Nanoparticles as Strategies for Modulating the Host’s Response in Periodontitis Treatment
Abstract
1. Introduction
2. Nanotechnology and Its Significance in the Field of Periodontology
2.1. Antimicrobial Nanotherapeutic Strategies
2.2. Antibiotic Nano-Antibacterial Agents
2.3. Metallic Nano-Antibacterial Agents
2.4. Application of Photothermal and Photodynamic Therapy
2.5. Immunomodulatory Nanotherapeutic Strategies: Macrophage Polarization Remodeling
3. Immunomodulatory and Regenerative Therapy for Periodontium
4. Challenges and Future Perspectives
5. Therapeutic Approaches for Periodontitis and Related Systemic Diseases
6. Therapeutic Potential of Natural Active Ingredients in Nanotechnology
7. Discussion
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| NSAIDs | Nonsteroidal anti-inflammatory drugs |
| PTT | Photothermal therapy |
| PDT | Photodynamic therapy |
| MIN-NPs | Minocycline-loaded nanoparticle delivery systems |
| MOX-PLGA | Moxifloxacin hydrochloride-loaded nanoparticles |
| ZnO | Zinc oxide |
| Au-NPs | Gold nanoparticles |
| ROS | Reactive oxygen species |
| AgNPs | Silver nanoparticles |
| AgNPs-CHL | AgNPs combined with chlorhexidine |
| AgNPs-PEG-MET | AgNPs combined with metronidazole |
| ZIF-8 | Zeolitic imidazole framework-8 |
| PLGA | Poly Lactic-Co-Glycolic Acid |
| CTP-SA | Sodium alginate hydrogel composite |
| TiO2@PDA | Polydopamine titanium dioxide |
| GTR | Guided tissue regeneration |
| BL | Blue light |
| NIR | Near-infrared light |
| CA | Carvacrol |
| Lipo-RSV | Resveratrol-loaded liposomes |
| STAT | Signal transducer and activator of transcription |
| PDLSCs | Periodontal ligament stem cells |
References
- Jiao, J.; Jing, W.; Si, Y.; Feng, X.; Tai, B.; Hu, D.; Lin, H.; Wang, B.; Wang, C.; Zheng, S.; et al. The prevalence and severity of periodontal disease in Mainland China: Data from the Fourth National Oral Health Survey (2015–2016). J. Clin. Periodontol. 2021, 48, 168–179. [Google Scholar] [CrossRef] [PubMed]
- Eke, P.I.; Wei, L.; Borgnakke, W.S.; Thornton-Evans, G.; Zhang, X.; Lu, H.; McGuire, L.C.; Genco, R.J. Periodontitis prevalence in adults ≥ 65 years of age, in the USA. Periodontology 2000 2016, 72, 76–95. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.S.; Luan, H.H.; Wu, L.; Shi, Y.J.; Wang, Y.B.; Huang, Q.; Xie, W.Z.; Zeng, X.T. Secular trends in severe periodontitis incidence, prevalence and disability-adjusted life years in five Asian countries: A comparative study from 1990 to 2017. J. Clin. Periodontol. 2021, 48, 627–637. [Google Scholar] [CrossRef] [PubMed]
- Hajishengallis, G. Periodontitis: From microbial immune subversion to systemic inflammation. Nat. Rev. Immunol. 2015, 15, 30–44. [Google Scholar] [CrossRef]
- Hajishengallis, G. Interconnection of periodontal disease and comorbidities: Evidence, mechanisms, and implications. Periodontology 2000 2022, 89, 9–18. [Google Scholar] [CrossRef]
- Zięba, M.; Chaber, P.; Duale, K.; Maksymiak, M.M.; Basczok, M.; Kowalczuk, M.; Adamus, G. Polymeric Carriers for Delivery Systems in the Treatment of Chronic Periodontal Disease. Polymers 2020, 12, 1574. [Google Scholar] [CrossRef]
- Mlachkova, A. Application of antibiotics in the treatment of periodontal diseases. Dent. Exam. 2005, 87, 58–65. [Google Scholar]
