Unlocking the Potential of Na2Ti3O7-C Hollow Microspheres in Sodium-Ion Batteries via Template-Free Synthesis
Abstract
1. Introduction
2. Materials and Methods
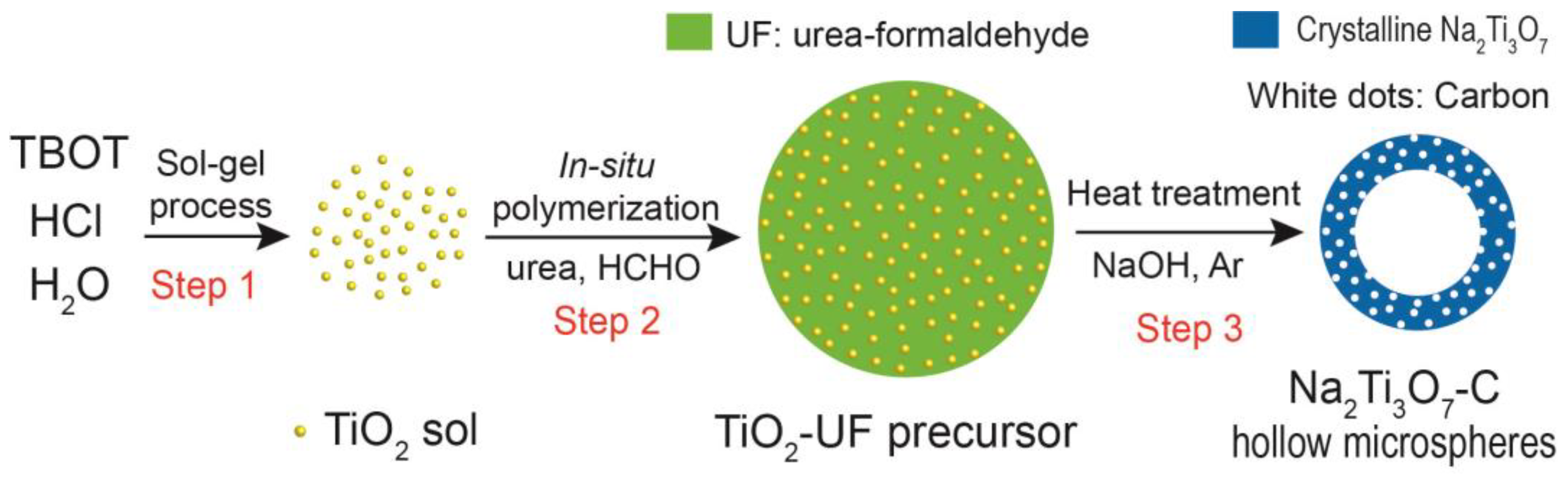
2.1. Synthesis of TiO2-UF Precursor
2.2. Synthesis of Na2Ti3O7-C Hollow Microspheres
2.3. Synthesis of Na2Ti3O7 Hollow Microspheres
2.4. Synthesis of Na2Ti3O7-C Solid Microspheres
2.5. Characterization
2.6. Electrochemical Measurements
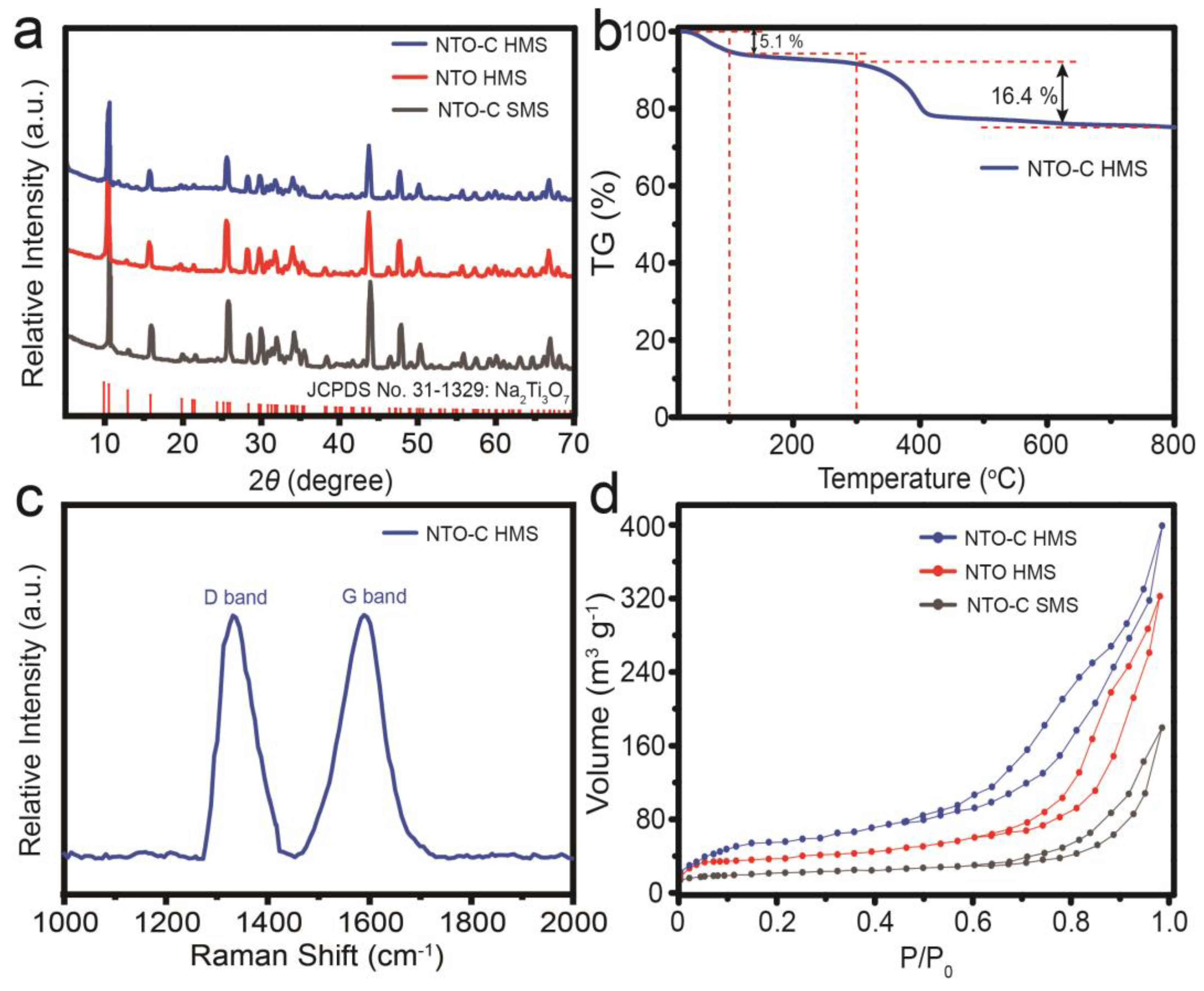
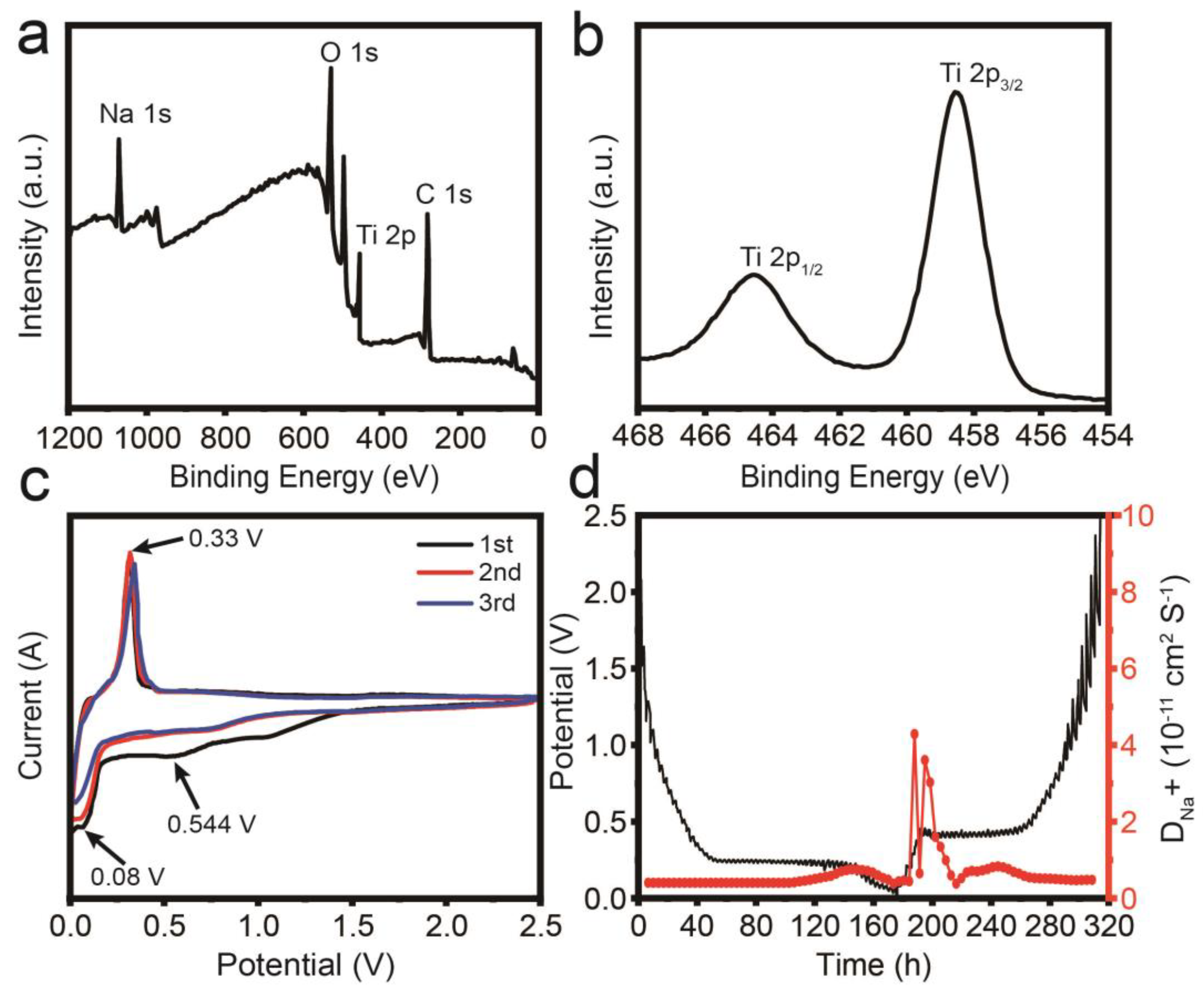
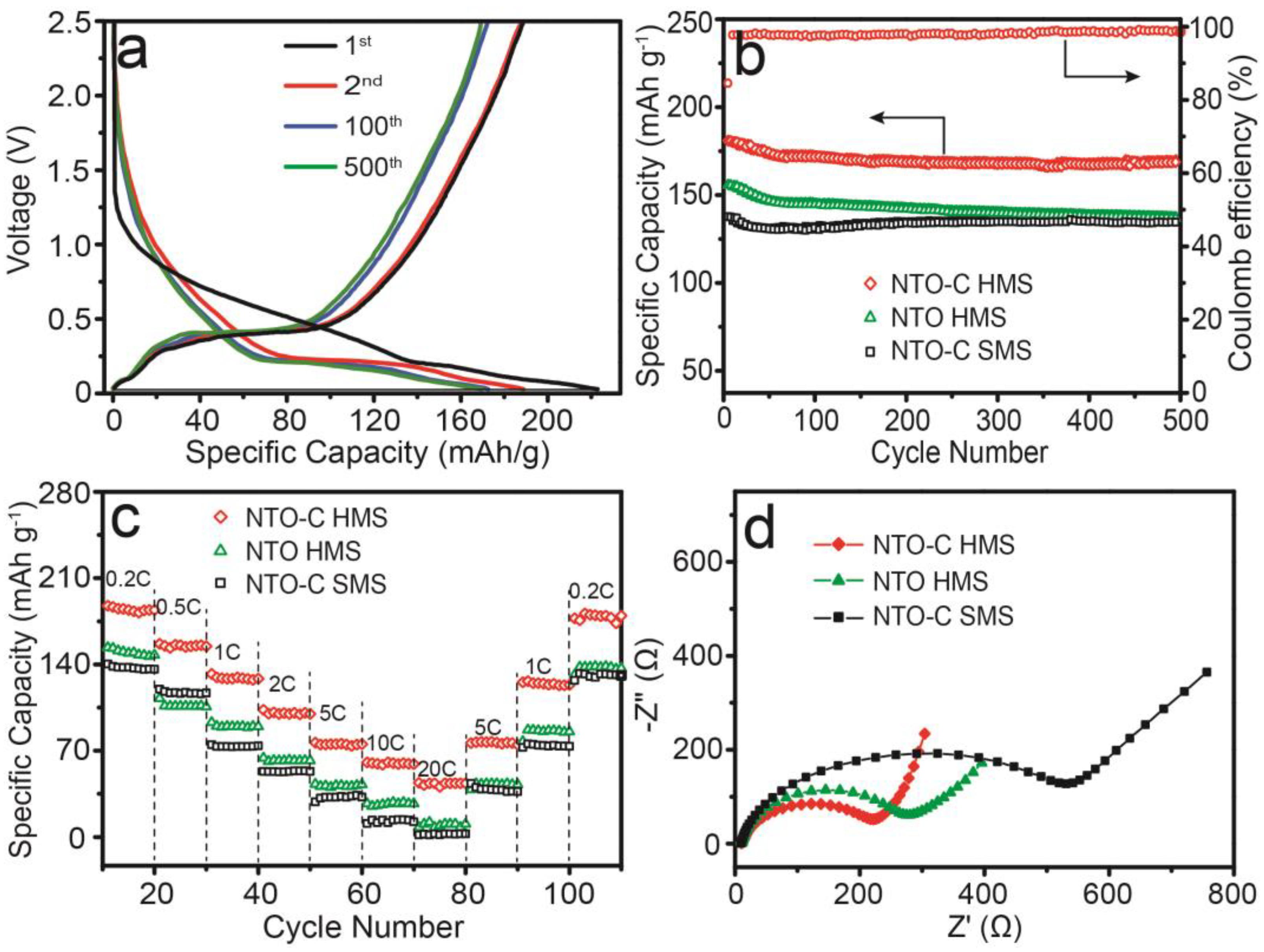
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schmuch, R.; Wagner, R.; Hörpel, G.; Placke, T.; Winter, M. Performance and cost of materials for lithium-based rechargeable automotive batteries. Nat. Energy 2018, 3, 267–278. [Google Scholar] [CrossRef]
- Fan, E.; Li, L.; Wang, Z.; Lin, J.; Huang, Y.; Yao, Y.; Chen, R.; Wu, F. Sustainable Recycling Technology for Li-Ion Batteries and Beyond: Challenges and Future Prospects. Chem. Rev. 2020, 120, 7020–7063. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Mao, B.; Stoliarov, S.I.; Sun, J. A review of lithium ion battery failure mechanisms and fire prevention strategies. Prog. Energy Combust. Sci. 2019, 73, 95–131. [Google Scholar] [CrossRef]
- Hwang, J.-Y.; Myung, S.-T.; Sun, Y.-K. Sodium-ion batteries: Present and future. Chem. Soc. Rev. 2017, 46, 3529–3614. [Google Scholar] [CrossRef]
