Carboxymethyl Cellulose Surface Modification Alleviates the Toxicity of Fe-MOFs to Rice and Improves Iron Absorption
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Design
2.3. Synthesis of MIL and MIL@CMC Experimental Design
2.4. Determination of Rice Shoot Length, Root Length, and Biomass
2.5. Determination of Chlorophyll Content
2.6. Determination of Antioxidant Enzyme Activity and MDA Content
2.7. Determination of the Content of Mineral Elements
2.8. Distribution of Fe-MOFs in Plants
2.9. Statistical Analysis
3. Results and Discussion
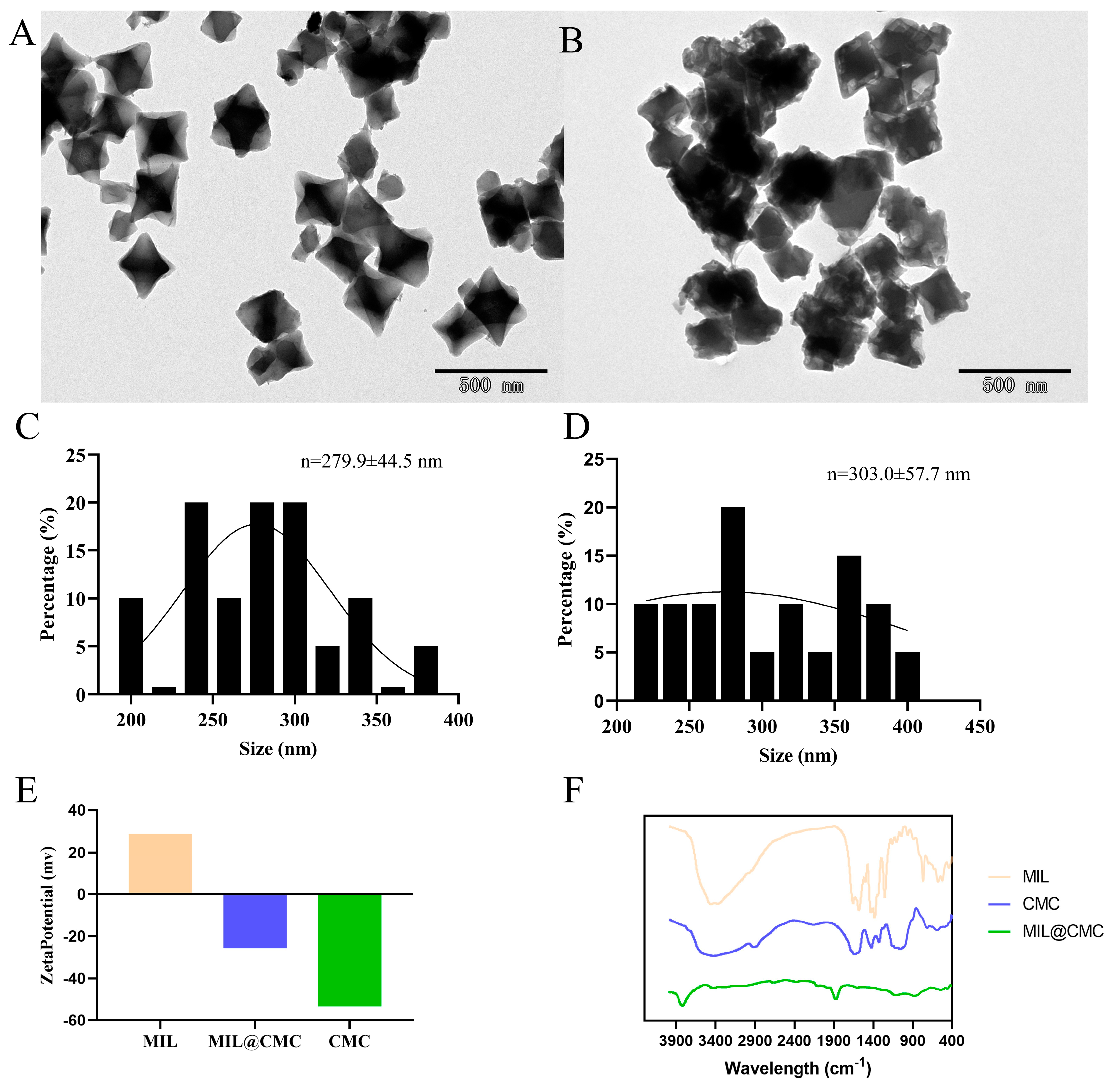
3.1. Synthesis and Characterization of MIL and MIL@CMC
3.2. Release of Fe Ions from Fe-MOFs in a Water Solution
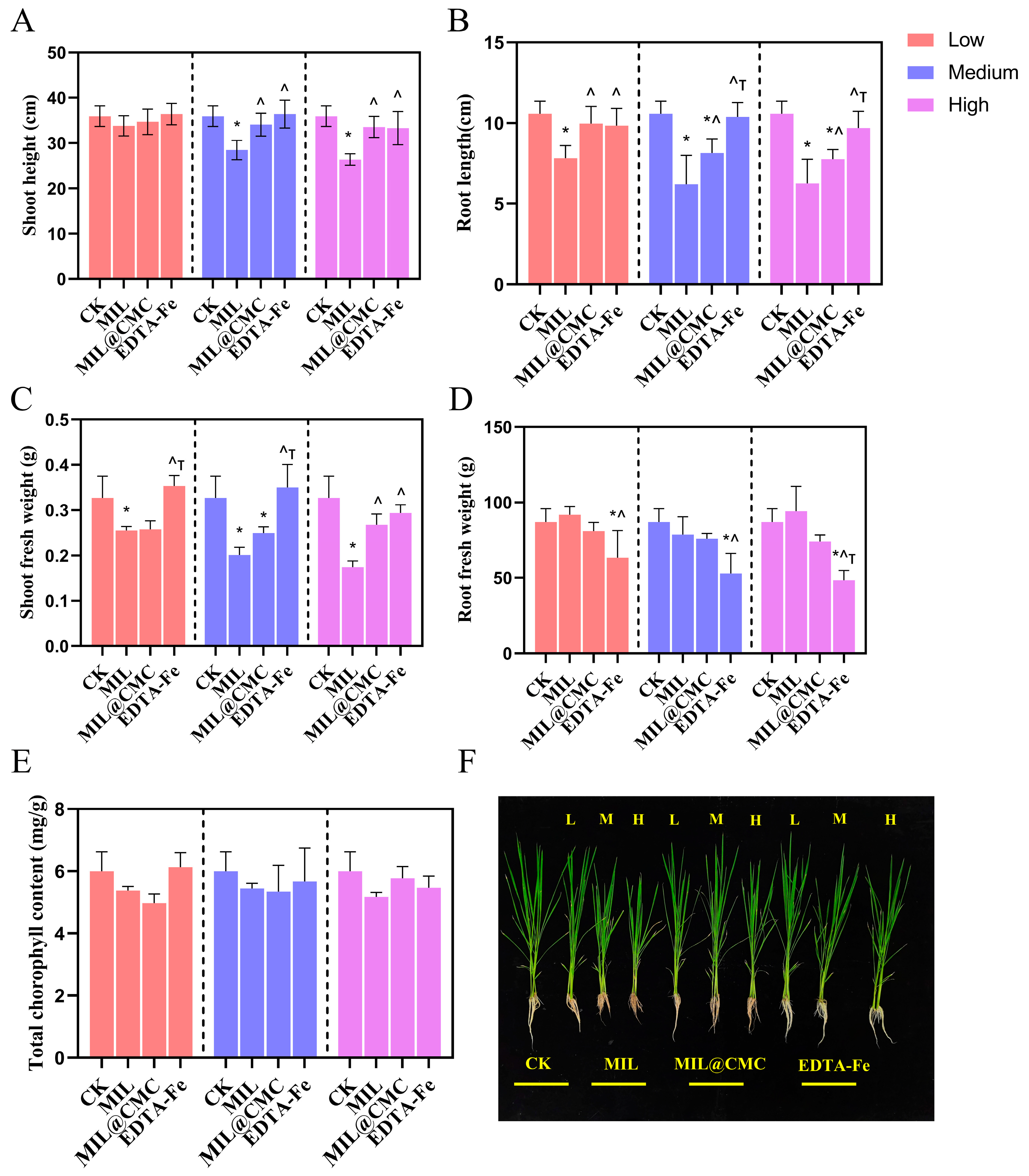
3.3. Effects of MIL and MIL@CMC on Rice Growth and Photosynthesis
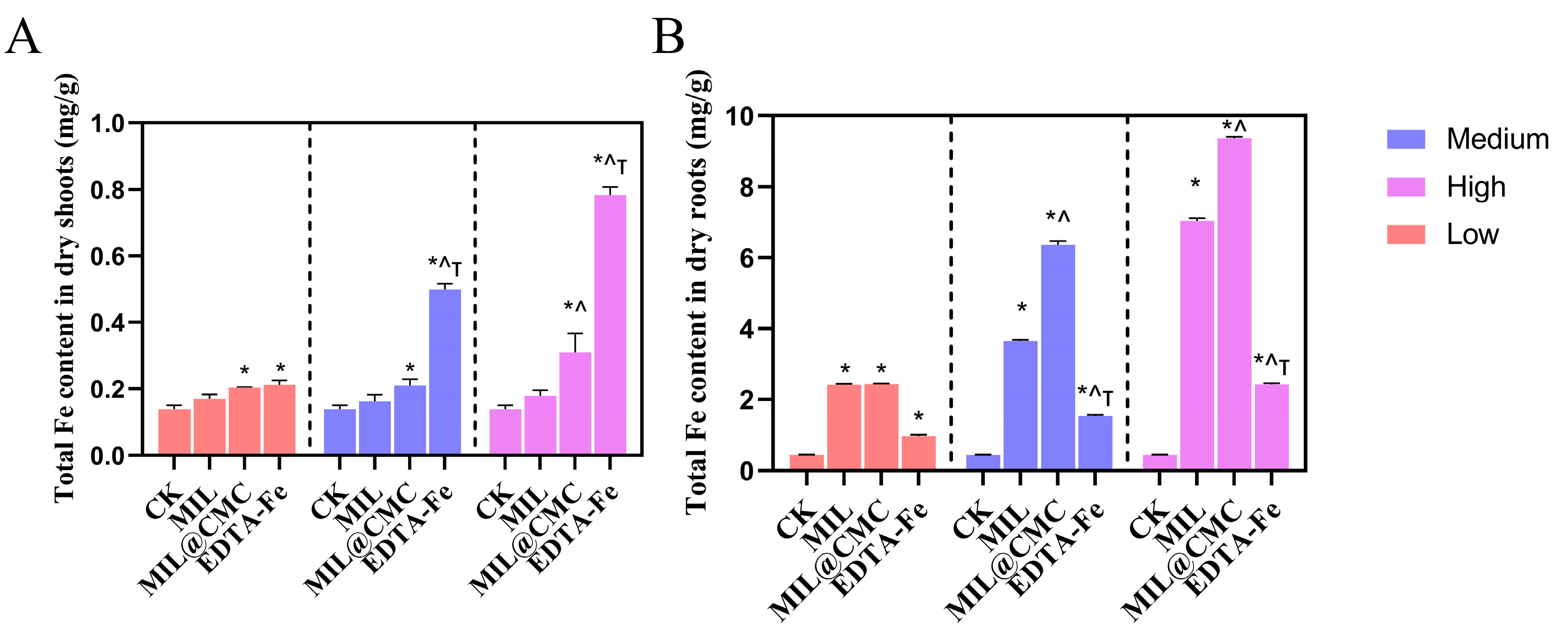
3.4. Uptake of Fe by Rice Aboveground and Root Systems
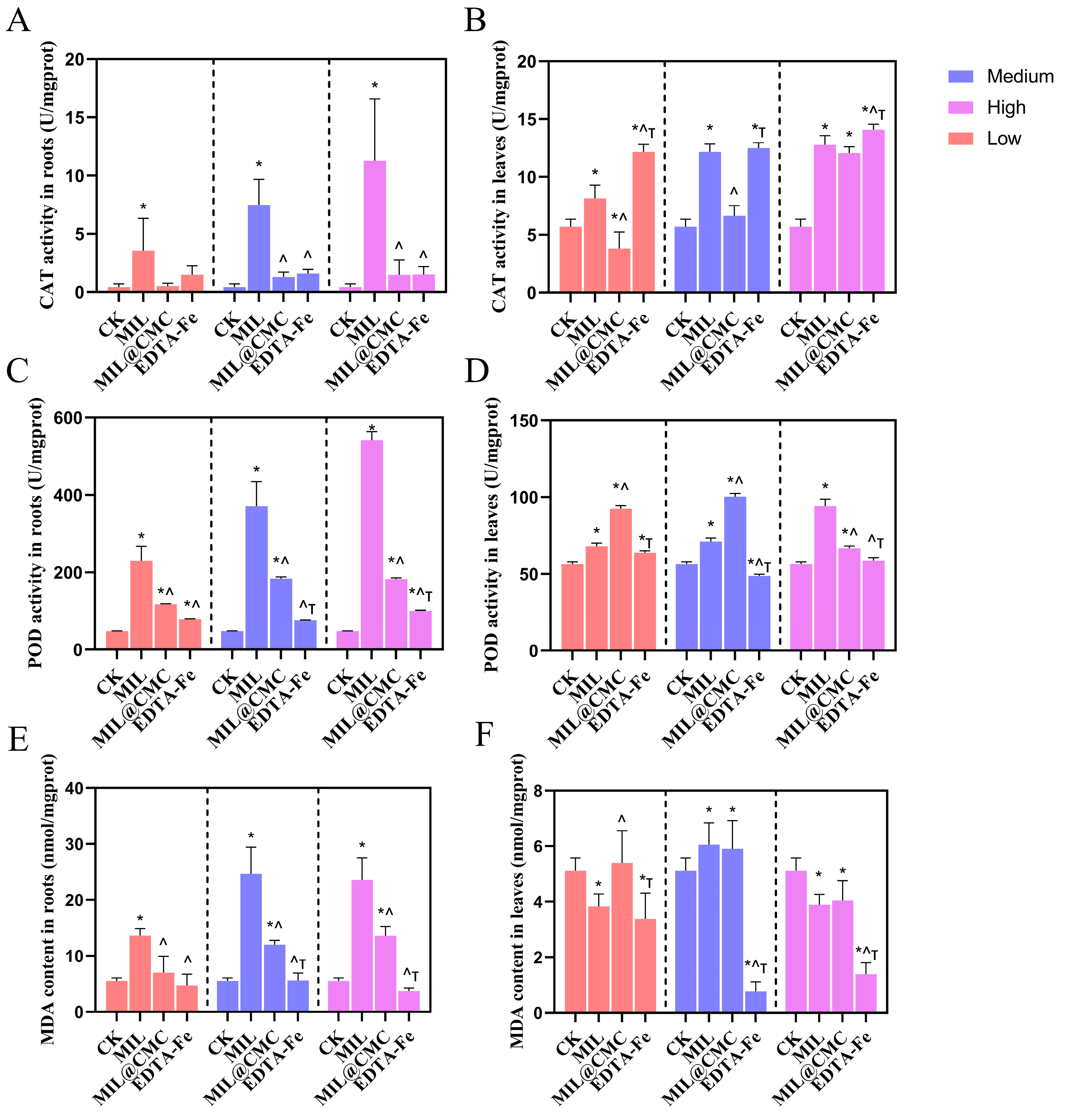
3.5. Effects of Fe-MOF Exposure on the Antioxidant System in Rice
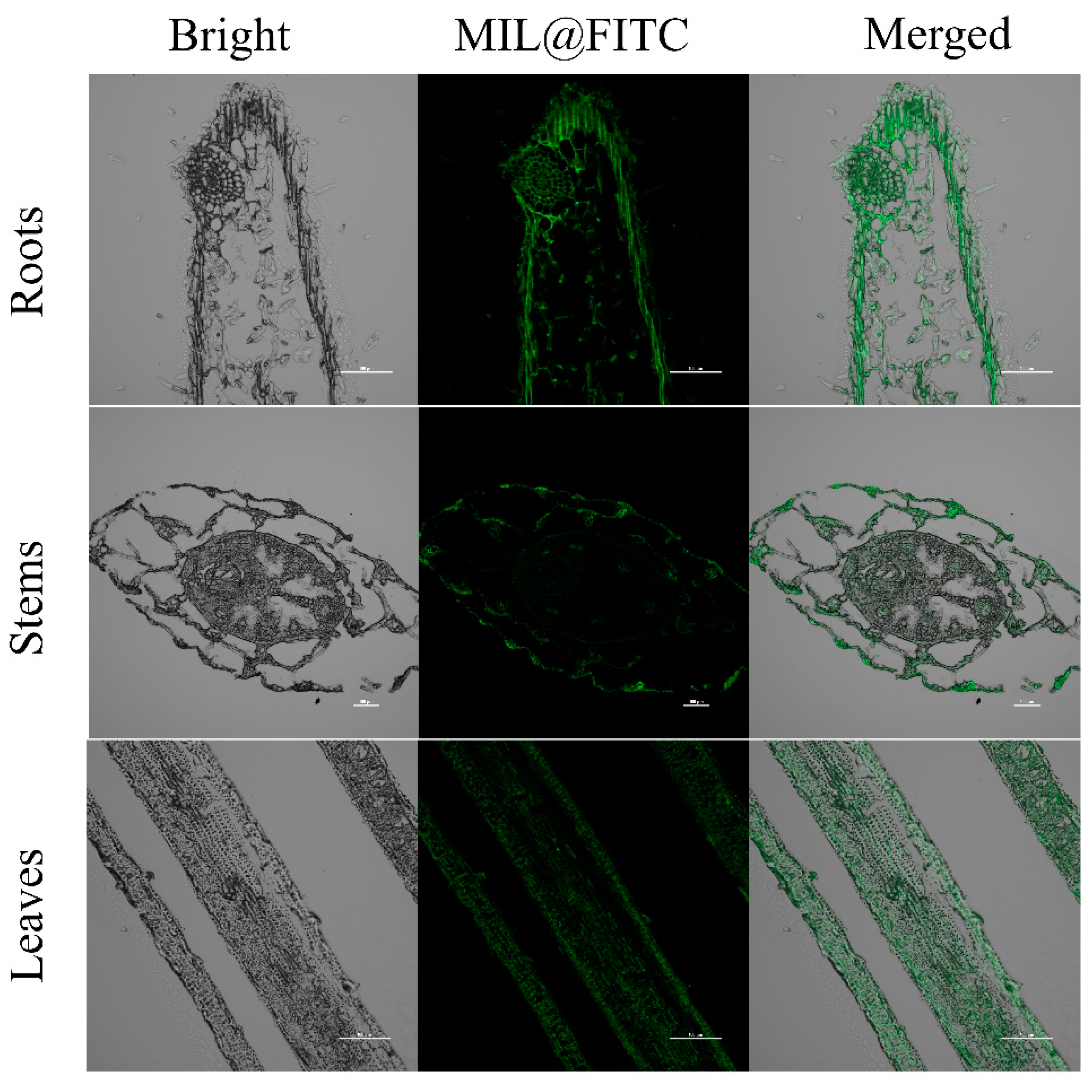
3.6. Distribution of Fe-MOFs in Rice Roots, Stems, and Leaves
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Altaf, M.A.; Hao, Y.Y.; Shu, H.Y.; Mumtaz, M.A.; Cheng, S.H.; Alyemeni, M.N.; Ahmad, P.; Wang, Z.W. Melatonin enhanced the heavy metal-stress tolerance of pepper by mitigating the oxidative damage and reducing the heavy metal accumulation. J. Hazard. Mater. 2023, 454, 131468. [Google Scholar] [CrossRef] [PubMed]
- Badwaik, L.S.; Borah, P.K.; Deka, S.C. Antimicrobial and enzymatic antibrowning film used as coating for bamboo shoot quality improvement. Carbohydr. Polym. 2014, 103, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Cai, M.K.; Li, Y.L.; Liu, Q.L.; Xue, Z.A.; Wang, H.P.; Fan, Y.N.; Zhu, K.L.; Ke, Z.F.; Su, C.Y.; Li, G.Q. One-Step Construction of Hydrophobic MOFs@COFs Core-Shell Composites for Heterogeneous Selective Catalysis. Adv. Sci. 2019, 6, 1802365. [Google Scholar] [CrossRef] [PubMed]
- Channab, B.E.; El Idrissi, A.; Essamlali, Y.; Zahouily, M. Nanocellulose: Structure, modification, biodegradation and applications in agriculture as slow/controlled release fertilizer, superabsorbent, and crop protection: A review. J. Environ. Manag. 2024, 352, 119928. [Google Scholar] [CrossRef]
- Chen, H.Y.; Yang, L.P.; Wu, P.Q.; Liu, P.P.; Xu, H.H.; Zhang, Z.X. Combined application of surfactants and iron-based metal-organic framework nanoparticles for targeted delivery of insecticides. Chem. Eng. J. 2024, 488, 151193. [Google Scholar] [CrossRef]
