Abstract
The continuous increase in global energy consumption has caused a considerable increase in CO2 emissions and environmental problems. To address these challenges, adsorbents and catalytic materials that can effectively reduce the CO2 levels in the atmosphere should be developed. Metal–organic frameworks (MOFs) have emerged as promising materials for CO2 capture owing to their high surface areas and tunable structures. Herein, the CO2 adsorption properties of MIL-100(Fe) and UiO-66(Zr) were investigated. Both MOFs exhibited excellent thermal stability and high CO2 adsorption capacities at 300 K, and they maintained good adsorption properties at 500 K compared to those of activated carbon fiber owing to their high adsorption potentials. A slight change in the UiO-66(Zr) structure and no change in the MIL-100(Fe) structure were observed under the CO2 atmosphere at 500 K. At that time, CO emissions and changes in the carboxyl and OCO functional groups were observed on MIL-100(Fe), suggesting a mechanism of CO2 reduction to CO on the bare Fe(II) sites. These findings confirm the potential of MOFs for the thermo-catalytic reduction of CO2 to achieve effective CO2 capture and conversion.
1. Introduction
Energy consumption has increased in recent decades to satisfy the increasing industrial requirements and daily needs caused by the rapid population growth. Fossil fuels are still one of the primary energy sources. As a result, the CO2 concentration in the atmosphere increased substantially from 340 ppm in 1980 to 408 ppm in 2019, leading to several negative environmental and global economic impacts [1]. For example, the total production of rice, maize, and wheat grains exhibited a 50–100% decrease due to global warming. In most climate models, global warming increases economic inequality because it affects developing countries more than developed countries [2]. Thus, reducing CO2 emissions and its atmospheric concentration is vital to mitigate these global problems.
Numerous extensive studies on CO2 capture and storage employing several techniques have been reported: absorption using aqueous amine solutions, adsorption using porous materials, and separation using polymeric membranes. However, there are still several challenges that should be overcome. For example, although the absorption techniques exhibit extremely high absorption performance, they require a substantially high amount of energy for regeneration [3,4]. Moreover, the pressure- and temperature-swing adsorption and membrane separation techniques exhibit relatively low CO2 selectivity and capacity. Nevertheless, CO2 capture and separation using porous materials, such as zeolites, activated carbons, carbon molecular sieves, porous alumina, carbon nanotubes, and silica gels, exhibit low energy consumption [5,6,7,8,9]. Metal–organic frameworks (MOFs) have high CO2 separation capacity and selectivity because of their uniform pore structures with large surface areas [1,10,11]. MOFs can have various designs while maintaining high chemical stability by choosing appropriate metals and ligands and applying various chemical modifications [12]. Extensive efforts have been dedicated to studying the adsorption of CO2 by several MOFs. Flexible MOFs have dynamic frameworks that can change their structures in response to external stimuli, such as temperature, pressure, and guest molecules, leading to high CO2 adsorption [13]. Mg-MOF-74 shows that CO2 selectivity against its N2 selectivity can be increased when water molecules are present in the system owing to water adsorption on the coordinatively unsaturated metal sites; however, this strategy decreases the CO2 adsorption capacity [14]. Moreover, MOF membranes exhibit long-term stability, high capacity, selectivity, and chemical resistance to acids, bases, and aqueous solutions. MOFs can be used in coupling reactions of CO2 and epoxides to form binary catalysts, such as propylene carbonate and butylene carbonate [15], and in optical media [16].
This study uses two MOFs, i.e., MIL-100(Fe) and UiO-66(Zr), which are representative MOFs with high porosity and thermal stability, as CO2 thermal-reducing catalysts. MIL-100(Fe) is a promising candidate for various applications in decontamination, drug delivery, and catalysis because of its superior capturing ability due to its mesoporous structure, which provides it with a large surface area and allows its post-synthetic modification [17,18,19]. Furthermore, UiO-66(Zr), in which zirconium-oxo clusters form the micropores with dicarboxylate ligands, exhibits excellent CO2 adsorption properties compared to its CH4 and N2 adsorption properties, which can be attributed to its polarity and small functional groups [20]. The modification and conjugation of the MOFs improved their CO2 adsorption capacity. Compositing UiO-66(Zr)-NH2 with GO increased its CO2 adsorption capacity by 50% [21]. Thus, we investigated the CO2 adsorption mechanism to clarify the physical adsorption and chemisorption mechanisms on MIL-100(Fe) and UiO-66(Zr) and the possibility of CO2 reduction on those representative MOFs at 300–500 K.
2. Materials and Methods
MIL-100(Fe) was prepared based on a method reported in the literature [22]. Briefly, a NaOH aqueous solution (1 M) was prepared by dissolving NaOH (23.7 g) in water, and 1,3,5-benzene tricarboxylic acid (7.6 mmol) was added dropwise to an aqueous solution of FeCl2·4H2O (11.4 mmol), which was prepared by dissolving FeCl2·4H2O (97.2 g) in water. After stirring for 24 h at ambient temperature, the mixture solution was centrifuged at 4000 rpm. The brown sediment was collected and washed several times with water and ethanol. UiO-66(Zr) was prepared according to procedures reported in the literature [23]. A HCl aqueous solution (10 M) and ZrCl4 (5.0 mmol) were added to dimethylformamide (100 mL) and stirred for 5 min at room temperature. 1,4-benzenedicarboxylic acid (7.5 mmol) was then added to the mixture under stirring for 15 min, and the final mixture was heated at 353 K for 24 h. The reaction mixture was finally cooled to room temperature, and fine white powder was collected by filtration, followed by washing with water and ethanol.
