Abstract
The rapid advancement of 3D packaging technology has emerged as a key solution to overcome the scaling-down limitation of advanced memory and logic devices. Redistribution layer (RDL) fabrication, a critical process in 3D packaging, requires the use of polyimide (PI) films with thicknesses in the micrometer range. However, these polyimide films present surface topography variations in the range of hundreds of nanometers, necessitating chemical–mechanical planarization (CMP) to achieve nanometer-level surface flatness. Polyimide films, composed of copolymers of pyromellitimide and diphenyl ether, possess strong covalent bonds such as C–C, C–O, C=O, and C–N, leading to inherently low polishing rates during CMP. To address this challenge, the introduction of Fe(NO3)3 into CMP slurries has been proposed as a polishing rate accelerator. During CMP, this Fe(NO3)3 deformed the surface of a polyimide film into strongly positively charged 1,2,4,5-benzenetetracarbonyliron and weakly negatively charged 4,4′-oxydianiline (ODA). The chemically dominant polishing rate enhanced with the concentration of the Fe(NO3)3 due to accelerated surface interactions. However, higher Fe(NO3)3 concentrations reduce the attractive electrostatic force between the positively charged wet ceria abrasives and the negatively charged deformed surface of the polyimide film, thereby decreasing the mechanically dominant polishing rate. A comprehensive investigation of the chemical and mechanical polishing rate dynamics revealed that the optimal Fe(NO3)3 concentration to achieve the maximum polyimide film removal rate was 0.05 wt%.
1. Introduction
With the advent of the Fourth Industrial Revolution, the rapid growth of markets such as artificial intelligence (AI), metaverse, the internet of things (IOT), and robots has driven increasing demand for semiconductors with a higher operation speed, a lower power consumption, and a higher bandwidth, requiring the rapid scaling-down of advanced memory and logic devices [1,2]. Recently, continuous scaling-down has been slowed down and limited because of the fabrication complexity of devices. As a solution, 2.5D and 3D heterogeneous package technology has been introduced and intensively researched, being essentially necessary to fabricate the redistribution layer (RDL). RDL plays a critical role by rearranging the input/output (I/O) terminals of chips and optimizing their electrical connections with the substrate [3,4,5,6]. To create the RDL, polyimide (PI) has been widely used as an insulation and surface topography planarization layer in RDL structures due to its excellent thermal stability and ductility [7,8,9,10]. Furthermore, in high-performance packaging, multiple-RDL structures have been essential to support a greater number of I/O pins, enhancing the importance of achieving a uniform PI thickness in multiple-RDL structures [11,12,13]. Generally, a polyimide film is spin-coated, and its thickness is approximately several μm, inducing a higher surface topography of several hundred nm. Thus, chemical–mechanical planarization (CMP) of the surface of a polyimide film with a high topography has been essentially utilized to achieve local and global planarization of its surface [8].
Historically, a mechanically dominant CMP method for polyimide films has been the primary method for planarizing the surface of polyimide films’ topography due to the hardness (i.e., 0.33 GPa) of the polyimide film having a strongly covalently bonded structure. However, in 2021, a groundbreaking study introduced a chemically dominant CMP method for polyimide films by using a hydrolysis reaction to deform the surface of the polyimide film into a softer surface structure, thereby improving the polyimide film’s polishing rate [8]. Despite this being a promising solution, this study employed amine-based polishing rate accelerators, which could be limited in their practical application because these polishing rate accelerators are now classified as environmentally regulated substances. Therefore, it is essential to design environmentally friendly polishing rate accelerators capable of converting the surface of polyimide films into a soft structure to improve the CMP polishing rate.
As a new solution for enhancing the polishing rate in CMP of polyimide films, a wet-ceria-abrasive-based polyimide film CMP slurry containing Fe(NO3)3 was designed. The Fe(NO3)3 acts as a polishing rate accelerator by breaking the strong covalent bonds between the pyromellitimide and diphenyl ether copolymers on the surface of the polyimide film, thereby deforming this surface into a soft film. Specifically, the Fe(NO3)3 facilitates a substitution nucleophilic bimolecular reaction (SN2 reaction) that breaks the C=O bonds on the surface of the polyimide film and forms O–C–Fe bonds, followed by ring-opening and a proton transfer reaction, ultimately transforming the surface of the polyimide film into a soft layer composed of strongly positively charged 1,2,4,5-benzenetetracarbonyliron and slightly negatively charged 4,4′-oxydianiline (ODA) [9,14,15,16,17,18,19,20,21,22,23,24]. The modified surface of the polyimide film was polished using 100 nm diameter wet ceria abrasives, achieving a high polishing rate of over 1000 nm/min.
To elucidate the mechanism behind the significant enhancement in the polyimide film polishing rate achieved by the designed wet ceria abrasive and the Fe(NO3)3 CMP slurry, both the chemically dominant polishing and mechanically dominant polishing properties of the CMP slurry were investigated. First, the dependency of polyimide film polishing on the Fe(NO3)3 concentration of the CMP slurry was evaluated. From a chemically dominant polishing perspective, the slurry adsorption degree on the surface of the polyimide film was measured according to the Fe(NO3)3 concentration in the CMP slurry. Additionally, the deformation of the polished surface of the polyimide film was observed via x-ray photoelectron spectroscopy (XPS), and the results were used to explain the mechanism of surface deformation after CMP.
2. Materials and Methods
2.1. Materials
A 2000 nm thick polyimide (PI) film (SAMSUNG Inc., Yongin, Republic of Korea) was thermally cured after being coated onto a glass substrate. At this time, the hardness of the polyimide film was 0.33 GPa [8]. To prepare for subsequent polishing, the glass substrate coated with the polyimide film was turned into 4 cm × 4 cm square pieces. The slurry was composed of 1.0 wt% 100 nm wet ceria abrasive (UB materials Inc., Yongin, Republic of Korea) 0 to 0.1 wt% Fe(NO3)3 (Sigma-Aldrich Inc., St. Louis, MO, USA) as an accelerator, and 0 to 0.2 wt% picolinic acid (Sigma-Aldrich Inc., St. Louis, MO, USA) as a dispersant. At this time, Fe(NO3)3 was used as the accelerator to increase the polishing rate of the surface of the polyimide film. The slurry’s viscosity was almost independent of the Fe(NO3)3 concentration, as shown in Figure S1. The presence of CMP debris on the surface of the polyimide film was investigated using an optical microscope.
2.2. CMP Conditions
The CMP was conducted using a CMP polisher (POLI-300, G&P Tech. Inc., Busan, Republic of Korea) with a rectangular-grooved CMP pad (SUBA 400, Nitta Haas Inc., Osaka, Japan) [8]. Before the main polishing, pad break-in was performed using brush conditioner for 30 min, and two dummy wafers were polished as part of the preparation. The head pressure of polishing was 4 psi, a head rotation speed of 87 rpm was applied, and the rotation speed of the CMP pad table was 93 rpm. CMP slurry flow rate of 100 mL/min and a polishing time of 60 s were adopted. Then, deionized water (DIW) buffing for 10 s was performed after to remove any remaining debris.
