Zeolitic Imidazolate Framework-67-Derived NiCoMn-Layered Double Hydroxides Nanosheets Dispersedly Grown on the Conductive Networks of Single-Walled Carbon Nanotubes for High-Performance Hybrid Supercapacitors
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
2.2. Preparation of the ZIF-67/SWCNTs Composite
2.3. Synthesis of the NiCoMn-LDH/SWCNTs Composite
2.4. Fabrication of Hybrid Supercapacitor (HSC) Devices
2.5. Material Characterization
2.6. Electrochemical Analyses
3. Results and Discussion
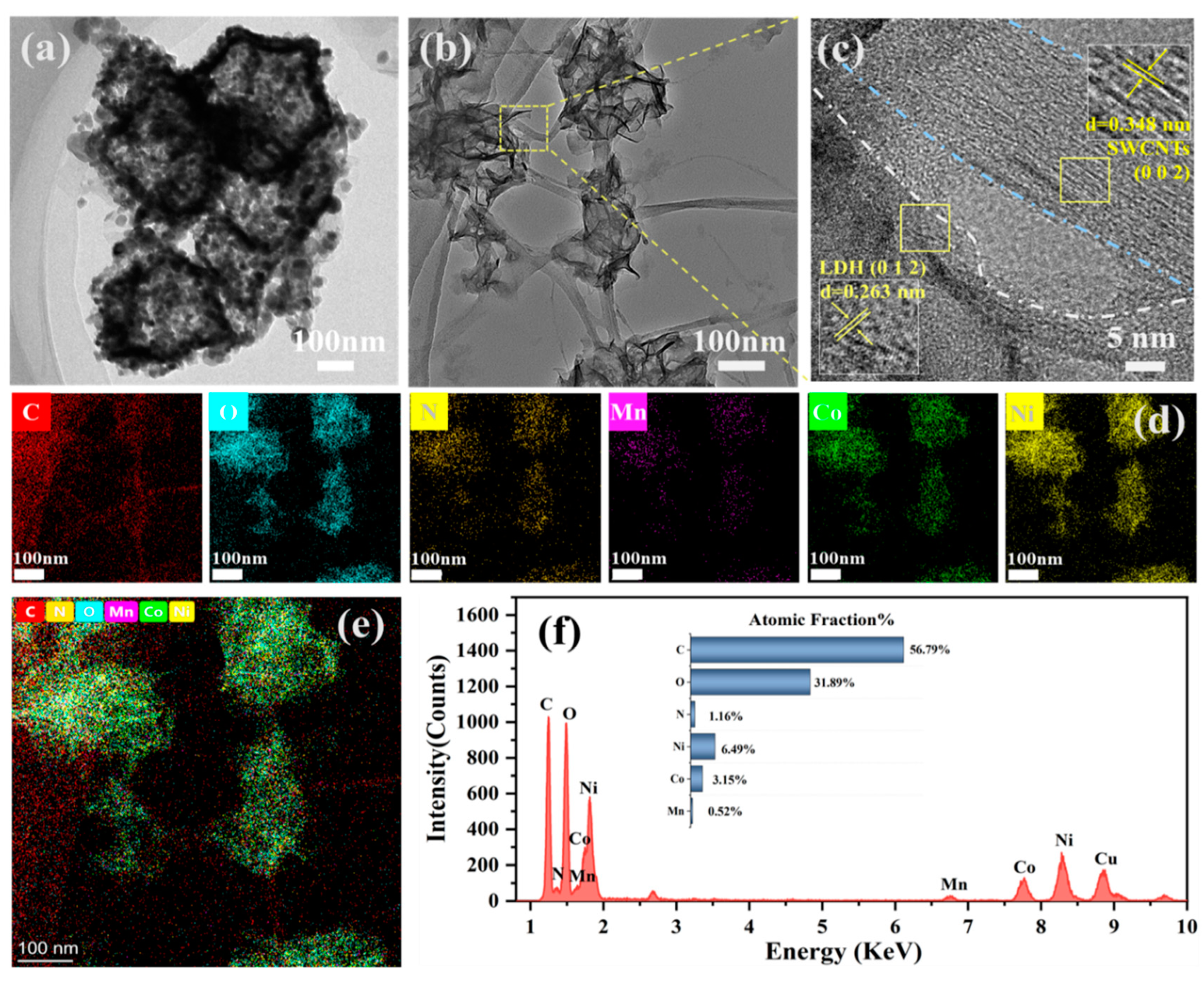
3.1. Morphology and Elemental Distribution
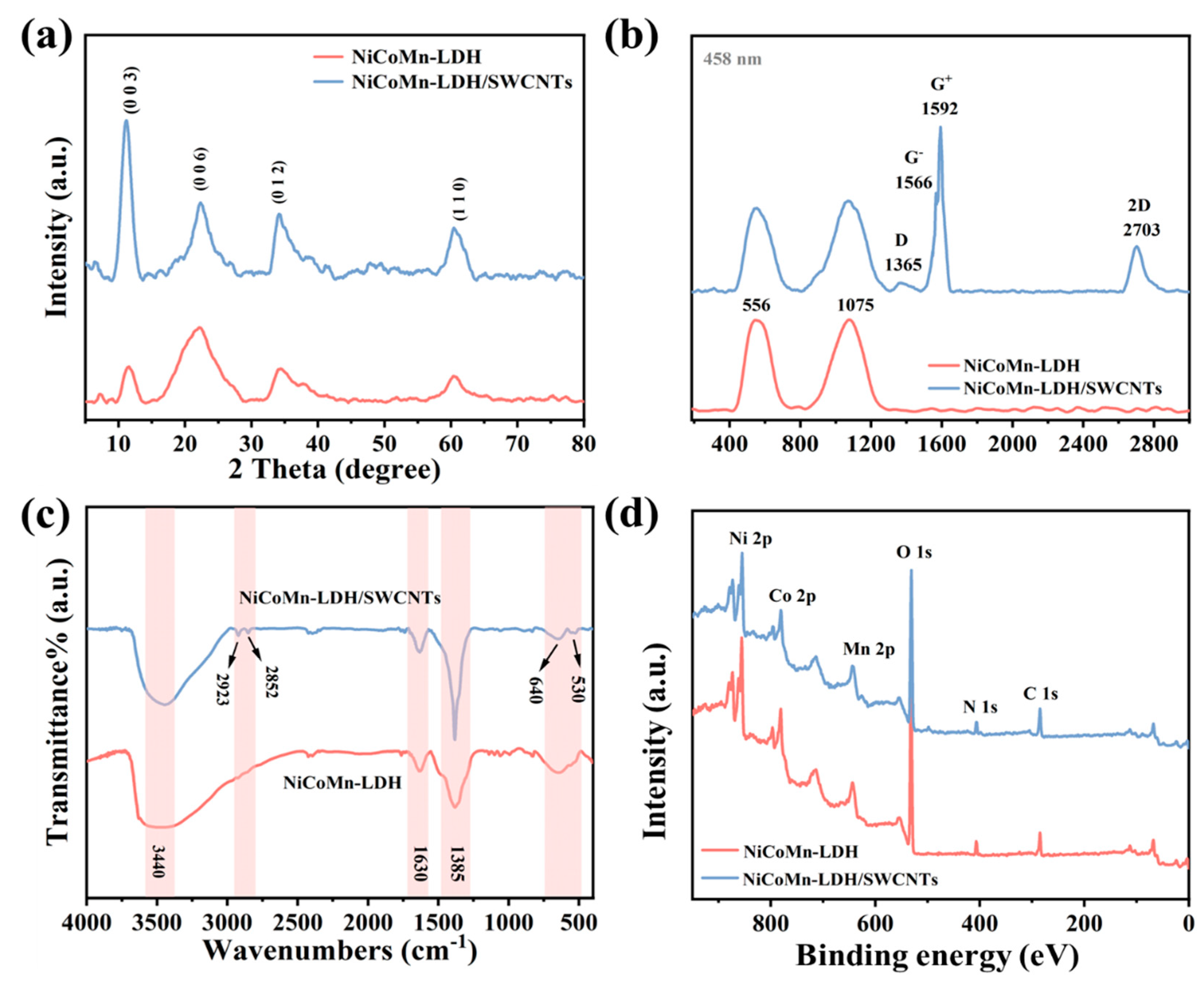
3.2. Material Structure
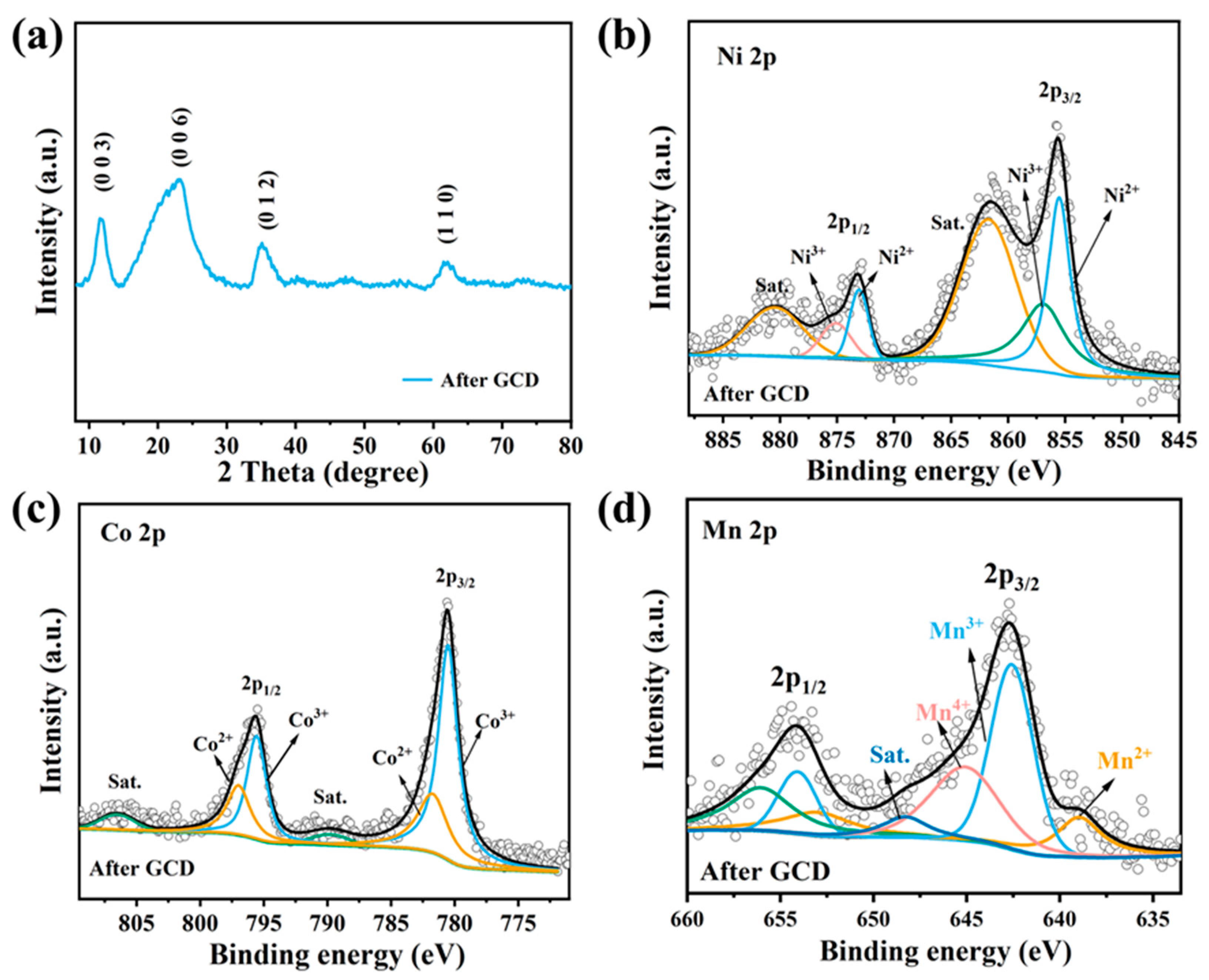
3.3. Surface Element State
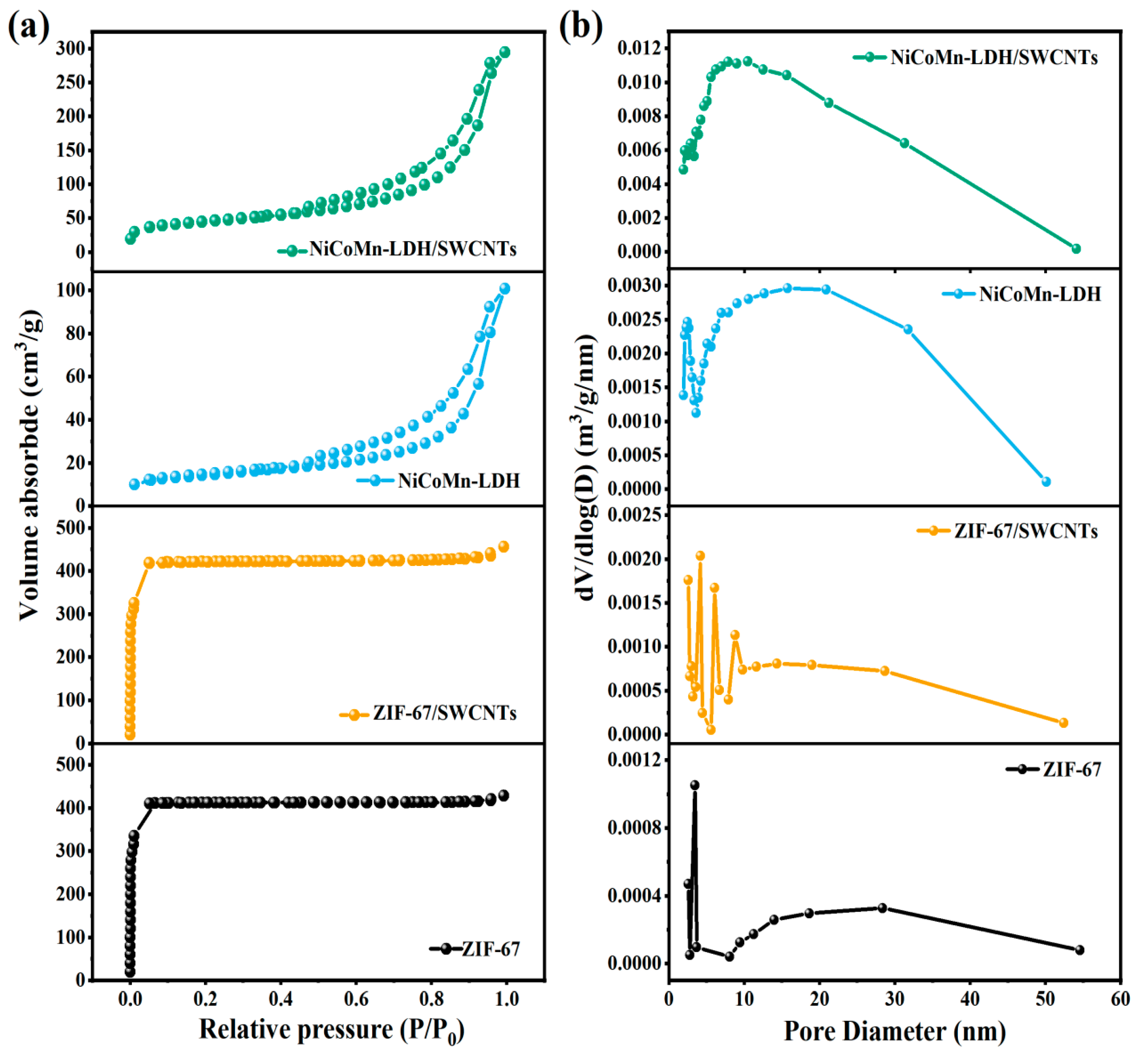
3.4. Specific Surface Area and Pore Structure
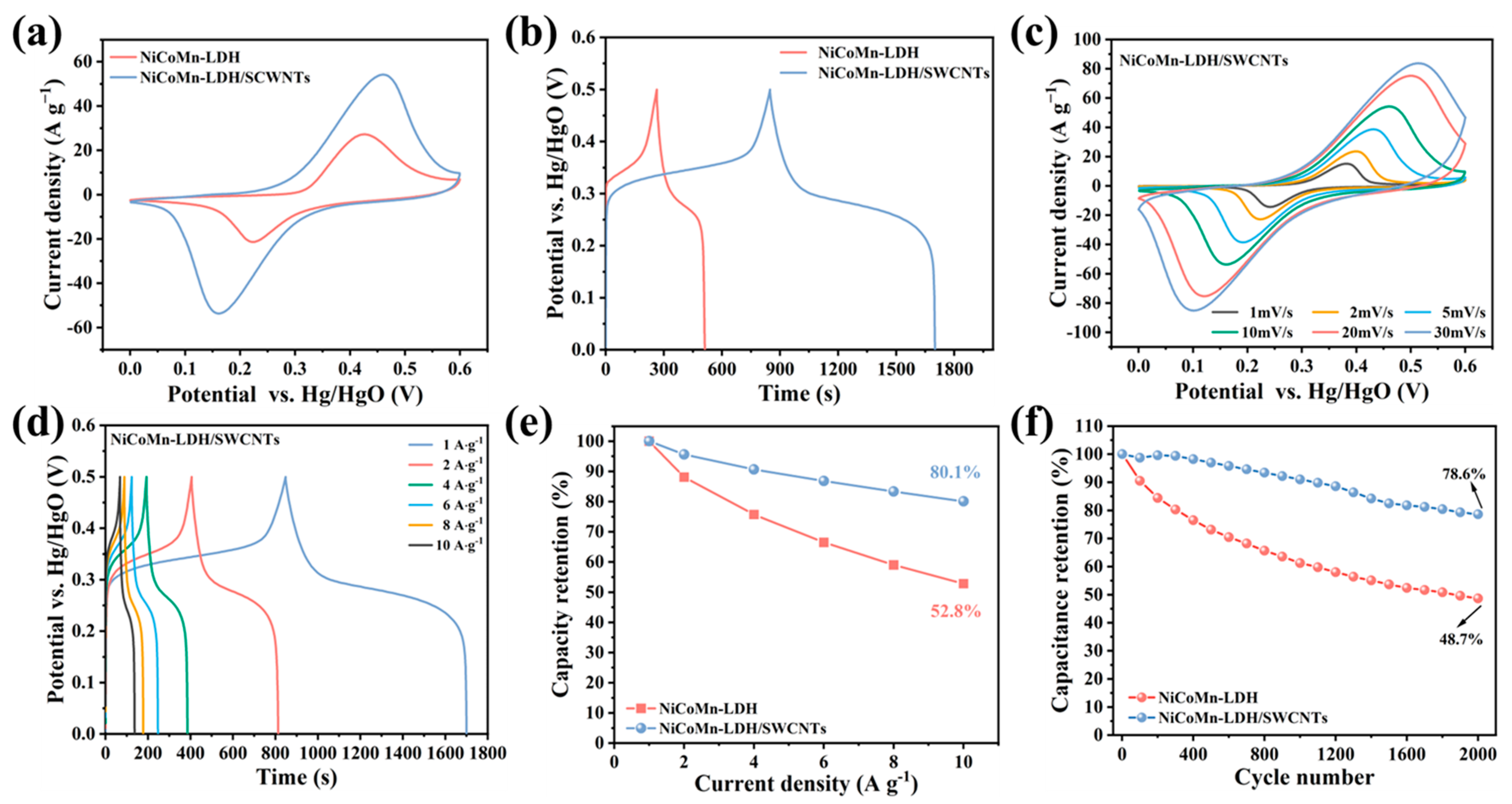
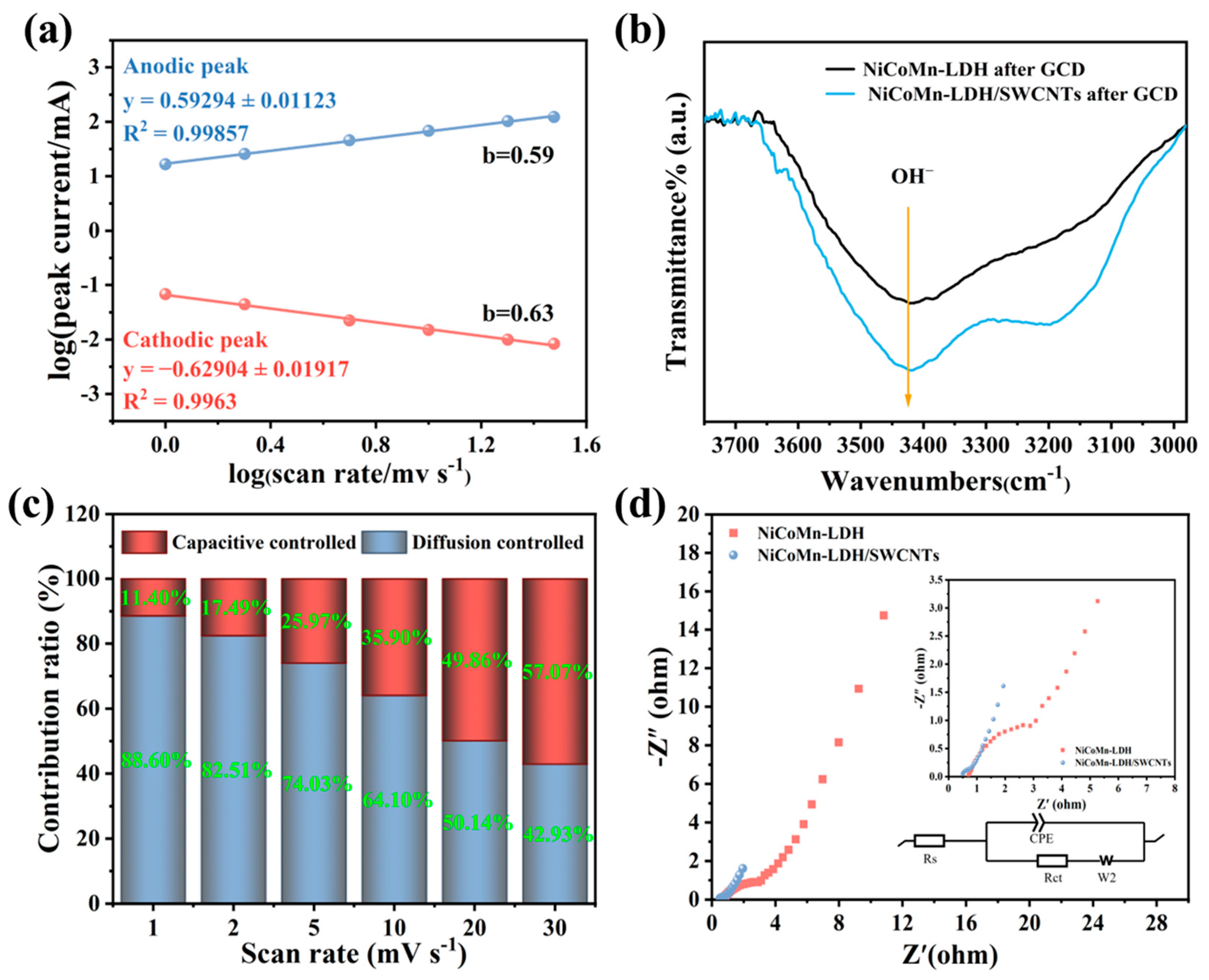
3.5. Electrochemical Performance of NiCoMn-LDH/SWCNTs
3.6. Supercapacitor Testing
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Simon, P.; Gogotsi, Y. Perspectives for electrochemical capacitors and related devices. Nat. Mater. 2020, 19, 1151–1163. [Google Scholar] [PubMed]
- Zhang, D.; Tan, C.; Zhang, W.; Pan, W.; Wang, Q.; Li, L. Expanded graphite-based materials for supercapacitors: A Review. Molecules 2022, 27, 716. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.C.; Hu, P.; Wei, H. Development of supercapacitor hybrid electric vehicle. J. Energy Storage 2023, 65, 107269. [Google Scholar]
- Liang, H.; Lin, T.; Wang, S.; Jia, H.; Li, C.; Cao, J.; Feng, J.; Fei, W.; Qi, J. A free-standing manganese cobalt sulfide@cobalt nickel layered double hydroxide core–shell heterostructure for an asymmetric supercapacitor. Dalton Trans. 2020, 49, 196–202. [Google Scholar]
- Naseri, F.; Karimi, S.; Farjah, E.; Schaltz, E. Supercapacitor management system: A comprehensive review of modeling, estimation, balancing, and protection techniques. Renew. Sustain. Energy Rev. 2022, 155, 111913. [Google Scholar]
- Chatterjee, D.P.; Nandi, A.K. A review on the recent advances in hybrid supercapacitors. J. Mater. Chem. A 2021, 9, 15880–15918. [Google Scholar]
- Choudhary, N.; Li, C.; Moore, J.; Nagaiah, N.; Zhai, L.; Jung, Y. Asymmetric supercapacitor electrodes and devices. Adv. Mater. 2017, 29, 1605336. [Google Scholar]