- Chen, J.; Pan, J.F.; Duan, M.J.; Fan, F.; Liu, J.B.; Hu, W.J.; Yu, D.; Sun, Z.Z.; Sheng, X.B.; Tan, Y.N.; et al. In situ images of Cd2+in rice reveal Cd2+protective mechanism using DNAzyme fluorescent probe. J. Hazard. Mater. 2025, 483, 136650. [Google Scholar] [CrossRef]
- Chen, J.Y.; Qin, C.C.; Mou, Y.; Cao, Y.X.; Chen, H.Y.; Yuan, X.Z.; Wang, H. Linker regulation of iron-based MOFs for highly effective Fenton-like degradation of refractory organic contaminants. Chem. Eng. J. 2023, 459, 141588. [Google Scholar] [CrossRef]
- Chen, Q.F.; Yao, M.Q.; Zhou, Y.Q.; Sun, Y.Y.; Zhang, G.X.; Pang, H. Etching MOF nanomaterials: Precise synthesis and electrochemical applications. Coord. Chem. Rev. 2024, 517, 216016. [Google Scholar] [CrossRef]
- Chu, F.H.; Wei, X.Y.; Lu, B.; Zhao, G.Y.; Li, Y.P.; Yang, K.X.; Zhu, Z.G.; Su, T.; Lü, H.Y. Flexible encapsulation polyoxometalate with electrostatically interaction into metal-organic framework for ultra-deep aerobic oxidation desulfurization of diesel via a biomimetic approach in deep eutectic solvents. Chem. Eng. J. 2024, 481, 148549. [Google Scholar] [CrossRef]
- Deng, G.; Zaman, Q.U.; Liu, C.; Luo, Y.; Xia, X.; Guo, L.H.; Sultan, K.; He, X.R.; Fahad, S.; Cheng, X. Phytoremediation of lead polluted mine soil by synergistic effect of chelating agents and nitrogen in hemp. Ind. Crops Prod. 2024, 222, 119815. [Google Scholar] [CrossRef]
- Dong, J.T.; Han, A.H.; Zhao, Y.L.; Li, H.M.; Yang, Y.; Yuan, B.W.; Wang, Y.S.; Liu, R.Q.; Yin, X.M.; Du, X.Z. Smart, degradable, and eco-friendly carboxymethyl cellulose-CaII hydrogel-like networks gated MIL-101(FeIII) nanoherbicides for paraquat delivery. Sci. Total Environ. 2023, 903, 166424. [Google Scholar] [CrossRef] [PubMed]
- Du, P.C.; Wen, T.T.; Gao, X.L.; Zhao, X.D.; Wang, M.H.; Huang, H.L. Metal cluster-dependent adsorption for rocephin antibiotic in MIL-101-NH2 metal-organic frameworks. Sep. Purif. Technol. 2025, 354, 129149. [Google Scholar] [CrossRef]
- Duan, H.T.; Shao, Z.Q.; Zhao, M.; Zhou, Z.W. Preparation and properties of environmental-friendly coatings based on carboxymethyl cellulose nitrate ester & modified alkyd. Carbohydr. Polym. 2016, 137, 92–99. [Google Scholar] [PubMed]
- Dutta, P.; Mukherjee, K.; Giri, T.K. Methacrylic acid grafted inulin and carboxymethyl cellulose as additives in the development of pH sensitive hydrogel matrix for colon targeting. J. Vinyl Addit. Technol. 2024, 30, 1485–1502. [Google Scholar] [CrossRef]
- Esmaeili, F.; Mehrabi, M.; Babapour, H.; Hassani, B.; Abedinia, A. Active coating based on carboxymethyl cellulose and flaxseed mucilage, containing burdock extract, for fresh-cut and fried potatoes. Lwt-Food Sci. Technol. 2024, 192, 115726. [Google Scholar] [CrossRef]
