Atomic Layer Deposition of Nickel Oxides as Electrocatalyst for Oxygen Evolution Reaction
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
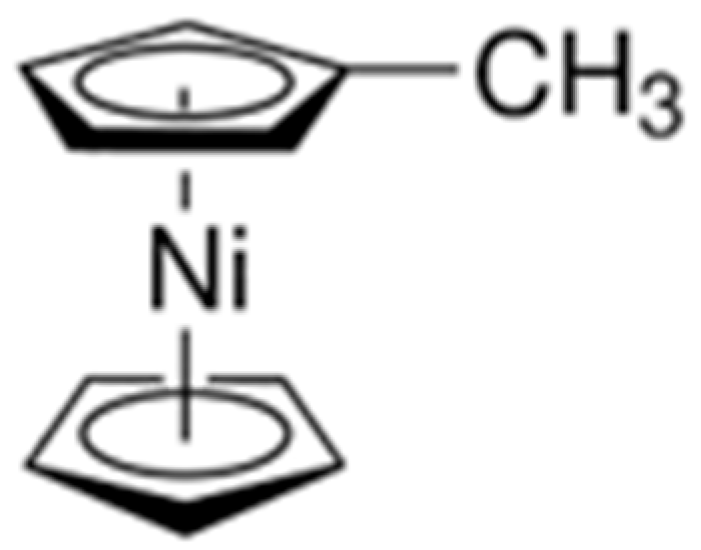
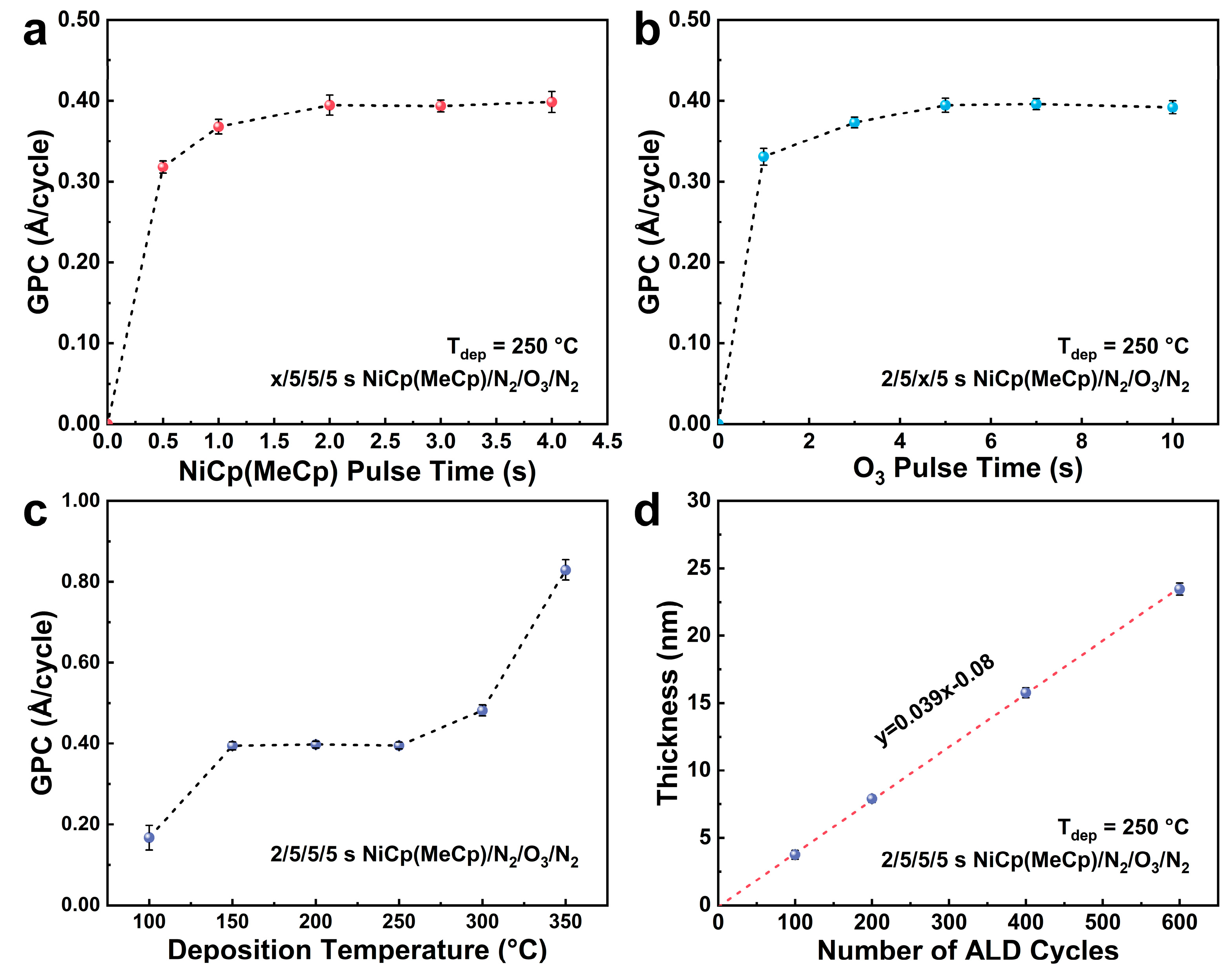
3.1. ALD Process Development
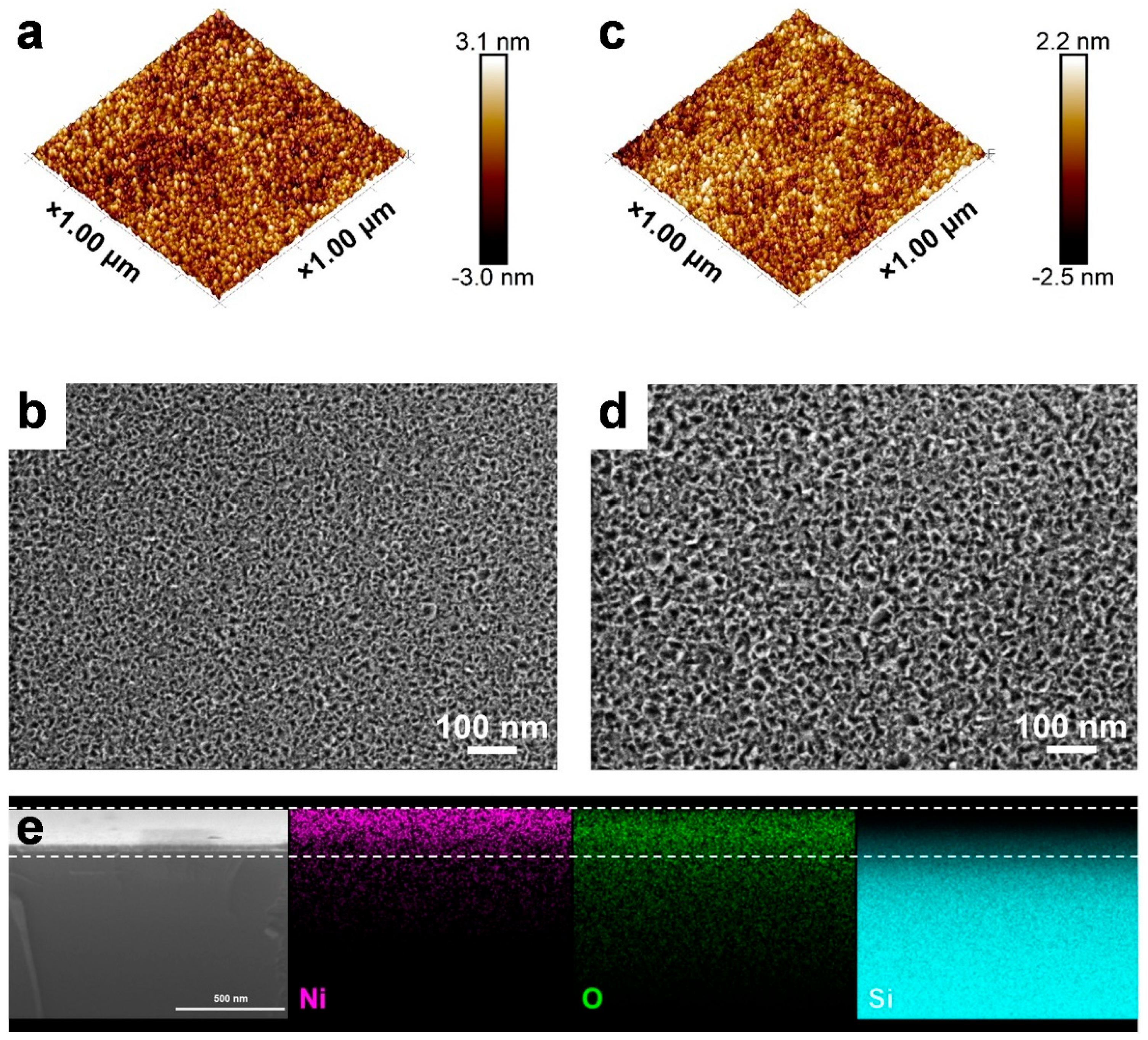
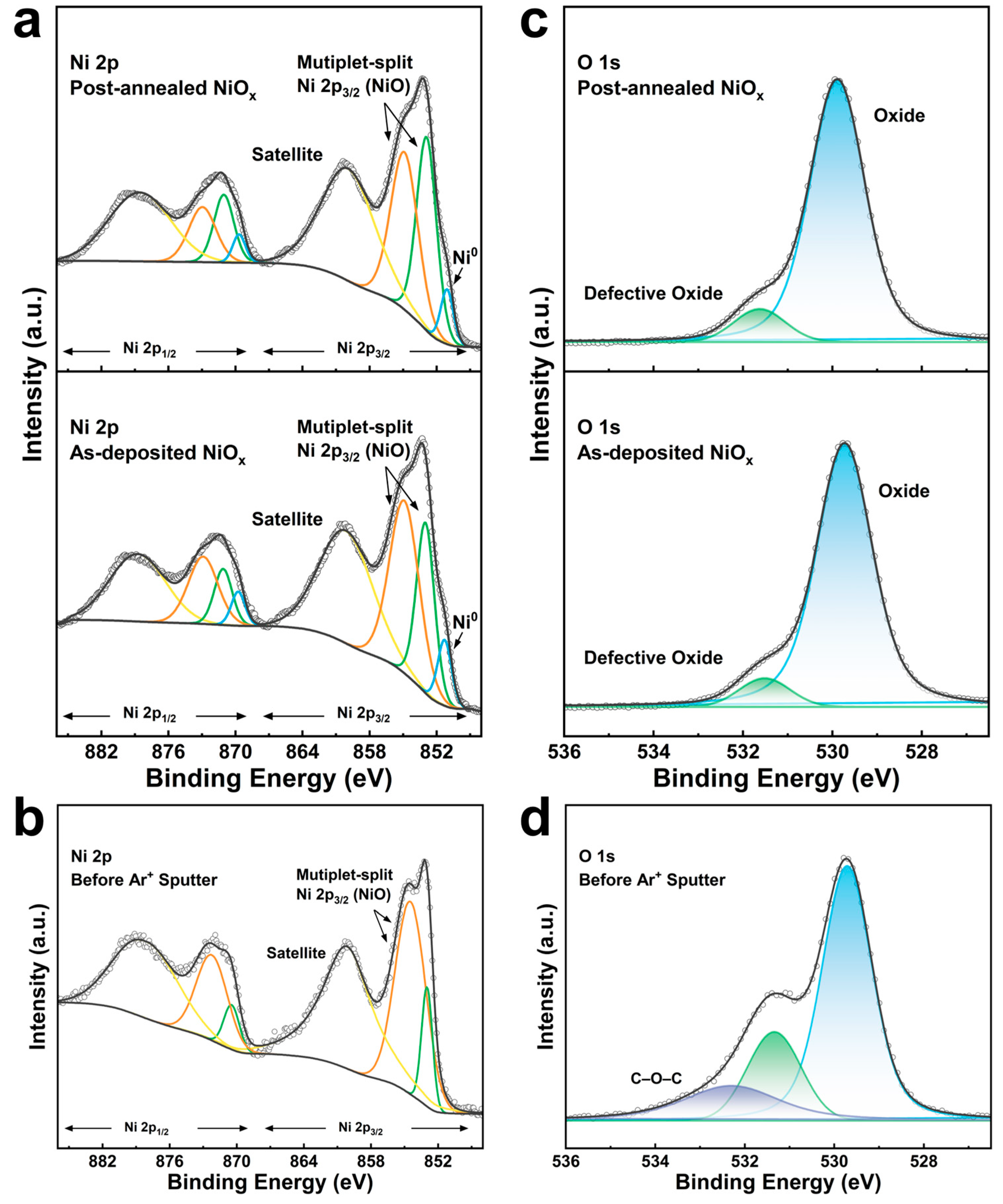
3.2. Characterization of ALD NiOx Films
3.3. Catalytic Performance on OER
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Odenweller, A.; Ueckerdt, F. The green hydrogen ambition and implementation gap. Nat. Energy 2025, 10, 110–123. [Google Scholar]
- Han, L.; Dong, S.; Wang, E. Transition-Metal (Co, Ni, and Fe)-Based Electrocatalysts for the Water Oxidation Reaction. Adv. Mater. 2016, 28, 9266–9291. [Google Scholar]
- Lu, F.; Zhou, M.; Zhou, Y.; Zeng, X. First-Row Transition Metal Based Catalysts for the Oxygen Evolution Reaction under Alkaline Conditions: Basic Principles and Recent Advances. Small 2017, 13, 1701931. [Google Scholar]
- Seitz, L.C.; Dickens, C.F.; Nishio, K.; Hikita, Y.; Montoya, J.; Doyle, A.; Kirk, C.; Vojvodic, A.; Hwang, H.Y.; Norskov, J.K.; et al. A highly active and stable IrOx/SrIrO3 catalyst for the oxygen evolution reaction. Science 2016, 353, 1011–1014. [Google Scholar]
- Concepcion, J.J.; Tsai, M.K.; Muckerman, J.T.; Meyer, T.J. Mechanism of water oxidation by single-site ruthenium complex catalysts. J. Am. Chem. Soc. 2010, 132, 1545–1557. [Google Scholar]
- Fan, Y.; Wu, Y.; Clavel, G.; Raza, M.H.; Amsalem, P.; Koch, N.; Pinna, N. Optimization of the Activity of Ni-Based Nanostructures for the Oxygen Evolution Reaction. ACS Appl. Energy Mater. 2018, 1, 4554–4563. [Google Scholar]
- Merrill, M.D.; Dougherty, R.C. Metal Oxide Catalysts for the Evolution of O2 from H2O. J. Phys. Chem. C 2008, 112, 3655–3666. [Google Scholar]
