Advanced Low-Dimensional Carbon Nanomaterials for Oxygen Electrocatalysis
Abstract
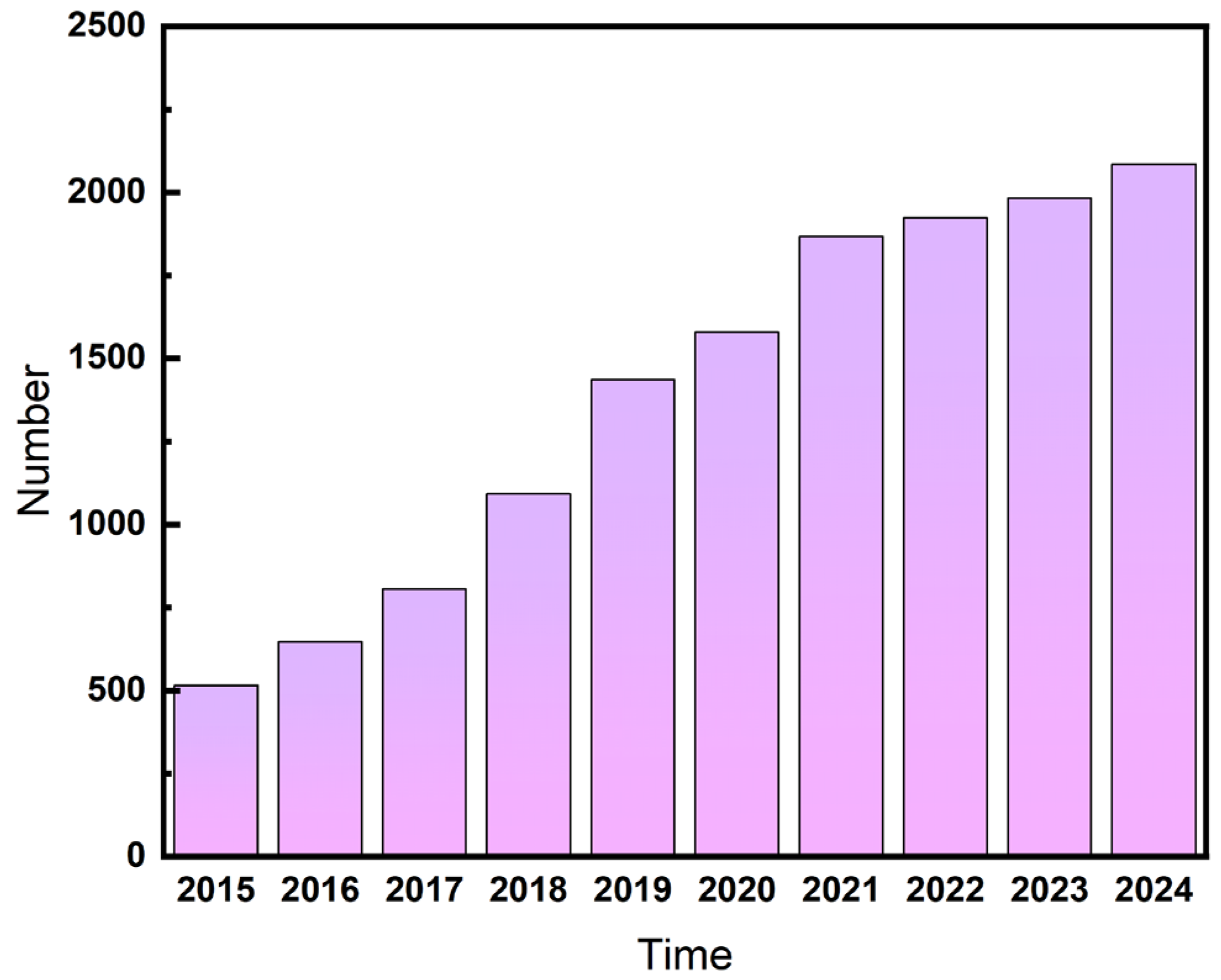
1. Introduction
2. Characteristics of Advanced Carbon Nanomaterials
2.1. Carbon Dots
2.1.1. Characteristics of Carbon Dots (CDs)
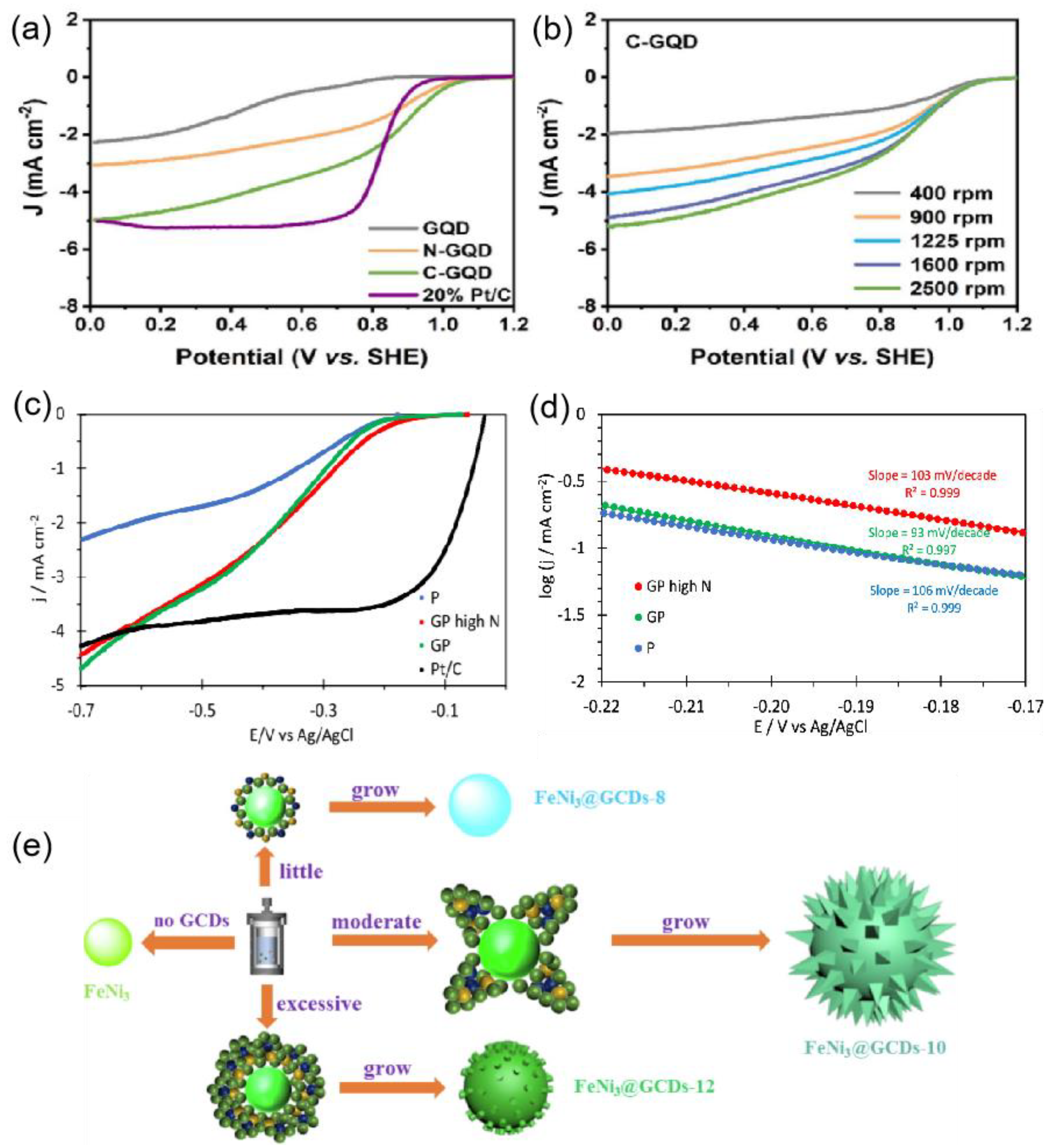
2.1.2. CD-Based Electrocatalysts
2.2. Carbon Nanotubes
2.2.1. Characteristics of Carbon Nanotubes (CNTS)
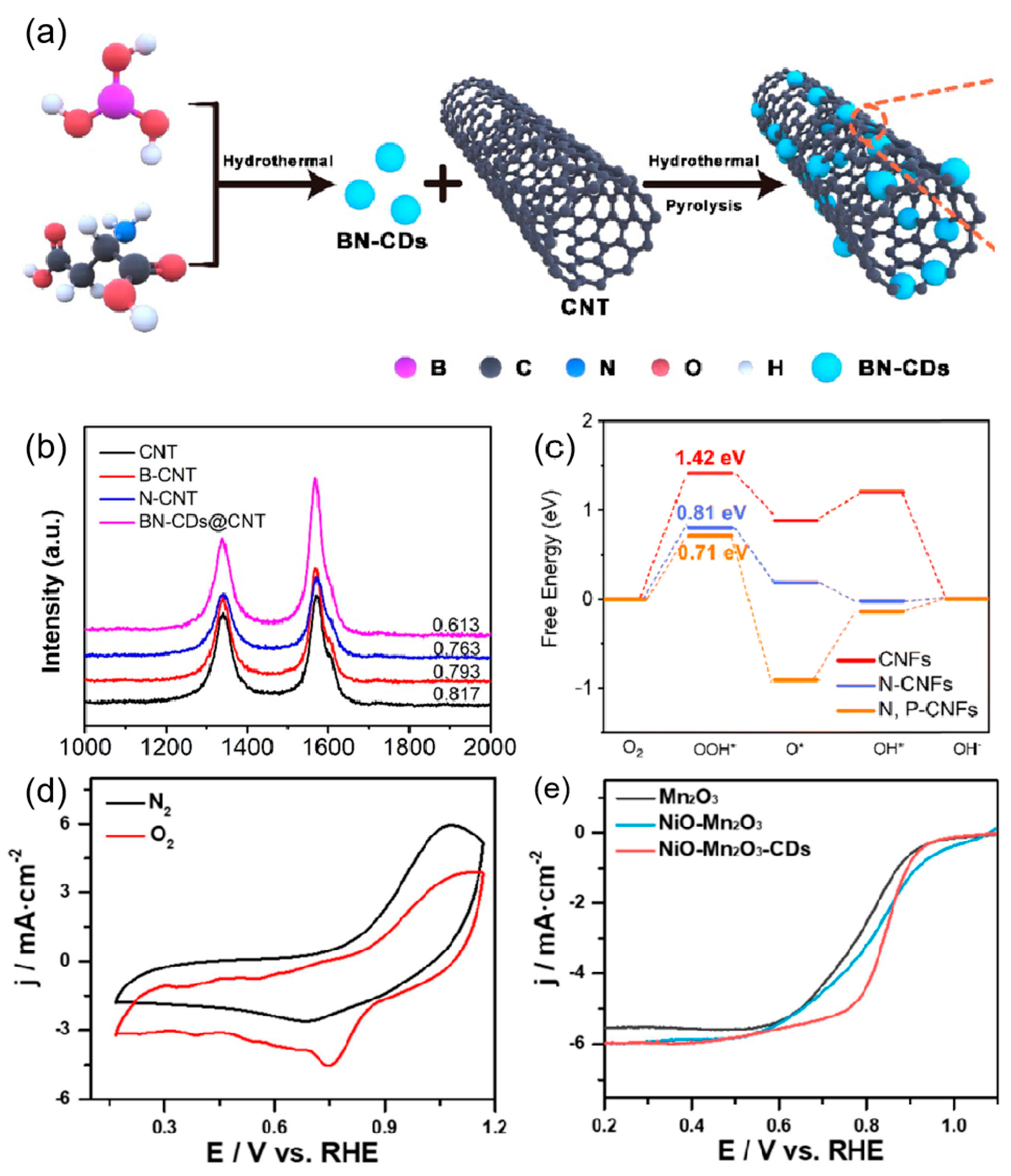
2.2.2. CNT-Based Electrocatalysts
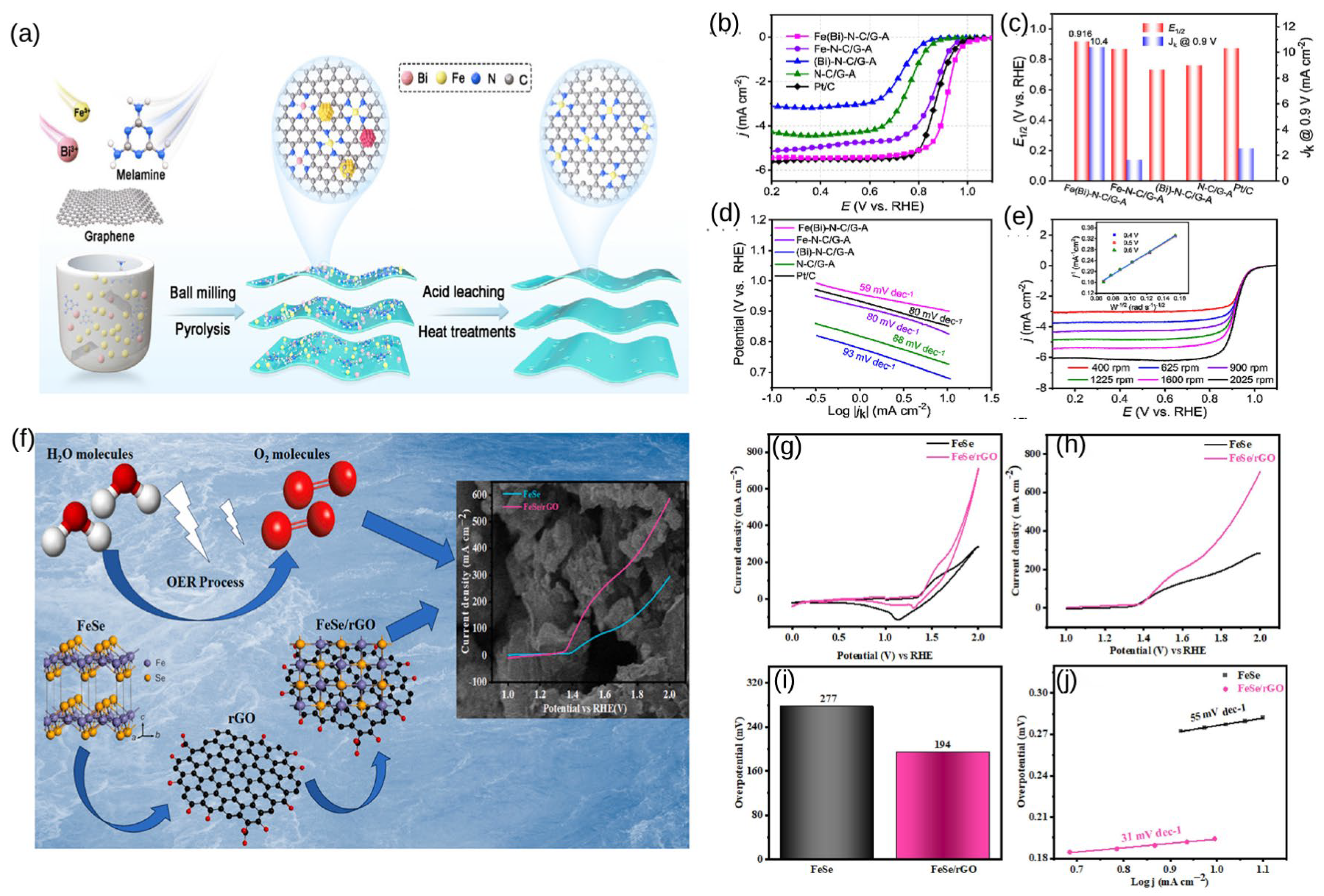
2.3. Graphene
2.3.1. Characteristics of Graphene
2.3.2. Graphene-Based Electrocatalysts
3. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhou, H.; Li, K.; Pan, Q.; Su, Z.; Wang, R. Application of Nanocomposites in Covalent Organic Framework-Based Electrocatalysts. Nanomaterials 2024, 14, 1907. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Pan, Z.; Wang, E.; An, L.; Sun, G. Aqueous metal-air batteries: Fundamentals and applications. Energy Storage Mater. 2020, 27, 478–505. [Google Scholar] [CrossRef]
- Javed, N.; Noor, T.; Iqbal, N.; Naqvi, S.R. A review on development of metal–organic framework-derived bifunctional electrocatalysts for oxygen electrodes in metal–air batteries. RSC Adv. 2023, 13, 1137–1161. [Google Scholar] [CrossRef]
- Tan, X.; Cao, F.; Han, X.; Wang, L.; He, Z.; Zhang, J.; Zhao, Y.; Deng, W.; Ning, H.; Li, Z.; et al. Engineering peripheral S-doped atomic Fe-N4 in defect-rich porous carbon nanoshells for durable oxygen reduction reaction and Zn-air batteries. J. Power Sources 2024, 623, 235477. [Google Scholar] [CrossRef]
- Xu, F.F.; Gao, Z.X.; Ge, Z.C.; Ma, H.; Ren, H.; Zhu, H.Y.; Chi, Y.H.; Guo, W.Y.; Zhao, W. Two-dimensional metal-organic frameworks as bifunctional electrocatalysts for the oxygen evolution reaction and oxygen reduction reaction (OER/ORR): A theoretical study. Phys. Chem. Chem. Phys. 2023, 25, 17508–17514. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y. Application of HTS in Green Hydrogen and Fuel Cells. In High Temperature Shock Technology: Ultra-Fast Micro-Nano Manufacturing; Chen, Y., Ed.; Springer Nature: Singapore, 2023; pp. 13–54. [Google Scholar]
- Santiago, A.P.R.; Fernandez-Delgado, O.; Gomez, A.; Ahsan, M.A.; Echegoyen, L. Fullerenes as Key Components for Low-Dimensional (Photo)Electrocatalytic Nanohybrid Materials. Angew. Chem. Int. Ed. 2021, 60, 122–141. [Google Scholar] [CrossRef]
- Ciftja, O. Novel Research in Low-Dimensional Systems. Nanomaterials 2023, 13, 364. [Google Scholar] [CrossRef]
- Zeng, X.J.; Zhang, Q.Q.; Jin, C.L.; Huang, H.; Gao, Y.F. Fe-Induced Electronic Transfer and Structural Evolution of Lotus Pod-Like CoNiFePx@P, N-C Heterostructure for Sustainable Oxygen Evolution. Energy Environ. Mater. 2024, 7, 12628. [Google Scholar] [CrossRef]
- Li, K.Y.; Guo, Y.Q.; Li, B.H.; Zhao, X.H.; Gong, M.; Liang, J.; Gan, J.N.; Xu, Z.Q.; Hou, M.D.; Huang, Y.L.; et al. Tailoring microstructure and conductivity of porous hollow carbon spheres to enhance their performance as electrode materials for supercapacitors. J. Alloys Compd. 2024, 1003, 175605. [Google Scholar] [CrossRef]
- Zhang, W.Z.; Van Dijk, B.; Wu, L.F.; Maheu, C.; Tudor, V.; Hofmann, J.P.; Jiang, L.; Hetterscheid, D.; Schneider, G. Role of Vacancy Defects and Nitrogen Dopants for the Reduction of Oxygen on Graphene. ACS Catal. 2024, 14, 11065–11075. [Google Scholar] [CrossRef]
- Prasankumar, T.; Bose, N.; Manikandan, M.; Mohana Suntharam, N.; Manoharan, K.; Farhana, N.K.; Bashir, S.; Ramesh, K.; Ramesh, S.; Ramachandaramurthy, V.K. Recent trends and challenges in heteroatom-rich carbon-based cathode for Zn-Ion hybrid supercapacitors. J. Ind. Eng. Chem. 2024, 142, 157–176. [Google Scholar] [CrossRef]
- Kalnin, A.; Kharisova, K.; Lukyanov, D.; Filippova, S.; Li, R.; Yang, P.; Levin, O.; Alekseeva, E. Impact of Metal Source Structure on the Electrocatalytic Properties of Polyacrylonitrile-Derived Co-N-Doped Oxygen Reduction Reaction Catalysts. Nanomaterials 2024, 14, 1924. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.Y.; Ray, R.; Gu, Y.L.; Ploehn, H.J.; Gearheart, L.; Raker, K.; Scrivens, W.A. Electrophoretic analysis and purification of fluorescent single-walled carbon nanotube fragments. J. Am. Chem. Soc. 2004, 126, 12736–12737. [Google Scholar] [CrossRef]
