Mussel-Inspired Hydrogels Incorporating Graphite Derivatives for Soft Tissue Regeneration
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
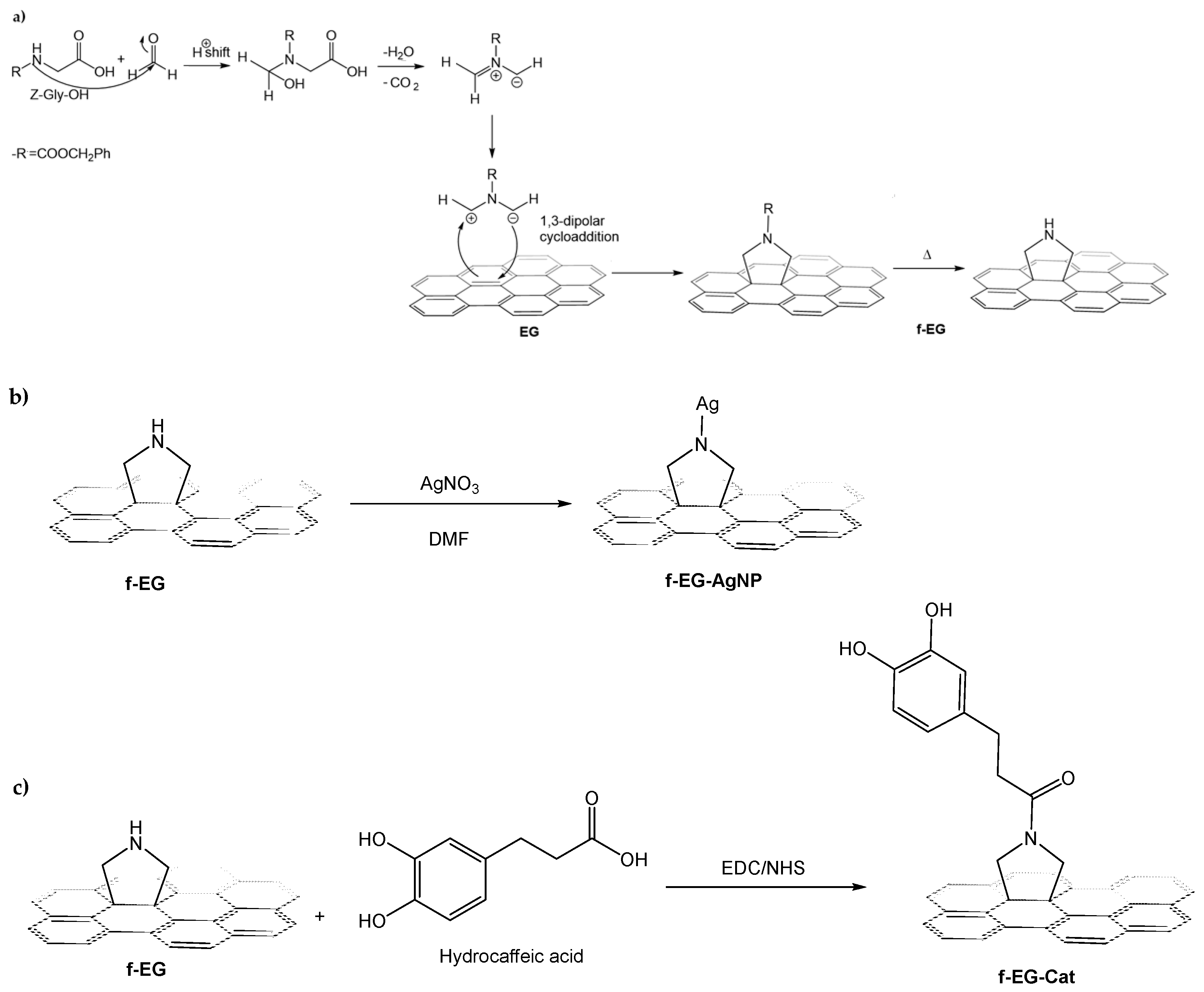
2.2. Functionalization of the EG
2.3. Anchoring Silver Nanoparticles on Functionalized EG
2.4. Synthesis of Catechol Conjugated with Functionalized EG
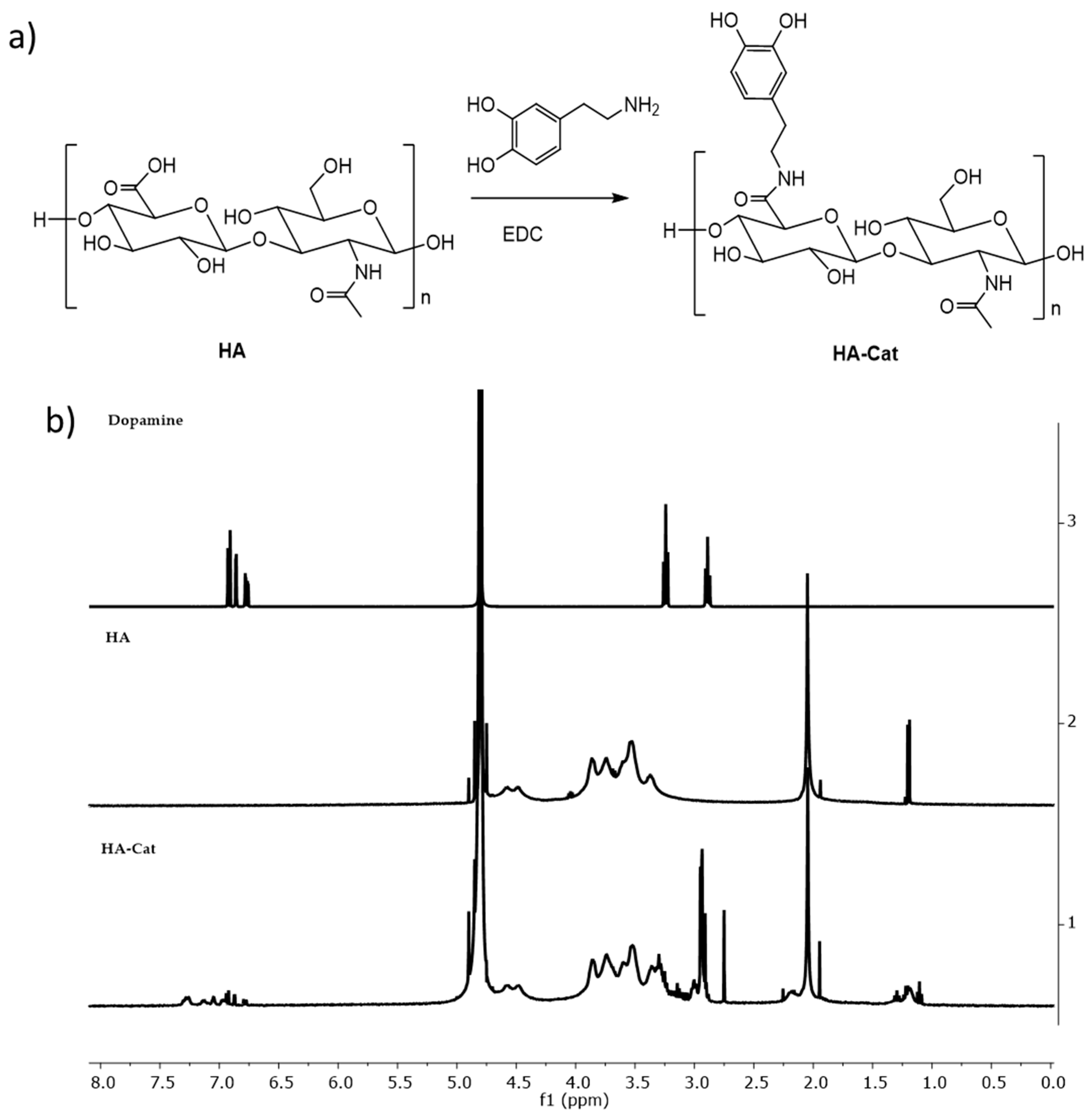
2.5. Synthesis of Dopamine-Conjugated Hyaluronic Acid
2.6. Characterization of the EG Derivatives
2.7. Preparation and Characterization of HA-Cat Composite Hydrogel
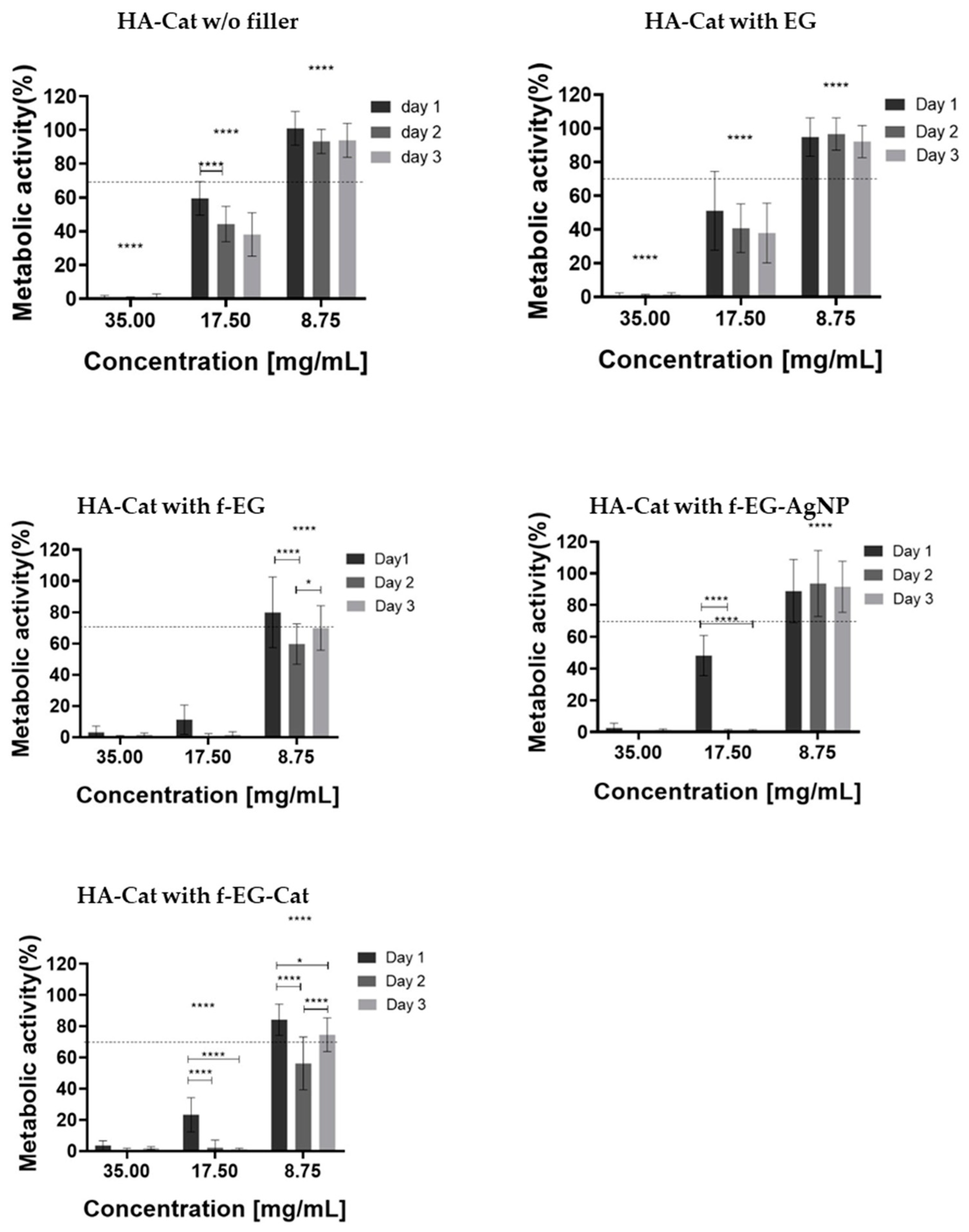
2.8. Biological Assays
3. Results
3.1. Production and Characterization of EG Derivatives
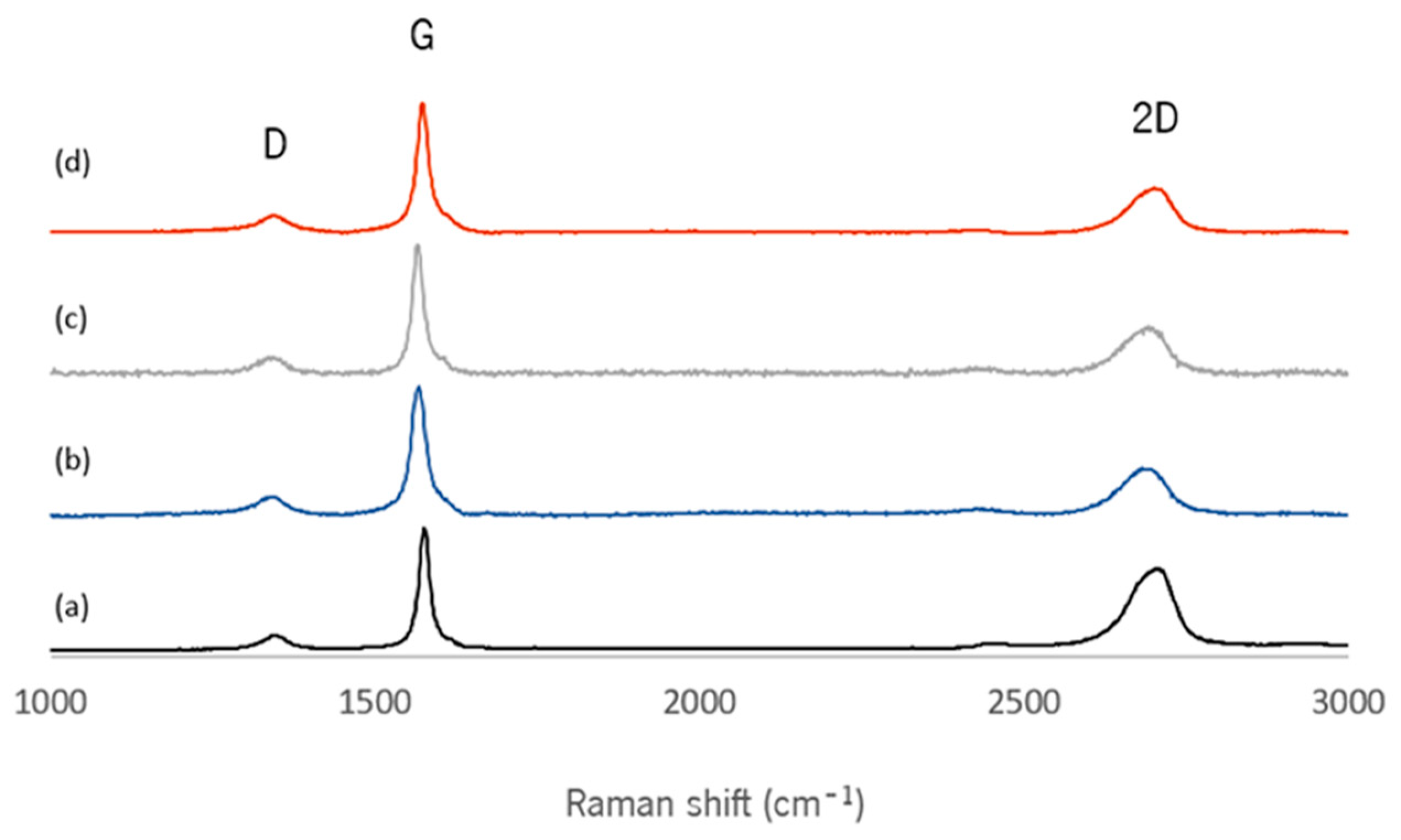
3.1.1. Raman Spectroscopy
3.1.2. X-Ray Photoelectron Spectroscopy
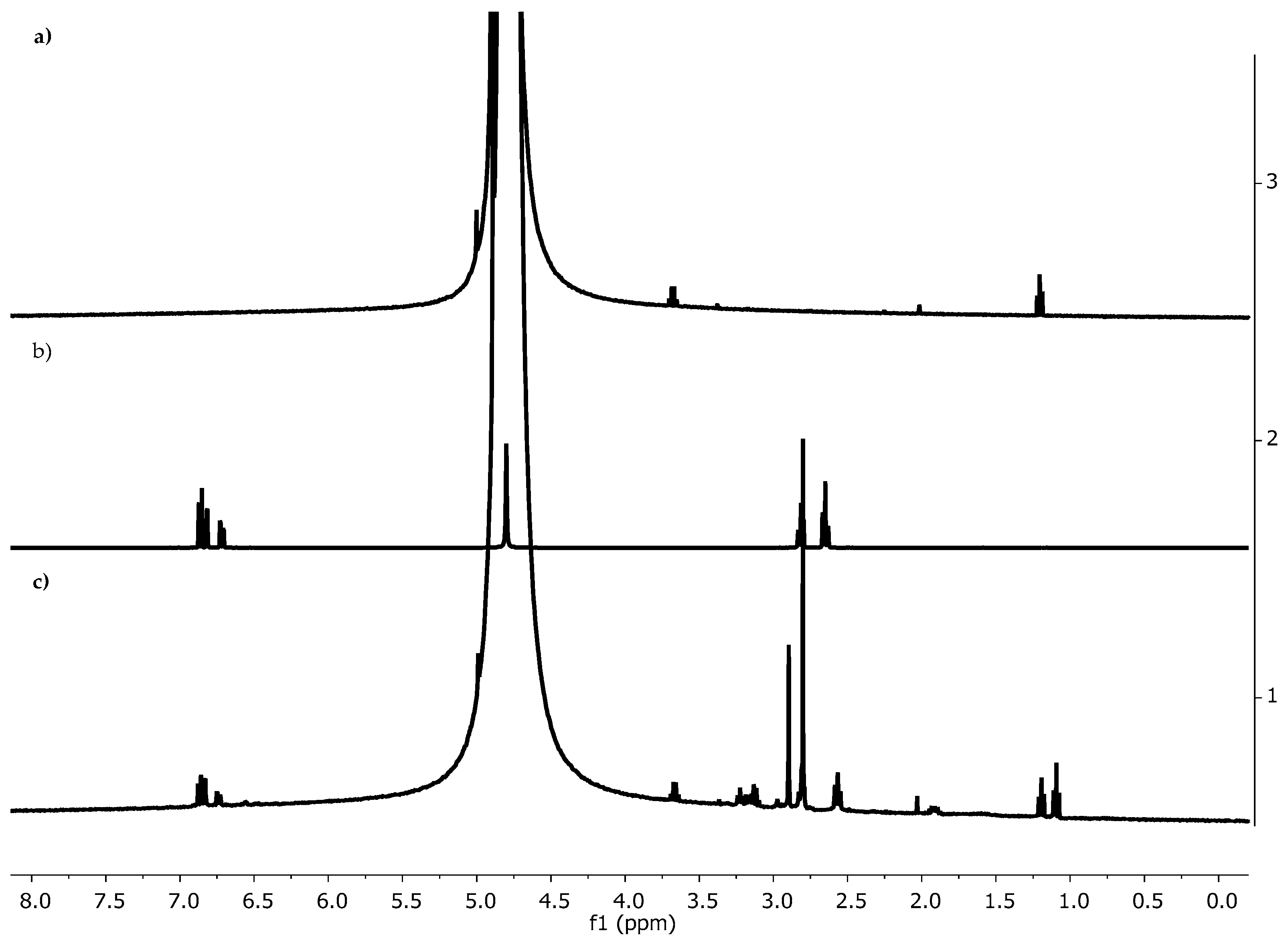
3.1.3. NMR Spectroscopy
3.2. Production and Characterization of HA-Cat Conjugate
3.3. Optimization of the Preparation of HA-Cat Hydrogels
3.4. Production and Characterization of Composite Hydrogels
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tiwari, S.; Bahadur, P. Modified Hyaluronic Acid Based Materials for Biomedical Applications. Int. J. Biol. Macromol. 2019, 121, 556–571. [Google Scholar] [CrossRef] [PubMed]
- Yasin, A.; Ren, Y.; Li, J.; Sheng, Y.; Cao, C.; Zhang, K. Advances in Hyaluronic Acid for Biomedical Applications. Front. Bioeng. Biotechnol. 2022, 10, 910290. [Google Scholar] [CrossRef] [PubMed]