- Pershaanaa, M.; Bashir, S.; Ramesh, S.; Ramesh, K. Every bite of supercap: A brief review on construction and enhancement of supercapacitor. J. Energy Storage 2022, 50, 104599. [Google Scholar]
- Wang, Q.; Yan, J.; Fan, Z. Carbon materials for high volumetric performance supercapacitors: Design, progress, challenges and opportunities. Energy Environ. Sci. 2016, 9, 729–762. [Google Scholar] [CrossRef]
- Muzaffar, A.; Ahamed, M.B.; Deshmukh, K.; Thirumalai, J. A review on recent advances in hybrid supercapacitors: Design, fabrication and applications. Renew. Sustain. Energy Rev. 2019, 101, 123–145. [Google Scholar]
- Zuo, W.; Li, R.; Zhou, C.; Li, Y.; Xia, J.; Liu, J. Battery-supercapacitor hybrid devices: Recent progress and future prospects. Adv. Sci. 2017, 4, 1600539. [Google Scholar] [CrossRef]
- Zhao, K.; Wang, Z.; Sun, X.; Guo, H.; Fu, H.; Lu, Z.; Liu, Z.; Liu, J. Metal-organic framework derived nickel-cobalt layered double hydroxide nanosheets cleverly constructed on interconnected nano-porous carbon for high-performance supercapacitors. J. Energy Storage 2023, 60, 106559. [Google Scholar] [CrossRef]
- Hao, L.; Li, X.; Zhi, L. Carbonaceous electrode materials for supercapacitors. Adv. Mater. 2013, 25, 3899–3904. [Google Scholar] [CrossRef]
- Liu, R.; Zhou, A.; Zhang, X.; Mu, J.; Che, H.; Wang, Y.; Wang, T.T.; Zhang, Z.; Kou, Z. Fundamentals, advances and challenges of transition metal compounds-based supercapacitors. Chem. Eng. J. 2021, 412, 128611. [Google Scholar]
- Chen, J.; Xu, W.; Wang, H.; Ren, X.; Zhan, F.; He, Q.; Wang, H.; Chen, L. Emerging two-dimensional nanostructured manganese-based materials for electrochemical energy storage: Recent advances, mechanisms, challenges, and prospects. J. Mater. Chem. A 2022, 10, 21197–21250. [Google Scholar]
- Wang, T.; Wang, Y.; Lei, J.; Chen, K.J.; Wang, H. Electrochemically induced surface reconstruction of Ni-Co oxide nanosheet arrays for hybrid supercapacitors. Exploration 2021, 1, 20210178. [Google Scholar] [CrossRef]
- Ding, C.; Wang, Y.; Wang, Y.; Dong, X.; Meng, C.; Zhang, Y. Mn-doping ensuring cobalt silicate hollow spheres with boosted electrochemical property for hybrid supercapacitors. Battery Energy 2023, 2, 20230042. [Google Scholar] [CrossRef]
