Design Strategies of S8 Molecule Cathodes for Room-Temperature Na-S Batteries
Abstract
1. Introduction
2. Nanostructure Engineering
3. Catalyst Strategies
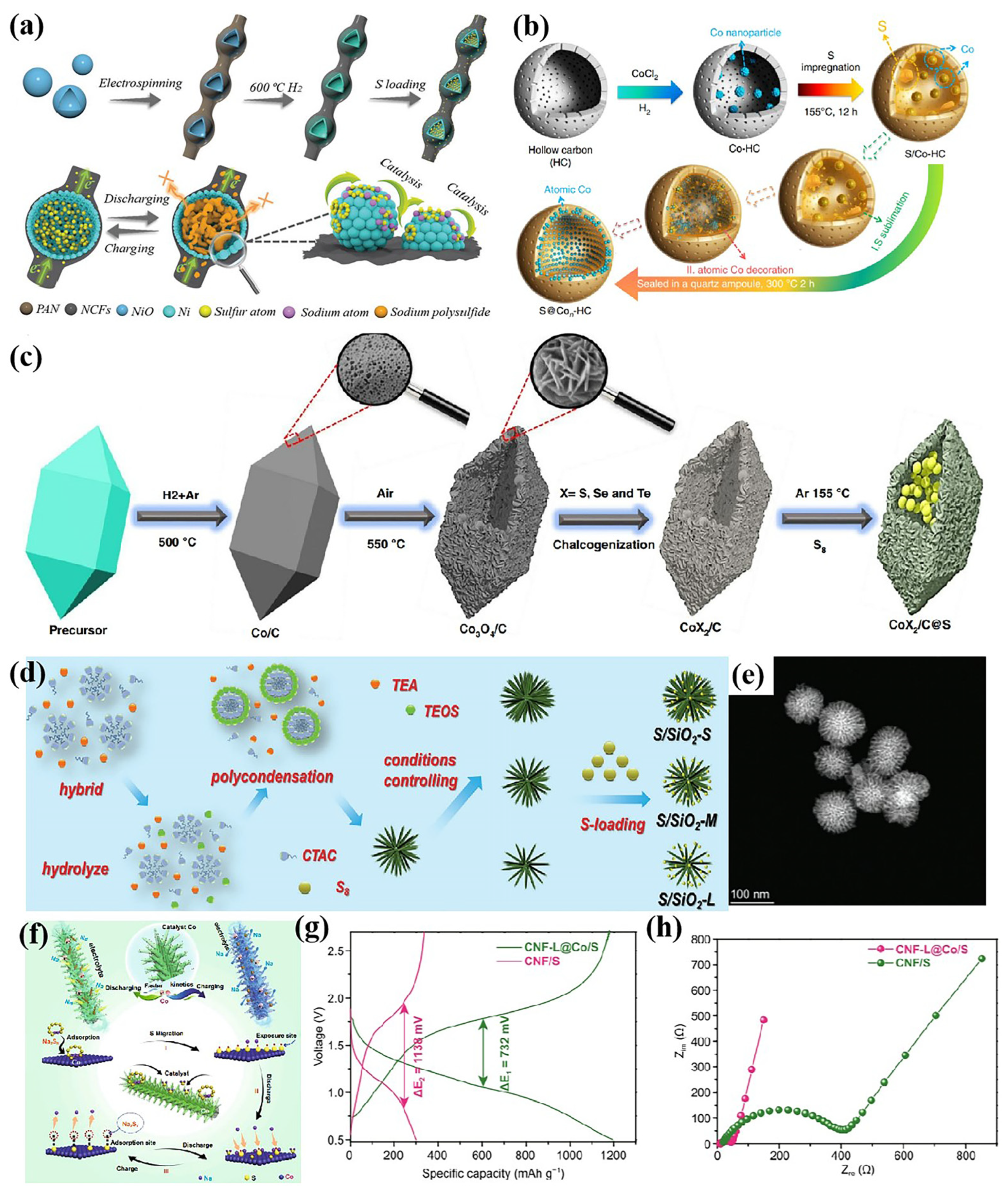
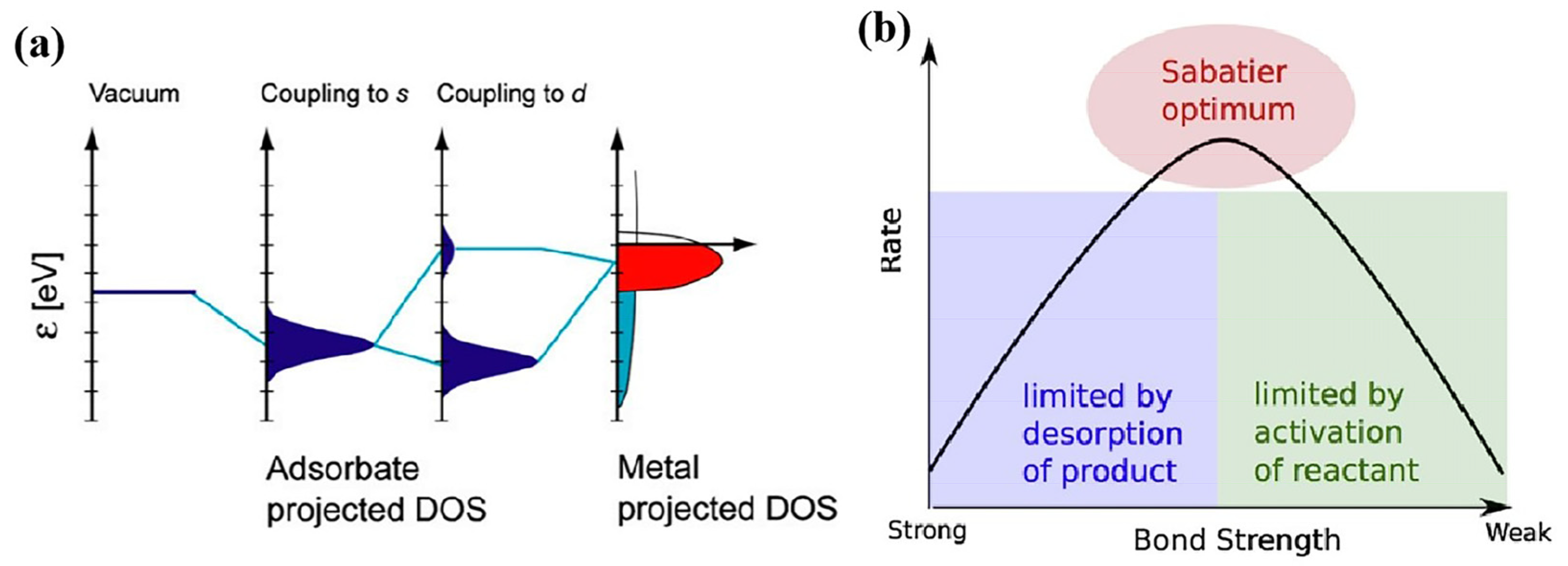
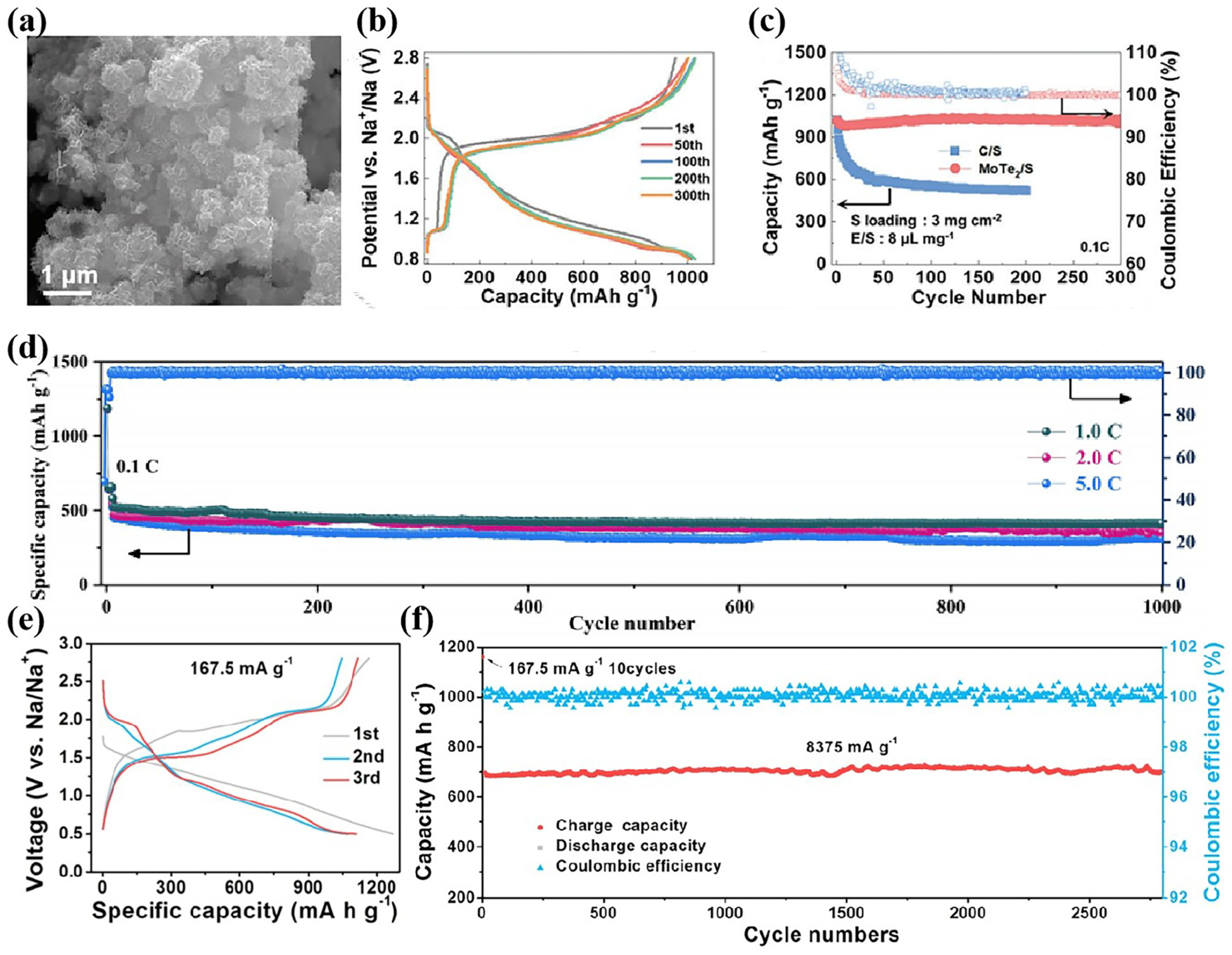
3.1. Metal Catalysts
3.2. Compound Catalysts
3.2.1. Adsorption Mode
3.2.2. Electronic State Regulation
3.2.3. Intercalation Modification
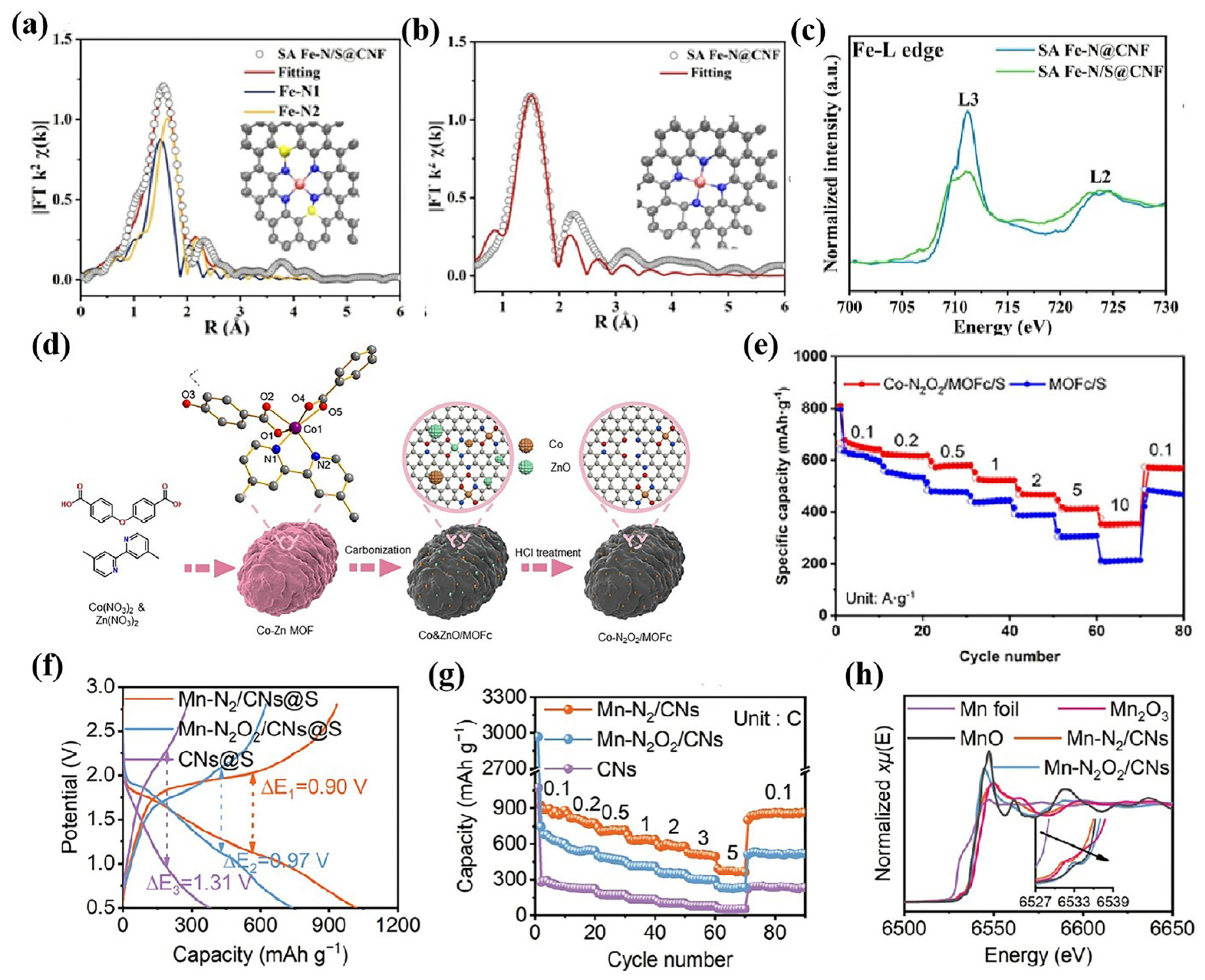
3.3. Atomically Dispersed Catalysts
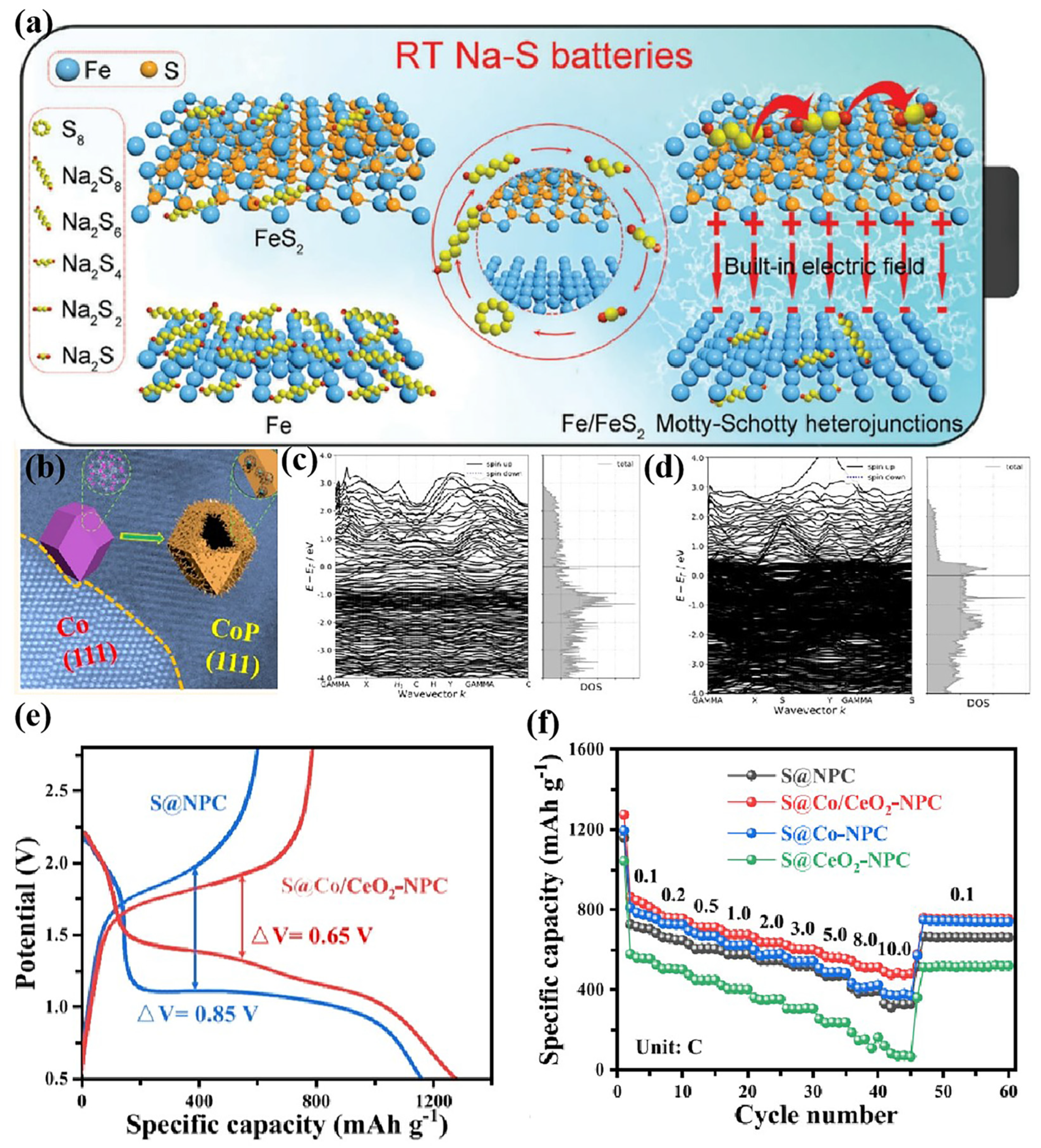
3.4. Heterojunctions

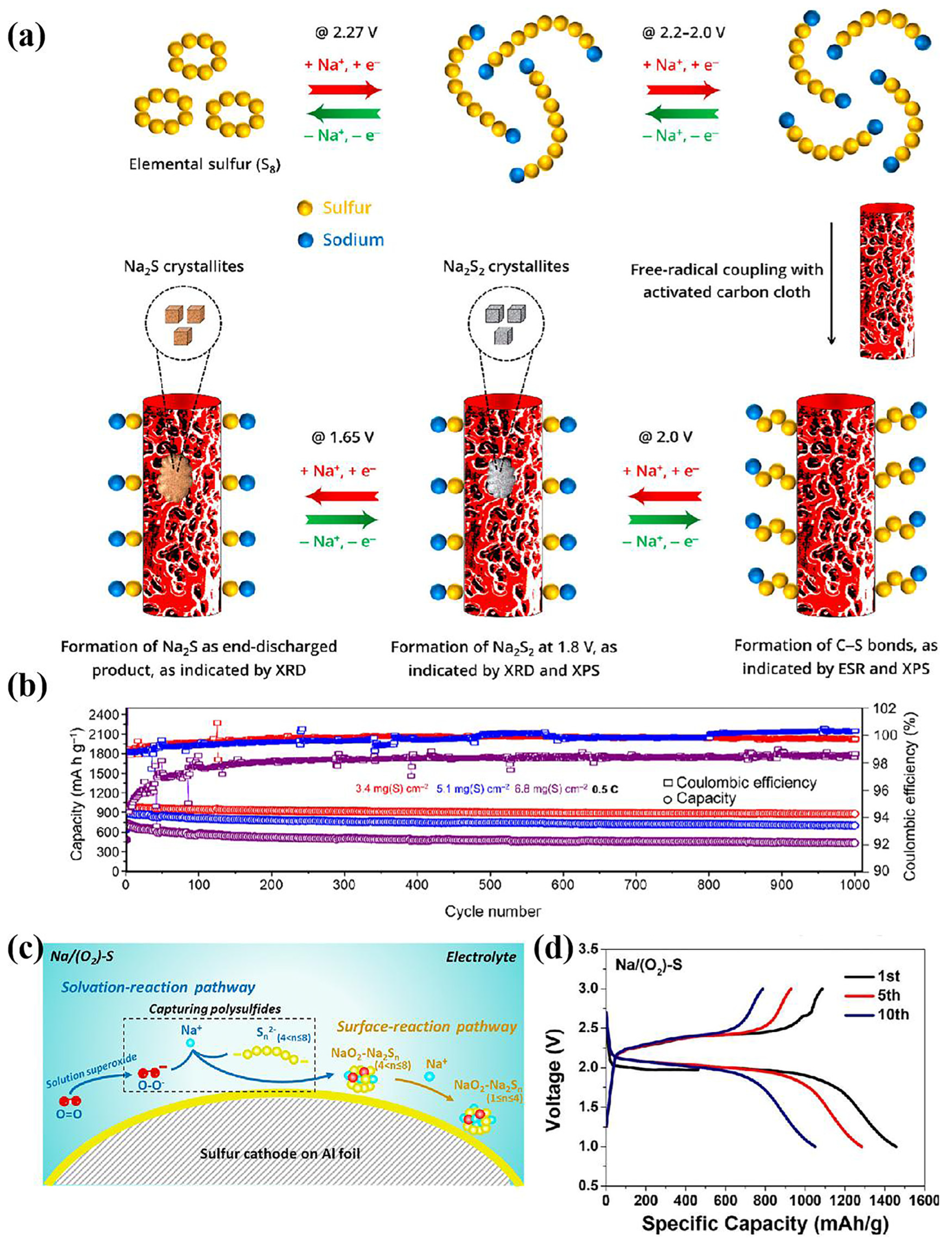
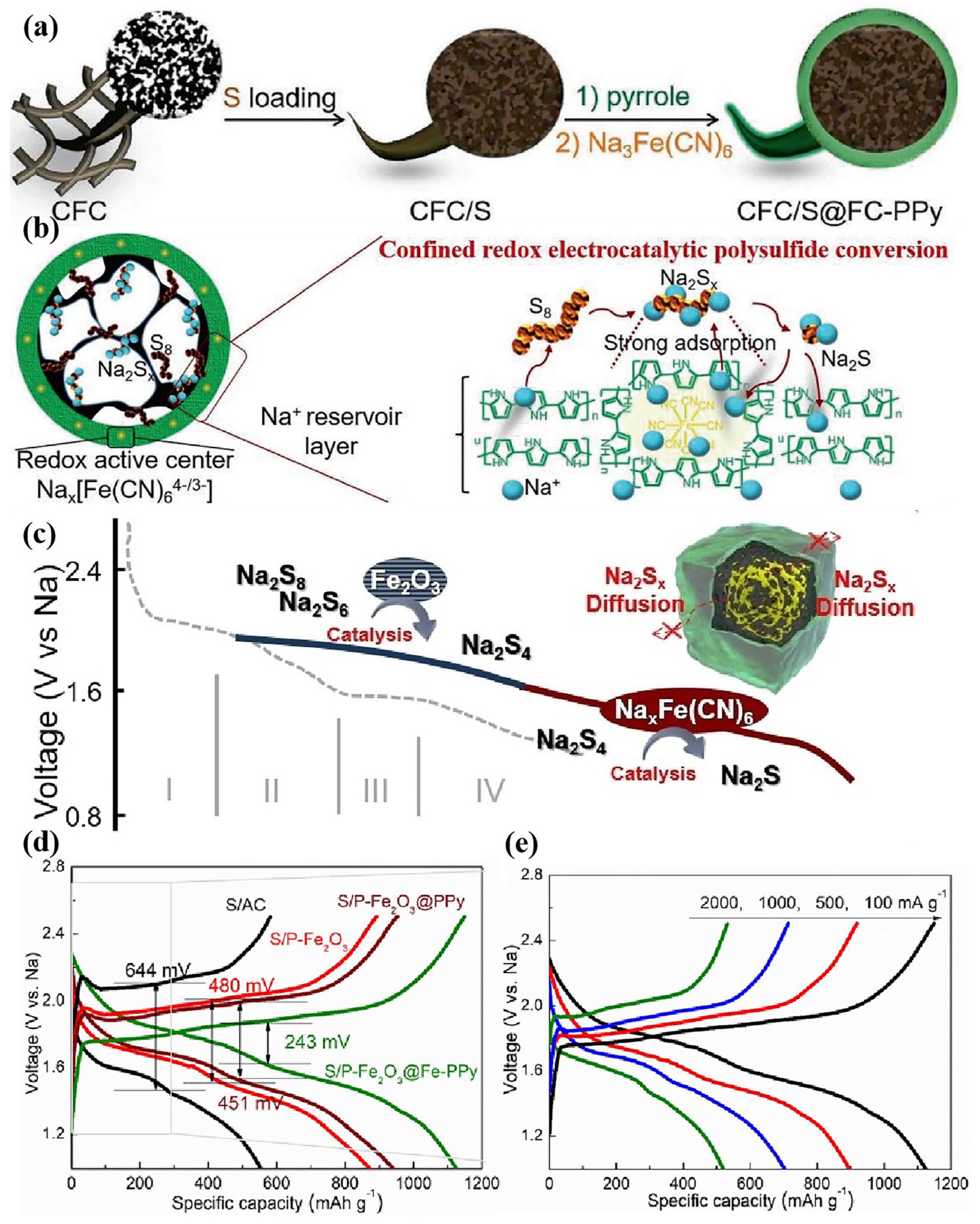
4. Regulating Sulfur Species Conversion Pathways
5. Conclusions and Outlook
| Host | Sulfur Loading Mass (mg cm−2) | Current Density | Cycle Number | Specifc Capacity (mAh g−1) | Ref. |
|---|---|---|---|---|---|
| Bipyramid prisms CoS2/C | 4.4 | 0.5 C | 800 | 675 | [12] |
| Ultrafine α-MoC1-x | 2 | 1 C 5 C | 1000 1000 | 418.4 312.6 | [73] |
| Urchin-Like SiO2 | 0.7–1.1 | 2 A g−1 5.0 A g−1 | 1050 500 | 1280.8 1132.6 | [4] |
| 2D/3D Co4N–NC@CC | 1 | 1 C | 1000 | 592 | [17] |
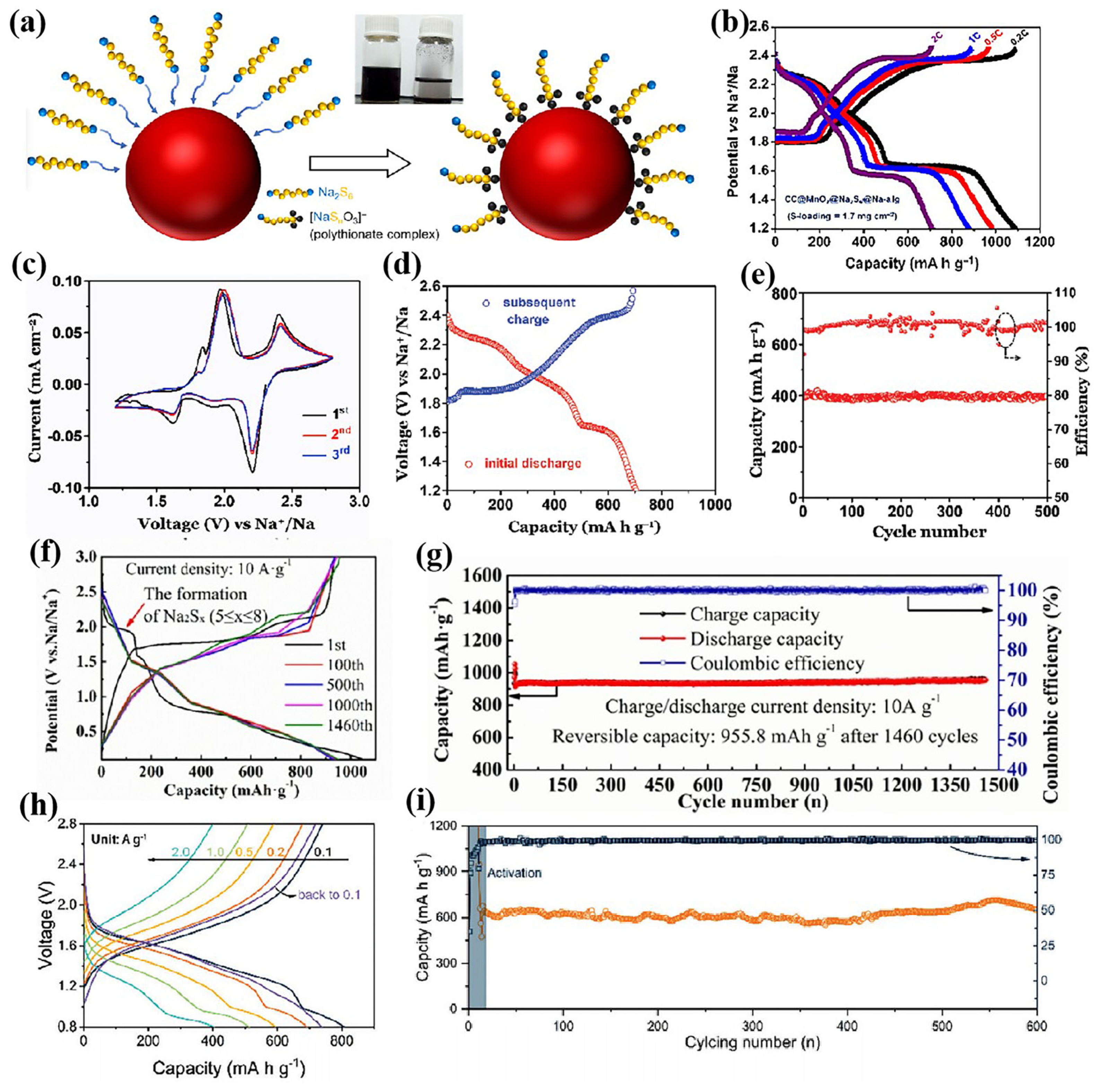
| Amorphous Silica | 0.7–1.1 | 10 A g−1 | 1460 | 955.8 | [62] |
| Fe3N/C | ~2.8 | 8375 mA g−1 | 2800 | 696 | [2] |
| Ta3N5-x@NMC | ~1 | 3.35 A g−1 | 2000 | 730 | [3] |
| TiO2 anatase/rutile (QVTRA) | - | 1 C | 1000 | 759 | [59] |
| CNF/CoFe2O4 | 0.5–0.6 | 1 C | 2700 | 542 | [81] |
| Sodiated MoS2 | 1.5 | 0.2 A g−1 1 A g−1 | 800 2800 | 774.2 360.7 | [87] |
| 3D-PNCV | - | 5 A g−1 | 800 | 445 | [91] |
| Y SAs/NC | - | 5 A g−1 | 1000 | 510 | [92] |
| Mn-N2/CNs | ~0.9 | 3 C | 2300 | 458 | [98] |
| Fe-Nx-Fe | 1.5–2 | 10 A g−1 | 5000 | 325 | [102] |
| Co-CeO2 | 1.5 | 5 C | 1000 | 561.1 | [13] |
| ZnS-NC@Ni-N4 | 1.4 | 5 A g−1 | 2000 | 650 | [118] |
| MoC-W2C-MCNFs | 2 | 0.2 A g−1 4 A g−1 | 500 3500 | 640 200 | [114] |
| BaTiO3-C-TiO2 | 1.2–1.4 | 1 A g−1 2 A g−1 | 1400 3000 | 524.8 382 | [104] |
| ITO@ACC | 6.8 | 0.5 C | 1000 | 445 | [79] |
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wen, Z.Y.; Hu, Y.Y.; Wu, X.W.; Han, J.D.; Gu, Z.H. Main Challenges for High Performance NAS Battery: Materials and Interfaces. Adv. Funct. Mater. 2013, 23, 1005–1018. [Google Scholar] [CrossRef]
- Qi, Y.R.; Li, Q.J.; Wu, Y.K.; Bao, S.J.; Li, C.M.; Chen, Y.M.; Wang, G.X.; Xu, M.W. A Fe3N/carbon composite electrocatalyst for effective polysulfides regulation in room-temperature Na-S batteries. Nat. Commun. 2021, 12, 6347. [Google Scholar] [CrossRef] [PubMed]
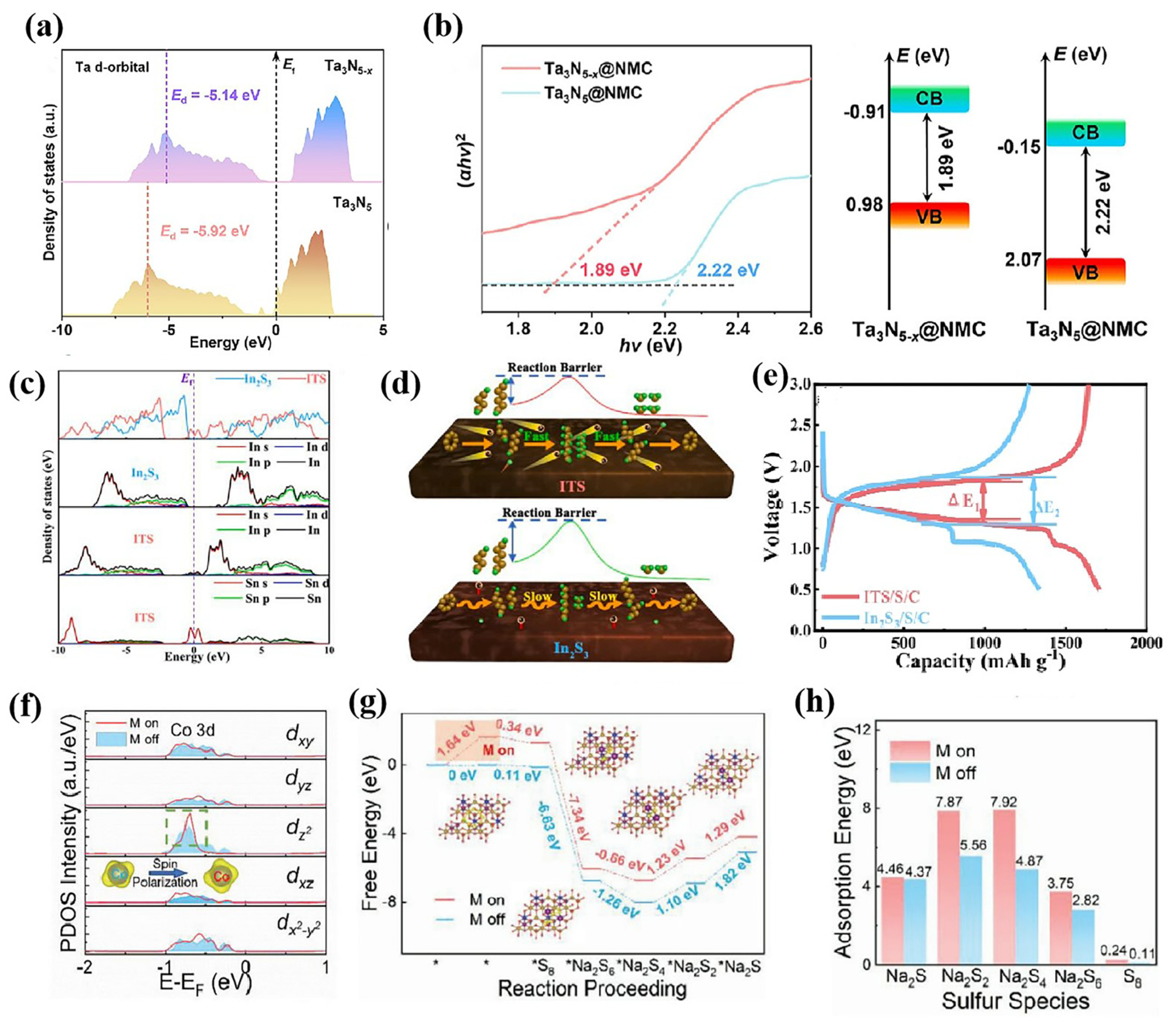
- Zhang, Z.; Luo, D.; Chen, J.; Ma, C.Y.; Li, M.T.; Zhang, H.Z.; Feng, R.F.; Gao, R.; Dou, H.Z.; Yu, A.P.; et al. Polysulfide regulation by defect-modulated Ta3N5−x electrocatalyst toward superior room-temperature sodium-sulfur batteries. Sci. Bull. 2024, 69, 197–208. [Google Scholar]
- Wang, X.; Zeng, Z.H.; Dong, Z.Y.; Ge, P.; Yang, Y. Designing Urchin-Like S/SiO2 with Regulated Pores Toward Ultra-Fast Room Temperature Sodium-Sulfur Battery. Small 2024, 20, 2400164. [Google Scholar] [CrossRef]
- Lei, Y.J.; Liu, H.W.; Yang, Z.; Zhao, L.F.; Lai, W.H.; Chen, M.Z.; Liu, H.K.; Dou, S.X.; Wang, Y.X. A Review on the Status and Challenges of Cathodes in Room-Temperature Na-S Batteries. Adv. Funct. Mater. 2023, 33, 2212600. [Google Scholar] [CrossRef]
- Huang, X.L.; Wang, Y.X.; Chou, S.L.; Dou, S.X.; Wang, Z.M.M. Materials engineering for adsorption and catalysis in room-temperature Na-S batteries. Energy Environ. Sci. 2021, 14, 3757–3795. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, X.L.; Liu, H.W.; Qiu, W.L.; Feng, C.; Li, C.; Zhang, S.H.; Liu, H.K.; Dou, S.X.; Wang, Z.M.M. Nanostructure Engineering Strategies of Cathode Materials for Room-Temperature Na-S Batteries. ACS Nano 2022, 16, 5103–5130. [Google Scholar] [CrossRef]
- Mou, J.R.; Liu, T.; Li, Y.J.; Zhang, W.J.; Li, M.; Xu, Y.T.; Huang, J.L.; Liu, M.L. Hierarchical porous carbon sheets for high-performance room temperature sodium-sulfur batteries: Integration of nitrogen-self-doping and space confinement. J. Mater. Chem. A 2020, 8, 24590–24597. [Google Scholar] [CrossRef]
- Huang, X.L.; Xiang, P.; Liu, H.W.; Feng, C.; Zhang, S.H.; Tian, Z.Q.; Liu, H.K.; Dou, S.X.; Wang, Z.M. In situ implanting MnO nanoparticles into carbon nanorod-assembled microspheres enables performance-enhanced room-temperature Na-S batteries. Inorg. Chem. Front. 2022, 9, 5486–5494. [Google Scholar] [CrossRef]
- Zhao, H.Y.; Li, Z.Z.; Liu, M.Z.; Ge, S.Y.; Ma, K.X.; Jiao, Q.Z.; Li, H.S.; Xie, H.J.; Zhao, Y.; Feng, C.H. Synergistically promoting the catalytic conversion of polysulfides via in-situ construction of Ni-MnO2 heterostructure on N-doped hollow carbon spheres towards high-performance sodium-sulfur batteries. Chem. Eng. J. 2024, 494, 153006. [Google Scholar] [CrossRef]
