Anal. Appl. Pyrolysis 2022, 164, 105531. [Google Scholar] [CrossRef]
- de Rezende Locatel, W.; Laurenti, D.; Schuurman, Y.; Guilhaume, N. Ex-situ catalytic upgrading of pyrolysis vapors using mixed metal oxides. J. Anal. Appl. Pyrolysis 2021, 158, 105241. [Google Scholar] [CrossRef]
- Zhang, X.; Sun, L.; Chen, L.; Xie, X.; Zhao, B.; Si, H.; Meng, G. Comparison of catalytic upgrading of biomass fast pyrolysis vapors over CaO and Fe(III)/CaO catalysts. J. Anal. Appl. Pyrolysis 2014, 108, 35–40. [Google Scholar] [CrossRef]
- Sun, L.; Zhang, X.; Chen, L.; Zhao, B.; Yang, S.; Xie, X. Effects of Fe contents on fast pyrolysis of biomass with Fe/CaO catalysts. J. Anal. Appl. Pyrolysis 2016, 119, 133–138. [Google Scholar] [CrossRef]
- Kantarelis, E.; Yang, W.; Blasiak, W. Effects of Silica-Supported Nickel and Vanadium on Liquid Products of Catalytic Steam Pyrolysis of Biomass. Energy Fuels 2014, 28, 591–599. [Google Scholar] [CrossRef]
- Ng, C.H.; Mistoh, M.A.; Teo, S.H.; Galassi, A.; Taufiq-Yap, Y.H.; Siambun, N.J.; Foo, J.; Sipaut, C.S.; Seay, J.; Janaun, J. The roles of carbonaceous wastes for catalysis, energy, and environmental remediation. Catal. Commun. 2024, 187, 106845. [Google Scholar] [CrossRef]
- Mateo, W.; Lei, H.; Villota, E.; Qian, M.; Zhao, Y.; Huo, E.; Zhang, Q.; Lin, X.; Wang, C.; Huang, Z. Synthesis and characterization of sulfonated activated carbon as a catalyst for bio-jet fuel production from biomass and waste plastics. Bioresour. Technol. 2020, 297, 122411. [Google Scholar] [CrossRef]
- Duan, D.; Chen, D.; Huang, L.; Zhang, Y.; Zhang, Y.; Wang, Q.; Xiao, G.; Zhang, W.; Lei, H.; Ruan, R. Activated carbon from lignocellulosic biomass as catalyst: A review of the applications in fast pyrolysis process. J. Anal. Appl. Pyrolysis 2021, 158, 105246. [Google Scholar] [CrossRef]
- Zhang, Y.; Lei, H.; Yang, Z.; Duan, D.; Villota, E.; Ruan, R. From glucose-based carbohydrates to phenol-rich bio-oils integrated with syngas production via catalytic pyrolysis over an activated carbon catalyst. Green Chem. 2018, 20, 3346–3358. [Google Scholar] [CrossRef]