2.3. Characterization
The polyimide film polishing rate was evaluated using a V-VASE ellipsometer (J.A. Woollam Co., Inc., Lincoln, NE, USA). The morphology and diameter of the primary wet ceria abrasives were measured using scanning electron microscopy (SEM) with an S-4800 (Hitachi High-Tech, Tokyo, Japan) using a 15 kV acceleration voltage. The secondary abrasive diameter and zeta potential of the wet ceria abrasives of the CMP slurry were estimated using an ELSZ2+ scattering particle analyzer, Otsuka Electronics (Tokyo, Japan). The pH and conductivity of the CMP slurries were evaluated using a pH meter from Thermo Fischer Scientific Inc., the ORIONSTAR A211 (Waltham, MA, USA). The formation of chemical covalent bonds on the polished surface of the polyimide film after CMP was analyzed using X-ray photoelectron spectroscopy with a K-Alpha+, Thermo Fisher Scientific Inc. (Waltham, MA, USA), at 12 keV and 6 Ma [8]. The adsorption degree of the CMP slurries onto the polished surface of the polyimide film (i.e., the contact angle) was measured via a contact angle meter from GBX Instruments Ltd. (Dublin, Ireland), the DIGIDROP, where 0.01 mL of the DIW or slurries was dropped onto the film surface.
3. Results
3.1. The Dependency of the Polyimide Film Polishing Rate on the Fe(NO3)3 Concentration of the CMP Slurry
The polyimide (PI) film consists of pyromellitimide and diphenyl ether, with strong C–C, C–O, C=O, and C–N bonds [8]. To enhance the polyimide film polishing rate, it is necessary to break these strong covalent bonds on the surface of the polyimide film, thereby deforming the surface into a soft surface of polyimide. This deformation increases the difference in hardness between the wet ceria abrasives and the deformed surface of the polyimide film, which ultimately boosts the polishing rate of the polyimide film. For example, the hardness of the wet ceria abrasive was 7.0 GPa, while the hardness of the polyimide film prior to CMP was ~0.33 GPa [8], resulting in a hardness difference of ~6.67 GPa. By incorporating the Fe(NO3)3 into the newly designed polyimide film CMP slurry, during CMP, the chemical reaction between the Fe(NO3)3 and the surface of the polyimide film caused the polished surface of the polyimide film to be deformed into a softer surface with a hardness below 0.33 GPa. Consequently, the difference in hardness between the wet ceria abrasives and the polished surface of the polyimide film exceeds ~6.67 GPa, thereby significantly enhancing the polishing rate. The polyimide film CMP slurry was formulated using 100 nm diameter wet ceria abrasives, along with Fe(NO3)3 as a polishing accelerator, picolinic acid as a dispersant, and a pH titrant. The dispersant concentration was increased at the same 1:2 ratio when the Fe(NO3)3 concentration of the CMP slurry was increased.
When the Fe(NO3)3 concentration of the CMP slurry increased up to 0.05 wt%, the polishing rate of the polyimide film dramatically rose from 725 nm/min to 1465 nm/min, as shown in Figure 1. However, above Fe(NO3)3 concentrations of 0.05 wt%, the polishing rate gradually decreased from 1465 nm/min to 1298 nm/min. These results indicate two distinct regions depending on the Fe(NO3)3 concentration: region I, where the polishing rate increases with the Fe(NO3)3 concentration, and region II, where the polishing rate decreases. Moreover, comparing the polyimide film polishing rates when using other accelerators, such as a hydrolysis accelerator (e.g., ethanolamine) and oxidants (e.g., ammonium persulfate and periodic acid), Fe(NO3)3 presented the highest polyimide film polishing rate, as shown in Figure S2. Also, the polishing rate of the PI film was almost independent of the frequency of CMP, indicating that the polishing rate of the PI film was not influenced by the by-products generated during CMP, as shown in Figure S3. To understand this dependency, based on a mechanically dominant polishing perspective, the following properties were measured, depending on the Fe(NO3)3 concentration, in the polyimide film CMP slurry: the secondary abrasive’s diameter, the abrasive’s zeta potential, and the polished polyimide film’s zeta potential. Otherwise, based on a chemically dominant polishing perspective, the following were estimated as a function of the Fe(NO3)3 concentration of the CMP slurry: the slurry adsorption degree on the surface of the polished polyimide film and the deformation of the polished surface of the polyimide film.
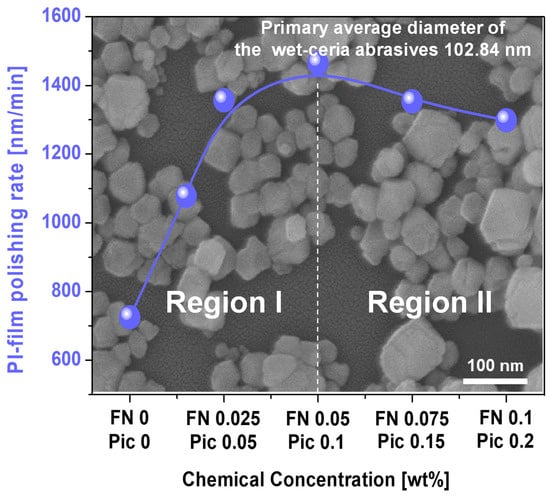
Figure 1.
Polyimide film polishing rate depending on concentration of Fe(NO3)3 and picolinic acid in the CMP slurry. The background SEM images in Figure 1 exhibit the primary abrasive morphology and diameter of the wet ceria abrasives.
