Photocatalytic Degradation of Ciprofloxacin by GO/ZnO/Ag Composite Materials
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Synthesis of Graphene Oxide (GO)
2.3. Synthesis of GO/ZnO Composite Materials
2.4. Synthesis of GO/ZnO/Ag Composite Materials
2.5. Sample Characterization
2.6. Catalytic Degradation
3. Results and Discussion
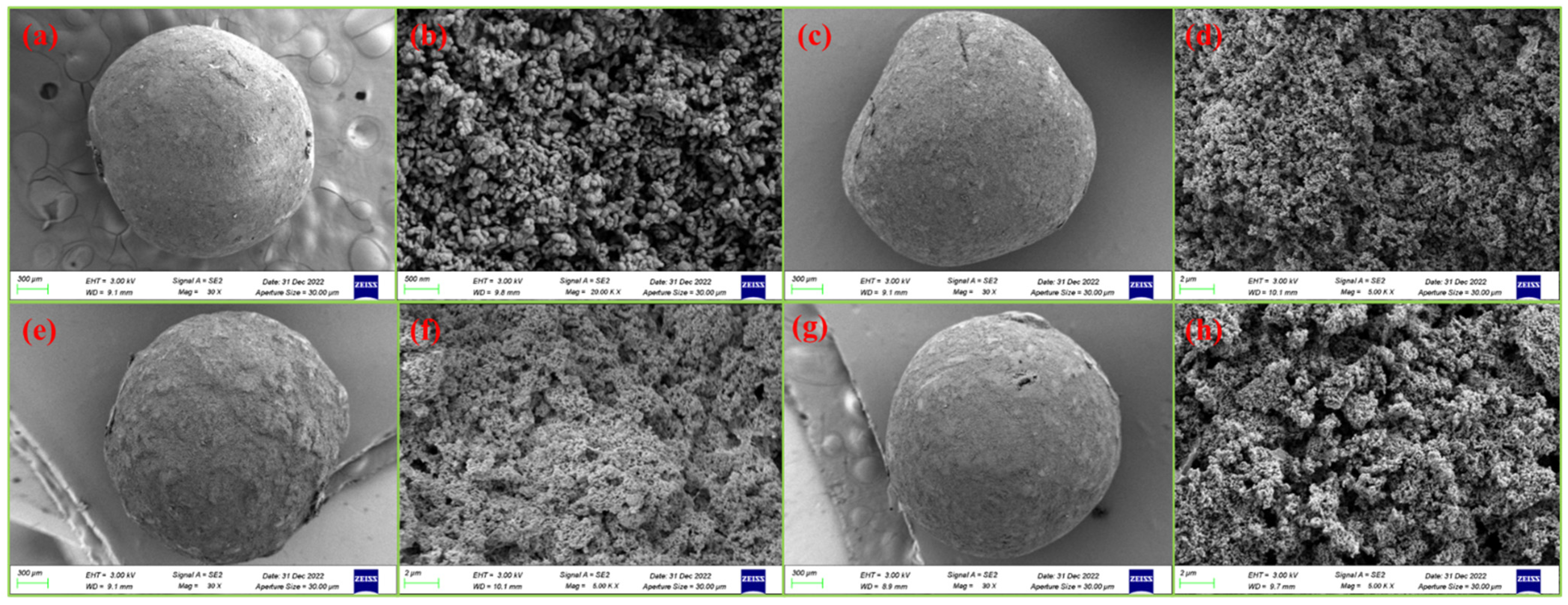
3.1. Surface Morphology Analysis (SEM)
3.2. X-Ray Photoelectron Spectroscopy (XPS) Analysis
3.3. X-Ray Diffraction (XRD) Analysis
3.4. FT-IR Analysis
3.5. Photocatalytic Activity Measurement
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, B.; Lai, C.; Zeng, G.; Huang, D. Black phosphorus, a rising star 2D nanomaterial in the post-graphene era: Synthesis, properties, modifications, and photocatalysis applications. Small 2022, 15, 1804565. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Ye, W.; Xie, M.; Seo, D.H.; Luo, J.; Wan, Y.; Bart, V.B. Environmental impacts and remediation of dye-containing wastewater. Nat. Rev. Earth Environ. 2023, 4, 785–803. [Google Scholar] [CrossRef]
- Asgharian, M.; Mehadipourghazi, M.; Khoshandam, B.; Keramati, N. Photocatalytic degradation of methylene blue with synthesized rGO/ZnO/Cu. Chem. Phys. Lett. 2019, 719, 1–7. [Google Scholar] [CrossRef]
- Motelica, L.; Oprea, O.C.; Vasile, B.S.; Ficai, A.; Ficai, D.; Andronescu, E.; Holban, A.M. Antibacterial Activity of Solvothermal Obtained ZnO Nanoparticles with Different Morphology and Photocatalytic Activity against a Dye Mixture: Methylene Blue, Rhodamine B and Methyl OrangeJournal. Int. J. Mol. Sci. 2023, 24, 5677. [Google Scholar] [CrossRef]
- Dai, C.; Liu, B. Conjugated polymers for visible-light-driven photocatalysis. Energy Environ. Sci. 2020, 13, 24–52. [Google Scholar] [CrossRef]
- Wang, S.; Guan, B.; Lou, X. Rction of ZnIn2S4-In2O3 hierarchical tubular heterostructures for efficient CO2 photoreduction. J. Am. Chem. Soc. 2018, 140, 5037–5040. [Google Scholar] [CrossRef]
- Yang, C.; Xu, H.; Shi, J.; Liu, Z.; Zhao, L. Preparation and Photocatalysis of CuO/Bentonite Based on Adsorption and Photocatalytic Activity. Materials 2021, 14, 5803. [Google Scholar] [CrossRef]
- Mourya, A.K.; Singh, R.P.; Kumar, T.; Talmale, A.S.; Gaikwad, G.S.; Wankhade, A.V. Tuning the morphologies of ZnO for enhanced photocatalytic activity. Inorg. Chem. Commun. 2023, 154, 110850. [Google Scholar] [CrossRef]