- Kherul Anuwar, A.H.; Saub, R.; Safii, S.H.; Ab-Murat, N.; Taib, M.S.M.; Mamikutty, R.; Ng, C.W. Systemic Antibiotics as an Adjunct to Subgingival Debridement: A Network Meta-Analysis. Antibiotics 2022, 11, 1716. [Google Scholar] [CrossRef]
- Golub, L.M.; Lee, H.M. Periodontal therapeutics: Current host-modulation agents and future directions. Periodontology 2000 2020, 82, 186–204. [Google Scholar] [CrossRef]
- Mlachkova, A. Modulation of host response—Use in periodontal diseases. Medinform 2022, 9, 1504–1510. [Google Scholar] [CrossRef]
- Popova, C.; Mlachkova, A. Effectiveness of additional therapy with NSAID (Aulin) on distribution of shallow and deep periodontal pockets in patients with chronic periodontitis (pilot study). J. IMAB 2009, 4, 55–57. [Google Scholar] [CrossRef][Green Version]
- Novak, M.J.; Dawson, D.R., 3rd; Magnusson, I.; Karpinia, K.; Polson, A.; Polson, A.; Ryan, M.E.; Ciancio, S.; Drisko, C.H.; Kinane, D.; et al. Combining host modulation and topical antimicrobial therapy in the management of moderate to severe periodontitis: A randomized multicenter trial. J. Periodontol. 2008, 79, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Deng, Y.; Ma, S.; Ran, M.; Jia, Y.; Meng, J.; Han, F.; Gou, J.; Yin, T.; He, H.; et al. Local drug delivery systems as therapeutic strategies against periodontitis: A systematic review. J. Control Release 2021, 333, 269–282. [Google Scholar] [CrossRef] [PubMed]
- Bako, J.; Toth, F.; Gall, J.; Kovacs, R.; Csík, A.; Varga, I.; Sculean, A.; Zelko, R.; Hegedus, C. Combined Release of Antiseptic and Antibiotic Drugs from Visible Light Polymerized Biodegradable Nanocomposite Hydrogels for Periodontitis Treatment. Pharmaceutics 2022, 14, 957. [Google Scholar] [CrossRef]
- Makabenta, J.M.V.; Nabawy, A.; Li, C.H.; Schmidt-Malan, S.; Patel, R.; Rotello, V.M. Nanomaterial-based therapeutics for antibiotic-resistant bacterial infections. Nat. Rev. Microbiol. 2021, 19, 23–36. [Google Scholar] [CrossRef]
- Gold, K.; Slay, B.; Knackstedt, M.; Gaharwar, A.K. Antimicrobial activity of metal and metal-oxide based nanoparticles. Adv. Ther. 2018, 1, 1700033. [Google Scholar] [CrossRef]
- Moothedath, M.; Moothedath, M.; Jairaj, A.; Harshitha, B.; Baba, S.M.; Khateeb, S.U. Role of Nanotechnology in Dentistry: Systematic Review. J. Int. Soc. Prev. Community Dent. 2019, 9, 535–541. [Google Scholar] [CrossRef]
- Vaishali, S.; Kareem, N. Nanotechnology in Periodontics: A Review. J. Res. Med. Dent. Sci. 2021, 9, 339–344. [Google Scholar]
- Steigmann, L.; Maekawa, S.; Sima, C.; Travan, S.; Wang, C.-W.; Giannobile, W.V. Biosensor and Lab-on-a-chip Biomarker-identifying Technologies for Oral and Periodontal Diseases. Front. Pharmacol. 2020, 11, 588480. [Google Scholar] [CrossRef]
- Fujihara, K.; Kotaki, M.; Ramakrishna, S. Guided bone regeneration membrane made of polycaprolactone/calcium carbonate composite nanofibers. Biomaterials 2005, 26, 4139–4147. [Google Scholar] [CrossRef]
- Kelotte, D.; Melath, A.; Kaykool, S.; Chandran, N. Nanotechnology and periodontics. J. Periodontal. Implant. Sci. 2023, 53, 245–247. [Google Scholar] [CrossRef] [PubMed]
- Tomisa, A.P.; Launey, M.E.; Lee, J.S.; Mankani, M.H.; Wegst, U.G.; Saiz, E. Nanotechnology approaches to improve dental implants. Int. J. Oral Maxillofac. Implants 2011, 26, 25–44. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3087979/ (accessed on 11 January 2025).