- Nayak, P.K.; Yang, L.; Brehm, W.; Adelhelm, P. From Lithium-Ion to Sodium-Ion Batteries: Advantages, Challenges, and Surprises. Angew. Chem. Int. Ed. 2018, 57, 102–120. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, Z.; Zheng, Y.; Luo, Y.; Jiang, X.; Wang, Y.; Wang, Z.; Wu, Y.; Zhang, Y.; Liu, X.; et al. Sodium-Ion Battery at Low Temperature: Challenges and Strategies. Nanomaterials 2024, 14, 1604. [Google Scholar] [CrossRef]
- Hu, J.; Li, X.; Liang, Q.; Xu, L.; Ding, C.; Liu, Y.; Gao, Y. Optimization Strategies of Na3V2(PO4)3 Cathode Materials for Sodium-Ion Batteries. Nano-Micro Lett. 2024, 17, 33. [Google Scholar] [CrossRef]
- Mosa, J.; García-García, F.J.; González-Elipe, A.R.; Aparicio, M. New Insights on the Conversion Reaction Mechanism in Metal Oxide Electrodes for Sodium-Ion Batteries. Nanomaterials 2021, 11, 966. [Google Scholar] [CrossRef]
- Wang, L.; Wei, Z.; Mao, M.; Wang, H.; Li, Y.; Ma, J. Metal oxide/graphene composite anode materials for sodium-ion batteries. Energy Storage Mater. 2019, 16, 434–454. [Google Scholar] [CrossRef]
- Wang, C.; Yan, J.; Li, T.; Tang, Y.; Zheng, Q.; Li, X. Single-atom generation inducing electrochemical transformation during cycling in transition metal sulfides for Na-ion batteries. Chem. Eng. J. 2025, 507, 160355. [Google Scholar] [CrossRef]
- Zhu, H.; Wang, K.; Liu, Q.; Wang, J.; Hu, T.; Hu, S. Defect-Enriched Heterojunction Coupling Nano-Scale Polyhedron with Cavity Structure to Promote Rapid and Stable Sodium Storage. Adv. Funct. Mater. 2025, 2025, 2419997. [Google Scholar] [CrossRef]
