Humidity-Triggered Reversible 0–1D Phase Transition in Hybrid Antimony Halides
Abstract
1. Introduction
2. Results and Discussion
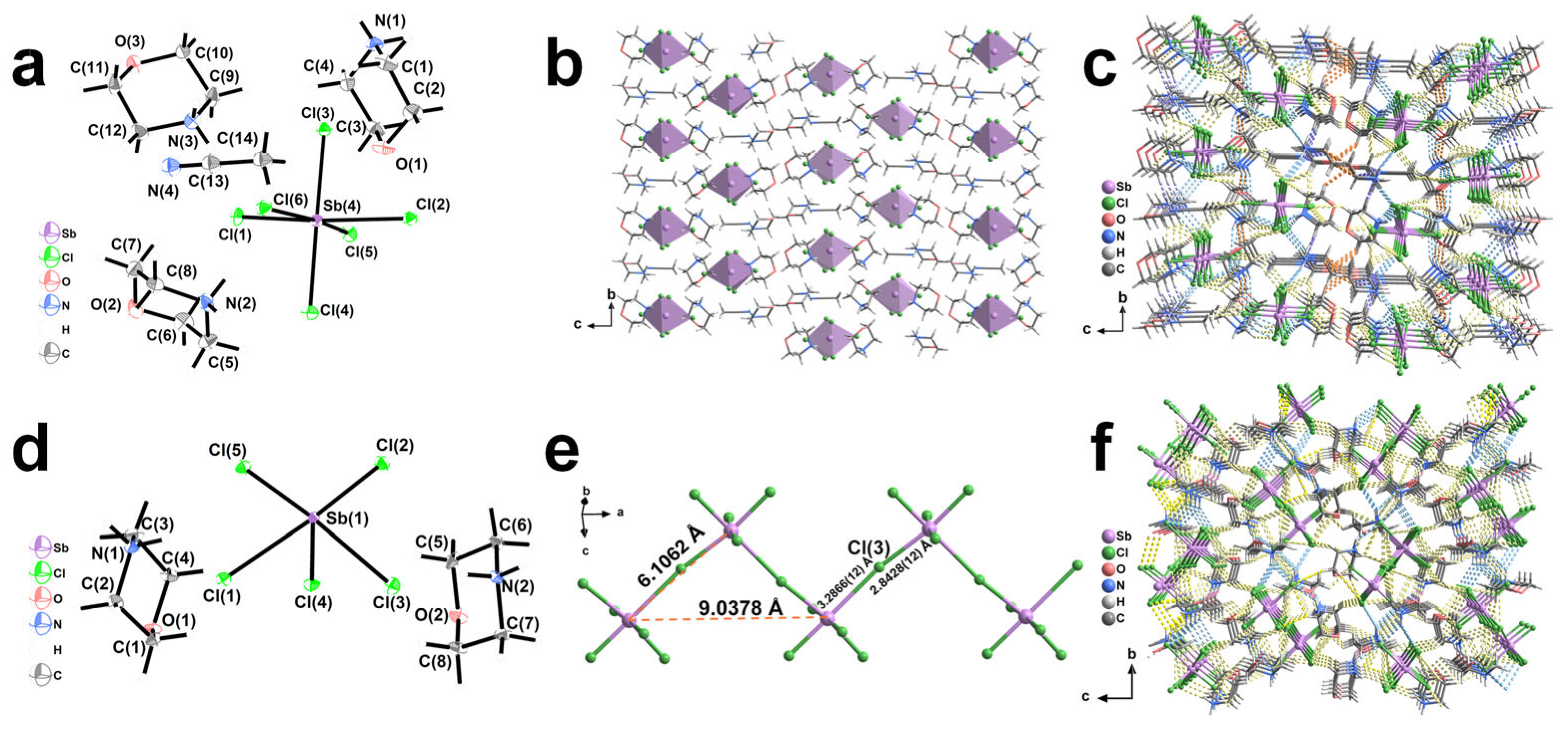
2.1. Description of the Structures
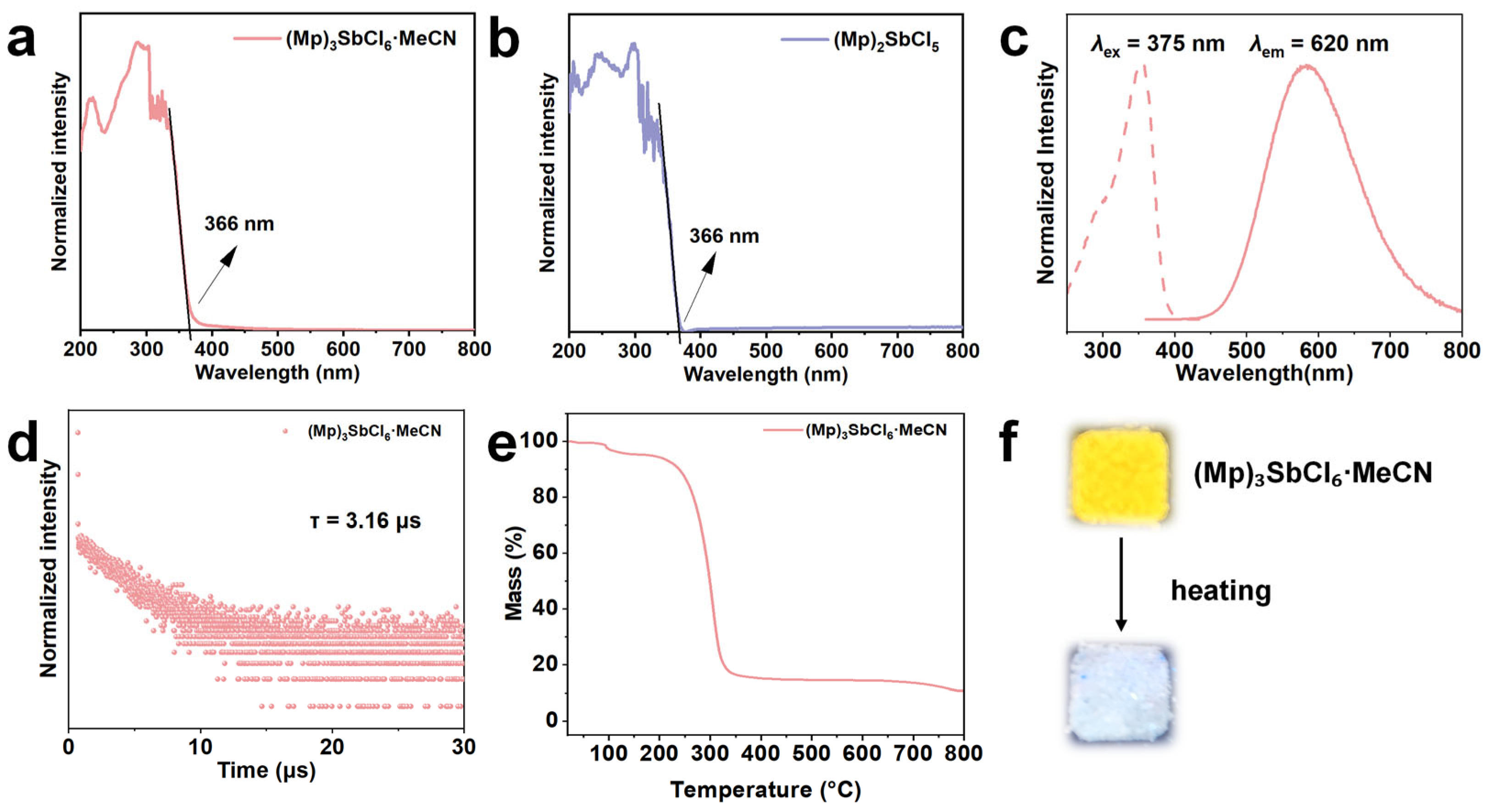
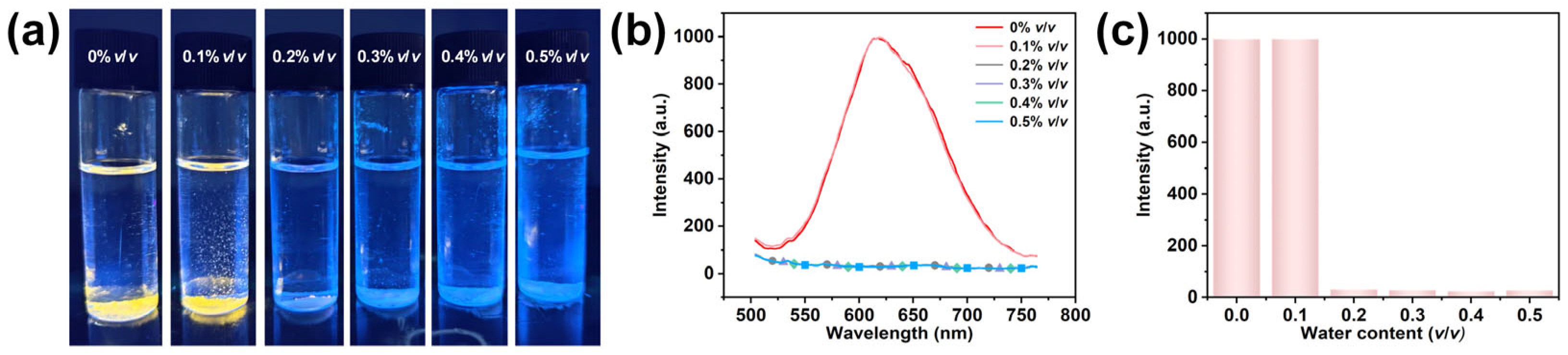
2.2. Photoluminescence Performance Description
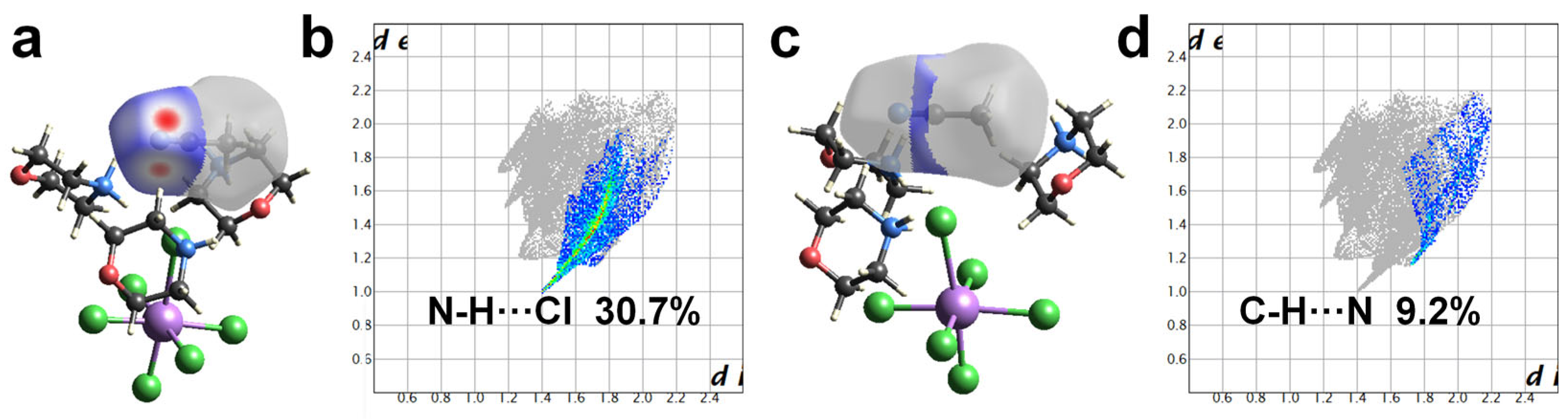
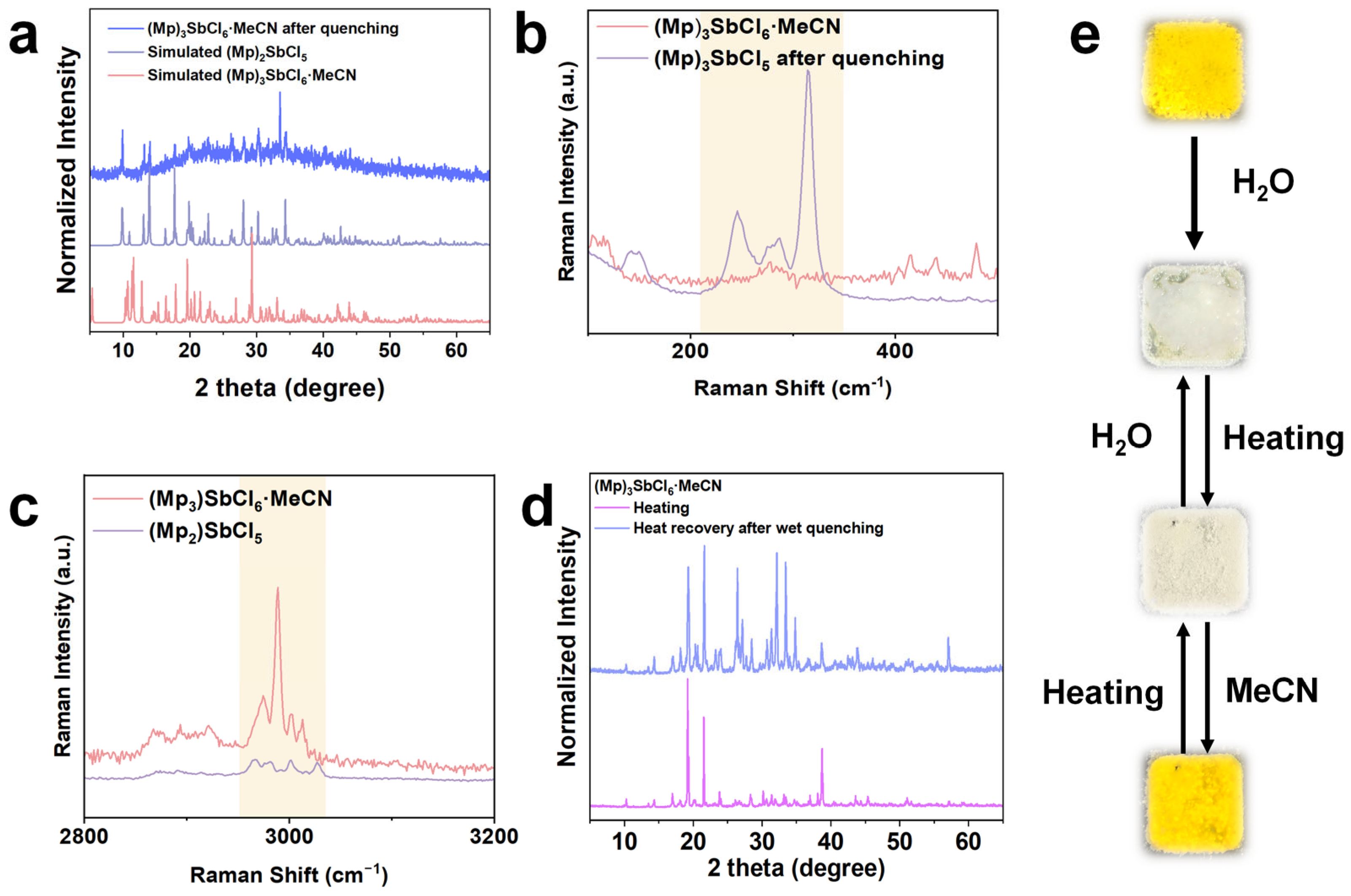
2.3. Mechanism Analysis
(Mp)3SbCl6 + xH2O ⇌ (Mp)2SbCl5 + MpCl·xH2O
2.4. Luminescent Water-Sensing
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jin, J.-C.; Lin, Y.-P.; Chen, D.-Y.; Lin, B.-Y.; Zhuang, T.-H.; Ma, W.; Gong, L.-K.; Du, K.-Z.; Jiang, J.; Huang, X.-Y. X-ray scintillation and photoluminescence of isomorphic ionic bismuth halides with [Amim]+ or [Ammim]+ cations. Inorg. Chem. Front. 2021, 8, 4474–4481. [Google Scholar] [CrossRef]
- Lin, H.-W.; Ablez, A.; Deng, Z.-H.; Chen, Z.-H.; Peng, Y.-C.; Wang, Z.-P.; Du, K.-Z.; Huang, X.-Y. A ligand-incorporating strategy towards single-component white light in ionic zero-dimensional indium chlorides. J. Mater. Chem. C 2024, 12, 5184–5190. [Google Scholar] [CrossRef]
- Peng, Y.-C.; Jin, J.-C.; Zhou, S.-H.; Lin, H.-W.; Huang, D.-D.; Deng, Z.-H.; Dong, Y.; Xu, H.-J.; Du, K.-Z.; Wang, Z.-P.; et al. Regulating photoluminescence through single-crystal-to-single-crystal transformation of solvent-containing zero-dimensional hybrid metal halide isomers. Chem. Eng. J. 2024, 488, 151026. [Google Scholar] [CrossRef]
- Zang, Z.G.; Liang, D.H.; Shi, Y.R.; Liu, Z.Y.; Li, R.; Qaid, S.M.H.; Cai, W.S. Ethanol-Induced Reversible Phase Transition in Antimony Halides for Morse Code Anti-Counterfeiting and Optical Logic Gates. Laser Photonics Rev. 2024, 19, 2401304. [Google Scholar] [CrossRef]
- Zhang, Z.-Z.; Jin, J.-C.; Gong, L.-K.; Lin, Y.-P.; Du, K.-Z.; Huang, X.-Y. Co-luminescence in a zero-dimensional organic–inorganic hybrid antimony halide with multiple coordination units. Dalton Trans. 2021, 50, 3586–3592. [Google Scholar] [CrossRef]
- Zhao, J.Q.; Wang, D.Y.; Yan, T.Y.; Wu, Y.F.; Gong, Z.L.; Chen, Z.W.; Yue, C.Y.; Yan, D.P.; Lei, X.W. Synchronously Improved Multiple Afterglow and Phosphorescence Efficiencies in 0D Hybrid Zinc Halides With Ultrahigh Anti-Water Stabilities. Angew. Chem. Int. Ed. 2024, 63, e202412350. [Google Scholar] [CrossRef]