- McCrory, C.C.; Jung, S.; Peters, J.C.; Jaramillo, T.F. Benchmarking heterogeneous electrocatalysts for the oxygen evolution reaction. J. Am. Chem. Soc. 2013, 135, 16977–16987. [Google Scholar]
- Nardi, K.L.; Yang, N.; Dickens, C.F.; Strickler, A.L.; Bent, S.F. Creating Highly Active Atomic Layer Deposited NiO Electrocatalysts for the Oxygen Evolution Reaction. Adv. Energy Mater. 2015, 5, 1500412. [Google Scholar]
- Hu, C.; Chu, K.; Zhao, Y.; Teoh, W.Y. Efficient photoelectrochemical water splitting over anodized p-type NiO porous films. ACS Appl. Mater. Interfaces 2014, 6, 18558–18568. [Google Scholar]
- Holden, K.E.K.; Dezelah, C.L.; Conley, J.F. Atomic Layer Deposition of Transparent p-Type Semiconducting Nickel Oxide Using Ni(tBu2DAD)2 and Ozone. ACS Appl. Mater. Interfaces 2019, 11, 30437–30445. [Google Scholar]
- Salunkhe, P.; Kekuda, D. p-channel NiO thin film transistors grown with high k ZrO2 gate oxide for low voltage operation. Phys. Scr. 2023, 98, 065913. [Google Scholar]
- Hufnagel, A.G.; Henß, A.K.; Hoffmann, R.; Zeman, O.E.O.; Häringer, S.; Fattakhova-Rohlfing, D.; Bein, T. Electron-Blocking and Oxygen Evolution Catalyst Layers by Plasma-Enhanced Atomic Layer Deposition of Nickel Oxide. Adv. Mater. Interfaces 2018, 5, 1701531. [Google Scholar]
- Zhao, F.; Chen, T.; Zeng, Y.; Chen, J.; Zheng, J.; Liu, Y.; Han, G. Nickel oxide electrochromic films: Mechanisms, preparation methods, and modification strategies—A review. J. Mater. Chem. C 2024, 12, 7126–7145. [Google Scholar]
- Fominykh, K.; Feckl, J.M.; Sicklinger, J.; Döblinger, M.; Böcklein, S.; Ziegler, J.; Peter, L.; Rathousky, J.; Scheidt, E.W.; Bein, T.; et al. Ultrasmall Dispersible Crystalline Nickel Oxide Nanoparticles as High-Performance Catalysts for Electrochemical Water Splitting. Adv. Funct. Mater. 2014, 24, 3123–3129. [Google Scholar]