- Fertahi, S.; Bertrand, I.; Ilsouk, M.; Oukarroum, A.; Amjoud, M.; Zeroual, Y.; Barakat, A. New generation of controlled release phosphorus fertilizers based on biological macromolecules: Effect of formulation properties on phosphorus release. Int. J. Biol. Macromol. 2020, 143, 153–162. [Google Scholar] [CrossRef]
- Fu, H.; Li, H.; He, Q.W.; Du, G.W.; Li, J.F.; Zhang, Y.C.; Liang, Y.T.; Lin, B.F. Flexible fiber composite functional materials based on Fe-MOFs nanozyme catalytic activity for efficient point-of-use water disinfection. Chem. Eng. J. 2024, 490, 151879. [Google Scholar] [CrossRef]
- Gao, L.Y.; Sun, H.J.; Nagassa, M.; Li, X.; Pei, H.; Liu, S.Y.; Gu, Y.Y.; He, S.D. Edible film preparation by anthocyanin extract addition into acetylated cassava starch/sodium carboxymethyl cellulose matrix for oxidation inhibition of pumpkin seeds. Int. J. Biol. Macromol. 2024, 267, 131439. [Google Scholar] [CrossRef]
- Hoang, Q.T.; Kim, D.; Park, H.S.; Jang, W.; Cao, T.G.N.; Kang, J.H.; Ko, Y.T.; Mun, S.J.; Bhang, S.H.; Shim, M.S.; et al. Oxygen-Supplying Piezocatalytic Therapy of Hypoxic Tumors by Intratumoral Delivery of pH-Responsive Multicompartmental Carriers with Sequential Drug Release Capability. Adv. Funct. Mater. 2024, 34, 2306078. [Google Scholar] [CrossRef]
- Huang, L.L.; Yao, Y.; Ruan, Z.R.; Zhang, S.Q.; Feng, X.J.; Lu, C.; Zhao, J.M.; Yin, F.Y.; Cao, C.W.; Zheng, L. Baicalin nanodelivery system based on functionalized metal-organic framework for targeted therapy of osteoarthritis by modulating macrophage polarization. J. Nanobiotechnol. 2024, 22, 221. [Google Scholar] [CrossRef]
- Jeffers, T.L.; Purvine, S.O.; Nicora, C.D.; McCombs, R.; Upadhyaya, S.; Stroumza, A.; Whang, K.; Gallaher, S.D.; Dohnalkova, A.; Merchant, S.S.; et al. Iron rescues glucose-mediated photosynthesis repression during lipid accumulation in the green alga Chromochloris zofingiensis. Nat. Commun. 2024, 15, 6046. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.X.; Jiang, P.P.; You, S.H.; Qiu, H.; Liu, J.; Zhang, X.H.; Chen, M.Y.X. Mechanisms of manganese uptake and long-distance transport in the hyperaccumulator Celosia argentea Linn. Ecotoxicol. Environ. Saf. 2025, 289, 117514. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.H.; Park, H.; Smit, B.; Kim, J. A multi-modal pre-training transformer for universal transfer learning in metal-organic frameworks. Nat. Mach. Intell. 2023, 5, 309–318. [Google Scholar] [CrossRef]