- Zhuang, T.; Lin, Y.; Jin, J.; Deng, Z.; Peng, Y.; Gong, L.; Wang, Z.; Du, K.; Huang, X. A Mechanochemically Synthesized Hybrid Bismuth Halide as Highly Efficient Red Phosphor for Blue Chip-Based WLED. Adv. Opt. Mater. 2023, 11, 2202951. [Google Scholar] [CrossRef]
- Zhuang, T.-H.; Lin, Y.-M.; Lin, H.-W.; Guo, Y.-L.; Li, Z.-W.; Du, K.-Z.; Wang, Z.-P.; Huang, X.-Y. Luminescence Enhancement and Temperature Sensing Properties of Hybrid Bismuth Halides Achieved via Tuning Organic Cations. Molecules 2023, 28, 2380. [Google Scholar] [CrossRef]
- Quan, M.Z.; Xiong, Y.; Xu, Y.L.; Li, M.Y.; Chen, M.Y.; Cao, J.D.; Zhao, J.; Liu, Q.L. Structural diversity and photoluminescence enhancement of indium-based hybrid metal halides. J. Alloys Compd. 2024, 972, 172818. [Google Scholar] [CrossRef]
- Wang, Z.P.; Huang, X.Y. Luminescent Organic-Inorganic Hybrid Metal Halides: An Emerging Class of Stimuli-Responsive Materials. Chem. Eur. J. 2022, 28, e202200609. [Google Scholar] [CrossRef]
- Zhou, Y.Y.; Padture, N.P. Gas-Induced Formation/Transformation of Organic Inorganic Halide Perovskites. ACS Energy Lett. 2017, 2, 2166–2176. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, D.-D.; Zhang, Z.-Z.; Lin, H.-W.; Du, K.-Z.; Wang, Z.-P.; Huang, X.-Y. Modulating organic functional groups in stimuli-responsive luminescent antimony chlorides. J. Mater. Chem. C 2024, 12, 11426–11432. [Google Scholar] [CrossRef]
- Zhang, Z.; Lin, Y.; Jin, J.; Gong, L.; Peng, Y.; Song, Y.; Shen, N.; Wang, Z.; Du, K.; Huang, X. Crystalline-Phase-Recognition-Induced Domino Phase Transition and Luminescence Switching for Advanced Information Encryption. Angew. Chem. Int. Ed. 2021, 60, 23373–23379. [Google Scholar] [CrossRef]
- Zhang, J.; Ren, M.P.; Xu, M.; Zhang, Z.H.; An, M.X.; Lu, Y.; Lei, X.W.; Gong, Z.L.; Yue, C.Y. Ultrafast Visual Detection of a Trace Amount of Water by Highly Efficient Hybrid Manganese Halides. ACS Appl. Mater. Interfaces 2024, 16, 33780–33788. [Google Scholar] [CrossRef]