- Malini, K.; Selvakumar, D.; Kumar, N.S. Activated carbon from biomass: Preparation, factors improving basicity and surface properties for enhanced CO2 capture capacity—A review. J. CO2 Util. 2023, 67, 102318. [Google Scholar] [CrossRef]
- Bu, Q.; Lei, H.; Wang, L.; Wei, Y.; Zhu, L.; Zhang, X.; Liu, Y.; Yadavalli, G.; Tang, J. Bio-based phenols and fuel production from catalytic microwave pyrolysis of lignin by activated carbons. Bioresour. Technol. 2014, 162, 142–147. [Google Scholar] [CrossRef] [PubMed]
- Bu, Q.; Lei, H.; Ren, S.; Wang, L.; Zhang, Q.; Tang, J.; Ruan, R. Production of phenols and biofuels by catalytic microwave pyrolysis of lignocellulosic biomass. Bioresour. Technol. 2012, 108, 274–279. [Google Scholar] [CrossRef] [PubMed]
- Mamaeva, A.; Tahmasebi, A.; Tian, L.; Yu, J. Microwave-assisted catalytic pyrolysis of lignocellulosic biomass for production of phenolic-rich bio-oil. Bioresour. Technol. 2016, 211, 382–389. [Google Scholar] [CrossRef] [PubMed]
- Mamaeva, A.; Tahmasebi, A.; Yu, J. The effects of mineral salt catalysts on selectivity of phenolic compounds in bio-oil during microwave pyrolysis of peanut shell. Korean J. Chem. Eng. 2017, 34, 672–680. [Google Scholar] [CrossRef]
- Peng, C.; Zhang, G.; Yue, J.; Xu, G. Pyrolysis of lignin for phenols with alkaline additive. Fuel Process. Technol. 2014, 124, 212–221. [Google Scholar] [CrossRef]
- Omoriyekomwan, J.E.; Tahmasebi, A.; Yu, J. Production of phenol-rich bio-oil during catalytic fixed-bed and microwave pyrolysis of palm kernel shell. Bioresour. Technol. 2016, 207, 188–196. [Google Scholar] [CrossRef]
- Yang, Z.; Lei, H.; Zhang, Y.; Qian, K.; Villota, E.; Qian, M.; Yadavalli, G.; Sun, H. Production of renewable alkyl-phenols from catalytic pyrolysis of Douglas fir sawdust over biomass-derived activated carbons. Appl. Energy 2018, 220, 426–436. [Google Scholar] [CrossRef]