3.2. The Properties of the Slurry in Polyimide Film CMP (i.e., the Zeta Potential of the Polished Surface of the Polyimide Film and the Slurry Adsorption Degree) and Mechanically Dominant Polishing Depending on the Fe(NO3)3 Concentration
In the polyimide film CMP slurry, the wet ceria abrasive’s secondary diameter remained approximately 170 nm when the Fe(NO3) 3 concentration increased from 0 to 0.05 wt%, as presented in Figure 2. However, as the Fe(NO3)3 concentration exceeded 0.05 wt%, the wet ceria abrasive’s secondary diameter increased from 170 nm to over 200 nm. Furthermore, when the Fe(NO3)3 concentration of the CMP slurry was enhanced from 0 to 0.1 wt%, the zeta potential of the wet ceria abrasives decreased from 51.7 mV to 39.0 mV. The zeta potential of the slurry without Fe(NO3)3 was 51.7 mV. However, when Fe(NO3)3 dissolved in the CMP slurry into Fe3+ cations and NO3− anions, the NO3− anions adsorbed onto the surface of the positively charged wet ceria abrasive, increasing the chemical double layer and thereby reducing the zeta potential. Therefore, when the Fe(NO3)3 concentration in the slurry was increased, the zeta potential of the wet ceria abrasive significantly decreased. The increase in the chemical double layer of the wet ceria abrasive due to the increased Fe(NO3)3 concentration of the slurry led to an increase in the secondary abrasive diameter. Moreover, when the Fe(NO3)3 concentration of the slurry was increased from 0 to 0.1 wt%, the zeta potential of the polished surface of the polyimide film noticeably increased from −15.0 mV to −3.3 mV. This increase was attributed to the adsorption of ionized Fe3+ from the Fe(NO3)3 in the CMP slurry onto the negatively charged surface of the polyimide film. As a result, the zeta potential of the polished surface of the polyimide film shifted positively when the Fe(NO3)3 concentration of the CMP slurry was increased. This was further confirmed through a surface chemical analysis of the polished polyimide film. By analyzing the dependence of the zeta potential of both the wet ceria abrasives and the polished surface of the polyimide film on the Fe(NO3)3 concentration, the attractive electrostatic force between the wet ceria abrasives and the polished surface of polyimide was calculated to evaluate the mechanically dominant CMP polishing rate during CMP of the polyimide film. When the Fe(NO3)3 concentration of the slurry was increased from 0 to 0.1 wt%, the attractive electrostatic force between the wet ceria abrasives and the polished surface of the polyimide film decreased from 764 abs. to 126 abs. This result implies that an increase in the Fe(NO3)3 concentration reduces the attractive electrostatic force between the positively charged wet ceria abrasives and the negatively charged surface of the polyimide film, thereby decreasing the mechanically dominant CMP polishing rate. It is generally known that a higher attractive electrostatic force between abrasives and a polished film surface leads to a higher mechanically dominant polishing rate [24,25,26,27,28,29,30,31,32]. Note that when the Fe(NO3)3 concentration increased from 0 to 0.1 wt%, the zeta potential of the PI film decreased from −15.0 to −3.3 mV, i.e., a 11.7 mV reduction, while the zeta potential of the abrasives decreased from ~51.7 to 39.0 mV, i.e., a 12.7 mV reduction. Thus, both the zeta potentials of the PI film and abrasives affected the electrostatic attractive force between the PI film surfaces and the abrasives. Thus, from a mechanically dominant polishing perspective, an increase in the Fe(NO3)3 concentration of the CMP slurry leads to a decrease in the mechanically dominant polishing rate. This result was not correlated with the dependence of the polishing rate on the Fe(NO3)3 concentration, as observed in Figure 1. Instead, it aligned with the trend observed in region II of Figure 1, where the mechanically dominant polishing rate decreased when the Fe(NO3)3 concentration was increased. Therefore, to interpret the polyimide film polishing rate depending on the Fe(NO3)3 concentration, as observed in Figure 1, further observation of the dependence of the chemically dominant polishing rate on the Fe(NO3)3 concentration is required.
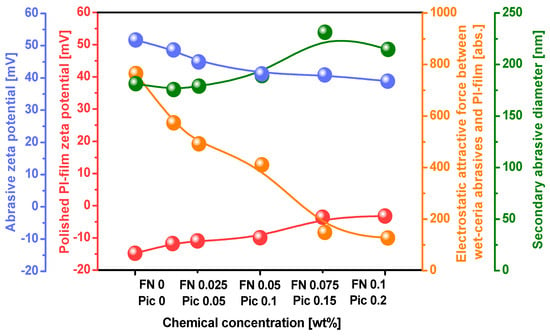
Figure 2.
Properties of polyimide film CMP slurry: the secondary abrasive diameter and zeta potentials of the wet ceria abrasives and the polished surface of the polyimide film, varying depending on the Fe(NO3)3 and dispersant (i.e., picolinic acid) concentrations. The polyimide film CMP slurry was titrated at a pH of 5. The electrostatic attractive force was calculated relative to the zeta potentials of the wet ceria abrasives and the polished surface of the polyimide film.
The chemically dominant polishing rate is primarily determined by the adsorption degree of the CMP slurry on the polished surface of the polyimide film (i.e., called contact angle or hydrophilicity) and the chemical reaction at the interface between the CMP slurry chemical and the polished surface of polyimide during CMP (i.e., the deformation of the polished surface of the polyimide film). To measure the slurry adsorption degree on the polished surface of the polyimide film, on changing the Fe(NO3)3 concentration in the CMP slurry, the contact angle was immediately estimated after dropping 0.01 mL of the slurry onto the polished surface of the polyimide film. The contact angle without Fe(NO3)3 was 36°, as presented in Figure 3(i). When the Fe(NO3)3 concentration of the CMP slurry was increased from 0 to 0.10 wt%, the contact angle decreased from 36° to 28°. This result indicates that the hydrophilicity of the polished surface of the polyimide film also increases when the Fe(NO3)3 concentration of the slurry increases. In general, a decrease in the contact angle of the CMP slurry on the polished film’s surface means an increase in the hydrophilicity. This result suggests that an increase in the Fe(NO3)3 concentration in the CMP slurry enhances the chemical reaction at the interface between the CMP slurry chemical and the polished surface of the polyimide film. To check this result, the dependency of the CMP slurry’s viscosity on the Fe(NO3)3 concentration was observed using a viscosity meter. The slurry’s viscosity remained constant despite the increase in the Fe(NO3)3 concentration, as presented in Figure S1. Therefore, the decrease in the contact angle of the CMP slurry on the surface of the polyimide film with an increasing Fe(NO3)3 concentration is due to an increase in the chemical reactions at the interface between the slurry and the polished surface of the polyimide film rather than any changes in the slurry’s viscosity.
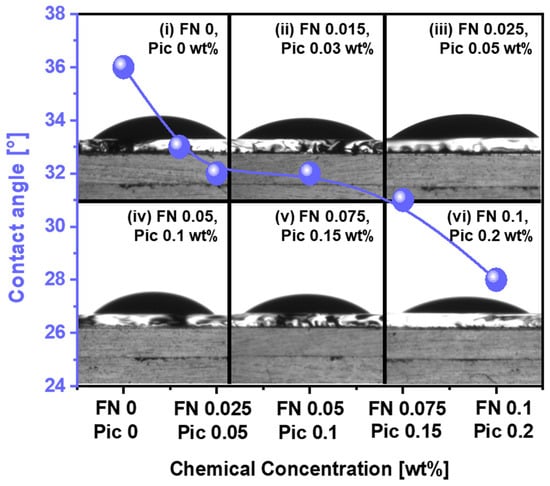
Figure 3.
The slurry adsorption degree (i.e., contact angle) depending on the Fe(NO3)3 concentration in the polyimide film CMP slurry. (i) Slurry without Fe(NO3)3 and slurries with (ii) 0.015, (iii) 0.025, (iv) 0.05, (v) 0.075, and (vi) 0.1 wt% of Fe(NO3)3 dropped onto the polished surface of polyimide.
The dependency of the contact angle on the Fe(NO3)3 concentration implies that when the Fe(NO3)3 concentration increases, the hydrophilicity of the polished surface of the polyimide film is also increased, leading to an increase in the chemically dominant polishing rate. Thus, during CMP of the polyimide film, the chemically dominant polishing rate increases with the Fe(NO3)3 concentration. However, this result was not consistent with the dependency of the polishing rate on the Fe(NO3)3 concentration shown in Figure 1. Rather, it was related to region I in Figure 1. By simultaneously considering both the dependency of the mechanically dominant polishing rate (i.e., the attractive electrostatic force) and that of the chemically dominant polishing rate (i.e., the contact angle) on the Fe(NO3)3 concentration, the dependency of the polyimide film polishing rate on the Fe(NO3)3 concentration in Figure 1 can be interpreted as follows. When the Fe(NO3)3 concentration of the CMP slurry is enhanced, the mechanically dominant polishing rate is reduced, while the chemically dominant polishing rate is increased. Consequently, the polyimide film polishing rate reaches its maximum with 0.05 wt% of Fe(NO3)3 in the CMP slurry. In region I of Figure 1, the chemically dominant polishing mechanism is more significant, whereas in region II, the mechanically dominant polishing mechanism plays a more dominant role.