- Wang, Z.P.; Zhang, Z.Z.; Tao, L.Q.; Shen, N.N.; Hu, B.; Gong, L.K.; Li, J.R.; Chen, X.P.; Huang, X.Y. Hybrid Chloroantimonates(III): Thermally Induced Triple-Mode Reversible Luminescent Switching and Laser- Printable Rewritable Luminescent Paper. Angew. Chem. Int. Ed. 2019, 58, 9974–9978. [Google Scholar] [CrossRef]
- Li, D.Y.; Wu, J.H.; Wang, X.Y.; Zhang, X.Y.; Yue, C.Y.; Lei, X.W. Reversible Triple-Mode Photo- and Radioluminescence and Nonlinear Optical Switching in Highly Efficient 0D Hybrid Cuprous Halides. Chem. Mater. 2023, 35, 6598–6611. [Google Scholar] [CrossRef]
- Liu, Y.H.; Yan, X.; Xiao, L.; Jiang, W.; Liu, Q.; Liu, T.C.; Yan, T.Y.; Yue, C.Y.; Lei, X.W. Water-Stable 0D Hybrid Manganese Halides with Adjustable Crystal Structure and Emission Color. Adv. Opt. Mater. 2023, 11, 2301010. [Google Scholar] [CrossRef]
- Li, D.Y.; Song, J.H.; Cheng, Y.; Wu, X.M.; Wang, Y.Y.; Sun, C.J.; Yue, C.Y.; Lei, X.W. Ultra-Sensitive, Selective and Repeatable Fluorescence Sensor for Methanol Based on a Highly Emissive 0D Hybrid Lead-Free Perovskite. Angew. Chem. Int. Ed. 2022, 61, e202206437. [Google Scholar] [CrossRef]
- Lu, X.Y.; Peng, H.; Wei, Q.L.; Lin, W.C.; Tian, Y.; Li, T.Z.; Zhou, S.C.; Zhao, J.L.; Zou, B.S. Bulk assemblies of organic antimony chloride with multiple reversible photoluminescence switching for anti-counterfeiting and information encryption. Mater. Today Phys. 2023, 35, 101085. [Google Scholar] [CrossRef]
- Sun, C.; Zhang, H.; Deng, Z.H.; Fan, C.; Liu, X.H.; Luo, M.M.; Zhao, Y.W.; Lian, K. Metal-Ion-Doped Manganese Halide Hybrids with Tunable Emission for Advanced Anti-Counterfeiting. Nanomaterials 2023, 13, 1890. [Google Scholar] [CrossRef]
- Ma, W.; Qian, Q.K.; Qaid, S.M.H.; Zhao, S.Y.; Liang, D.H.; Cai, W.S.; Zang, Z.G. Water-Molecule-Induced Reversible Fluorescence in a One-Dimensional Mn-Based Hybrid Halide for Anticounterfeiting and Digital Encryption-Decryption. Nano Lett. 2023, 23, 8932–8939. [Google Scholar] [CrossRef] [PubMed]
- Jiang, R.; Peng, G.Q.; Li, Q.J.; Wang, H.X.; Ci, Z.P.; Wang, Q. Manganese (II) Halides for X-Ray Imaging and Moisture Detection. Adv. Mater. Technol. 2024, 9, 2301894. [Google Scholar] [CrossRef]
- Song, Z.X.; Chen, D.P.; Yu, B.Y.; Liu, G.K.; Li, H.Y.; Wei, Y.Y.; Wang, S.H.; Meng, L.Q.; Dang, Y.Y. Thermal/Water-Induced Phase Transformation and Photoluminescence of Hybrid Manganese(II)-Based Chloride Single Crystals. Inorg. Chem. 2023, 62, 17931–17939. [Google Scholar] [CrossRef]