- Sun, Y.P.; Zhou, B.; Lin, Y.; Wang, W.; Fernando, K.S.; Pathak, P.; Meziani, M.J.; Harruff, B.A.; Wang, X.; Wang, H.; et al. Quantum-sized carbon dots for bright and colorful photoluminescence. J. Am. Chem. Soc. 2006, 128, 7756–7757. [Google Scholar] [CrossRef]
- Zhao, C.X.; Liu, J.N.; Li, B.Q.; Ren, D.; Chen, X.; Yu, J.; Zhang, Q. Multiscale Construction of Bifunctional Electrocatalysts for Long-Lifespan Rechargeable Zinc-Air Batteries. Adv. Funct. Mater. 2020, 30, 2003619. [Google Scholar] [CrossRef]
- Xia, C.L.; Zhu, S.J.; Feng, T.L.; Yang, M.X.; Yang, B. Evolution and Synthesis of Carbon Dots: From Carbon Dots to Carbonized Polymer Dots. Adv. Sci. 2019, 6, 1901316. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Lei, Y.; Yu, X.; Wu, Y. Ratiometric fluorometric and colorimetric dual-signal sensing platform for rapid analyzing Cr(VI), Ag(I) and HCHO in food and environmental samples based on N-doped carbon nanodots and o-phenylenediamine. Food Chem. 2024, 437 Pt 2, 137945. [Google Scholar] [CrossRef]
- Rajoriya, K.; Pratibha Abhijeet Meena, R.; Kumari, A. Synthesis of fluorometric carbon nano dots (CNDs) for selective sensing of biologically important Fe3+ and Cu2+ metal ions and evaluating their antioxidant capacity. J. Fluoresc. 2024, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.J.; Zhang, R.Y.; Ma, X.Y.; Xu, Z.H.; Ma, M.Z.; Zhang, T.Y.; Ma, Y.; Shi, F. Carbon dots@noble metal nanoparticle composites: Research progress report. Analyst 2024, 149, 665–688. [Google Scholar] [CrossRef]
- Liu, Y.M.; Roy, S.; Sarkar, S.; Xu, J.Q.; Zhao, Y.F.; Zhang, J.J. A review of carbon dots and their composite materials for electrochemical energy technologies. Carbon Energy 2021, 3, 795–826. [Google Scholar] [CrossRef]
- Sheikh, M.A.; Chandok, R.S.; Abida, K. High energy density storage, antifungal activity and enhanced bioimaging by green self-doped heteroatom carbon dots. Discov. Nano 2023, 18, 132. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Mi, X.; Qian, J.; Ma, N.; Dai, W. CdS Nanoparticles Supported on a Dual Metal–Organic Framework as a Catalyst for the Photodegradation of Tetracycline. ACS Appl. Nano Mater. 2024, 7, 3154–3167. [Google Scholar] [CrossRef]
- Wang, S.Y.; Jing, W.; Chang, J.W.; Lu, S.Y. Recent Progress on Carbon Dots Preparation and Electrochemical Energy Application. Chem. J. Chin. Univ. 2023, 44, 20220733. [Google Scholar]
- Sikiru, S.; Oladosu, T.L.; Kolawole, S.Y.; Mubarak, L.A.; Soleimani, H.; Afolabi, L.O.; Oluwafunke Toyin, A.-O. Advance and prospect of carbon quantum dots synthesis for energy conversion and storage application: A comprehensive review. J. Energy Storage 2023, 60, 106556. [Google Scholar] [CrossRef]
- Li, T.Z.; Dong, Y.Y.; Zhang, J.J.; Su, Y.W.Q. A short review of carbon dots-based composites as advanced electrocatalysis. Int. J. Hydrog. Energy 2024, 87, 76–88. [Google Scholar] [CrossRef]
- Sun, M.; Chu, S.Y.; Guo, D.F.; Jiao, X.Y.; Wang, H.Y.; Wang, L.L.; Li, Z.J.; Jiang, L.Y. Improved Electron Distribution in Graphene Quantum Dots Carrier by F, N Co-Doping for Electrocatalytic ORR. Catal. Lett. 2024, 154, 4078–4087. [Google Scholar] [CrossRef]
- Mahato, D.; Kharwar, Y.P.; Ramanujam, K.; Haridoss, P.; Thomas, T. S, N co-doped graphene quantum dots decorated TiO2 and supported with carbon for oxygen reduction reaction catalysis. Int. J. Hydrog. Energy 2021, 46, 21549–21565. [Google Scholar] [CrossRef]
- Syahputra, S.; Sgreccia, E.; Nallayagari, A.R.; Vacandio, F.; Kaciulis, S.; Di Vona, M.L.; Knauth, P. Influence of Nitrogen Position on the Electrocatalytic Performance of B, N-Codoped Carbon Quantum Dots for the Oxygen Reduction Reaction. J. Electrochem. Soc. 2024, 171, 066510. [Google Scholar] [CrossRef]