MIL-100(Fe) and UiO-66(Zr) powders were characterized using powder X-ray diffraction (XRD, SmartLab, Rigaku Co., Tokyo, Japan) with Cu Kα radiation (λ = 0.1541 nm) at 40 kV and 40 mA. Their microstructures were observed directly using high-resolution transmission electron microscopy (HR-TEM, ARM200CF, JEOL Ltd., Tokyo, Japan). The acceleration voltage was set to 120 kV to minimize electron irradiation damage and obtain a clear microstructural image. The thermal stabilities of these MOFs were evaluated using thermogravimetric (TG) analysis (ThermoEVO2, Rigaku Co., Tokyo, Japan), in which each MOF was placed in an alumina pan and heated to 900 K at a heating rate of 10 K min−1 under an O2 atmosphere (flow rate = 100 mL min−1), and the alumina powder was used as a reference. The pore structures and specific surface areas were evaluated based on N2 adsorption isotherms at 77 K using the BELSORP MAX apparatus (MicrotracBEL Co., Osaka, Japan). The samples were preheated at 500 K at a pressure lower than 10−3 Pa in an N2 atmosphere (gas purity > 99.995%) to remove guest molecules. The pore size distributions were determined based on the desorption branch of the N2 adsorption isotherms using the grand canonical Monte Carlo simulation models in the BELMaster 7 software (MicrotracBEL Co., Osaka, Japan). The adsorbed CO2 amounts at 300, 400, and 500 K were determined using BELSORP MAX, as mentioned in the N2 adsorption measurements. CO2 gas (purity > 99.9%) was used in the experiments. Activated carbon fiber (A5, AD’ALL Co., Kyoto, Japan) was used as a reference sample for determining adsorbed CO2 amounts. The adsorption–chemisorption dynamics of CO2 were evaluated using TG analysis. For this analysis, the samples were preheated at 500 K under an Ar atmosphere (flow rate of CO2 and Ar gases = 100 mL min−1) for eight hours, and the TG analysis was then conducted at 500 K for four hours. Moreover, CO emissions were evaluated using mass spectroscopy during the TG measurement (BEL-MASS II, MicrotracBEL Co., Osaka, Japan) combined with TG analysis. The CO2-CO adsorbed structures on MOFs were evaluated using Fourier-transform infrared (IR) spectroscopy (FT/IR-4000, IRT-5000 JASCO Co., Tokyo, Japan).
3. Results and Discussion
Figure 1a,b show the prepared UiO-66(Zr) and MIL-100(Fe) XRD patterns, respectively. The peaks corresponded to the reported UiO-66(Zr) [24] and MIL-100(Fe) crystals [25,26]. The HR-TEM images in Figure 2 indicated that the interference fringes were observed with distances of 1.0 ± 0.2 nm and 2.4 ± 0.2 nm for UiO-66(Zr) and MIl-100(Fe), respectively. The uniform lattice fringes confirm that the prepared MOF materials are highly crystalline. Those distances were assigned by the (111) lattice plane on UiO-66(Zr) at 7.1 (1.2 nm distance) and the (220) lattice plane on MIL-100(Fe) at 3.4 (2.6 nm distance) in the XRD patterns. UiO-66(Zr) contains octahedral and tetrahedral cages (size = 1.2 and 0.6 nm, respectively) [27], whereas MIL-100(Fe) contains two types of mesoporous cages (size = 2.5 and 2.9 nm) [28]. The N2 adsorption isotherms on UiO-66(Zr) and MIL-100(Fe) at 77 K are shown in Figure 1c, which indicates that both isotherms are typical Langmuir isotherms and are in good agreement with each other, indicating the similar micropore volumes. However, the adsorption jump on UiO-66(Zr) was steeper than on MIL-100(Fe). The steep adsorption jumps at low pressure indicated the presence of narrower micropores, leading to micropore filling. Moreover, the adsorption and desorption isotherms completely matched, indicating few mesopores from the MIL-100(Fe) crystal structure. UiO-66(Zr) and MIL-100(Fe) pore size distributions were similar despite the different pore sizes determined based on their crystal structures. The discrepancy between the crystal and pore structures is due to the strong adsorption potential in the MIL-100(Fe) nanopores. The structural parameters are shown in Table 1. The specific surface area of UiO-66(Zr) was slightly larger than that of MIL-100(Fe), calculated using the Brunauer–Emmett–Teller (BET) equation [29]. In contrast, the pore volumes of the two materials, which were estimated based on the amounts at P/P0 = 0.8, were close. The average pore diameter of UiO-66(Zr) was smaller than that of MIL-100(Fe), which agreed with the estimation based on the crystal structures. However, the pore volume of UiO-66(Zr) 0.50 cm3 g−1 estimated from the N2 adsorption was larger than the value of 0.42 cm3 g−1, which was estimated from the crystal structure, implying the presence of defects and/or interparticle pores.
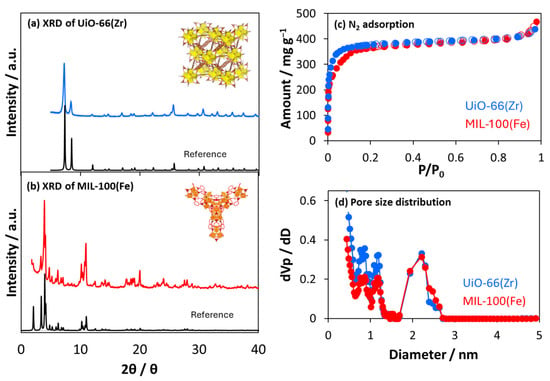
Figure 1.
XRD patterns of (a) UiO-66(Zr) and (b) MIL-100(Fe): the blue, red, and black curves represent the prepared UiO-66(Zr), the prepared MIL-100(Fe), and the references. (c) N2 adsorption isotherms on UiO-66(Zr) and MIL-100(Fe) at 77 K. The closed and open symbols represent adsorption and desorption branches, respectively. (d) Pore size distributions were obtained based on the N2 adsorption isotherms at 77 K.