- Xia, Y.; Yang, T.; Wang, Z.; Mao, T.; Hong, Z.; Han, J.; Peng, D.-L.; Yue, G. Van der Waals Forces between S and P Ions at the CoP-C@MoS2/C Heterointerface with Enhanced Lithium/Sodium Storage. Adv. Funct. Mater. 2023, 33, 2302830. [Google Scholar] [CrossRef]
- Zhao, W.; Ma, X.; Gao, L.; Li, Y.; Wang, G.; Sun, Q. Engineering carbon-nanochain concatenated hollow Sn4P3 nanospheres architectures as ultrastable and high-rate anode materials for sodium ion batteries. Carbon 2020, 167, 736–745. [Google Scholar] [CrossRef]
- Zhang, W.; Mao, J.; Li, S.; Chen, Z.; Guo, Z. Phosphorus-Based Alloy Materials for Advanced Potassium-Ion Battery Anode. J. Am. Chem. Soc. 2017, 139, 3316–3319. [Google Scholar] [CrossRef]
- Yin, B.; He, H.; Lin, J.; Hong, Y.; Cheng, B.; Zhu, L.; He, H.; Ma, M.; Wang, J. Bichannel design inspired by membrane pump: A rate booster for the conversion-type anode of sodium-ion battery. J. Mater. Chem. A 2022, 10, 3373–3381. [Google Scholar] [CrossRef]
- Lao, M.; Zhang, Y.; Luo, W.; Yan, Q.; Sun, W.; Dou, S.X. Alloy-Based Anode Materials toward Advanced Sodium-Ion Batteries. Adv. Mater. 2017, 29, 1700622. [Google Scholar] [CrossRef]
- Dong, J.; Jiang, Y.; Wang, R.; Wei, Q.; An, Q.; Zhang, X. Review and prospects on the low-voltage Na2Ti3O7 anode materials for sodium-ion batteries. J. Energy Chem. 2024, 88, 446–460. [Google Scholar] [CrossRef]