- Li, Z.; Xu, X.L.; Lu, X.H.; He, C.C.; Huang, J.J.; Sun, W.; Tian, L. Synergistic coupling of FeNi alloy with graphene carbon dots for advanced oxygen evolution reaction electrocatalysis. J. Colloid Interface Sci. 2022, 615, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Yin, K.; Ma, M.; Sun, Q.; Liao, F.; Huang, H.; Chen, J.; Feng, K.; Yang, H.; Zhong, J.; et al. Carbon-dots-facilitated oxygen migration in heterophase iridium oxide for enhanced acidic water oxidation. Chin. Chem. Lett. 2024, 110749. [Google Scholar] [CrossRef]
- Pei, Y.; Song, H.; Liu, Y.; Cheng, Y.; Li, W.; Chen, Y.; Fan, Y.; Liu, B.; Lu, S. Boron–nitrogen-doped carbon dots on multi-walled carbon nanotubes for efficient electrocatalysis of oxygen reduction reactions. J. Colloid Interface Sci. 2021, 600, 865–871. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.W.; Liu, P.H.; Ren, J.; Jiang, Z.; Wang, X.H.; Zhao, H.G. Carbon dot hybrid porous carbon nanofibers as efficient electrocatalysts for the oxygen reduction reaction. Mater. Chem. Front. 2024, 8, 1643–1650. [Google Scholar] [CrossRef]
- Shao, C.R.; Liao, F.; Zhu, W.X.; Zhang, Y.; Ma, M.J.; Yang, J.J.; Yin, K.; Shao, M.W.; Jiang, B.B. Carbon dots bridge NiO and Mn2O3 as highly efficient bifunctional oxygen electrocatalysts for rechargeable zinc-air batteries. Appl. Surf. Sci. 2022, 596, 153642. [Google Scholar] [CrossRef]
- Wu, N.N.; Hu, Q.; Wei, R.B.; Mai, X.M.; Naik, N.; Pan, D.; Guo, Z.H.; Shi, Z.J. Review on the electromagnetic inter-ference shielding properties of carbon based materials and their novel composites: Recent progress, challenges and prospects. Carbon 2021, 176, 88–105. [Google Scholar] [CrossRef]
- Wang, P.; Dong, Q.P.; Gao, C.Y.; Bai, W.J.; Chu, D.M.; He, Y. A comprehensive review of carbon nanotubes: Growth mechanisms, preparation and applications. Fuller. Nanotub. Carbon Nanostruct. 2024, 32, 415–429. [Google Scholar] [CrossRef]
- Cui, S.; Xu, D.; Wang, Z.; Wang, L.; Zhao, Y.; Deng, W.; Zhao, Q.; Wu, M. Pd Nanoparticles Immobilized on Pyridinic N-Rich Carbon Nanosheets for Promoting Suzuki Cross-Coupling Reactions. Nanomaterials 2024, 14, 1690. [Google Scholar] [CrossRef]
- Iijima, S.; Ichihashi, T. Single-Shell Carbon Nanotubes of 1-nm Diameter. Nature 1993, 363, 603–605. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, J.; Chen, S.; Zhang, X.; Li, Y.; Yang, Z. Vapor-Responsive Shape-Memory Material Based on Carbon Nanotube Sponge Dominated by Pressure-Induced Conformational Transition of Spidroin. ACS Appl. Polym. Mater. 2023, 5, 2490–2500. [Google Scholar] [CrossRef]
- Auclair, J.; Roubeau-Dumont, E.; Gagné, F. Lethal and Sublethal Toxicity of Nanosilver and Carbon Nanotube Composites to Hydra vulgaris—A Toxicogenomic Approach. Nanomaterials 2024, 14, 1955. [Google Scholar] [CrossRef]
- Sun, J.C.; Su, L.; Zheng, L.Q. Pressure-driven flow behavior of small molecules through a carbon nanotube. J. Mol. Liq. 2023, 374, 121276. [Google Scholar] [CrossRef]
- Wu, K.J.; Wang, B.; Niu, Y.T.; Wang, W.J.; Wu, C.; Zhou, T.; Chen, L.; Zhan, X.H.; Wan, Z.Y.; Wang, S.; et al. Carbon nanotube fibers with excellent mechanical and electrical properties by structural realigning and densification. Nano Res. 2023, 16, 12762–12771. [Google Scholar] [CrossRef]
- Men, Q.Q.; Wang, S.; Yan, Z.K.; Zhao, B.; Guan, L.; Chen, G.Y.; Guo, X.Q.; Zhang, R.; Che, R.C. Iron-encapsulated CNTs on carbon fiber with high-performance EMI shielding and electrocatalytic activity. Adv. Compos. Hybrid Mater. 2022, 5, 2429–2439. [Google Scholar] [CrossRef]
- Zhang, D.Y.; Song, W.H.; Lv, L.; Gao, C.Q.; Gao, F.; Guo, H.; Diao, R.M.; Dai, W.; Niu, J.; Chen, X.C.; et al. Mono-dispersion decorated ultra-long single-walled carbon nanotube/aramid nanofiber for high-strength electromagnetic interference shielding film with Joule heating properties. Carbon 2023, 214, 118315. [Google Scholar] [CrossRef]