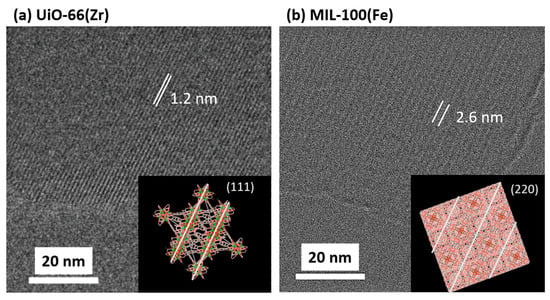
Figure 2.
HR-TEM images of UiO-66(Zr) (a) and MIL-100(Fe) (b) and their structure images. The insets represent the unit cells of the MOFs. Green, yellow, brown, and red spheres depict Zr, Fe, C, and O, respectively.

Table 1.
Structural parameters of the two MOF samples.
The thermal stability of the MOFs was assessed using thermal decomposition analysis under an oxygen atmosphere at 300–800 K (Figure 3). The weight decreases at temperatures less than 400 K, which can be attributed to the removal of guest molecules. At 400–550 K, the MOFs were stable. Finally, the MOFs started to decompose at temperatures higher than 550 K. The thermal stability of the MOFs at 500 K was evaluated based on their crystallinity (Figure 4). Figure 4 shows the XRD patterns before and after heating for 4 and 8 h under CO2 and Ar atmospheres, respectively. The peak positions of UiO-66(Zr) did not change, indicating that the UiO-66(Zr) crystal structure was generally maintained throughout the heating process. At the same time, the peaks were broadened, suggesting that the crystallinity was slightly decreased compared to that in the pristine one. The MIL-100(Fe) crystal structure was also hardly changed by heating. This indicated that the structure degradation probably caused by pulverization occurred on the UiO-66(Zr) compared with MIL-100(Fe) despite the higher stability in Figure 3. However, the crystalline structures of both MOFs were maintained because of unchanged XRD peak positions. When heating for longer than 10 h, the MOF structures were unchanged. Thus, we concluded that UiO-66(Zr) and MIL-100(Fe) were thermally stable at temperatures less than 550 K.
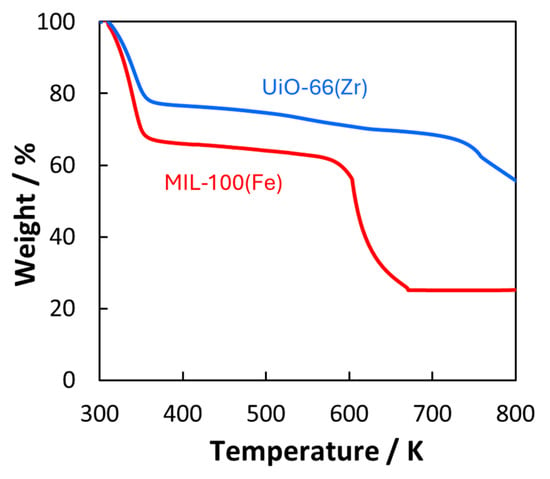
Figure 3.
Thermal decomposition of MOFs. UiO-66(Zr) and MIL-100(Fe) are depicted in blue and red curves.
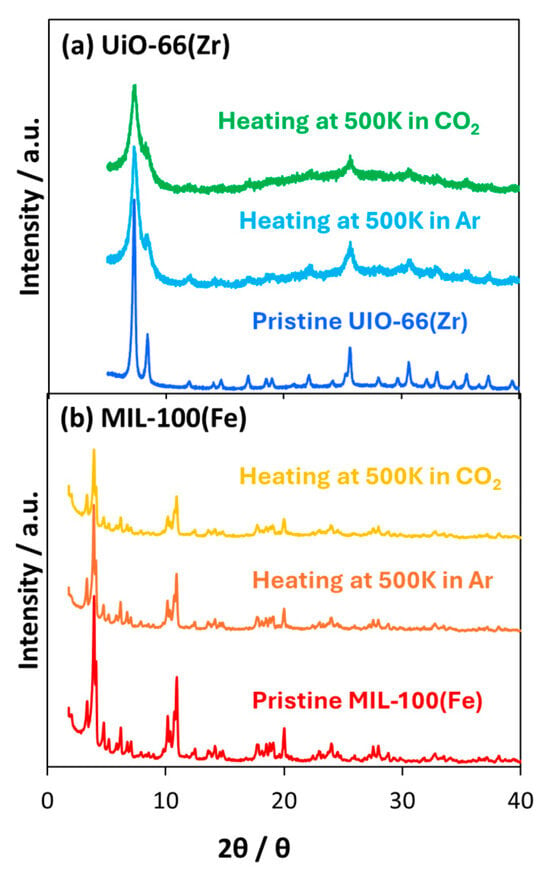
Figure 4.
XRD patterns of (a) UiO-66(Zr) and (b) MIL-100(Fe) after heating at 500 K under CO2 and Ar atmospheres.
The relationship between the thermal stability and specific surface areas of various MOFs is illustrated in Figure 5 [17,30,31,32,33,34,35,36,37,38,39,40,41]. Some MOFs exhibit high thermal stability and specific surface areas. The previously reported UiO-66(Zr) and MIL-100(Fe) also showed higher stability and specific surface areas compared to the prepared ones, which is likely due to variations in the synthesis techniques, solvents used, and distinct analytical methods employed for characterization [17,36]. The thermal stabilities of UiO-66(Zr) and MIL-100(Fe) were confirmed until 750 K and 550 K, respectively, and their specific surface areas were both 1500 ± 100 m2 g−1. Although the prepared UiO-66(Zr) and MIL-100(Fe) do not show the best thermal stability and specific surface areas among MOFs, they are common materials that exhibit relatively high thermal stability, specific surface areas, and numerous nanopores. Therefore, this study used the two MOFs, UiO-66(Zr) and MIL-100(Fe), to evaluate further CO2 thermal chemisorption and reduction properties, although the other highly thermally stabilized MOFs are also candidates for catalytic reactions of CO2 reduction.