- Zhao, X.; Li, H.; Zhang, M.; Pan, W.; Luo, Z.; Sun, X. Hierarchical nanocages assembled by NiCo-Layered double hydroxide nanosheets for a high-performance hybrid Supercapacitor. ACS Appl. Mater. Interfaces 2022, 14, 34781–34792. [Google Scholar] [CrossRef]
- Das, A.K.; Pan, U.N.; Sharma, V.; Kim, N.H.; Lee, J.H. Nanostructured CeO2/NiV–LDH composite for energy storage in asymmetric supercapacitor and as methanol oxidation electrocatalyst. Chem. Eng. J. 2021, 417, 128019. [Google Scholar] [CrossRef]
- Liu, Z.J.; Zhao, Z.H.; Wang, Y.Y.; Dou, S.; Yan, D.F.; Liu, D.D.; Xia, Z.H.; Wang, S.Y. In situ exfoliated, edge-rich, oxygen-functionalized graphene from carbon fibers for oxygen electrocatalysis. Adv. Mater. 2017, 29, 1606207. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Zhao, K.; Guo, H.; Duan, L.; Sun, H.; Chen, K.; Liu, J. In situ construction of NiCoMn-LDH derived from zeolitic imidazolate framework on eggshell-like carbon skeleton for high-performance flexible supercapacitors. Small 2023, 20, 2309814. [Google Scholar] [CrossRef]
- Luo, X.; Abazari, R.; Tahir, M.; Fan, W.K.; Kumar, A.; Kalhorizadeh, T.; Kirillov, A.M.; Amani-Ghadim, A.R.; Chen, J.; Zhou, Y. Trimetallic metal–organic frameworks and derived materials for environmental remediation and electrochemical energy storage and conversion. Coord. Chem. Rev. 2022, 461, 214505. [Google Scholar] [CrossRef]
- Hao, C.; Wang, X.; Wu, X.; Guo, Y.; Zhu, L.; Wang, X. Composite material CCO/Co-Ni-Mn LDH made from sacrifice template CCO/ZIF-67 for high-performance supercapacitor. Appl. Surf. Sci. 2022, 572, 151373. [Google Scholar] [CrossRef]
- Chen, Y.; Pang, W.K.; Bai, H.; Zhou, T.; Liu, Y.; Li, S.; Guo, Z. Enhanced structural stability of nickel–cobalt hydroxide via intrinsic pillar effect of metaborate for high-power and long-life supercapacitor electrodes. Nano Lett. 2017, 17, 429–436. [Google Scholar]
- Fu, H.; Zhang, A.; Jin, F.; Guo, H.; Liu, J. Ternary NiCeCo-Layered double hydroxides grown on CuBr2@ZIF-67 nanowire arrays for high-performance supercapacitors. ACS Appl. Mater. Interfaces 2022, 14, 16165–16177. [Google Scholar] [CrossRef] [PubMed]
