Sponge Morphology of Osteosarcoma Finds Origin in Synergy Between Bone Synthesis and Tumor Growth
Abstract
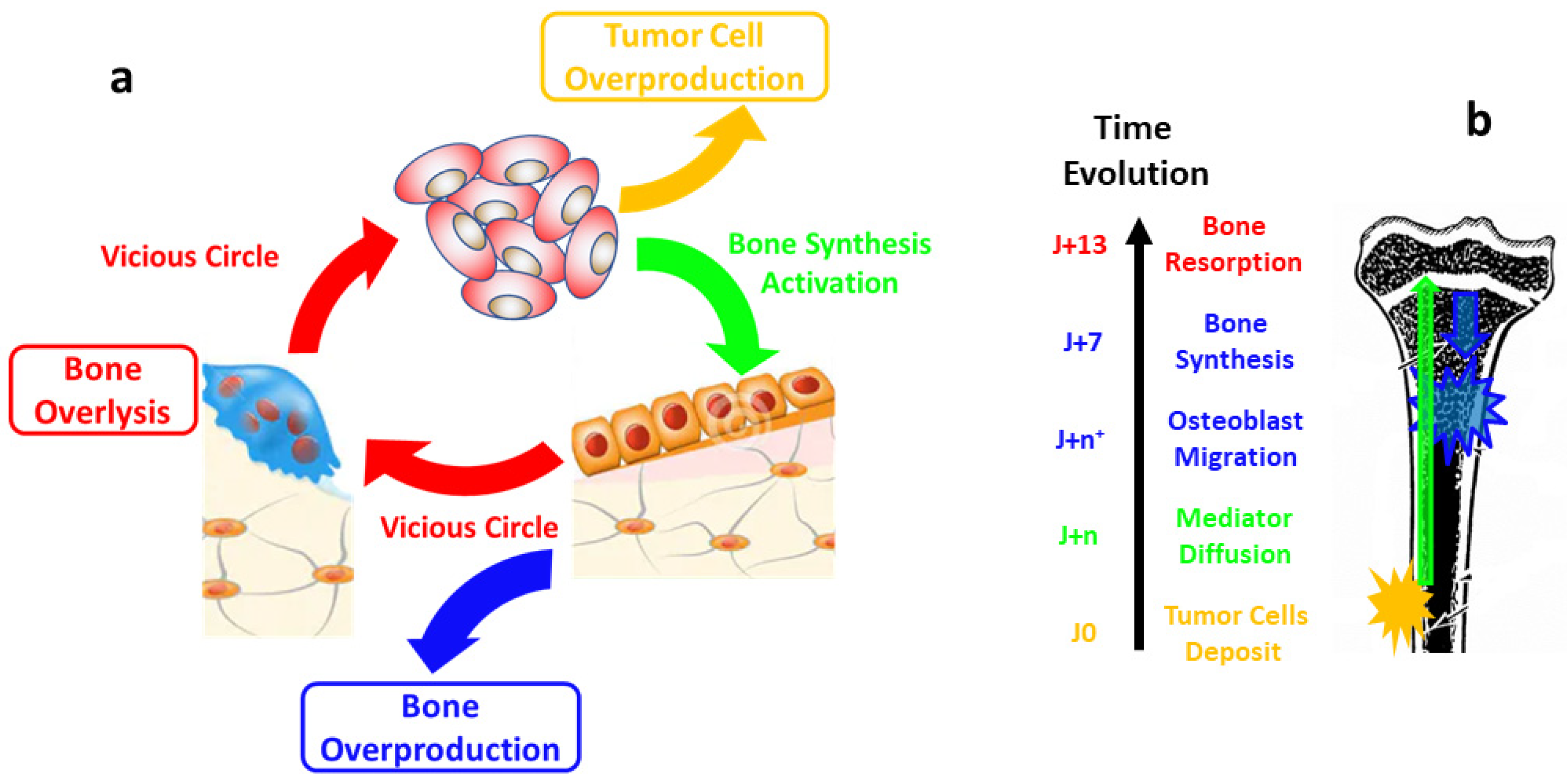
1. Introduction
2. Materials and Methods
2.1. Syngenic Model of Osteoblastic Osteosarcoma in Mouse (MOS-J) and Cryosection
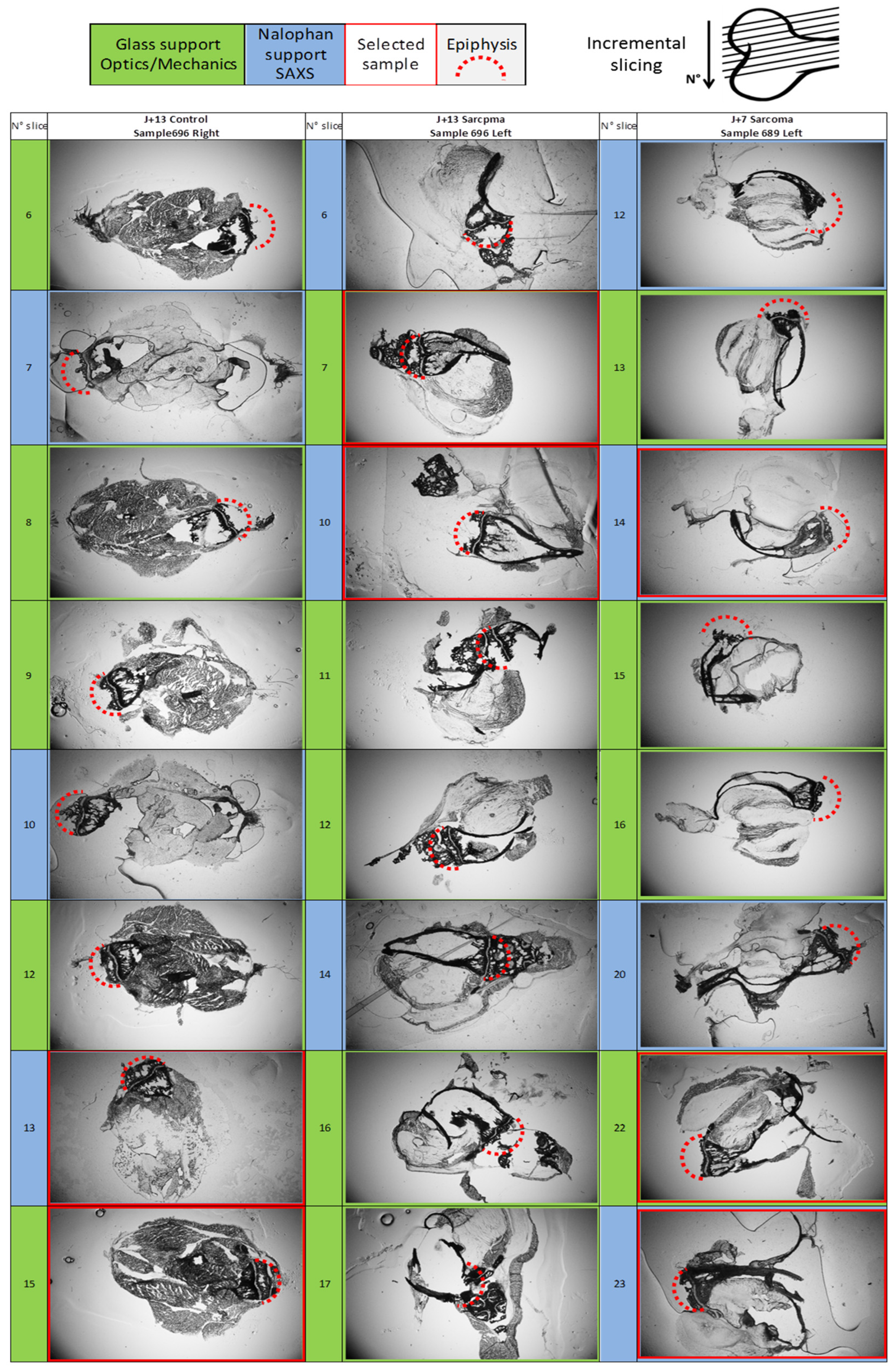
2.2. Optical Microscopy of 100 μm Thick Slices
2.3. Microfocus SWING Beamline
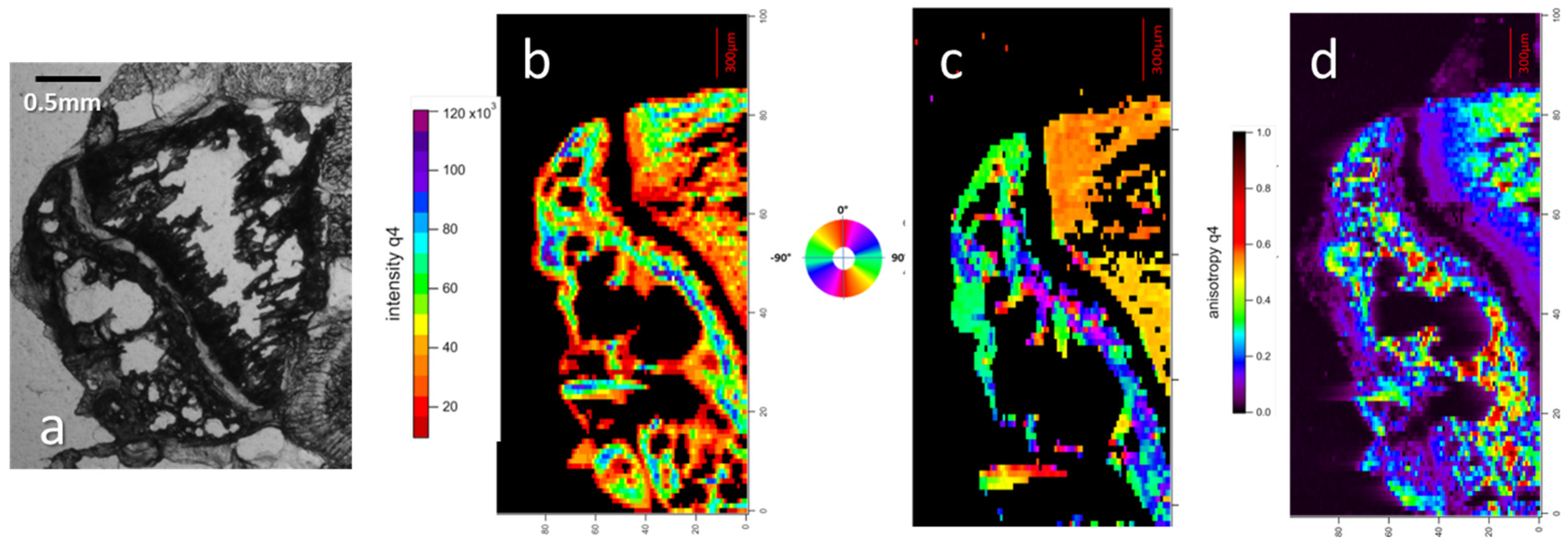
2.4. SAXS Parameters Mapping
2.5. Martens Hardness Indentation
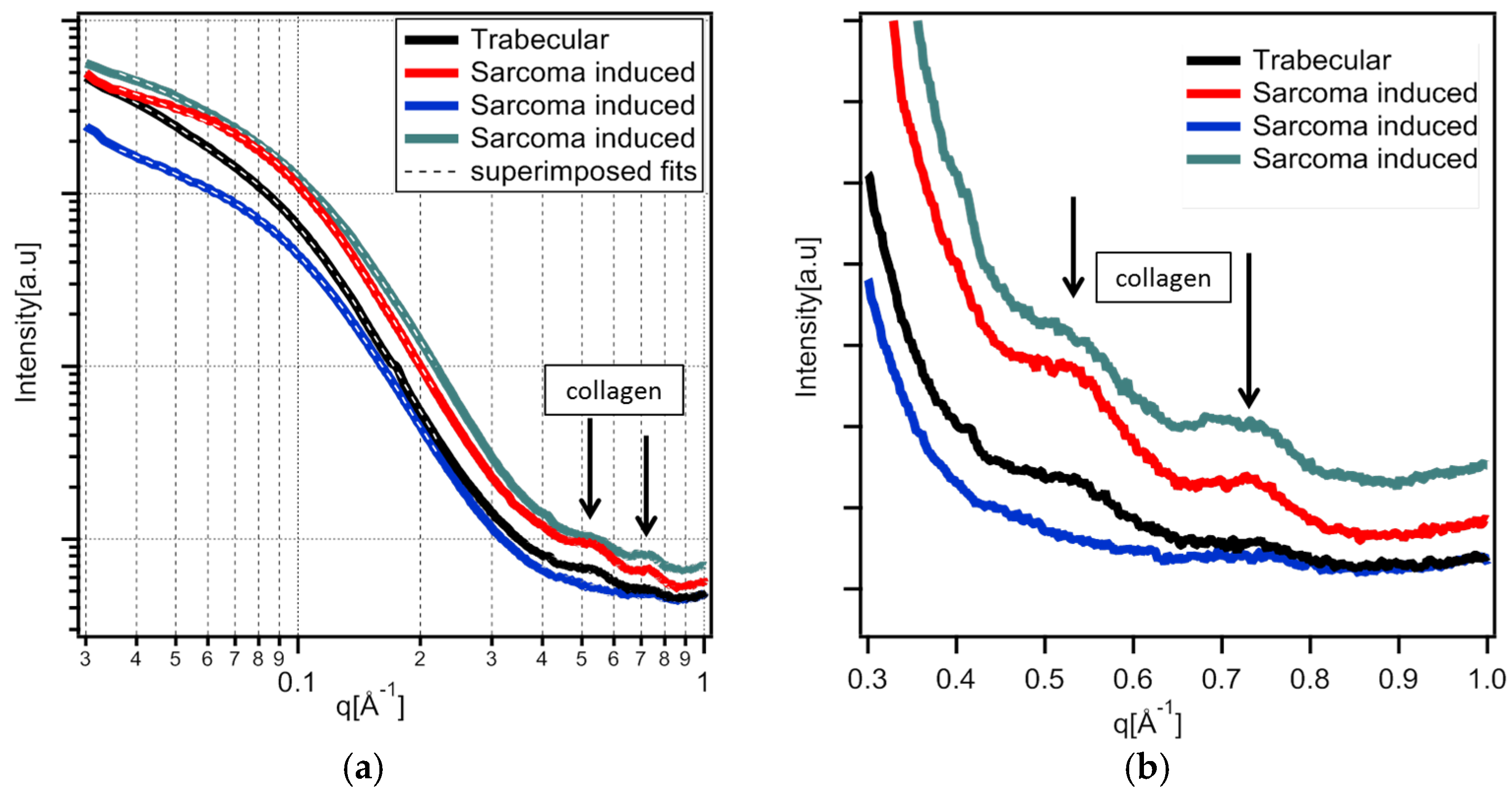
2.6. Wide-Angle X-Ray Scattering
2.7. Thin Section for Microscopies
2.8. Advanced Osteosarcoma MicroCT
2.9. Simulation
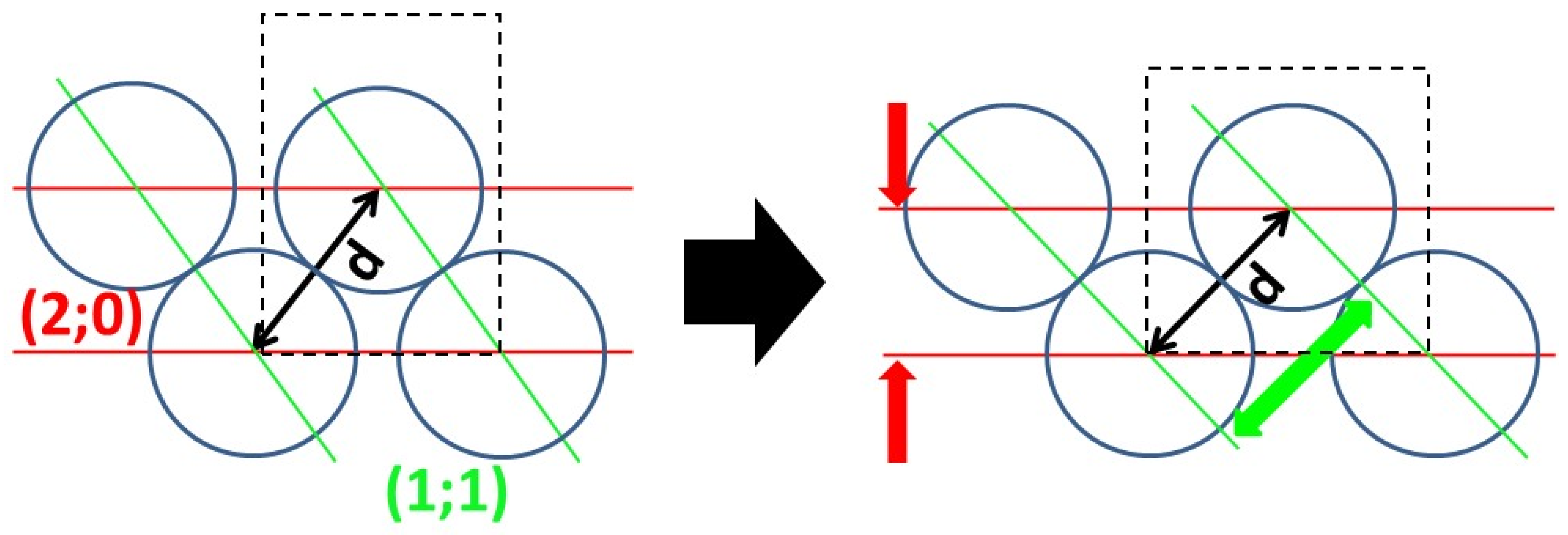
2.9.1. Topology Modeling
- -
- The structures formed are not static and subject to remodeling. Sarcoma s and bone cells b (p type cells) propagate at speed .
- -
- Bone and sarcoma are the two cell types capable of propagation at the interface in this simulation, while healthy tissue is incapable of it, since it would correspond to tumor regression, which is not considered here.
- -
- Propagation of bone (b) and sarcoma (s) are each exclusively dependent on the presence of the other. As such, depends on the presence of s and depends on the presence of b as both elements progress together.
- -
- Propagation happens through contact, but under a sum influence exerted at a distance, as the model we are trying to ascertain is one of the propagation being enabled by the diffusion of a mediator. Sum influence takes the form of with (i;j) coordinates of cell propagation. d is the distance to (i;j), γp(i′;j′) a binary function of presence of species p at coordinates (i′;j′). f(d) is a law of distance influence, such as the distance of maximum influence, and the ratio between peak influence and long range can be adjusted.where d0 is the distance of maximum influence and η is long-range influence.
- -
- The resulting domains must be cohesive, and interface roughness in the system is controlled by limiting growth that creates too much interface and clearing out all isolated cells. The limiting function appears in, for example, with the binary function If there is a function the following comparison is true , i.e., the number of same-species neighbor cells to (i;j) is superior or equal to that of one of its different species neighbor cells.