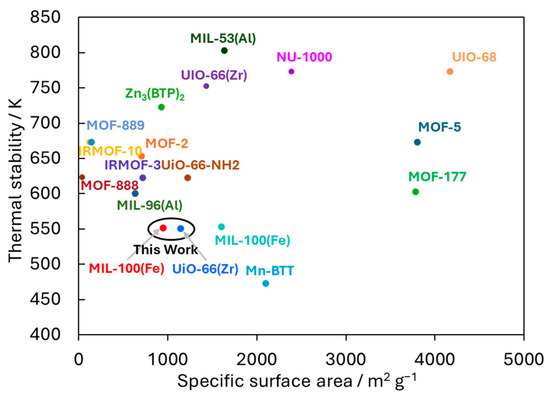
Figure 5.
Relationship between the thermal stabilities and specific surface areas of various MOFs. The UiO-66(Zr) and MIL-100(Fe) prepared in this work are circled in black.
The temperature-dependent CO2 adsorption on MOFs was evaluated based on the adsorption isotherms of CO2 at 300, 400, and 500 K (Figure 6a,b). The CO2 adsorption capacities of UiO-66(Zr) and MIL-100(Fe) at 1 atm and 300 K were 1200 and 810 μmol g−1, respectively. The higher adsorption on UiO-66(Zr) can be attributed to its more substantial adsorption potential, i.e., smaller pore sizes and a more substantial adsorption site such as a bare Zr metal site. On the other hand, the adsorption capacity of MIL-100(Fe) at 500 K (115 μmol g−1) was higher than that of UiO-66(Zr) (21 μmol g−1). The maintenance of the adsorption amount on MIL-100(Fe) was especially anomalous because physical adsorption capacities are proportional to their adsorption potentials. This was obvious for an activated carbon fiber; the adsorption on activated carbon fiber drastically decreased from 4400 to 20 μmol g−1 by elevating the temperature from 300 K to 500 K (Figure 6c). Therefore, MIL-100(Fe) has more substantial adsorption sites than UiO-66(Zr) and activated carbon fiber. The high adsorption potential on MIL-100(Fe) has also been reported to be approximately 29 cm3 g−1 under low pressure at 298 K, 12.2 mmol g−1 under high pressure at 303 K [42,43], and 1.43 mmol g−1 at 1 bar and 298 K [44]. The strong adsorption potential of CO2 on MIL-100(Fe) primarily occurs at the Fe-metal sites, distinguishing MIL-100(Fe) from other MOF materials [43,45,46]. The slight hysteresis in the adsorption and desorption curves of MIL-100(Fe) suggests CO2 chemisorption on the Fe-metal sites at elevated temperatures.
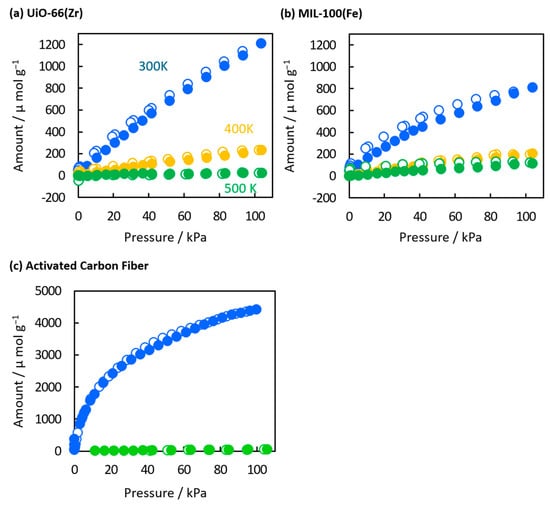
Figure 6.
CO2 adsorption isotherms of (a) UiO-66(Zr), (b) MIL-100(Fe), and (c) activated carbon fiber at 300 K (blue circles), 400 K (orange circles), and 500 K (green circles). The closed and open symbols represent adsorption and desorption branches, respectively.
The CO2 adsorptions on UiO-66(Zr) and MIL-100(Fe) at 500 K were also investigated using TG analysis (Figure 7a) after their pretreatment under an Ar atmosphere for eight hours to eliminate contamination and guest molecules. The sample weights considerably increased after switching the gas flow from Ar to CO2, which can be attributed to the adsorption of CO2. The maximum CO2 amounts on UiO-66(Zr) and MIL-100(Fe) were 50 and 80 μmol g−1, respectively. A rapid adsorption was observed on the two MOFs, which can be attributed to the strong affinity of CO₂ molecules to the active sites (probably the metal sites) on the MOFs. However, the weights related to CO2 adsorption decreased to approximately 20 μmol g−1, which was rarely observed in common porous materials such as activated carbon fiber. In addition, the adsorption amounts were 20 and 110 μmol g−1 for UiO-66(Zr) and MIL-100(Fe), as shown in Figure 6. The amounts evaluated from the adsorption and TG measurements were similar for the UiO-66(Zr). In contrast, those were considerably different for the MIL-100(Fe). Therefore, we consider that any chemical reactions were involved in the CO2 adsorption process, especially for MIL-100(Fe). We assumed that the weight reductions were attributed to the decomposition of the adsorbed CO2 into CO and O2, which were hardly observed in the adsorption measurements, especially using a volumetric adsorption apparatus. CO and O2 emissions during the adsorption of the CO2 flow on UiO-66(Zr) and MIL-100(Fe) at 500 K were then examined using mass spectroscopy (Figure 7b,c). Considerable CO emissions were observed on MIL-100(Fe) compared to those on UiO-66(Zr), proving the thermo-catalytic reduction of CO2 on the MOFs. In addition, tiny amounts of O2 emissions were also observed. Thus, considerable CO2 decomposition into CO occurred, especially on MIL-100(Fe), via the thermo-catalytic reduction of CO2 on their surfaces. The CO2 reduction reaction rate was hardly estimated in the current work. However, the weight changes in Figure 7a suggested that the reduced amounts were 20–60 μmol g−1 in terms of the difference between the weight at the top peak and the equilibrium weight.
