Surface Grafting of Graphene Flakes with Fluorescent Dyes: A Tailored Functionalization Approach
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
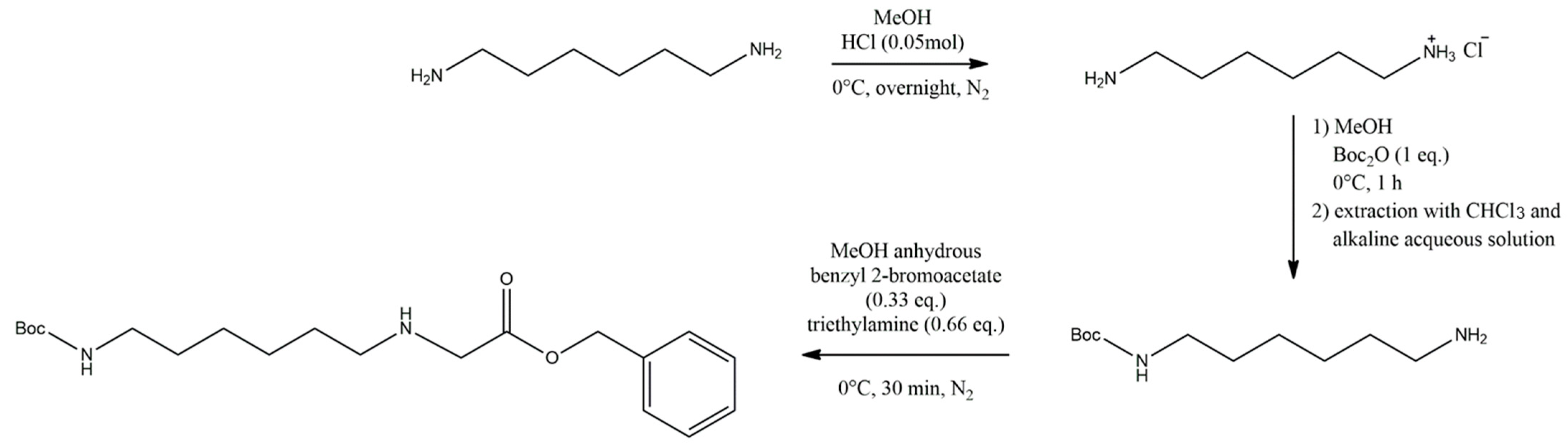
2.2. Synthesis of the Linker Molecule
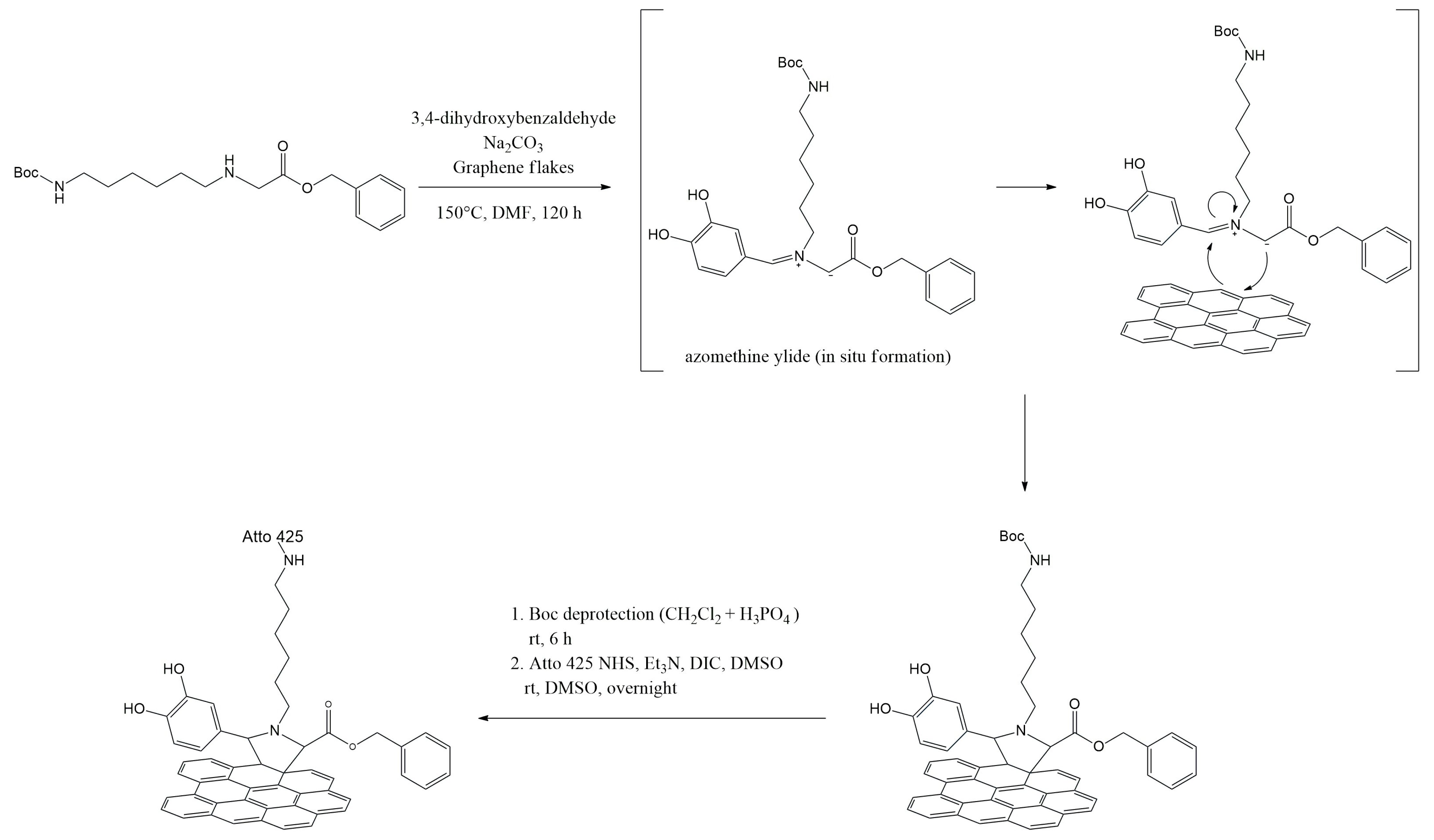
2.3. Covalent Functionalization of Graphene Flakes by 1,3-Dipolar Cycloaddition
2.4. Spectroscopic, Optical, and Morphological Characterization Techniques
3. Results and Discussion
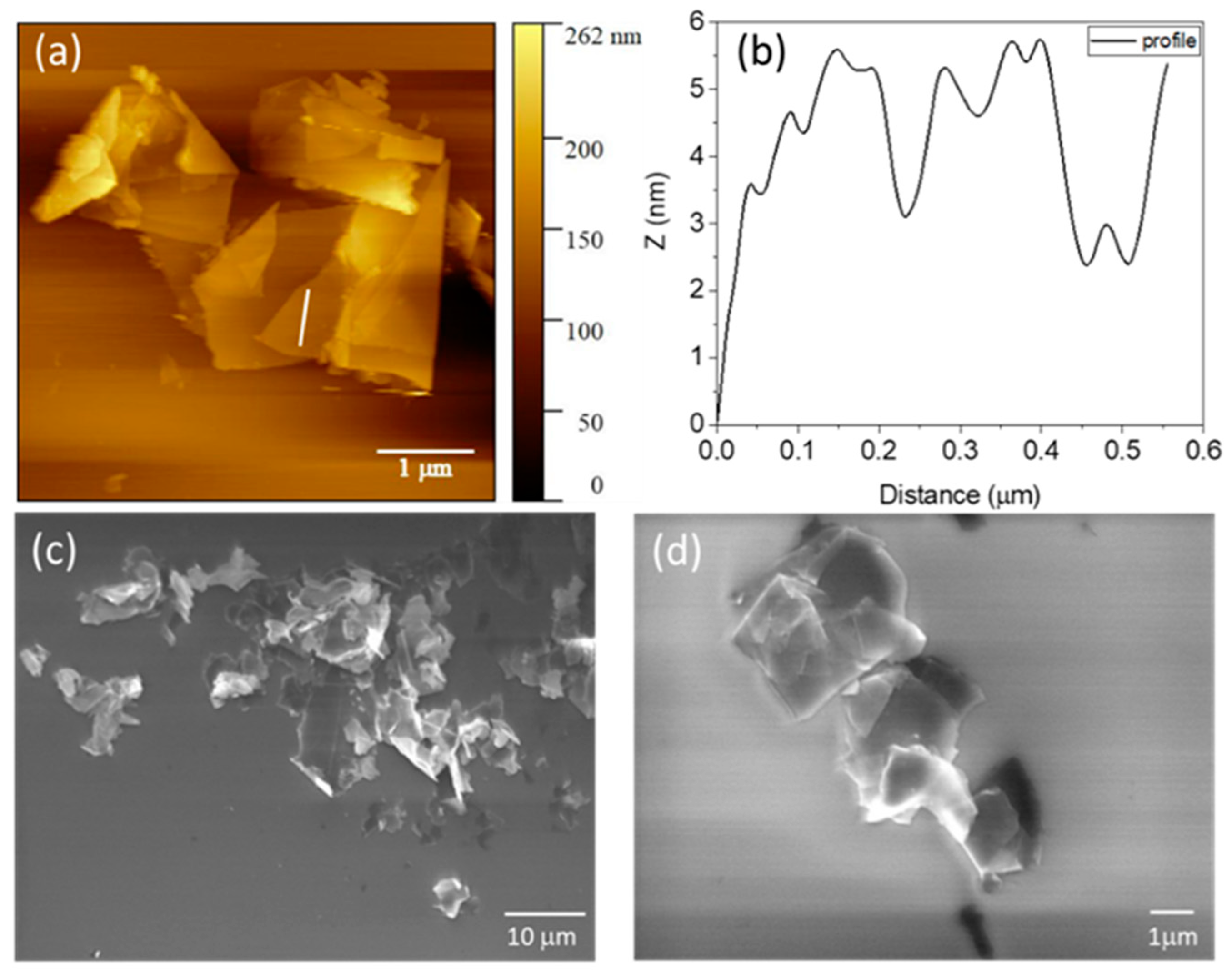
3.1. Surface Characterization
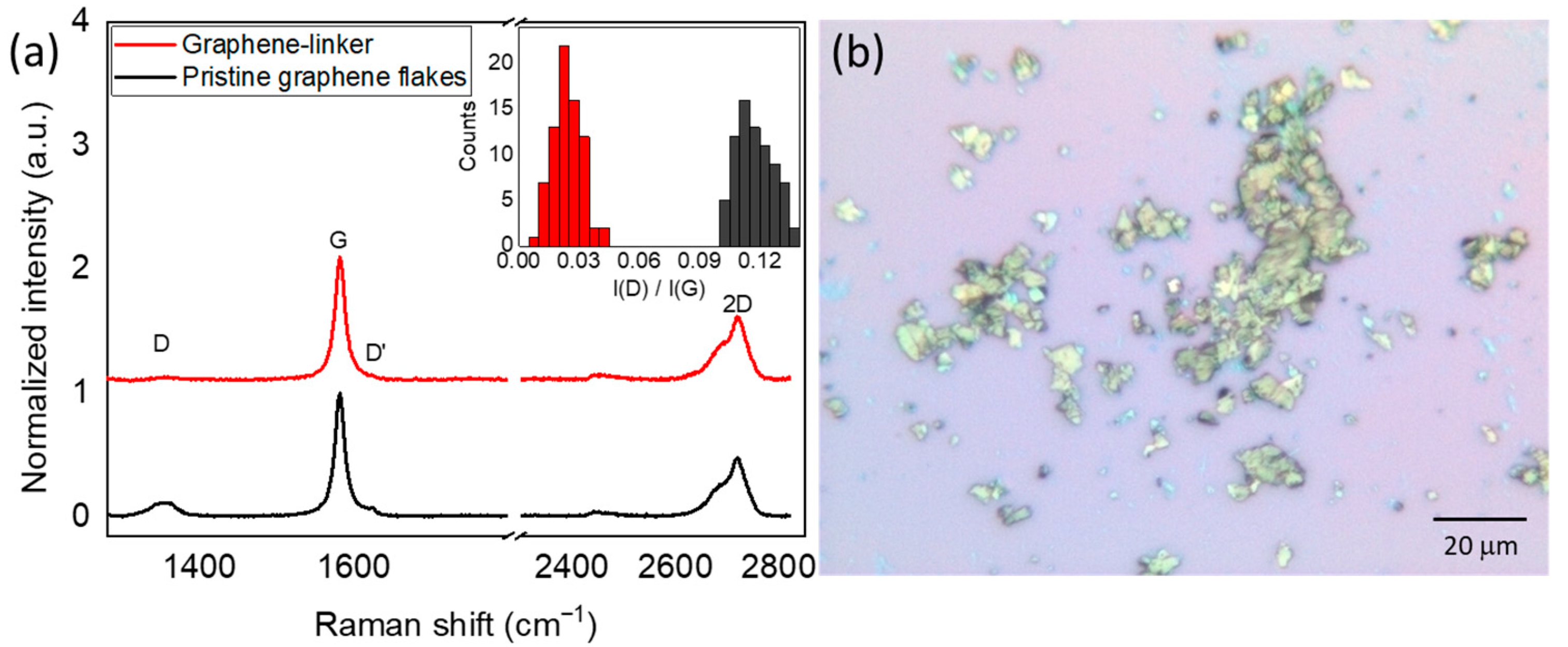
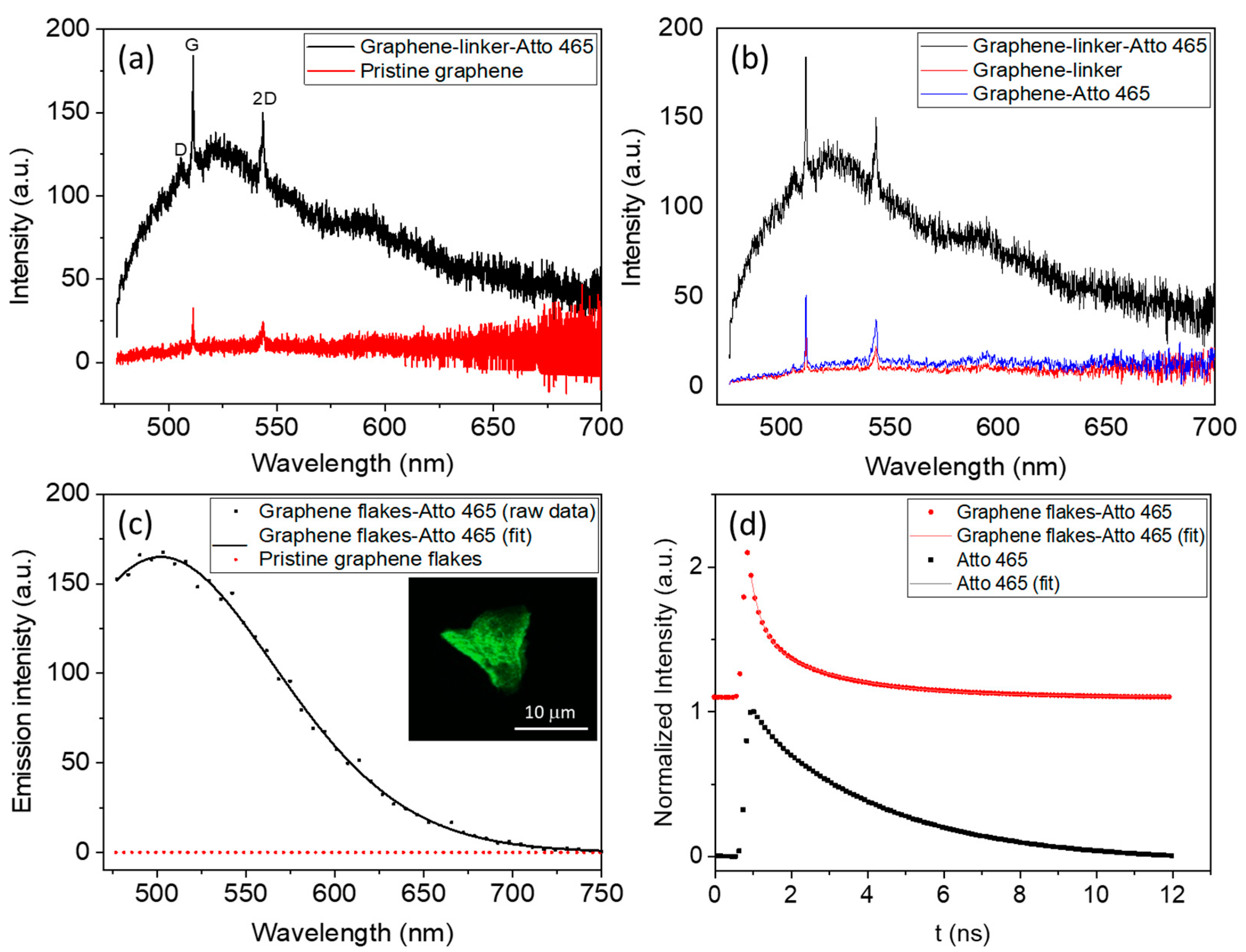
3.2. Raman Characterization of Graphene Functionalized with the Linker by 1,3-Dipolar Cycloaddition
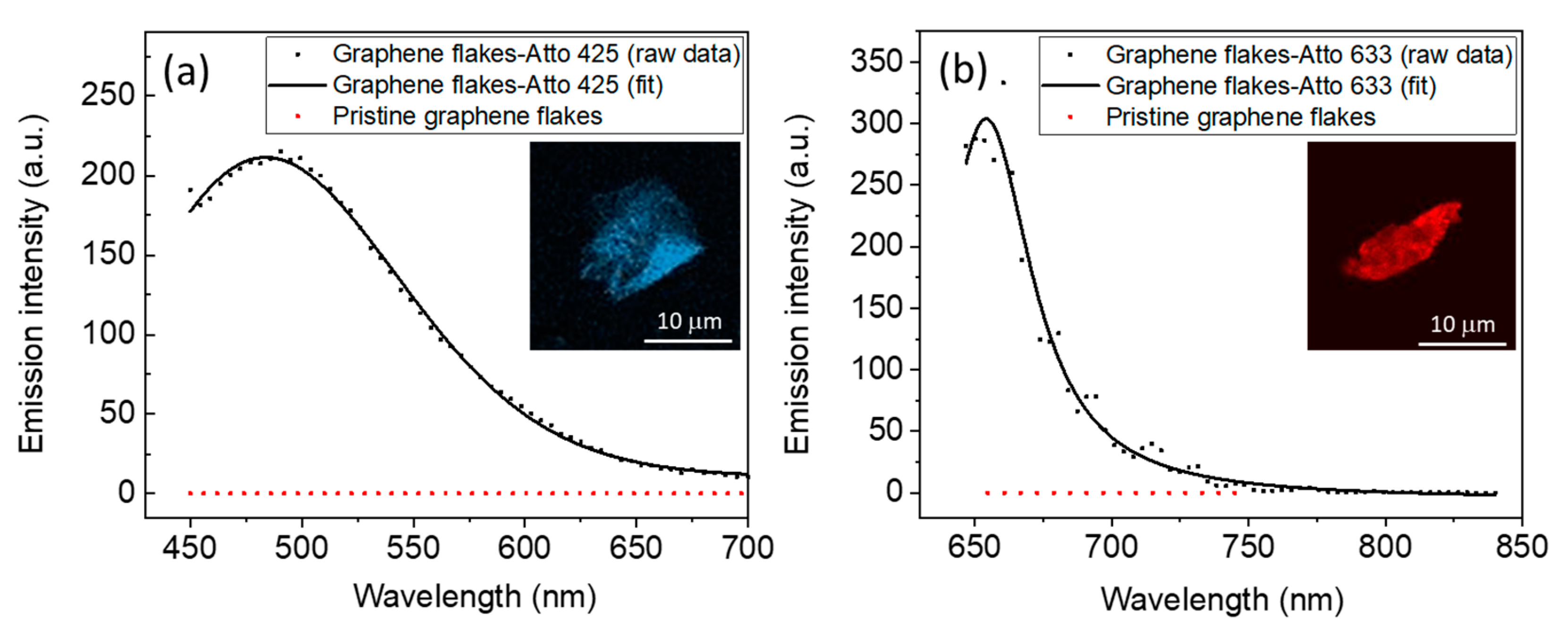
3.3. Optical Characterization of Graphene Flakes Functionalized with the Fluorophores
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric Field Effect in Atomically Thin Carbon Films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [PubMed]
- Soldano, C.; Mahmood, A.; Dujardin, E. Production, properties and potential of graphene. Carbon 2010, 48, 2127–2150. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Morozov, S.V.; Mohinddin, T.M.G.; Ponomarenko, L.A.; Elias, D.C.; Yang, R.; Barbolina, I.I.; Blake, P.; Booth, T.J.; Jiang, D.; et al. Electronic properties of graphene. Phys. Status Solidi Basic Res. 2007, 244, 4106–4111. [Google Scholar] [CrossRef]
- Sun, X.; Huang, C.; Wang, L.; Liang, L.; Cheng, Y.; Fei, W.; Li, Y. Recent Progress in Graphene/Polymer Nanocomposites. Adv. Mater. 2021, 33, 2001105. [Google Scholar] [CrossRef] [PubMed]
- Ke, Q.; Wang, J. Graphene-based materials for supercapacitor electrodes—A review. J. Mater. 2016, 2, 37–54. [Google Scholar] [CrossRef]
- Goenka, S.; Sant, V.; Sant, S. Graphene-based nanomaterials for drug delivery and tissue engineering. J. Control. Release 2014, 173, 75–88. [Google Scholar] [CrossRef]