- Sood, R.; Sharma, E.; Garg, R.; Kaur, S. Artificial intelligence (AI) and recent advancements in periodontology. IP Int. J. Periodontol. Implant. 2022, 7, 99–102. [Google Scholar] [CrossRef]
- Cao, J.H.; Xue, R.; He, B. Quercetin protects oral mucosal keratinocytes against lipopolysaccharide-induced inflammatory toxicity by suppressing the AKT/AMPK/mTOR pathway. Immunopharmacol. Immunotoxicol. 2021, 43, 519–526. [Google Scholar] [CrossRef] [PubMed]
- Taskan, M.M.; Gevrek, F. Quercetin Decreased Alveolar Bone Loss and Apoptosis in Experimentally Induced Periodontitis Model in Wistar Rats. Anti-Inflamm. Anti-Allergy Agents Med. Chem. 2020, 19, 436–448. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, C.; Wan, Y.; Qi, M.; Chen, Q.; Sun, Y.; Sun, X.; Fang, J.; Fu, L.; Xu, L.; et al. Quercetin-Loaded Ceria Nanocomposite Potentiate Dual-Directional Immunoregulation via Macrophage Polarization against Periodontal Inflammation. Small 2021, 17, e2101505. [Google Scholar] [CrossRef]
- Tian, Y.; Li, Y.; Liu, J.; Lin, Y.; Jiao, J.; Chen, B.; Wang, W.; Wu, S.; Li, C. Photothermal therapy with regulated Nrf2/NF-κB signaling pathway for treating bacteria-induced periodontitis. Bioact. Mater. 2021, 9, 428–445. [Google Scholar] [CrossRef]
- Chen, Y.; Gao, Y.; Chen, Y.; Liu, L.; Mo, A.; Peng, Q. Nanomaterials-based photothermal therapy and its potentials in antibacterial treatment. J. Control Release 2020, 328, 251–262. [Google Scholar] [CrossRef]
- Mi, G.; Shi, D.; Wang, M.; Webster, T.J. Reducing Bacterial Infections and Biofilm Formation Using Nanoparticles and Nanostructured Antibacterial Surfaces. Adv. Healthc. Mater. 2018, 7, e1800103. [Google Scholar] [CrossRef]
- Mehta, D.; Saini, V.; Aggarwal, B.; Khan, A.; Bajaj, A. Unlocking the bacterial membrane as a therapeutic target for next-generation antimicrobial amphiphiles. Mol. Asp. Med. 2021, 81, 100999. [Google Scholar] [CrossRef]
- Mudgil, M.; Pawar, P.K. Preparation and In Vitro/Ex Vivo Evaluation of Moxifloxacin-Loaded PLGA Nanosuspensions for ophthalmic application. Sci. Pharm. 2013, 81, 591–606. [Google Scholar] [CrossRef]
- Beg, S.; Dhiman, S.; Sharma, T.; Jain, A.; Sharma, R.K.; Jain, A.; Singh, B. Stimuli responsive in situ gelling systems loaded with PLGA nanoparticles of moxifloxacin hydrochloride for effective treatment of periodontitis. AAPS PharmSciTech. 2020, 21, 76. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Malkmes, M.J.; Jiang, C.; Wang, P.; Zhu, L.; Zhang, H.; Zhang, Y.; Huang, H.; Jiang, L. Antibacterial mechanism and transcriptome analysis of ultra-small gold nanoclusters as an alternative of harmful antibiotics against Gram-negative bacteria. J. Hazard. Mater. 2021, 416, 126236. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, R.; Wang, Y.; Wang, P.; Pu, J.; Xu, X.; Chen, F.; Jiang, L.; Jiang, Q.; Yan, F. Antibiofilm activity of ultra-small gold nanoclusters against Fusobacterium nucleatum in dental plaque biofilms. J. Nanobiotechnol. 2022, 20, 470. [Google Scholar] [CrossRef]