- Duan, D.; Lei, H.; Wang, Y.; Ruan, R.; Liu, Y.; Ding, L.; Zhang, Y.; Liu, L. Renewable phenol production from lignin with acid pretreatment and ex-situ catalytic pyrolysis. J. Clean. Prod. 2019, 231, 331–340. [Google Scholar] [CrossRef]
- Ren, S.; Lei, H.; Wang, L.; Bu, Q.; Chen, S.; Wu, J. Hydrocarbon and hydrogen-rich syngas production by biomass catalytic pyrolysis and bio-oil upgrading over biochar catalysts. RSC Adv. 2014, 4, 10731–10737. [Google Scholar] [CrossRef]
- Zhang, Y.; Duan, D.; Lei, H.; Villota, E.; Ruan, R. Jet fuel production from waste plastics via catalytic pyrolysis with activated carbons. Appl. Energy 2019, 251, 113337. [Google Scholar] [CrossRef]
- Zhang, Z.-b.; Lu, Q.; Ye, X.-n.; Li, W.-t.; Hu, B.; Dong, C.-q. Production of phenolic-rich bio-oil from catalytic fast pyrolysis of biomass using magnetic solid base catalyst. Energy Convers. Manag. 2015, 106, 1309–1317. [Google Scholar] [CrossRef]
- Zhang, S.; Ma, J.; Liu, X.; Ma, Y.; Wang, J. Enhanced levoglucosan production by graphene oxide-catalyzed pyrolysis of biomass. Environ. Chem. Lett. 2024, 22, 2635–2639. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, S.; Ma, J.; Liu, X.; Ma, Y.; Fan, Z.; Wang, J. Augmenting levoglucosan production through catalytic pyrolysis of biomass exploiting Ti3C2Tx MXene. Chin. Chem. Lett. 2024, 35, 109725. [Google Scholar] [CrossRef]
- Sudarsanam, P.; Peeters, E.; Makshina, E.V.; Parvulescu, V.I.; Sels, B.F. Advances in porous and nanoscale catalysts for viable biomass conversion. Chem. Soc. Rev. 2019, 48, 2366–2421. [Google Scholar] [CrossRef]
- Li, C.; Yue, X.; Yang, J.; Yang, Y.; Gu, H.; Peng, W. Catalytic Fast Pyrolysis of Forestry Wood Waste for Bio-Energy Recovery Using Nano-Catalysts. Energies 2019, 12, 3972. [Google Scholar] [CrossRef]
- Gupta, S.; Lanjewar, R.; Mondal, P. Enhancement of hydrocarbons and phenols in catalytic pyrolysis bio-oil by employing aluminum hydroxide nanoparticle based spent adsorbent derived catalysts. Chemosphere 2022, 287, 132220. [Google Scholar] [CrossRef]