- Li, F.; Gao, L.; Lv, Y.; Cai, B.; Wang, C.; Zhao, X.S. Doping of potassium by partial substitution of sodium in sodium trititanate for improved sodium-ion storage properties. J. Power Sources 2024, 623, 235393. [Google Scholar] [CrossRef]
- Xia, J.; Zhao, H.; Pang, W.K.; Yin, Z.; Zhou, B.; He, G.; Guo, Z.; Du, Y. Lanthanide doping induced electrochemical enhancement of Na2Ti3O7 anodes for sodium-ion batteries. Chem. Sci. 2018, 9, 3421–3425. [Google Scholar] [CrossRef]
- Jin, X.; Li, F.; Zhang, X.; Zeng, G.; Liu, X.; Cai, B.; Wang, C.; Zhao, X.S. Isovalent doping of tin in sodium trititanate for enhanced sodium-ion battery performance. J. Energy Chem. 2025, 103, 324–332. [Google Scholar] [CrossRef]
- Li, M.; Xiao, X.; Fan, X.; Huang, X.; Liu, Y.; Chen, L. Carbon coated sodium-titanate nanotube as an advanced intercalation anode material for sodium-ion batteries. J. Alloys Compd. 2017, 712, 365–372. [Google Scholar] [CrossRef]
- Zou, W.; Fan, C.; Li, J. Sodium Titanate/Carbon (NaTiO/C) Nanofibers via Electrospinning Technique as the Anode of Sodium-ion Batteries. Chin. J. Chem. 2017, 35, 79–85. [Google Scholar] [CrossRef]
- Yan, X.; Sun, D.; Jiang, J.; Yan, W.; Jin, Y. Self-assembled twine-like Na2Ti3O7 nanostructure as advanced anode for sodium-ion batteries. J. Alloys Compd. 2017, 697, 208–214. [Google Scholar] [CrossRef]