- Rabchinskii, M.K.; Sysoev, V.V.; Brzhezinskaya, M.; Solomatin, M.A.; Gabrelian, V.S.; Kirilenko, D.A.; Stolyarove, D.Y.; Saveliev, S.D.; Shvidchenko, A.V.; Cherviakova, P.D.; et al. Rationalizing Graphene–ZnO Composites for Gas Sensing via Functionalization with Amines. Nanomaterials 2024, 14, 735. [Google Scholar] [CrossRef]
- Rabell, G.O.; Cruz, M.A.; Ramirez, I.J. Hydrogen production of ZnO and ZnO/Ag films by photocatalysis and photoelectrocatalysis. Mater. Sci. Semicond. Process. 2021, 134, 105985. [Google Scholar] [CrossRef]
- Tan, X.; Zhou, S.; Tao, H.J.; Wang, W.Y.; Wan, Q.W.; Zhang, K.C. Influence of Ag on photocatalytic performance of Ag/ZnO nanosheet photocatalysts. J. Cent. South Univ. 2019, 26, 2011–2018. [Google Scholar] [CrossRef]
- Ahmad, M.; Ahmed, E.; Hong, Z.L.; Khalid, N.R.; Ahmed, W.; Elhissi, A. Graphene-Ag/ZnO nanocomposites as high performance photocatalysts under visible light irradiation. J. Alloys Compd. 2013, 577, 717–727. [Google Scholar] [CrossRef]
- Viet, H.T.T.; The, H.C.; Pham, T.N.; Pham, T.T.; Le, M.C. Synergistic Adsorption and Photocatalytic Activity under Visible Irradiation Using Ag-ZnO/GO Nanoparticles Derived at Low Temperature. J. Chem. 2019, 2019, 2979517. [Google Scholar]
- Tan, L.L.; Ong, W.J.; Chai, S.P.; Mohamed, A.R. Reduced graphene oxide-TiO2 nanocomposite as a promising visiblelight-active photocatalyst for the conversion of carbon dioxide. Nanoscale Res. Lett. 2013, 8, 465. [Google Scholar] [CrossRef] [PubMed]
- Motelica, L.; Vasile, S.; Fical, A.; Surdu, A.V.; Ficai, D.; Oprea, O.C.; Andronescu, E.; Jinga, D.C.; Holban, A.M. Influence of the Alcohols on the ZnO Synthesis and Its Properties: The Photocatalytic and Antimicrobial Activities. Pharmaceutics 2022, 14, 2842. [Google Scholar] [CrossRef]
- Gebretsadik, A.; Kafale, B.; Sori, C.; Tsegaye, D.; Murthy, H.C.A.; Abebe, B. Cu-doped ZnO/Ag/CuO heterostructure: Superior photocatalysis and charge transfer. RSC Adv. 2024, 14, 29763–29773. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Shah, A.A.; Allehabi, S.O.; Ahmed, F.; Alsulami, A.; Azam, A. Enhanced degradation of Ciprofloxacin (CIP) antibiotic and methylene blue (MB) dye using ZnO/GO nanocomposites under solar irradiation. Sci. Rep. 2024, 14, 30696. [Google Scholar] [CrossRef]