- Chen, S.; Duan, J.; Ran, J.; Jaroniec, M.; Qiao, S.Z. N-doped graphene film-confined nickel nanoparticles as a highly efficient three-dimensional oxygen evolution electrocatalyst. Energy Environ. Sci. 2013, 6, 3693–3699. [Google Scholar]
- Fan, K.; Ji, Y.; Zou, H.; Zhang, J.; Zhu, B.; Chen, H.; Daniel, Q.; Luo, Y.; Yu, J.; Sun, L. Hollow Iron-Vanadium Composite Spheres: A Highly Efficient Iron-Based Water Oxidation Electrocatalyst without the Need for Nickel or Cobalt. Angew. Chem. Int. Ed. 2017, 56, 3289–3293. [Google Scholar]
- Li, Y.; Liu, C.; Wang, G.; Pei, Y.L. Investigation of solution combustion-processed nickel oxide p-channel thin film transistors. Semicond. Sci. Technol. 2017, 32, 085004. [Google Scholar]
- Guo, W.; Hui, K.N.; Hui, K.S. High conductivity nickel oxide thin films by a facile sol–gel method. Mater. Lett. 2013, 92, 291–295. [Google Scholar]
- Manojreddy, P.; Itapu, S.; Ravali, J.K.; Sakkarai, S. Tuning the Electrical Parameters of p-NiOx-Based Thin Film Transistors (TFTs) by Pulsed Laser Irradiation. Condens. Matter 2021, 6, 21. [Google Scholar] [CrossRef]
- Tanaka, M.; Mukai, M.; Fujimori, Y.; Kondoh, M.; Tasaka, Y.; Baba, H.; Usami, S. Transition metal oxide films prepared by pulsed laser deposition for atomic beam detection. Thin Solid Film. 1996, 281–282, 453–456. [Google Scholar]
- Lin, C.W.; Chung, W.C.; Zhang, Z.D.; Hsu, M.C. P-channel transparent thin-film transistor using physical-vapor-deposited NiO layer. Jpn. J. Appl. Phys. 2018, 57, 01AE01. [Google Scholar]
- George, S.M. Atomic Layer Deposition: An Overview. Chem. Rev. 2009, 110, 111–131. [Google Scholar]
- Kim, H.; Lee, H.-B.-R.; Maeng, W.J. Applications of atomic layer deposition to nanofabrication and emerging nanodevices. Thin Solid Film. 2009, 517, 2563–2580. [Google Scholar]