- Zhou, X.F.; Yu, Z.X.; Yao, Y.; Jiang, Y.; Rui, X.H.; Liu, J.Q.; Yu, Y. A High-Efficiency Mo2C Electrocatalyst Promoting the Polysulfide Redox Kinetics for Na-S Batteries. Adv. Mater. 2022, 34, 2200479. [Google Scholar] [CrossRef] [PubMed]
- Aslam, M.K.; Seymour, I.D.; Katyal, N.; Li, S.; Yang, T.T.; Bao, S.J.; Henkelman, G.; Xu, M.W. Metal chalcogenide hollow polar bipyramid prisms as efficient sulfur hosts for Na-S batteries. Nat. Commun. 2020, 11, 5242. [Google Scholar] [CrossRef] [PubMed]
- Ou, L.Q.; Mou, J.R.; Peng, J.Y.; Zhang, Y.; Chen, Y.; Huang, J.L. Hetero structured Co/CeO2-Decorating N-Doped Porous Carbon Nanocubes as Efficient Sulfur Hosts with Enhanced Rate Capability and Cycling Durability toward Room-Temperature Na-S Batteries. ACS Appl. Mater. Interfaces 2024, 16, 3302–3310. [Google Scholar] [CrossRef]
- Gao, W.J.; Song, B.Y.; Zhang, Q.Y.; He, J.R.; Wu, Y.P. 3D Flower-like Nanospheres Constructed by Transition Metal Telluride Nanosheets as Sulfur Immobilizers for High-Performance Room-Temperature Na-S Batteries. Small 2024, 20, 2310225. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.N.; Ruan, J.F.; Wang, Y.; Hu, J.M.; Song, Y.; Chen, M.; Wu, L.M. Flower-Like Interlayer-Expanded MoS2−x Nanosheets Confined in Hollow Carbon Spheres with High-Efficiency Electrocatalysis Sites for Advanced Sodium-Sulfur Battery. Small 2021, 17, 2101879. [Google Scholar] [CrossRef]
- Kumar, A.; Ghosh, A.; Ghosh, A.; Ahuja, A.; Forsyth, M.; MacFarlane, D.R.; Mitra, S. Approach to Increase the Utilization of Active Material in a High Sulfur-Loaded Cathode for High Areal Capacity Room-Temperature Sodium-Sulfur Batteries. Acs Appl. Energy Mater. 2021, 4, 384–393. [Google Scholar] [CrossRef]
- Li, Y.; Wang, X.Z.; Sun, M.H.; Ai, L.S.; Qin, L.; Zhao, Z.B.; Qiu, J.S. Co4N embedded nitrogen doped carbon with 2D/3D hybrid structure as sulfur host for room-temperature sodium-sulfur batteries. Electrochim. Acta 2023, 451, 142288. [Google Scholar] [CrossRef]
- Du, W.Y.; Shen, K.Q.; Qi, Y.R.; Gao, W.; Tao, M.L.; Du, G.Y.; Bao, S.J.; Chen, M.Y.; Chen, Y.M.; Xu, M.W. Efficient Catalytic Conversion of Polysulfides by Biomimetic Design of “Branch-Leaf” Electrode for High-Energy Sodium-Sulfur Batteries. Nano Micro Lett. 2021, 13, 50. [Google Scholar] [CrossRef]
- Yang, H.L.; Zhou, S.; Zhang, B.W.; Chu, S.Q.; Guo, H.P.; Gu, Q.F.; Liu, H.W.; Lei, Y.J.; Konstantinov, K.; Wang, Y.X.; et al. Architecting Freestanding Sulfur Cathodes for Superior Room-Temperature Na-S Batteries. Adv. Funct. Mater. 2021, 31, 2102280. [Google Scholar] [CrossRef]
- Saroha, R.; Heo, J.; Liu, Y.; Angulakshmi, N.; Lee, Y.; Cho, K.K.; Ahn, H.J.; Ahn, J.H. V2O3-decorated carbon nanofibers as a robust interlayer for long-lived, high-performance, room-temperature sodium-sulfur batteries. Chem. Eng. J. 2022, 431, 134205. [Google Scholar] [CrossRef]
- Tang, W.W.; Zhong, W.; Wu, Y.K.; Qi, Y.R.; Guo, B.S.; Liu, D.Y.; Bao, S.J.; Xu, M.W. Vanadium carbide nanoparticles incorporation in carbon nanofibers for room-temperature sodium sulfur batteries: Confining, trapping, and catalyzing. Chem. Eng. J. 2020, 395, 124978. [Google Scholar] [CrossRef]
- Yang, T.T.; Gao, W.; Guo, B.S.; Zhan, R.M.; Xu, Q.J.; He, H.; Bao, S.J.; Li, X.Y.; Chen, Y.M.; Xu, M.W. A railway-like network electrode design for room temperature Na-S battery. J. Mater. Chem. A 2019, 7, 150–156. [Google Scholar] [CrossRef]
- Liu, H.; Wu, Y.K.; Liu, P.; Wang, H.; Xu, M.W.; Bao, S.J. Anthozoan-like porous nanocages with nano-cobalt-armed CNT multifunctional layers as a cathode material for highly stable Na-S batteries. Inorg. Chem. Front. 2022, 9, 645–651. [Google Scholar] [CrossRef]
- Du, W.Y.; Wu, Y.K.; Yang, T.T.; Guo, B.S.; Liu, D.Y.; Bao, S.J.; Xu, M.W. Rational construction of rGO/VO2 nanoflowers as sulfur multifunctional hosts for room temperature Na-S batteries. Chem. Eng. J. 2020, 379, 122359. [Google Scholar] [CrossRef]
- Ye, X.; Luo, S.A.; Li, Z.Q.; Ruan, J.F.; Pang, Y.P.; Yang, J.H.; Wang, J.H.; Zheng, S.Y. Engineering CoMoO4 in reduced graphene oxide as superior cathode hosts for advanced room-temperature sodium-sulfur batteries. J. Energy Chem. 2023, 86, 620–627. [Google Scholar] [CrossRef]
- Reddy, B.S.; Premasudha, M.; Oh, K.M.; Reddy, N.S.; Ahn, H.J.; Ahn, J.H.; Cho, K.K. Hydrothermal synthesis of MoS2/rGO composite as sulfur hosts for room temperature sodium-sulfur batteries and its electrochemical properties. J. Energy Storage 2021, 39, 102660. [Google Scholar] [CrossRef]
- Samriddhi; Patel, A.; Tiwari, A.; Singh, S.P.; Yadav, V.; Tiwari, R.K.; Singh, R.K. Hydrothermal assisted RGO wrapped fumed silica-sulfur composite for an advanced room-temperature sodium-sulfur battery. J. Energy Storage 2024, 99, 113260. [Google Scholar] [CrossRef]
- Bao, W.Z.; Shuck, C.E.; Zhang, W.X.; Guo, X.; Gogotsi, Y.; Wang, G.X. Boosting Performance of Na-S Batteries Using Sulfur-Doped Ti3C2Tx MXene Nanosheets with a Strong Affinity to Sodium Polysulfides. ACS Nano 2019, 13, 11500–11509. [Google Scholar] [CrossRef]