- Yan, Z.; Liu, L.; Shu, H.; Yang, X.; Wang, H.; Tan, J.; Zhou, Q.; Huang, Z.; Wang, X. A tightly integrated sodium titanate-carbon composite as an anode material for rechargeable sodium ion batteries. J. Power Sources 2015, 274, 8–14. [Google Scholar] [CrossRef]
- Chen, J.; Zhou, X.; Mei, C.; Xu, J.; Wong, C.-P. Improving the sodiation performance of Na2Ti3O7 through Nb-doping. Electrochim. Acta 2017, 224, 446–451. [Google Scholar] [CrossRef]
- Chandel, S.; Zulkifli; Kim, J.; Rai, A.K. Effect of vanadium doping on the electrochemical performances of sodium titanate anode for sodium ion battery application. Dalton Trans. 2022, 51, 11797–11805. [Google Scholar] [CrossRef]
- Song, T.; Ye, S.; Liu, H.; Wang, Y.-G. Self-doping of Ti3+ into Na2Ti3O7 increases both ion and electron conductivity as a high-performance anode material for sodium-ion batteries. J. Alloys Compd. 2018, 767, 820–828. [Google Scholar] [CrossRef]
- Pan, D.; Chen, W.; Sun, S.; Lu, X.; Wu, X.; Yu, C.; Hu, Y.-S.; Bai, Y. A high-rate capability and energy density sodium ion full cell enabled by F-doped Na2Ti3O7 hollow spheres. J. Mater. Chem. A 2022, 10, 23232–23243. [Google Scholar] [CrossRef]
- Meng, W.; Dang, Z.; Li, D.; Jiang, L. Long-Cycle-Life Sodium-Ion Battery Fabrication via a Unique Chemical Bonding Interface Mechanism. Adv. Mater. 2023, 35, e2301376. [Google Scholar] [CrossRef]
- Sun, Y.-G.; Piao, J.-Y.; Hu, L.-L.; Bin, D.-S.; Lin, X.-J.; Duan, S.-Y.; Cao, A.-M.; Wan, L.-J. Controlling the Reaction of Nanoparticles for Hollow Metal Oxide Nanostructures. J. Am. Chem. Soc. 2018, 140, 9070–9073. [Google Scholar] [CrossRef]