3.3. The Surface Deformation of the Polyimide Film Depending on the Fe(NO3)3 Concentration of the CMP Slurry
To prove that the chemical reaction at the interface between the Fe(NO3)3 and the surface of the polyimide film (i.e., the deformation of the polished surface of polyimide) increased with the Fe(NO3)3 concentration of the CMP slurry, the dependency of the chemically reacted bonds in the polished surface of the polyimide film was investigated using XPS. First, the C 1s XPS intensity peak of the polished surface of the polyimide film was analyzed to measure the relative intensities of covalent bonds of C–C, C–O, and O–C–Fe. The binding energies for C–C, C–O, and O–C–Fe bonds were found to be 284.8 eV, 288.52 eV, and 282.46 eV, respectively, as presented in Figure 4a [33,34]. When the Fe(NO3)3 concentration was increased up to 0.1 wt%, the relative bonding intensity of the C–C bond significantly decreased from 73,658 a.u. to 26,415 a.u., while the relative intensity of the C–O bond slightly increased from 7242 a.u. to 8523 a.u. In addition, the relative bonding intensity of the O–C–Fe bond noticeably increased from 0 a.u. to 5041 a.u., as presented in Figure 4b. In general, the surface of the polyimide film prior to CMP was composed of copolymers of pyromellitimide and diphenyl ether, with C–C, C–O, and C=O covalent bonds. Due to the covalent bonds in the polyimide film’s surface, the chemical reaction at the interface between the surface of the polyimide film and the CMP slurry was limited, resulting in a low polishing rate during CMP. However, the newly designed CMP slurry containing the Fe(NO3)3 ionized toward Fe3+ and NO3−. During CMP, the Fe3+ diffused toward the polished surface of the polyimide film and chemically reacted with the polished surface of the polyimide film, resulting in the formation of O–C–Fe bonds on the polished surface of the polyimide film. Thus, the relative intensities of the O–C–Fe and C–O bonds increased with the Fe(NO3)3 concentration, while that of the C–C bonds decreased with the increasing Fe(NO3)3 concentration. Second, the O 1s XPS spectra of the polished surface of the polyimide film were observed to measure the relative intensity of the C–O and C=O bonds. The binding energies for the C–O and C=O bonds were found to be 532.58 eV and 530.2 eV, respectively, as presented in Figure 4c [35,36]. When the Fe(NO3)3 concentration was increased up to 0.1 wt%, the relative intensity of the C–O bond significantly increased from 63,122 a.u. to 77,539 a.u., while the relative intensity of the C=O bond slightly decreased from 41,593 a.u. to 35,329 a.u., as presented in Figure 4d. Comparing the C 1s XPS spectra of the C–C, C–O, and O–C–Fe bonds in Figure 4b with the O 1s XPS spectra of the C–O and C=O bonds in Figure 4d, it is evident that when the Fe(NO3)3 concentration was increased, the relative intensities of the C–C bond and the C=O bond evidently decreased, whereas the relative intensities of the C–O bond and the O–C–Fe bond obviously increased. This result indicates that during CMP, the Fe3+ in the CMP slurry would undergo chemical reactions with the surface of the polyimide film, i.e., forming O–C–Fe bonds, reducing the C–C bonds, and increasing the C–O bonds via an SN2 reaction, a ring-opening reaction, and a proton transfer reaction [14,15,16,17,18,19,20,21,22,23]. This mechanism will be explained in detail later. Third, the N 1s XPS spectra of the polished surface of the polyimide film were investigated to estimate the relative intensity of the C–NH bond with a binding energy of 399.5 eV, as presented in Figure 4e [37,38]. When the Fe(NO3)3 concentration was increased up to 0.1 wt%, the relative intensity of the C-NH bond notably increased from 1903 a.u. to 7658 a.u., as presented in Figure 4f. This result indicates that prior to CMP, the surface of the polyimide film contains C–N–C bonds within the pyromellitimide structure. During CMP, the Fe3+ in the slurry chemically reacts with the surface of the polyimide film, breaking the C–N–C bonds and forming strongly positively charged 1,2,4,5-benzenetetracarbony iron and slightly negatively charged 4,4′-oxydianiline (ODA). The H+ in the CMP slurry then reacts with the nitrogen atoms in ODA, forming C–NH bonds on the polished surface of the polyimide film. Thus, the relative intensity of the C–NH bond increases with the Fe(NO3)3 concentration. Finally, the Fe 2p XPS spectra of the polished surface of the polyimide film were analyzed to calculate the relative intensities of Fe–O @ Fe 2p 3/2, Fe–C and Fe @ Fe 2p 3/2, and Fe–O @ Fe 2p 1/2 bonds. The binding energies were found to be 710.16 eV, 708.45 eV, 716.06 eV, and 724.11 eV, respectively, as presented in Figure 4g [39,40]. These bonds were not observed in the CMP slurry without Fe(NO3)3, but their relative intensities increased almost linearly when the Fe(NO3)3 concentration was increased, as presented in Figure 4h. The sequence of a higher XPS peak was followed by Fe–O @ Fe 2p 3/2, Fe–C and Fe @ Fe 2p 3/2, and Fe–O @ Fe 2p 1/2. This result implies that the dissolved Fe3+ diffuses to the surface of the polyimide film during CMP and undergoes chemical reactions, forming Fe-O @ Fe 2p 3/2 and Fe–C bonds. Thus, the XPS peak intensities of Fe–O @ Fe 2p 3/2, Fe–C and Fe @ Fe 2p 3/2, and Fe–O @ Fe 2p 1/2 increased with the Fe(NO3)3 concentration. In summary, when the CMP slurry contains Fe(NO3)3 in a slightly acidic environment (i.e., at a pH of 5), the Fe(NO3)3 dissociates into Fe3+ and NO3−, which diffuse and adsorb onto the surface of the polyimide film. During CMP, the Fe3+ chemically reacts with the C–C, C–O, C=O, and C–N bonds in the copolymer structure of the pyromellitimide and diphenyl ether on the surface of the polyimide film. These changes lead to the surface deformation of the polyimide film into strongly positively charged 1,2,4,5-benzenetetracarbonyliron and slightly negatively charged ODA, thereby increasing the chemically dominant polishing rate of the surface of the polyimide film. This mechanism will be reviewed in detail later.