- Wang, X.; Zhi, L.; Müllen, K. Transparent, conductive graphene electrodes for dye-sensitized solar cells. Nano Lett. 2008, 8, 323–327. [Google Scholar] [CrossRef]
- Ji, Y.; Lee, S.; Cho, B.; Song, S.; Lee, T. Flexible organic memory devices with multilayer graphene electrodes. ACS Nano 2011, 5, 5995–6000. [Google Scholar] [CrossRef]
- Lai, S.; Vlamidis, Y.; Mishra, N.; Cosseddu, P.; Mišeikis, V.; Ricci, P.C.; Voliani, V.; Coletti, C.; Bonfiglio, A. A Flexible, Transparent Chemosensor Integrating an Inkjet-Printed Organic Field-Effect Transistor and a Non-Covalently Functionalized Graphene Electrode. Adv. Mater. Technol. 2021, 6, 2100481. [Google Scholar] [CrossRef]
- Alwarappan, S.; Erdem, A.; Liu, C.; Li, C.Z. Probing the electrochemical properties of graphene nanosheets for biosensing applications. J. Phys. Chem. C 2009, 113, 8853–8857. [Google Scholar] [CrossRef]
- Mao, H.Y.; Lu, Y.H.; Lin, J.D.; Zhong, S.; Wee, A.T.S.; Chen, W. Manipulating the electronic and chemical properties of graphene via molecular functionalization. Prog. Surf. Sci. 2013, 88, 132–159. [Google Scholar] [CrossRef]
- Guo, S.; Garaj, S.; Bianco, A.; Ménard-Moyon, C. Controlling covalent chemistry on graphene oxide. Nat. Rev. Phys. 2022, 4, 247–262. [Google Scholar] [CrossRef]
- Rabchinskii, M.K.; Ryzhkov, S.A.; Besedina, N.A.; Brzhezinskaya, M.; Malkov, M.N.; Stolyarova, D.Y.; Arutyunyan, A.F.; Struchkov, N.S.; Saveliev, S.D.; Diankin, I.D.; et al. Guiding graphene derivatization for covalent immobilization of aptamers. Carbon 2022, 196, 264–279. [Google Scholar] [CrossRef]
- Rabchinskii, M.K.; Sysoev, V.V.; Varezhnikov, A.S.; Solomatin, M.A.; Struchkov, N.S.; Stolyarova, D.Y.; Ryzhkov, S.A.; Antonov, G.A.; Gabrelian, V.S.; Cherviakova, P.D.; et al. Toward On-Chip Multisensor Arrays for Selective Methanol and Ethanol Detection at Room Temperature: Capitalizing the Graphene Carbonylation. ACS Appl. Mater. Interfaces 2023, 15, 28370–28386. [Google Scholar] [CrossRef]
