Advancements in the Preparation and Application of Ni-Co System (Alloys, Composites, and Coatings): A Review
Abstract
1. Introduction
2. Preparation of Nickel–Cobalt Alloys
2.1. Sol–Gel Method
2.2. Liquid-Phase Reduction
2.3. Brush Plating Method
2.4. Mechanical Alloying Method
2.5. Composite Electrodeposition
3. The Effect of Process Conditions on Nickel–Cobalt Alloy Plating in Electrodeposition
3.1. The Effect of Cobalt Content on Nickel–Cobalt Alloy Plating Layer
3.2. Type of Current and Density Magnitude
3.3. Other Process Parameters
4. Research Status of Composite Electrodeposition Method in Nickel–Cobalt Composites
4.1. Metals or Metallic Compounds
4.2. Non-Metallic Substances
4.3. Silicon Carbide (SiC)
5. Summary and Outlook
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Luo, J.K.; Flewitt, A.J.; Spearing, S.M.; Fleck, N.A.; Milne, W.I. Young’s modulus of electroplated Ni thin film for MEMS applications. Mater. Lett. 2004, 58, 2306–2309. [Google Scholar] [CrossRef]
- Allameh, S.M.; Lou, J.; Kavishe, F.; Buchheit, T.; Soboyejo, W.O. An investigation of fatigue in LIGA Ni MEMS thin films. Mater. Sci. Eng. A 2004, 371, 256–266. [Google Scholar] [CrossRef]
- Yang, Y.; Imasogie, B.I.; Allameh, S.M.; Boyce, B.; Lian, K.; Lou, J.; Soboyejo, W.O. Mechanisms of fatigue in LIGA Ni MEMS thin films. Mater. Sci. Eng. A 2007, 444, 39–50. [Google Scholar] [CrossRef]
- Teh, K.S.; Cheng, Y.T.; Lin, L. MEMS fabrication based on nickel-nanocomposite: Film deposition and characterization. J. Micromech. Microengin. 2005, 15, 2205. [Google Scholar] [CrossRef][Green Version]
- Cohen, A.L.; Frodis, U.; Tseng, F.G.; Zhang, G.; Mansfeld, F.; Will, P.M. EFAB: Low-cost automated electrochemical batch fabrication of arbitrary 3D microstructures. Micromach. Microfabr. Process Technol. V SPIE 1999, 3874, 236–247. [Google Scholar] [CrossRef]
- Khan, F.; Zhu, Y.; Lu, J.; Pal, J. MEMS-based tunable meander inductor. Electron. Lett. 2015, 51, 1582–1583. [Google Scholar] [CrossRef]
- Huang, M.; Ruoff, R.S. Growth of single-layer and multilayer graphene on Cu/Ni alloy substrates. Acc. Chem. Res. 2020, 53, 800–811. [Google Scholar] [CrossRef]
- Deng, J.; Chen, C.; Liu, X.; Li, Y.; Zhou, K.; Guo, S. A high-strength heat-resistant Al–5.7Ni eutectic alloy with spherical Al3Ni nano-particles by selective laser melting. Scr. Mater. 2021, 203, 114034. [Google Scholar] [CrossRef]
- Kong, F.P.; Ren, Z.H.; Banis, M.N.; Du, L.; Zhou, X.; Chen, G.; Zhang, L.; Li, J.; Wang, S.; Li, M.; et al. Active and stable Pt–Ni alloy octahedra catalyst for oxygen reduction via near-surface atomical engineering. ACS Catal. 2020, 10, 4205–4214. [Google Scholar] [CrossRef]
- Bhat, R.S.; Shetty, S.M.; Kumar, N.V.A. Electroplating of Zn-Ni alloy coating on mild steel and its electrochemical studies. J. Mater. Eng. Perform. 2021, 30, 8188–8195. [Google Scholar] [CrossRef]
- Kumaraswamy, J.; Kumar, V.; Purushotham, G.; Suresh, R. Thermal analysis of nickel alloy/Al2O3/TiO2 hybrid metal matrix composite in automotive engine exhaust valve using FEA method. J. Therm. Eng. 2021, 7, 415–428. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, Y.; Pan, J.; Liu, E.; Hao, J.; Han, J. Facile Fabrication of High-White and Robust Superhydrophobic Ni/Al2O3 Composite Coating on Al Alloy. Adv. Eng. Mater. 2023, 25, 2300920. [Google Scholar] [CrossRef]
- Dwivedi, S.P.; Sharma, S. The behaviour of AZ91 magnesium-based alloy with Ni–Al2O3 reinforcement. Mater. Sci. Technol. 2024, 40, 851–863. [Google Scholar] [CrossRef]
- Fahrenholtz, W.G.; Ellerby, D.T.; Loehman, R.E. Al2O3–Ni composites with high strength and fracture toughness. J. Am. Ceram. Soc. 2000, 83, 1279–1280. [Google Scholar] [CrossRef]
- Zhou, Y.; Sun, Z.P.; Yu, Y.; Li, L.; Song, J.L.; Xie, F.Q.; Wu, X.Q. Tribological behavior of Ni–SiC composite coatings produced by circulating-solution electrodeposition technique. Tribol. Int. 2021, 159, 106933. [Google Scholar] [CrossRef]
- Khazrayie, M.A.; Aghdam, A.R.S. Si3N4/Ni nanocomposite formed by electroplating: Effect of average size of nanoparticulates. Trans. Nonferrous Met. Soc. China 2010, 20, 1017–1023. [Google Scholar] [CrossRef]
- Lai, L.Y.; Wu, Y.P.; Yang, Y.; Wang, H.; Yang, Z.Q.; Ding, G.F. Microfabrication and characterization of high tensile strength SiC whisker-reinforced nickel composite coatings by electrodeposition. J. Electrochem. Soc. 2019, 166, D726. [Google Scholar] [CrossRef]
- Matsui, N.; Mashimo, K.; Egami, A.; Konishi, A.; Okada, O.; Tsukada, T. Etching characteristics of magnetic materials (Co, Fe, Ni) using CO/NH3 gas plasma for hardening mask etching. Vacuum 2002, 66, 479–485. [Google Scholar] [CrossRef]
- Gu, Q. Application of Cobalt in High Performance Magnetic Materials. World Nonferrous Met. 2012, 10, 58–59. [Google Scholar]
- Majeed, A.; Khan, M.A.; Ahmad, R.; Lodhi, M.Y.; Ahmad, I. Tuning the properties of novel magnetic oxide via Co–Bi Co-substitution including theoretical background of characterization techniques. J. Supercond. Nov. Magn. 2021, 34, 2313–2329. [Google Scholar] [CrossRef]