- Haghverdi Khamene, S.; van Helvoirt, C.; Tsampas, M.N.; Creatore, M. Electrochemical Activation of Atomic-Layer-Deposited Nickel Oxide for Water Oxidation. J. Phys. Chem. C 2023, 127, 22570–22582. [Google Scholar]
- O’Neill, B.J.; Jackson, D.H.K.; Lee, J.; Canlas, C.; Stair, P.C.; Marshall, C.L.; Elam, J.W.; Kuech, T.F.; Dumesic, J.A.; Huber, G.W. Catalyst Design with Atomic Layer Deposition. ACS Catal. 2015, 5, 1804–1825. [Google Scholar]
- Marichy, C.; Bechelany, M.; Pinna, N. Atomic Layer Deposition of Nanostructured Materials for Energy and Environmental Applications. Adv. Mater. 2012, 24, 1017–1032. [Google Scholar]
- Cao, K.; Cai, J.; Liu, X.; Chen, R. Review Article: Catalysts design and synthesis via selective atomic layer deposition. J. Vac. Sci. Technol. A 2018, 36, 010801. [Google Scholar]
- Chae, J.; Park, H.-S.; Kang, S.-w. Atomic Layer Deposition of Nickel by the Reduction of Preformed Nickel Oxide. ECS Solid State Lett. 2002, 5, C64–C66. [Google Scholar]
- Koushik, D.; Jošt, M.; Dučinskas, A.; Burgess, C.; Zardetto, V.; Weijtens, C.; Verheijen, M.A.; Kessels, W.M.M.; Albrecht, S.; Creatore, M. Plasma-assisted atomic layer deposition of nickel oxide as hole transport layer for hybrid perovskite solar cells. J. Mater. Chem. C 2019, 7, 12532–12543. [Google Scholar]
- Dashjav, E.; Lipińska-Chwałek, M.; Grüner, D.; Mauer, G.; Luysberg, M.; Tietz, F. Atomic layer deposition and high-resolution electron microscopy characterization of nickel nanoparticles for catalyst applications. Surf. Coat. Technol. 2016, 307, 428–435. [Google Scholar]
- Lu, H.L.; Scarel, G.; Wiemer, C.; Perego, M.; Spiga, S.; Fanciulli, M.; Pavia, G. Atomic Layer Deposition of NiO Films on Si(100) Using Cyclopentadienyl-Type Compounds and Ozone as Precursors. J. Electrochem. Soc. 2008, 155, H807–H811. [Google Scholar]