- Chen, S.Q.; Huang, M.L.; Yin, Y.L.; Shi, J.L. Paper-based sensor based on lead-free manganese halide for the determination of water content in organic solvents. Microchim. Acta 2023, 190, 329. [Google Scholar] [CrossRef]
- Xu, W.; Li, F.M.; Cai, Z.X.; Wang, Y.R.; Luo, F.; Chen, X. An ultrasensitive and reversible fluorescence sensor of humidity using perovskite CH3NH3PbBr3. J. Mater. Chem. C 2016, 4, 9651–9655. [Google Scholar] [CrossRef]
- Qiu, Y.X.; Ma, Z.M.; Li, Z.W.; Sun, H.Y.; Dai, G.K.; Fu, X.H.; Jiang, H.; Ma, Z.Y. Solely 3-Coordinated Organic-Inorganic Hybrid Copper(I) Halide: Hexagonal Channel Structure, Turn-On Response to Mechanical Force, Moisture, and Amine. Inorg. Chem. 2022, 61, 8320–8327. [Google Scholar] [CrossRef]
- Gao, W.R.; Leng, M.Y.; Hu, Z.X.; Li, J.Z.; Li, D.H.; Liu, H.; Gao, L.; Niu, G.D.; Tang, J. Reversible luminescent humidity chromism of organic-inorganic hybrid PEA2MnBr4 single crystals. Dalton Trans. 2020, 49, 5662–5668. [Google Scholar] [CrossRef]
- Cai, W.D.; Qin, J.J.; Pang, T.Q.; Cai, X.Y.; Jia, R.X.; Gao, F. Chirality Induced Crystal Structural Difference in Metal Halide Composites. Adv. Opt. Mater. 2022, 10, 2102140. [Google Scholar] [CrossRef]
- Li, D.Y.; Song, J.H.; Xu, Z.Y.; Gao, Y.J.; Yin, X.; Hou, Y.H.; Feng, L.J.; Yue, C.Y.; Fei, H.H.; Lei, X.W. Reversible Triple-Mode Switching in Photoluminescence from 0D Hybrid Antimony Halides. Chem. Mater. 2022, 34, 6985–6995. [Google Scholar] [CrossRef]
- Liu, H.L.; Ru, H.Y.; Sun, M.E.; Wang, Z.Y.; Zang, S.Q. Organic-Inorganic Manganese Bromide Hybrids with Water-Triggered Luminescence for Rewritable Paper. Adv. Opt. Mater. 2022, 10, 2101700. [Google Scholar] [CrossRef]
- Sharma, S.K.; Phadnis, C.; Das, T.K.; Kumar, A.; Kayaipatti, B.; Chowdhury, A.; Yella, A. Reversible Dimensionality Tuning of Hybrid Perovskites with Humidity: Visualization and Application to Stable Solar Cells. Chem. Mater. 2019, 31, 3111–3117. [Google Scholar] [CrossRef]