- Pan, Q.; Zheng, F.; Deng, D.; Chen, B.; Wang, Y. Interlayer spacing regulation of NiCo-LDH nanosheets with ultrahigh specific capacity for battery-type supercapacitors. ACS Appl. Mater. Interfaces 2021, 13, 56692–56703. [Google Scholar] [PubMed]
- Zang, Y.; Luo, H.; Zhang, H.; Xue, H. Polypyrrole nanotube-interconnected NiCo-LDH nanocages derived by ZIF-67 for supercapacitors. ACS Appl. Energy Mater. 2021, 4, 1189–1198. [Google Scholar]
- Yao, Y.; Yu, Y.; Wan, L.; Du, C.; Zhang, Y.; Chen, J.; Xie, M. Structurally-stable Mg-Co-Ni LDH grown on reduced graphene by ball-milling and ion-exchange for highly-stable asymmetric supercapacitor. J. Colloid Interface Sci. 2023, 649, 519–527. [Google Scholar]
- Dinari, M.; Allami, H.; Momeni, M.M. Construction of Ce-doped NiCo-LDH@CNT nanocomposite electrodes for high-performance supercapacitor application. Energy Fuels 2021, 35, 1831–1841. [Google Scholar]
- Zhang, D.; Zhao, M.; Zhang, H.; Terrones, M.; Wang, Y. A novel electro-synthesis of hierarchical Ni–Al LDH nanostructures on 3D carbon nanotube networks for hybrid-capacitors. Carbon 2023, 201, 1081–1089. [Google Scholar] [CrossRef]
- Wu, W.; Liu, J.; Chen, G.; Chen, Y.; Xu, C. Thin porous nanosheets of NiFe layered-double hydroxides toward a highly efficient electrocatalyst for water oxidation. Int. J. Hydrogen Energy 2020, 45, 1948–1958. [Google Scholar] [CrossRef]
- Li, Y.Y.; Li, C.; Zhou, Q.; Lian, Y.F. Pyridinic-N-enriched mesoporous carbon coated carbon cloth for efficient oxygen evolution reactions. Appl. Surf. Sci. 2025, 689, 162444. [Google Scholar] [CrossRef]
- Jiang, X.X.; Zhou, Q.; Lian, Y.F. Efficient photocatalytic degradation of tetracycline on the MnFe2O4/BGA composite under visible light. Int. J. Mol. Sci. 2023, 24, 9378. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Tang, J.; Qian, H.; Hou, S.; Bando, Y.; Hossain, M.S.A.; Pan, L.; Yamauchi, Y. Three-dimensional networked metal–organic frameworks with conductive polypyrrole tubes for flexible supercapacitors. ACS Appl. Mater. Interfaces 2017, 9, 38737–38744. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Wang, X.; He, H.; Chen, W. ZIF-67 derived hollow OCS/NiCo-LDH nanocages as binder-free electrodes for high performance supercapacitors. Appl. Clay Sci. 2020, 198, 105820. [Google Scholar] [CrossRef]