2.9.2. Three-Dimensional Cellular Automaton
3. Results
3.1. Early Stage of Osteosarcoma Development
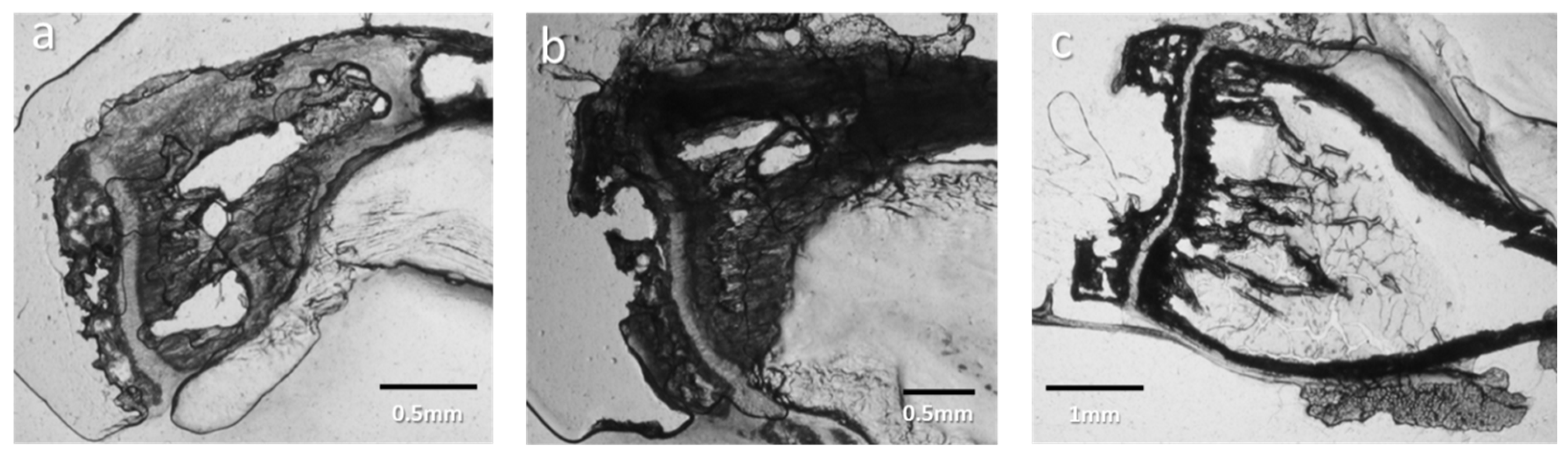
3.1.1. Optical Observation of Intracortical Bone Overproduction
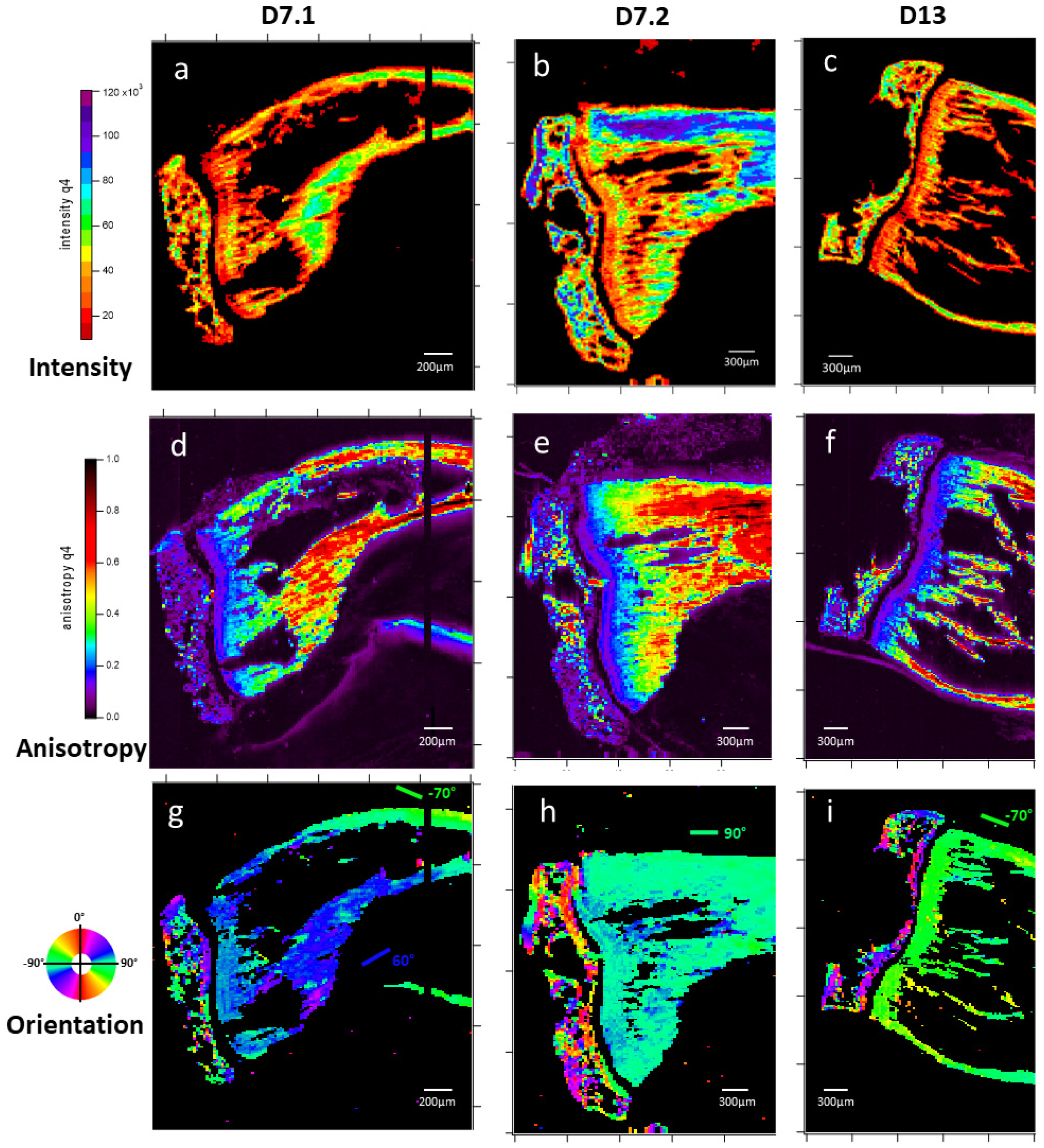
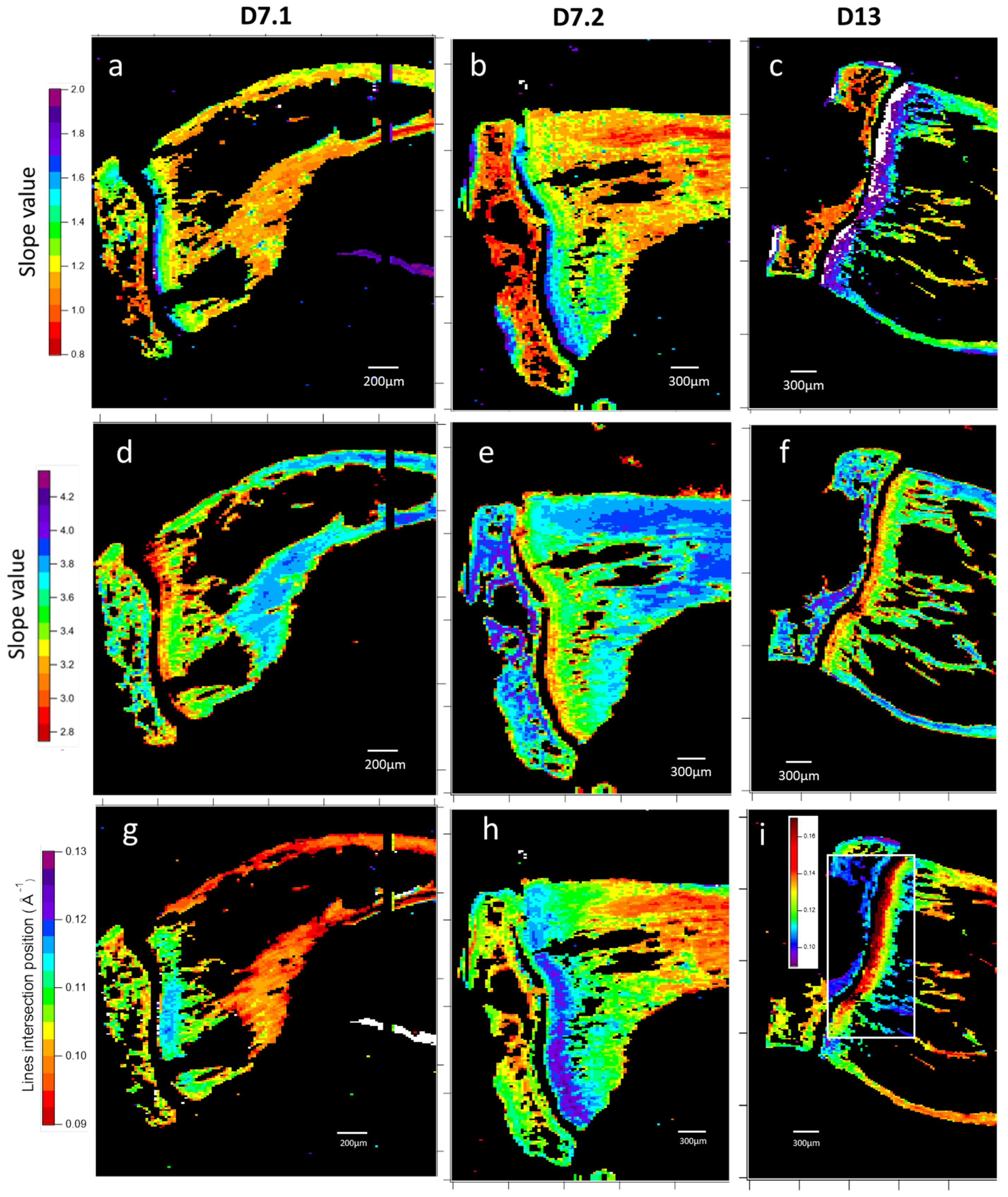
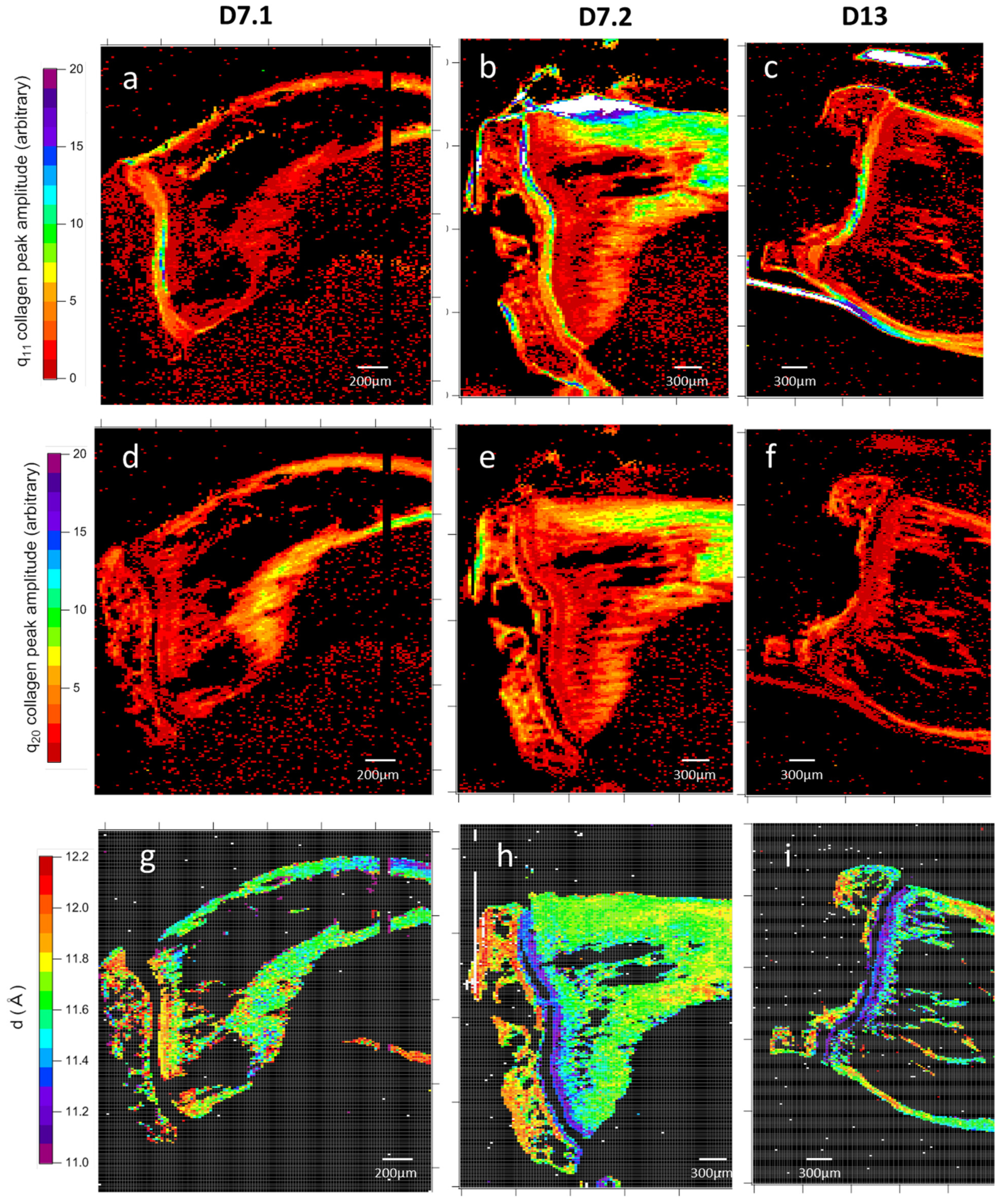
3.1.2. Microfocus Small-Angle X-Ray Scattering of Bone Structure
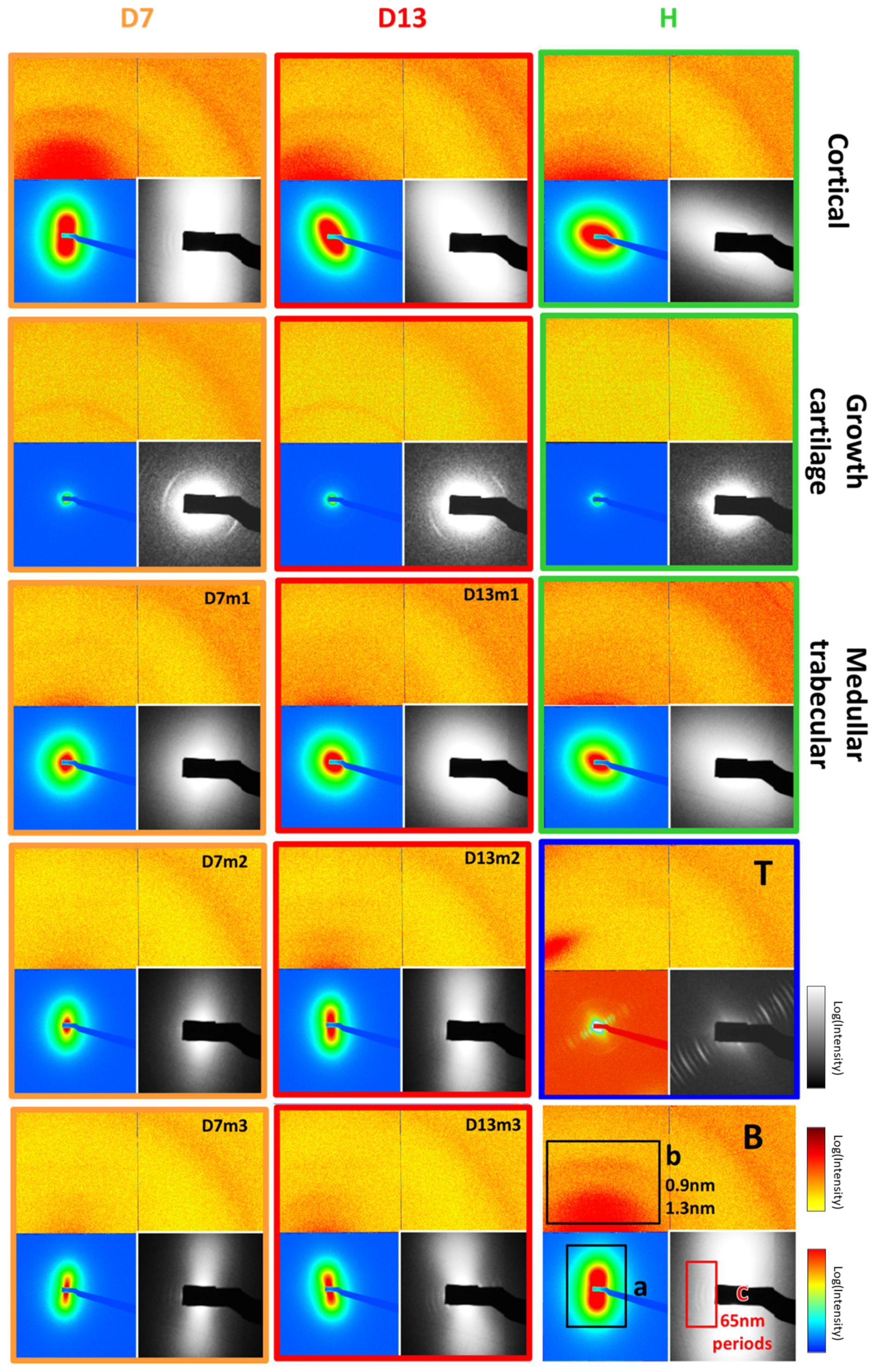
3.1.3. Microfocus SAXS Mapping of Bone Structure
3.1.4. Wide-Angle X-Ray Scattering of Overproduced Hydroxy-Apatite
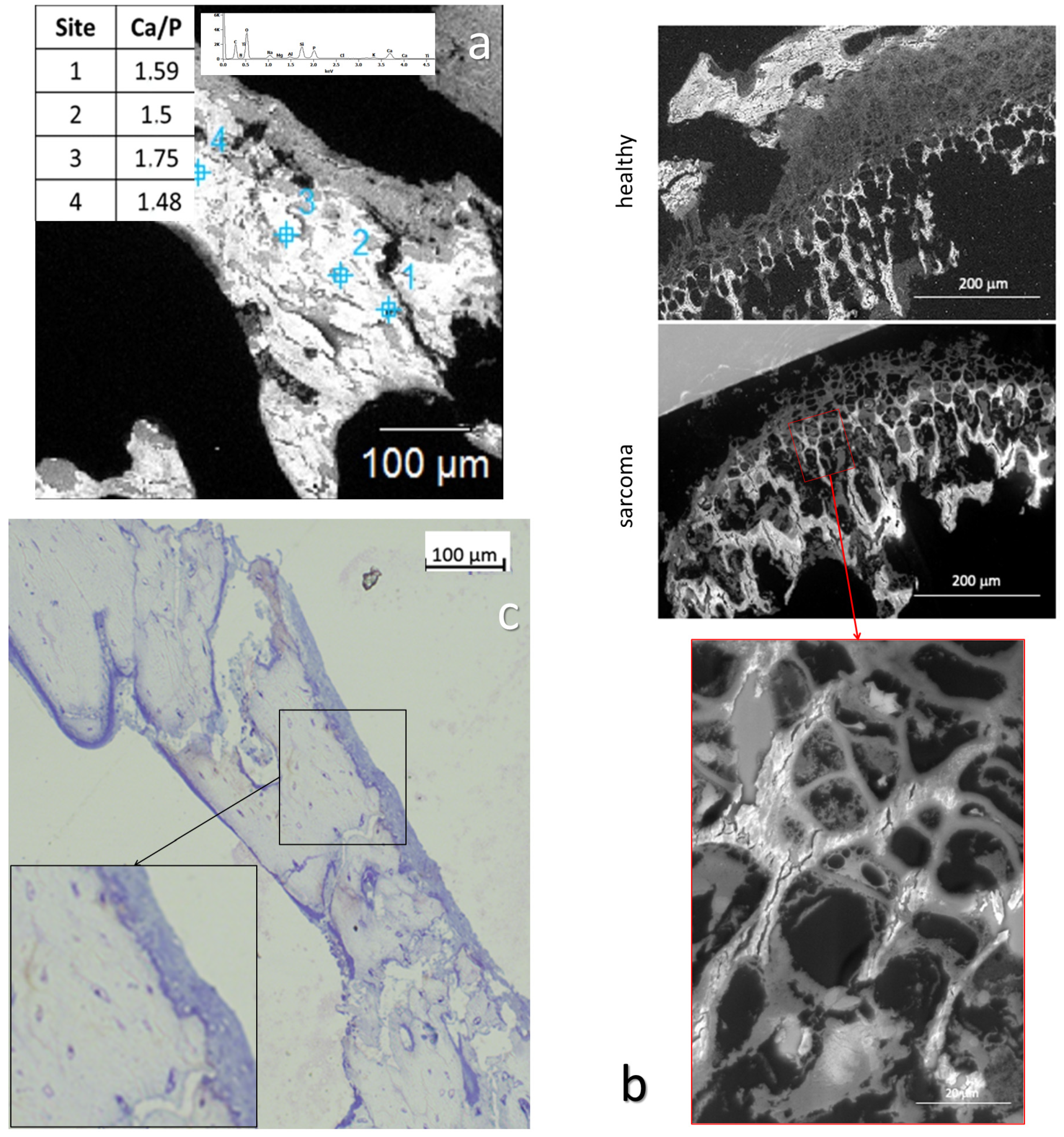
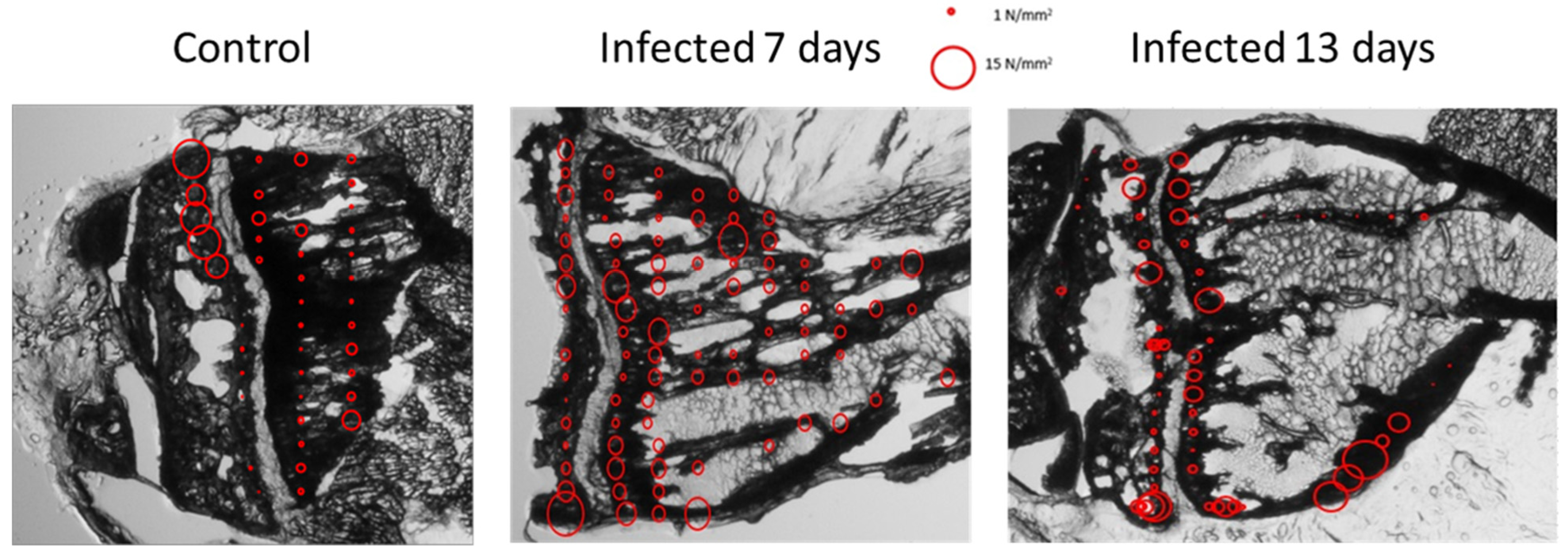
3.1.5. Mechanical Properties
3.1.6. Cell Identification by Microscopies of Overproduced Bone-like Materials
3.2. Topology at Late Stage of Osteosarcoma Development
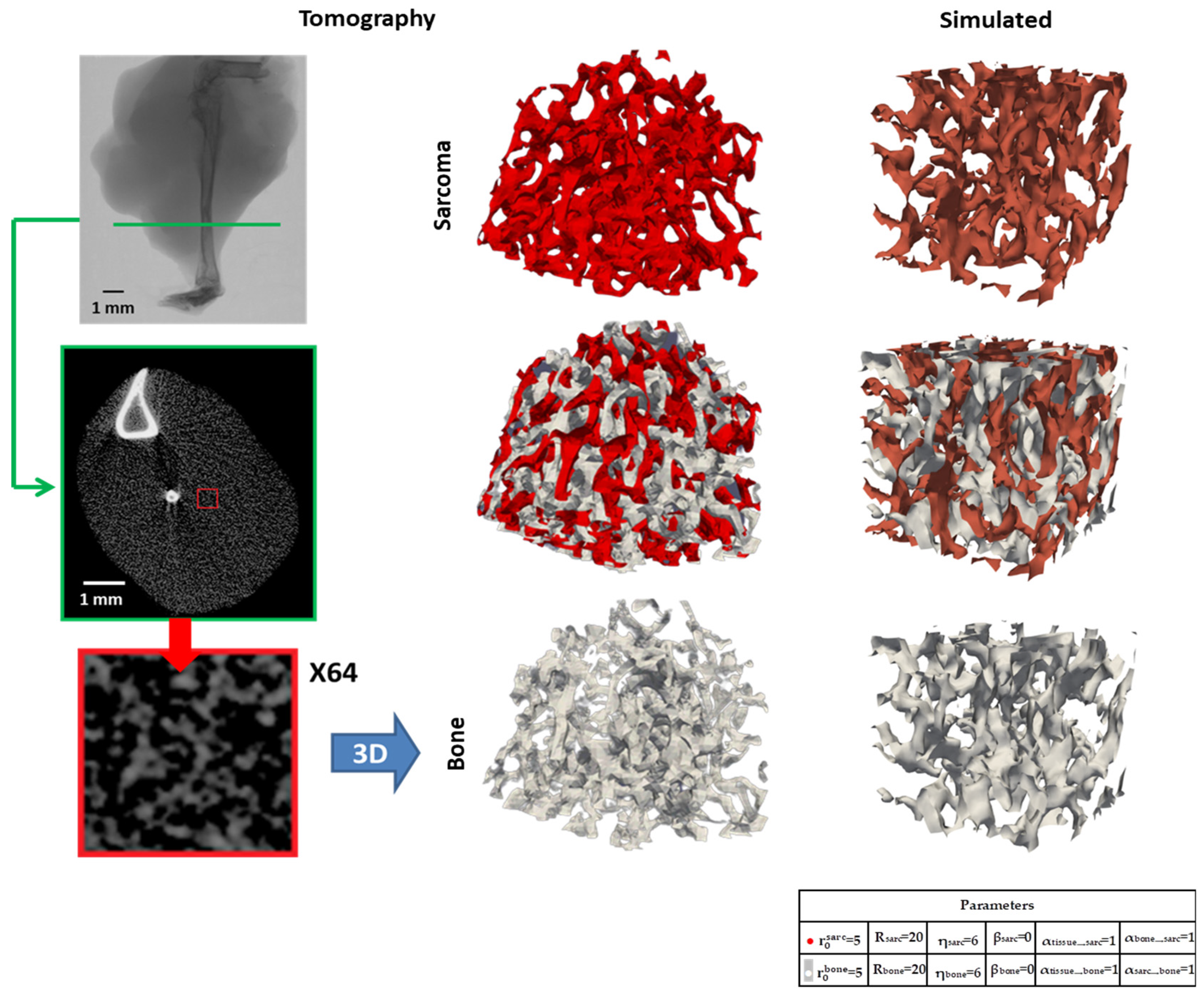
3.2.1. Micro-CT Reconstructions
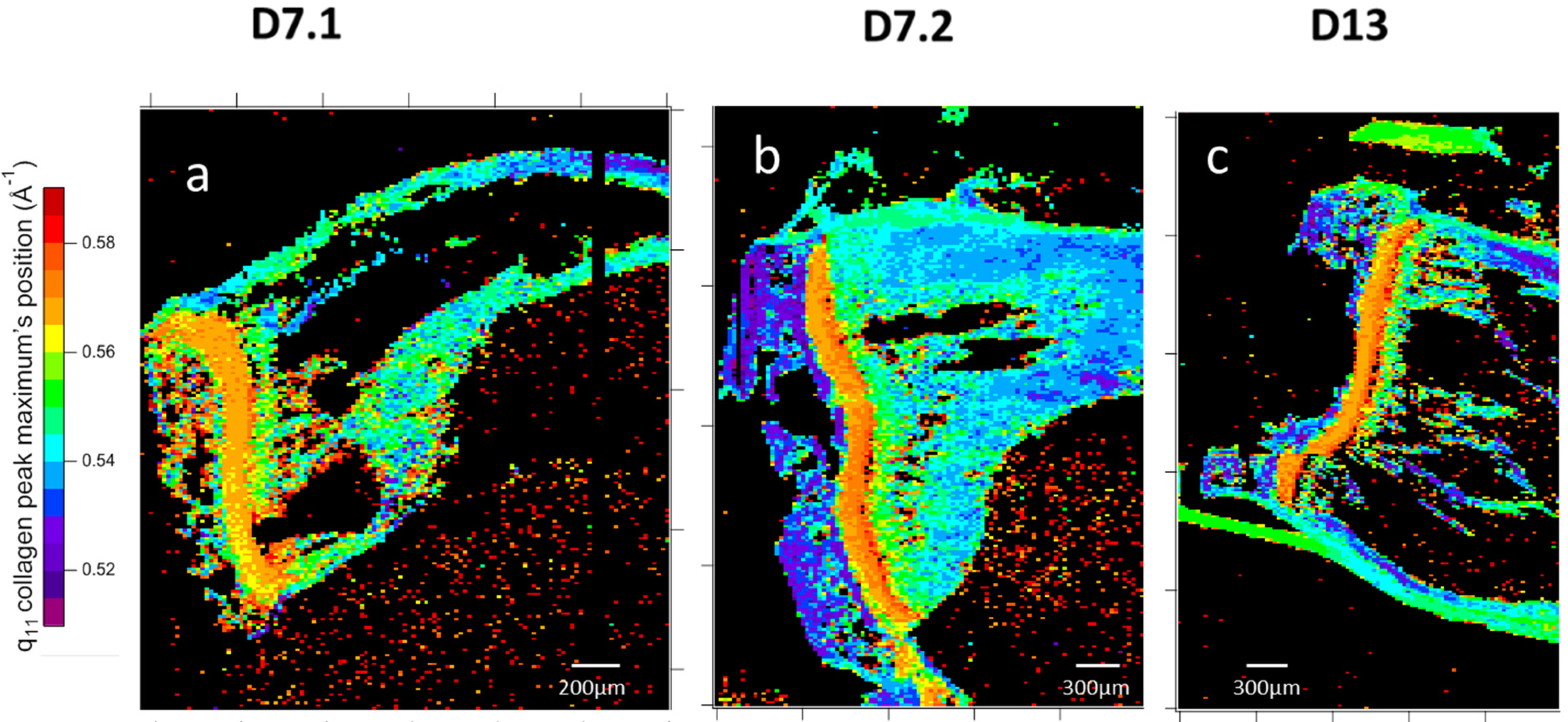
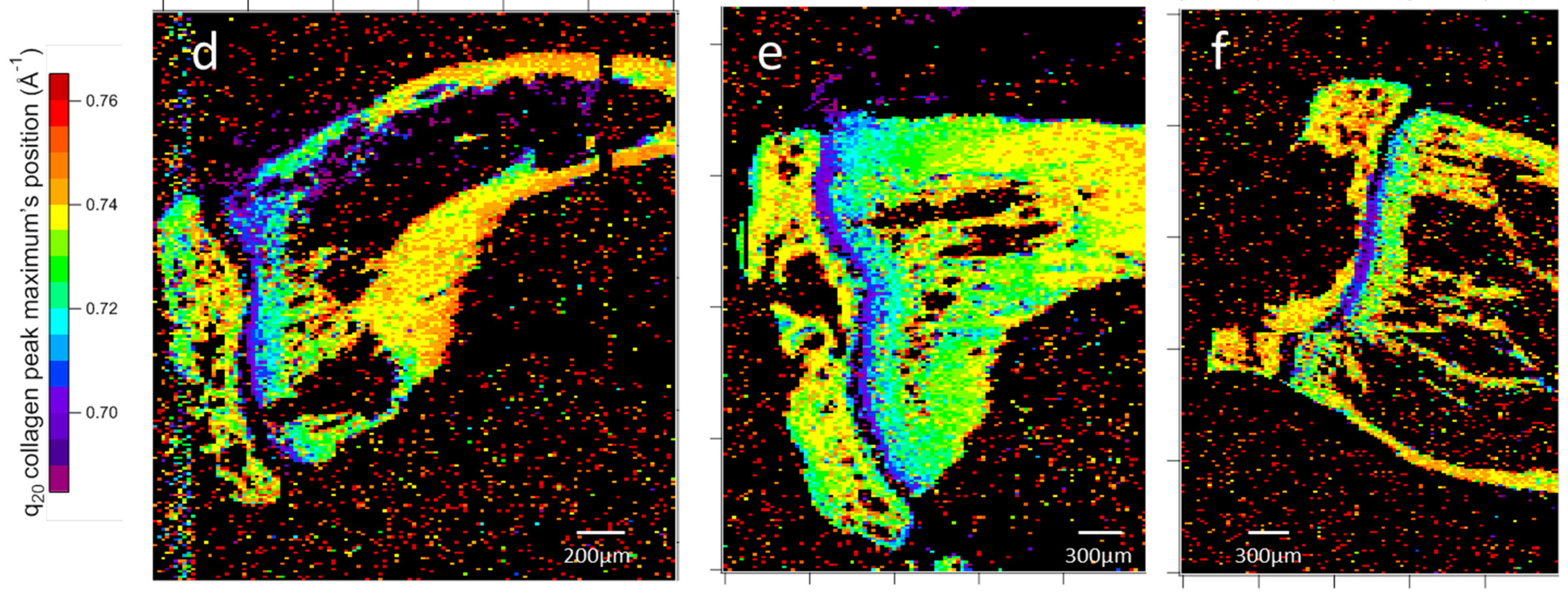
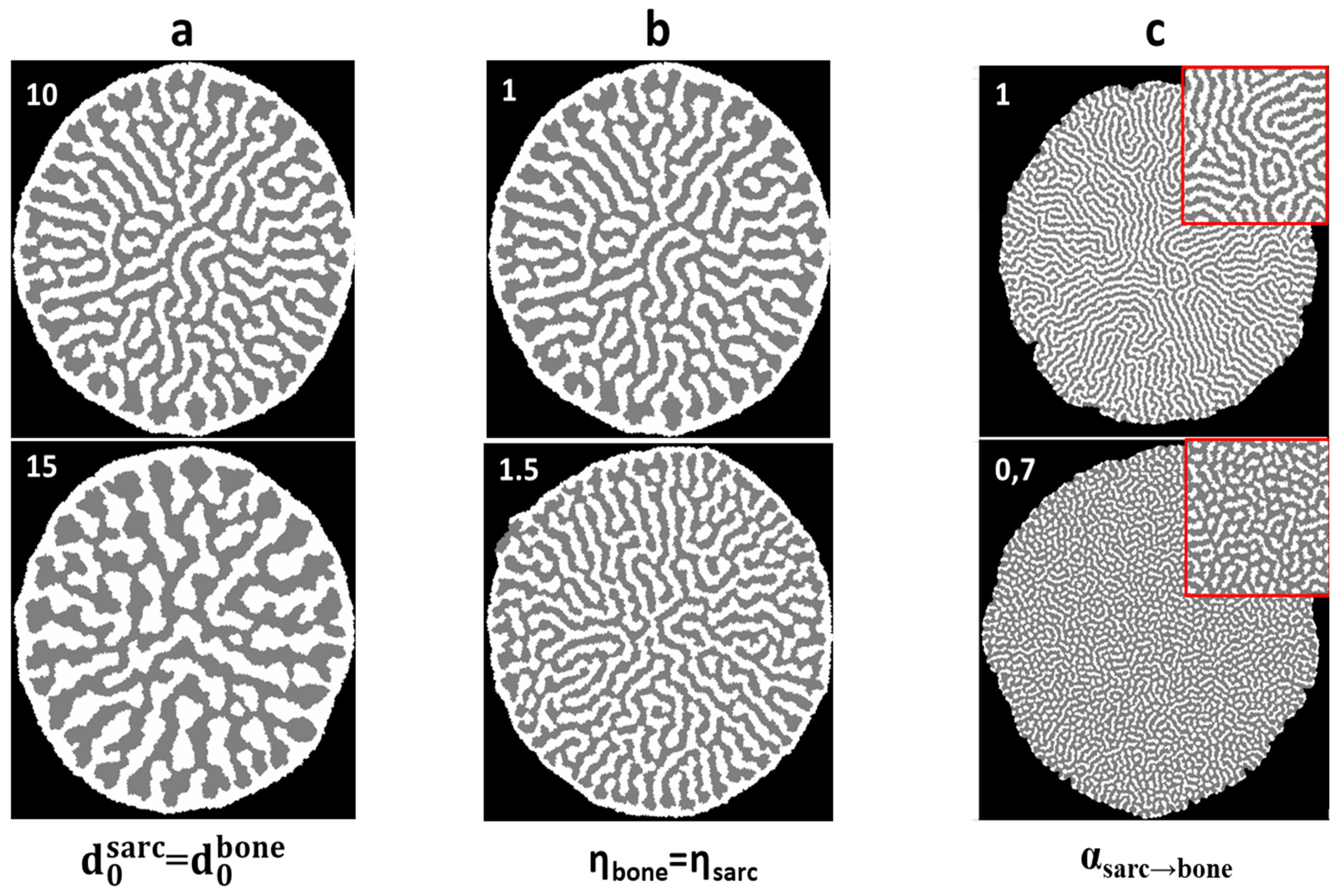
3.2.2. Two-Dimensional Topology Modeling
3.2.3. Three-Dimensional Topology Modeling
4. Discussion
4.1. Bone-like Material Surplus at D7
4.1.1. Structure of the Bone-like Material Surplus at D7
4.1.2. Structural Gradient Within the Medullary Cavity at D7
4.1.3. Structural Evolution Induced by the Tumor in the Growth Cartilage
4.2. De Novo Bone Synthesis Activation Mechanism
4.3. Bone-like Material Degradation at D13
4.4. Sponge-Morphology
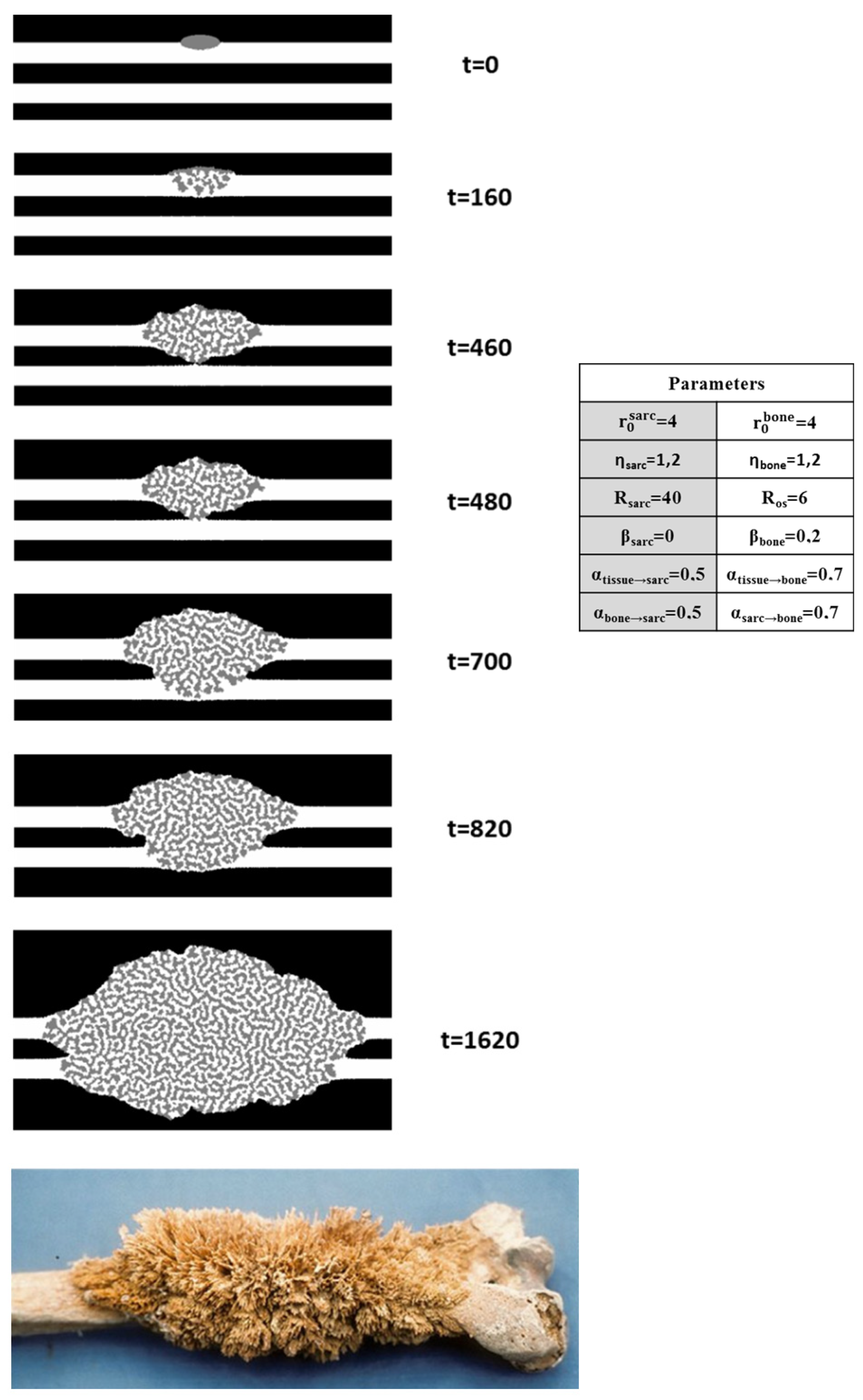
4.4.1. Osteosarcoma Morphology Model with a Minimalist Model
4.4.2. Biological Steps Included in Each Automaton Step
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wegst, U.G.K.; Bai, H.; Saiz, E.; Tomsia, A.P.; Ritchie, R.O. Bioinspired Structural Materials. Nat. Mater. 2015, 14, 23–36. [Google Scholar] [CrossRef] [PubMed]