- Snetkov, P.; Zakharova, K.; Morozkina, S.; Olekhnovich, R.; Uspenskaya, M. Hyaluronic Acid: The Influence of Molecular Weight on Structural, Physical, Physico-Chemical, and Degradable Properties of Biopolymer. Polymers 2020, 12, 1800. [Google Scholar] [CrossRef] [PubMed]
- Hintze, V.; Schnabelrauch, M.; Rother, S. Chemical Modification of Hyaluronan and Their Biomedical Applications. Front. Chem. 2022, 10, 830671. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, W.; Li, H.; Huo, P.; Teng, P.; Ding, H.; Shen, X. Recent Progress in Fabrications, Properties and Applications of Multifunctional Conductive Hydrogels. Eur. Polym. J. 2024, 208, 112895. [Google Scholar] [CrossRef]
- Cholewinski, A.; Yang, F.; Zhao, B. Algae-Mussel-Inspired Hydrogel Composite Glue for Underwater Bonding. Mater. Horiz. 2019, 6, 285–293. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, R.; Sun, Z.M.; Zhu, X.; Zhao, Q.; Zhang, T.; Cholewinski, A.; Yang, F.; Zhao, B.; Pinnaratip, R.; et al. Catechol-Functionalized Hydrogels: Biomimetic Design, Adhesion Mechanism, and Biomedical Applications. Chem. Soc. Rev. 2020, 49, 433–464. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Peng, K.; Wu, Y.; Fu, F. Functional Adhesive Hydrogels for Biological Interfaces. Smart Med. 2023, 2, e20230024. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Cheng, J.; Delparastan, P.; Wang, H.; Sigg, S.J.; DeFrates, K.G.; Cao, Y.; Messersmith, P.B. Molecular Design Principles of Lysine-DOPA Wet Adhesion. Nat. Commun. 2020, 11, 3895. [Google Scholar] [CrossRef] [PubMed]