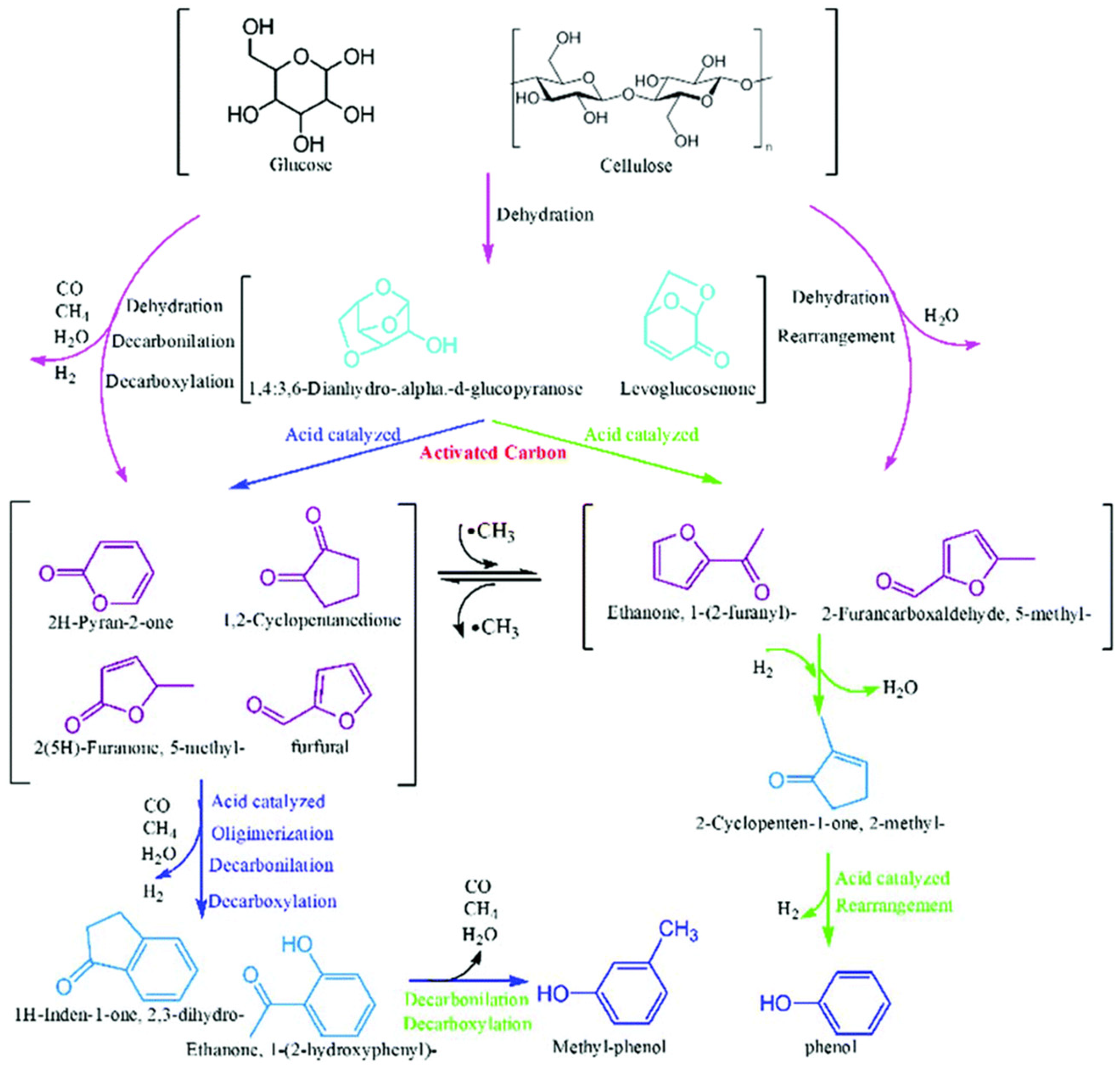

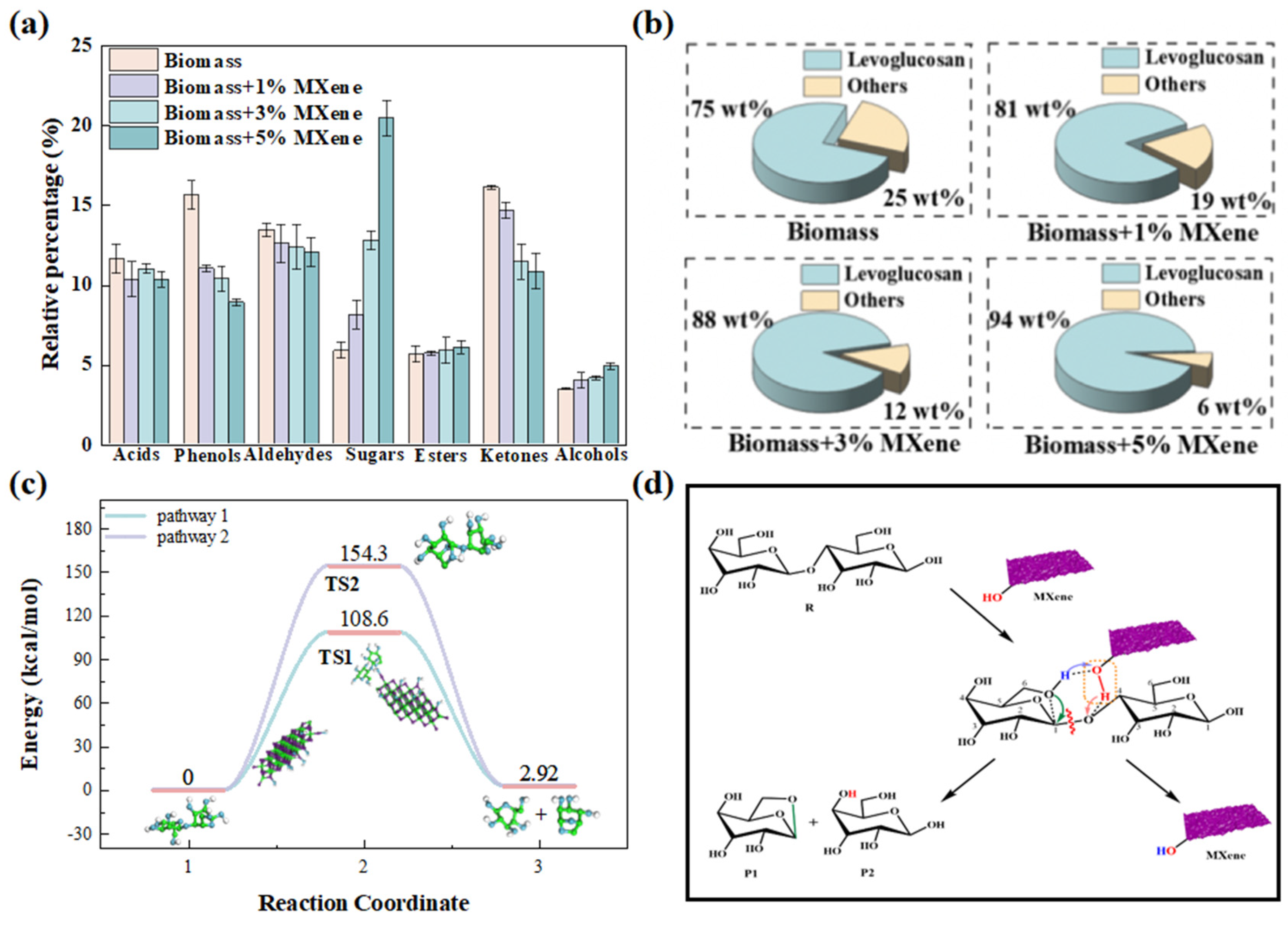

| Feedstock | Catalyst | Pore Size (nm) | BET (m2/g) | Selectively | Refs. |
|---|---|---|---|---|---|
| Pinewood sawdust | ZSM-5 | / | 350 | Aromatic hydrocarbon | [48] |
| Sawdust | Metal modified ZSM-5 | 3.88–4.19 | 248.46–288.33 | BTX production | [57] |
| Rice straw | Hexadecyl trimethyl ammonium bromide modified hierarchical HZSM-5 | 2–8 | 333–462 | Aromatics | [58] |
| Rape straw | Parent and hierarchical HZSM-5 | 3.3–7.6 | / | Aromatic hydrocarbons | [59] |
| Soybean straw and soapstock | ZSM-5 and composite catalyst | 3.77 and 42.915 | 290.76 and 6.004 | Aromatics | [60] |
| Corn stover and low-density polyethylene | HZSM-5 | / | 425 | Hydrocarbon | [61] |
| Torrefied rice husk | Parent and Fe-modified ZSM-5 | 2.014–2.024 | 334.81–381.82 | Upgrading bio-oil | [62] |
| Sewage sludge | Ni and Co loaded HZSM-5 | 4.88–5.12 | 258–306 | Aromatic rich bio-oil | [63] |
| Green microalgae | Mg-Ce/ZSM-5 | 1.418–1.924 | 110.08–311.69 | High quality bio-oil | [64] |
| Cellulose | Parent and metal-modified hierarchical HZSM-5 | / | 156–366 | Aromatics | [50] |
| Cellulose | ZSM-5 and dual catalyst bed of CaO and ZSM-5 | 3.49 | 363.7 | Light olefins and aromatics | [65] |
| Lignin | HZSM-5 | / | 425 | Upgrading of pyrolytic vapor | [66] |
| Hydrolysis lignin | ZSM-5 and other zeolite catalysts | 1.3 | 425 | Hydrocarbon rich bio-oil | [67] |
| Hydrolysis lignin | Acid-modified and Ni-loaded ZSM-5 | 1.3–1.6 | 381–401 | Mono-aromatics/phenol | [68] |
| Ethanol and oleic acid | Mesoporous ZSM-5 | 4.8–22 | 405–469 | Olefin production | [69] |
| Biomass | Catalysts | BET (m2/g) | Selectively | Refs. |
|---|---|---|---|---|
| Cotton stalk | CaO | 3.20 | Furans and carboxylic acids (decreased) | [72] |
| Palm empty fruit bunches | MgO | 19.84 | Levoglucosan (decreased) | [73] |