- Gao, J.Y.; Li, M.X.; Chen, H.; Guo, L.P.; Li, Z.Z.; Wang, X.P. Microstructure regulation of graphitic carbon nitride nanotubes via quick thermal polymerization process for photocatalytic hydrogen evolution. J. Photochem. Photobiol. A Chem. 2023, 441, 114747. [Google Scholar] [CrossRef]
- Zhang, F.R.; Zhang, L.; Wang, J.Q.; Zhang, B.L. Thickness-controlled HsGDY/N-doped double-shell hollow carbon tubes for broadband high-performance microwave absorption. Carbon 2025, 231, 119740. [Google Scholar] [CrossRef]
- Zhang, H.F.; Zhang, J.H.; Liu, Y.R.; Feng, F.; Zhang, Y.G.; Sun, L.; Zhang, Y.H. Functional porous carbons for zinc ion energy storage: Structure-Function relationship and future perspectives. Coord. Chem. Rev. 2023, 482, 215056. [Google Scholar] [CrossRef]
- Cheng, X.; Tian, W.; Gao, J.F.; He, L.; Zhang, J.H.; Wang, X.H. Effect of multiwalled carbon nanotube size on electrical properties of cement mortar composites. Mag. Concr. Res. 2022, 75, 595–606. [Google Scholar] [CrossRef]
- Mykhailova, H.Y.; Kovalchuk, B.V.; Bozbey, Y.F.; Dekhtyarenko, V.A. Electrical conductivity of nanocomposites metal-carbon nanotubes. MRS Commun. 2023, 13, 320–323. [Google Scholar] [CrossRef]
- Benhamou, S.M.; Houbad, M. Assessment of effective electrical conductivity in spherocylindrical carbon nanotube composites incorporating intrinsic properties of CNTs and interphase layer. Phys. B-Condens. Matter. 2024, 691, 416323. [Google Scholar] [CrossRef]
- Awasthi, S.; Yadav, T.P.; Awasthi, K. Notable electrical and mechanical properties of polyacrylamide (PAM) with graphene oxide (GO) and single-walled carbon nanotubes (SWCNTs). Int. Polym. Process. 2023, 38, 290–299. [Google Scholar] [CrossRef]
- Chaturvedy, G.K.; Bashar, N.; Pandey, U.K. Incorporating multi-walled carbon nanotubes in rubberized concrete: Impact on physical, mechanical, and fire resistance properties. Fuller. Nanotub. Carbon Nanostruct. 2024, 32, 1121–1134. [Google Scholar] [CrossRef]
- Hao, G.Q.; Li, X.; Wang, S.C.; Wang, S.R.; Ryu, M.; Yang, J.X. Surface Modification of Carbon Nanotubes in Silicone-Polyurethane for Improved Mechanical and Anticorrosion Properties. Coatings 2023, 13, 634. [Google Scholar] [CrossRef]
- Miao, Y.; Chen, Q.H.; Li, Y.G.; Zhuo, D.X.; Wang, R. Tribological properties of carbon nanotube/polymer composites: A mini-review. Front. Mater. 2023, 10, 1129676. [Google Scholar] [CrossRef]
- Nie, X.W.; Bahrami, A. Effect of carbon nanotubes on mechanical properties of aluminum matrix composites: A review. Sci. Eng. Compos. Mater. 2024, 31, 20240009. [Google Scholar] [CrossRef]
- Imran, M.; Amjad, H.; Khan, S.; Ali, S. Machine learning assisted prediction of the mechanical properties of carbon nanotube-incorporated concrete. Struct. Concr. 2024, 1–23. [Google Scholar] [CrossRef]
- Khan, S.; Ashraf, S.; Ali, S.; Khan, K. Experimental investigations on the mechanical properties and damage detection of carbon nanotubes modified crumb rubber concrete. J. Build. Eng. 2023, 75, 106937. [Google Scholar] [CrossRef]
- Ren, W.; Qian, W.; Zhang, Z.X.; Wang, Z.; Wang, J.J.; Cui, L.M.; Zhao, X.; He, D.P. Intercross-linked aramid nanofibers/graphene hybrid films toward high mechanical strength and electrical conductivity. J. Alloys Compd. 2024, 976, 173390. [Google Scholar] [CrossRef]
- Reis, E.D.; Borges, L.A.; Camargos JS, F.; Gatuingt, F.; Poggiali FS, J.; Bezerra, A.C.S. A systematic review on the engineering properties of concrete with carbon nanotubes. J. Braz. Soc. Mech. Sci. Eng. 2023, 45, 205. [Google Scholar] [CrossRef]
- Casto, A.; Bellussi, F.M.; Diego, M.; Del Fatti, N.; Banfi, F.; Maioli, P.; Fasano, M. Water filling in carbon nanotubes with different wettability and implications on nanotube/water heat transfer via atomistic simulations. Int. J. Heat Mass Transf. 2023, 205, 123868. [Google Scholar] [CrossRef]
- Morita, S.I.; Saito, T.; Takai, K.; Hayamizu, Y.; Haruki, N. Natural Convection Melting in a Rectangular Heat Storage Tank of Carbon Nanotube Dispersed Latent Heat Storage Material. J. Therm. Sci. 2024, 33, 847–855. [Google Scholar] [CrossRef]