- Rahimnejad, M.; Zhong, W. Mussel-Inspired Hydrogel Tissue Adhesives for Wound Closure. RSC Adv. 2017, 7, 47380–47396. [Google Scholar] [CrossRef]
- Maier, G.P.; Bernt, C.M.; Butler, A. Catechol Oxidation: Considerations in the Design of Wet Adhesive Materials. Biomater. Sci. 2018, 6, 332–339. [Google Scholar] [CrossRef] [PubMed]
- Pinnataip, R.; Lee, B.P. Oxidation Chemistry of Catechol Utilized in Designing Stimuli-Responsive Adhesives and Antipathogenic Biomaterials. ACS Omega 2021, 6, 5113–5118. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Wu, B.; Zhou, Y.; Zhou, F.; Liu, W.; Wang, Z. Mussel-Inspired Hydrogels: From Design Principles to Promising Applications. Chem. Soc. Rev. 2020, 49, 3605–3637. [Google Scholar] [CrossRef]
- Silva, M.; Alves, N.M.; Paiva, M.C. Graphene-Polymer Nanocomposites for Biomedical Applications. Polym. Adv. Technol. 2018, 29, 687–700. [Google Scholar] [CrossRef]
- Silva, M.; Gomes, S.; Correia, C.; Peixoto, D.; Vinhas, A.; Rodrigues, M.T.; Gomes, M.E.; Covas, J.A.; Paiva, M.C.; Alves, N.M. Biocompatible 3D-Printed Tendon/Ligament Scaffolds Based on Polylactic Acid/Graphite Nanoplatelet Composites. Nanomaterials 2023, 13, 2518. [Google Scholar] [CrossRef] [PubMed]
- Paiva, M.C.; Simon, F.; Novais, R.M.; Ferreira, T.; Proenĉa, M.F.; Xu, W.; Besenbacher, F. Controlled Functionalization of Carbon Nanotubes by a Solvent-Free Multicomponent Approach. ACS Nano 2010, 4, 7379–7386. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.M.; Ribeiro, D.; Cunha, E.; Fernanda Proença, M.; Young, R.J.; Paiva, M.C. A Simple Method for Anchoring Silver and Copper Nanoparticles on Single Wall Carbon Nanotubes. Nanomaterials 2019, 9, 1416. [Google Scholar] [CrossRef]
- Neto, A.I.; Cibrão, A.C.; Correia, C.R.; Carvalho, R.R.; Luz, G.M.; Ferrer, G.G.; Botelho, G.; Picart, C.; Alves, N.M.; Mano, J.F. Nanostructured Polymeric Coatings Based on Chitosan and Dopamine-Modified Hyaluronic Acid for Biomedical Applications. Small 2014, 10, 2459–2469. [Google Scholar] [CrossRef]
- Duarte, D.; Correia, C.; Reis, R.L.; Pashkuleva, I.; Peixoto, D.; Alves, N.M. Bioadhesive Hyaluronic Acid-Based Hydrogels for Spinal Cord Injury. Biomacromolecules 2024, 25, 1592–1601. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Li, S.; Pei, M.; Yang, H.; Gu, S.; Tao, Y.; Ye, D.; Zhou, Y.; Xu, W.; Xiao, P. Dopamine-Modified Hyaluronic Acid Hydrogel Adhesives with Fast-Forming and High Tissue Adhesion. ACS Appl. Mater. Interfaces 2020, 12, 18225–18234. [Google Scholar] [CrossRef] [PubMed]
- Brandão, A.T.S.C.; Costa, R.; Silva, A.F.; Pereira, C.M. Sustainable Preparation of Nanoporous Carbons via Dry Ball Milling: Electrochemical Studies Using Nanocarbon Composite Electrodes and a Deep Eutectic Solvent as Electrolyte. Nanomaterials 2021, 11, 3258. [Google Scholar] [CrossRef] [PubMed]
- Roscher, S.; Hoffmann, R.; Ambacher, O. Determination of the Graphene-Graphite Ratio of Graphene Powder by Raman 2D Band Symmetry Analysis. Anal. Methods 2019, 11, 1180–1191. [Google Scholar] [CrossRef]