- Maio, A.; Pibiri, I.; Morreale, M.; La Mantia, F.P.; Scaffaro, R. An Overview of Functionalized Graphene Nanomaterials for Advanced Applications. Nanomaterials 2021, 11, 1717. [Google Scholar] [CrossRef]
- Boukhvalov, D.W.; Katsnelson, M.I. Chemical Functionalization of Graphene with Defects. Nano Lett. 2008, 8, 4373–4379. [Google Scholar] [CrossRef]
- Ioniţă, M.; Vlăsceanu, G.M.; Watzlawek, A.A.; Voicu, S.I.; Burns, J.S.; Iovu, H. Graphene and functionalized graphene: Extraordinary prospects for nanobiocomposite materials. Compos. Part B Eng. 2017, 121, 34–57. [Google Scholar] [CrossRef]
- Shan, C.; Yang, H.; Han, D.; Zhang, Q.; Ivaska, A.; Niu, L. Water-soluble graphene covalently functionalized by biocompatible poly-L-lysine. Langmuir 2009, 25, 12030–12033. [Google Scholar] [CrossRef]
- Hu, H.; Allan, C.C.K.; Li, J.; Kong, Y.; Wang, X.; Xin, J.H.; Hu, H. Multifunctional organically modified graphene with super-hydrophobicity. Nano Res. 2014, 7, 418–433. [Google Scholar] [CrossRef]
- Loh, K.P.; Bao, Q.; Ang, P.K.; Yang, J. The chemistry of graphene. J. Mater. Chem. 2010, 20, 2277–2289. [Google Scholar] [CrossRef]
- Yang, G.-h.; Bao, D.-d.; Liu, H.; Zhang, D.-q.; Wang, N.; Li, H.-t. Functionalization of Graphene and Applications of the Derivatives. J. Inorg. Organomet. Polym. Mater. 2017, 27, 1129–1141. [Google Scholar] [CrossRef]
- Criado, A.; Melchionna, M.; Marchesan, S.; Prato, M. The Covalent Functionalization of Graphene on Substrates. Angew. Chem. Int. Ed. 2015, 54, 10734–10750. [Google Scholar] [CrossRef] [PubMed]
- Wetzl, C.; Silvestri, A.; Garrido, M.; Hou, H.L.; Criado, A.; Prato, M. The Covalent Functionalization of Surface-Supported Graphene: An Update. Angew. Chem. Int. Ed. 2023, 62, e202212857. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Kim, K.J.; Kim, B.; Lee, H.; Kim, S. Covalent functionalization of epitaxial graphene by azidotrimethylsilane. J. Phys. Chem. C 2009, 113, 9433–9435. [Google Scholar] [CrossRef]