- Wang, Z.P.; Huang, D.D.; Liu, Y.; Lin, H.W.; Zhang, Z.Z.; Ablez, A.; Zhuang, T.H.; Du, K.Z.; Li, J.; Huang, X.Y. Vacancy Effect on the Luminescent and Water Responsive Properties of Vacancy-Ordered Double Perovskite Derivatives. Angew. Chem. Int. Ed. 2024, 63, e202412346. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.J.; Qi, J.L.; Guo, Y.; Yan, S.F.; Liu, W.L.; Guo, S.P. Reversible tri-state structural transitions of hybrid copper(i) bromides toward tunable multiple emissions. Inorg. Chem. Front. 2023, 11, 156–163. [Google Scholar] [CrossRef]
- Wang, Z.P.; Xie, D.L.; Zhang, F.; Yu, J.B.; Chen, X.P.; Wong, C.P. Controlling information duration on rewritable luminescent paper based on hybrid antimony (III) chloride/small-molecule absorbates. Sci. Adv. 2020, 6, eabc2181. [Google Scholar] [CrossRef]
- Zhao, J.Q.; Han, M.F.; Zhao, X.J.; Ma, Y.Y.; Jing, C.Q.; Pan, H.M.; Li, D.Y.; Yue, C.Y.; Lei, X.W. Structural Dimensionality Modulation toward Enhanced Photoluminescence Efficiencies of Hybrid Lead-Free Antimony Halides. Adv. Opt. Mater. 2021, 9, 2100556. [Google Scholar] [CrossRef]
- Sun, C.; Deng, Z.Y.; Li, Z.Y.; Chen, Z.W.; Zhang, X.Y.; Chen, J.; Lu, H.P.; Canepa, P.; Chen, R.; Mao, L.L. Achieving Near-unity Photoluminescence Quantum Yields in Organic-Inorganic Hybrid Antimony (III) Chlorides with the SbCl5 Geometry. Angew. Chem. Int. Ed. 2023, 62, e202216720. [Google Scholar] [CrossRef]
- Liao, J.F.; Zhang, Z.P.; Zhou, L.; Tang, Z.K.; Xing, G.C. Achieving Near-Unity Red Light Photoluminescence in Antimony Halide Crystals via Polyhedron Regulation. Angew. Chem. Int. Ed. 2024, 63, e202404100. [Google Scholar] [CrossRef]
- Lei, Y.S.; Li, Y.H.; Lu, C.C.F.; Yan, Q.Z.; Wu, Y.L.; Babbe, F.; Gong, H.X.; Zhang, S.; Zhou, J.Y.; Wang, R.T.; et al. Perovskite superlattices with efficient carrier dynamics. Nature 2022, 608, 317. [Google Scholar] [CrossRef]
- Lin, J.; Zhang, Q.; Wang, L.; Liu, X.C.; Yan, W.B.; Wu, T.; Bu, X.H.; Feng, P.Y. Atomically Precise Doping of Monomanganese Ion into Coreless Supertetrahedral Chalcogenide Nanocluster Inducing Unusual Red Shift in Mn2+ Emission. J. Am. Chem. Soc. 2014, 136, 4769–4779. [Google Scholar] [CrossRef]
- Lin, F.; Wang, H.; Liu, W.; Li, J. Zero-dimensional ionic antimony halide inorganic-organic hybrid with strong greenish yellow emission. J. Mater. Chem. C 2020, 8, 7300–7303. [Google Scholar] [CrossRef]