- Liang, Y.; Wang, S.J.; Jia, H.J.; Yao, Y.J.; Song, J.H.; Yang, W.C.; Cao, Y.S.; Zhu, F.; Huo, Z.Y. pH/redox/α-amylase triple responsive metal-organic framework composites for pest management and plant growth promotion. Microporous Mesoporous Mater. 2022, 344, 112230. [Google Scholar] [CrossRef]
- Kudarha, R.; Dhas, N.; Mutalik, S. Distinct features of iron based metal organic frameworks (MOFs) for ferroptosis mediated cancer therapy: A comprehensive review. Coord. Chem. Rev. 2023, 494, 215330. [Google Scholar] [CrossRef]
- Liang, J.Y.; Guo, F.; Cao, S.F.; Zhao, K.; Zhao, K.X.; Wang, H.F.; Shao, X.F.; Wei, Y.Y.; Zhang, C.D.; Zheng, Y.H.; et al. γ-aminobutyric acid (GABA) alleviated oxidative damage and programmed cell death in fresh-cut pumpkins. Plant Physiol. Biochem. 2022, 180, 9–16. [Google Scholar] [CrossRef]
- Lin, R.F.; Zhao, T.C.; Chen, L.; Liu, M.C.; Yu, H.Y.; Wang, R.C.; Yuan, M.J.; Li, X.M.; Zhao, D.Y. Amphipathicity mediated endocytosis of mesoporous silica nanoparticles with tunable frameworks. Nano Res. 2024, 17, 8350–8359. [Google Scholar] [CrossRef]
- Liu, J.C.; Xu, D.J.; Xu, G.C.; Li, X.A.; Dong, J.T.; Luan, X.K.; Du, X.Z. Smart controlled-release avermectin nanopesticides based on metal-organic frameworks with large pores for enhanced insecticidal efficacy. Chem. Eng. J. 2023, 475, 146312. [Google Scholar] [CrossRef]
- Liu, Q.; Kawai, T.; Inukai, Y.; Aoki, D.; Feng, Z.H.; Xiao, Y.H.; Fukushima, K.; Lin, X.Y.; Shi, W.M.; Busch, W.; et al. A lignin-derived material improves plant nutrient bioavailability and growth through its metal chelating capacity. Nat. Commun. 2023, 14, 4866. [Google Scholar] [CrossRef]
- Liu, Y.Z.; Liu, D.; Han, X.; Chen, Z.P.; Li, M.; Jiang, L.W.; Zeng, J.G. Magnesium-Doped Carbon Quantum Dot Nanomaterials Alleviate Salt Stress in Rice by Scavenging Reactive Oxygen Species to Increase Photosynthesis. Acs Nano 2024, 18, 31188–31203. [Google Scholar] [CrossRef]
- Lu, Y.B.; Zhang, G.X.; Zhou, H.J.; Cao, S.; Zhang, Y.; Wang, S.L.; Pang, H. Enhanced Active Sites and Stability in Nano-MOFs for Electrochemical Energy Storage through Dual Regulation by Tannic Acid. Angew. Chem.-Int. Ed. 2023, 62, e202311075. [Google Scholar] [CrossRef] [PubMed]
- Luczak, J.; Kroczewska, M.; Baluk, M.; Sowik, J.; Mazierski, P.; Zaleska-Medynska, A. Morphology control through the synthesis of metal-organic frameworks. Adv. Colloid Interface Sci. 2023, 314, 102864. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.D.; Li, C.G.; Liu, Z.; Guo, W.; Sun, C.Z.; Zhao, S.S.; Wang, Q.; Li, Y.Y.; Chen, L.Y.; Zheng, H.; et al. Photocatalytic activation of peroxymonosulfate (PMS) by CNN@NH2-MIL-101(Fe) Z-scheme heterojunction for phthalates degradation under visible light irradiation. Chem. Eng. J. 2024, 481, 148683. [Google Scholar] [CrossRef]