- Król, A.; Pomastowski, P.; Rafińska, K.; Railean-Plugaru, V.; Buszewski, B. Zinc oxide nanoparticles: Synthesis, antiseptic activity and toxicity mechanism. Adv. Colloid. Interface Sci. 2017, 249, 37–52. [Google Scholar] [CrossRef]
- Sirelkhatim, A.; Mahmud, S.; Seeni, A.; Kaus, N.H.M.; Ann, L.C.; Bakhori, S.K.M.; Hasan, H.; Mohamad, D. Review on Zinc Oxide Nanoparticles: Antibacterial Activity and Toxicity Mechanism. Nanomicro Lett. 2015, 7, 219–242. [Google Scholar] [CrossRef]
- Münchow, E.A.; Albuquerque, M.T.; Zero, B.; Kamocki, K.; Piva, E.; Gregory, R.L.; Bottino, M.C. Development and characterization of novel ZnO-loaded electrospun membranes for periodontal regeneration. Dent. Mater. 2015, 31, 1038–1051. [Google Scholar] [CrossRef]
- Augustine, R.; Dominic, E.A.; Reju, I.; Kaimal, B.; Kalarikkal, N.; Thomas, S. Electrospun polycaprolactone membranes incorporated with ZnO nanoparticles as skin substitutes with enhanced fibroblast proliferation and wound healing. RSC Adv. 2014, 4, 24777–24785. [Google Scholar] [CrossRef]
- Wang, D.; Li, Q.; Xiao, C.; Wang, H.; Dong, S. Nanoparticles in Periodontitis Therapy: A Review of the Current Situation. Int. J. Nanomed. 2024, 19, 6857–6893. [Google Scholar] [CrossRef]
- Steckiewicz, K.P.; Cieciórski, P.; Barcińska, E.; Jaśkiewicz, M.; Narajczyk, M.; Bauer, M.; Kamysz, W.; Megiel, E.; Inkielewicz-Stepniak, I. Silver Nanoparticles as Chlorhexidine and Metronidazole Drug Delivery Platforms: Their Potential Use in Treating Periodontitis. Int. J. Nanomed. 2022, 17, 495–517, Erratum in Int. J. Nanomed. 2023, 18, 1613–1614. [Google Scholar] [CrossRef]
- Liu, Y.; Li, T.; Sun, M.; Cheng, Z.; Jia, W.; Jiao, K.; Wang, S.; Jiang, K.; Yang, Y.; Dai, Z.; et al. ZIF-8 modified multifunctional injectable photopolymerizable GelMA hydrogel for the treatment of periodontitis. Acta Biomater. 2022, 146, 37–48. [Google Scholar] [CrossRef]
- Desai, H.; Mahmoud, M.Y.; Tan, J.; Minooei, F.; Demuth, D.R.; Steinbach-Rankins, J.M. Assessment of CafA Targeted BAR-Encapsulated Nanoparticles against Oral Biofilms. Pharmaceutics 2020, 12, 835. [Google Scholar] [CrossRef]
- de Freitas, L.M.; Calixto, G.M.; Chorilli, M.; Giusti, J.S.M.; Bagnato, V.S.; Soukos, N.S.; Amiji, M.M.; Fontana, C.R. Polymeric Nanoparticle-Based Photodynamic Therapy for Chronic Periodontitis in Vivo. Int. J. Mol. Sci. 2016, 17, 769. [Google Scholar] [CrossRef] [PubMed]
- Da, H.; Decheng, W. Biodegradable dendrimers for drug delivery. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 90, 713–727. [Google Scholar] [CrossRef]
- Wang, L.; Li, Y.; Ren, M.; Wang, X.; Li, L.; Liu, F.; Lan, Y.; Yang, S.; Song, J. pH and lipase-responsive nanocarrier-mediated dual drug delivery system to treat periodontitis in diabetic rats. Bioact. Mater. 2022, 18, 254–266. [Google Scholar] [CrossRef] [PubMed]
- Large, D.; Abdelmessih, R.; Fink, E.; Auguste, D.T. Liposome composition in drug delivery design, synthesis, characterization, and clinical application. Adv. Drug Deliv. Rev. 2021, 176, 113851. [Google Scholar] [CrossRef]