- Kim, K.H.; Seo, S.E.; Park, S.J.; Kim, J.; Park, C.S.; Le, T.H.; Lee, C.S.; Kim, Y.K.; Kim, H.Y.; Jun, S.; et al. N-Heterocyclic Carbene–Graphene Nanotransistor Based on Covalent Bond for Ultrastable Biosensors. Adv. Funct. Mater. 2024, 34, 2310377. [Google Scholar] [CrossRef]
- Sulleiro, M.V.; Quiroga, S.; Peña, D.; Pérez, D.; Guitián, E.; Criado, A.; Prato, M. Microwave-induced covalent functionalization of few-layer graphene with arynes under solvent-free conditions. Chem. Commun. 2018, 54, 2086–2089. [Google Scholar] [CrossRef]
- Georgakilas, V.; Bourlinos, A.B.; Zboril, R.; Steriotis, T.A.; Dallas, P.; Stubos, A.K.; Trapalis, C. Organic functionalisation of graphenes. Chem. Commun. 2010, 46, 1766–1768. [Google Scholar] [CrossRef]
- Wu, S.; Sun, W.; Zhang, D.; Shu, L.; Wu, H.; Xu, J.; Lao, X. 1,3-Dipolar cycloaddition of several azomethine ylides to [60]fullerene: Synthesis of derivatives of 2′,5′-dihydro-1′H-pyrrolo[3′,4′:1,2][60]fullerene. J. Chem. Soc., Perkin Trans. 1998, 1, 1733–1738. [Google Scholar] [CrossRef]
- Bayazit, M.K.; Coleman, K.S. Fluorescent single-walled carbon nanotubes following the 1,3-dipolar cycloaddition of pyridinium ylides. J. Am. Chem. Soc. 2009, 131, 10670–10676. [Google Scholar] [CrossRef]
- Filippone, S.; Maroto, E.E.; Martín-Domenech, Á.; Suarez, M.; Martín, N. An efficient approach to chiral fullerene derivatives by catalytic enantioselective 1,3-dipolar cycloadditions. Nat. Chem. 2009, 1, 578–582. [Google Scholar] [CrossRef]
- Basta, L.; Moscardini, A.; Fabbri, F.; Bellucci, L.; Tozzini, V.; Rubini, S.; Griesi, A.; Gemmi, M.; Heun, S.; Veronesi, S. Covalent organic functionalization of graphene nanosheets and reduced graphene oxide via1,3-dipolar cycloaddition of azomethine ylide. Nanoscale Adv. 2021, 3, 5841–5852. [Google Scholar] [CrossRef] [PubMed]
- Basta, L.; Bianco, F.; Moscardini, A.; Fabbri, F.; Bellucci, L.; Tozzini, V.; Heun, S.; Veronesi, S. Deterministic Covalent Organic Functionalization of Monolayer Graphene with 1,3-Dipolar Cycloaddition Via High Resolution Surface Engineering. J. Mater. Chem. C 2022, 11, 2630–2639. [Google Scholar] [CrossRef]