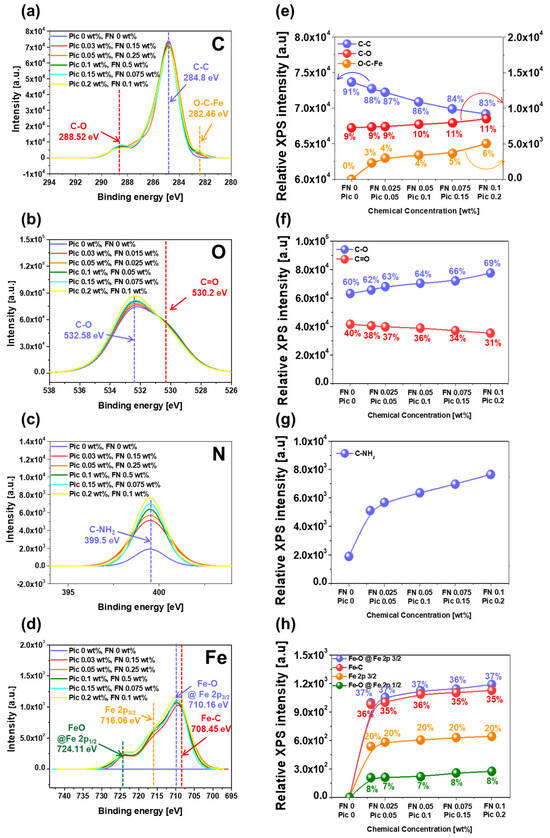
Figure 4.
Chemical deformation of the polished surface of polyimide depending on the Fe(NO3)3 concentration of the slurry. (a) C 1s, (b) O 1s, (c) N 1s, and (d) Fe 2p XPS spectra. (e) Relative C–C, C–O, and O–C–Fe, (f) C–O and C=O, (g) C–NH, and (h) Fe–O @ Fe 2p 3/2, Fe–C, Fe 2p 3/2, and Fe–O @ Fe 2p 2/1 bond intensities.
3.4. The Deformation Mechanism of the Surface of the Polyimide Film During CMP and the Polishing Rate Enhancement via the Effect of Fe(No3)3
To explain the mechanism according to which the chemical reaction at the interface between the Fe(NO3)3 and the surface of the polyimide film increased the chemically dominant polishing rate in CMP of the polyimide film, a step-by-step analysis of the reaction process was conducted. First, when Fe(NO3)3 is added to the CMP slurry, which consists of H2O, H+, and positively charged (~51.7 mV) wet ceria abrasives at a pH of 5, the Fe(NO3)3 dissociates into Fe3+ and NO3−, as presented in Figure 5a. Notably, when the Fe(NO3)3 concentration is increased, the concentration of Fe3+ in the slurry also increases. Second, before CMP, the polyimide film is a copolymer composed of pyromellitimide and diphenyl ether, containing strong C–C, C–O, C=O, and C–N covalent bonds, as presented in Figure 5b. During CMP, the positively charged Fe3+ diffuses to the negatively charged polished surface of the polyimide film through attractive electrostatic interactions and adsorbs onto the surface. After adsorption, the Fe3+ encounters the wet ceria abrasives and engages in the rubbing process, leading to polishing, as presented in Figure 5b. Third, the friction energy generated during polishing causes the adsorbed Fe3+ ions to react with the C=O bonds in the pyromellitimide, which is an SN2 reaction. In this reaction, the Fe3+ ion donates an electron to the oxygen atom in the C=O bond, creating a singly negatively charged O–C–Fe4+ bond, as presented in Figure 5c. Fourth, the oxygen atom in the O–C–Fe4+ bond transfers its non-bonding electrons to the nitrogen atom in the O–C–N–C bond, resulting in the de-bonding of the O–C–N bond and the formation of a ring-opening reaction, creating a singly negatively charged pyromellitimide structure composed of N–C2 bonds and O=C–Fe4+ bonds. The negatively charged N–C2 bond then forms a covalent bond with the diphenyl ether, as presented in Figure 5d. Fifth, the negatively charged N–C2 bond in pyromellitimide reacts with the H+ ions in the slurry through a proton transfer reaction, forming a H–N–C2 bond. One of the carbon atoms in the H–N–C2 bond forms a double covalent bond with an oxygen atom, resulting in the H–N–C2=O bond. The doubly covalently bonded oxygen atom in the H–N–C2=O bond undergoes further reactions through the SN2 reaction, as presented in Figure 5e,f. Sixth, a similar chemical reaction occurs, as presented in Figure 5c,d, leading to the de-bonding of the negatively charged diphenyl ether to the positively charged 4,5-dicarbonyl-iron phthalimide, as presented in Figure 5g. This reaction results in the formation of the negatively charged 4-aminophenyl ether and the positively charged 4,5-dicarbonyl-iron-phthalimide. Since the polyimide film is composed of the copolymers of pyromellitimide and diphenyl ether, the reactions described in Figure 5a–g are repeated in Figure 5h,i. As a result, the polished surface of the polyimide film is converted into slightly negatively charged ODA (4,4′-oxydianiline) and strong positively charged 1,2,4,5-benzenetetracabonyliron with covalent bonds to four Fe4+ ions. The 1,2,4,5-benzenetetracarbonyliron exhibits a strong positive charge due to its covalent bonding with four Fe4+ ions, whereas the ODA remains doubly negatively charged, reducing the zeta potential of the polished surface of the polyimide film. Comparing the film before CMP in Figure 5b with it after CMP in Figure 5j, the C–C, C–O, C=O, and C-N bonds on the surface of the polyimide film prior to CMP were deformed into C–C, C–O, C=O, C–N, and O–C–Fe bonds by CMP. Particularly, comparing between before CMP in Figure 5b and after CMP in Figure 5j, the number of C=O bonds decreased from 4 to 2 after CMP; thus, the relative intensity of the C=O bonds decreased after CMP, and it decreased noticeably with the Fe(NO3)3 concentration in the CMP slurry. Otherwise, the number of C–O bonds increased from 1 to 3 after CMP; thus, the relative intensity of the C–O bonds increased after CMP, and it increased significantly with the Fe(NO3)3 concentration. Moreover, O–C–Fe bonds were newly produced after CMP; thus, they increased significantly with the Fe(NO3)3 concentration in the CMP slurry. Furthermore, the number of C–N–H bonds increased from 0 to 2; thus, the relative intensity of the C–N–H bonds increased after CMP, and it increased remarkably with the Fe(NO3)3 concentration. All of these changes in the covalent bonds on the surface of the polyimide film after CMP are referred to as polyimide film deformation. As a result, after CMP using the slurry including Fe(NO3)3, the surface of the polyimide film, consisting of pyromellitimide–diphenyl and diphenyl ether copolymers before CMP, was deformed into slightly negatively charged 4,4′-oxydianiline (ODA) and strong positively charged 1,2,4,5-benzenetetracarbonyliron, with four Fe4+ ions.
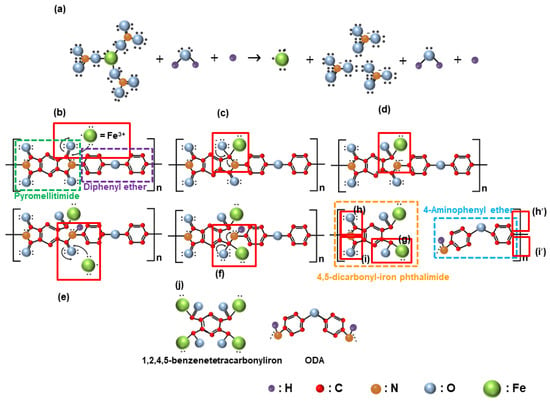
Figure 5.
The mechanism of the chemical decomposition of the hard surface of the polyimide film into a soft surface (i.e., positively charged 1,2,4,5-benzenetetracarbonyliron and negatively charged ODA) through CMP using the slurry containing Fe(NO3)3 (a–j).
