Anisotropic Swelling Behavior of Liquid Crystal Elastomers in Isotropic Solvents
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Fabrication of Liquid Crystal Cells
2.3. Synthesis and Preparation of the Aligned LCEs
2.4. Gel Fraction
2.5. Mechanical Properties
2.6. Optical Microscopy
2.7. Wide-Angle X-Ray Diffraction
3. Results and Discussion
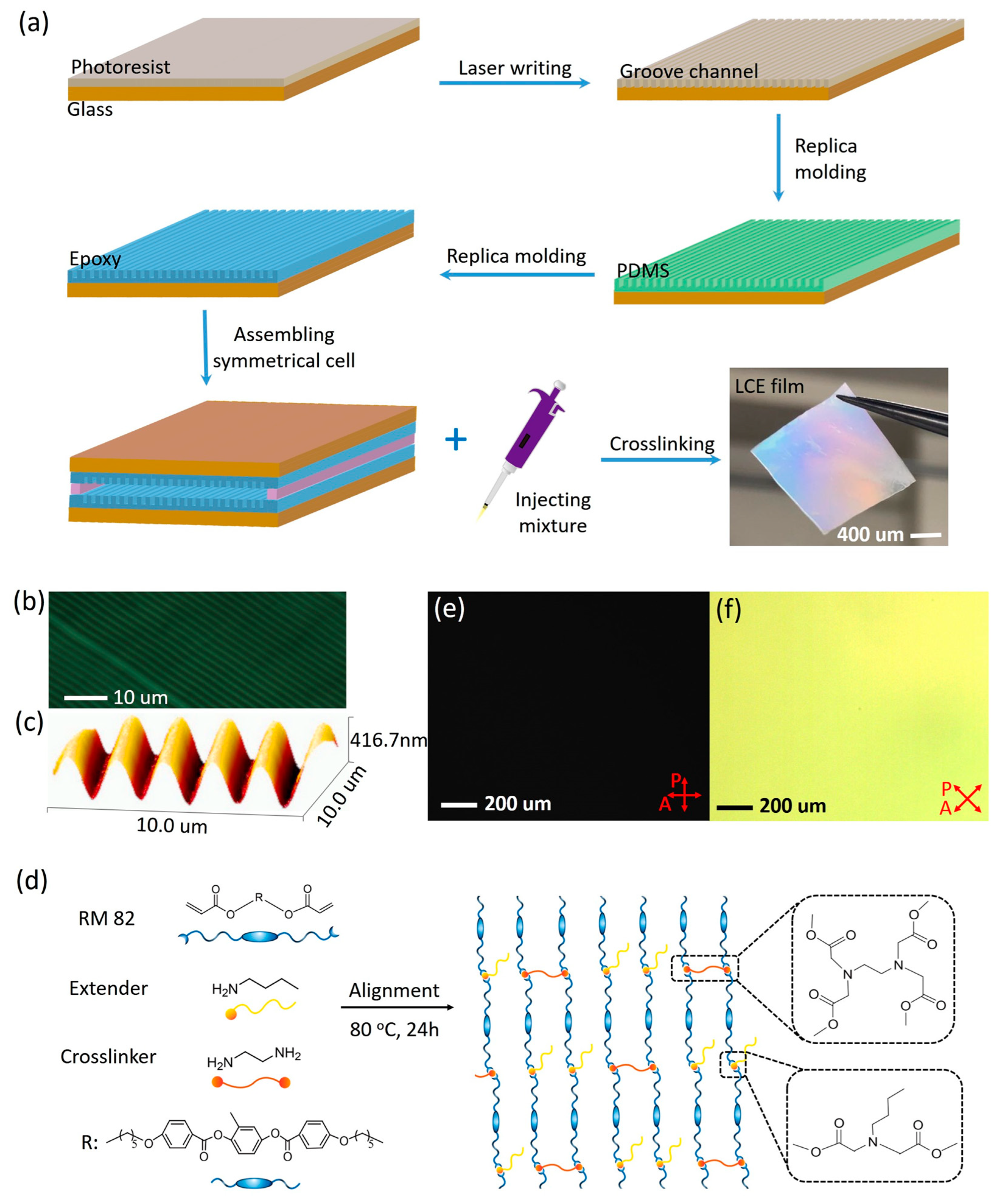
3.1. Fabrication of the Liquid Crystal Cells
3.2. Preparation of the Aligned LCEs
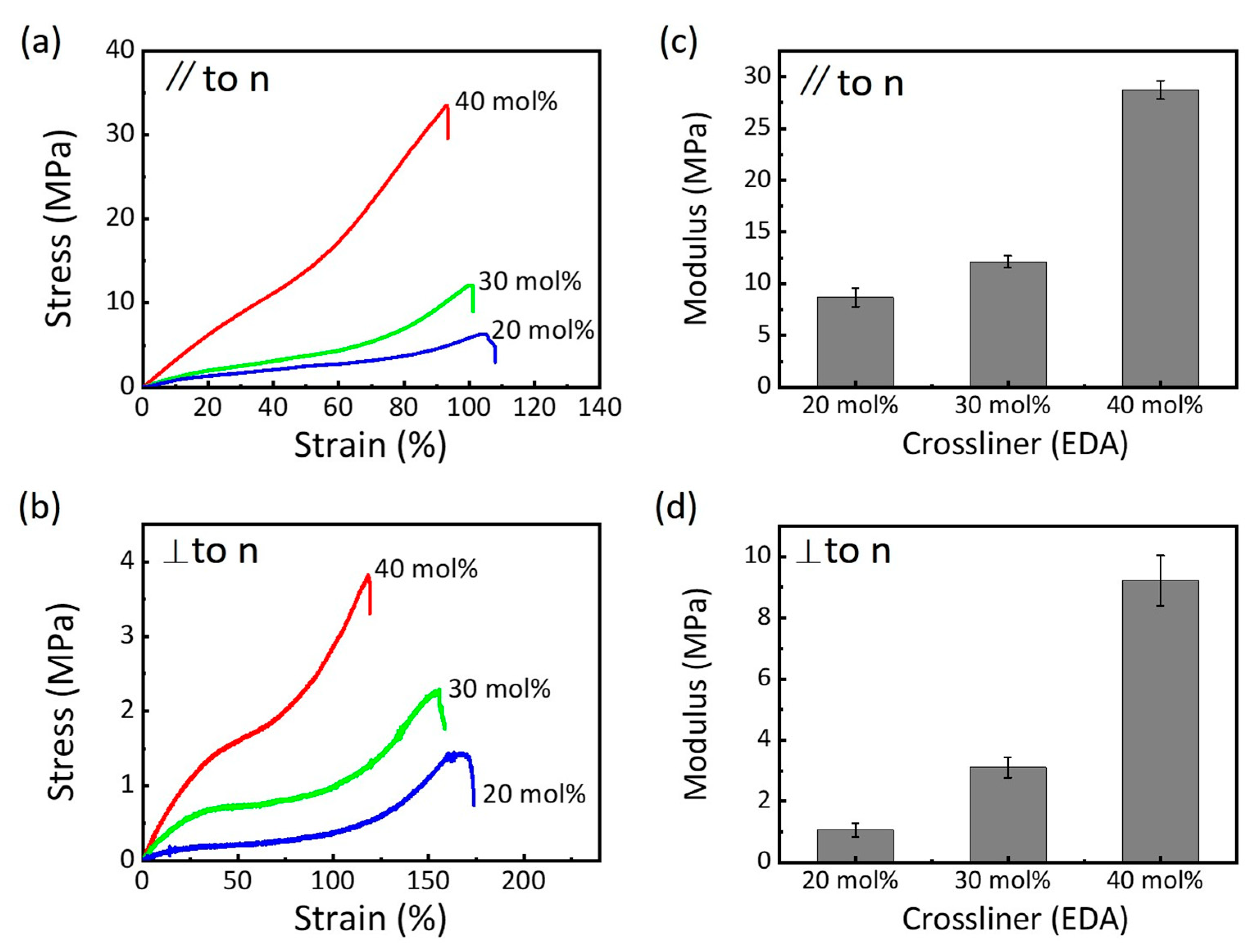
3.3. Mechanical Properties of the LCEs
3.4. Anisotropic Swelling Behavior of the LCEs
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Son, H.; Yoon, C. Advances in Stimuli-Responsive Soft Robots with Integrated Hybrid Materials. Actuators 2020, 9, 115. [Google Scholar] [CrossRef]
- Hu, J.; Yu, M.; Wang, M.; Choy, K.-L.; Yu, H. Design, Regulation, and Applications of Soft Actuators Based on Liquid-Crystalline Polymers and Their Composites. ACS Appl. Mater. Interfaces 2022, 14, 12951–12963. [Google Scholar] [CrossRef]
- Zhao, Y.; Hua, M.; Yan, Y.; Wu, S.; Alsaid, Y.; He, X. Stimuli-Responsive Polymers for Soft Robotics. Annu. Rev. Control Rob. Auton. Syst. 2022, 5, 515–545. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, Y.; Ma, Y.; Wang, J. Bioinspired Stimuli-Responsive Materials for Soft Actuators. Biomimetics 2024, 9, 128. [Google Scholar] [CrossRef]
- Forterre, Y.; Skotheim, J.M.; Dumais, J.; Mahadevan, L. How the Venus flytrap snaps. Nature 2005, 433, 421–425. [Google Scholar] [CrossRef] [PubMed]
- Dumais, J.; Forterre, Y. “Vegetable Dynamicks”: The Role of Water in Plant Movements. Annu. Rev. Fluid Mech. 2012, 44, 453–478. [Google Scholar] [CrossRef]
- Shen, Z.; Chen, F.; Zhu, X.; Yong, K.-T.; Gu, G. Stimuli-responsive functional materials for soft robotics. J. Mater. Chem. B 2020, 8, 8972–8991. [Google Scholar] [CrossRef]
- Nordendorf, G.; Jünnemann-Held, G.; Lorenz, A.; Kitzerow, H.-S. Effects of Composition and Polymerization Conditions on the Electro-Optic Performance of Liquid Crystal-Polymer Composites Doped with Ferroelectric Nanoparticles. Nanomaterials 2024, 14, 961. [Google Scholar] [CrossRef] [PubMed]