- Ma, Y.J.; Yu, M.; Wang, Y.M.; Pan, S.H.; Sun, X.L.; Zhao, R.; Sun, Z.; Gao, R.; Guo, X.Y.; Xu, Y.; et al. A pH/cellulase dual stimuli-responsive cellulose-coated metal-organic framework for eco-friendly fungicide delivery. Chem. Eng. J. 2023, 462, 142190. [Google Scholar] [CrossRef]
- Marcuello, C.; Foulon, L.; Chabbert, B.; Aguié-Béghin, V.; Molinari, M. Atomic force microscopy reveals how relative humidity impacts the Young’s modulus of lignocellulosic polymers and their adhesion with cellulose nanocrystals at the nanoscale. Int. J. Biol. Macromol. 2020, 147, 1064–1075. [Google Scholar] [CrossRef]
- Moghadam, P.Z.; Chung, Y.G.; Snurr, R.Q. Progress toward the computational discovery of new metal-organic framework adsorbents for energy applications. Nat. Energy 2024, 9, 121–133. [Google Scholar] [CrossRef]
- Muhammad, I.; Yang, L.; Ahmad, S.; Farooq, S.; Khan, A.; Muhammad, N.; Ullah, S.; Adnan, M.; Ali, S.; Liang, Q.P.; et al. Melatonin-priming enhances maize seedling drought tolerance by regulating the antioxidant defense system. Plant Physiol. 2023, 191, 2301–2315. [Google Scholar] [CrossRef]
- Nair, K.M.; Thomas, N.; Pallilavalappil, S.; Mathew, S.; Deignan, K.; Hinder, S.J.; Brennan, B.; McArdle, F.; Pillai, S.C. Unravelling the impact of lower vacuum activation temperature on Fe2+/ Fe3+ mixed-valence unsaturated iron centres in MIL-101(Fe) and its impact on Fenton degradation of acetaminophen. J. Environ. Chem. Eng. 2024, 12, 113615. [Google Scholar] [CrossRef]
- Nikiforova, T.; Kozlov, V.; Razgovorov, P.; Politaeva, N.; Velmozhina, K.; Shinkevich, P.; Chelysheva, V. Heavy Metal Ions(II) Sorption by a Cellulose-Based Sorbent Containing Sulfogroups. Polymers 2023, 15, 4212. [Google Scholar] [CrossRef]
- Pandey, A.; Devi, L.L.; Gupta, S.; Prasad, P.; Agrwal, K.; Asif, M.H.; Pandey, A.K.; Bandyopadhyay, K.; Singh, A.P. Jasmonate signaling modulates root growth by suppressing iron accumulation during ammonium stress. Plant Physiol. 2024, 196, 2213–2231. [Google Scholar] [CrossRef]
- Pandey, K.; Omar, R.A.; Verma, N.; Gupta, G. Fe-carbon nanofiber-modified Mo-MOF for the controlled release and translocation of micronutrients in plants. Environ. Sci. Nano 2024, 11, 1597–1611. [Google Scholar] [CrossRef]
- Peng, X.X.; Cheng, K.; Yuan, Y.; Chen, S.; Chen, Y.L.; Qiao, H.; Liu, Z.Q.; Yang, F. Foliar application of iron strengthened- artificial humic acid promotes nitrogen fixation and improves soybean yield. Ind. Crops Prod. 2025, 224, 120368. [Google Scholar] [CrossRef]