- Pouget, E.; Dujardin, E.; Cavalier, A.; Moreac, A.; Valery, C.; Marchi-Artzner, V.; Weiss, T.; Renault, A.; Paternostre, M.; Artzner, F. Hierarchical Architectures by Synergy between Dynamical Template Self-Assembly and Biomineralization. Nat. Mater. 2007, 6, 434–439. [Google Scholar] [CrossRef] [PubMed]
- Brunner, E. Double-Walled Silica Nanotubes. Nat. Mater. 2007, 6, 398–399. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Guo, X.; Tao, W.; Chen, L.; Peng, L.; Ding, W. Inorganic Nanotubes Formation through the Synergic Evolution of Dynamic Templates and Metallophosphates: From Vesicles to Nanotubes. Chem. Commun. 2011, 47, 10061–10063. [Google Scholar] [CrossRef]
- Choi, H.; Kim, J.-J.; Mo, Y.-H.; Reddy, B.M.; Park, S.-E. Novelty of Dynamic Process in the Synthesis of Biocompatible Silica Nanotubes by Biomimetic Glycyldodecylamide as a Soft Template. Langmuir 2017, 33, 10707–10714. [Google Scholar] [CrossRef]
- Rao, A.; Seto, J.; Berg, J.K.; Kreft, S.G.; Scheffner, M.; Cölfen, H. Roles of Larval Sea Urchin Spicule SM50 Domains in Organic Matrix Self-Assembly and Calcium Carbonate Mineralization. J. Struct. Biol. 2013, 183, 205–215. [Google Scholar] [CrossRef]
- Tyszka, J.; Bickmeyer, U.; Raitzsch, M.; Bijma, J.; Kaczmarek, K.; Mewes, A.; Topa, P.; Janse, M. Form and Function of F-Actin during Biomineralization Revealed from Live Experiments on Foraminifera. Proc. Natl. Acad. Sci. USA 2019, 116, 4111–4116. [Google Scholar] [CrossRef]
- Chen, T.; Shi, P.; Li, Y.; Duan, T.; Yu, Y.; Li, X.; Zhu, W. Biomineralization of Varied Calcium Carbonate Crystals by the Synergistic Effect of Silk Fibroin/Magnesium Ions in a Microbial System. Crystengcomm 2018, 20, 2366–2373. [Google Scholar] [CrossRef]
- Wang, Y.; Azaïs, T.; Robin, M.; Vallée, A.; Catania, C.; Legriel, P.; Pehau-Arnaudet, G.; Babonneau, F.; Giraud-Guille, M.-M.; Nassif, N. The Predominant Role of Collagen in the Nucleation, Growth, Structure and Orientation of Bone Apatite. Nat. Mater. 2012, 11, 724–733. [Google Scholar] [CrossRef]