- Chen, D.; Dai, F.L.; Hao, S.Q.; Zhou, G.J.; Liu, Q.L.; Wolverton, C.; Zhao, J.; Xia, Z.G. Crystal structure and luminescence properties of lead-free metal halides (C6H5CH2NH3)3MBr6(M = Bi and Sb). J. Mater. Chem. C 2020, 8, 7322–7329. [Google Scholar] [CrossRef]
- McCall, K.M.; Stoumpos, C.C.; Kostina, S.S.; Kanatzidis, M.G.; Wessels, B.W. Strong Electron-Phonon Coupling and Self-Trapped Excitons in the Defect Halide Perovskites A3M2I9 (A = Cs, Rb; M = Bi, Sb). Chem. Mater. 2017, 29, 4129–4145. [Google Scholar] [CrossRef]
- Li, Z.Y.; Li, Y.; Liang, P.; Zhou, T.L.; Wang, L.; Xie, R.J. Dual-Band Luminescent Lead-Free Antimony Chloride Halides with Near-Unity Photoluminescence Quantum Efficiency. Chem. Mater. 2019, 31, 9363–9371. [Google Scholar] [CrossRef]
- Feng, L.J.; Liu, H.R.; Wang, L.L.; Yang, C.C.; Ding, Y.W.; Lei, X.W.; Chen, Z.W. Blue light emissive zero-dimensional hybrid cadmium bromide as fluorescence sensor toward benzaldehyde. J. Lumin. 2024, 270, 120538. [Google Scholar] [CrossRef]
- Wang, X.C.; Bai, T.X.; Sun, J.L.; Liu, J.Y.; Su, Y.; Chen, J.S. The effect of solvent on the formation of low-dimensional metal halides and their self-trapped exciton emission. Chem. Eng. J. 2024, 486, 150257. [Google Scholar] [CrossRef]
- Xie, P.R.; Wang, P.; Zhou, J.Q.; Guo, Z.; Mao, L.L. Increasing the Structural Rigidity and Quantum Yield of Highly Emissive Hybrid Antimony Chlorides Using a Diverse Set of Solvent Guests. Adv. Opt. Mater. 2024, 12, 2303230. [Google Scholar] [CrossRef]
- Lin, J.W.; Zhang, M.W.; Sun, N.; He, S.H.; Zhang, X.S.; Guo, Z.N.; Zhao, J.; Liu, Q.L.; Yuan, W.X. Narrowing the band of green emission in manganese hybrids by reducing the hydrogen bond strength and structural distortion. J. Mater. Chem. C 2022, 10, 16773–16780. [Google Scholar] [CrossRef]
- Zhang, Q.; Huang, T.W.; Liu, Z.Y.; Feng, Y.N.; Yu, Y.; Li, L.Y. Hydrogen bonding evolution and efficient blue light emission in a series of Zn-based organic-inorganic hybrid metal halide crystals. Sci. China Mater. 2025, 1–8. [Google Scholar] [CrossRef]
- Huang, Z.H.; Wang, Y.X.; Du, P.; Gao, W.; Niu, P.; Xu, D.M.; Wang, L.M.; Deng, Y.C.; Song, A.X. Structural Design of Hybrid Manganese(II) Halides for High Quantum Efficiency and Specific Response to Methanol. Inorg. Chem. 2024, 63, 21059–21069. [Google Scholar] [CrossRef]