- Wu, J.B.; Lin, M.L.; Cong, X.; Liu, H.N.; Tan, P.H. Raman Spectroscopy of Graphene-Based Materials and Its Applications in Related Devices. Chem. Soc. Rev. 2018, 47, 1822–1873. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Kumar, A.; Lee, D.J.; Park, S.S. Estimation of Number of Graphene Layers Using Different Methods: A Focused Review. Materials 2021, 14, 4590. [Google Scholar] [CrossRef] [PubMed]
- Amiri, A.; Naraghi, M.; Ahmadi, G.; Soleymaniha, M.; Shanbedi, M. A Review on Liquid-Phase Exfoliation for Scalable Production of Pure Graphene, Wrinkled, Crumpled and Functionalized Graphene and Challenges. FlatChem 2018, 8, 40–71. [Google Scholar] [CrossRef]
- Lavin-Lopez, M.P.; Valverde, J.L.; Cuevas, M.C.; Garrido, A.; Sanchez-Silva, L.; Martinez, P.; Romero-Izquierdo, A. Synthesis and Characterization of Graphene: Influence of Synthesis Variables. Phys. Chem. Chem. Phys. 2014, 16, 2962–2970. [Google Scholar] [CrossRef]
- Cunha, E.; Ren, H.; Lin, F.; Kinloch, I.A.; Sun, Q.; Fan, Z.; Young, R.J. The Chemical Functionalization of Graphene Nanoplatelets through Solvent-Free Reaction. RSC Adv. 2018, 8, 33564–33573. [Google Scholar] [CrossRef]
- Ren, H.; Cunha, E.; Sun, Q.; Li, Z.; Kinloch, I.A.; Young, R.J.; Fan, Z. Surface Functionality Analysis by Boehm Titration of Graphene Nanoplatelets Functionalized: Via a Solvent-Free Cycloaddition Reaction. Nanoscale Adv. 2019, 1, 1432–1441. [Google Scholar] [CrossRef] [PubMed]