- Bao, W.Z.; Wang, R.H.; Qian, C.F.; Zhang, Z.R.; Wu, R.J.; Zhang, Y.H.; Liu, F.Y.; Li, J.F.; Wang, G.X. Porous Heteroatom-Doped Ti3C2Tx MXene Microspheres Enable Strong Adsorption of Sodium Polysulfides for Long-Life Room-Temperature Sodium-Sulfur Batteries. ACS Nano 2021, 15, 16207–16217. [Google Scholar] [CrossRef]
- Yang, Q.J.; Yang, T.T.; Gao, W.; Qi, Y.R.; Guo, B.S.; Zhong, W.; Jiang, J.; Xu, M.W. An MXene-based aerogel with cobalt nanoparticles as an efficient sulfur host for room-temperature Na-S batteries. Inorg. Chem. Front. 2020, 7, 4396–4403. [Google Scholar] [CrossRef]
- Du, W.Y.; Gao, W.; Yang, T.T.; Guo, B.S.; Zhang, L.Z.; Bao, S.J.; Chen, Y.M.; Xu, M.W. Cobalt nanoparticles embedded into free-standing carbon nanofibers as catalyst for room-temperature sodium-sulfur batteries. J. Colloid Interface Sci. 2020, 565, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.W.; Sheng, T.; Liu, Y.D.; Wang, Y.X.; Zhang, L.; Lai, W.H.; Wang, L.; Yang, J.P.; Gu, Q.F.; Chou, S.L.; et al. Atomic cobalt as an efficient electrocatalyst in sulfur cathodes for superior room-temperature sodium-sulfur batteries. Nat. Commun. 2018, 9, 4082. [Google Scholar] [CrossRef] [PubMed]
- Guo, B.S.; Du, W.Y.; Yang, T.T.; Deng, J.H.; Liu, D.Y.; Qi, Y.R.; Jiang, J.; Bao, S.J.; Xu, M.W. Nickel Hollow Spheres Concatenated by Nitrogen-Doped Carbon Fibers for Enhancing Electrochemical Kinetics of Sodium-Sulfur Batteries. Adv. Sci. 2020, 7, 1902617. [Google Scholar] [CrossRef] [PubMed]
- Aslam, M.K.; Hussain, T.; Tabassum, H.; Wei, Z.; Tang, W.W.; Li, S.; Bao, S.J.; Zhao, X.S.; Xu, M.W. Sulfur encapsulation into yolk-shell Fe2N@nitrogen doped carbon for ambient-temperature sodium-sulfur battery cathode. Chem. Eng. J. 2022, 429, 132389. [Google Scholar] [CrossRef]
- Wu, Y.F.; Xu, Q.Q.; Huang, L.; Huang, B.; Hu, P.; Xiao, F.P.; Li, N. Encapsulation of sulfur in MoS2-modified metal-organic framework-derived N, O-codoped carbon host for sodium-sulfur batteries. J. Colloid Interface Sci. 2024, 654, 649–659. [Google Scholar] [CrossRef]
- Qiang, Z.; Chen, Y.M.; Xia, Y.F.; Liang, W.F.; Zhu, Y.; Vogt, B.D. Ultra-long cycle life, low-cost room temperature sodium-sulfur batteries enabled by highly doped (N,S) nanoporous carbons. Nano Energy 2017, 32, 59–66. [Google Scholar] [CrossRef]
- Norskov, J.K.; Abild-Pedersen, F.; Studt, F.; Bligaard, T. Density functional theory in surface chemistry and catalysis. Proc. Natl. Acad. Sci. USA 2011, 108, 937–943. [Google Scholar] [CrossRef]
- Zhou, J.B.; Liu, X.J.; Zhu, L.Q.; Zhou, J.; Guan, Y.; Chen, L.; Niu, S.W.; Cai, J.Y.; Sun, D.; Zhu, Y.C.; et al. Deciphering the Modulation Essence of p Bands in Co-Based Compounds on Li-S Chemistry. Joule 2018, 2, 2681–2693. [Google Scholar] [CrossRef]
- Medford, A.J.; Vojvodic, A.; Hummelshoj, J.S.; Voss, J.; Abild-Pedersen, F.; Studt, F.; Bligaard, T.; Nilsson, A.; Norskov, J.K. From the Sabatier principle to a predictive theory of transition-metal heterogeneous catalysis. J. Catal. 2015, 328, 36–42. [Google Scholar] [CrossRef]
- Zhu, J.H.; Abdelkader, A.; Demko, D.; Deng, L.B.; Zhang, P.X.; He, T.S.; Wang, Y.Y.; Huang, L.C. Electrocatalytic Assisted Performance Enhancement for the Na-S Battery in Nitrogen-Doped Carbon Nanospheres Loaded with Fe. Molecules 2020, 25, 1585. [Google Scholar] [CrossRef]
- Zhang, B.W.; Sheng, T.; Wang, Y.X.; Chou, S.L.; Davey, K.; Dou, S.X.; Qiao, S.Z. Long-Life Room-Temperature Sodium-Sulfur Batteries by Virtue of Transition-Metal-Nanocluster-Sulfur Interactions. Angew. Chem. Int. Ed. 2019, 58, 1484–1488. [Google Scholar] [CrossRef] [PubMed]
- Mei, T.H.; Li, X.; Lin, X.T.; Bai, L.X.; Xu, M.W.; Qi, Y.R. Cobalt Catalytic Regulation Engineering in Room-Temperature Sodium-Sulfur Batteries: Facilitating Rapid Polysulfides Conversion and Delicate Na2S Nucleation. Adv. Funct. Mater. 2024, 10, 2418126. [Google Scholar] [CrossRef]
- Jin, F.; Ning, Y.; Wang, B.; Ren, Z.H.; Luo, H.; Zhang, Z.K.; Zhang, N.; Wang, D.L. Cobalt, nitrogen co-doped microporous carbon matrix derived from metal organic frameworks toward high specific capacity room temperature sodium sulfur batteries. J. Power Sources 2023, 565, 232917. [Google Scholar] [CrossRef]
- Zhi, P.P.; Qi, Y.R.; Zhao, J.; Ding, H.F.; Zhao, Q.; Li, Y.; Xu, M.W. Cobalt nanoparticles embedded in nitrogen-doped carbon nanofibers to enhance redox kinetics for long-cycling sodium-sulfur batteries. Mater. Today Energy 2024, 41, 101536. [Google Scholar] [CrossRef]
- Ma, Q.R.; Du, G.Y.; Zhong, W.; Du, W.Y.; Bao, S.J.; Xu, M.W.; Li, C.M. Template method for fabricating Co and Ni nanoparticles/porous channels carbon for solid-state sodium-sulfur battery. J. Colloid Interface Sci. 2020, 578, 710–716. [Google Scholar] [CrossRef]