- Barr, M.K.S.; Assaud, L.; Wu, Y.; Laffon, C.; Parent, P.; Bachmann, J.; Santinacci, L. Engineering a three-dimensional, photoelectrochemically active p-NiO/i-Sb2S3 junction by atomic layer deposition. Electrochim. Acta 2015, 179, 504–511. [Google Scholar] [CrossRef]
- Xie, L.-Y.; Xiao, D.-Q.; Pei, J.-X.; Huo, J.; Wu, X.; Liu, W.-J.; Ding, S.-J. Growth, physical and electrical characterization of nickel oxide thin films prepared by plasma-enhanced atomic layer deposition using nickelocene and oxygen precursors. Mater. Res. Express. 2020, 7, 046401. [Google Scholar] [CrossRef]
- Zhao, R.; Xiao, S.; Yang, S.; Wang, X. Surface Thermolytic Behavior of Nickel Amidinate and Its Implication on the Atomic Layer Deposition of Nickel Compounds. Chem. Mater. 2019, 31, 5172–5180. [Google Scholar] [CrossRef]
- Balogun, M.S.; Qiu, W.; Huang, Y.; Yang, H.; Xu, R.; Zhao, W.; Li, G.R.; Ji, H.; Tong, Y. Cost-Effective Alkaline Water Electrolysis Based on Nitrogen- and Phosphorus-Doped Self-Supportive Electrocatalysts. Adv. Mater. 2017, 29, 1702095. [Google Scholar] [CrossRef]
- Knoops, H.C.M.; Potts, S.E.; Bol, A.A.; Kessels, W.M.M. Atomic Layer Deposition. In Handbook of Crystal Growth; Elsevier: Amsterdam, The Netherlands, 2015; pp. 1101–1134. [Google Scholar]
- Eggers, S.H.; Kopf, J.; Fischer, R.D. On the metal coordination in base-free tris(cyclopentadienyl) complexes of the lanthanoids. 2. The x-ray structure of tris(cyclopentadienyl)lanthanum(III): A notably stable polymer displaying more than three different lanthanum-carbon interactions. Organometallics 2002, 5, 383–385. [Google Scholar]
- Hämäläinen, J.; Ritala, M.; Leskelä, M. Atomic Layer Deposition of Noble Metals and Their Oxides. Chem. Mater. 2013, 26, 786–801. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Payne, B.P.; Grosvenor, A.P.; Lau, L.W.M.; Gerson, A.R.; Smart, R.S.C. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Cr, Mn, Fe, Co and Ni. Appl. Surf. Sci. 2011, 257, 2717–2730. [Google Scholar]