- Dong, Y.; Zhang, T.L.; Wang, H.; Liu, X.; Jiang, C.B. Chemical synthesis and characterization of SmCo5/Co magnetic nanocomposite particles. Rare Met. 2021, 40, 1224–1231. [Google Scholar] [CrossRef]
- Zhang, Y.Y.; Ma, R.; Feng, S.H.; Cheng, L.; Davies, P.A.; Yu, P. Microstructures and magnetic properties of Fe-35% Co alloy fabricated by metal injection molding. J. Magn. Magn. Mater. 2020, 497, 165982. [Google Scholar] [CrossRef]
- Wu, X.; Liu, X.; Liao, J. The Effect of WC Carbon Content on the Properties and Hard Phase Grain Size of High Cobalt Hard Alloys. Cem. Carbides 2015, 32, 31–35. [Google Scholar]
- Tikkanen, M.H.; Taskinen, A.; Taskinen, P. Characteristic properties of cobalt powder suitable for hard metal production. Powder Met. 1975, 18, 259–282. [Google Scholar] [CrossRef]
- Klünsner, T.; Mitterhuber-Gressl, L.; Maier, K.; Beckstein, F.; Czettl, C. Thermal conductivity loss in WC-Co hard metal due to high-temperature cyclic loading damage as a function of microstructure and temperature. Int. J. Refract. Met. Hard Mater. 2024, 123, 106797. [Google Scholar] [CrossRef]
- da Silva, E.N.; dos Santos, A.A.A.; do Nascimento, R.M.; Alves, S.M.; Guimarães, R.S.; Filgueira, M. Investigation of characteristics and properties of spark plasma sintered ultrafine WC-6.4 Fe3.6Ni alloy as potential alternative WC-Co hard metals. Int. J. Refract. Met. Hard Mater. 2021, 101, 105669. [Google Scholar] [CrossRef]
- Yang, Y.K.; Zhang, C.Q.; Wang, D.; Nie, L.P.; Wellmann, D.; Tian, Y.T.; Ju, J.; Shen, Z.; Kang, M.D.; Zhang, J.Q.; et al. Additive manufacturing of WC-Co hardmetals: A review. Int. J. Adv. Manuf. Technol. 2020, 108, 1653–1673. [Google Scholar] [CrossRef]
- Ju, J.; Shen, Z.; Kang, M.; Zhang, J.; Wang, J. On the preferential grain boundary oxidation of a Ni-Co-based superalloy. Corros. Sci. 2022, 199, 110203. [Google Scholar] [CrossRef]
- Knop, M.; Mulvey, P.; Ismail, F.; Radecka, A.; Rahman, K.M.; Lindley, T.C.; Shollock, B.A.; Hardy, M.C.; Moody, M.P.; Martin, T.L.; et al. A new polycrystalline Co-Ni superalloy. JOM 2014, 66, 2495–2501. [Google Scholar] [CrossRef]
- Wei, W.; Xiao, J.C.; Wang, C.F.; Cheng, Q.; Guo, F.J.; He, Q.; Wang, M.S.; Jiang, S.Z.; Huang, C.X. Hierarchical microstructure and enhanced mechanical properties of SLM-fabricated GH5188 Co-superalloy. Mater. Sci. Eng. A 2022, 831, 142276. [Google Scholar] [CrossRef]
- Murray, S.P.; Pusch, K.M.; Polonsky, A.T.; Torbet, C.J.; Seward, G.G.E.; Zhou, N.; Forsik, S.A.J.; Nandwana, P.; Kirka, M.M.; Dehoff, R.R.; et al. A defect-resistant Co–Ni superalloy for 3D printing. Nat. Commun. 2020, 11, 4975. [Google Scholar] [CrossRef] [PubMed]
- Song, J.Z.; Chen, Y.H.; Hao, X.C.; Wang, M.; Ma, Y.C.; Xie, J.L. Microstructure and mechanical properties of novel Ni–Cr–Co-based superalloy GTAW joints. J. Mater. Res. Technol. 2024, 29, 2758–2767. [Google Scholar] [CrossRef]
- Gao, Q.Z.; Jiang, Y.J.; Liu, Z.Y.; Zhang, H.L.; Jiang, C.C.; Zhang, X.; Li, H.J. Effects of alloying elements on microstructure and mechanical properties of Co–Ni–Al–Ti superalloy. Mater. Sci. Eng. A 2020, 779, 139139. [Google Scholar] [CrossRef]
- Liu, P.; Zhang, R.; Yuan, Y.; Cui, C.Y.; Zhou, Y.Z.; Sun, X.F. Hot deformation behavior and workability of a Ni–Co based superalloy. J. Alloys Compd. 2020, 831, 154618. [Google Scholar] [CrossRef]
- Li, J.H.; Li, X.H.; Hu, Q.Y.; Wang, Z.X.; Zheng, J.C.; Wu, L.; Zhang, L.X. Study of extraction and purification of Ni, Co and Mn from spent battery material. Hydrometallurgy 2009, 99, 7–12. [Google Scholar] [CrossRef]
- Iqbal, M.Z.; Faisal, M.M.; Ali, S.R.; Farid, S.; Afzal, A.M. Co-MOF/polyaniline-based electrode material for high performance supercapattery devices. Electrochim. Acta 2020, 346, 136039. [Google Scholar] [CrossRef]
- Zhang, D.; Guo, X.M.; Tong, X.Z.; Chen, Y.F.; Duan, M.T.; Shi, J.; Jiang, C.W.; Hu, L.L.; Kong, Q.H.; Zhang, J.H.; et al. High-performance battery-type supercapacitor based on porous biocarbon and biocarbon supported Ni–Co layered double hydroxide. J. Alloys Compd. 2020, 837, 155529. [Google Scholar] [CrossRef]
- Zhou, J.N.; Yang, Q.Y.; Xie, Q.Y.; Ou, H.; Lin, X.M.; Zeb, A.; Hu, L.; Wu, Y.B.; Ma, G.Z. Recent progress in Co–based metal–organic framework derivatives for advanced batteries. J. Mater. Sci. Technol. 2022, 96, 262–284. [Google Scholar] [CrossRef]
- Sun, J.L.; Tian, X.D.; Xu, C.J.; Chen, H.Y. Porous CuCo2O4 microtubes as a promising battery-type electrode material for high-performance hybrid supercapacitors. J. Mater. 2021, 7, 1358–1368. [Google Scholar] [CrossRef]
- Wang, H.; Liu, Y.; Zhang, J. Preparation of nitrogen doped mesoporous carbon supported cobalt catalyst and its performance in removing hydrogen rich gas CO. Power Gener. Technol. 2023, 44, 373–381. [Google Scholar]