- Xing, Z.; Ju, Z.; Zhao, Y.; Wan, J.; Zhu, Y.; Qiang, Y.; Qian, Y. One-Pot Hydrothermal Synthesis of Nitrogen-Doped Graphene as High-Performance Anode Materials for Lithium Ion Batteries. Sci. Rep. 2016, 6, 6146. [Google Scholar] [CrossRef] [PubMed]
- Jing, X.; Mi, H.Y.; Napiwocki, B.N.; Peng, X.F.; Turng, L.S. Mussel-Inspired Electroactive Chitosan/Graphene Oxide Composite Hydrogel with Rapid Self-Healing and Recovery Behavior for Tissue Engineering. Carbon 2017, 125, 557–570. [Google Scholar] [CrossRef]
- Aliyev, E.; Filiz, V.; Khan, M.M.; Lee, Y.J.; Abetz, C.; Abetz, V. Structural Characterization of Graphene Oxide: Surface Functional Groups and Fractionated Oxidative Debris. Nanomaterials 2019, 9, 1180. [Google Scholar] [CrossRef] [PubMed]
- Díez, N.; Śliwak, A.; Gryglewicz, S.; Grzyb, B.; Gryglewicz, G. Enhanced Reduction of Graphene Oxide by High-Pressure Hydrothermal Treatment. RSC Adv. 2015, 5, 81831–81837. [Google Scholar] [CrossRef]
- Moreira, V.R.; Lebron, Y.A.R.; da Silva, M.M.; de Souza Santos, L.V.; Jacob, R.S.; de Vasconcelos, C.K.B.; Viana, M.M. Graphene Oxide in the Remediation of Norfloxacin from Aqueous Matrix: Simultaneous Adsorption and Degradation Process. Environ. Sci. Pollut. Res. 2020, 27, 34513–34528. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Lee, C.; Ryu, J.H. Adhesive Catechol-conjugated Hyaluronic Acid for Biomedical Applications: A Mini Review. Appl. Sci. 2021, 11, 21. [Google Scholar] [CrossRef]