- Grosvenor, A.P.; Biesinger, M.C.; Smart, R.S.C.; McIntyre, N.S. New interpretations of XPS spectra of nickel metal and oxides. Surf. Sci. 2006, 600, 1771–1779. [Google Scholar] [CrossRef]
- Chen, Y.S.; Kang, J.F.; Chen, B.; Gao, B.; Liu, L.F.; Liu, X.Y.; Wang, Y.Y.; Wu, L.; Yu, H.Y.; Wang, J.Y.; et al. Microscopic mechanism for unipolar resistive switching behaviour of nickel oxides. J. Phys. D 2012, 45, 065303. [Google Scholar]
- Kim, K.S.; Winograd, N. X-ray photoelectron spectroscopic studies of nickel-oxygen surfaces using oxygen and argon ion-bombardment. Surf. Sci. 1974, 43, 625–643. [Google Scholar]
- Umezawa, Y.; Reilley, C.N. Effect of argon ion bombardment on metal complexes and oxides studied by X-ray photoelectron spectroscopy. Anal. Chem. 2002, 50, 1290–1295. [Google Scholar]
- Biesinger, M.C.; Payne, B.P.; Lau, L.W.M.; Gerson, A.; Smart, R.S.C. X-ray photoelectron spectroscopic chemical state quantification of mixed nickel metal, oxide and hydroxide systems. Surf. Interface Anal. 2009, 41, 324–332. [Google Scholar]
- Timoshnev, S.; Kazakin, A.; Shubina, K.; Andreeva, V.; Fedorenko, E.; Koroleva, A.; Zhizhin, E.; Koval, O.; Kurinnaya, A.; Shalin, A.; et al. Annealing Temperature Effect on the Physical Properties of NiO Thin Films Grown by DC Magnetron Sputtering. Adv. Mater. Interfaces 2024, 11, 2300815. [Google Scholar]
- Hassanzadeh-Tabrizi, S.A. Precise calculation of crystallite size of nanomaterials: A review. J. Alloys Compd. 2023, 968, 171914. [Google Scholar]
- Farva, U.; Kim, J. Temperature optimization of NiO hole transport layer prepared by atomic layer deposition. Vacuum 2023, 207, 111674. [Google Scholar]