- Herard, N.; Annapooranan, R.; Henry, T.; Kröger, M.; Cai, S.; Boechler, N.; Sliozberg, Y. Modeling nematic phase main-chain liquid crystal elastomer synthesis, mechanics, and thermal actuation via coarse-grained molecular dynamics. Soft Matter 2024, 20, 9219–9231. [Google Scholar] [CrossRef]
- Hussain, M.; Jull, E.I.L.; Mandle, R.J.; Raistrick, T.; Hine, P.J.; Gleeson, H.F. Liquid Crystal Elastomers for Biological Applications. Nanomaterials 2021, 11, 813. [Google Scholar] [CrossRef]
- Pilz da Cunha, M.; Debije, M.G.; Schenning, A.P.H.J. Bioinspired light-driven soft robots based on liquid crystal polymers. Chem. Soc. Rev. 2020, 49, 6568–6578. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Lee, J.A.; Ambulo, C.P.; Lee, H.B.; Kim, S.H.; Naik, V.V.; Haines, C.S.; Aliev, A.E.; Ovalle-Robles, R.; Baughman, R.H.; et al. Intelligently Actuating Liquid Crystal Elastomer-Carbon Nanotube Composites. Adv. Funct. Mater. 2019, 29, 1905063. [Google Scholar] [CrossRef]
- Rajan, D.; Nandha, S.; Venkatachalam, G.; Shivakumar, U. Polyaniline-Incorporated Liquid Crystal Elastomers for Muscle-like Actuation. ACS Appl. Polym. Mater. 2024, 6, 13089–13101. [Google Scholar] [CrossRef]
- White, T.J.; Broer, D.J. Programmable and adaptive mechanics with liquid crystal polymer networks and elastomers. Nat. Mater. 2015, 14, 1087–1098. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Yu, K.H.; Garcia, I.; Nah, S.H.; Chui, H.N.T.; Tian, Z.; Yang, S. Direct Ink Writing of Cephalopod Skin-Like Core-Shell Fibers From Cholesteric Liquid Crystal Elastomers and Dyed Solutions. Adv. Funct. Mater. 2024, 35, 2413965. [Google Scholar] [CrossRef]
- Jiang, J.; Ma, Y.; Cheng, R.; Zhao, Y. A Porous Multi-Stimuli-Responsive Liquid Crystal Elastomer Actuator Enabled by Mof Loading. Adv. Funct. Mater. 2024, 34, 2313625. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, G.; Zhang, K.; Jin, B.; Zhao, Q.; Xie, T. Repeatedly Programmable Liquid Crystal Dielectric Elastomer with Multimodal Actuation. Adv. Mater. 2024, 36, 2313078. [Google Scholar] [CrossRef]
- Nandha, S.; Rajan, D.; Shivakumar, U. Nanocellulose Incorporated Liquid Crystal Elastomers as Soft Actuators. ACS Appl. Polym. Mater. 2024, 6, 12842–12853. [Google Scholar] [CrossRef]