| Forest residues | CaO and MgO | 1.97 and 8.14 | Deoxygenation | [74] |
| Oakwood | CaO | 8.7 ± 0.2 | Ketones and light phenols | [75] |
| Switchgrass | CaO | / | Phenols and hydrocarbons | [76] |
| Poplar wood | CoO, Cr2O3, CuO, Mn2O3, NiO, TiO2, V2O5 and CeO2 | / | Alcohol, furans, ketones, acetic acid and phenolic compounds | [77] |
| Wood | CaO and CaO/MgO | / | Decreased acidity and oxygen content | [78] |
| Biomass | Catalyst | BET (m2/g) | Bio-Oil (%) | Selectivity (%) | Refs. |
|---|---|---|---|---|---|
| Palm kernel shell | Commercial AC | 707.65 | 45 | 64.58 | [101] |
| Glucose | AC with H3PO4 activation | 1125.6 | 49.6 | 100 | [94] |
| Douglas fir sawdust | AC with H3PO4 activation | 559.82–889.67 | 25 | 43.24 | [102] |
| Lignin | AC with H3PO4 activation | / | 27 | 95.5 | [103] |
| Acid pretreated lignin | AC with H3PO4 activation | / | 22.3 | 98.2 | [103] |
| Douglas fir pellet | Lignite coal-DARCO 830 | / | 28.97 | 74.77 | [97] |
| Wood-DARCO MRX | Douglas fir pellet | / | 26.5 | 74.61 | [97] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, W.; Yan, Y.; Wei, Y.; Ma, J.; Niu, Z.; Hu, G. Catalytic Pyrolysis of Biomass: A Review of Zeolite, Carbonaceous, and Metal Oxide Catalysts. Nanomaterials 2025, 15, 493. https://doi.org/10.3390/nano15070493
Sun W, Yan Y, Wei Y, Ma J, Niu Z, Hu G. Catalytic Pyrolysis of Biomass: A Review of Zeolite, Carbonaceous, and Metal Oxide Catalysts. Nanomaterials. 2025; 15(7):493. https://doi.org/10.3390/nano15070493
Chicago/Turabian StyleSun, Weiqiang, Yihong Yan, Yuxin Wei, Jingjing Ma, Zhenchuan Niu, and Guang Hu. 2025. "Catalytic Pyrolysis of Biomass: A Review of Zeolite, Carbonaceous, and Metal Oxide Catalysts" Nanomaterials 15, no. 7: 493. https://doi.org/10.3390/nano15070493
APA StyleSun, W., Yan, Y., Wei, Y., Ma, J., Niu, Z., & Hu, G. (2025). Catalytic Pyrolysis of Biomass: A Review of Zeolite, Carbonaceous, and Metal Oxide Catalysts. Nanomaterials, 15(7), 493. https://doi.org/10.3390/nano15070493