- Xie, J.; Liu, L.; Xia, J.; Zhang, Y.; Li, M.; Ouyang, Y.; Nie, S.; Wang, X. Template-Free Synthesis of Sb2S3 Hollow Microspheres as Anode Materials for Lithium-Ion and Sodium-Ion Batteries. Nano-Micro Lett. 2017, 10, 12. [Google Scholar] [CrossRef] [PubMed]
- Song, G.; Ryu, J.; Kim, J.C.; Lee, J.H.; Kim, S.; Wang, C.; Kwak, S.K.; Park, S. Revealing salt-expedited reduction mechanism for hollow silicon microsphere formation in bi-functional halide melts. Commun. Chem. 2018, 1, 42. [Google Scholar] [CrossRef]
- Xie, F.; Zhang, L.; Su, D.; Jaroniec, M.; Qiao, S.-Z. Na2Ti3O7@N-Doped Carbon Hollow Spheres for Sodium-Ion Batteries with Excellent Rate Performance. Adv. Mater. 2017, 29, 1700989. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Pang, Y.; Liang, J.; Ding, S. Red blood cell-like hollow carbon sphere anchored ultrathin Na2Ti3O7 nanosheets as long cycling and high rate-performance anodes for sodium-ion batteries. J. Mater. Chem. A 2018, 6, 13164–13170. [Google Scholar] [CrossRef]
- Yang, L.-P.; Lin, X.-J.; Zhang, X.; Zhang, W.; Cao, A.-M.; Wan, L.-J. General Synthetic Strategy for Hollow Hybrid Microspheres through a Progressive Inward Crystallization Process. J. Am. Chem. Soc. 2016, 138, 5916–5922. [Google Scholar] [CrossRef]