- Plaeyao, K.; Talodthaisong, C.; Yingyuen, W.; Kaewbundit, R.; Tun, W.S.T.; Saenchoopa, A.; Kayunkid, N.; Wiwattananukul, R.; Sakulsombat, M.; Kulchat, S. Biodegradable antibacterial food packaging based on carboxymethyl cellulose from sugarcane bagasse/cassava starch/chitosan/gingerol extract stabilized silver nanoparticles (Gin-AgNPs) and vanillin as cross-linking agent. Food Chem. 2025, 466, 2235. [Google Scholar] [CrossRef] [PubMed]
- Qin, L.Z.; Xiong, X.H.; Wang, S.H.; Zhang, L.; Meng, L.L.; Yan, L.; Fan, Y.N.; Yan, T.A.; Liu, D.H.; Wei, Z.W.; et al. MIL-101-Cr/Fe/Fe-NH2 for Efficient Separation of CH4 and C3H8 from Simulated Natural Gas. Acs Appl. Mater. Interfaces 2022, 14, 45444. [Google Scholar] [CrossRef] [PubMed]
- Shan, Y.P.; Cao, L.D.; Muhammad, B.; Xu, B.; Zhao, P.Y.; Cao, C.; Huang, Q.L. Iron-based porous metal-organic frameworks with crop nutritional function as carriers for controlled fungicide release. J. Colloid Interface Sci. 2020, 566, 383–393. [Google Scholar] [CrossRef]
- Shi, X.C.; Wang, Z.B.; Liu, S.Y.; Xia, Q.Q.; Liu, Y.Z.; Chen, W.S.; Yu, H.P.; Zhang, K. Scalable production of carboxylated cellulose nanofibres using a green and recyclable solvent. Nat. Sustain. 2024, 7, s41893-024-01267-0. [Google Scholar] [CrossRef]
- Song, S.J.; Wan, M.H.; Feng, W.L.; Tian, Y.; Jiang, X.F.; Luo, Y.; Shen, J. Environmentally Friendly Zr-Based MOF for Pesticide Delivery: Ultrahigh Loading Capacity, pH-Responsive Release, Improved Leaf Affinity, and Enhanced Antipest Activity. Langmuir 2022, 38, 10867–10874. [Google Scholar] [CrossRef]
- Sun, L.; Hou, C.Q.; Wei, N.; Tan, Y.F.; Liang, Q.W.; Feng, J.G. pH/cellulase dual environmentally responsive nano-metal organic frameworks for targeted delivery of pesticides and improved biosafety. Chem. Eng. J. 2023, 478, 147294. [Google Scholar] [CrossRef]
- Vuong, M.D.L.; Horbenko, Y.; Frégnaux, M.; Christodoulou, I.; Martineau-Corcos, C.; Levitz, P.; Rollet, A.L.; Gref, R.; Haouas, M. Degradation and Erosion of Metal-Organic Frameworks: Comparative Study of a NanoMIL-100 Drug Delivery System. Acs Appl. Mater. Interfaces 2024, 16, 2086–2100. [Google Scholar] [CrossRef]
- Wang, C.Y.; Wang, C.C.; Chu, H.Y.; Wang, P.; Zhao, C.; Fu, H.F. In situ growth of MIL-101 (Fe) on waste PET plastic slices for effective arsenic removal. Sep. Purif. Technol. 2024, 331, 125589. [Google Scholar] [CrossRef]