- Kaiser, A.; Winkler, M.; Krause, S.; Finkelmannb, H.; Schmidt, A.M. Magnetoactive liquid crystal elastomer nanocomposites. J. Mater. Chem. 2009, 19, 538–543. [Google Scholar] [CrossRef]
- Guan, Z.; Wang, L.; Bae, J. Advances in 4D printing of liquid crystalline elastomers: Materials, techniques, and applications. Mater. Horiz. 2022, 9, 1825–1849. [Google Scholar] [CrossRef]
- Marshall, J.E.; Gallagher, S.; Terentjev, E.M.; Smoukov, S.K. Anisotropic Colloidal Micromuscles from Liquid Crystal Elastomers. J. Am. Chem. Soc. 2014, 136, 474–479. [Google Scholar] [CrossRef] [PubMed]
- Lan, R.; Gao, Y.; Shen, C.; Huang, R.; Bao, J.; Zhang, Z.; Wang, Q.; Zhang, L.; Yang, H. Humidity-Responsive Liquid Crystalline Network Actuator Showing Synergistic Fluorescence Color Change Enabled by Aggregation Induced Emission Luminogen. Adv. Funct. Mater. 2021, 31, 2010578. [Google Scholar] [CrossRef]
- Boothby, J.M.; Kim, H.; Ware, T.H. Shape changes in chemoresponsive liquid crystal elastomers. Sens. Actuators B 2017, 240, 511–518. [Google Scholar] [CrossRef]
- Velasco Abadia, A.; Schwartz, D.K.; Kaar, J.L. Chemically and biochemically responsive liquid crystal polymer networks and elastomers. Polym. Int. 2023, 72, 977–983. [Google Scholar] [CrossRef]
- Torras, N.; Zinoviev, K.E.; Camargo, C.J.; Campo, E.M.; Campanella, H.; Esteve, J.; Marshall, J.E.; Terentjev, E.M.; Omastová, M.; Krupa, I.; et al. Tactile device based on opto-mechanical actuation of liquid crystal elastomers. Sens. Actuators A 2014, 208, 104–112. [Google Scholar] [CrossRef]
- Urayama, K.; Arai, Y.O.; Takigawa, T. Anisotropic Swelling and Phase Behavior of Monodomain Nematic Networks in Nematogenic Solvents. Macromolecules 2005, 38, 5721–5728. [Google Scholar] [CrossRef]
- Yusuf, Y.; Hashimoto, S.; Cladis, P.E.; Brand, H.R.; Krause, S.; Finkelmann, H.; Kai, S. Main Chain Liquid-Crystalline Elastomers: Swelling Dynamics and Electromechanical Effects. Mol. Cryst. Liq. Cryst. 2009, 508, 367–729. [Google Scholar] [CrossRef]
- Yusuf, Y.; Hashimoto, S.; Cho, D.-U.; Brand, H.; Finkelmann, H.; Kai, S. Electromechanical and Electrooptical Effects of Liquid Crystal Elastomers Swollen with a Low Molecular Weight Liquid Crystal. Mol. Cryst. Liq. Cryst. 2007, 477, 127–135. [Google Scholar] [CrossRef]