- Hu, F.; Zhou, Z.; Xu, Q.; Fan, C.; Wang, L.; Ren, H.; Xu, S.; Ji, Q.; Chen, X. A novel pH-responsive quaternary ammonium chitosan-liposome nanoparticles for periodontal treatment. Int. J. Biol. Macromol. 2019, 129, 1113–1119. [Google Scholar] [CrossRef]
- Ding, Y.; Wang, Y.; Li, J.; Tang, M.; Chen, H.; Wang, G.; Guo, J.; Gui, S. Microemulsion-thermosensitive gel composites as in situ-forming drug reservoir for periodontitis tissue repair through alveolar bone and collagen regeneration strategy. Pharm. Dev. Technol. 2023, 28, 30–39. [Google Scholar] [CrossRef]
- Lin, J.; He, Z.; Liu, F.; Feng, J.; Huang, C.; Sun, X.; Deng, H. Hybrid Hydrogels for Synergistic Periodontal Antibacterial Treatment with Sustained Drug Release and NIR-Responsive Photothermal Effect. Int. J. Nanomed. 2020, 15, 5377–5387. [Google Scholar] [CrossRef]
- Zhang, T.; Ying, D.; Qi, M.; Li, X.; Fu, L.; Sun, X.; Wang, L.; Zhou, Y. Anti-Biofilm Property of Bioactive Upconversion Nanocomposites Containing Chlorin e6 against Periodontal Pathogens. Molecules 2019, 24, 2692. [Google Scholar] [CrossRef]
- Xu, Y.; Zhao, S.; Weng, Z.; Zhang, W.; Wan, X.; Cui, T.; Ye, J.; Liao, L.; Wang, X. Jelly-Inspired Injectable Guided Tissue Regeneration Strategy with Shape Auto-Matched and Dual-Light-Defined Antibacterial/Osteogenic Pattern Switch Properties. ACS Appl. Mater. Interfaces 2020, 12, 54497–54506. [Google Scholar] [CrossRef]
- Hu, D.; Zhang, C.; Sun, C.; Bai, H.; Xie, J.; Gu, Y.; Li, M.; Jiang, J.; Le, A.; Qiu, J.; et al. Carvacrol combined with NIR light-responsive nano-drug delivery system with specific anti-bacteria, anti-inflammation, and immunomodulation for periodontitis. Nano Res. 2023, 16, 7199–7215. [Google Scholar] [CrossRef]
- Huang, X.; Li, Y.; Fu, M.; Xin, H.B. Polarizing Macrophages In Vitro. Methods Mol. Biol. 2018, 1784, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.X.; Zhang, S.X.; Wu, H.J.; Rong, X.L.; Guo, J. M2b macrophage polarization and its roles in diseases. J. Leukoc. Biol. 2019, 106, 345–358. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Zhang, Y.; Zhang, X.; Chen, R.; Wei, J.; Hou, J.; Wang, B.; Lai, H.; Huang, Y. Remodeling immune microenvironment in periodontitis using resveratrol liposomes as an antibiotic-free therapeutic strategy. J. Nanobiotechnol. 2021, 19, 429. [Google Scholar] [CrossRef]
- Yang, B.; Chen, Y.; Shi, J. Reactive Oxygen Species (ROS)-Based Nanomedicine. Chem. Rev. 2019, 119, 4881–4985. [Google Scholar] [CrossRef]
- Liu, J.; Ouyang, Y.; Zhang, Z.; Wen, S.; Pi, Y.; Chen, D.; Su, Z.; Liang, Z.; Guo, L.; Wang, Y. The role of Th17 cells: Explanation of relationship between periodontitis and COPD? Inflamm. Res. 2022, 71, 1011–1024. [Google Scholar] [CrossRef]
- Beklen, A.; Ainola, M.; Hukkanen, M.; Gürgan, C.; Sorsa, T.; Konttinen, Y. MMPs, IL-1, and TNF are regulated by IL-17 in periodontitis. J. Dent. Res. 2007, 86, 347–351. [Google Scholar] [CrossRef]
- Cavalla, F.; Hernández, M. Polarization Profiles of T Lymphocytes and Macrophages Responses in Periodontitis. Adv. Exp. Med. Biol. 2022, 1373, 195–208. [Google Scholar] [CrossRef]
- Papathanasiou, E.; Conti, P.; Carinci, F.; Lauritano, D.; Theoharides, T. IL-1 Superfamily Members and Periodontal Diseases. J. Dent. Res. 2020, 99, 1425–1434. [Google Scholar] [CrossRef]