- Ma, Q.R.; Zhong, W.; Du, G.Y.; Qi, Y.R.; Bao, S.J.; Xu, M.W.; Li, C.M. Multi-step Controllable Catalysis Method for the Defense of Sodium Polysulfide Dissolution in Room-Temperature Na-S Batteries. Acs Appl. Mater. Interfaces 2021, 13, 11852–11860. [Google Scholar] [CrossRef]
- He, Q.C.; Zhu, Y.P.; Li, Y.; Ma, S.; Wang, D.L.; Xie, J.Y. Etch-driven N, P co-doped hierarchical porous carbon embedded with Ni nanoparticles as an efficient dynamic carrier for room-temperature Na-S battery. J. Energy Storage 2023, 74, 109353. [Google Scholar] [CrossRef]
- Ma, Q.R.; Du, G.Y.; Guo, B.S.; Tang, W.W.; Li, Y.T.; Xu, M.W.; Li, C.M. Carbon-wrapped cobalt nanoparticles on graphene aerogel for solid-state room sodium-sulfur batteries. Chem. Eng. J. 2020, 388, 124210. [Google Scholar] [CrossRef]
- Wei, X.L.; Zhang, Z.; Luo, D.; Wang, X. Construction of Heterointerfaced Nanoreactor Electrocatalyst via In Situ Evolution Toward Practical Room-Temperature Sodium-Sulfur Batteries. Adv. Funct. Mater. 2024, 9, 2414172. [Google Scholar] [CrossRef]
- Wang, N.N.; Wang, Y.X.; Bai, Z.C.; Fang, Z.W.; Zhang, X.; Xu, Z.F.; Ding, Y.; Xu, X.; Du, Y.; Dou, S.X.; et al. High-performance room-temperature sodium-sulfur battery enabled by electrocatalytic sodium polysulfides full conversion. Energy Environ. Sci. 2020, 13, 562–570. [Google Scholar] [CrossRef]
- Liu, Y.P.; Ma, S.Y.; Rosebrock, M.; Rusch, P.; Barnscheidt, Y.; Wu, C.Q.; Nan, P.F.; Bettels, F.; Lin, Z.H.; Li, T.R.; et al. Tungsten Nanoparticles Accelerate Polysulfides Conversion: A Viable Route toward Stable Room-Temperature Sodium-Sulfur Batteries. Adv. Sci. 2022, 9, 2105544. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.F.; Wang, H.Y.; Zhang, S.P.; Ren, N.Q.; Wu, Y.; Wu, L.; Zhou, X.F.; Yao, Y.; Wu, X.J.; Yu, Y. Manipulating the Electronic Structure of Nickel via Alloying with Iron: Toward High-Kinetics Sulfur Cathode for Na-S Batteries. ACS Nano 2021, 15, 15218–15228. [Google Scholar] [CrossRef]
- Tang, K.J.; Peng, X.Q.; Zhang, Z.Y.; Li, G.H.; Wang, J.; Wang, Y.X.J.; Chen, C.; Zhang, N.; Xie, X.Q.; Wu, Z.J. A Highly Dispersed Cobalt Electrocatalyst with Electron-Deficient Centers Induced by Boron toward Enhanced Adsorption and Electrocatalysis for Room-Temperature Sodium-Sulfur Batteries. Small 2024, 20, 2311151. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.; Kumar, A.; Roy, A.; Panda, M.R.; Kar, M.; MacFarlane, D.R.; Mitra, S. Three-Dimensionally Reinforced Freestanding Cathode for High Energy Room-Temperature Sodium-Sulfur Batteries. ACS Appl. Mater. Interfaces 2019, 11, 14101–14109. [Google Scholar] [CrossRef]
- Kumar, A.; Ghosh, A.; Roy, A.; Panda, M.R.; Forsyth, M.; MacFarlane, D.R.; Mitra, S. High-energy density room temperature sodium-sulfur battery enabled by sodium polysulfide catholyte and carbon cloth current collector decorated with MnO2 nanoarrays. Energy Storage Mater. 2019, 20, 196–202. [Google Scholar] [CrossRef]
- Ma, Q.Y.; Liu, Q.Q.; Li, Z.Y.; Pu, J.; Mujtaba, J.; Fang, Z. Oxygen vacancy-mediated amorphous GeOx assisted polysulfide redox kinetics for room-temperature sodium-sulfur batteries. J. Colloid Interface Sci. 2023, 629, 76–86. [Google Scholar] [CrossRef]
- Li, W.Q.; Wang, T.; Yang, G.; Ma, R.Z.; Li, W.Z.; Fu, Y.J.; Wu, L.; Liu, Z.J.; Liu, D.Q.; Wu, Y.; et al. Oxygen vacancy modified cerium dioxide with excellent catalytic activity for room-temperature Na-S batteries. J. Energy Storage 2024, 91, 112141. [Google Scholar] [CrossRef]
- Zhu, J.H.; Zeng, L.C.; Li, X.Y.; Zhao, N.; Wang, Y.Y.; Lin, Z.L.; He, T.S.; Deng, L.B.; Zhang, P.X. An extraordinary Na-S battery enabled by a carbon nanosheet host loaded with Fe-accelerator and TiO2-inhibitor. Appl. Surf. Sci. 2022, 604, 154496. [Google Scholar] [CrossRef]
- Xiao, Y.; Zheng, Y.L.; Yao, G.; Zhang, Y.H.; Li, Z.Q.; Liu, S.J.; Zheng, F.C. Defect engineering of a TiO2 anatase/rutile homojunction accelerating sulfur redox kinetics for high-performance Na-S batteries. Dalton Trans. 2024, 53, 8168–8176. [Google Scholar] [CrossRef]
- Ye, X.; Li, Z.Q.; Sun, H.; Wu, M.X.; An, Z.X.; Pang, Y.P.; Yang, J.H.; Zheng, S.Y. Incorporating TiO2 nanoparticles into the multichannels of electrospun carbon fibers to increase the adsorption of polysulfides in room temperature sodium-sulfur batteries. New Carbon Mater. 2022, 37, 1116–1122. [Google Scholar] [CrossRef]
- Huang, X.L.; Zhang, X.F.; Zhou, L.J.; Guo, Z.P.; Liu, H.K.; Dou, S.X.; Wang, Z.M. Orthorhombic Nb2O5 Decorated Carbon Nanoreactors Enable Bidirectionally Regulated Redox Behaviors in Room-Temperature Na-S Batteries. Adv. Sci. 2023, 10, 2206558. [Google Scholar] [CrossRef] [PubMed]
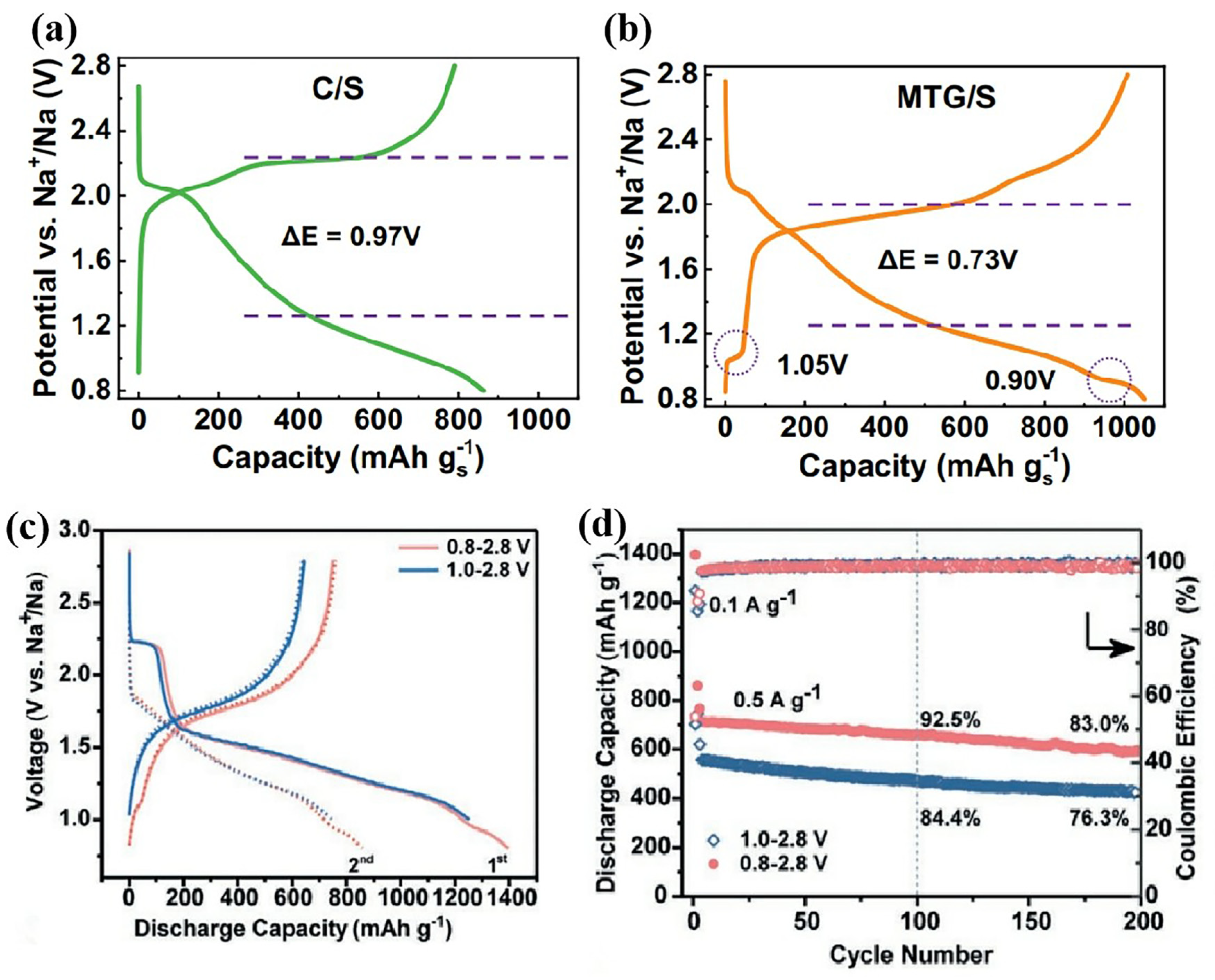