- Henry, E.; Dif, A.; Schmutz, M.; Legoff, L.; Amblard, F.; Marchi-Artzner, V.; Artzner, F. Crystallization of Fluorescent Quantum Dots within a Three-Dimensional Bio-Organic Template of Actin Filaments and Lipid Membranes. Nano Lett. 2011, 11, 5443–5448. [Google Scholar] [CrossRef]
- Kerschnitzki, M.; Wagermaier, W.; Roschger, P.; Seto, J.; Shahar, R.; Duda, G.N.; Mundlos, S.; Fratzl, P. The Organization of the Osteocyte Network Mirrors the Extracellular Matrix Orientation in Bone. J. Struct. Biol. 2011, 173, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Eriksen, E.F. Cellular Mechanisms of Bone Remodeling. Rev. Endocr. Metab. Disord. 2010, 11, 219–227. [Google Scholar] [CrossRef]
- Kameo, Y.; Adachi, T.; Hojo, M. Effects of Loading Frequency on the Functional Adaptation of Trabeculae Predicted by Bone Remodeling Simulation. J. Mech. Behav. Biomed. Mater. 2011, 4, 900–908. [Google Scholar] [CrossRef] [PubMed]
- Turner, C.H. Three Rules for Bone Adaptation to Mechanical Stimuli. Bone 1998, 23, 399–407. [Google Scholar] [CrossRef] [PubMed]
- Mackie, E.J.; Ahmed, Y.A.; Tatarczuch, L.; Chen, K.-S.; Mirams, M. Endochondral Ossification: How Cartilage Is Converted into Bone in the Developing Skeleton. Int. J. Biochem. Cell Biol. 2008, 40, 46–62. [Google Scholar] [CrossRef] [PubMed]
- Corre, I.; Verrecchia, F.; Crenn, V.; Redini, F.; Trichet, V. The Osteosarcoma Microenvironment: A Complex but Targetable Ecosystem. Cells 2020, 9, 976. [Google Scholar] [CrossRef]
- Lamora, A.; Talbot, J.; Mullard, M.; Brounais-Le Royer, B.; Redini, F.; Verrecchia, F. TGF-β Signaling in Bone Remodeling and Osteosarcoma Progression. J. Clin. Med. 2016, 5, E96. [Google Scholar] [CrossRef]
- Fletcher, C.; Bridge, J.A.; Hogendoorn, P.C.W.; Mertens, F. WHO Classification of Tumours of Soft Tissue and Bone; IARC: Lyon, France, 2013; ISBN 978-92-832-2434-1. [Google Scholar]