- Lukumbuzya, M.; Schmid, M.; Pjevac, P.; Daims, H. A multicolor fluorescence in situ hybridization approach using an extended set of fluorophores to visualize microorganisms. Front. Microbiol. 2019, 10, 1383. [Google Scholar] [CrossRef] [PubMed]
- Dodge, J.T.; Doyle, A.D.; Costa-da-Silva, A.C.; Hogden, C.T.; Mezey, E.; Mays, J.W. Atto 465 Derivative Is a Nuclear Stain with Unique Excitation and Emission Spectra Useful for Multiplex Immunofluorescence Histochemistry. J. Histochem. Cytochem. 2022, 70, 211–223. [Google Scholar] [CrossRef]
- Hübner, K.; Joshi, H.; Aksimentiev, A.; Stefani, F.D.; Tinnefeld, P.; Acuna, G.P. Determining the In-Plane Orientation and Binding Mode of Single Fluorescent Dyes in DNA Origami Structures. ACS Nano 2021, 15, 5109–5117. [Google Scholar] [CrossRef]
- Krasley, A.T.; Li, E.; Galeana, J.M.; Bulumulla, C.; Beyene, A.G.; Demirer, G.S. Carbon Nanomaterial Fluorescent Probes and Their Biological Applications. Chem. Rev. 2024, 124, 3085–3185. [Google Scholar] [CrossRef]
- Peng, C.; Hu, W.; Zhou, Y.; Fan, C.; Huang, Q. Intracellular imaging with a graphene-based fluorescent probe. Small 2010, 6, 1686–1692. [Google Scholar] [CrossRef]
- Halbig, C.E.; Lasch, R.; Krüll, J.; Pirzer, A.S.; Wang, Z.; Kirchhof, J.N.; Bolotin, K.I.; Heinrich, M.R.; Eigler, S. Selective Functionalization of Graphene at Defect-Activated Sites by Arylazocarboxylic tert-Butyl Esters. Angew. Chem. Int. Ed. 2019, 58, 3599–3603. [Google Scholar] [CrossRef]
- Lim, C.Y.; Owens, N.A.; Wampler, R.D.; Ying, Y.; Granger, J.H.; Porter, M.D.; Takahashi, M.; Shimazu, K. Succinimidyl ester surface chemistry: Implications of the competition between aminolysis and hydrolysis on covalent protein immobilization. Langmuir 2014, 30, 12868–12878. [Google Scholar] [CrossRef]