- Zhou, Y.; Jia, Z.; Shi, L.; Wu, Z.; Jie, B.; Zhao, S.; Wei, L.; Zhou, A.; Zhu, J.; Wang, X.; et al. Pressure difference-induced synthesis of P-doped carbon nanobowls for high-performance supercapacitors. Chem. Eng. J 2020, 385, 123858. [Google Scholar] [CrossRef]
- Chen, Y.; He, J.; Ye, S.; Guan, J.; Liu, X.; Wang, J.; Xu, S.; Gu, J.; Chen, K.; Zhang, L.; et al. A hierarchical heterostructure NiCo-layered double hydroxide nanosheets/CoP nanowires/Ni foam for high-efficiency oxygen evolution reaction. Inorg. Chem. Commun. 2023, 157, 111347. [Google Scholar] [CrossRef]
- Yadav, D.K.; Uma, S.; Nagarajan, R. Microwave-assisted synthesis of ternary Li-M-Al LDHs (M = Mg, Co, Ni, Cu, Zn, and Cd) and examining their use in phenol oxidation. Appl. Clay Sci. 2022, 228, 106655. [Google Scholar] [CrossRef]
- Chen, X.; Luo, B.; Ding, J.; Yang, Q.; Xu, D.; Zhou, P.; Ying, Y.; Li, L.; Liu, Y. Kinetics-favorable heterojunctional CNTs@CuCo-LDH/BPQD electrode with boosted charge storage capability for supercapacitor. Appl. Surf. Sci. 2023, 609, 155287. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, J.; Gao, B. Raman spectroscopy of strained single-walled carbon nanotubes. Chem. Commun. 2009, 45, 6902–6918. [Google Scholar] [CrossRef]
- Ouyang, Y.; Xing, T.; Chen, Y.; Zheng, L.; Peng, J.; Wu, C.; Chang, B.; Luo, Z.; Wang, X. Hierarchically structured spherical nickel cobalt layered double hydroxides particles grown on biomass porous carbon as an advanced electrode for high specific energy asymmetric supercapacitor. J. Energy Storage 2020, 30, 101454. [Google Scholar]
- Tian, J.; Shan, Q.; Yin, X.L.; Wu, W. A facile preparation of graphene/reduced graphene oxide/Ni(OH)2 two dimension nanocomposites for high performance supercapacitors. Adv. Powder Technol. 2019, 30, 3118–3126. [Google Scholar]
- Huang, C.; Ding, Y.; Hao, C.; Zhou, S.; Wang, X.; Gao, H.; Zhu, L.; Wu, J. PVP-assisted growth of Ni-Co oxide on N-doped reduced graphene oxide with enhanced pseudocapacitive behavior. Chem. Eng. J. 2019, 378, 122202. [Google Scholar]
- Xu, X.; Yang, Y.; Wang, M.; Dong, P.; Baines, R.; Shen, J.; Ye, M. Straightforward synthesis of hierarchical Co3O4@CoWO4/rGO core–shell arrays on Ni as hybrid electrodes for asymmetric supercapacitors. Ceram. Int. 2016, 42, 10719–10725. [Google Scholar] [CrossRef]