- Fratzl, P.; Weinkamer, R. Nature’s Hierarchical Materials. Prog. Mater. Sci. 2007, 52, 1263–1334. [Google Scholar] [CrossRef]
- Murray, J.D. Mathematical Biology I: An Introduction, 3rd Corrected ed. 2002.; Corr. 3rd printing 2008 ed.; Springer-Verlag New York Inc.: New York, NY, USA, 2011; ISBN 978-0-387-95223-9. [Google Scholar]
- Murray, J.D. Mathematical Biology II: Spatial Models and Biomedical Applications, 3rd Corrected ed. 2003.; Corr. 3rd printing 2008 ed.; Springer-Verlag New York Inc.: New York, NY, USA, 2011; ISBN 978-0-387-95228-4. [Google Scholar]
- Banerjee, S.; Rondoni, L. (Eds.) Applications of Chaos and Nonlinear Dynamics in Science and Engineering—Volume 4; Understanding Complex Systems; Springer International Publishing: Cham, Switzerland, 2015; ISBN 978-3-319-17036-7. [Google Scholar]
- Alonso, D.; Bartumeus, F.; Catalan, J. Mutual Interference Between Predators Can Give Rise to Turing Spatial Patterns. Ecology 2002, 83, 28–34. [Google Scholar] [CrossRef]
- Reznikov, N.; Hoac, B.; Buss, D.J.; Addison, W.N.; Barros, N.M.T.; McKee, M.D. Biological Stenciling of Mineralization in the Skeleton: Local Enzymatic Removal of Inhibitors in the Extracellular Matrix. Bone 2020, 138, 115447. [Google Scholar] [CrossRef]
- Crenn, V.; Biteau, K.; Amiaud, J.; Dumars, C.; Guiho, R.; Vidal, L.; Le Nail, L.-R.; Heymann, D.; Moreau, A.; Gouin, F.; et al. Bone Microenvironment Has an Influence on the Histological Response of Osteosarcoma to Chemotherapy: Retrospective Analysis and Preclinical Modeling. Am. J. Cancer Res. 2017, 7, 2333–2349. [Google Scholar] [PubMed]
- Fratzl, P.; Groschner, M.; Vogl, G.; Plenk, H.; Eschberger, J.; Fratzl-Zelman, N.; Koller, K.; Klaushofer, K. Mineral Crystals in Calcified Tissues: A Comparative Study by SAXS. J. Bone Miner. Res. 1992, 7, 329–334. [Google Scholar] [CrossRef] [PubMed]