- Zhou, J.H.; Yang, Y.; Zhang, Y.C.; Duan, S.K.; Zhou, X.; Sun, W.; Xu, S.M. Sulfur in Amorphous Silica for an Advanced Room-Temperature Sodium-Sulfur Battery. Angew. Chem. Int. Ed. 2021, 60, 10129–10136. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.L.; Dou, S.X.; Wang, Z.M.M. Metal-based electrocatalysts for room-temperature Na-S batteries. Mater. Horiz. 2021, 8, 2870–2885. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.C.; Xiao, J.; Lai, W.H.; Wang, L.; Gebert, F.; Wang, Y.X.; Gu, Q.F.; Liu, H.; Chou, S.L.; Liu, H.K.; et al. Nickel sulfide nanocrystals on nitrogen-doped porous carbon nanotubes with high-efficiency electrocatalysis for room-temperature sodium-sulfur batteries. Nat. Commun. 2019, 10, 4793. [Google Scholar] [CrossRef]
- Ma, Q.Y.; Ai, J.; Zou, H.D.; He, H.L.; Li, Z.Y.; Mujtaba, J.; Fang, Z. Gallate-MOF derived CoS2/C composites as an accelerated catalyst for room-temperature sodium-sulfur batteries. Chem. Commun. 2022, 58, 13612–13615. [Google Scholar] [CrossRef]
- Xiao, F.P.; Wang, H.K.; Yao, T.H.; Zhao, X.; Yang, X.M.; Yu, D.Y.W.; Rogach, A.L. MOF-Derived CoS2/N-Doped Carbon Composite to Induce Short-Chain Sulfur Molecule Generation for Enhanced Sodium-Sulfur Battery Performance. ACS Appl. Mater. Interfaces 2021, 13, 18010–18020. [Google Scholar] [CrossRef]
- Wang, H.; Qi, Y.R.; Xiao, F.Y.; Liu, P.; Li, Y.; Bao, S.J.; Xu, M.W. Tessellated N-doped carbon/CoSe2 as trap-catalyst sulfur hosts for room-temperature sodium-sulfur batteries. Inorg. Chem. Front. 2022, 9, 1743–1751. [Google Scholar] [CrossRef]
- Yan, Z.C.; Liang, Y.R.; Xiao, J.; Lai, W.H.; Wang, W.L.; Xia, Q.B.; Wang, Y.X.; Gu, Q.F.; Lu, H.M.; Chou, S.L.; et al. A High-Kinetics Sulfur Cathode with a Highly Efficient Mechanism for Superior Room-Temperature Na-S Batteries. Adv. Mater. 2020, 32, 1906700. [Google Scholar] [CrossRef]
- Wang, H.M.; Deng, C.; Li, X.L.; Yan, D.K.; Xie, M.X.; Zhang, S.; Huang, B. Designing dual-defending system based on catalytic and kinetic iron Pyrite@C hybrid fibers for long-life room-temperature sodium-sulfur batteries. Chem. Eng. J. 2021, 420, 129681. [Google Scholar] [CrossRef]
- Ma, F.; Hu, P.; Wang, T.Y.; Liang, J.S.; Han, R.; Han, J.T.; Li, Q. Yolk@Shell Structured MnS@Nitrogen-Doped Carbon as a Sulfur Host and Polysulfide Conversion Booster for Lithium/Sodium Sulfur Batteries. ACS Appl. Energy Mater. 2021, 4, 3487–3494. [Google Scholar]
- Yan, Z.C.; Tian, Q.; Liang, Y.R.; Jing, L.Y.; Hu, Z.; Hua, W.B.; Tayal, A.; Lai, W.H.; Wang, W.L.; Peng, J.; et al. Electrochemical release of catalysts in nanoreactors for solid sulfur redox reactions in room-temperature sodium-sulfur batteries. Cell Rep. Phys. Sci. 2021, 2, 100539. [Google Scholar] [CrossRef]
- Dong, C.W.; Zhou, H.Y.; Jin, B.; Gao, W.; Lang, X.Y.; Li, J.C.; Jiang, Q. Enabling high-performance room-temperature sodium/sulfur batteries with few-layer 2H-MoSe2 embellished nitrogen-doped hollow carbon spheres as polysulfide barriers. J. Mater. Chem. A 2021, 9, 3451–3463. [Google Scholar] [CrossRef]
- Mou, J.R.; Li, Y.J.; Ou, L.Q.; Huang, J.L. A highly-efficient electrocatalyst for room temperature sodium-sulfur batteries: Assembled nitrogen-doped hollow porous carbon spheres decorated with ultrafine α-MoC1-x nanoparticles. Energy Storage Mater. 2022, 52, 111–119. [Google Scholar] [CrossRef]
- Huang, X.L.; Hussain, T.; Liu, H.W.; Kaewmaraya, T.; Xu, M.W.; Liu, H.K.; Dou, S.X.; Wang, Z.M. Dredging sodium polysulfides using a Fe3C electrocatalyst to realize improved room-temperature Na-S batteries. Inorg. Chem. Front. 2023, 10, 4241–4251. [Google Scholar] [CrossRef]
- Ye, C.; Jin, H.Y.; Shan, J.Q.; Jiao, Y.; Li, H.; Gu, Q.F.; Davey, K.; Wang, H.H.; Qiao, S.Z. A Mo5N6 electrocatalyst for efficient Na2S electrodeposition in room-temperature sodium-sulfur batteries. Nat. Commun. 2021, 12, 7195. [Google Scholar] [CrossRef]
- Li, Z.; Wang, C.L.; Ling, F.X.; Wang, L.F.; Bai, R.L.; Shao, Y.; Chen, Q.W.; Yuan, H.; Yu, Y.; Tan, Y.Q. Room-Temperature Sodium-Sulfur Batteries: Rules for Catalyst Selection and Electrode Design. Adv. Mater. 2022, 34, 2204214. [Google Scholar] [CrossRef]
- Ghosh, A.; Kumar, A.; Dos, T.; Ghosh, A.; Chakrabory, S.; Kar, M.; MacFarlane, D.R.; Mitra, S. Lewis Acid-Base Interactions between Polysulfides and Boehmite Enables Stable Room-Temperature Sodium-Sulfur Batteries. Adv. Funct. Mater. 2020, 30, 2005669. [Google Scholar] [CrossRef]
- Zhu, J.H.; Zeng, L.C.; Song, Y.M.; Peng, F.; Wang, Y.Y.; He, T.S.; Deng, L.B.; Zhang, P.X. High performance sulfur/carbon cathode for Na-S battery enabled by electrocatalytic effect of Sn-doped In2S3. J. Colloid Interface Sci. 2023, 647, 546–553. [Google Scholar] [CrossRef]
- Kumar, A.; Ghosh, A.; Ghosh, A.; Ahuja, A.; Sengupta, A.; Forsyth, M.; MacFarlane, D.R.; Mitra, S. Sub-zero and room-temperature sodium-sulfur battery cell operations: A rational current collector, catalyst and sulphur-host design and study. Energy Storage Mater. 2021, 42, 608–617. [Google Scholar] [CrossRef]
- Li, J.X.; Yu, Y.; Xu, S.R.; Yan, W.F.; Mu, S.C.; Zhang, J.N. Function of Electron Spin Effect in Electrocatalysts. Acta Phys. Chim. Sin. 2023, 39, 2302049. [Google Scholar] [CrossRef]