- Xu, W.; Zhang, J.; Li, Y.; Zhang, L.; Chen, L.; Zhu, D.; Cao, P.; Liu, W.; Han, S.; Liu, X.; et al. p-Type transparent amorphous oxide thin-film transistors using low-temperature solution-processed nickel oxide. J. Alloys Compd. 2019, 806, 40e51. [Google Scholar]
- Park, H.; Nandi, P.; In, Y.; Shin, H. Practical and Thermal Atomic Layer Deposition of NiO as Hole-Transporting Layers for Inverted Perovskite Solar Cells. Sol. RRL 2024, 8, 2300858. [Google Scholar] [CrossRef]
- Jiang, W.J.; Niu, S.; Tang, T.; Zhang, Q.H.; Liu, X.Z.; Zhang, Y.; Chen, Y.Y.; Li, J.H.; Gu, L.; Wan, L.J.; et al. Crystallinity-Modulated Electrocatalytic Activity of a Nickel(II) Borate Thin Layer on Ni3B for Efficient Water Oxidation. Angew. Chem. Int. Ed. 2017, 56, 6572–6577. [Google Scholar]
- Poulain, R.; Klein, A.; Proost, J. Electrocatalytic Properties of (100)-, (110)-, and (111)-Oriented NiO Thin Films toward the Oxygen Evolution Reaction. J. Phys. Chem. C 2018, 122, 22252–22263. [Google Scholar] [CrossRef]
- Dong, H.; Tang, P.; Zhang, S.; Xiao, X.; Jin, C.; Gao, Y.; Yin, Y.; Li, B.; Yang, S. Excellent oxygen evolution reaction of NiO with a layered nanosphere structure as the cathode of lithium-oxygen batteries. RSC Adv. 2018, 8, 3357–3363. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.A.; Rashid, N.; Junaid, M.; Zafar, M.N.; Faheem, M.; Ahmad, I. NiO/NiS Heterostructures: An Efficient and Stable Electrocatalyst for Oxygen Evolution Reaction. ACS Appl. Energy Mater. 2019, 2, 3587–3594. [Google Scholar] [CrossRef]
- Bucci, A.; García-Tecedor, M.; Corby, S.; Rao, R.R.; Martin-Diaconescu, V.; Oropeza, F.E.; de la Peña O’Shea, V.A.; Durrant, J.R.; Giménez, S.; Lloret-Fillol, J. Self-supported ultra-active NiO-based electrocatalysts for the oxygen evolution reaction by solution combustion. J. Mater. Chem. C 2021, 9, 12700–12710. [Google Scholar] [CrossRef]