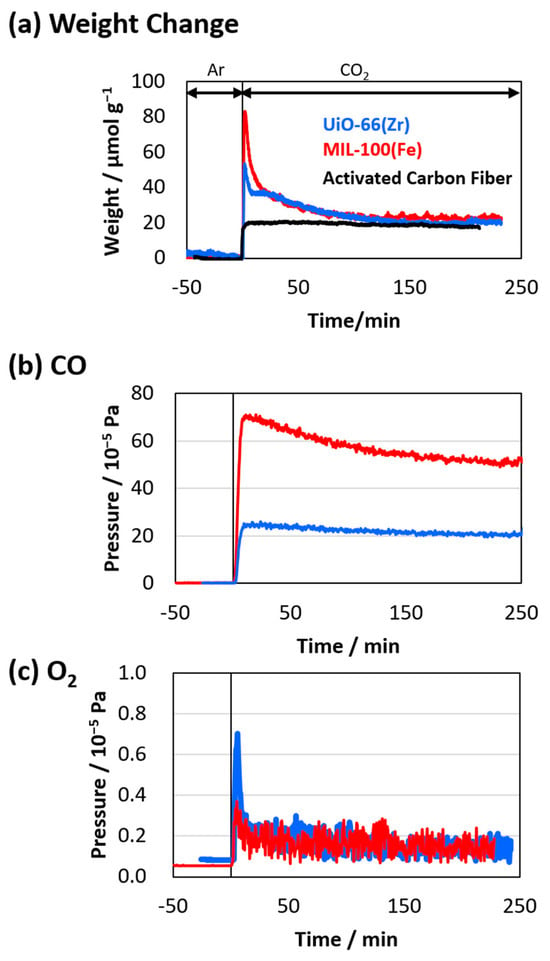
Figure 7.
(a) CO2 adsorption on UiO-66(Zr) (blue curve) and MIL-100(Fe) (red curve) at 500 K. CO2 adsorption on activated carbon fiber is also shown as a reference (black curve). (b) CO and (c) O2 emissions are evaluated based on mass spectroscopies.
The IR spectroscopies of CO2 and the related species were measured to analyze the chemisorbed and decomposed structures of CO2 adsorbed on the MOFs (Figure 8). Sharp reflectance bands were observed at 1460, 1500, and 1560 cm−1, which are attributed to asymmetric COO stretching for both UiO-66(Zr) and MIL-100(Fe) [47]. The reflectance band transformed into the other reflectance bands on UiO-66(Zr) when heated at 500 K under an Ar atmosphere. Moreover, the reflectance band of the asymmetric COO groups was recovered after the CO2 adsorption at 500 K, indicating the physical adsorption and/or chemisorption of CO2 on UiO-66(Zr). The reflectance bands of MIL-100(Fe) at 1600–1400 cm−1 were hardly observed after heating at 500 K. However, the broad band of the symmetric COO stretching at 1300–1400 cm−1 was weakly observed after the CO2 adsorption at 500 K compared to heating in the Ar atmosphere. These results suggest different forms of CO2 adsorption on the UiO-66(Zr) and MIL-100(Fe) surfaces. The electrochemical CO2 reduction on the two-dimensional MOF, PcNi-Co-O, had the intermediate state of *COOH, which was determined from the ATR-FTIR peak at 1400 cm−1 [48]. The CoO4 active sites worked as the substrate for the intermediate structure formation of CO2. The iron (II) site could be active in this work, as depicted in Figure 9. The CO2 adsorption and decomposition mechanism on MIL-100(Fe) was proposed and is shown in Figure 9, although the transition process of CO2 reduction was hardly observed. Fe(III) on MIL-100(Fe) is reduced to Fe(II) at 500 K [25,46,49]. Fe(II) moves to a bare site and interacts with CO2 gas. Next, the chemisorbed CO2 molecule is attached to Fe(II) in its symmetric OCO form and released as CO. The rest of the CO2 molecules are chemisorbed as carbonates on Fe(II), finally releasing CO and O2, confirmed by detecting CO and a slight amount of O2 in the mass spectra (Figure 7a,b).
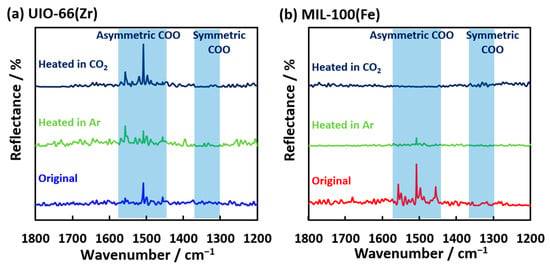
Figure 8.
IR spectra of (a) UiO-66(Zr) and (b) MIL-100(Fe) before and after CO2 adsorption at 500 K.
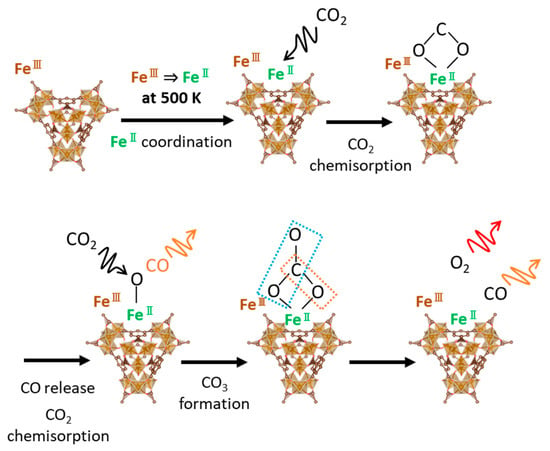
Figure 9.