- Wisniewska, M.; Laptev, A.M.; Marczewski, M.; Leshchynsky, V.; Lota, G.; Acznik, I.; Celotti, L.; Sullivan, A.; Szybowicz, M.; Garbiec, D. Influence of carbon nanotubes on thermal and electrical conductivity of zirconia-based composite. Ceram. Int. 2023, 49, 15442–15450. [Google Scholar] [CrossRef]
- Zhang, X.; Tan, W.; Carey, T.; Wen, B.; He, D.L.; Arbab, A.; Groombridge, A.; Smail, F.; De La Verpilliere, J.; Yao, C.N.; et al. Enhanced composite thermal conductivity by percolated networks of in situ confined-grown carbon nanotubes. Nano Res. 2023, 16, 12821–12829. [Google Scholar] [CrossRef]
- Yi, S.C.; Xin, R.; Li, X.X.; Sun, Y.Y.; Yang, M.; Liu, B.; Chen, H.B.; Li, H.M.; Liu, Y.J. -like cobalt complex derived Co/N-doped carbon nanotubes as efficient ORR/OER electrocatalysts for long-life rechargeable Zn-air batteries. Nanoscale 2023, 15, 16612–16618. [Google Scholar] [CrossRef]
- Zhao, W.Q.; Yang, J.W.; Yang, B.Y.; Gao, Z.; Han, D.; Su, Q.M.; Xu, B.S.; Du, G.H. N-Doped Carbon Knotting Carbon Nanotube Sponge Networks for Lithium-Ion Capacitor Anodes. ACS Appl. Nano Mater. 2023, 6, 9306–9314. [Google Scholar] [CrossRef]
- Gong, K.P.; Du, F.; Xia, Z.H.; Durstock, M.; Dai, L.M. Nitrogen-Doped Carbon Nanotube Arrays with High Electrocatalytic Activity for Oxygen Reduction. Science 2009, 323, 760–764. [Google Scholar] [CrossRef]
- Xie, A.; Tai, X.F.; Guo, Y.J.; Miao, J.Y.; Liu, D.Y.; Zhang, R.F.; Cheng, L.T.; Zhao, Z.M.; Tang, Y.; Yang, X.J.; et al. Elaborate construction of honeycomb-like porous carbon with embedment of N-doped carbon nanotubes as efficient electrocatalyst for zinc-air battery. Carbon 2024, 228, 119299. [Google Scholar] [CrossRef]
- Ding, R.X.; Zhang, D.; Bi, L.S.; Shi, S.; Tang, X.Q.; Zhang, Z.M.; He, Y. N/P-Codoped Carbon Nanotubes for Efficient Oxygen Reduction Reaction. ACS Appl. Nano Mater. 2023, 6, 21887–21896. [Google Scholar] [CrossRef]
- Xu, Y.L.; Wang, J.Y.; Wang, S.S.; Zhai, Z.Z.; Ren, B.; Tian, Z.; Zhang, L.H.; Liu, Z.F. Lattice defective and N, S co-doped carbon nanotubes for trifunctional electrocatalyst application. Int. J. Hydrog. Energy 2023, 48, 34340–34354. [Google Scholar] [CrossRef]
- Liu, C.Y.; Wu, S.; Tian, S.; Yang, J.C.; Li, J.K.; Wang, X.; Wang, L.H.; Chen, C.; Zhang, P.; Yang, Q.L. Nanoporous Fe and Co Dually Doped Carbon Nanotube-Based Oxygen Electrocatalysts for Efficient Zinc-Air Batteries. ACS Appl. Nano Mater. 2024, 7, 13536–13546. [Google Scholar] [CrossRef]
- Meng, X.; Xie, J.H.; Liu, J.; Liu, B.; Wang, R.Y.; Gu, P.; Ma, F.W.; Wang, X.Y.; Zou, J.L. Co3C-stabilized-CoFe heterostructure wrapped by bamboo-like N-doped carbon nanotubes for highly-efficient oxygen electrocatalysis. Chem. Eng. J. 2023, 459, 141591. [Google Scholar] [CrossRef]
- Pan, S.Y.; Shi, H.; Yu, Y.J.; Li, Y.F.; Chen, Y.Z.; Li, C.S.; Sun, Y.; Yang, Z.H.; Luo, F. High entropy alloy nanoparticles encapsulated into carbon nanotubes enable high performance of zinc air batteries. Chem. Commun. 2024, 60, 11778–11781. [Google Scholar] [CrossRef]
- Choi, E.Y.; Kim, D.E.; Lee, S.Y.; Park, C.B.; Kim, C.K. Cobalt nanoparticles-encapsulated holey nitrogen-doped carbon nanotubes for stable and efficient oxygen reduction and evolution reactions in rechargeable Zn-air batteries. Appl. Catal. B-Environ. Energy 2023, 325, 122386. [Google Scholar] [CrossRef]
- Wang, M.; Liu, B.L.; Zhang, H.Y.; Lu, Z.J.; Xie, J.; Cao, Y.L. High quality bifunctional cathode for rechargeable zinc-air batteries using N-doped carbon nanotubes constrained CoFe alloy. J. Colloid Interface Sci. 2024, 661, 681–689. [Google Scholar] [CrossRef] [PubMed]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric Field Effect in Atomically Thin Carbon Films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef]
- Kalinić, A.; Radović, I.; Karbunar, L.; Despoja, V.; Mišković, Z.L. Phonon-Induced Wake Potential in a Graphene-Insulator-Graphene Structure. Nanomaterials 2024, 14, 1951. [Google Scholar] [CrossRef] [PubMed]