- Yang, L.-P.; Wang, Y.; Zhao, X.-Q.; Lin, X.-J.; Zhang, B.-B.; Sun, Y.-G.; Chen, S. Controlled synthesis of hollow Si-C microspheres and its potential as an anode material in lithium-ion batteries. J. Alloys Compd. 2023, 968, 171860. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Y.-G.; Hu, Y.; Dong, L.; Zhou, T.-T.; Qian, X.-Y.; Zhang, F.-J.; Shen, J.-Q.; Shan, Z.-Y.; Yang, L.-P.; Lin, X.-J. Unlocking the Potential of Na2Ti3O7-C Hollow Microspheres in Sodium-Ion Batteries via Template-Free Synthesis. Nanomaterials 2025, 15, 423. https://doi.org/10.3390/nano15060423
Sun Y-G, Hu Y, Dong L, Zhou T-T, Qian X-Y, Zhang F-J, Shen J-Q, Shan Z-Y, Yang L-P, Lin X-J. Unlocking the Potential of Na2Ti3O7-C Hollow Microspheres in Sodium-Ion Batteries via Template-Free Synthesis. Nanomaterials. 2025; 15(6):423. https://doi.org/10.3390/nano15060423
Chicago/Turabian StyleSun, Yong-Gang, Yu Hu, Li Dong, Ting-Ting Zhou, Xiang-Yu Qian, Fa-Jia Zhang, Jia-Qi Shen, Zhi-Yang Shan, Li-Ping Yang, and Xi-Jie Lin. 2025. "Unlocking the Potential of Na2Ti3O7-C Hollow Microspheres in Sodium-Ion Batteries via Template-Free Synthesis" Nanomaterials 15, no. 6: 423. https://doi.org/10.3390/nano15060423
APA StyleSun, Y.-G., Hu, Y., Dong, L., Zhou, T.-T., Qian, X.-Y., Zhang, F.-J., Shen, J.-Q., Shan, Z.-Y., Yang, L.-P., & Lin, X.-J. (2025). Unlocking the Potential of Na2Ti3O7-C Hollow Microspheres in Sodium-Ion Batteries via Template-Free Synthesis. Nanomaterials, 15(6), 423. https://doi.org/10.3390/nano15060423