- Ni, C.; Zhou, J.; Kong, N.; Bian, T.; Zhang, Y.; Huang, X.; Xiao, Y.; Yang, W.; Yan, F. Gold nanoparticles modulate the crosstalk between macrophages and periodontal ligament cells for periodontitis treatment. Biomaterials 2019, 206, 115–132. [Google Scholar] [CrossRef]
- Li, S.; Wang, L.; Gu, Y.; Lin, L.; Zhang, M.; Jin, M.; Mao, C.; Zhou, J.; Zhang, W.; Huang, X.; et al. Biomimetic immunomodulation by crosstalk with nanoparticulate regulatory T cells. Matter 2021, 4, 3621–3645. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, X.; Li, H.; Ni, C.; Du, Z.; Yan, F. Human oral microbiota and its modulation for oral health. Biomed. Pharmacother. 2018, 99, 883–893. [Google Scholar] [CrossRef] [PubMed]
- Bustamante, M.; Oomah, B.D.; Mosi-Roa, Y.; Rubilar, M.; Burgos-Díaz, C. Probiotics as an Adjunct Therapy for the Treatment of Halitosis, Dental Caries and Periodontitis. Probiotics Antimicrob. Proteins 2020, 12, 325–334. [Google Scholar] [CrossRef] [PubMed]
- Zidar, A.; Kristl, J.; Kocbek, P.; Zupančič, Š. Treatment challenges and delivery systems in immunomodulation and probiotic therapies for periodontitis. Expert. Opin. Drug Deliv. 2021, 18, 1229–1244. [Google Scholar] [CrossRef]
- Wang, J.; Liu, Y.; Wang, W.; Ma, J.; Zhang, M.; Lu, X.; Liu, J.; Kou, Y. The rationale and potential for using Lactobacillus in the management of periodontitis. J. Microbiol. 2022, 60, 355–363, Erratum in J. Microbiol. 2022, 60, 450. [Google Scholar] [CrossRef]
- Esteban-Fernández, A.; Ferrer, M.D.; Zorraquín-Peña, I.; López-López, A.; Moreno-Arribas, M.V.; Mira, A. In vitro beneficial effects of Streptococcus dentisani as potential oral probiotic for periodontal diseases. J. Periodontol. 2019, 90, 1346–1355. [Google Scholar] [CrossRef]
- Mittal, M.; Siddiqui, M.R.; Tran, K.; Reddy, S.P.; Malik, A.B. Reactive oxygen species in inflammation and tissue injury. Antioxid. Redox Signal. 2014, 20, 1126–1167. [Google Scholar] [CrossRef]
- Tan, D.Q.; Suda, T. Reactive Oxygen Species and Mitochondrial Homeostasis as Regulators of Stem Cell Fate and Function. Antioxid. Redox Signal. 2018, 29, 149–168. [Google Scholar] [CrossRef]
- González-Febles, J.; Sanz, M. Periodontitis and rheumatoid arthritis: What have we learned about their connection and their treatment? Periodontology 2000 2021, 87, 181–203. [Google Scholar] [CrossRef]
- Ray, R.R. Periodontitis: An Oral Disease with Severe Consequences. Appl. Biochem. Biotechnol. 2023, 195, 17–32. [Google Scholar] [CrossRef]
- Newman, K.L.; Kamada, N. Pathogenic associations between oral and gastrointestinal diseases. Trends Mol. Med. 2022, 28, 1030–1039. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Xin, Z.; Gao, S.; Li, Y.; Guo, S.; Fu, Y.; Xu, R.; Wang, D.; Cheng, J.; Liu, L.; et al. SIRT6-regulated macrophage efferocytosis epigenetically controls inflammation resolution of diabetic periodontitis. Theranostics 2023, 13, 231–249. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.-Z.; Yuan, Y.-H.; Liu, H.-H.; Li, S.-S.; Zhang, B.-W.; Chen, W.; An, Z.-J.; Chen, S.-Y.; Wu, Y.-Z.; Han, B.; et al. Epidemiologic relationship between periodontitis and type 2 diabetes mellitus. BMC Oral Health 2020, 20, 204. [Google Scholar] [CrossRef]