CO2 chemisorption and reduction mechanism on MIL-100(Fe) at 500 K.
4. Conclusions
The CO2 adsorption on UiO-66(Zr) and MIL-100(Fe) and their thermo-catalytic activities for CO2 reduction at 300–500 K were investigated. Both MOFs exhibit large specific surface areas owing to their nanoporous crystal structures, which were maintained after heating at 500 K and under a CO2 atmosphere. The CO₂ adsorption capacity of UiO-66(Zr) (1200 μmol g−1) was considerably higher than that of MIL-100(Fe) (810 μmol g−1) at 300 K despite their similar specific surface areas and pore volumes. However, the adsorption amount of MIL-100(Fe), 115 μmol g−1, was much larger than UiO-66(Zr) (21 μmol g−1) at 500 K. In addition, the CO2 adsorption on an activated carbon fiber at 500 K was drastically decreased to 0.4% of its original value at 300 K. The better performance of MOFs is due to their strong adsorption potential, resulting in unique CO2 adsorption properties, including CO2 chemisorption and reduction into CO, which was especially evident on MIL-100(Fe). The changes in the IR spectra corresponding to the carbonyl and OCO groups indicated the chemisorption, reduction, and release cycles of CO2 during the thermo-catalytic process on the bare Fe(II) sites of MIL-100(Fe). The success of MOFs as thermo-catalysts for CO2 reduction can be a basis for further advancing MOF thermo-catalysts for efficient and sustainable CO₂ capture strategies. Further studies on the quantitative analysis of CO2 reduction on MOFs should be conducted to develop MOF-based CO2 reduction catalysts.
Author Contributions
S.T. and M.M.: investigation, data curation, formal analysis, and writing—original draft. A.K.: methodology, resources, and writing—review and editing. K.U.: investigation and writing—review and editing. T.O.: conceptualization, writing—original draft, writing—review and editing, and supervision. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by JSPS KAKENHI, Grant Number 23H01999.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Acknowledgments
Jiang Weihua and Risa Nakajima conducted some experiments. IR measurements were performed at the Center for the Chiba Iodine Resource Innovation Center at Chiba University.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Ghanbari, T.; Abnisa, F.; Wan Daud, W.M.A. A review on production of metal organic frameworks (MOF) for CO2 adsorption. Sci. Total Environ. 2020, 707, 135090. [Google Scholar] [CrossRef] [PubMed]
- Diffenbaugh, N.S.; Burke, M. Global warming has increased global economic inequality. Proc. Natl. Acad. Sci. USA 2019, 116, 9808–9813. [Google Scholar] [CrossRef]
- Notz, R.J.; Tönnies, I.; McCann, N.; Scheffknecht, G.; Hasse, H. CO2 Capture for Fossil Fuel-Fired Power Plants. Chem. Eng. Technol. 2011, 34, 163–172. [Google Scholar] [CrossRef]
- Yeh, J.T.; Resnik, K.P.; Rygle, K.; Pennline, H.W. Semi-batch absorption and regeneration studies for CO2 capture by aqueous ammonia. Fuel Process. Technol. 2005, 86, 1533–1546. [Google Scholar] [CrossRef]
- Chue, K.T.; Kim, J.N.; Yoo, Y.J.; Cho, S.H.; Yang, R.T. Comparison of activated carbon and zeolite 13X for CO2 recovery from flue gas by pressure swing adsorption. Ind. Eng. Chem. Res. 1995, 34, 591–598. [Google Scholar] [CrossRef]
- Wright, P.A. Microporous Framework Solids; RSC Publishing: Cambridge, UK, 2008; pp. 8–78. [Google Scholar]
- Xu, R.; Pang, W.; Yu, J.; Huo, Q.; Chen, J. Chemistry of Zeolites and Related Porous Materials: Synthesis and Structure; Wiley: New York, NY, USA, 2007; pp. 19–116. [Google Scholar]
- Guo, B.; Chang, L.; Xie, K. Adsorption of Carbon Dioxide on Activated Carbon. J. Nat. Gas Chem. 2006, 15, 223–229. [Google Scholar] [CrossRef]
- Wahby, A.; Silvestre-Albero, J.; Sepúlveda-Escribano, A.; Rodriguez-Reinoso, F. CO2 Adsorption on Carbon Molecular Sieves. Micropor. Mesopor. Mater. 2012, 164, 280. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Z.; Zhou, H.-C. Recent advances in carbon dioxide capture with metal-organic frameworks. Greenh. Gases Sci. Technol. 2012, 2, 239. [Google Scholar] [CrossRef]
- Lawson, H.D.; Walton, S.P.; Chan, C. Metal-Organic Frameworks for Drug Delivery: A Design Perspective. ACS Appl. Mater. Interfaces 2021, 13, 7004–7020. [Google Scholar] [CrossRef]
- Furukawa, H.; Cordova, K.E.; O’Keeffe, M.; Yaghi, O.M. The chemistry and Applications of metal-organic frameworks. Science 2013, 341, 1230444. [Google Scholar] [CrossRef]