- Hong, S.; Yang, K.; Kang, B.; Lee, C.; Song, I.T.; Byun, E.; Park, K.I.; Cho, S.W.; Lee, H. Hyaluronic Acid Catechol: A Biopolymer Exhibiting a PH-Dependent Adhesive or Cohesive Property for Human Neural Stem Cell Engineering. Adv. Funct. Mater. 2013, 23, 1774–1780. [Google Scholar] [CrossRef]
- Guo, W.; Pan, X.; Zhang, Y.; Cai, H.; Zhang, W. A Hyaluronic Acid/Graphene Oxide Hydrogel for Enhanced Ex Vivo Expansion of Cord Blood-Derived CD34+ Cells. Mater. Lett. 2017, 205, 253–256. [Google Scholar] [CrossRef]
- Correia, C.; Sousa, R.O.; Vale, A.C.; Peixoto, D.; Silva, T.H.; Reis, R.L.; Pashkuleva, I.; Alves, N.M. Adhesive and Biodegradable Membranes Made of Sustainable Catechol-Functionalized Marine Collagen and Chitosan. Colloids Surf. B Biointerfaces 2022, 213, 112409. [Google Scholar] [CrossRef] [PubMed]
- Correia, C.; Da Costa, D.S.; Inácio, A.R.; Vale, A.C.; Peixoto, D.; Silva, T.H.; Reis, R.L.; Pashkuleva, I.; Alves, N.M. Adhesive and Antibacterial Films Based on Marine-Derived Fucoidan and Chitosan. ACS Sustain. Chem. Eng. 2022, 10, 16770–16779. [Google Scholar] [CrossRef]
- Han, Y.; Li, D.; Li, D.; Chen, W.; Mu, S.E.; Chen, Y.; Chai, J. Impact of refractive index increment on the determination of molecular weight of hyaluronic acid by muti-angle laser light-scattering technique. Sci. Rep. 2020, 10, 1858. [Google Scholar] [CrossRef] [PubMed]





| Atomic % | ||||
|---|---|---|---|---|
| C 1s | O 1s | N 1s | Ag 3d | |
| EG | 97.48 | 2.52 | - | - |
| f-EG | 89.43 | 4.85 | 5.32 | - |