Before CMP, the surface of the polyimide film had a strong negative zeta potential of −15.0 mV. However, during CMP of the polyimide film, when the Fe(NO3)3 concentration was increased up to 0.1 wt%, the polished surface of the polyimide film became more positively charged, reducing the zeta potential from −15.0 mV to −10.0 mV, as presented in Figure 2. Moreover, before CMP, the wet ceria abrasive in the CMP slurry had a strong positive zeta potential of +51.7 mV. During CMP of the polyimide film, when the Fe(NO3)3 concentration in the slurry was increased up to 0.1 wt%, the wet ceria abrasives in the CMP slurry became more negatively charged, reducing the zeta potential from +51.7 mV to +39.0 mV, as presented in Figure 2. Thus, the attractive electrostatic force at the interface between the positively charged wet ceria abrasives and the negatively charged surface of the polyimide film during CMP evidently decreased with an increasing Fe(NO3)3 concentration. This result showed that the mechanically dominant polishing rate of the polyimide film decreased noticeably with the increasing Fe(NO3)3 concentration in the CMP slurry. In addition, before CMP, the surface of the polyimide film was composed of strong covalent bonds (i.e., C–C, C–O, C=O, and C–N bonds) in the pyromellitimide–diphenyl ether copolymer, resulting in a low polishing rate of approximately 700 nm/min. However, during CMP, the addition of the Fe(NO3)3 to the slurry caused the surface of the polyimide film to undergo surface deformation, converting the copolymer structure into slightly negatively charged ODA and strong positively charged 1,2,4,5-benzenetetracarbonyliron. As a result, the hardness of the polished surface of the polyimide film reduced so that the chemically dominant polishing rate increased. Therefore, when the Fe(NO3)3 concentration in the polyimide film CMP slurry was increased, the chemically dominant polishing rate also increased significantly. However, as a trade-off between the mechanically dominant and chemically dominant polishing rates depending on the Fe(NO3)3 concentration, the maximum polishing rate of approximately 1500 nm/min occurred at a specific Fe(NO3)3 concentration of 0.05 wt%, as presented in Figure 1. Note that the amount of passivation of Fe3+, i.e., reducing the PI film polishing rate, increased slightly with the concentration of the ferric catalyst in the CMP slurry, as shown in Figure 4. Otherwise, the electrostatic repulsive force, i.e., decreasing the PI film polishing rate, decreased remarkably with the ferric catalyst concentration in the CMP slurry, as shown in Figure 2. Thus, in region II, the decrease in the PI film polishing rate is mainly associated with a decrease in the electrostatic repulsive force rather than an increase in the amount of passivation.
4. Conclusions
The continuous scaling-down of memory and logic devices has been essential to improve their operation speeds and reduce their power consumption. However, the rapid increase in the complexity of the fabrication process has slowed down the scaling process, pushing advanced memory and logic devices toward their physical limits. To address these challenges, heterogeneous 2.5D and 3D packaging technology has emerged as a promising solution. In 2.5 and 3D packaging, the redistribution layer (RDL) requires the use of polyimide films with a micrometer-scale thickness. However, spin-coating polyimide films at such thicknesses results in a significant surface topography, necessitating chemical–mechanical planarization (CMP) for effective planarization. Achieving high polishing rates (e.g., >1000 nm/min) is critical for the CMP of polyimide films to meet the industrial requirements. Polyimide films, composed of pyromellitimide and diphenyl ether copolymers, possess strong covalent bonds that contribute to their high surface hardness (i.e., 0.33 GPa). As a result, relying solely on mechanically dominant polishing yields insufficient removal rates, typically below 800 nm/min. Thus, it is essential to design CMP slurry with effective polishing rate accelerators to enhance the polyimide film polishing rate. In this study, Fe(NO3)3 was introduced into the polyimide film CMP slurry as a polishing rate accelerator. The Fe3+ was adsorbed onto the surface of the polyimide film due to the attractive electrostatic force between the positively charged Fe3+ ions and the negatively charged polyimide film. During polishing, the wet ceria abrasives induce further surface reactions, including SN2 substitution, ring-opening, and proton transfer reactions, which deform the surface of the polyimide film by converting the pyromellitimide and diphenyl ether groups into strongly positively charged 1,2,4,5-benzenetetracarbonyliron and weakly negatively charged ODA moieties. This deformation reduces the surface hardness of the polyimide film, thereby increasing the chemically dominant polishing rate. However, the NO3− ions generated from the dissociation of Fe(NO3)3 adsorbed onto the positively charged wet ceria abrasives, reducing their positive zeta potential. Additionally, the formation of O–C–Fe4+ bonds on the deformed surface of the polyimide film diminishes the negative zeta potential of the polyimide film. As a result, the attractive electrostatic force at the interface between the positively charged wet ceria abrasives and the negatively charged deformed surface of the polyimide film weakens, thereby decreasing the mechanically dominant polishing rate. This study demonstrates that increasing the Fe(NO3)3 concentration in the CMP slurry enhances the chemically dominant polishing rate while reducing the mechanically dominant polishing rate. Therefore, the overall polishing performance depends on achieving an optimal balance between these two rates. The results show that at a specific Fe(NO3)3 concentration (i.e., 0.05 wt%), the maximum polyimide film removal rate (i.e., 1465 nm/min) can be achieved. This finding provides a valuable basis for optimizing CMP slurries in advanced RDL fabrication, enabling the efficient planarization of polyimide films in heterogeneous 3D packaging applications.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/nano15060425/s1. Figure S1: Dependency of the slurry viscosity on the ferric catalyst concentration for the PI film CMP slurry; Figure S2: Dependency of PI film polishing rates on the accelerator types in the slurries including ethanol amine, ammonium persulfate, periodic acid, and Fe(NO3)3; Figure S3: Dependency of the PI film polishing rates on the frequency of CMP.
Author Contributions
Conceptualization: H.-S.K., M.-H.H. and J.-G.P. Methodology: I.-H.H. Software: M.-H.K. Validation: P.-S.K. Formal analysis: M.-U.J. Investigation: H.-S.K., M.-H.H., M.-H.K., W.-H.J., K.-C.C. and M.-J.K. Data curation: M.-J.K. Writing—original draft preparation: M.-H.H. Writing—review and editing: H.-S.K. and J.-G.P. Supervision: J.-G.P. and J.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the MOTIE (Ministry of Trade, Industry & Energy) (1415180388) and the KSRC (Korea Semiconductor Research Consortium) (20019474) support program for the development of future semiconductor devices, as well as by the BK21 FOUR (Fostering Outstanding Universities for Research) program through the National Research Foundation (NRF) funded by the Ministry of Education of Korea, under the artificial intelligence semiconductor support program to nurture the best talents (IITP-(2023)-RS-2023-00253914) grant funded by the Korean government (MSIT) and the Samsung Electronics University R&D program.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Conflicts of Interest
The authors declare no conflicts of interest. The funding sponsors had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, and in the decision to publish the results.