- Iacono, V.; Mirabella, S.; Ruffino, F. Efficient Oxygen Evolution Reaction Catalyzed by Ni/NiO Nanoparticles Produced by Pulsed Laser Ablation in Liquid Environment. Phys. Status Solidi B 2023, 260, 2200590. [Google Scholar] [CrossRef]
- Solomon, G.; Landström, A.; Mazzaro, R.; Jugovac, M.; Moras, P.; Cattaruzza, E.; Morandi, V.; Concina, I.; Vomiero, A. NiMoO4@Co3O4 Core–Shell Nanorods: In Situ Catalyst Reconstruction toward High Efficiency Oxygen Evolution Reaction. Adv. Energy Mater. 2021, 11, 2101324. [Google Scholar] [CrossRef]
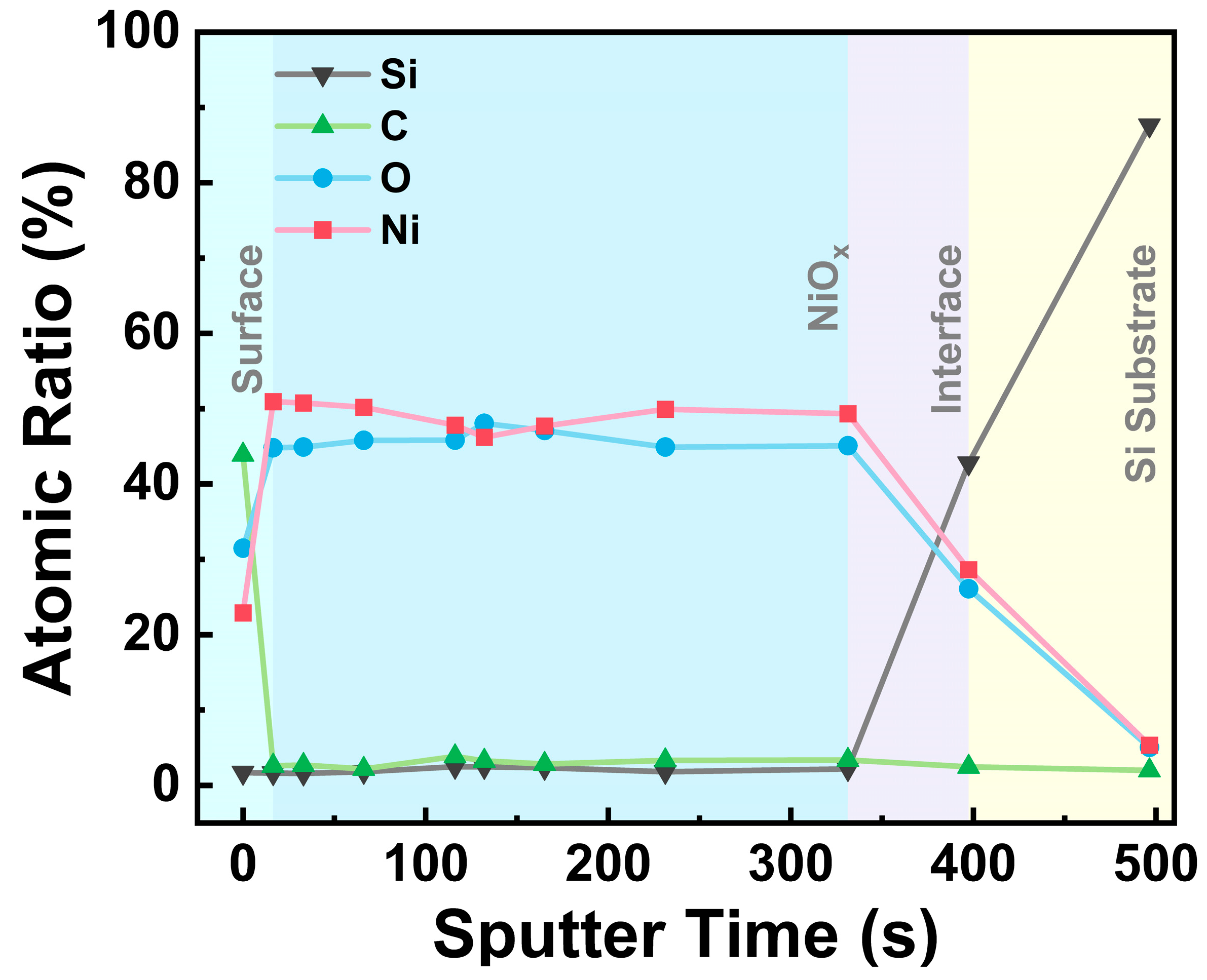
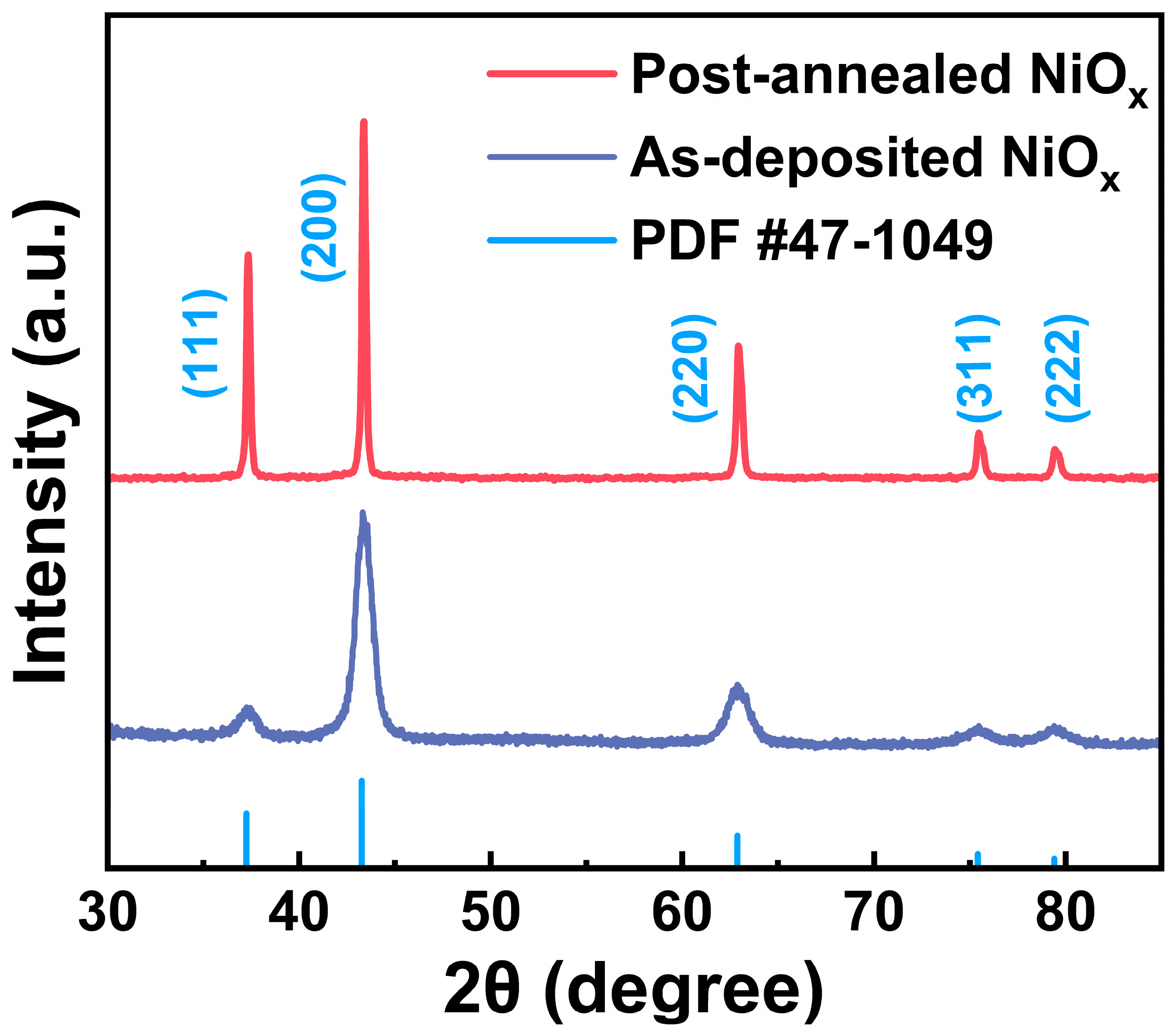
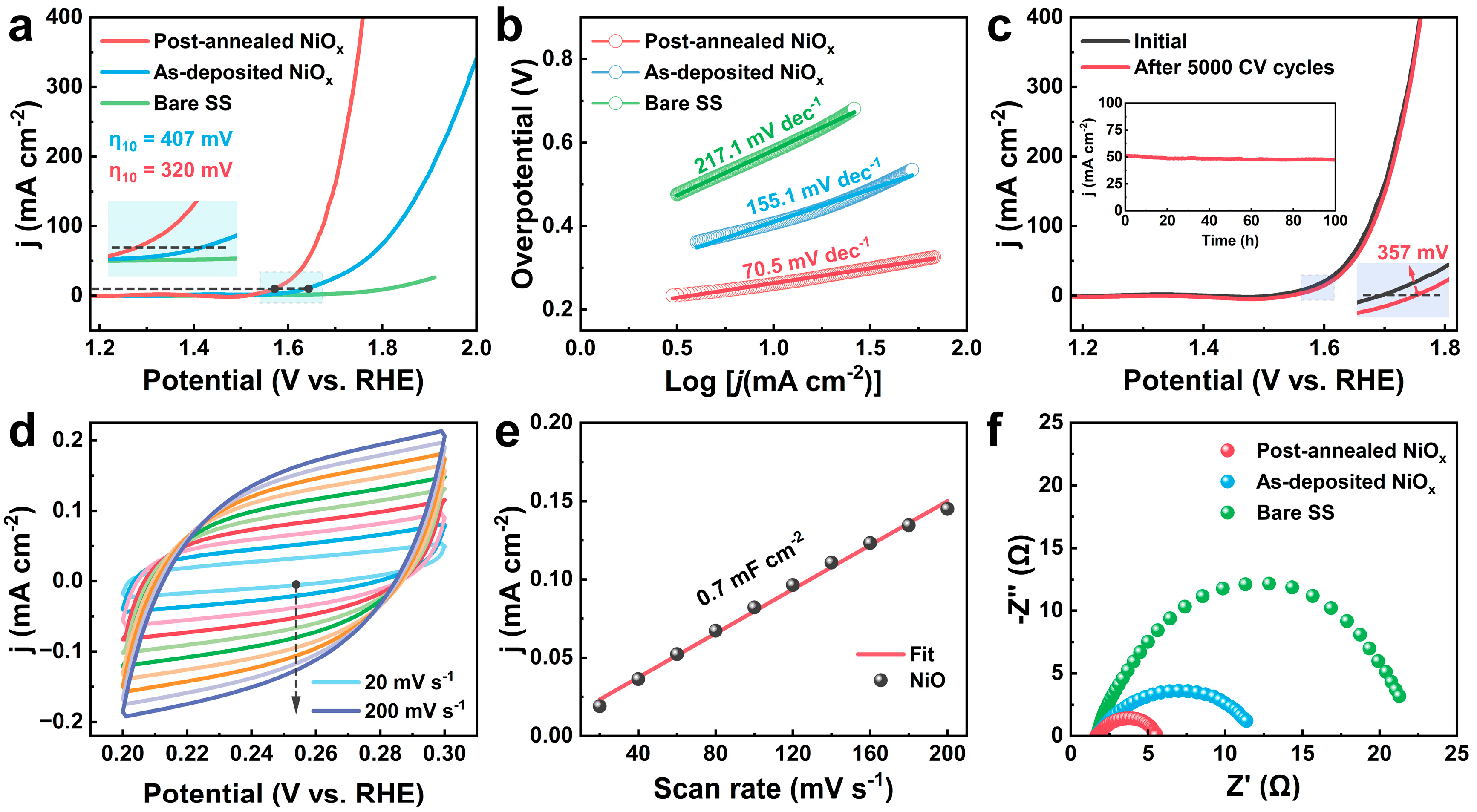
| Samples | Tdep (°C) | Ni (%) | O (%) | C (%) | Ni/O Ratio |
|---|---|---|---|---|---|
| As-deposited | 150 | 51.1 | 45.7 | 3.2 | 1.12 |
| 200 | 52.3 | 44.8 | 2.9 | 1.17 | |
| 250 | 52.3 | 45.1 | 2.6 | 1.16 | |
| Post-annealed | 250 | 52.1 | 45.5 | 2.4 | 1.15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.; Dai, R.; Ma, H.; Lin, Z.; Li, Y.; Xi, B. Atomic Layer Deposition of Nickel Oxides as Electrocatalyst for Oxygen Evolution Reaction. Nanomaterials 2025, 15, 474. https://doi.org/10.3390/nano15070474
Chen J, Dai R, Ma H, Lin Z, Li Y, Xi B. Atomic Layer Deposition of Nickel Oxides as Electrocatalyst for Oxygen Evolution Reaction. Nanomaterials. 2025; 15(7):474. https://doi.org/10.3390/nano15070474
Chicago/Turabian StyleChen, Jueyu, Ruijie Dai, Hongwei Ma, Zhijie Lin, Yuanchao Li, and Bin Xi. 2025. "Atomic Layer Deposition of Nickel Oxides as Electrocatalyst for Oxygen Evolution Reaction" Nanomaterials 15, no. 7: 474. https://doi.org/10.3390/nano15070474
APA StyleChen, J., Dai, R., Ma, H., Lin, Z., Li, Y., & Xi, B. (2025). Atomic Layer Deposition of Nickel Oxides as Electrocatalyst for Oxygen Evolution Reaction. Nanomaterials, 15(7), 474. https://doi.org/10.3390/nano15070474