- Bouchikhi, N.; Semdani, F.; Bedjaoui, L.A.; Maschke, U. Elaboration of Side-Chain Liquid-Crystalline Elastomers and Study of Their Swelling Behavior in Anisotropic Solvents. Mol. Cryst. Liq. Cryst. 2012, 560, 159–169. [Google Scholar] [CrossRef]
- Chambers, M.; Verduzco, R.; Gleeson, J.T.; Sprunt, S.; Jákli, A. Calamitic Liquid-Crystalline Elastomers Swollen in Bent-Core Liquid-Crystal Solvents. Adv. Mater. 2009, 21, 1622–1626. [Google Scholar] [CrossRef]
- Aharoni, H.; Xia, Y.; Zhang, X.; Kamien, R.D.; Yang, S. Universal inverse design of surfaces with thin nematic elastomer sheets. PNAS 2018, 115, 7206–7211. [Google Scholar] [CrossRef] [PubMed]
- Lin, R.; Rogers, J.A. Molecular-Scale Soft Imprint Lithography for Alignment Layers in Liquid Crystal Devices. Nano Lett. 2007, 7, 1613–1621. [Google Scholar] [CrossRef]
- Wang, Z.; Servio, P.; Rey, A.D. Complex Nanowrinkling in Chiral Liquid Crystal Surfaces: From Shaping Mechanisms to Geometric Statistics. Nanomaterials 2022, 12, 1555. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, L.; Hu, J. Varied Alignment Methods and Versatile Actuations for Liquid Crystal Elastomers: A Review. Adv. Intell. Syst. 2021, 4, 2100065. [Google Scholar] [CrossRef]
- Xia, Y.; Cedillo-Servin, G.; Kamien, R.D.; Yang, S. Guided Folding of Nematic Liquid Crystal Elastomer Sheets into 3D via Patterned 1D Microchannels. Adv. Mater. 2016, 28, 9637–9643. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, J.; Yang, S. Multi-functional liquid crystal elastomer composites. Appl. Phys. Rev. 2022, 9, 011301. [Google Scholar] [CrossRef]
- Jiang, Z.-C.; Liu, Q.; Xiao, Y.-Y.; Zhao, Y. Liquid crystal elastomers for actuation: A perspective on structure-property-function relation. Prog. Polym. Sci. 2024, 153, 101829. [Google Scholar] [CrossRef]
- Ren, W.; McMullan, P.J.; Griffin, A.C. Stress-strain behavior in main chain liquid crystalline elastomers: Effect of crosslinking density and transverse rod incorporation on “Poisson’s ratio”. Phys. Status Solidi B Basic Res. 2009, 246, 2124–2130. [Google Scholar] [CrossRef]