- Tohidi, M.M.; Paymard, B.; Vasquez-García, S.R.; Fernández-Quiroz, D. Recent progress in applications of cobalt catalysts in organic reactions. Tetrahedron 2023, 136, 133352. [Google Scholar] [CrossRef]
- Dong, Z.L.; Jiang, T.; Xu, B.; Zhang, B.S.; Liu, G.Q.; Li, Q.; Yang, Y.B. A systematic and comparative study of copper, nickel and cobalt-ammonia catalyzed thiosulfate processes for eco-friendly and efficient gold extraction from an oxide gold concentrate. Sep. Purif. Technol. 2021, 272, 118929. [Google Scholar] [CrossRef]
- Yong, H.; Wei, X.; Hu, J.; Yuan, Z.; Guo, S.; Zhao, D.; Zhang, Y. Hydrogen storage behavior of Mg-based alloy catalyzed by carbon-cobalt composites. J. Magnes. Alloys 2021, 9, 1977–1988. [Google Scholar] [CrossRef]
- Paksoy, A.; Kurtoğlu, S.F.; Dizaji, A.K.; Altıntaş, Z.; Khoshsima, S.; Uzun, A.; Balcı, Ö. Nanocrystalline cobalt–nickel–boron (metal boride) catalysts for efficient hydrogen production from the hydrolysis of sodium borohydride. Int. J. Hydrogen Energy 2021, 46, 7974–7988. [Google Scholar] [CrossRef]
- Kim, J.; Kim, H.; Han, G.H.; Hong, S.; Park, J.; Bang, J.; Kim, S.Y.; Ahn, S.H. Electrodeposition: An efficient method to fabricate self-supported electrodes for electrochemical energy conversion systems. Exploration 2022, 2, 20210077. [Google Scholar] [CrossRef]
- Choi, K.H.; Kim, H.S.; Lee, T.H. Electrode fabrication for proton exchange membrane fuel cells by pulse electrodeposition. J. Power Sources 1998, 75, 230–235. [Google Scholar] [CrossRef]
- Li, X.M.; Du, X.; Ma, X.L.; Wang, Z.D.; Hao, X.G.; Abudula, A.; Yoshida, A.; Guan, G.Q. CuO nanowire@ Co3O4 ultrathin nanosheet core-shell arrays: An effective catalyst for oxygen evolution reaction. Electrochim. Acta 2017, 250, 77–83. [Google Scholar] [CrossRef]
- Woo, S.; Kim, I.; Lee, J.K.; Bong, S.; Lee, J.; Kim, H. Preparation of cost-effective Pt–Co electrodes by pulse electrodeposition for PEMFC electrocatalysts. Electrochim. Acta 2011, 56, 3036–3041. [Google Scholar] [CrossRef]
- Aliofkhazraei, M.; Walsh, F.C.; Zangari, G.; Köçkar, H.; Alper, M.; Rizal, C.; Magagnin, L.; Protsenko, V.; Arunachalam, R.; Rezvanian, A.; et al. Development of electrodeposited multilayer coatings: A review of fabrication, microstructure, properties and applications. Appl. Surf. Sci. Adv. 2021, 6, 100141. [Google Scholar] [CrossRef]
- Liu, Y.; Song, Q.M.; Zhang, L.G.; Xu, Z.M. Targeted recovery of Ag-Pd alloy from polymetallic electronic waste leaching solution via green electrodeposition technology and its mechanism. Sep. Purif. Technol. 2022, 280, 118944. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, J.; Jiang, S.; Li, P. Recent Progress in Sol-Gel Method for Designing and Preparing Metallic and Alloy Nanocrystals. Acta Phys.-Chim. Sin. 2019, 35, 1186–1206. [Google Scholar] [CrossRef]
- Jiang, Y.W.; Yang, S.G.; Hua, Z.H.; Huang, H.B. Sol–gel autocombustion synthesis of metals and metal alloys. Angew. Chem. 2009, 121, 8681–8683. [Google Scholar] [CrossRef]
- Hua, Z.H.; Cao, Z.W.; Deng, Y.; Jiang, Y.W.; Yang, S.G. Sol–gel autocombustion synthesis of Co–Ni alloy powder. Mater. Chem. Phys. 2011, 126, 542–545. [Google Scholar] [CrossRef]
- Bokov, D.; Jalil, A.T.; Chupradit, S.; Suksatan, W.; Ansari, M.J.; Shewael, I.H.; Valiev, G.H.; Kianfar, E. Nanomaterial by sol-gel method: Synthesis and application. Adv. Mater. Sci. Eng. 2021, 2021, 5102014. [Google Scholar] [CrossRef]
- Wang, Y.D.; Jie, S.; Zhang, G.; Mu, P.X.; Wang, X.Q.; Li, S.Y.; Qiao, L.L.; Mu, H.B. Advances in sol-gel-based superhydrophobic coatings for wood: A review. Int. J. Mol. Sci. 2023, 24, 9675. [Google Scholar] [CrossRef]
- Zhan, J.; Yue, J.; Zhang, C.; Fan, Y. Research on preparation and application of quasi one-dimensional Ni-Co alloy materials. Mater. Guide 2010, 24, 93–96+100. [Google Scholar]
- Tian, M.B. Introduction to Materials Science; Tsinghua University Press: Beijing, China, 2005; pp. 25–26. [Google Scholar] [CrossRef]
- Yang, X.; Lu, X.F.; Zhang, W.; Guo, X.; Ren, J.Q.; Xue, H.T.; Tang, F.L. Preparation and application of nano-Ni–Co alloy. J. Nanoparticle Res. 2023, 25, 152. [Google Scholar] [CrossRef]
- Usami, T.; Salman, S.A.; Kuroda, K.; Gouda, M.K.; Mahdy, A.; Okido, M. Synthesis of Cobalt-Nickel Nanoparticles via a Liquid-Phase Reduction Process. J. Nanotechnol. 2021, 2021, 9401024. [Google Scholar] [CrossRef]
- He, N.; He, Z.D.; Liu, L.; Lu, Y.; Wang, F.Q.; Wu, W.H.; Tong, G.X. Ni2+ guided phase/structure evolution and ultra-wide bandwidth microwave absorption of CoxNi1−x alloy hollow microspheres. Chem. Eng. J. 2020, 381, 122743. [Google Scholar] [CrossRef]
- Eom, H.; Hwang, I.-H.; Lee, D.Y.; Lee, S.M.; Kim, S.S. Preparation of liquid-phase reduction method-based Pt/TiO2 catalyst and reaction characteristics during HCHO room-temperature oxidation. Ind. Eng. Chem. Res. 2020, 59, 15489–15496. [Google Scholar] [CrossRef]
- Dennis, J.K.; Jones, D. Brush plating. Surf. Technol. 1981, 12, 57–73. [Google Scholar] [CrossRef]