- Choi, M.H.; Moon, T.H.; Kuk, Y.; Ok, K.M. Green and Red Photoluminescent Manganese Bromides with Aminomethylpyridine Isomers. Inorg. Chem. 2023, 62, 12058–12066. [Google Scholar] [CrossRef]
- Hu, W.H.; Xu, W.J.; Meng, Q.R.; Zhang, X.W.; He, C.T.; Zhang, W.X.; Chen, X.M. Switching hydrogen bonds to readily interconvert two room-temperature long-term stable crystalline polymorphs in chiral molecular perovskites. Chem. Commun. 2019, 55, 11555–11558. [Google Scholar] [CrossRef] [PubMed]
- Ye, H.; Hu, W.H.; Chen, X.X.; Zhao, B.Q.; Zhang, W.X.; Chen, X.M. Heat- and Pressure-driven Room-temperature Polymorphic Transition Accompanied with Switchable SHG Signal in a New Chiral Hexagonal Perovskite. Chem. Asian J. 2023, 18, e202300608. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.H.; Wang, X.Q.; Luo, J.H.; Zhang, S.Q.; Yuan, D.Q.; Hong, M.C. Ferroelastic phase transition and switchable dielectric behavior associated with ordering of molecular motion in a perovskite-like architectured supramolecular cocrystal. J. Mater. Chem. C 2013, 1, 2561–2567. [Google Scholar] [CrossRef]
- Gong, Z.L.; Zheng, W.; Huang, P.; Cheng, X.W.; Zhang, W.; Zhang, M.R.; Han, S.Y.; Chen, X.Y. Highly efficient Sb3+ emitters in 0D cesium indium chloride nanocrystals with switchable photoluminescence through water-triggered structural transformation. Nano Today 2022, 44, 101460. [Google Scholar] [CrossRef]
- Song, G.M.; Li, M.Z.; Zhang, S.Z.; Wang, N.Z.; Gong, P.F.; Xia, Z.G.; Lin, Z.S. Enhancing Photoluminescence Quantum Yield in 0D Metal Halides by Introducing Water Molecules. Adv. Funct. Mater. 2020, 30, 2002468. [Google Scholar] [CrossRef]
- Zhou, L.; Liao, J.F.; Huang, Z.G.; Wei, J.H.; Wang, X.D.; Li, W.G.; Chen, H.Y.; Kuang, D.B.; Su, C.Y. A Highly Red-Emissive Lead-Free Indium-Based Perovskite Single Crystal for Sensitive Water Detection. Angew. Chem. Int. Ed. 2019, 58, 5277–5281. [Google Scholar] [CrossRef]