- Zhang, C.Y.; Gong, L.; Zhang, C.Q.; Cheng, X.; Balcells, L.; Zeng, G.F.; Biendicho, J.J.; Li, J.S.; Sun, G.Z.; Zhou, J.Y.; et al. Sodium-Sulfur Batteries with Unprecedented Capacity, Cycling Stability and Operation Temperature Range Enabled by a CoFe2O4 Catalytic Additive Under an External Magnetic Field. Adv. Funct. Mater. 2023, 33, 2305908. [Google Scholar] [CrossRef]
- Wang, J.; Yang, G.; Ghosh, T.; Bai, Y.; Lim, C.Y.J.; Zhang, L.; Seng, D.H.L.; Goh, W.P.; Xing, Z.; Liu, Z.; et al. Hierarchical FeS2 cathode with suppressed shuttle effect for high performance magnesium-ion batteries. Nano Energy 2024, 119, 109082. [Google Scholar] [CrossRef]
- Chong, P.; Zhou, Z.; Wang, K.; Zhai, W.; Li, Y.; Wang, J.; Wei, M. The Stabilizing of 1T-MoS2 for All-Solid-State Lithium-Ion Batteries. Batteries 2023, 9, 26. [Google Scholar] [CrossRef]
- Li, Z.N.; Sami, I.; Yang, J.U.; Li, J.T.; Kumar, R.V.; Chhowalla, M. Lithiated metallic molybdenum disulfide nanosheets for high-performance lithium-sulfur batteries. Nat. Energy 2023, 8, 84–93. [Google Scholar] [CrossRef]
- Xue, W.J.; Shi, Z.; Suo, L.M.; Wang, C.; Wang, Z.A.; Wang, H.Z.; So, K.P.; Maurano, A.; Yu, D.W.; Chen, Y.M.; et al. Intercalation-conversion hybrid cathodes enabling Li-S full-cell architectures with jointly superior gravimetric and volumetric energy densities. Nat. Energy 2019, 4, 374–382. [Google Scholar] [CrossRef]
- Ma, C.S.; Wang, X.; Lan, J.Q.; Zhang, J.Y.; Song, K.M.; Chen, J.C.; Ge, J.M.; Chen, W.H. Dynamic Multistage Coupling of FeS2/S Enables Ultrahigh Reversible Na-S Batteries. Adv. Funct. Mater. 2023, 33, 2211821. [Google Scholar] [CrossRef]
- Wang, Y.X.; Lai, Y.Y.; Chu, J.; Yan, Z.C.; Wang, Y.X.; Chou, S.L.; Liu, H.K.; Dou, S.X.; Ai, X.P.; Yang, H.X.; et al. Tunable Electrocatalytic Behavior of Sodiated MoS2 Active Sites toward Efficient Sulfur Redox Reactions in Room-Temperature Na-S Batteries. Adv. Mater. 2021, 33, 2100229. [Google Scholar] [CrossRef]
- He, J.R.; Bhargav, A.; Su, L.S.; Charalambous, H.; Manthiram, A. Intercalation-type catalyst for non-aqueous room temperature sodium-sulfur batteries. Nat. Commun. 2023, 14, 6568. [Google Scholar] [CrossRef]
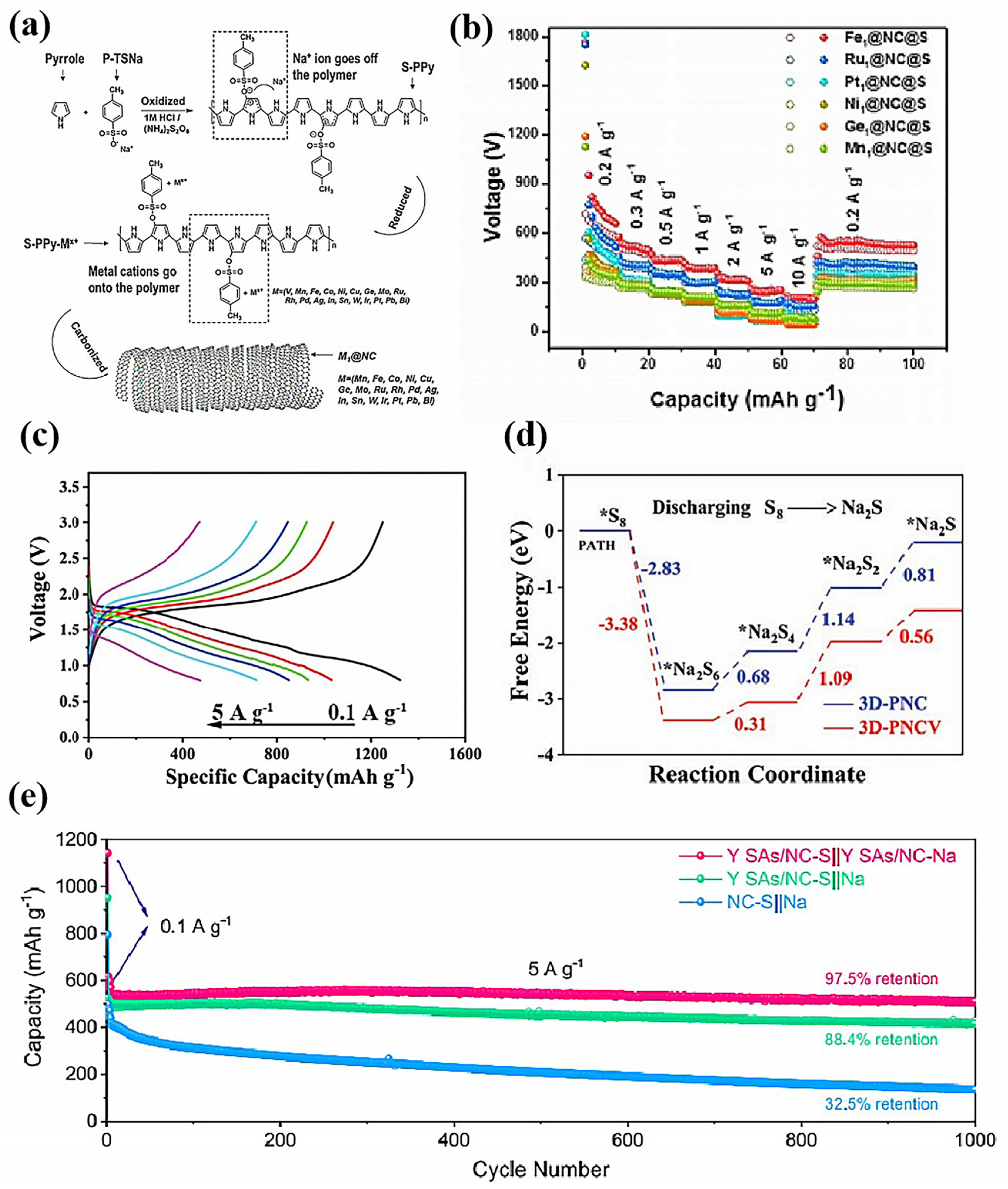
- Lai, W.H.; Wang, H.; Zheng, L.R.; Jiang, Q.; Yan, Z.C.; Wang, L.; Yoshikawa, H.; Matsumura, D.; Sun, Q.; Wang, Y.X.; et al. General Synthesis of Single-Atom Catalysts for Hydrogen Evolution Reactions and Room-Temperature Na-S Batteries. Angew. Chem. Int. Ed. 2020, 59, 22171–22178. [Google Scholar] [CrossRef]
- Lei, Y.J.; Lu, X.X.; Yoshikawa, H.; Matsumura, D.; Fan, Y.M.; Zhao, L.F.; Li, J.; Wang, S.; Gu, Q.F.; Liu, H.K.; et al. Understanding the charge transfer effects of single atoms for boosting the performance of Na-S batteries. Nat. Commun. 2024, 15, 3325. [Google Scholar] [CrossRef]
- Jiang, Y.; Yu, Z.X.; Zhou, X.F.; Cheng, X.L.; Huang, H.J.; Liu, F.F.; Yang, Y.X.; He, S.N.; Pan, H.G.; Yang, H.; et al. Single-Atom Vanadium Catalyst Boosting Reaction Kinetics of Polysulfides in Na-S Batteries. Adv. Mater. 2023, 35, 2208873. [Google Scholar] [CrossRef] [PubMed]
- Zhang, E.R.; Hu, X.; Meng, L.Z.; Qiu, M.; Chen, J.X.; Liu, Y.J.; Liu, G.Y.; Zhuang, Z.C.; Zheng, X.B.; Zheng, L.R.; et al. Single-Atom Yttrium Engineering Janus Electrode for Rechargeable Na-S Batteries. J. Am. Chem. Soc. 2022, 144, 18995–19007. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wang, M.L.; Huang, X.L.; Lu, S.T.; Lu, K.; Wu, X.H. Atomic Manganese Manipulating Polysulfide Speciation Pathway for Room-Temperature Na-S Batteries. CCS Chem. 2024, 6, 2289–2304. [Google Scholar] [CrossRef]
- Wu, G.; Liu, T.; Lao, Z.; Cheng, Y.; Wang, T.; Mao, J.; Zhang, H.; Liu, E.; Shi, C.; Zhou, G.; et al. Optimizing s-p Orbital Overlap between Sodium Polysulfides and Single-Atom Indium Catalyst for Efficient Sulfur Redox Reaction. Angew. Chem. Int. Ed. 2024, e202422208. [Google Scholar] [CrossRef]
- Bai, R.L.; Lin, Q.S.; Li, X.Y.; Ling, F.X.; Wang, H.J.; Tan, S.; Hu, L.X.; Ma, M.Z.; Wu, X.J.; Shao, Y.; et al. Toward Complete Transformation of Sodium Polysulfides by Regulating the Second-Shell Coordinating Environment of Atomically Dispersed Fe. Angew. Chem. Int. Ed. 2023, 62, e202218165. [Google Scholar] [CrossRef]
- Hu, P.; Wu, Y.F.; Gao, X.P.; Huang, L.; Cai, B.B.; Liu, Y.X.; Ma, Y.; Jiang, S.; Wang, F.; Xiao, F.P. N/O dual coordination of cobalt single atom for fast kinetics sodium-sulfur batteries. Rare Met. 2025, 44, 288–299. [Google Scholar] [CrossRef]
- Xiao, F.P.; Wang, H.K.; Xu, J.; Yang, W.Q.; Yang, X.M.; Yu, D.Y.W.; Rogach, A.L. Generating Short-Chain Sulfur Suitable for Efficient Sodium-Sulfur Batteries via Atomic Copper Sites on a N,O-Codoped Carbon Composite. Adv. Energy Mater. 2021, 11, 2100989. [Google Scholar] [CrossRef]
- Li, Z.Q.; Chen, X.; Yao, G.; Wei, L.Z.; Chen, Q.W.; Luo, Q.Q.; Zheng, F.C.; Wang, H. Strengthening d-p Orbital-Hybridization via Coordination Number Regulation of Manganese Single-Atom Catalysts Toward Fast Kinetic and Long-Life Sodium-Sulfur Batteries. Adv. Funct. Mater. 2024, 34, 2400859. [Google Scholar] [CrossRef]