- Guo, Z.; Chakraborty, S.; Monikh, F.A.; Varsou, D.-D.; Chetwynd, A.J.; Afantitis, A.; Lynch, I.; Zhang, P. Surface functionalization of graphene-based materials: Biological behavior, toxicology, and safe-by-design aspects. Adv. Biol. 2021, 5, 2100637. [Google Scholar] [CrossRef]
- Isidro-Llobet, A.; Álvarez, M.; Albericio, F. Amino Acid-Protecting Groups. Chem. Rev. 2009, 109, 2455–2504. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Ma, J.; Imler, G.H.; Parrish, D.A.; Shreeve, J.M. Versatile functionalization of 3,5-diamino-4-nitropyrazole for promising insensitive energetic compounds. Dalt. Trans. 2019, 48, 14490–14496. [Google Scholar] [CrossRef]
- Bütikofer, A.; Chen, P. Zwitterionic Halido Cyclopentadienone Iron Complexes and Their Catalytic Performance in Hydrogenation Reactions. Inorg. Chem. 2023, 62, 4188–4196. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.W.; Ha, H.J.; Lee, W.K. Selective mono-BOC protection of diamines. Synth. Commun. 2007, 37, 737–742. [Google Scholar] [CrossRef]
- George, N.; Ofori, S.; Parkin, S.; Awuah, S.G. Mild deprotection of the: N-tert-butyloxycarbonyl (N-Boc) group using oxalyl chloride. RSC Adv. 2020, 10, 24017–24026. [Google Scholar] [CrossRef] [PubMed]
- Fattahi, N.; Ayubi, M.; Ramazani, A. Amidation and esterification of carboxylic acids with amines and phenols by N,N′-diisopropylcarbodiimide: A new approach for amide and ester bond formation in water. Tetrahedron 2018, 74, 4351–4356. [Google Scholar] [CrossRef]
- Datta, R.; Heaster, T.M.; Sharick, J.T.; Gillette, A.A.; Skala, M.C. Fluorescence lifetime imaging microscopy: Fundamentals and advances in instrumentation, analysis, and applications. J. Biomed. Opt. 2020, 25, 071203. [Google Scholar] [CrossRef]
- Del Rio Castillo, A.E.; Pellegrini, V.; Ansaldo, A.; Ricciardella, F.; Sun, H.; Marasco, L.; Buha, J.; Dang, Z.; Gagliani, L.; Lago, E.; et al. High-yield production of 2D crystals by wet-jet milling. Mater. Horiz. 2018, 5, 890–904. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Meyer, J.C.; Scardaci, V.; Casiraghi, C.; Lazzeri, M.; Mauri, F.; Piscanec, S.; Jiang, D.; Novoselov, K.S.; Roth, S.; et al. Raman Spectrum of Graphene and Graphene Layers. Phys. Rev. Lett. 2006, 97, 187401. [Google Scholar] [CrossRef]