- Luo, J.B.; Wei, J.H.; Zhang, Z.Z.; Kuang, D.B. Water-Molecule-Induced Emission Transformation of Zero-Dimension Antimony-Based Metal Halide. Inorg. Chem. 2022, 61, 338–345. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. C-Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Spackman, M.A.; McKinnon, J.J. Fingerprinting intermolecular interactions in molecular crystals. CrystEngComm 2002, 4, 378–392. [Google Scholar] [CrossRef]
- McKinnon, J.J.; Spackman, M.A.; Mitchell, A.S. Novel tools for visualizing and exploring intermolecular interactions in molecular crystals. Acta. Crystallogr. B. Struct. Sci. Cryst. Eng. Mater. 2004, 60, 627–668. [Google Scholar] [CrossRef]
- McKinnon, J.J.; Jayatilaka, D.; Spackman, M.A. Towards quantitative analysis of intermolecular interactions with Hirshfeld surfaces. Chem. Commun. 2007, 7, 3814–3816. [Google Scholar] [CrossRef] [PubMed]
- Spackman, M.A.; McKinnon, J.J.; Jayatilaka, D. Electrostatic potentials mapped on Hirshfeld surfaces provide direct insight into intermolecular interactions in crystals. CrystEngComm 2008, 10, 377–388. [Google Scholar] [CrossRef]
- Spackman, M. Rationalising molecular crystal structures using Hirshfeld surfaces. Acta Cryst. 2014, 70, C34. [Google Scholar] [CrossRef]
- Turner, M.J.; McKinnon, J.J.; Jayatilaka, D.; Spackman, M.A. Visualisation and characterisation of voids in crystalline materials. CrystEngComm 2011, 13, 1804–1813. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Luo, J.; Ablez, A.; Liu, J.; Wang, N.; Lin, H.; Wang, Z.; Huang, X. Humidity-Triggered Reversible 0–1D Phase Transition in Hybrid Antimony Halides. Nanomaterials 2025, 15, 442. https://doi.org/10.3390/nano15060442
Liu Y, Luo J, Ablez A, Liu J, Wang N, Lin H, Wang Z, Huang X. Humidity-Triggered Reversible 0–1D Phase Transition in Hybrid Antimony Halides. Nanomaterials. 2025; 15(6):442. https://doi.org/10.3390/nano15060442
Chicago/Turabian StyleLiu, Yi, Jiahua Luo, Abdusalam Ablez, Jinmei Liu, Nianhao Wang, Haowei Lin, Zeping Wang, and Xiaoying Huang. 2025. "Humidity-Triggered Reversible 0–1D Phase Transition in Hybrid Antimony Halides" Nanomaterials 15, no. 6: 442. https://doi.org/10.3390/nano15060442
APA StyleLiu, Y., Luo, J., Ablez, A., Liu, J., Wang, N., Lin, H., Wang, Z., & Huang, X. (2025). Humidity-Triggered Reversible 0–1D Phase Transition in Hybrid Antimony Halides. Nanomaterials, 15(6), 442. https://doi.org/10.3390/nano15060442