- Zheng, W.; Zhuang, Z.; Dai, P.; Zhao, Y.; Xie, Y. Study on the microstructure and properties of nanocrystalline Ni Co alloy coating by electric brush plating. J. Fuzhou Univ. (Nat. Sci. Ed.) 2010, 38, 91–94. [Google Scholar]
- Fang, X.Q.; Xu, H.; Jin, G.Q.; Wang, Y. Effect of magnetic field on brush plating Ni–Co alloy. Surf. Eng. 2017, 33, 142–148. [Google Scholar] [CrossRef]
- Jin, G.; Ding, X.L.; Hu, Z.F.; Lv, B.; Wang, X.H. Corrosion resistance of Ni graphene composite coating by electric brush plating. Rare Met. Mater. Eng. 2019, 48, 2002–2008. [Google Scholar]
- Wang, X.H.; Hu, Z.F.; Lv, B.; Yang, Y.R.; Liu, X.B.; Zhang, H. Corrosion behavior of nickel cobalt nano alumina composite electric brush coating in NaCl solution. Electroplat. Finish. 2019, 38, 405–410. [Google Scholar]
- Suryanarayana, C. Mechanical alloying and milling. Prog. Mater. Sci. 2001, 46, 1–184. [Google Scholar] [CrossRef]
- Vaidya, M.; Muralikrishna, G.M.; Murty, B.S. High-entropy alloys by mechanical alloying: A review. J. Mater. Res. 2019, 34, 664–686. [Google Scholar] [CrossRef]
- Benjamin, J.S. Dispersion strengthened superalloys by mechanical alloying. Metall. Trans. 1970, 1, 2943–2951. [Google Scholar] [CrossRef]
- Olvera, S.; Sánchez-Marcos, J.; Palomares, F.J.; Salas, E.; Arce, E.M.; Herrasti, P. Characterization and corrosion behaviour of CoNi alloys obtained by mechanical alloying. Mater. Charact. 2014, 93, 79–86. [Google Scholar] [CrossRef]
- García-Contreras, M.A.; Fernández-Valverde, S.M.; Vargas-Garcia, J.R. Oxygen reduction reaction on cobalt–nickel alloys prepared by mechanical alloying. J. Alloys Compd. 2007, 434, 522–524. [Google Scholar] [CrossRef]
- Shuai, C.J.; He, C.X.; Peng, S.P.; Qi, F.W.; Wang, G.Y.; Min, A.J.; Yang, W.J.; Wang, W.G. Mechanical alloying of immiscible metallic systems: Process, microstructure, and mechanism. Adv. Eng. Mater. 2021, 23, 2001098. [Google Scholar] [CrossRef]
- Suryanarayana, C. Mechanical alloying: A novel technique to synthesize advanced materials. Research 2019, 2019, 4219812. [Google Scholar] [CrossRef] [PubMed]
- Burakowski, T.; Wierzchon, T. Surface Engineering of Metals: Principles, Equipment, Technologies; CRC Press: Boca Raton, FL, USA, 1998. [Google Scholar]
- Golodnitsky, D.; Rosenberg, Y.; Ulus, A. The role of anion additives in the electrodeposition of nickel–cobalt alloys from sulfamate electrolyte. Electrochim. Acta 2002, 47, 2707–2714. [Google Scholar] [CrossRef]
- Benea, L.; Bonora, P.L.; Borello, A.; Martelli, S. Wear corrosion properties of nano-structured SiC–nickel composite coatings obtained by electroplating. Wear 2001, 249, 995–1003. [Google Scholar] [CrossRef]
- Baghery, P.; Farzam, M.; Mousavi, A.B.; Hosseini, M. Ni–TiO2 nanocomposite coating with high resistance to corrosion and wear. Surf. Coat. Technol. 2010, 204, 3804–3810. [Google Scholar] [CrossRef]
- Wang, X.; Zhou, Z.; Huang, D. Research progress on the preparation of metal based particle thin films by composite electrodeposition technology. Hot Work. Technol. 2018, 47, 16–20+26. [Google Scholar]
- Tian, Z.J.; Wang, D.S.; Wang, G.F.; Shen, L.D.; Liu, Z.D.; Huang, Y.H. Microstructure and properties of nanocrystalline nickel coatings prepared by pulse jet electrodeposition. Trans. Nonferrous Met. Soc. China 2010, 20, 1037–1042. [Google Scholar] [CrossRef]
- Low, C.T.J.; Wills, R.G.A.; Walsh, F.C. Electrodeposition of composite coatings containing nanoparticles in a metal deposit. Surf. Coat. Technol. 2006, 201, 371–383. [Google Scholar] [CrossRef]
- Borkar, T.; Harimkar, S.P. Effect of electrodeposition conditions and reinforcement content on microstructure and tribological properties of nickel composite coatings. Surf. Coat. Technol. 2011, 205, 4124–4134. [Google Scholar] [CrossRef]
- Kerr, C.; Barker, D.; Walsh, F.; Archer, J. The electrodeposition of composite coatings based on metal matrix-included particle deposits. Trans. IMF 2000, 78, 171–178. [Google Scholar] [CrossRef]
- Benea, L.; Bonora, P.L.; Borello, A.; Martelli, S.; Wenger, F.; Ponthiaux, P.; Galland, J. Composite electrodeposition to obtain nanostructured coatings. J. Electrochem. Soc. 2001, 148, C461. [Google Scholar] [CrossRef]
- Zhang, Y.C.; Wan, F.Q.; Qi, Q.X.; Wang, J.Y. Research on Electrodeposited Ni-Co Alloy. Plat. Finish. 1995, 4–7. (In Chinese) [Google Scholar]
- Wang, D.; Gao, J.C.; Zhang, J.Q.; Cheng, Y.F.; Jin, H.M. Study on the Influence of Deposition Parameters on the Composition and Microstructure of Ni Co Coatings. J. Sichuan Univ. (Nat. Sci. Ed.) 2013, 50, 118–124. [Google Scholar]
- Chen, Y.L.; Yang, H.S.; Feng, H.; Yang, P.; Zhang, J.; Shu, B.P. Electrodeposition and corrosion performance of Ni-Co alloys with different cobalt contents. Mater. Today Commun. 2023, 35, 106058. [Google Scholar] [CrossRef]
- Ling, W.; Wang, H. Study on electrochemical properties of cobalt-nickel alloy prepared by pulsed electrodeposition. Int. J. Electrochem. Sci. 2023, 18, 100053. [Google Scholar] [CrossRef]