- Reddy, A.L.M.; Shaijumon, M.M.; Gowda, S.R.; Ajayan, P.M. Coaxial MnO2/Carbon Nanotube Array Electrodes for High-Performance Lithium Batteries. Nano Lett. 2009, 9, 1002–1006. [Google Scholar] [PubMed]
- Zampieri, G.; Abbate, M.; Prado, F.; Caneiro, A. Mn-2p XPS spectra of differently hole-doped Mn perovskites. Solid State Commun. 2002, 123, 81–85. [Google Scholar]
- Fang, S.; Chen, K.; Yao, H.; Cao, Y.; Guo, S.; Wang, L.; Wang, Y.; Yu, S.; Wang, N. Preparation of gallic acid intercalated layered double hydroxide for enhanced corrosion protection of epoxy coatings. Coatings 2023, 13, 128. [Google Scholar] [CrossRef]
- Lockett, V.; Sedev, R.; Bassell, C.; Ralston, J. Angle-resolved X-ray photoelectron spectroscopy of the surface of imidazolium ionic liquids. Phys. Chem. Chem. Phys. 2008, 10, 1330–1335. [Google Scholar]
- Lu, Q.; Zou, X.; Liao, K.; Ran, R.; Zhou, W.; Ni, M.; Shao, Z. Direct growth of ordered N-doped carbon nanotube arrays on carbon fiber cloth as a free-standing and binder-free air electrode for flexible quasi-solid-state rechargeable Zn-Air batteries. Carbon Energy 2020, 2, 461–471. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Y.; Shi, C.; Chen, Y.; Li, D.; He, Z.; Wang, C.; Guo, L.; Ma, J. Co-ZIF derived porous NiCo-LDH nanosheets/N doped carbon foam for high-performance supercapacitor. Carbon 2020, 165, 129–138. [Google Scholar]
- Wang, M.; Wang, S.; Zhao, J.; Li, S.; Wang, J.; Mi, J.; Feng, Y. Multifunctional-regions integrated with Mn-Co-Ni ternary hydroxides as self-assembled electrodes for high-performance hybrid supercapacitors. Chem. Eng. J. 2024, 480, 148206. [Google Scholar]
- Zan, G.; Jiang, W.; Kim, H.; Zhao, K.; Li, S.; Lee, K.; Jang, J.; Kim, G.; Shin, E.; Kim, W.; et al. A core-shell fiber moisture-driven electric generator enabled by synergetic complex coacervation and built-in potential. Nat. Commun. 2024, 15, 10056. [Google Scholar] [PubMed]
- Arbaz, S.J.; Ramulu, B.; Yu, J.S. Micro-supercapacitors based on fungi-derived biocarbon microfibers infused with NiMoO nanoparticles for biomedical and E-skin applications. Adv. Fiber Mater. 2024, 6, 1008–1025. [Google Scholar]