- Wang, Q.L.; Zhu, G.K.; Wang, Q.B.; Zhao, W.C.; Li, Y.B.; Shakoor, N.; Tan, Z.Q.; Wang, F.Y.; Zhang, P.; Rui, Y.K. The fate and impact of Co3O4 nanoparticles in the soil environment: Observing the dose effect of nanoparticles on soybeans. J. Environ. Manag. 2024, 368, 122186. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.X.; Li, M.S.; Ying, J.H.; Shen, J.; Dou, D.L.; Yin, M.Z.; Whisson, S.C.; Birch, P.R.J.; Yan, S.; Wang, X.D. High-efficiency green management of potato late blight by a self-assembled multicomponent nano-bioprotectant. Nat. Commun. 2023, 14, 5622. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.Y.; Wang, L.Q.; Ma, C.X.; Wang, K.X.; Hao, Y.; Chen, Q.; Mo, Y.; Rui, Y.K. Effects of cerium oxide on rice seedlings as affected by co-exposure of cadmium and salt. Environ. Pollut. 2019, 252, 1087–1096. [Google Scholar] [CrossRef] [PubMed]
- Wisniewska, P.; Haponiuk, J.; Saeb, M.R.; Rabiee, N.; Bencherif, S.A. Mitigating metal-organic framework (MOF) toxicity for biomedical applications. Chem. Eng. J. 2023, 471, 144400. [Google Scholar] [CrossRef]
- Xie, M.Z.; Zhan, Z.H.; Zhang, C.Q.; Xu, W.Q.; Zhang, C.; Chen, Y.P.; Dong, Z.C.; Wang, Z.L. Programmable Microfluidics Enabled by 3D Printed Bionic Janus Porous Matrics for Microfluidic Logic Chips. Small 2023, 19, 2300047. [Google Scholar] [CrossRef]
- Xu, C.L.; Cao, L.D.; Liu, T.T.; Chen, H.P.; Li, Y.B. pH-responsive copper-doped ZIF-8 MOF nanoparticles for enhancing the delivery and translocation of pesticides in wheat plants. Environ. Sci. Nano 2023, 10, 2578–2590. [Google Scholar] [CrossRef]
- Xu, Q.Z.; Sun, Y.T.; Lv, T.; Liu, H. Selective CO2 photoreduction into CO over Ti3C2 quantum dots decorated NH2-MIL-101(Fe) heterostructures. J. Alloys Compd. 2023, 954, 170088. [Google Scholar] [CrossRef]
- Xu, W.J.; Lin, Z.X.; Pan, S.J.; Chen, J.Q.; Wang, T.Z.; Cortez-Jugo, C.; Caruso, F. Direct Assembly of Metal-Phenolic Network Nanoparticles for Biomedical Applications. Angew. Chem. Int. Ed. 2023, 62, e202312925. [Google Scholar] [CrossRef]
- Yang, L.P.; Chen, H.Y.; Du, P.R.; Miao, X.R.; Huang, S.Q.; Cheng, D.M.; Xu, H.H.; Zhang, Z.X. Inhibition mechanism of Rhizoctonia solani by pectin-coated iron metal-organic framework nanoparticles and evidence of an induced defense response in rice. J. Hazard. Mater. 2024, 474, 134807. [Google Scholar] [CrossRef]
- Yang, L.P.; Chen, H.Y.; Kaziem, A.E.; Miao, X.R.; Huang, S.Q.; Cheng, D.M.; Xu, H.H.; Zhang, Z.X. Effects of Exposure to Different Types of Metal-Organic Framework Nanoparticles on the Gut Microbiota and Liver Metabolism of Adult Zebrafish. Acs Nano 2024, 18, 25425–25445. [Google Scholar] [CrossRef]
- Yang, L.P.; Chen, H.Y.; Zhu, S.Q.; Zhao, S.J.; Huang, S.Q.; Cheng, D.M.; Xu, H.H.; Zhang, Z.X. Pectin-Coated Iron-Based Metal-Organic Framework Nanoparticles for Enhanced Foliar Adhesion and Targeted Delivery of Fungicides. Acs Nano 2024, 18, 6533–6549. [Google Scholar] [CrossRef] [PubMed]