- Hajialilou, E.; Rezanezhad, A.; Hanif, M.B.; Motola, M. Two-Dimensional Carbon Graphenylene. In Handbook of Functionalized Carbon Nanostructures: From Synthesis Methods to Applications; Springer: Berlin/Heidelberg, Germany, 2023; pp. 1–37. [Google Scholar]
- Ma, D.L.; Lee, T.R. Meeting a Rising Need of Electromagnetic Interference Shielding with Nanomaterials. ACS Appl. Nano Mater. 2024, 7, 2414–2416. [Google Scholar] [CrossRef]
- Esteve-Adell, I.; Porcel-Valenzuela, M.; Zubizarreta, L.; Gil-Agusti, M.; Garcia-Pellicer, M.; Quijano-Lopez, A. Influence of the Specific Surface Area of Graphene Nanoplatelets on the Capacity of Lithium-Ion Batteries. Front. Chem. 2022, 10, 807980. [Google Scholar] [CrossRef] [PubMed]
- Efimov, M.; Vasilev, A.; Muratov, D.; Panin, A.; Malozovskaya, M.; Karpacheva, G. Application of Infrared Pyrolysis and Chemical Post-Activation in the Conversion of Polyethylene Terephthalate Waste into Porous Carbons for Water Purification. Polymers 2024, 16, 891. [Google Scholar] [CrossRef] [PubMed]
- Asghar, S.A.; Jalil, A.; Ul Ain, N.; Kanwal, A. Computational prediction of phosphorene and graphene-like AsP3 monolayers. New J. Chem. 2024, 48, 10599–10606. [Google Scholar] [CrossRef]
- Yang, D.Y.; Liu, J.X.; Shi, F.; Li, Y.; Wan, J.X.; Yuan, X.Y.; Tian, Z.W.; Wang, M.Y.; Ma, C.C. One-step solvothermal synthesis of CsxWO3: Crystal growth regulation by halogen acids with generating oxygen vacancies and W5+ for improving transparent thermal insulation performance. J. Alloys Compd. 2023, 942, 169119. [Google Scholar] [CrossRef]
- Bo, Z.; Ying, C.; Yang, H.; Wu, S.; Yang, J.; Kong, J.; Yang, S.; Zhou, Y.; Yan, J.; Cen, K. Highly thermo-conductive three-dimensional graphene aqueous medium. Nano-Micro Lett. 2020, 12, 138. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.J.; Li, Z.; Liu, Y.; Shi, Y.H.; Zhu, M.Y. Advances in the synthesis and modification of two-dimensional antimonene. Phys. Chem. Chem. Phys. 2023, 25, 21773–21786. [Google Scholar] [CrossRef]
- Khan, M.U.; Shaida, M.A. Reduction mechanism of graphene oxide including various parameters affecting the C/O ratio. Mater. Today Commun. 2023, 36, 106577. [Google Scholar] [CrossRef]
- Jiang, T.; Amadei, C.A.; Lin, Y.; Gou, N.; Rahman, S.M.; Lan, J.; Vecitis, C.D.; Gu, A.Z. Dependence of Graphene Oxide (GO) Toxicity on Oxidation Level, Elemental Composition, and Size. Int. J. Mol. Sci. 2021, 22, 10578. [Google Scholar] [CrossRef]
- Zhao, K.; Hu, Q.X.; Cao, J.Z.; Qi, Y.; Wei, P.; Lu, Y.Y.; Cheng, J.; Xie, Y.H. Enhancing Efficiency and Stability in Carbon-Based Perovskite Solar Cells by Double Passivation with Ultralow-Cost Coal-Derived Graphene and Its Derivatives. ACS Appl. Mater. Interfaces 2024, 16, 20577–20586. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Zhao, Y.; Zou, W.; Lu, Q.; Liao, J.; Li, F.; Shang, M.; Lin, L.; Liu, Z. Doping of Graphene Films: Open the way to Applications in Electronics and Optoelectronics. Adv. Funct. Mater. 2022, 32, 2203179. [Google Scholar] [CrossRef]
- Zhuo, H.-Y.; Zhang, X.; Liang, J.-X.; Yu, Q.; Xiao, H.; Li, J. Theoretical Understandings of Graphene-based Metal Single-Atom Catalysts: Stability and Catalytic Performance. Chem. Rev. 2020, 120, 12315–12341. [Google Scholar] [CrossRef]
- Basiuk, E.V.; Prezhdo, O.V.; Basiuk, V.A. Strong Bending Distortion of a Supercoronene Graphene Model upon Adsorption of Lanthanide Atoms. J. Phys. Chem. Lett. 2023, 14, 2910–2916. [Google Scholar] [CrossRef] [PubMed]
- Batool, S.; Guo, W.; Gill, R.; Xin, W.; Hu, Y. Chitin based multi-layered coatings with flame retardancy an approach to mimic nacre: Synthesis, characterization and mechanical properties. Carbohydr. Polym. 2022, 291, 119488. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Li, C.; Yang, Z.; Wang, J.; Yang, P. Conductivity and interaction mechanism of polydopamine-graphene oxide: A combined experimental and density functional theory investigation. J. Polym. Res. 2024, 31, 22–36. [Google Scholar] [CrossRef]