- Yao, S.W.; Liu, B.; Zhang, W.G.; Guo, H.T.; Shan, X.X. Study on Electrodeposited Ni-Co Alloy and Its Structure. Plat. Finish. 1996, 18, 7–10. [Google Scholar]
- Wu, G.; Li, N.; Du, M.H.; Zhou, D.R. Study on the Structure and Hardness of Electrodeposited Co Ni Alloy Coating. Mater. Sci. Technol. 2002, 419–423. [Google Scholar] [CrossRef]
- Wang, L.P.; Gao, Y.; Xue, Q.J.; Liu, H.W.; Xu, T. Microstructure and tribological properties of electrodeposited Ni–Co alloy deposits. Appl. Surf. Sci. 2005, 242, 326–332. [Google Scholar] [CrossRef]
- Wu, Z.W.; Lei, Y.P.; Wang, Y.; Fu, H.G. Effect of cobalt content on microstructure and property of electroplated nickel-cobalt alloy coatings. Mater. Und Werkst. 2013, 44, 593–600. [Google Scholar] [CrossRef]
- Hong, S.H.; Ahn, S.H.; Choi, I.; Pyo, S.G.; Kim, H.J.; Jang, J.H.; Kim, S.K. Fabrication and evaluation of nickel cobalt alloy electrocatalysts for alkaline water splitting. Appl. Surf. Sci. 2014, 307, 146–152. [Google Scholar] [CrossRef]
- Srivastava, M.; Selvi, V.E.; Grips, V.K.W.; Rajam, K.S. Corrosion resistance and microstructure of electrodeposited nickel–cobalt alloy coatings. Surf. Coat. Technol. 2006, 201, 3051–3060. [Google Scholar] [CrossRef]
- Zamani, M.; Amadeh, A.; Baghal, S.M.L. Effect of Co content on electrodeposition mechanism and mechanical properties of electrodeposited Ni–Co alloy. Trans. Nonferrous Met. Soc. China 2016, 26, 484–491. [Google Scholar] [CrossRef]
- Karpuz, A.; Kockar, H.; Alper, M.; Karaagac, O.; Haciismailoglu, M. Electrodeposited Ni–Co films from electrolytes with different Co contents. Appl. Surf. Sci. 2012, 258, 4005–4010. [Google Scholar] [CrossRef]
- Tebbakh, S.; Mentar, L.; Messaoudi, Y.; Khelladi, M.R.; Belhadj, H.; Azizi, A. Effect of cobalt content on electrodeposition and properties of Co–Ni alloy thin films. Inorg. Nano-Met. Chem. 2021, 51, 1796–1802. [Google Scholar] [CrossRef]
- Hagarova, M.; Jakubéczyová, D.; Cervová, J. Microstructure and properties of electroplated Ni-Co alloy coatings. Int. J. Electrochem. Sci. 2015, 10, 9968–9974. [Google Scholar] [CrossRef]
- Tury, B.; Lakatos-Varsányi, M.; Roy, S. Ni–Co alloys plated by pulse currents. Surf. Coat. Technol. 2006, 200, 6713–6717. [Google Scholar] [CrossRef]
- Tury, B.; Lakatos-Varsányi, M.; Roy, S. Effect of pulse parameters on the passive layer formation on pulse plated Ni–Co alloys. Appl. Surf. Sci. 2007, 253, 3103–3108. [Google Scholar] [CrossRef]
- Chen, Y.R.; Long, J.M.; Pei, H.Z.; Hong, J.P.; Shi, X.Z. Study on Internal Stress and Cobalt Content of Pulse Plated Ni Co Alloy Coating. Plat. Finish. 2009, 31, 1–3, 17. [Google Scholar]
- Liu, X.W.; Xu, Y.H.; Hu, P.; Qu, Y.; Sun, L.L. Wear resistance of high-frequency pulse electroplated nickel cobalt alloy coatings. Electroplat. Finish. 2013, 32, 18–22. [Google Scholar]
- Chen, Y.L.; Wen, X.Y.; Li, H.G.; Zhu, F.K.; Fang, C.; Li, Z.Q.; Zhou, Z.; Jiang, W. Effects of deposition current density, time and scanning velocity on scanning jet electrodeposition of Ni-Co alloy coating. J. Manuf. Process. 2023, 101, 458–468. [Google Scholar] [CrossRef]
- Li, Y.D.; Jiang, H.; Huang, W.H.; Tian, H. Effects of peak current density on the mechanical properties of nanocrystalline Ni–Co alloys produced by pulse electrodeposition. Appl. Surf. Sci. 2008, 254, 6865–6869. [Google Scholar] [CrossRef]
- Liu, G.Z.; Chen, Z.L.; Luo, F.; Liu, T.; Xi, X.L.; Wang, Z.H.; Gao, Z.; Shao, P.H.; Wu, D.S.; Luo, X.B.; et al. One-step nickel-cobalt alloy electrodeposition from spent lithium-ion battery via synergistic pH adjustment and Mn2+ supplementation. Sep. Purif. Technol. 2023, 314, 123581. [Google Scholar] [CrossRef]
- Tian, L.; Xu, J.; Xiao, S. The influence of pH and bath composition on the properties of Ni–Co coatings synthesized by electrodeposition. Vacuum 2011, 86, 27–33. [Google Scholar] [CrossRef]
- Vazquez-Arenas, J.; Altamirano-Garcia, L.; Treeratanaphitak, T.; Pritzker, M.; Luna-Sánchez, R.; Cabrera-Sierra, R. Co–Ni alloy electrodeposition under different conditions of pH, current and composition. Electrochim. Acta 2012, 65, 234–243. [Google Scholar] [CrossRef]
- Liang, Y.; Xu, Y.H.; Li, X.; Zheng, F.C.; Zheng, H.Q. Effect of process parameters on the corrosion resistance of high-frequency pulse electroplating nickel cobalt alloy in NaOH solution. Corros. Prot. 2010, 31, 338–341. [Google Scholar]
- Idris, J.; Christian, C.; Gaius, E. Nanocrystalline Ni-Co Alloy Synthesis by High Speed Electrodeposition. J. Nanomater. 2013, 2013, 841260. [Google Scholar] [CrossRef]
- Qiao, G.Y.; Jing, T.F.; Wang, N.; Gao, Y.W.; Zhao, X.; Zhou, J.F.; Wang, W. High-speed jet electrodeposition and microstructure of nanocrystalline Ni–Co alloys. Electrochim. Acta 2005, 51, 85–92. [Google Scholar] [CrossRef]
- Zhang, Y. Electrodeposited MCrAlY coatings for gas turbine engine applications. JOM 2015, 67, 2599–2607. [Google Scholar] [CrossRef]
- Huang, L.F.; Liu, J.M.; Wang, S.; Liu, T.; Shen, J.; Zhang, D.M. Development Trends of Composite Electroplating Technology and Applications. Therm. Spray Technol. 2019, 11, 1–6. [Google Scholar]