- Jorio, A.; Ferreira, E.H.M.; Stavale, F.; Achete, C.A.; Capaz, R.B.; Moutinho, M.V.O.; Lombardo, A.; Kulmala, T.S.; Ferrari, A.C. Quantifying Defects in Graphene via Raman Spectroscopy at Different Excitation Energies. Nano Lett. 2011, 12, 3190–3196. [Google Scholar] [CrossRef]
- Eckmann, A.; Felten, A.; Mishchenko, A.; Britnell, L.; Krupke, R.; Novoselov, K.S.; Casiraghi, C. Probing the nature of defects in graphene by Raman spectroscopy. Nano Lett. 2012, 12, 3925–3930. [Google Scholar] [CrossRef] [PubMed]
- Eckmann, A.; Felten, A.; Verzhbitskiy, I.; Davey, R.; Casiraghi, C. Raman study on defective graphene: Effect of the excitation energy, type, and amount of defects. Phys. Rev. B 2013, 88, 035426. [Google Scholar] [CrossRef]
- MacHáň, R.; Hof, M.; Chernovets, T.; Zhmak, M.N.; Ovchinnikova, T.V.; Sýkora, J. Formation of arenicin-1 microdomains in bilayers and their specific lipid interaction revealed by Z-scan FCS. Anal. Bioanal. Chem. 2011, 399, 3547–3554. [Google Scholar] [CrossRef] [PubMed]
- Sethurajaperumal, A.; Ravichandran, V.; Merenkov, I.; Ostrikov, K.K.; Varrla, E. Delamination and defects in graphene nanosheets exfoliated from 3D precursors. Carbon 2023, 213, 118306. [Google Scholar] [CrossRef]
- Acik, M.; Chabal, Y.J. Nature of graphene edges: A review. Jpn. J. Appl. Phys. 2011, 50, 070101. [Google Scholar] [CrossRef]
- Chmyrov, A.; Arden-Jacob, J.; Zilles, A.; Drexhage, K.H.; Widengren, J. Characterization of new fluorescent labels for ultra-high resolution microscopy. Photochem. Photobiol. Sci. 2008, 7, 1378–1385. [Google Scholar] [CrossRef]
- Arden-Jacob, J.; Drexhage, K.H.; Druzhinin, S.I.; Ekimova, M.; Flender, O.; Lenzer, T.; Oum, K.; Scholz, M. Ultrafast photoinduced dynamics of the 3,6-diaminoacridinium derivative ATTO 465 in solution. Phys. Chem. Chem. Phys. 2013, 15, 1844–1853. [Google Scholar] [CrossRef]