- Abdelghafar, F.; Xu, X.; Guan, D.; Lin, Z.; Hu, Z.; Ni, M.; Huang, H.; Bhatelia, T.; Jiang, S.P.; Shao, Z. New nanocomposites derived from cation-nonstoichiometric Bax(Co, Fe, Zr, Y)O3−δ as efficient electrocatalysts for water oxidation in alkaline olution. ACS Mater. Lett. 2024, 6, 2985–2994. [Google Scholar]
- Lukatskaya, M.R.; Dunn, B.; Gogotsi, Y. Multidimensional materials and device architectures for future hybrid energy storage. Nat. Commun. 2016, 7, 12647. [Google Scholar] [PubMed]
- Wu, X.; Huang, B.; Wang, Q.; Wang, Y. Wide potential and high energy density for an asymmetric aqueous supercapacitor. J. Mater. Chem. A 2019, 7, 19017–19025. [Google Scholar]
- Xue, Y.; Huo, J.; Wang, X.; Zhao, Y. ZnxMnO2/PPy Nanowires Composite as Cathode Material for Aqueous Zinc-Ion Hybrid Supercapacitors. Battery Energy 2024, 3, 20240035. [Google Scholar] [CrossRef]
- Guan, D.; Xu, H.; Huang, Y.C.; Jing, C.; Tsujimoto, Y.; Xu, X.; Lin, Z.; Tang, J.; Wang, Z.; Sun, X.; et al. Operando studies redirect spatiotemporal restructuration of model coordinated oxides in electrochemical oxidation. Adv. Mater. 2025, 37, 2413073. [Google Scholar]
- Zhang, R.; Dong, J.; Zhang, W.; Ma, L.; Jiang, Z.; Wang, J.; Huang, Y. Synergistically coupling of 3D FeNi-LDH arrays with Ti3C2Tx-MXene nanosheets toward superior symmetric supercapacitor. Nano Energy 2022, 91, 106633. [Google Scholar]
- Moysiadou, A.; Lee, S.; Hsu, C.S.; Chen, H.M.; Hu, X. Mechanism of oxygen evolution catalyzed by cobalt oxyhydroxide: Cobalt superoxide species as a key intermediate and dioxygen release as a rate-determining step. J. Am. Chem. Soc. 2020, 142, 11901–11914. [Google Scholar]
- Zhao, Y.; He, X.; Chen, R.; Liu, Q.; Liu, J.; Yu, J.; Li, J.; Zhang, H.; Dong, H.; Zhang, M.; et al. A flexible all-solid-state asymmetric supercapacitors based on hierarchical carbon cloth@CoMoO4@NiCo layered double hydroxide core-shell heterostructures. Chem. Eng. J. 2018, 352, 29–38. [Google Scholar]
- Wang, G.; Li, Y.; Xu, L.; Jin, Z.; Wang, Y. Facile synthesis of difunctional NiV LDH@ZIF-67 p-n junction: Serve as prominent photocatalyst for hydrogen evolution and supercapacitor electrode as well. Renew. Energy 2020, 162, 535–549. [Google Scholar]
- Zhang, H.; Xiong, T.; Chen, R.; Wang, Y.; Fang, C.; Xu, L.; Liu, C. High electrochemical performance of MnCo2O4.5 nanoneedles/NiCo LDH nanosheets as advanced electrodes of supercapacitor. Electrochim. Acta 2023, 455, 142412. [Google Scholar]