- Walsh, F.C.; Larson, C. Towards improved electroplating of metal-particle composite coatings. Trans. IMF 2020, 98, 288–299. [Google Scholar] [CrossRef]
- Walsh, F.C.; Wang, S.; Zhou, N. The electrodeposition of composite coatings: Diversity, applications and challenges. Curr. Opin. Electrochem. 2020, 20, 8–19. [Google Scholar] [CrossRef]
- Huang, J.L.; Wang, Q.W.; Yang, Y.F.; Wang, X.M.; Zhao, Y.; Zhu, S.; Li, W. Research progress on silicon carbide composite electroplating technology as an alternative to chromium electroplating. Surf. Technol. 2021, 50, 130–137. [Google Scholar]
- Gao, H.; Bu, L.; Wang, W. A Brief Discussion on the Development and Application of Electroplating Technology. Plat. Finish. 2016, 38, 29–33+40. [Google Scholar]
- Wang, G.F.; Jiang, S.S.; Lu, Z.; Zhang, K.F. Preparation and tensile properties of Al2O3/Ni-Co nanocomposites. Trans. Nonferrous Met. Soc. China 2011, 21, s374–s379. [Google Scholar] [CrossRef]
- Tian, Y.; Chengzhang, P.; Wei, X. Ni Co Cr alloy co deposition process. J. Hunan Univ. Sci. Technol. (Nat. Sci. Ed.) 2015, 30, 36–40. [Google Scholar]
- Zhang, Z.; Li, B.S.; Chen, S.Q.; Yuan, Z.W.; Xu, C.Y.; Zhang, W.W. Influences of Al particles and current density on structural, mechanical and anti-corrosion properties of electrodeposited Ni–Co/Al composite coatings. Ceram. Int. 2024, 50, 11804–11816. [Google Scholar] [CrossRef]
- Li, B.; Zhang, W.; Li, D. Synthesis and properties of a novel Ni–Co and Ni–Co/ZrO2 composite coating by DC electrodeposition. J. Alloys Compd. 2020, 821, 153258. [Google Scholar] [CrossRef]
- Wu, G.; Li, N.; Zhou, D.R.; Mitsuo, K. Electrodeposited Co–Ni–Al2O3 composite coatings. Surf. Coat. Technol. 2004, 176, 157–164. [Google Scholar] [CrossRef]
- Elkhoshkhany, N.; Hafnway, A.; Khaled, A. Electrodeposition and corrosion behavior of nano-structured Ni-WC and Ni-Co-WC composite coating. J. Alloys Compd. 2017, 695, 1505–1514. [Google Scholar] [CrossRef]
- Ma, L.; Zhou, K.C.; Li, Z.Y.; Wei, Q.P. Electrodeposition of Ni-Co-Fe2O3 composite coatings. J. Cent. South Univ. Technol. 2010, 17, 708–714. [Google Scholar] [CrossRef]
- Torabinejad, V.; Aliofkhazraei, M.; Rouhaghdam, A.S.; Allahyarzadeh, M.H. Tribological properties of Ni-Fe-Co multilayer coatings fabricated by pulse electrodeposition. Tribol. Int. 2017, 106, 34–40. [Google Scholar] [CrossRef]
- Kumaraguru, S.; Gnanamuthu, R.M. An efficient corrosion protection activity of electrodeposited Ni/Ni-Co-Mn oxide composite for surface modification of steel. Mater. Lett. 2021, 295, 129804. [Google Scholar] [CrossRef]
- Apelt, S.; Zhang, Y.; Zhu, J.H.; Leyens, C. Electrodeposition of Co–Mn3O4 composite coatings. Surf. Coat. Technol. 2015, 280, 208–215. [Google Scholar] [CrossRef]
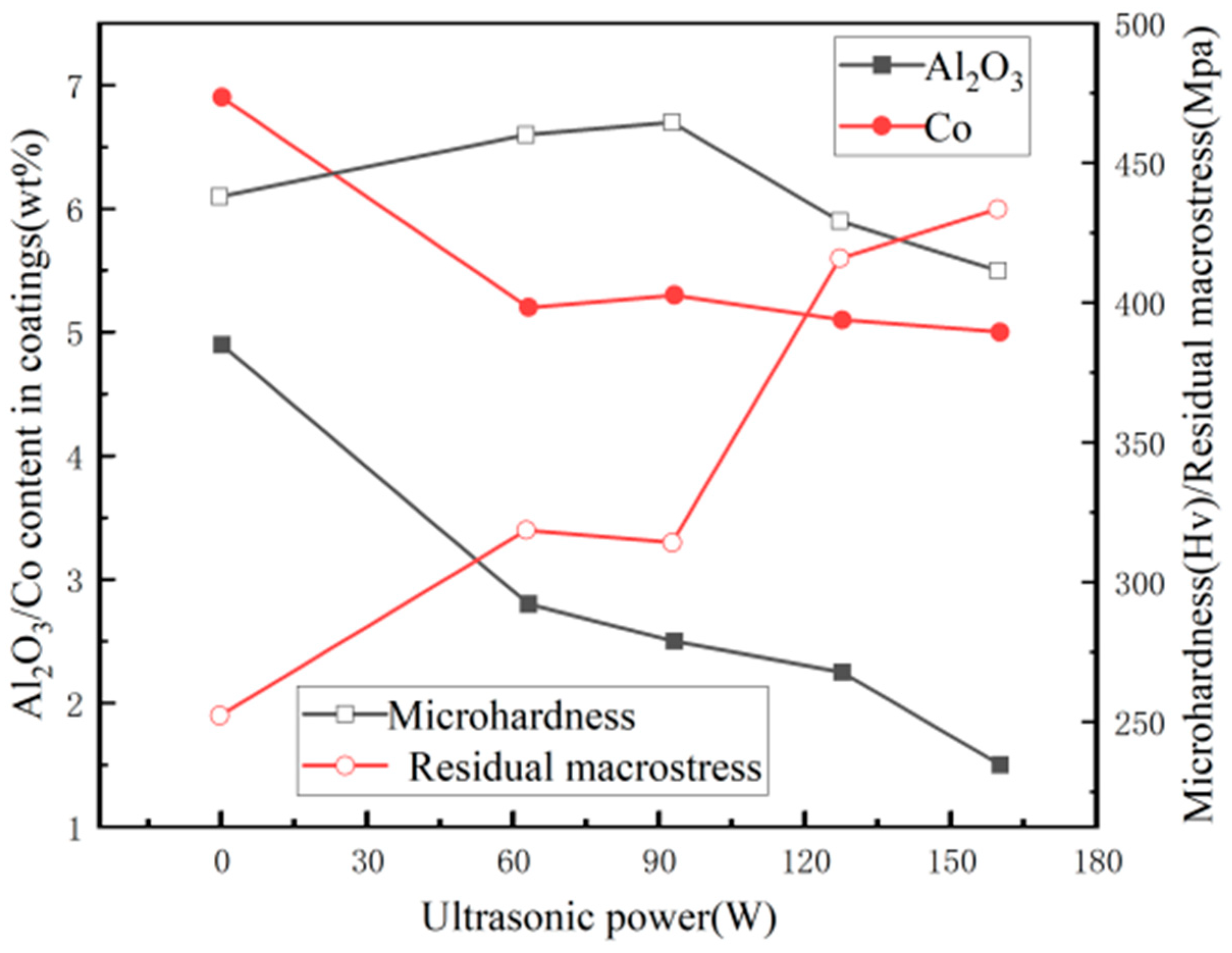
- Chang, L.M.; Guo, H.F.; An, M.Z. Electrodeposition of Ni–Co/Al2O3 composite coating by pulse reverse method under ultrasonic condition. Mater. Lett. 2008, 62, 3313–3315. [Google Scholar] [CrossRef]
- Shi, L.; Sun, C.; Liu, W. Electrodeposited nickel–cobalt composite coating containing MoS2. Appl. Surf. Sci. 2008, 254, 6880–6885. [Google Scholar] [CrossRef]
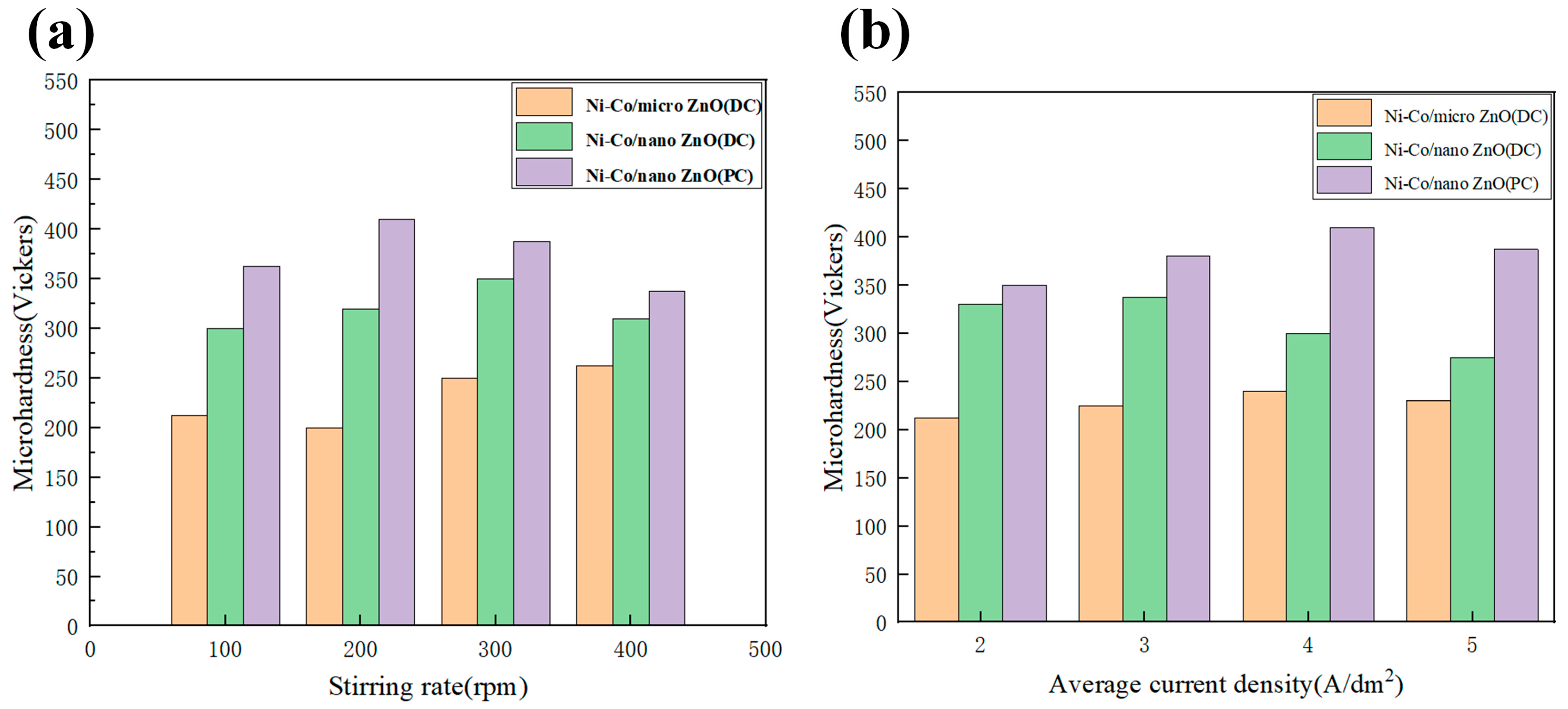
- Ghazanlou, S.I.; Farhood, A.H.S.; Ahmadiyeh, S.; Ziyaei, E.; Rasooli, A.; Hosseinpour, S. Characterization of pulse and direct current methods for electrodeposition of Ni-Co composite coatings reinforced with nano and micro ZnO particles. Metall. Mater. Trans. A 2019, 50, 1922–1935. [Google Scholar] [CrossRef]
- Choudhary, R.K.; Mishra, P.; Kain, V. Pulse DC electrodeposition of Zn–Ni–Co coatings. Surf. Eng. 2017, 33, 90–93. [Google Scholar] [CrossRef]
- Arzumanova, A.V.; Starunov, A.V.; Shpanova, K.A. Wear Resistance of a Composite Galvanic Coating Based on the Nickel-Cobalt Alloy. Mater. Sci. Forum 2019, 945, 735–739. [Google Scholar] [CrossRef]
- Wang, L.P.; Gao, Y.; Xue, Q.J.; Liu, H.W.; Xu, T. Study on the Wear Resistance of Ni Co/Nanodiamond Composite Coating. China Surface Eng. 2005, 24–26. [Google Scholar] [CrossRef]
- Xu, W.; Xu, T.; Wang, X.L.; Wang, L.L.; Tan, Y.F. Effect of Diamond Particle Size on the Microstructure and Wear Resistance of Ni Co/Diamond Composite Coating. Equip. Manuf. Technol. 2015, 9–11. [Google Scholar] [CrossRef]
- Shi, L.; Sun, C.F.; Gao, P.; Zhou, F.; Liu, W.M. Electrodeposition and characterization of Ni–Co–carbon nanotubes composite coatings. Surf. Coat. Technol. 2006, 200, 4870–4875. [Google Scholar] [CrossRef]
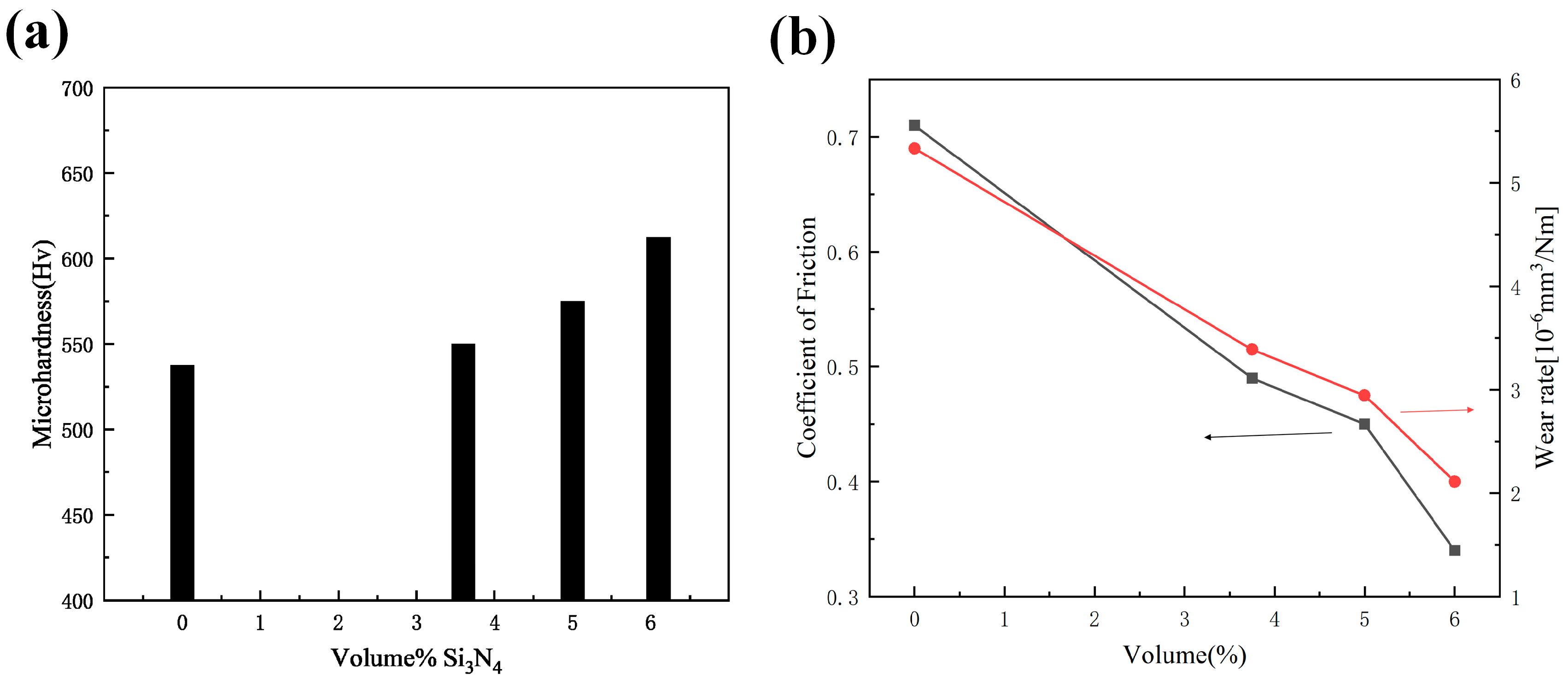
- Shi, L.; Sun, C.F.; Zhou, F.; Liu, W.M. Electrodeposited nickel–cobalt composite coating containing nano-sized Si3N4. Mater. Sci. Eng. A 2005, 397, 190–194. [Google Scholar] [CrossRef]
- Ghazanlou, S.I.; Farhood, A.H.S.; Hosouli, S.; Ahmadiyeh, S.; Rasooli, A. Pulse and direct electrodeposition of Ni–Co/micro and nanosized SiO2 particles. Mater. Manuf. Process. 2018, 33, 1067–1079. [Google Scholar] [CrossRef]