- Thi, P.T.; Vuong, V.D.; Le, T.V.; Phong, M.T.; Thanh, P.N. Understanding adsorption of divalent metals ions (Mg, Ca) on Nitrogen-, Boron- doped, and defective graphene in nanofiltration process using van der Waals density functional method. Mater. Res. Express 2023, 10, 095602. [Google Scholar]
- Helsel, N.; Choudhury, P. Investigation of bifunctionality of FePc-functionalized graphene for enhanced ORR/OER activity. Mol. Catal. 2023, 545, 113213. [Google Scholar] [CrossRef]
- Ye, S.; Chen, W.; Ou, Z.; Zhang, Q.; Zhang, J.; Li, Y.; Ren, X.; Ouyang, X.; Zheng, L.; Yan, X.; et al. Harnessing the Synergistic Interplay between Atomic-Scale Vacancies and Ligand Effect to Optimize the Oxygen Reduction Activity and Tolerance Performance. Angew. Chem. 2025, 137, e202414989. [Google Scholar] [CrossRef]
- Kumar, P.; Kannimuthu, K.; Zeraati, A.S.; Roy, S.; Wang, X.; Wang, X.; Samanta, S.; Miller, K.A.; Molina, M.; Trivedi, D.; et al. High-Density Cobalt Single-Atom Catalysts for Enhanced Oxygen Evolution Reaction. J. Am. Chem. Soc. 2023, 145, 8052–8063. [Google Scholar] [CrossRef]
- Chen, W.; Yuan, F.; Guo, X.; Chen, F.; Fan, L.; Zheng, H.; Guo, X.; Zheng, P.; Zheng, L.; Zhang, Y. Enhancing performance of nitrogen-doped graphene nano-catalyst for oxygen reduction reaction by Ag loading. Int. J. Hydrog. Energy 2024, 59, 375–382. [Google Scholar] [CrossRef]
- Byeon, A.; Yun, W.C.; Kim, J.M.; Lee, J.W. Recent progress in heteroatom-doped carbon electrocatalysts for the two-electron oxygen reduction reaction. Chem. Eng. J. 2023, 456, 141042. [Google Scholar] [CrossRef]
- San Roman, D.; Krishnamurthy, D.; Garg, R.; Hafiz, H.; Lamparski, M.; Nuhfer, N.T.; Meunier, V.; Viswanathan, V.; Co-hen-Karni, T. Engineering Three-Dimensional (3D) Out-of-Plane Graphene Edge Sites for Highly Selective Two-Electron Oxygen Reduction Electrocatalysis. ACS Catal. 2020, 10, 1993–2008. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, B.W.; Wu, N. Active sites and structure regulation of non-radical pathways in N-doped gra-phene-activated persulfate. Catal. Commun. 2024, 187, 106910. [Google Scholar] [CrossRef]
- Wu, X.; Yan, Q.; Wang, H.; Wu, D.; Zhou, H.; Li, H.; Yang, S.; Ma, T.; Zhang, H. Heterostructured Catalytic Materials as Advanced Electrocatalysts: Classification, Synthesis, Characterization, and Application. Adv. Funct. Mater. 2024, 34, 2404535. [Google Scholar] [CrossRef]
- Chen, J.; Li, H.; Fan, C.; Meng, Q.; Tang, Y.; Qiu, X.; Fu, G.; Ma, T. Dual Single-Atomic Ni-N4 and Fe-N4 Sites Constructing Janus Hollow Graphene for Selective Oxygen Electrocatalysis. Adv. Mater. 2020, 32, 2003134. [Google Scholar] [CrossRef]
- Lyu, Z.; Yu, S.; Wang, M.Y.; Tieu, P.; Zhou, J.C.; Shi, Q.R.; Du, D.; Feng, Z.X.; Pan, X.Q.; Lin, H.F.; et al. NiFe Nanoparticle Nest Supported on Graphene as Electrocatalyst for Highly Efficient Oxygen Evolution Reaction. Small 2023, 20, 2308278. [Google Scholar] [CrossRef]
- Yang, Z.Y.; Zhang, Y.; Xiao, J.D.; Wang, J.Y.; Wang, J.Z. Enhancing oxygen reduction of Fe-N-C active sites onto graphene via bismuth sacrifice for Zn-air batteries. Chin. J. Catal. 2024, 59, 272–281. [Google Scholar] [CrossRef]
- Perveen, W.; Alyousef, H.A.; Alotaibi, B.M.; Alrowaily, A.W.; Hussein, K.I.; Henaish, A.M.A. Hydrothermally synthesized rGO based FeSe nanocomposite as electrocatalyst for oxygen evolution reaction. J. Phys. Chem. Solids 2024, 191, 112055. [Google Scholar] [CrossRef]
- Xie, T.; Wang, Q.; Wallace, R.M.; Gong, C. Understanding and optimization of graphene gas sensors. Appl. Phys. Lett. 2021, 119, 013104. [Google Scholar] [CrossRef]