- Yong, J.X.; Xu, W.; Wu, M.M.; Zhang, R.; Mann, C.W.G.; Liu, G.Q.; Brosnan, C.A.; Mitter, N.; Carroll, B.J.; Xu, Z.P. Lysozyme-coated nanoparticles for active uptake and delivery of synthetic RNA and plasmid-encoded genes in plants. Nat. Plants 2025, 11, 131–144. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.F.; Zhang, P.; He, M.K.; Cao, Y.; Adeel, M.; Shakoor, N.; Jiang, Y.Q.; Zhao, W.C.; Li, Y.B.; Li, M.S.; et al. Iron-based nanomaterials reduce cadmium toxicity in rice (Oryza sativa L.) by modulating phytohormones, phytochelatin, cadmium transport genes and iron plaque formation. Environ. Pollut. 2023, 320, 121063. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Lu, S.X.; Xu, P.W.; Niu, D.Y.; Puglia, D.; Yang, W.J.; Ma, P.M. Tendon-inspired robust ionic conductive hydrogels with multi-hierarchical structures towards asthmatic patients’ medication monitoring. Green Chem. 2025, 27, 684–695. [Google Scholar] [CrossRef]
- Zhu, G.K.; Tang, Y.Y.; Ding, Y.R.; Zhao, W.C.; Wang, Q.L.; Li, Y.B.; Wang, Q.B.; Zhang, P.; Tan, Z.Q.; Rui, Y.K. Synergistic effect of nano-iron phosphide and wood vinegar on soybean production and grain quality. Environ. Sci. Nano 2024, 11, 4634–4643. [Google Scholar] [CrossRef]
- Zuo, X.; Guo, X.P.; Gu, Y.N.; Zhao, D.; Zou, Z.; Shen, Y.Y.; He, C.L.; Rong, Y.; Xu, C.N.; Wang, F. A Novel Catalase Nanogels for Effective Treatment of Neutrophilic Asthma. Adv. Funct. Mater. 2024, 34, 2316496. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Tang, Y.; Ding, Y.; Lyu, Y.; Su, W.; Nadeem, M.; Zhang, P.; Rui, Y. Carboxymethyl Cellulose Surface Modification Alleviates the Toxicity of Fe-MOFs to Rice and Improves Iron Absorption. Nanomaterials 2025, 15, 336. https://doi.org/10.3390/nano15050336
Li Y, Tang Y, Ding Y, Lyu Y, Su W, Nadeem M, Zhang P, Rui Y. Carboxymethyl Cellulose Surface Modification Alleviates the Toxicity of Fe-MOFs to Rice and Improves Iron Absorption. Nanomaterials. 2025; 15(5):336. https://doi.org/10.3390/nano15050336
Chicago/Turabian StyleLi, Yuanbo, Yuying Tang, Yanru Ding, Yaping Lyu, Wenhao Su, Muhammad Nadeem, Peng Zhang, and Yukui Rui. 2025. "Carboxymethyl Cellulose Surface Modification Alleviates the Toxicity of Fe-MOFs to Rice and Improves Iron Absorption" Nanomaterials 15, no. 5: 336. https://doi.org/10.3390/nano15050336
APA StyleLi, Y., Tang, Y., Ding, Y., Lyu, Y., Su, W., Nadeem, M., Zhang, P., & Rui, Y. (2025). Carboxymethyl Cellulose Surface Modification Alleviates the Toxicity of Fe-MOFs to Rice and Improves Iron Absorption. Nanomaterials, 15(5), 336. https://doi.org/10.3390/nano15050336