- Li, J.-R.; Ma, Y.; McCarthy, M.C.; Sculley, J.; Yu, J.; Jeong, H.-K.; Balbuena, P.B.; Zhou, H.-C. Carbon dioxide capture-related gas adsorption and separation in metal-organic frameworks. Coord. Chem. Rev. 2011, 255, 1791–1823. [Google Scholar] [CrossRef]
- Yu, J.; Balbuena, P.B. Water Effects on Postcombustion CO2 Capture in Mg-MOF-74. J. Phys. Chem. C 2013, 117, 3383–3388. [Google Scholar] [CrossRef]
- Wang, H.H.; Hou, L.; Li, Y.Z.; Jiang, C.Y.; Wang, Y.Y.; Zhu, Z. Porous MOF with Highly Efficient Selectivity and Chemical Conversion for CO2. ACS Appl. Mater. Interfaces 2017, 9, 17969–17976. [Google Scholar] [CrossRef]
- Emam, H.E.; Abdelhamid, H.N.; Abdelhameed, R.M. Self-cleaned photoluminescent viscose fabric incorporated lanthanide-organic framework (Ln-MOF). Dyes Pigm. 2018, 159, 491–498. [Google Scholar] [CrossRef]
- Fang, Y.; Yang, Z.; Li, H.; Liu, X. MIL-100(Fe) and its derivatives: From synthesis to application for wastewater decontamination. Environ. Sci. Pollut. Res. Int. 2020, 27, 4703–4724. [Google Scholar] [CrossRef] [PubMed]
- Quijia, C.R.; Lima, C.; Silva, C.; Alves, R.C.; Frem, R.; Chorilli, M. Application of MIL-100(Fe) in drug delivery and biomedicine. Drug Deliv. Technol. 2021, 61, 102217. [Google Scholar] [CrossRef]
- Huang, S.; Yang, K.-L.; Liu, X.-F.; Pan, H.; Zhang, H.; Yang, S. MIL-100(Fe)-catalyzed efficient conversion of hexoses to lactic acid. RSC Adv. 2017, 7, 5621–5627. [Google Scholar] [CrossRef]
- Cmarik, G.E.; Kim, M.; Cohen, S.M.; Walton, K.S. Tuning the Adsorption Properties of UiO-66 via Ligand Functionalization. Langmuir 2012, 28, 15606–15613. [Google Scholar] [CrossRef]
- Cao, Y.; Zhang, H.; Song, F.; Huang, T.; Ji, J.; Zhong, Q.; Chu, W.; Xu, Q. UiO-66-NH2/GO Composite: Synthesis, Characterization and CO2 Adsorption Performance. Materials 2018, 11, 589. [Google Scholar] [CrossRef]
- Guesh, K.; Caiuby, C.A.D.; Mayoral, Á.; Díaz-García, M.; Díaz, I.; Sanchez-Sanchez, M. Sustainable Preparation of MIL-100(Fe) and Its Photocatalytic Behavior in the Degradation of Methyl Orange in Water. Cryst. Growth Des. 2017, 17, 1806–1813. [Google Scholar] [CrossRef]
- Katz, M.J.; Brown, Z.J.; Colón, Y.J.; Siu, P.W.; Scheidt, K.A.; Snurr, R.Q.; Hupp, J.T.; Farha, O.K. A facile synthesis of UiO-66, UiO-67 and their derivatives. Chem. Commun. 2013, 49, 9449–9451. [Google Scholar] [CrossRef]
- Cavka, J.H.; Jakobsen, S.; Olsbye, U.; Guillou, N.; Lamberti, C.; Bordiga, S.; Lillerud, K.P. A New Zirconium Inorganic Building Brick Forming Metal Organic Frameworks with Exceptional Stability. J. Am. Chem. Soc. 2008, 130, 13850–13851. [Google Scholar] [CrossRef]
- Horcajada, P.; Surblé, S.; Serre, C.; Hong, D.-Y.; Seo, Y.-K.; Chang, J.-S.; Grenèche, J.-M.; Margiolaki, I.; Férey, G. Synthesis and catalytic properties of MIL-100(Fe), an iron(III) carboxylate with large pores. Chem. Commun. 2007, 2820–2822. [Google Scholar] [CrossRef]
- Dhakshinamoorthy, A.; Alvaro, M.; Hwang, Y.K.; Seo, Y.-K.; Corma, A.; Garcia, H. Intracrystalline diffusion in Metal Organic Framework during heterogeneous catalysis: Influence of particle size on the activity of MIL-100 (Fe) for oxidation reactions. Dalton Trans. 2011, 40, 10719–10724. [Google Scholar] [CrossRef]
- Ramsahye, N.A.; Trens, P.; Shepherd, C.; Gonzalez, P.; Trung, T.K.; Ragon, F.; Serre, C. The effect of pore shape on hydrocarbon selectivity on UiO-66(Zr), HKUST-1 and MIL-125(Ti) metal organic frameworks: Insights from molecular simulations and chromatography. Micropor. Mesopor. Mater. 2014, 189, 222–231. [Google Scholar] [CrossRef]
- Ragon, F.; Campo, B.; Yang, Q.; Martineau, C.; Wiersum, A.D.; Lago, A.; Guillerm, V.; Hemsley, C.; Eubank, J.F.; Vishnuvarthan, M.; et al. Acid-functionalized UiO-66(Zr) MOFs and their evolution after intra-framework cross-linking: Structural features and sorption properties. J. Mater. Chem. A 2015, 3, 3294–3309. [Google Scholar] [CrossRef]
- Ladavos, A.K.; Katsoulidis, A.P.; Iosifidis, A.; Triantafyllidis, K.S.; Pinnavaia, T.J.; Pomonis, P.J. The BET equation, the inflection points of N2 adsorption isotherms and the estimation of specific surface area of porous solids. Micropor. Mesopor. Mater. 2012, 151, 126–133. [Google Scholar] [CrossRef]
- Asghar, A.; Iqbal, N.; Noor, T. Ultrasonication treatment enhances MOF surface area and gas uptake capacity. Polyhedron 2020, 181, 114463. [Google Scholar] [CrossRef]
- Yin, D.; Hu, X.; Cai, M.; Wang, K.; Peng, H.; Bai, J.; Xv, Y.; Fu, T.; Dong, X.; Ni, J.; et al. Preparation, Characterization, and In Vitro Release of Curcumin-Loaded IRMOF-10 Nanoparticles and Investigation of Their Pro-Apoptotic Effects on Human Hepatoma HepG2 Cells. Molecules 2022, 27, 3940. [Google Scholar] [CrossRef] [PubMed]