- Ghazanlou, S.I.; Ahmadiyeh, S.; Yavari, R. Investigation of pulse electrodeposited Ni–Co/SiO2 nanocomposite coating. Surf. Eng. 2017, 33, 337–347. [Google Scholar] [CrossRef]
- Ghazanlou, S.I.; Shokuhfar, A.; Navazani, S.; Yavari, R. Influence of pulse electrodeposition parameters on microhardness, grain size and surface morphology of Ni–Co/SiO2 nanocomposite coating. Bull. Mater. Sci. 2016, 39, 1185–1195. [Google Scholar] [CrossRef][Green Version]
- Akbarpour, M.R.; Asl, F.G.; Rashedi, H. Pulse-reverse electrodeposition of Ni-Co/graphene composite films with high hardness and electrochemical behaviour. Diam. Relat. Mater. 2023, 133, 109720. [Google Scholar] [CrossRef]
- Ramesh, C.S.; Seshadri, S.K. Tribological characteristics of nickel based composite coatings. Wear 2003, 255, 893–902. [Google Scholar] [CrossRef]
- Garcia, I.; Fransaer, J.; Celis, J.P. Electrodeposition and sliding wear resistance of nickel composite coatings containing micron and submicron SiC particles. Surf. Coat. Technol. 2001, 148, 171–178. [Google Scholar] [CrossRef]
- Benea, L.; Bonora, P.L.; Borello, A.; Martelli, S. Effect of SiC size dimensions on the corrosion wear resistance of the electrodeposited composite coating. Mater. Corros. 2002, 53, 23–29. [Google Scholar] [CrossRef]
- Carter, C.H., Jr.; Davis, R.F.; Bentley, J. Kinetics and mechanisms of high-temperature creep in silicon carbide: II, chemically vapor deposited. J. Am. Ceram. Soc. 1984, 67, 732–740. [Google Scholar] [CrossRef]
- Gulbransen, E.A.; Jansson, S.A. The high-temperature oxidation, reduction, and volatilization reactions of silicon and silicon carbide. Oxid. Met. 1972, 4, 181–201. [Google Scholar] [CrossRef]
- Price, R.J. Properties of silicon carbide for nuclear fuel particle coatings. Nucl. Technol. 1977, 35, 320–336. [Google Scholar] [CrossRef]
- Snead, L.L.; Nozawa, T.; Katoh, Y.; Byun, T.S.; Kondoa, S.; Petti, D.A. Handbook of SiC properties for fuel performance modeling. J. Nucl. Mater. 2007, 371, 329–377. [Google Scholar] [CrossRef]
- Bakhit, B.; Akbari, A.; Nasirpouri, F.; Hosseini, M.G. Corrosion resistance of Ni–Co alloy and Ni–Co/SiC nanocomposite coatings electrodeposited by sediment codeposition technique. Appl. Surf. Sci. 2014, 307, 351–359. [Google Scholar] [CrossRef]
- Dai, P.Q.; Zhong, Y.H.; Zhou, X. Corrosion characteristic of pulsed electrodeposition Ni–Co/SiC nanocomposite coating. Surf. Eng. 2011, 27, 71–76. [Google Scholar] [CrossRef]
- Shi, L.; Sun, C.; Gao, P.; Zhou, F.; Liu, W. Mechanical properties and wear and corrosion resistance of electrodeposited Ni–Co/SiC nanocomposite coating. Appl. Surf. Sci. 2006, 252, 3591–3599. [Google Scholar] [CrossRef]
- Srivastava, M.; William Grips, V.K.; Jain, A.; Rajam, K.S. Influence of SiC particle size on the structure and tribological properties of Ni–Co composites. Surf. Coat. Technol. 2007, 202, 310–318. [Google Scholar] [CrossRef]
- Shi, L.; Zhou, F.; Sun, C.F.; Liu, W.M. Corrosion resistance and tribological properties of Ni Co SiC nanocomposite coating. Chin. J. Nonferrous Met. 2005, 15, 5. [Google Scholar]
- Kamel, M.M.; Mohsen, Q.; Abdel Hamid, Z.; Rashwan, S.M.; Ibrahim, I.S.; El-Sheikh, S.M. Electrodeposition of Ni-Co/Nano SiC Composites from a Citrate Bath and their Characterization. Int. J. Electrochem. Sci. 2021, 16, 210563. [Google Scholar] [CrossRef]
- Ababsa, A.; Ben Temam, H.; Hasan, G.G.; Althamthami, M.; Malfi, A.N. Effect of sodium dodecyl sulfate and different SiC quantities on electrodeposited Ni-Co alloy coatings. Surface Topogr. Metrol. Prop. 2022, 10, 015038. [Google Scholar] [CrossRef]
- Yang, Y.; Cheng, Y.F. Mechanistic aspects of electrodeposition of Ni–Co–SiC composite nano-coating on carbon steel. Electrochim. Acta 2013, 109, 638–644. [Google Scholar] [CrossRef]
- Bahadormanesh, B.; Dolati, A. The kinetics of Ni–Co/SiC composite coatings electrodeposition. J. Alloys Compd. 2010, 504, 514–518. [Google Scholar] [CrossRef]

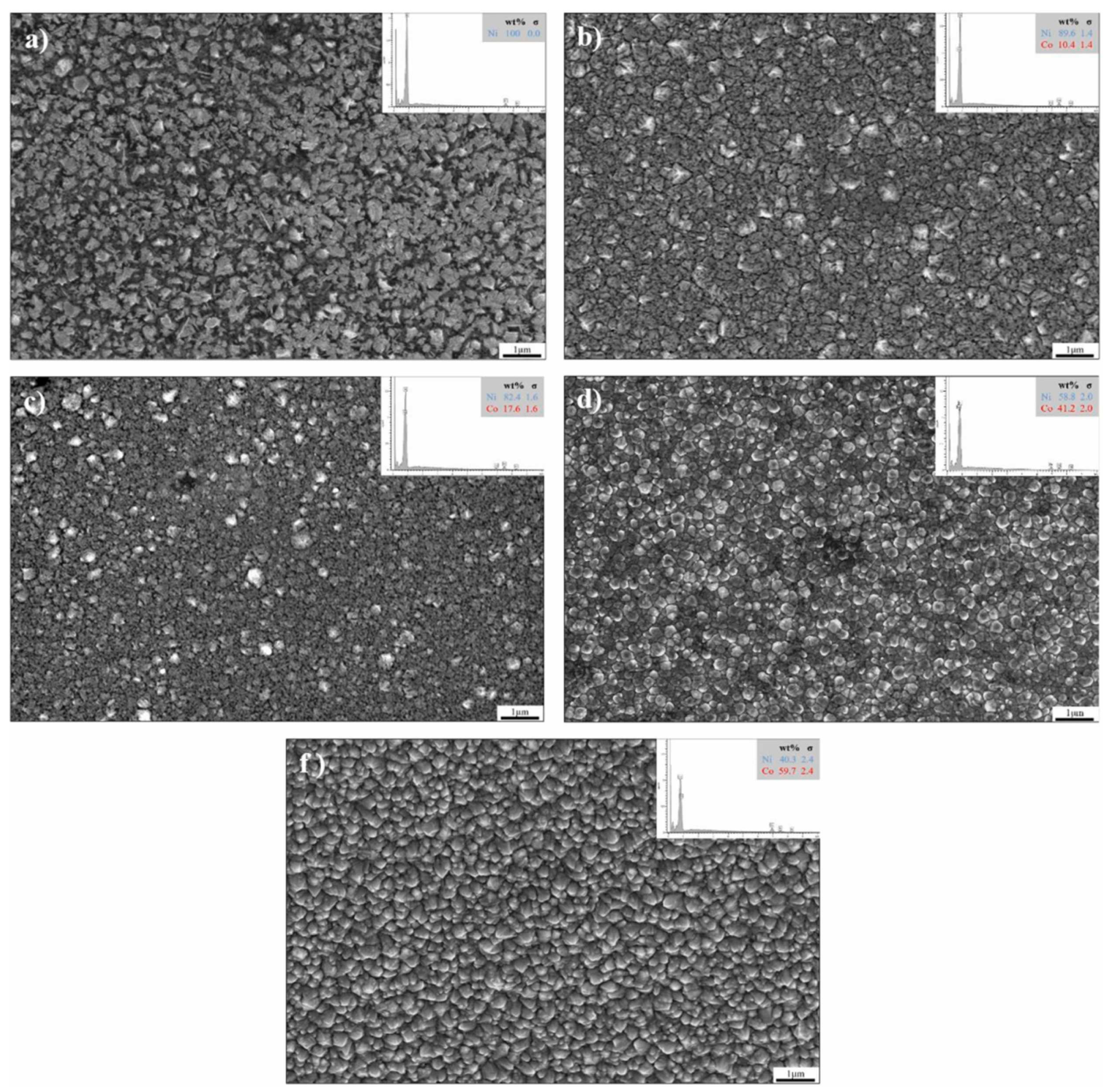
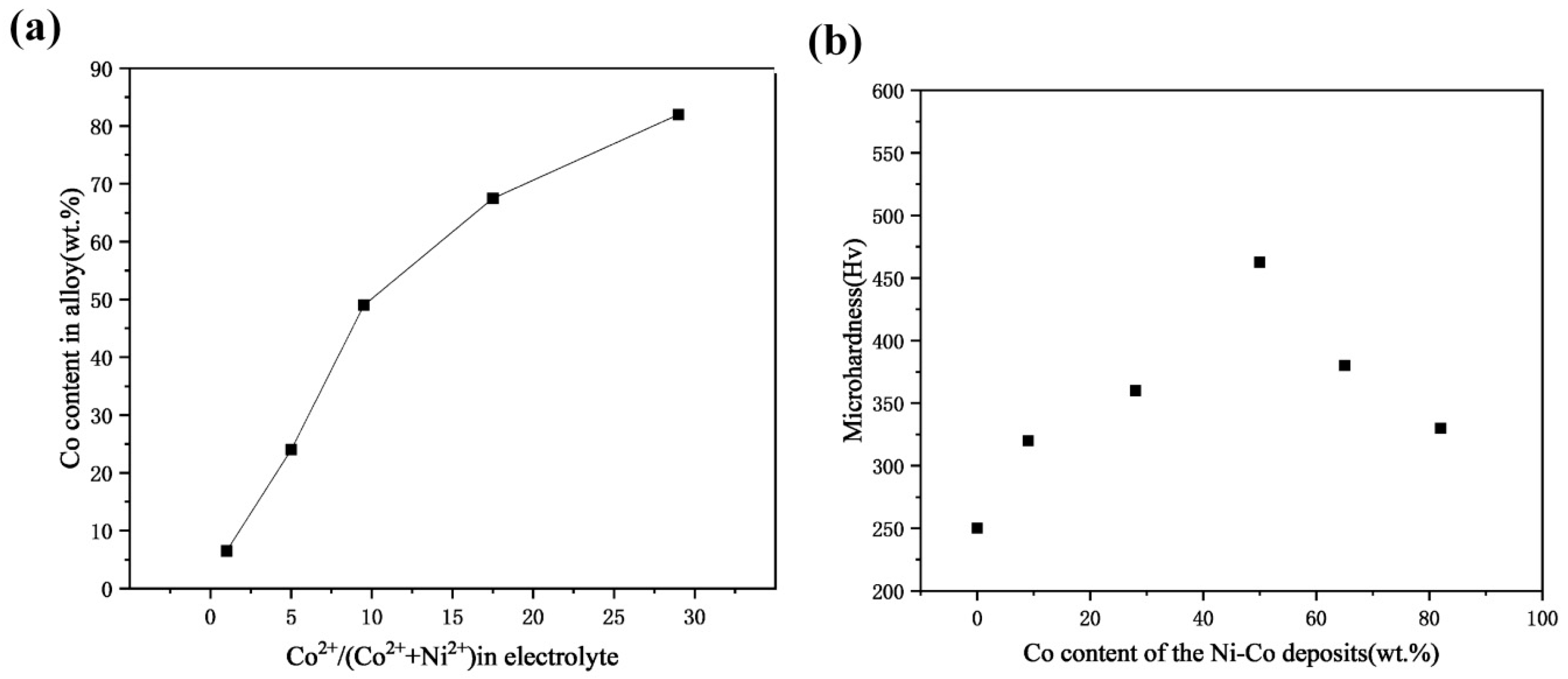
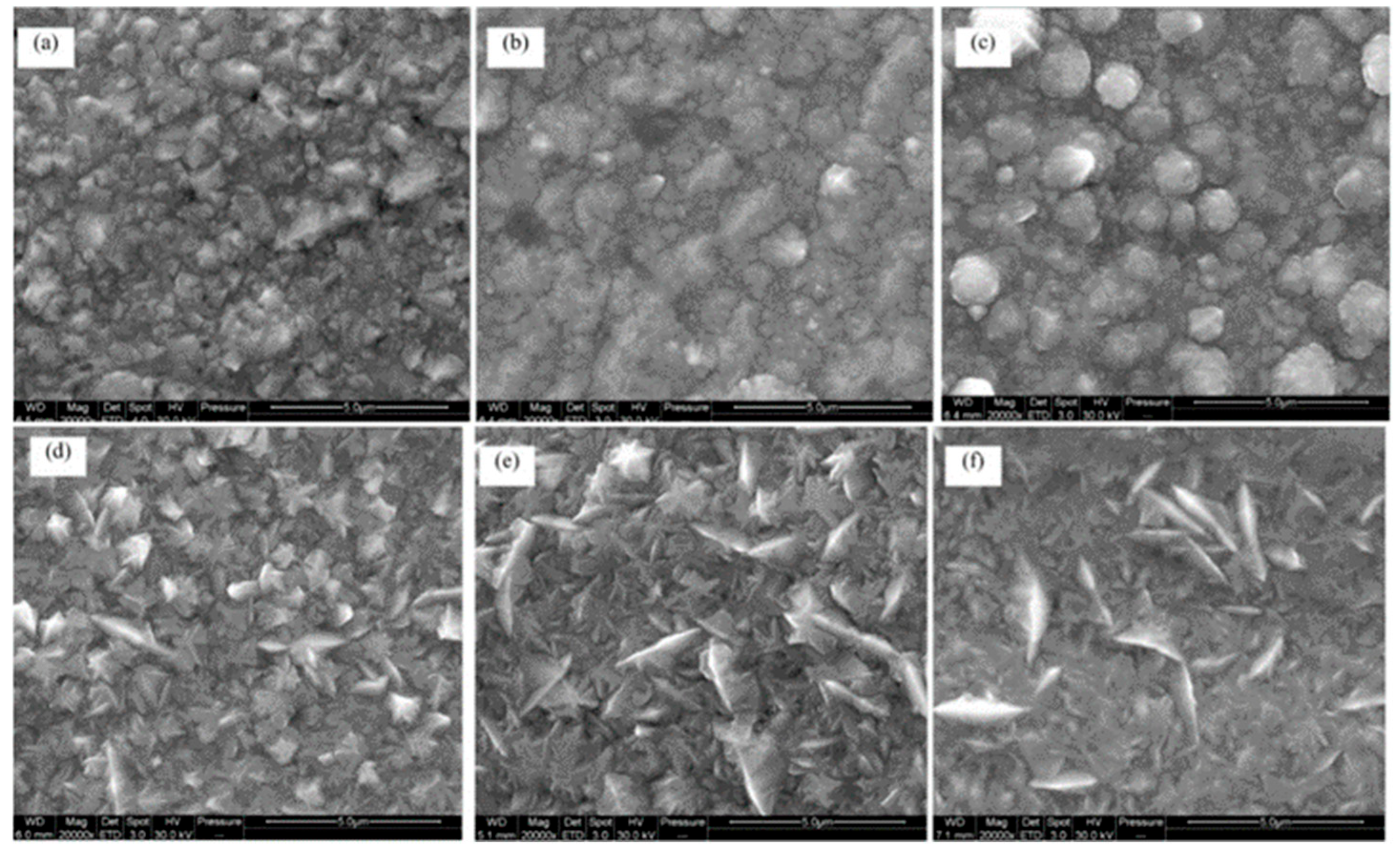
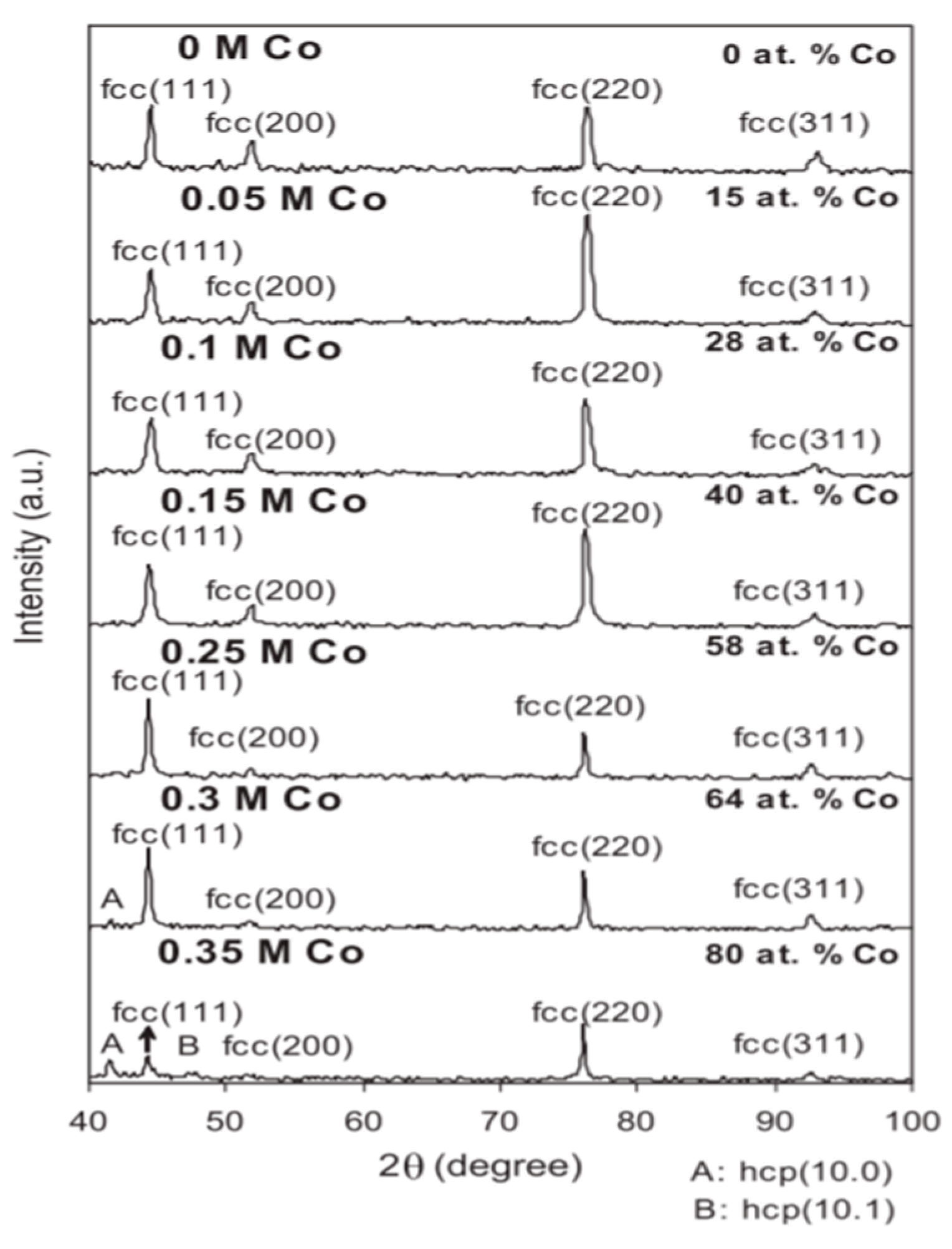

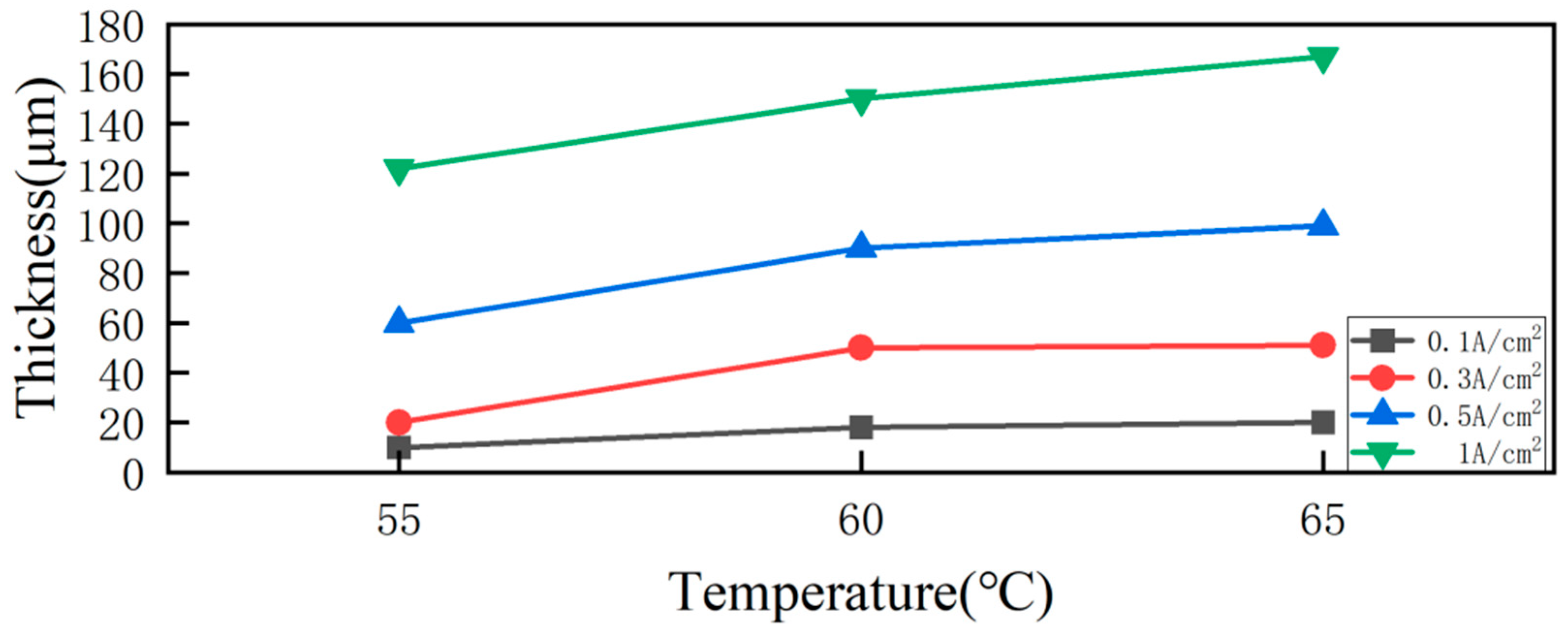
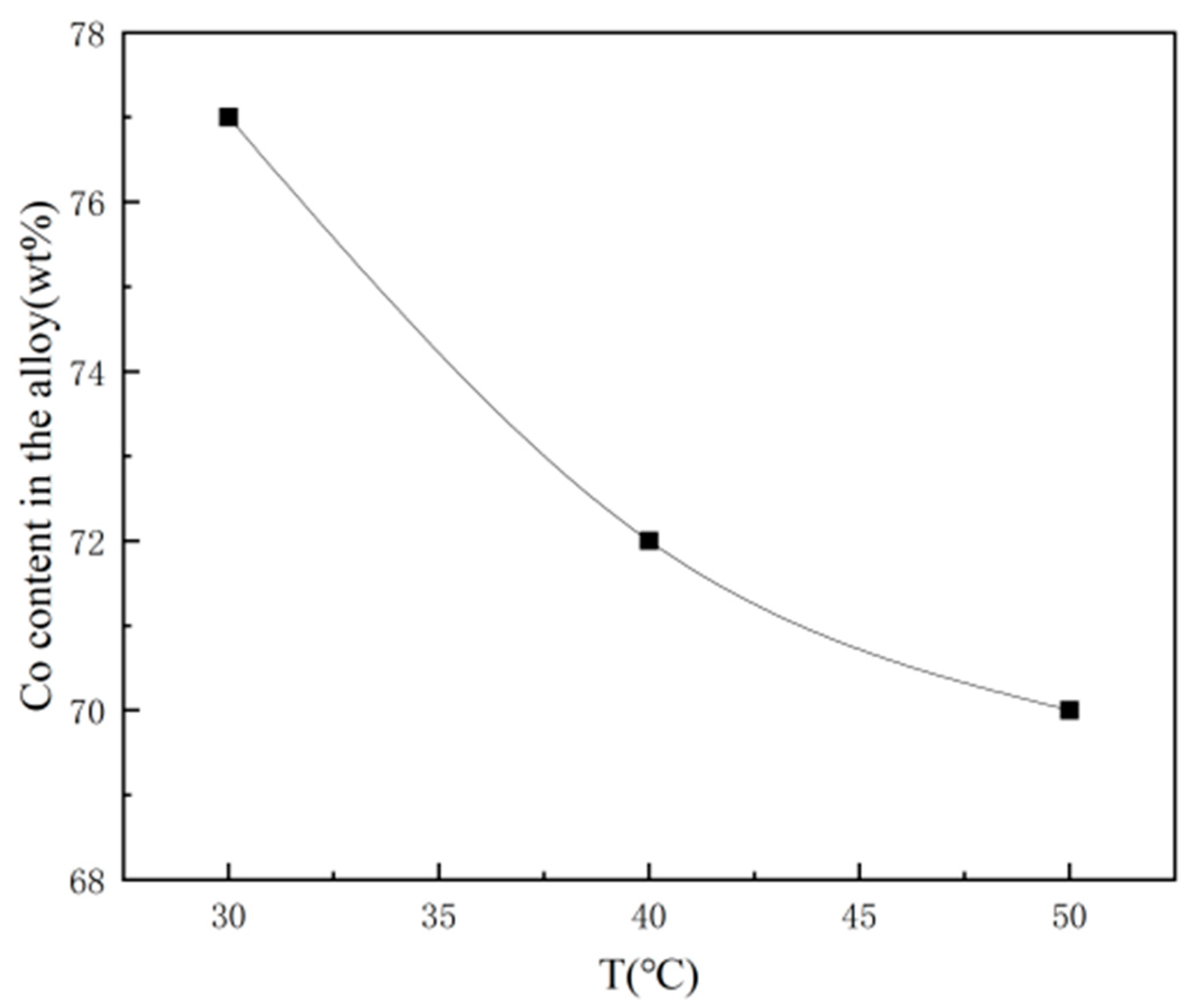
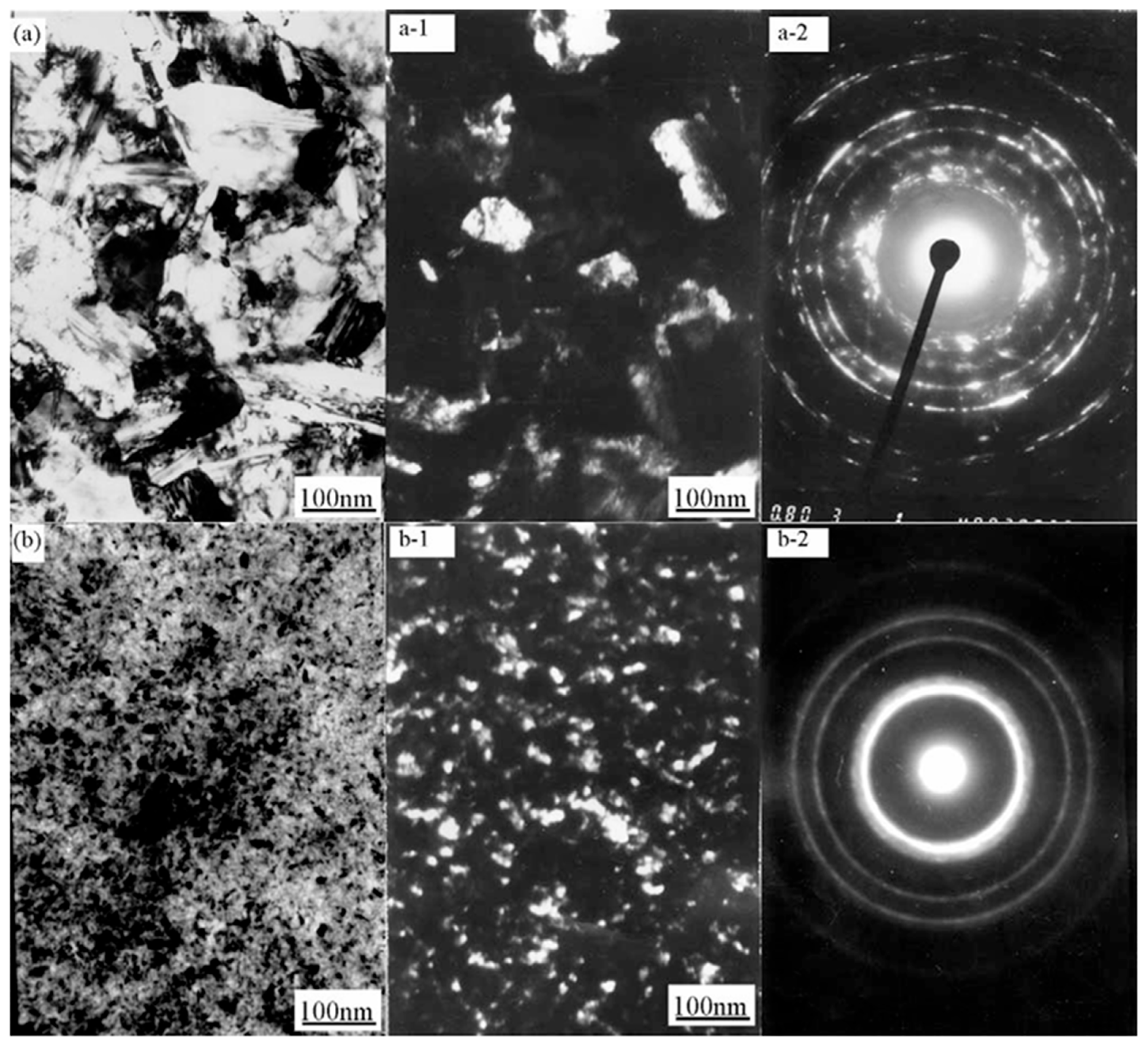
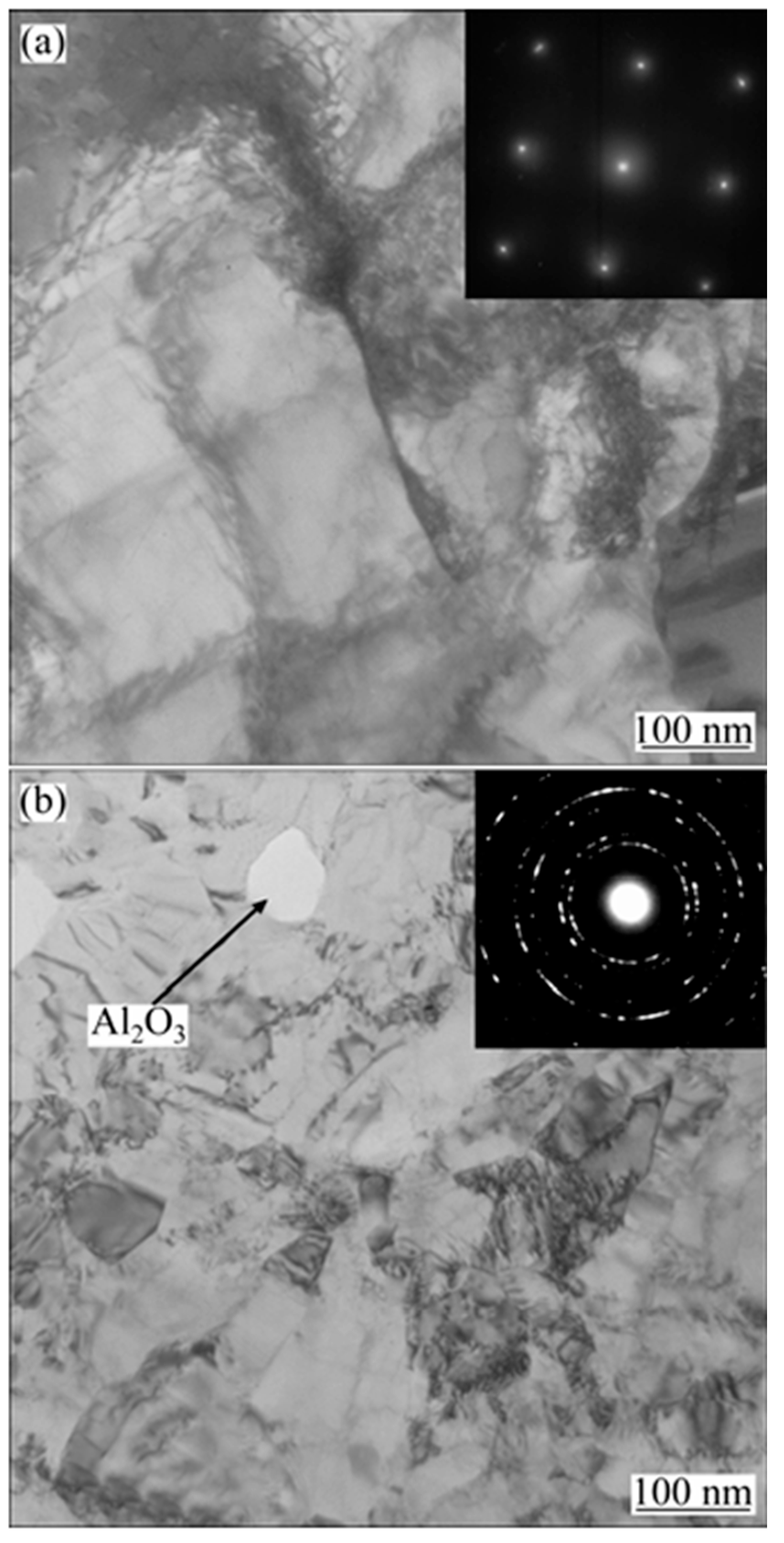

| Comparison Aspect | Sol–Gel Method | Phase Reduction | Electroplating | Mechanical Alloying | Composite Electrodeposition |
|---|---|---|---|---|---|
| Preparation method | Sol–gel process using metal alkoxides | Reduction using a metal salt and reducing agent | Electrochemical deposition onto a substrate | Ball milling and mechanical energy input from | Electrochemical co-deposition with additives |
| Equipment | Furnace for heat treatment | Reaction vessel, stirring | Power supply, substrate | ball mill, protective atmosphere | Electroplating setup |
| Cost | Moderate | Moderate to high | Low | High | Moderate |
| Environmental impact | Low | Moderate | Moderate | Low | Moderate |