- Hammler, D.; Marx, A.; Zumbusch, A. Fluorescence-Lifetime-Sensitive Probes for Monitoring ATP Cleavage. Chem. Eur. J. 2018, 24, 15329–15335. [Google Scholar] [CrossRef]
- Chen, T.; Karedla, N.; Enderlein, J. Measuring sub-nanometer undulations at microsecond temporal resolution with metal- and graphene-induced energy transfer spectroscopy. Nat. Commun. 2024, 15, 1789. [Google Scholar] [CrossRef]
- Xin, G.; Gong, S.; Kim, N.; Kim, J.; Hwang, W.; Nam, J.; Cho, Y.H.; Cho, S.M.; Chae, H. Graphene oxide/N-methyl-2-pyrrolidone charge-transfer complexes for molecular detection. Sens. Actuators B Chem. 2013, 176, 81–85. [Google Scholar] [CrossRef]
- Berezin, M.Y.; Achilefu, S. Fluorescence lifetime measurements and biological imaging. Chem. Rev. 2010, 110, 2641–2684. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vlamidis, Y.; Marinelli, C.; Moscardini, A.; Faraci, P.; Heun, S.; Veronesi, S. Surface Grafting of Graphene Flakes with Fluorescent Dyes: A Tailored Functionalization Approach. Nanomaterials 2025, 15, 329. https://doi.org/10.3390/nano15050329
Vlamidis Y, Marinelli C, Moscardini A, Faraci P, Heun S, Veronesi S. Surface Grafting of Graphene Flakes with Fluorescent Dyes: A Tailored Functionalization Approach. Nanomaterials. 2025; 15(5):329. https://doi.org/10.3390/nano15050329
Chicago/Turabian StyleVlamidis, Ylea, Carmela Marinelli, Aldo Moscardini, Paolo Faraci, Stefan Heun, and Stefano Veronesi. 2025. "Surface Grafting of Graphene Flakes with Fluorescent Dyes: A Tailored Functionalization Approach" Nanomaterials 15, no. 5: 329. https://doi.org/10.3390/nano15050329
APA StyleVlamidis, Y., Marinelli, C., Moscardini, A., Faraci, P., Heun, S., & Veronesi, S. (2025). Surface Grafting of Graphene Flakes with Fluorescent Dyes: A Tailored Functionalization Approach. Nanomaterials, 15(5), 329. https://doi.org/10.3390/nano15050329