- Sharda, P.; Satendra, K.C.; Anchal, S.; Shukla, R.K. Synergy of Fe dopant and graphite oxide in ZnO based nanocomposites for efficient photocatalytic degradation of indigo carmine dye. Ceram. Int. 2024, 51, 4929–4945. [Google Scholar]
- Rahman, M.S.; Suvo, M.A.H.; Islam, M.M.T.; Noor, A.R.; Yeachin, N.; Bhuiyan, M. Fast and efficient removal of metronidazole from aqueous solution using graphene oxide (GO) supported nitrogen (N) doped zinc oxide (ZnO) nanoparticles. Colloid Surf. A. 2024, 690, 133660. [Google Scholar] [CrossRef]
- Abdullah, S.A.; Muhammad, M.A.; Alaa, A.A.; Mohamed, M. Optimizing pollutant removal ability of GO/ZnO nanocomposites via controlled GO treatment and ZnO NPs content. Mater. Sci. Eng. B 2024, 302, 117202. [Google Scholar]
- Handan, B.; Durmus, A.; Colak, H.; Kurban, R.; Sahmetlioglu, E.; Karakose, E. Investigation of the performance and properties of ZnO/GO double-layer supercapacitor. J. Phys. Chem. Solids 2024, 191, 11984. [Google Scholar]
- Wang, P.Q.; Bai, Y.; Luo, P.Y.; Liu, J.Y. Graphene-WO3 nanobelt composite: Elevated conduction band toward photocatalytic reduction of CO2 into hydrocarbon fuels. Catal. Commun. 2013, 38, 82–85. [Google Scholar] [CrossRef]
- Nasrollahzadeh, M.; Jaleh, B.; Jabbari, A. Synthesis, characterization and catalytic activity of graphene oxide/ZnO nanocomposites. RSC Adv. 2014, 4, 36713–36720. [Google Scholar] [CrossRef]
- Gao, P.; Ng, K.; Sun, D. Sulfonated graphene oxide ZnO-Ag photocatalyst for fast photodegradation and disinfection under visible light. J. Hazard. Mater. 2013, 262, 826–835. [Google Scholar] [CrossRef]
- Jaleh, B.; Jabbari, A. Evaluation of reduced graphene oxide/ZnO effect on properties of PVDF nanocomposite films. Appl. Surf. Sci. 2014, 320, 339–347. [Google Scholar] [CrossRef]
- Deepthi, V.; Anju, S.; Vidhya, B. Influence of GO content on ZnO: GO composite thin films for visible light driven photocatalytic degradation of model pollutants. J. Sol-Gel Sci. Technol. 2023, 105, 673–682. [Google Scholar] [CrossRef]
- Ravichandran, K.; Uma, R.; Sriram, S.; Balamurgan, D. Fabrication of ZnO:Ag/GO composite thin films for enhanced photocatalytic activity. Ceram. Int. 2017, 43, 10041–10051. [Google Scholar] [CrossRef]
- Doluel, E.C.; Kartal, U.; Dikici, T.; Yurddaskal, M. Effect of Ag Content on Photocatalytic Activity of Ag@TiO2/rGO Hybrid Photocatalysts. J. Electron. Mater. 2020, 49, 3849–3859. [Google Scholar] [CrossRef]