- Biswas, S.; Maes, M.; Dhakshinamoorthy, A.; Feyand, M.; De Vos, D.E.; Garcia, H.; Stock, N. Fuelpurification, Lewis acid and aerobic oxidation catalysis performed by a microporous Co-BTT (BTT3− = 1,3,5-benzenetristetrazolate) framework having coordinatively unsaturated sites. J. Mater. Chem. 2012, 22, 10200–10209. [Google Scholar] [CrossRef]
- Ta, H.; Pham Ngoc, T.; Nguyen, X.H.; Son, D. Metal—Organic Frameworks: State-of-the-art Material for Gas Capture and Storage. VNU J. Sci. Math. Phys. 2016, 32, 67–85. [Google Scholar]
- Yari Kalashgrani, M.; Babapoor, A.; Mousavi, S.M.; Feizpoor, S.; Hashemi, S.A.; Chiang, W.-H.; Lai, C.w. Synthesis of Isoreticular Metal Organic Framework-3 (IRMOF-3) Porous Nanostructure and Its Effect on Naphthalene Adsorption: Optimized by Response Surface Methodology. Separations 2023, 10, 261. [Google Scholar] [CrossRef]
- Colombo, V.; Galli, S.; Choi, H.J.; Han, G.D.; Maspero, A.; Palmisano, G.; Masciocchi, N.; Long, J.R. High thermal and chemical stability in pyrazolate-bridged metal–organic frameworks with exposed metal sites. Chem. Sci. 2011, 2, 1311–1319. [Google Scholar] [CrossRef]
- Abid, H.R.; Azhar, M.R.; Iglauer, S.; Rada, Z.H.; Al-Yaseri, A.; Keshavarz, A. Physicochemical Characterization of metal organic framework materials: A mini review. Heliyon 2024, 10, e23840. [Google Scholar] [CrossRef] [PubMed]
- Abid, H.R.; Rada, Z.H.; Li, Y.; Mohammed, H.A.; Wang, Y.; Wang, S.; Arandiyan, H.; Tan, X.; Liu, S. Boosting CO2 adsorption and selectivity in metal–organic frameworks of MIL-96(Al) via second metal Ca coordination. RSC Adv. 2020, 10, 8130–8139. [Google Scholar] [CrossRef]
- Halder, A.; Lee, S.; Yang, B.; Pellin, M.; Vajda, S.; Li, Z.; Yang, Y.; Farha, O.; Hupp, J. Structural reversibility of Cu doped NU-1000 MOFs under hydrogenation conditions. J. Chem. Phys. 2020, 152, 084703. [Google Scholar] [CrossRef]
- Nguyen, P.T.; Nguyen, H.T.; Pham, H.Q.; Kim, J.; Cordova, K.E.; Furukawa, H. Synthesis and Selective CO2 Capture Properties of a Series of Hexatopic Linker-Based Metal-Organic Frameworks. Inorg. Chem. 2015, 54, 10065–10072. [Google Scholar] [CrossRef]
- Ye, X.; Liu, D. Metal–Organic Framework UiO-68 and Its Derivatives with Sufficiently Good Properties and Performance Show Promising Prospects in Potential Industrial Applications. Cryst. Growth Des. 2021, 21, 4780–4804. [Google Scholar] [CrossRef]
- Saha, D.; Deng, S. Structural Stability of Metal Organic Framework MOF-177. J. Phys. Chem. Lett. 2010, 1, 73–78. [Google Scholar] [CrossRef]
- Mutyala, S.; Yakout, S.M.; Ibrahim, S.S.; Jonnalagadda, M.; Mitta, H. Enhancement of CO2 capture and separation of CO2/N2 using post-synthetic modified MIL-100(Fe). New J. Chem. 2019, 43, 9725–9731. [Google Scholar] [CrossRef]
- Oliveira, L.T.; Gonçalves, R.V.; Gonçalves, D.V.; de Azevedo, D.C.S.; Pereira de Lucena, S.M. Superior Performance of Mesoporous MOF MIL-100 (Fe) Impregnated with Ionic Liquids for CO2 Adsorption. J. Chem. Eng. Data 2019, 64, 2221–2228. [Google Scholar] [CrossRef]
- Mei, L.; Jiang, T.; Zhou, X.; Li, Y.; Wang, H.; Li, Z. A novel DOBDC-functionalized MIL-100(Fe) and its enhanced CO2 capacity and selectivity. Chem. Eng. J. 2017, 321, 600–607. [Google Scholar] [CrossRef]
- Ahmed, H.E.; Albolkany, M.K.; El-Khouly, M.E.; El-Moneim, A.A. Tailoring MIL-100(Fe)-derived catalyst for controlled carbon dioxide conversion and product selectivity. RSC Adv. 2024, 14, 13946–13956. [Google Scholar] [CrossRef] [PubMed]
- Lv, H.; Zhao, H.; Cao, T.; Qian, L.; Wang, Y.; Zhao, G. Efficient degradation of high concentration azo-dye wastewater by heterogeneous Fenton process with iron-based metal-organic framework. J. Mol. Catal. A Chem. 2015, 400, 81–89. [Google Scholar] [CrossRef]
- Du, J.; Zhang, F.; Jiang, L.; Guo, Z.; Song, H. Enhanced cobalt MOF electrocatalyst for oxygen evolution reaction via morphology regulation. Inorg. Chem. Commun. 2023, 158, 111661. [Google Scholar] [CrossRef]
- Zhang, M.-D.; Huang, J.-R.; Shi, W.; Liao, P.-Q.; Chen, X.-M. Synergistic Effect in a Metal–Organic Framework Boosting the Electrochemical CO2 Overall Splitting. J. Am. Chem. Soc. 2023, 145, 2439–2447. [Google Scholar] [CrossRef]
- Cheng, R.; Debroye, E.; Hofkens, J.; Roeffaers, M.B.J. Efficient Photocatalytic CO2 Reduction with MIL-100(Fe)-CsPbBr3 Composites. Catalysts 2020, 10, 1352. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).