- Azoug, A.; Vasconcellos, V.; Dooling, J.; Saed, M.; Yakacki, C.M.; Nguyen, T.D. Viscoelasticity of the polydomain-monodomain transition in main-chain liquid crystal elastomers. Polymer 2016, 98, 165–171. [Google Scholar] [CrossRef]
- Mito, K.; Haque, M.A.; Nakajima, T.; Uchiumi, M.; Kurokawa, T.; Nonoyama, T.; Gong, J.P. Supramolecular hydrogels with multi-cylindrical lamellar bilayers: Swelling-induced contraction and anisotropic molecular diffusion. Polymer 2017, 128, 373–378. [Google Scholar] [CrossRef]
- Flory, P.J.; Rehner, J. Statistical Mechanics of Cross-Linked Polymer Networks II. Swelling. J. Chem. Phys. 1943, 11, 521–526. [Google Scholar] [CrossRef]
- Doi, M. Gel Dynamics. J. Phys. Soc. Jpn. 2009, 78, 052001. [Google Scholar] [CrossRef]
- Urayama, K. Selected Issues in Liquid Crystal Elastomers and Gels. Macromolecules 2007, 40, 2277–2288. [Google Scholar] [CrossRef]
- Lucantonio, A.; DeSimone, A. Coupled swelling and nematic reordering in liquid crystal gels. Soft Matter 2017, 13, 7907–7915. [Google Scholar] [CrossRef]
- Harris, K.D.; Bastiaansen, C.W.M.; Broer, D.J. A Glassy Bending-Mode Polymeric Actuator Which Deforms in Response to Solvent Polarity. Macromol. Rapid Commun. 2006, 27, 1323–1329. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Li, H.; Zheng, W.; Zhao, Y.; Pan, W.; Zhang, N.; Xu, J.; Liu, X. Anisotropic Swelling Behavior of Liquid Crystal Elastomers in Isotropic Solvents. Nanomaterials 2025, 15, 443. https://doi.org/10.3390/nano15060443
Zhang L, Li H, Zheng W, Zhao Y, Pan W, Zhang N, Xu J, Liu X. Anisotropic Swelling Behavior of Liquid Crystal Elastomers in Isotropic Solvents. Nanomaterials. 2025; 15(6):443. https://doi.org/10.3390/nano15060443
Chicago/Turabian StyleZhang, Limei, Hong Li, Wenjiang Zheng, Yu Zhao, Weimin Pan, Niankun Zhang, Jing Xu, and Xuewei Liu. 2025. "Anisotropic Swelling Behavior of Liquid Crystal Elastomers in Isotropic Solvents" Nanomaterials 15, no. 6: 443. https://doi.org/10.3390/nano15060443
APA StyleZhang, L., Li, H., Zheng, W., Zhao, Y., Pan, W., Zhang, N., Xu, J., & Liu, X. (2025). Anisotropic Swelling Behavior of Liquid Crystal Elastomers in Isotropic Solvents. Nanomaterials, 15(6), 443. https://doi.org/10.3390/nano15060443