- Zhao, X.; Yang, Y.; Yu, J.; Ding, R.; Pei, D.; Zhang, Y.; He, G.; Cheng, Y.; Li, A. Injectable hydrogels with high drug loading through B-N coordination and ROS-triggered drug release for efficient treatment of chronic periodontitis in diabetic rats. Biomaterials 2022, 282, 121387. [Google Scholar] [CrossRef]
- Wang, H.; Chang, X.; Ma, Q.; Sun, B.; Li, H.; Zhou, J.; Hu, Y.; Yang, X.; Li, J.; Chen, X.; et al. Bioinspired drug-delivery system emulating the natural bone healing cascade for diabetic periodontal bone regeneration. Bioact. Mater. 2022, 21, 324–339. [Google Scholar] [CrossRef]
- Inchingolo, A.D.; Inchingolo, A.M.; Malcangi, G.; Avantario, P.; Azzollini, D.; Buongiorno, S.; Viapiano, F.; Campanelli, M.; Ciocia, A.M.; De Leonardis, N.; et al. Effects of Resveratrol, Curcumin and Quercetin Supplementation on Bone Metabolism-A Systematic Review. Nutrients 2022, 14, 3519. [Google Scholar] [CrossRef]
- Bhattarai, G.; Poudel, S.B.; Kook, S.H.; Lee, J.-C. Resveratrol prevents alveolar bone loss in an experimental rat model of periodontitis. Acta Biomater. 2016, 29, 398–408. [Google Scholar] [CrossRef]
- Li, Y.; Jiao, J.; Qi, Y.; Yu, W.; Yang, S.; Zhang, J.; Zhao, J. Curcumin: A review of experimental studies and mechanisms related to periodontitis treatment. J. Periodontal. Res. 2021, 56, 837–847. [Google Scholar] [CrossRef]
- Zheng, X.Y.; Mao, C.Y.; Qiao, H.; Zhang, X.; Yu, L.; Wang, T.Y.; Lu, E.Y. Plumbagin suppresses chronic periodontitis in rats via down-regulation of TNF-α, IL-1β and IL-6 expression. Acta Pharmacol. Sin. 2017, 38, 1150–1160. [Google Scholar] [CrossRef]
- Martínez, G.; Merinero, M.; Pérez-Aranda, M.; Pérez- Soriano, E.M.; Ortiz, T.; Begines, B.; Alcudia, A. Environmental impact of Nanoparticles’ Application as an Emerging Technology: A Review. Materials 2021, 14, 166. [Google Scholar] [CrossRef]
- Kou, L.; Bhutia, Y.D.; Yao, Q.; He, Z.; Sun, J.; Ganapathy, V. Transporter-guided delivery of nanoparticles to improve drug permeation across cellular barriers and drug exposure to selective cell types. Front. Pharmacol. 2018, 9, 27. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.; Chevvuri, R.; Sharma, H. Nanotechnology in dentistry: Unleashing the hidden gems. J. Indian Soc. Periodontol. 2018, 22, 196–200. [Google Scholar] [CrossRef]
- D’Amico, E.; Aceto, G.M.; Petrini, M.; Cinquini, C.; D’Ercole, S.; Iezzi, G.; Pierfelice, T.V. How Will Nanomedicine Revolutionize Future Dentistry and Periodontal Therapy? Int. J. Mol. Sci. 2025, 26, 592. [Google Scholar] [CrossRef]
- Zong, C.; Bronckaers, A.; Willems, G.; He, H.; Cadenas de Llano-Pérula, M. Nanomaterials for Periodontal Tissue Regeneration: Progress, Challenges and Future Perspectives. J. Funct. Biomater. 2023, 14, 290. [Google Scholar] [CrossRef]
- Lenders, V.; Koutsoumpou, X.; Sargsian, A.; Manshian, B.B. Biomedical nanomaterials for immunological applications: Ongoing research and clinical trials. Nanoscale Adv. 2020, 2, 5046–5089. [Google Scholar] [CrossRef]