References
- Yoneda, S.; Adachi, K.; Kobayashi, K.; Matsukawa, D.; Sasaki, M.; Itabashi, T.; Shirasaka, T.; Shibata, T. A Novel Photosensitive Polyimide Adhesive Material for Hybrid Bonding Processing. In Proceedings of the 2021 IEEE 71st Electronic Components and Technology Conference (ECTC), Virtual Event, 1 June–4 July 2021; pp. 680–686. [Google Scholar] [CrossRef]
- Kim, J.-Y.; Kim, T.; You, J.; Kim, K.; Moon, B.M.; Sohn, K.; Jung, S.-O. An Energy-Efficient Design of TSV I/O for HBM with a Data Rate up to 10 Gb/s. IEEE J. Solid-State Circuits 2023, 58, 1–11. [Google Scholar] [CrossRef]
- Kim, M.J.; Lee, S.H.; Suk, K.L.; Jang, J.G.; Jeon, G.J.; Choi, J.I.; Yun, H.J.; Hong, J.; Choi, J.Y.; Lee, W.J.; et al. Novel 2.5D RDL Interposer Packaging: A Key Enabler for the New Era of Heterogenous Chip Integration. In Proceedings of the 2021 IEEE 71st Electronic Components and Technology Conference (ECTC), Virtual Event, 1 June–4 July 2021; pp. 321–326. [Google Scholar] [CrossRef]
- Yu, C.H.; Yen, L.J.; Hsieh, C.Y.; Hsieh, J.S.; Chang, V.C.Y.; Hsieh, C.H.; Liu, C.S.; Wang, C.T.; Yee, K.; Yu, D.C.H. High Performance, High Density RDL for Advanced Packaging. In Proceedings of the 2018 IEEE 68th Electronic Components and Technology Conference (ECTC), San Diego, CA, USA, 28 May–1 June 2018; pp. 587–593. [Google Scholar] [CrossRef]
- Son, K.; Kim, G.; Park, Y.B.; Ryu, H.K. Effect of Dielectric Process on the Interfacial Adhesion of RDL for FOWLP. In Proceedings of the 2020 IEEE 70th Electronic Components and Technology Conference (ECTC), Orlando, FL, USA, 3–30 June 2020; pp. 348–353. [Google Scholar] [CrossRef]
- You, S.H.; Jeon, S.; Oh, D.; Kim, K.; Kim, J.; Cha, S.Y.; Kim, G. Advanced Fan-Out Package SI/PI/Thermal Performance Analysis of Novel RDL Packages. In Proceedings of the 2018 IEEE 68th Electronic Components and Technology Conference (ECTC), San Diego, CA, US, 29 May–1 June 2018; pp. 1295–1301. [Google Scholar] [CrossRef]
- Kim, H.-G.; An, Y.-M.; Moon, D.-K.; Park, J.-G. Effect of Chemicals and Slurry Particles on Chemical Mechanical Polishing of Polyimide. Jpn. J. Appl. Phys. 2000, 39, 1085–1090. [Google Scholar] [CrossRef]
- Jeong, G.-P.; Park, J.-S.; Lee, S.-J.; Kim, P.-S.; Han, M.-H.; Hong, S.-W.; Kim, E.-S.; Park, J.-H.; Choo, B.-K.; Kang, S.-B.; et al. Polymer link breakage of polyimide-film-surface using hydrolysis reaction accelerator for enhancing chemical–mechanical-planarization polishing-rate. Sci. Rep. 2022, 12, 1–10. [Google Scholar] [CrossRef]
- Huang, S.; Zhang, Y.; Lai, X.; Lv, X.; Li, J.; Qiu, S.; Zhang, G.; Sun, R. A comprehensive study on the effect of molecular chain flexibility on the low-temperature curing ability of polyimides. J. Mater. Chem. C 2023, 12, 177–186. [Google Scholar] [CrossRef]
- Sang, H.; Zhou, J.; Hu, F.; Geng, K.; Feng, S.; Wang, H.; Zhuang, Y. Highly Selective Soluble Polyimides Simultaneously Containing Benzimidazole and Hydroxyl Groups for Membrane-Based Gas Separation. Macromolecules 2024, 57, 5929–5940. [Google Scholar] [CrossRef]
- Lin, Y.H.; Yew, M.C.; Chen, S.M.; Liu, M.S.; Kavle, P.; Lai, T.M.; Yu, C.T.; Hsu, F.C.; Chen, C.S.; Fang, T.J.; et al. Multilayer RDL Interposer for Heterogeneous Device and Module Integration. In Proceedings of the 2019 IEEE 69th Electronic Components and Technology Conference (ECTC), Las Vegas, NV, USA, 28–31 May 2019; pp. 931–936. [Google Scholar] [CrossRef]
- Lia, J.; Tsai, F.L.; Li, J.; Pan, G.; Chan, M.H.; Zheng, L.; Chen, S.; Kao, N.; Lai, D.; Wan, K.; et al. Large Size Multilayered Fan-Out RDL Packaging for Heterogeneous Integration. In Proceedings of the 2021 IEEE 23rd Electronics Packaging Technology Conference (EPTC), Singapore, 7–9 December 2021; pp. 239–243. [Google Scholar] [CrossRef]
- Rao, V.S.; Chong, C.T.; Ho, D.; Zhi, D.M.; Choong, C.S.; Ps, S.L.; Ismael, D.; Liang, Y.Y. Development of High Density Fan Out Wafer Level Package (HD FOWLP) with Multi-Layer Fine Pitch RDL for Mobile Applications. In Proceedings of the 2016 IEEE 66th Electronic Components and Technology Conference (ECTC), Las Vegas, NV, USA, 31 May–3 June 2016; pp. 1522–1529. [Google Scholar] [CrossRef]
- Capurso, M.; Gette, R.; Radivoy, G.; Dorn, V. The SN2 Reaction: A Theoretical-Computational Analysis of a Simple and Very Interesting Mechanism. Proceedings 2019, 41, 81. [Google Scholar] [CrossRef]
- Zhang, X.; Ren, J.; Tan, S.M.; Tan, D.; Lee, R.; Tan, C.H. An Enantioconvergent Halogenophilic Nucleophilic Substitution Reaction. Science 2019, 363, 400–404. [Google Scholar] [CrossRef]
- Hagan, D.; Schmidberger, J.W. Enzymes that catalyse SN2 reaction mechanisms. Nat. Prod. Rep. 2010, 27, 900–918. [Google Scholar] [CrossRef] [PubMed]
- Hennig, C.; Schmatz, S. Mechanisms of SN2 reactions: Insights from a nearside/farside analysis. Phys. Chem. Chem. Phys. 2015, 17, 26670–26676. [Google Scholar] [CrossRef]
- Hamlin, T.A.; Swart, M.; Bickelhaupt, F.M. Nucleophilic Substitution (SN2): Dependence on Nucleophile, Leaving Group, Central Atom, Substituents, and Solvent. ChemPhysChem 2018, 19, 1315–1330. [Google Scholar] [CrossRef]
- Jain, I.H.; Zazzeron, L.; Gvozdenovic-Jeremic, J.; Guarani, V.; Collins, S.R.; Di Paulo, A.; Murphy, M.P.; Kampf, G.; Kocher, T.; Broaddus, C.C.; et al. Hypoxia as a Therapy for Mitochondrial Disease. Science 2016, 352, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Hansen, T.; Vermeeren, P.; De Jong, L.; Bickelhaupt, F.M.; Hamlin, T.A. SN2 versus SN2′ Competition. J. Org. Chem. 2022, 87, 8892–8901. [Google Scholar] [CrossRef] [PubMed]