| f-EG-AgNP | 89.45 | 5.57 | 4.46 | 0.52 |
| f-EG-Cat | 76.04 | 15.47 | 5.25 | - |
| Sample | Method |
|---|---|
| 1 | Dissolution of HA-Cat in PBS at pH 8–9, and posterior addition of f-EG to the pre-gel solution |
| 2 | Dispersion of f-EG in PBS solution at pH 8–9, followed by the dissolution of the HA-Cat in the dispersion |
| 3 | Mixing of the solids of HA-Cat and f-EG, and then the addition of PBS solution at pH 8–9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernandes, F.; Peixoto, D.; Correia, C.; Silva, M.; Paiva, M.C.; Alves, N.M. Mussel-Inspired Hydrogels Incorporating Graphite Derivatives for Soft Tissue Regeneration. Nanomaterials 2025, 15, 276. https://doi.org/10.3390/nano15040276
Fernandes F, Peixoto D, Correia C, Silva M, Paiva MC, Alves NM. Mussel-Inspired Hydrogels Incorporating Graphite Derivatives for Soft Tissue Regeneration. Nanomaterials. 2025; 15(4):276. https://doi.org/10.3390/nano15040276
Chicago/Turabian StyleFernandes, Filipa, Daniela Peixoto, Cátia Correia, Magda Silva, Maria C. Paiva, and Natália M. Alves. 2025. "Mussel-Inspired Hydrogels Incorporating Graphite Derivatives for Soft Tissue Regeneration" Nanomaterials 15, no. 4: 276. https://doi.org/10.3390/nano15040276
APA StyleFernandes, F., Peixoto, D., Correia, C., Silva, M., Paiva, M. C., & Alves, N. M. (2025). Mussel-Inspired Hydrogels Incorporating Graphite Derivatives for Soft Tissue Regeneration. Nanomaterials, 15(4), 276. https://doi.org/10.3390/nano15040276