- Yao, G.; Li, Z.Q.; Zhang, Y.H.; Xiao, Y.; Wei, L.Z.; Niu, H.L.; Chen, Q.W.; Yang, Y.; Zheng, F.C. Highly Flexible Carbon Film Implanted with Single-Atomic Zn-N2 Moiety for Long-Life Sodium-Sulfur Batteries. Adv. Funct. Mater. 2024, 34, 2214353. [Google Scholar] [CrossRef]
- Fang, D.L.; Huang, S.Z.; Xu, T.T.; Sun, P.; Li, X.L.; Lim, Y.V.; Yan, D.; Shang, Y.; Su, B.J.; Juang, J.Y.; et al. Low-Coordinated Zn-N2 Sites as Bidirectional Atomic Catalysis for Room-Temperature Na-S Batteries. ACS Appl. Mater. Interfaces 2023, 15, 26650–26659. [Google Scholar] [CrossRef]
- Cui, K.; Wang, T.S.; Zhang, Q.Y.; Zhang, H.P. Multi-Functional Descriptor Design of V-Based Double Atomic Catalysts for Room Temperature Sodium-Sulfur Batteries. Small 2024, 11, 2409866. [Google Scholar] [CrossRef] [PubMed]
- Ruan, J.F.; Lei, Y.J.; Fan, Y.M.; Borras, M.C.; Luo, Z.X.; Yan, Z.C.; Johannessen, B.; Gu, Q.F.; Konstantinov, K.; Pang, W.K.; et al. Linearly Interlinked Fe-Nx-Fe Single Atoms Catalyze High-Rate Sodium-Sulfur Batteries. Adv. Mater. 2024, 36, 2312207. [Google Scholar] [CrossRef] [PubMed]
- Zhong, X.; Huang, Y.J.; Cai, J.M.; Li, Y.J.; He, Z.D.; Cai, D.Y.; Geng, Z.L.; Deng, W.T.; Zou, G.Q.; Hou, H.S.; et al. Origin of the High Catalytic Activity of MoS2 in Na-S Batteries: Electrochemically Reconstructed Mo Single Atoms. J. Am. Chem. Soc. 2024, 146, 32124–32134. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.T.; Li, Y.L.; Yang, J.B.; Mi, H.W.; Luo, S.; Deng, L.B.; Yan, C.Y.; Rauf, M.; Zhang, P.X.; Sun, X.L.; et al. New Strategy for Polysulfide Protection Based on Atomic Layer Deposition of TiO2 onto Ferroelectric-Encapsulated Cathode: Toward Ultrastable Free-Standing Room Temperature Sodium-Sulfur Batteries. Adv. Funct. Mater. 2018, 28, 1705537. [Google Scholar] [CrossRef]
- Zhang, S.P.; Yao, Y.; Jiao, X.J.; Ma, M.Z.; Huang, H.J.; Zhou, X.F.; Wang, L.F.; Bai, J.T.; Yu, Y. Mo2N-W2N Heterostructures Embedded in Spherical Carbon Superstructure as Highly Efficient Polysulfide Electrocatalysts for Stable Room-Temperature Na-S Batteries. Adv. Mater. 2021, 33, 2103846. [Google Scholar] [CrossRef]
- Reddy, B.S.; Cho, G.B.; Reddy, N.S.; Ahn, H.J.; Ahn, J.H.; Maiyalagan, T.; Cho, K.K. Layered-like structure of TiO2-T3C2 Mxene as an efficient sulfur host for room-temperature sodium-sulfur batteries. J. Alloys Compd. 2021, 883, 160910. [Google Scholar] [CrossRef]
- Wang, T.; Li, W.Q.; Fu, Y.J.; Wang, D.J.; Wu, L.; Sun, K.; Liu, D.Q.; Ma, R.Z.; Shi, Y.J.; Yang, G.; et al. A Mott-Schottky Heterojunction with Strong Chemisorption and Fast Conversion Effects for Room-Temperature Na-S Batteries. Small 2024, 20, 2311180. [Google Scholar] [CrossRef]
- Yan, Z.C.; Liang, Y.R.; Hua, W.B.; Zhang, X.G.; Lai, W.H.; Hu, Z.; Wang, W.L.; Peng, J.; Indris, S.; Wang, Y.X.; et al. Multiregion Janus-Featured Cobalt Phosphide-Cobalt Composite for Highly Reversible Room-Temperature Sodium-Sulfur Batteries. ACS Nano 2020, 14, 10284–10293. [Google Scholar] [CrossRef]
- Li, Y.; Wang, X.Z.; Wang, L.X.; Jia, D.Z.; Yang, Y.Z.; Liu, X.G.; Sun, M.H.; Zhao, Z.B.; Qiu, J.S. Ni@Ni3N Embedded on Three-Dimensional Carbon Nanosheets for High-Performance Lithium/Sodium-Sulfur Batteries. ACS Appl. Mater. Interfaces 2021, 13, 48536–48545. [Google Scholar] [CrossRef]
- Qin, G.H.; Liu, Y.T.; Han, P.Y.; Cao, S.X.; Guo, X.Y.; Guo, Z.Y. High performance room temperature Na-S batteries based on FCNT modified Co3C-Co nanocubes. Chem. Eng. J. 2020, 396, 125295. [Google Scholar] [CrossRef]
- Ye, X.; Ruan, J.F.; Pang, Y.P.; Yang, J.H.; Liu, Y.F.; Huang, Y.Z.; Zheng, S.Y. Enabling a Stable Room-Temperature Sodium-Sulfur Battery Cathode by Building Heterostructures in Multichannel Carbon Fibers. ACS Nano 2021, 15, 5639–5648. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.H.; Yu, Z.X.; Yao, Y.; Wang, L.F.; Wu, Y.; Cheng, X.L.; Ali, Z.; Yu, Y. Bifunctional Catalyst for Liquid-Solid Redox Conversion in Room-Temperature Sodium-Sulfur Batteries. Small Struct. 2022, 3, 2200020. [Google Scholar] [CrossRef]
- Qian, S.Y.; Yuan, Z.Y.; Li, G.S.; Li, D.D.; Li, J.Z.; Han, W. 3D layered structure Ti3C2Tx MXene/Ni(OH)2/C with strong catalytic and adsorption capabilities of polysulfides for high-capacity Sodium-Sulfur battery. Chem. Eng. J. 2023, 471, 144528. [Google Scholar] [CrossRef]
- Zhang, S.Q.; Huang, M.; Wang, Y.Y.; Wang, Z.P.; Wang, H.; Liu, X.J. Achieving a Quasi-Solid-State Conversion of Polysulfides via Building High Efficiency Heterostructure for Room Temperature Na-S Batteries. Adv. Energy Mater. 2024, 14, 2303925. [Google Scholar] [CrossRef]
- Wu, S.C.; Huang, Y.H.; Liao, C.R.; Tang, S.Y.; Yang, T.Y.; Wang, Y.C.; Yu, Y.J.; Perng, T.P.; Chueh, Y.L. Rational design of a polysulfide catholyte electrocatalyst by interfacial engineering based on novel MoS2/MoN heterostructures for superior room-temperature Na-S batteries. Nano Energy 2021, 90, 106590. [Google Scholar] [CrossRef]