| Sample | Ni 20%Co | Ni 40%Co | Ni 60%Co | Ni 80%Co |
|---|---|---|---|---|
| Co2+ concentration percentage (%) | 2.5 | 9.2 | 12.5 | 28.7 |
| Co atomic percentage (%) | 20.14 | 40.33 | 59.97 | 80.14 |
| Coating | Crystallite Size (nm) | Coating | Crystallite Size (nm) |
|---|---|---|---|
| Pure Ni | 23.2 | Nickel–70% cobalt | 17.6 |
| Nickel–20% cobalt | 23.1 | Nickel–80% cobalt | 17.6 |
| Nickel–50% cobalt | 11.5 | Pure Co | 17.6 |
| Process Parameter | Effect on Coating Properties | Specific Impact |
|---|---|---|
| Cobalt Content | Alloy composition | Higher cobalt content increases hardness and wear resistance. Excessive cobalt may make the coating brittle and affect corrosion resistance. Affects electrical conductivity, oxidation resistance, and magnetism. |
| Current Density | Coating quality, uniformity, and thickness | High current density leads to thicker coatings but may cause unevenness. Low current density results in more uniform coatings. Very low current density may result in thin coatings with poor durability. |
| Current Type | Deposition rate and coating performance | DC current yields thicker, uniform coatings. Pulse current improves crystal structure, hardness, and corrosion resistance. Different current types affect coating morphology and microstructure. |
| pH Value | Crystallization behavior and composition | Low pH (acidic) increases brittleness and solubility of the coating. High pH (alkaline) may hinder cobalt deposition, leading to poor coating quality. Moderate pH helps achieve uniform coatings with controlled composition. |
| Temperature | Deposition rate and composition stability | High temperature accelerates deposition but may lead to coarse grains, affecting mechanical properties. Low temperature reduces deposition rate and can cause uneven coatings. Optimal temperature improves microstructure stability, hardness, and corrosion resistance. |
| NCM | Jcorr (A·cm−2) | Ecorr (mV vs. SCE) | Rp | Corrosion Rate (CR) | Rs | Rct | Thickness | |
|---|---|---|---|---|---|---|---|---|
| (kΩcm2) | (mpy) | (mmpy) | (Ωcm2) | (kΩcm2) | (μm) | |||
| MS | 5.350 × 10−6 | −525.2 | 1.357 | 2.4446 | 6.210 × 10−2 | 10.46 | 0.916 | - |
| Ni | 2.163 × 10−6 | −475.7 | 3.042 | 0.9180 | 2.332 × 10−2 | 11.80 | 2.106 | 50.04 |
| 5 | 2.419 × 10−7 | −302.9 | 38.670 | 0.1026 | 2.608 × 10−3 | 10.61 | 90.951 | 58.13 |
| 10 | 2.325 × 10−6 | −843.7 | 4.118 | 0.9868 | 2.507 × 10−2 | 8.24 | 31.453 | 60.89 |
| 15 | 6.603 × 10−7 | −759.3 | 12.387 | 0.2802 | 7.118 × 10−3 | 9.11 | 23.845 | 62.82 |
| Composite | Wear Coefficient | Coefficient of Friction (avg.) |
|---|---|---|
| Ni-SiC nano | 8.33 × 10−6 | 0.835 |
| Ni-SiC micron | 2.42 × 10−5 | 0.707 |
| Ni-rich Ni-Co composite | ||
| Ni-Co-SiC nano | 3.35 × 10–6 | 0.834 |
| Ni-Co-SiC micron | 1.31 × 10−6 | 0.801 |
| Co-rich Ni-Co composite | ||
| Ni-Co-SiC nano | 1.77 × 10−6 | 0.821 |
| Ni-Co-SiC micron | 1.36 × 10−6 | 0.847 |
| Research Variable | Metals or Metal Compounds | Non-Metal Substances | Silicon Carbide |
|---|---|---|---|
| Metal/Compound Types | Common metals include nickel, cobalt, copper, silver, and aluminum, and compounds may involve oxides or nitrides. | Non-metallic substances like nitrides, borides, carbons, and fluorides are often used in composite materials | Silicon carbide, a common ceramic material, is used to enhance physical properties when combined with nickel–cobalt metals. |
| Advantages | Good electroplating properties, forming uniform coatings. Enhanced electrochemical performance, such as conductivity and corrosion resistance. | Improved high-temperature resistance and wear resistance. Enhanced mechanical strength and corrosion resistance. | Increased hardness, wear resistance, and thermal expansion resistance of the nickel–cobalt composite material. Enhanced oxidation resistance and chemical stability of the material. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lai, L.; Qian, F.; Bi, Y.; Niu, B.; Yu, G.; Li, Y.; Ding, G. Advancements in the Preparation and Application of Ni-Co System (Alloys, Composites, and Coatings): A Review. Nanomaterials 2025, 15, 312. https://doi.org/10.3390/nano15040312
Lai L, Qian F, Bi Y, Niu B, Yu G, Li Y, Ding G. Advancements in the Preparation and Application of Ni-Co System (Alloys, Composites, and Coatings): A Review. Nanomaterials. 2025; 15(4):312. https://doi.org/10.3390/nano15040312
Chicago/Turabian StyleLai, Liyan, Feng Qian, Yuxiao Bi, Bing Niu, Guanliang Yu, Yigui Li, and Guifu Ding. 2025. "Advancements in the Preparation and Application of Ni-Co System (Alloys, Composites, and Coatings): A Review" Nanomaterials 15, no. 4: 312. https://doi.org/10.3390/nano15040312
APA StyleLai, L., Qian, F., Bi, Y., Niu, B., Yu, G., Li, Y., & Ding, G. (2025). Advancements in the Preparation and Application of Ni-Co System (Alloys, Composites, and Coatings): A Review. Nanomaterials, 15(4), 312. https://doi.org/10.3390/nano15040312