- Tiwari, N.; Osorio-Blanco, E.R.; Sonzogni, A.; Esporrín-Ubieto, D.; Wang, H.; Calderón, M. Nanocarriers for Skin Applications: Where DoWe Stand? Angew. Chem. Int. Ed. Engl. 2022, 61, e202107960. [Google Scholar] [CrossRef]
- Brannon, E.R.; Guevara, M.V.; Pacifici, N.J.; Lee, J.K.; Lewis, J.S.; Eniola-Adefeso, O. Polymeric particle-based therapies for acute inflammatory diseases. Nat. Rev. Mater. 2022, 7, 796–813. [Google Scholar] [CrossRef]
- Budală, D.G.; Luchian, I.; Tatarciuc, M.; Butnaru, O.; Armencia, A.O.; Virvescu, D.I.; Scutariu, M.M.; Rusu, D. Are local drug delivery systems a challenge in clinical Periodontology? J. Clin. Med. 2023, 12, 4137. [Google Scholar] [CrossRef]

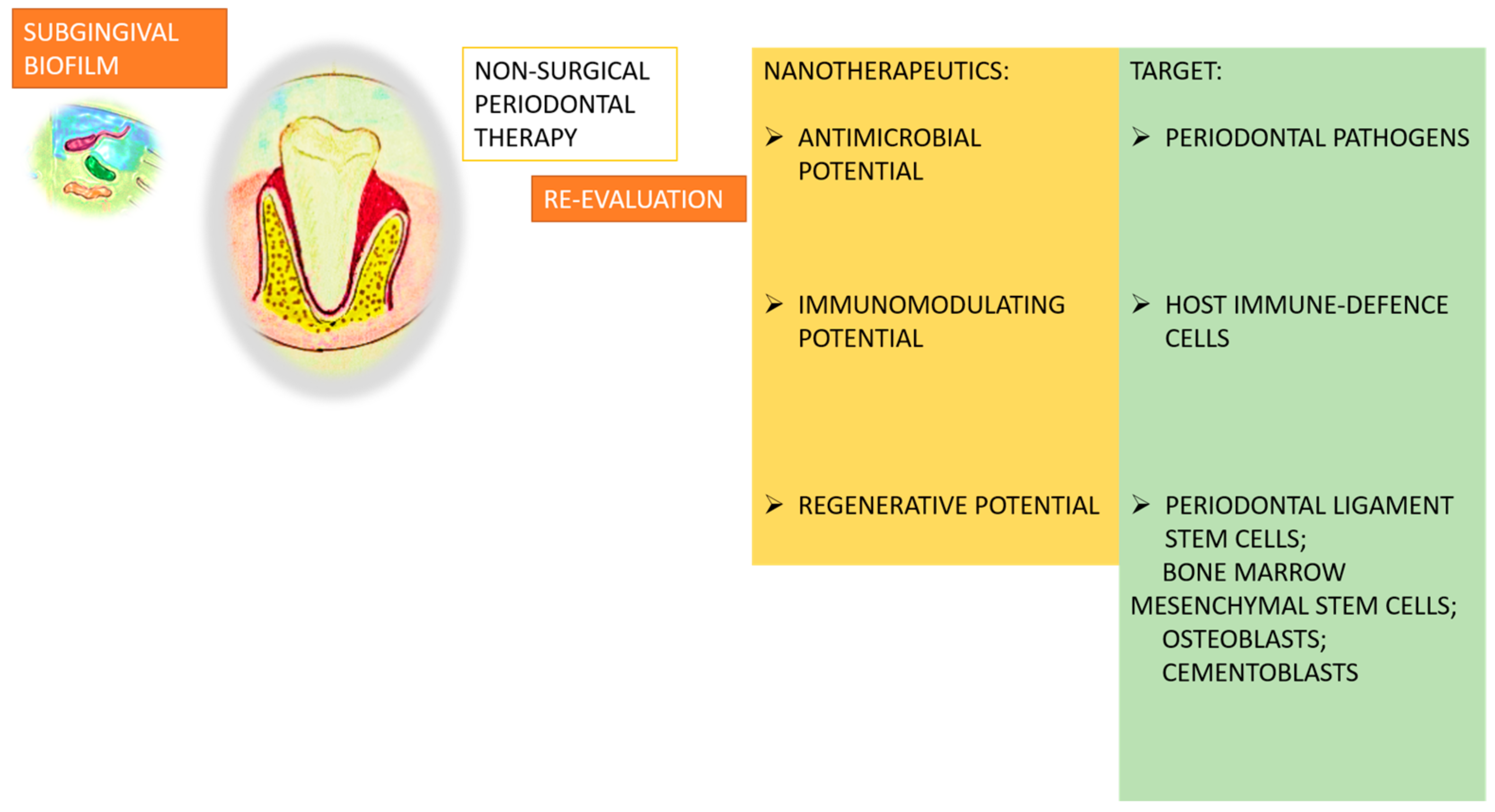
| Type of Nanoparticle | Origin | Classification | Properties | Action |
|---|---|---|---|---|
| Phenethyl Caffeate Ester (CAPE) | Propolis | Belongs to phenolic compounds with an ester group | Antioxidant and anti-inflammatory properties | Clears free radicals that cause oxidative stress and cell damage |
| Quercetin | Vegetables, herbs, grains, and wine | Flavonoid, belonging to the polyphenol class | Antioxidant action | Acts against free radicals, which damage cells and increase the risk of disease |
| Baicalin | From the mint family (Lamiaceae) | Flavonoid | Immunostimulant, anti-cancer, and antiviral properties | Antioxidant and anti-inflammatory action by suppressing the release of pro-inflammatory cytokines |
| Nanoparticles | ||
|---|---|---|
| Inorganic | Organic | Composite |
|
|
|
| Mechanism | Mean and Media | Application Form | Biodegradability | Trade Name |
|---|---|---|---|---|
| Protein synthesis inhibitors |
| gel | yes | Atridox |
| microspheres | yes | Arestin | |
| ointment | yes | Dentomycin | |
| fiber | no | Actisite | |
| film | yes | Gelcide | |
| Bactericidal action |
| gel | yes | Chlosite |
| film | yes | PerioChip | |
| Nucleic acid metabolism interference |
| gel | yes | Elyzol |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mlachkova, A.; Dosseva-Panova, V.; Maynalovska, H.; Pashova-Tasseva, Z. Nanoparticles as Strategies for Modulating the Host’s Response in Periodontitis Treatment. Nanomaterials 2025, 15, 476. https://doi.org/10.3390/nano15070476
Mlachkova A, Dosseva-Panova V, Maynalovska H, Pashova-Tasseva Z. Nanoparticles as Strategies for Modulating the Host’s Response in Periodontitis Treatment. Nanomaterials. 2025; 15(7):476. https://doi.org/10.3390/nano15070476
Chicago/Turabian StyleMlachkova, Antoaneta, Velitchka Dosseva-Panova, Hristina Maynalovska, and Zdravka Pashova-Tasseva. 2025. "Nanoparticles as Strategies for Modulating the Host’s Response in Periodontitis Treatment" Nanomaterials 15, no. 7: 476. https://doi.org/10.3390/nano15070476
APA StyleMlachkova, A., Dosseva-Panova, V., Maynalovska, H., & Pashova-Tasseva, Z. (2025). Nanoparticles as Strategies for Modulating the Host’s Response in Periodontitis Treatment. Nanomaterials, 15(7), 476. https://doi.org/10.3390/nano15070476