- Seidel, R.; Gourrier, A.; Kerschnitzki, M.; Burghammer, M.; Fratzl, P.; Gupta, H.; Wagermaier, W. Synchrotron 3D SAXS Analysis of Bone Nanostructure. Bioinspired Biomim. Nanobiomater. 2011, 1, 123–131. [Google Scholar] [CrossRef]
- Pabisch, S.; Wagermaier, W.; Zander, T.; Li, C.; Fratzl, P. Imaging the Nanostructure of Bone and Dentin through Small- and Wide-Angle X-Ray Scattering. Methods Enzymol. 2013, 532, 391–413. [Google Scholar] [CrossRef]
- Grunow, O.H. Radiating Spicules, a Non-Specific Sign of Bone Disease. Radiology 1955, 65, 200–205. [Google Scholar] [CrossRef]
- Yoshikawa, H.; Shimizu, K.; Nakase, T.; Takaoka, K. Periosteal Sunburst Spiculation in Osteosarcoma. A Possible Role for Bone Morphogenetic Protein. Clin. Orthop. 1994, 308, 213–219. [Google Scholar] [CrossRef]
- Nazmov, V.; Reznikova, E.; Mohr, J.; Snigirev, A.; Snigireva, I.; Achenbach, S.; Saile, V. Fabrication and Preliminary Testing of X-Ray Lenses in Thick SU-8 Resist Layers. Microsyst. Technol. 2004, 10, 716–721. [Google Scholar] [CrossRef]
- Krywka, C.; Last, A.; Marschall, F.; Márkus, O.; Georgi, S.; Müller, M.; Mohr, J. Polymer Compound Refractive Lenses for Hard X-Ray Nanofocusing. AIP Conf. Proc. 2016, 1764, 020001. [Google Scholar] [CrossRef]
- Leclercq, N.; Bisou, J.; Blache, F.; Langlois, F.; Lê, S.; Medjoubi, K.; Mocuta, C.; Poirier, S. Flyscan: A Fast and Multi-Technique Data Acquisition Platform for the SOLEIL Beamlines. In Proceedings of the 15th International Conference on Accelerator and Large Experimental Physics Control Systems, Melbourne, Australia, 17–23 October 2015; p. WEPGF056. [Google Scholar]
- Gang, O. Small Angle X-Ray Scattering (SAXS) from Bulks and Surfaces 2015. Available online: https://www.diamond.ac.uk/Instruments/Techniques/Scattering/SAXS.html (accessed on 20 February 2025).