- Fu, G.C. Transition-Metal Catalysis of Nucleophilic Substitution Reactions: A Radical Alternative to SN1 and SN2 Processes. ACS Central Sci. 2017, 3, 692–700. [Google Scholar] [CrossRef]
- Bahrami, F.; Zhao, Y. Rational Design and Synthesis of an Artificial Enzyme for SN2 Reactions through Micellar Imprinting. Org. Lett. 2023, 26, 73–77. [Google Scholar] [CrossRef] [PubMed]
- Samunual, P.; Bergbreiter, D.E. SN2 Reactions in Hydrocarbon Solvents Using Ammonium-Terminated Polyisobutylene Oligomers as Phase-Solubilizing Agents and Catalysts. J. Org. Chem. 2018, 83, 11101–11107. [Google Scholar] [CrossRef]
- Tamai, K.; Morinaga, H.; Doi, T.K.; Kurokawa, S.; Ohnishi, O. Analysis of Chemical and Mechanical Factors in CMP Processes for Improving Material Removal Rate. J. Electrochem. Soc. 2011, 158, H333–H337. [Google Scholar] [CrossRef]
- Chen, S.-W.; Kung, T.-M.; Liu, C.-P.; Chang, S.-C.; Cheng, Y.-L.; Wang, Y.-L. Effect of Electric Potential and Mechanical Force on Copper Electro-Chemical Mechanical Planarization. Jpn. J. Appl. Phys. 2012, 51, 036504. [Google Scholar] [CrossRef]
- Choi, S.-H.; Yim, J.; Lim, J.; Kim, S.; Jeong, Y.; Bae, K.; Seo, J.; Lee, K. Tailored electrostatic attraction force between anionic polymer and Si3N4 film in consecutive gate poly open CMP. Mater. Sci. Semicond. Process. 2024, 184, 108761. [Google Scholar] [CrossRef]
- Busnaina, A.; Lin, H.; Moumen, N.; Feng, J.-W.; Taylor, J. Particle adhesion and removal mechanisms in post-CMP cleaning processes. IEEE Trans. Semicond. Manuf. 2002, 15, 374–382. [Google Scholar] [CrossRef]
- Basim, G.; Vakarelski, I.U.; Moudgil, B.M. Role of interaction forces in controlling the stability and polishing performance of CMP slurries. J. Colloid Interface Sci. 2003, 263, 506–515. [Google Scholar] [CrossRef]
- Bae, J.-Y.; Han, M.-H.; Lee, S.-J.; Kim, E.-S.; Lee, K.; Lee, G.-S.; Bae, J.-Y.; Han, M.-H.; Lee, S.-J.; Kim, E.-S.; et al. Citation: Silicon Wafer CMP Slurry Using a Hydrolysis Reaction Accelerator with an Amine Functional Group Remarkably Enhances Polishing Rate. Nanomaterials 2022, 12, 3893. [Google Scholar] [CrossRef]
- Bae, J.-Y.; Han, M.-H.; Shim, T.-H.; Park, J.-G. Chemical mechanical planarization mechanism of epitaxially grown Ge-film for sequential integrating 3D-structured transistor cells. J. Korean Phys. Soc. 2022, 81, 1262–1268. [Google Scholar] [CrossRef]
- Yun, S.-S.; Son, Y.-H.; Jeong, G.-P.; Lee, J.-H.; Jeong, J.-H.; Bae, J.-Y.; Kim, S.-I.; Park, J.-H.; Park, J.-G. Dishing-free chemical mechanical planarization for copper films. Colloids Surf. A Physicochem. Eng. Asp. 2021, 616, 126143. [Google Scholar] [CrossRef]
- Lee, J.M.; Lee, J.C.; Kim, S.I.; Lee, S.J.; Bae, J.Y.; Park, J.H.; Park, J.G. Surface Transformation of Spin-on-Carbon Film via Forming Carbon Iron Complex for Remarkably Enhanced Polishing Rate. Nanomaterials 2022, 12, 969. [Google Scholar] [CrossRef]
- Davydov, V.; Rakhmanina, A.; Kireev, I.; Alieva, I.; Zhironkina, O.; Strelkova, O.; Dianova, V.; Samani, T.D.; Mireles, K.; Hocine Yahia, L.; et al. Solid state synthesis of carbon-encapsulated iron carbide nanoparticles and their interaction with living cells. J. Mater. Chem. B 2014, 2, 4250–4261. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Feng, J.; Liu, W.; Yin, L.; Chen, W.; Shi, C.; Song, J. Fe3C Decorated Folic Acid-Derived Graphene-Like Carbon as a Polysulfide Catalyst for High-Performance Lithium-Sulfur Battery. Batteries 2023, 9, 296. [Google Scholar] [CrossRef]
- Roy, A.; Mukhopadhyay, A.K.; Das, S.C.; Bhattacharjee, G.; Majumdar, A.; Hippler, R. Surface Stoichiometry and Optical Properties of Cux-TiyCz Thin Films Deposited by Magnetron Sputtering. Coatings 2019, 9, 551. [Google Scholar] [CrossRef]
- Díez, N.; Śliwak, A.; Gryglewicz, S.; Grzyb, B.; Gryglewicz, G. Enhanced Reduction of Graphene Oxide by High-Pressure Hydrothermal Treatment. RSC Adv. 2015, 5, 81831–81837. [Google Scholar] [CrossRef]
- Lara, G.G.; Andrade, G.F.; Cipreste, M.F.; da Silva, W.M.; Gastelois, P.L.; Gomes, D.A.; de Miranda, M.C.; Macedo, W.A.d.A.; Neves, M.J.; de Sousa, E.M.B. Protection of normal cells from irradiation bystander effects by silica-flufenamic acid nanoparticles. J. Mater. Sci. Mater. Med. 2018, 29, 1–14. [Google Scholar] [CrossRef]
- Ravi, S.; Zhang, S.; Lee, Y.-R.; Kang, K.-K.; Kim, J.-M.; Ahn, J.-W.; Ahn, W.-S. EDTA-functionalized KCC-1 and KIT-6 mesoporous silicas for Nd3+ ion recovery from aqueous solutions. J. Ind. Eng. Chem. 2018, 67, 210–218. [Google Scholar] [CrossRef]
- Lee, H.; Lee, W.-J.; Park, Y.-K.; Ki, S.J.; Kim, B.-J.; Jung, S.-C. Liquid Phase Plasma Synthesis of Iron Oxide Nanoparticles on Nitrogen-Doped Activated Carbon Resulting in Nanocomposite for Supercapacitor Applications. Nanomaterials 2018, 8, 190. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Xiao, D.; Ma, J.; Chen, C.; Li, K.; Ma, J.; Liao, Y.; Zheng, L.; Zuo, X. The self-template synthesis of highly efficient hollow structure Fe/N/C electrocatalysts with Fe-N coordination for the oxygen reduction reaction. RSC Adv. 2018, 8, 24509–24516. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).