- Prajapati, M.; Kant, C.R.; Jacob, M.V. Binder free cobalt Manganese layered double hydroxide anode conjugated with bioderived rGO cathode for sustainable, high-performance asymmetric supercapacitors. J. Electroanal. Chem. 2024, 961, 118242. [Google Scholar]
- Li, Y.; Yan, X.; Zhang, W.; Zhou, W.; Zhu, Y.; Zhang, M.; Zhu, W.; Cheng, X. Hierarchical micro-nano structure based NiCoAl-LDH nanosheets reinforced by NiCo2S4 on carbon cloth for asymmetric supercapacitor. J. Electroanal. Chem. 2022, 905, 115982. [Google Scholar]
- Padalkar, N.S.; Sadavar, S.V.; Shinde, R.B.; Patil, A.S.; Patil, U.M.; Dhawale, D.S.; Bulakhe, R.N.; Kim, H.; Im, H.; Vinu, A.; et al. Layer-by-layer nanohybrids of Ni-Cr-LDH intercalated with 0D polyoxotungstate for highly efficient hybrid supercapacitor. J. Colloid Interface Sci. 2022, 616, 548–559. [Google Scholar]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Zhou, Q.; Lian, Y. Zeolitic Imidazolate Framework-67-Derived NiCoMn-Layered Double Hydroxides Nanosheets Dispersedly Grown on the Conductive Networks of Single-Walled Carbon Nanotubes for High-Performance Hybrid Supercapacitors. Nanomaterials 2025, 15, 481. https://doi.org/10.3390/nano15070481
Li Y, Zhou Q, Lian Y. Zeolitic Imidazolate Framework-67-Derived NiCoMn-Layered Double Hydroxides Nanosheets Dispersedly Grown on the Conductive Networks of Single-Walled Carbon Nanotubes for High-Performance Hybrid Supercapacitors. Nanomaterials. 2025; 15(7):481. https://doi.org/10.3390/nano15070481
Chicago/Turabian StyleLi, Yingying, Qin Zhou, and Yongfu Lian. 2025. "Zeolitic Imidazolate Framework-67-Derived NiCoMn-Layered Double Hydroxides Nanosheets Dispersedly Grown on the Conductive Networks of Single-Walled Carbon Nanotubes for High-Performance Hybrid Supercapacitors" Nanomaterials 15, no. 7: 481. https://doi.org/10.3390/nano15070481
APA StyleLi, Y., Zhou, Q., & Lian, Y. (2025). Zeolitic Imidazolate Framework-67-Derived NiCoMn-Layered Double Hydroxides Nanosheets Dispersedly Grown on the Conductive Networks of Single-Walled Carbon Nanotubes for High-Performance Hybrid Supercapacitors. Nanomaterials, 15(7), 481. https://doi.org/10.3390/nano15070481