- Li, G.; Yang, C.; He, Q.; Liu, J. Ag-based photocatalytic heterostructures: Construction and photocatalytic energy conversion application. J. Environ. Chem. Eng. 2022, 10, 107343. [Google Scholar] [CrossRef]
- Fan, Z.; Li, C.; Xu, M. Fabrication of ZnO/Ag photocatalyst and its photocatalytic degradation properties on trimethylamine. J. Iran. Chem. Soc. 2024, 21, 2121–2126. [Google Scholar] [CrossRef]
- Feng, C.; Chen, Z.; Jing, J.; Hou, J. The photocatalytic phenol degradation mechanism of Ag-modified ZnO nanorods. J. Mater. Chem. C. 2020, 8, 3000–3009. [Google Scholar] [CrossRef]
- Mishakav, I.V.; Bauman, Y.I.; Brzhezinskaya, M.; Netskina, O.V.; Shubin, Y.V.; Kibis, L.S.; Stoyanovskii, V.O.; Larionov, K.B.; Serkova, A.N.; Vedyagin, A.A. Water purification from chlorobenzenes using heteroatom-functionalized carbon nanofibers produced on self-organizing Ni-Pd catalyst. J. Environ. Chem. Eng. 2022, 10, 107873. [Google Scholar] [CrossRef]
- Sobaszek, M.; Brzhezinskaya, M.; Olejnik, A.; Mortet, V.; Alam, M.; Sawczak, M.; Ficek, M.; Gazda, M.; Weiss, Z.; Bogdanowica, R. Highly Occupied Surface States at Deuterium-Grown Boron-Doped Diamond Interfaces for Efficient Photoelectrochemistry. Small 2023, 19, 2208265. [Google Scholar] [CrossRef]
- Rabchinskii, M.K.; Ryzhkov, S.A.; Besedina, N.A.; Brzhezinskaya, M.; Malkov, M.N.; Stolyarova, D.Y.; Arutyunyan, A.F.; Struchkov, N.S.; Saveliev, S.D.; Diankin, I.D.; et al. Guiding graphene derivatization for covalent immobilization of aptamers. Carbon 2022, 196, 264–279. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chi, H.; Cao, P.; Shi, Q.; Song, C.; Lv, Y.; Peng, T. Photocatalytic Degradation of Ciprofloxacin by GO/ZnO/Ag Composite Materials. Nanomaterials 2025, 15, 383. https://doi.org/10.3390/nano15050383
Chi H, Cao P, Shi Q, Song C, Lv Y, Peng T. Photocatalytic Degradation of Ciprofloxacin by GO/ZnO/Ag Composite Materials. Nanomaterials. 2025; 15(5):383. https://doi.org/10.3390/nano15050383
Chicago/Turabian StyleChi, Haonan, Pan Cao, Qi Shi, Chaoyu Song, Yuguang Lv, and Tai Peng. 2025. "Photocatalytic Degradation of Ciprofloxacin by GO/ZnO/Ag Composite Materials" Nanomaterials 15, no. 5: 383. https://doi.org/10.3390/nano15050383
APA StyleChi, H., Cao, P., Shi, Q., Song, C., Lv, Y., & Peng, T. (2025). Photocatalytic Degradation of Ciprofloxacin by GO/ZnO/Ag Composite Materials. Nanomaterials, 15(5), 383. https://doi.org/10.3390/nano15050383