- Liu, H.W.; Lai, W.H.; Liang, Y.R.; Liang, X.; Yan, Z.C.; Yang, H.L.; Lei, Y.J.; Wei, P.; Zhou, S.; Gu, Q.F.; et al. Sustainable S cathodes with synergic electrocatalysis for room-temperature Na-S batteries. J. Mater. Chem. A 2021, 9, 566–574. [Google Scholar] [CrossRef]
- Wang, B.; Wang, L.; Guo, B.N.; Kong, Y.Y.; Wang, F.B.; Jing, Z.X.; Qu, G.M.; Mamoor, M.; Wang, D.D.; He, X.Y.; et al. In Situ Electrochemical Evolution of Amorphous Metallic Borides Enabling Long Cycling Room-/Subzero-Temperature Sodium-Sulfur Batteries. Adv. Mater. 2024, 36, 2411725. [Google Scholar] [CrossRef]
- Fang, D.L.; Ghosh, T.; Huang, S.Z.; Wang, Y.; Qiu, J.B.; Xu, X.H.; Yang, H.Y. Core-Shell Tandem Catalysis Coupled with Interface Engineering For High-Performance Room-Temperature Na-S Batteries. Small 2023, 19, 2302461. [Google Scholar] [CrossRef]
- Liu, H.W.; Pei, W.; Lai, W.H.; Yan, Z.C.; Yang, H.L.; Lei, Y.J.; Wang, Y.X.; Gu, Q.F.; Zhou, S.; Chou, S.L.; et al. Electrocatalyzing S Cathodes via Multisulfiphilic Sites for Superior Room-Temperature Sodium-Sulfur Batteries. ACS Nano 2020, 14, 7259–7268. [Google Scholar] [CrossRef]
- Lei, Y.J.; Wu, C.; Lu, X.X.; Hua, W.B.; Li, S.B.; Liang, Y.R.; Liu, H.W.; Lai, W.H.; Gu, Q.F.; Cai, X.L.; et al. Streamline Sulfur Redox Reactions to Achieve Efficient Room-Temperature Sodium-Sulfur Batteries. Angew. Chem. Int. Ed. 2022, 61, e202200384. [Google Scholar] [CrossRef]
- Wang, M.L.; Zhang, H.; Zhang, W.L.; Chen, Q.W.; Lu, K. Electrocatalysis in Room Temperature Sodium-Sulfur Batteries: Tunable Pathway of Sulfur Speciation. Small Methods 2022, 6, 2200335. [Google Scholar] [CrossRef] [PubMed]
- Jin, F.; Wang, B.; Wang, J.L.; Wang, Y.X.; Ning, Y.; Yang, J.; Zhang, Z.K.; Liu, P.; Zhou, Y.; Wang, D.A.L.; et al. Boosting electrochemical kinetics of S cathodes for room temperature Na/S batteries. Matter 2021, 4, 1768–1800. [Google Scholar] [CrossRef]
- Huang, Z.P.; Song, B.; Zhang, H.; Feng, F.; Zhang, W.L.; Lu, K.; Chen, Q.W. High-Capacity and Stable Sodium-Sulfur Battery Enabled by Confined Electrocatalytic Polysulfides Full Conversion. Adv. Funct. Mater. 2021, 31, 2100666. [Google Scholar] [CrossRef]
- Zhang, H.; Song, B.; Zhang, W.W.; An, B.W.; Fu, L.; Lu, S.T.; Cheng, Y.W.; Chen, Q.W.; Lu, K. Bidirectional Tandem Electrocatalysis Manipulated Sulfur Speciation Pathway for High-Capacity and Stable Na-S Battery. Angew. Chem. Int. Ed. 2023, 62, e202217009. [Google Scholar] [CrossRef]
- Meng, R.J.; He, X.; Ong, S.J.H.; Cui, C.X.; Song, S.F.; Paoprasert, P.; Pang, Q.Q.; Xu, Z.C.J.; Liang, X. A Radical Pathway and Stabilized Li Anode Enabled by Halide Quaternary Ammonium Electrolyte Additives for Lithium-Sulfur Batteries. Angew. Chem. Int. Ed. 2023, 62, e202309046. [Google Scholar] [CrossRef]
- Wang, Q.; Zheng, J.M.; Walter, E.; Pan, H.L.; Lv, D.P.; Zuo, P.J.; Chen, H.H.; Deng, Z.D.; Liaw, B.Y.; Yu, X.Q.; et al. Direct Observation of Sulfur Radicals as Reaction Media in Lithium Sulfur Batteries. J. Electrochem. Soc. 2015, 162, A474–A478. [Google Scholar] [CrossRef]
- Kumar, A.; Ghosh, A.; Forsyth, M.; MacFarlane, D.R.; Mitra, S. Free-Radical Catalysis and Enhancement of the Redox Kinetics for Room-Temperature Sodium-Sulfur Batteries. ACS Energy Lett. 2020, 5, 2112–2121. [Google Scholar] [CrossRef]
- Zhang, S.P.; Pollard, T.P.; Feng, X.; Borodin, O.; Xu, K.; Li, Z. Altering the Electrochemical Pathway of Sulfur Chemistry with Oxygen for High Energy Density and Low Shuttling in a Na/S Battery. ACS Energy Lett. 2020, 5, 1070–1076. [Google Scholar] [CrossRef]
- Luo, S.N.; Ruan, J.F.; Wang, Y.; Chen, M.; Wu, L.M. Enhancing Conversion Kinetics through Electron Density Dual-Regulation of Catalysts and Sulfur toward Room-/Subzero-Temperature Na-S Batteries. Adv. Sci. 2024, 11, 2308180. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, S.-S.; Cai, Z.-Q.; Lu, C.-K.; Li, J.; Geng, N.-N.; Lin, D.-T.; Yang, T.; Liu, T. Design Strategies of S8 Molecule Cathodes for Room-Temperature Na-S Batteries. Nanomaterials 2025, 15, 330. https://doi.org/10.3390/nano15050330
Shi S-S, Cai Z-Q, Lu C-K, Li J, Geng N-N, Lin D-T, Yang T, Liu T. Design Strategies of S8 Molecule Cathodes for Room-Temperature Na-S Batteries. Nanomaterials. 2025; 15(5):330. https://doi.org/10.3390/nano15050330
Chicago/Turabian StyleShi, Sha-Sha, Zi-Qi Cai, Chen-Kai Lu, Jing Li, Nan-Nan Geng, Dong-Tao Lin, Tao Yang, and Tao Liu. 2025. "Design Strategies of S8 Molecule Cathodes for Room-Temperature Na-S Batteries" Nanomaterials 15, no. 5: 330. https://doi.org/10.3390/nano15050330
APA StyleShi, S.-S., Cai, Z.-Q., Lu, C.-K., Li, J., Geng, N.-N., Lin, D.-T., Yang, T., & Liu, T. (2025). Design Strategies of S8 Molecule Cathodes for Room-Temperature Na-S Batteries. Nanomaterials, 15(5), 330. https://doi.org/10.3390/nano15050330