- Lamoureux, F.; Richard, P.; Wittrant, Y.; Battaglia, S.; Pilet, P.; Trichet, V.; Blanchard, F.; Gouin, F.; Pitard, B.; Heymann, D.; et al. Therapeutic Relevance of Osteoprotegerin Gene Therapy in Osteosarcoma: Blockade of the Vicious Cycle between Tumor Cell Proliferation and Bone Resorption. Cancer Res. 2007, 67, 7308–7318. [Google Scholar] [CrossRef]
- Cedola, A.; Mastrogiacomo, M.; Lagomarsino, S.; Cancedda, R.; Giannini, C.; Guagliardi, A.; Ladisa, M.; Burghammer, M.; Rustichelli, F.; Komlev, V. Orientation of Mineral Crystals by Collagen Fibers during in Vivo Bone Engineering: An X-Ray Diffraction Imaging Study. Spectrochim. Acta Part B At. Spectrosc. 2007, 62, 642–647. [Google Scholar] [CrossRef]
- Fratzl, P.; Fratzl-Zelman, N.; Klaushofer, K. Collagen Packing and Mineralization. An x-Ray Scattering Investigation of Turkey Leg Tendon. Biophys. J. 1993, 64, 260–266. [Google Scholar] [CrossRef] [PubMed]
- Albéric, M.; Gourrier, A.; Wagermaier, W.; Fratzl, P.; Reiche, I. The Three-Dimensional Arrangement of the Mineralized Collagen Fibers in Elephant Ivory and Its Relation to Mechanical and Optical Properties. Acta Biomater. 2018, 72, 342–351. [Google Scholar] [CrossRef] [PubMed]
- Gourrier, A.; Wagermaier, W.; Burghammer, M.; Lammie, D.; Gupta, H.; Fratzl, P.; Riekel, C.; Wess, T.; Paris, O. Scanning X-Ray Imaging with Small-Angle Scattering Contrast. J. Appl. Cryst. 2007, 40, 78–82. [Google Scholar] [CrossRef]
- Breßler, I.; Kohlbrecher, J.; Thünemann, A.F. SASfit: A Tool for Small-Angle Scattering Data Analysis Using a Library of Analytical Expressions. J. Appl. Crystallogr. 2015, 48, 1587–1598. [Google Scholar] [CrossRef]
- Martinez-Zelaya, V.R.; Archilha, N.L.; Calasans-Maia, M.; Farina, M.; Rossi, A.M. Trabecular Architecture during the Healing Process of a Tibial Diaphysis Defect. Acta Biomater. 2021, 120, 181–193. [Google Scholar] [CrossRef]
- Markovic, M.; Fowler, B.O.; Tung, M.S. Preparation and Comprehensive Characterization of a Calcium Hydroxyapatite Reference Material. J. Res. Natl. Inst. Stand. Technol. 2004, 109, 553–568. [Google Scholar] [CrossRef]
- Zanghellini, B.; Grünewald, T.A.; Burghammer, M.; Rennhofer, H.; Liegl-Atzwanger, B.; Leithner, A.; Lichtenegger, H.C. High-Resolution Large-Area Imaging of Nanoscale Structure and Mineralization of a Sclerosing Osteosarcoma in Human Bone. J. Struct. Biol. 2019, 207, 56–66. [Google Scholar] [CrossRef]
- Dorozhkin, S.V.; Epple, M. Biological and Medical Significance of Calcium Phosphates. Angew. Chem. Int. Ed Engl. 2002, 41, 3130–3146. [Google Scholar] [CrossRef]
- Kourkoumelis, N.; Balatsoukas, I.; Tzaphlidou, M. Ca/P Concentration Ratio at Different Sites of Normal and Osteoporotic Rabbit Bones Evaluated by Auger and Energy Dispersive X-Ray Spectroscopy. J. Biol. Phys. 2012, 38, 279–291. [Google Scholar] [CrossRef]
- Maldonado, S.; Findeisen, R. Force-Induced Bone Growth and Adaptation: A System Theoretical Approach to Understanding Bone Mechanotransduction. IOP Conf. Ser. Mater. Sci. Eng. 2010, 10, 012127. [Google Scholar] [CrossRef]
- Odgaard, A.; Weinans, H. Bone Structure and Remodeling; Recent Advances in Human Biology; World Scientific: Singapore, 1995; Volume 2, ISBN 978-981-02-2190-4. [Google Scholar]
- Panizza, P.; Algaba, H.; Postic, M.; Raffy, G.; Courbin, L.; Artzner, F. Order-Disorder Structural Transitions in Mazes Built by Evaporating Drops. Phys. Rev. Lett. 2018, 121, 078002. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Gojgini, S.; Chen, T.-H.; Fei, P.; Dong, S.; Ho, C.-M.; Segura, T. Directing Three-Dimensional Multicellular Morphogenesis by Self-Organization of Vascular Mesenchymal Cells in Hyaluronic Acid Hydrogels. J. Biol. Eng. 2017, 11, 12. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.-H.; Zhu, X.; Pan, L.; Zeng, X.; Garfinkel, A.; Tintut, Y.; Demer, L.L.; Zhao, X.; Ho, C.-M. Directing Tissue Morphogenesis via Self-Assembly of Vascular Mesenchymal Cells. Biomaterials 2012, 33, 9019–9026. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Capasso, L. Antiquity of Cancer. Int. J. Cancer J. Int. Cancer 2005, 113, 2–13. [Google Scholar] [CrossRef]
- Nudelman, F.; Lausch, A.J.; Sommerdijk, N.A.J.M.; Sone, E.D. In Vitro Models of Collagen Biomineralization. J. Struct. Biol. 2013, 183, 258–269. [Google Scholar] [CrossRef]
- Aghajanian, P.; Mohan, S. The Art of Building Bone: Emerging Role of Chondrocyte-to-Osteoblast Transdifferentiation in Endochondral Ossification. Bone Res. 2018, 6, 19. [Google Scholar] [CrossRef]
- Robin, M.; Almeida, C.; Azaïs, T.; Haye, B.; Illoul, C.; Lesieur, J.; Giraud-Guille, M.-M.; Nassif, N.; Hélary, C. Involvement of 3D Osteoblast Migration and Bone Apatite during in Vitro Early Osteocytogenesis. Bone 2016, 88, 146–156. [Google Scholar] [CrossRef]
- Florencio-Silva, R.; Sasso, G.R.d.S.; Sasso-Cerri, E.; Simões, M.J.; Cerri, P.S. Biology of Bone Tissue: Structure, Function, and Factors That Influence Bone Cells. BioMed Res. Int. 2015, 2015, 421746. [Google Scholar] [CrossRef]
- Lamoureux, F.; Baud’huin, M.; Rodriguez Calleja, L.; Jacques, C.; Berreur, M.; Rédini, F.; Lecanda, F.; Bradner, J.E.; Heymann, D.; Ory, B. Selective Inhibition of BET Bromodomain Epigenetic Signalling Interferes with the Bone-Associated Tumour Vicious Cycle. Nat. Commun. 2014, 5, 3511. [Google Scholar] [CrossRef]
- Gleave, M.; Hsieh, J.; Gao, C.; Voneschenbach, A.; Chung, L. Acceleration of Human Prostate-Cancer Growth-Invivo by Factors Produced by Prostate and Bone Fibroblasts. Cancer Res. 1991, 51, 3753–3761. [Google Scholar]
- Ribelli, G.; Simonetti, S.; Iuliani, M.; Rossi, E.; Vincenzi, B.; Tonini, G.; Pantano, F.; Santini, D. Osteoblasts Promote Prostate Cancer Cell Proliferation Through Androgen Receptor Independent Mechanisms. Front. Oncol. 2021, 11, 789885. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.B.; Im, J.G.; Goo, J.M.; Chung, M.J.; Kim, M.Y. Atypical Pulmonary Metastases: Spectrum of Radiologic Findings. Radiographics 2001, 21, 403–417. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, C.F.; Custódio, C.A.; Mano, J.F. Three-Dimensional Osteosarcoma Models for Advancing Drug Discovery and Development. Adv. Ther. 2019, 2, 1800108. [Google Scholar] [CrossRef]
- Kovar, H.; Bierbaumer, L.; Radic-Sarikas, B. The YAP/TAZ Pathway in Osteogenesis and Bone Sarcoma Pathogenesis. Cells 2020, 9, 972. [Google Scholar] [CrossRef]
- Boyé, P.; David, E.; Serres, F.; Pascal, Q.; Floch, F.; Geeraert, K.; Coste, V.; Marescaux, L.; Cagnol, S.; Goujon, J.-Y.; et al. Phase I Dose Escalation Study of 12b80 (Hydroxybisphosphonate Linked Doxorubicin) in Naturally Occurring Osteosarcoma. Oncotarget 2020, 11, 4281–4292. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bardouil, A.; Bizien, T.; Amiaud, J.; Fautrel, A.; Battaglia, S.; Almarouk, I.; Rouxel, T.; Panizza, P.; Perez, J.; Last, A.; et al. Sponge Morphology of Osteosarcoma Finds Origin in Synergy Between Bone Synthesis and Tumor Growth. Nanomaterials 2025, 15, 374. https://doi.org/10.3390/nano15050374
Bardouil A, Bizien T, Amiaud J, Fautrel A, Battaglia S, Almarouk I, Rouxel T, Panizza P, Perez J, Last A, et al. Sponge Morphology of Osteosarcoma Finds Origin in Synergy Between Bone Synthesis and Tumor Growth. Nanomaterials. 2025; 15(5):374. https://doi.org/10.3390/nano15050374
Chicago/Turabian StyleBardouil, Arnaud, Thomas Bizien, Jérome Amiaud, Alain Fautrel, Séverine Battaglia, Iman Almarouk, Tanguy Rouxel, Pascal Panizza, Javier Perez, Arndt Last, and et al. 2025. "Sponge Morphology of Osteosarcoma Finds Origin in Synergy Between Bone Synthesis and Tumor Growth" Nanomaterials 15, no. 5: 374. https://doi.org/10.3390/nano15050374
APA StyleBardouil, A., Bizien, T., Amiaud, J., Fautrel, A., Battaglia, S., Almarouk, I., Rouxel, T., Panizza, P., Perez, J., Last, A., Djediat, C., Bessot, E., Nassif, N., Rédini, F., & Artzner, F. (2025). Sponge Morphology of Osteosarcoma Finds Origin in Synergy Between Bone Synthesis and Tumor Growth. Nanomaterials, 15(5), 374. https://doi.org/10.3390/nano15050374