- Li, X.F.; Su, F.Y.; Xie, L.J.; Tian, Y.R.; Yi, Z.L.; Cheng, J.Y.; Chen, C.M. Carbon Corrosion Induced by Surface Defects Accelerates Degradation of Platinum/Graphene Catalysts in Oxygen Reduction Reaction. Small 2024, 20, 2310940. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.; Wang, J.; Wang, L.; Xu, C.; Luo, R.; Shao, F.; Zhang, X.; Fan, Y. Three-Dimensional Cadmium–Organic Framework with Dual Functions of Oxygen Evolution in Water Splitting and Fenton-like Photocatalytic Removal of Organic Pollutants. Inorg. Chem. 2023, 62, 6339–6351. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.-X.; Chen, X.-L.; Fan, W.-J.; Wang, X.-F.; Li, Y.-F.; Jiang, Y.; Jiang, Z.-Q.; Wen, T. Advanced BIFs with Co, B, N, and S for Electrocatalytic Oxygen Reduction and Oxygen Evolution Reactions. Inorg. Chem. 2023, 62, 11287–11290. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-H.; Wang, X.-Y.; Liu, Y.-Q.; Dai, S.-Y.; Jin, N.-H.; Chen, H.; Liu, X.-M.; Zhao, Y.; Luo, H.-L.; Li, W. Eight semiconducting MOFs constructed with conjugated ligands and d-metals (Cd, Zn, Co and Ni) serve as functional materials for oxygen evolution reactions, photocatalytic degradation of dyes and photoluminescence. CrystEngComm 2022, 24, 8407–8426. [Google Scholar] [CrossRef]
- Shah, S.S.; Albadrani, A.; Fettouhi, M.; Aziz, M.A.; Helal, A. Synthesis and Oxygen Evolution Reaction Application of a Co−Cd Based Bimetallic Metal-Organic Framework. Chem. Asian J. 2024, 19, e202301039. [Google Scholar] [CrossRef]
- Lu, Z.; Xiong, Q.; Fu, R.; Wang, W.; Zhang, L.; Yan, M.; Wu, C.; Sun, M.; Su, G.; Wang, Y.; et al. In situ construction of N-doped hollow carbon nanotubes anchored Co nanoparticles for bifunctional ORR/OER electrocatalyst. Int. J. Hydrog. Energy 2024, 61, 203–209. [Google Scholar] [CrossRef]
- Liu, X.; Huo, S.; Xu, X.; Wang, X.; Zhang, W.; Chen, Y.; Wang, C.; Jiahaoxie Liu, X.; Chang, H.; Zou, J. Carbon nanotube-encapsulated Co/Co3Fe7 heterojunctions as a highly-efficient bifunctional electrocatalyst for rechargeable zinc-air batteries. J. Colloid Interface Sci. 2024, 666, 296–306. [Google Scholar] [CrossRef]
- Li, G.; Tang, Y.; Fu, T.; Xiang, Y.; Xiong, Z.; Si, Y.; Guo, C.; Jiang, Z. S, N co-doped carbon nanotubes coupled with CoFe nanoparticles as an efficient bifunctional ORR/OER electrocatalyst for rechargeable Zn-air batteries. Chem. Eng. J. 2022, 429, 132174. [Google Scholar] [CrossRef]
- Chang, H.; Liu, X.; Zhao, S.; Liu, Z.; Lv, R.; Zhang, Q.; Yi, T.-F. Self-Assembled 3D N/P/S-Tridoped Carbon Nanoflower with Highly Branched Carbon Nanotubes as Efficient Bifunctional Oxygen Electrocatalyst Toward High-Performance Rechargeable Zn-Air Batteries. Adv. Funct. Mater. 2024, 34, 2313491. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, J.; Zhang, T.; Liu, F.; Zheng, M.; Shi, K.; Liu, J.; Zhang, Y.; Wang, H. Synthesis of novel hollow carbon nanotube@Co-Fe alloy/iron phthalocyanine electrocatalyst by self-assembly method for OER and ORR study. Colloids Surf. A Physicochem. Eng. Asp. 2024, 684, 133093. [Google Scholar] [CrossRef]
- Khairani, I.Y.; Jin, B.; Palardonio, S.M.; Hagemann, U.; Alonso, B.; Ortega, A.; Doñate-Buendía, C.; Martorell, J.; Ros, C.; Kallio, T.; et al. FeNi nanoparticle-modified reduced graphene oxide as a durable electrocatalyst for oxygen evolution. J. Catal. 2024, 439, 115771. [Google Scholar] [CrossRef]
- Madampadi, R.; Patel, A.B.; Vinod, C.P.; Gupta, R.; Jagadeesan, D. Facile synthesis of nanostructured Ni/NiO/N-doped graphene electrocatalysts for enhanced oxygen evolution reaction. Nanoscale Adv. 2024, 6, 2813–2822. [Google Scholar] [CrossRef]
- Guo, R.; Fang, M.; Chen, Q.; Wang, N.; Wang, B.; Liu, N.; Mo, Z. Self-assembled graphene quantum dots-Co3O4 nanocomposite for highly efficient oxygen evolution reaction electrocatalyst. Carbon Lett. 2023, 33, 1591–1600. [Google Scholar] [CrossRef]
- Hu, Y.; Zhao, X.; Li, F.; Dong, Q.; Wen, B.; Sun, D.; Liang, W.; Lyu, X. Spherical ZnFe2O4 Nanoparticles on Nitrogen-Doped Graphene: A Synergistic Effect on Efficient Electrocatalytic Oxygen Evolution Reaction. ACS Appl. Energy Mater. 2023, 6, 9985–9993. [Google Scholar] [CrossRef]
- Chu, S.; Sun, M.; Li, Z.; Wang, H.; Miao, F. Coordination engineering of defective F and N co-doped carbon dots anchored silver nanoparticles for boosting oxygen reduction reaction. J. Alloys Compd. 2024, 1002, 175256. [Google Scholar] [CrossRef]
- Shen, M.; Hu, W.; Yang, G.; Wang, Q.; Duan, C.; Ni, Y. TEMPO-mediated oxidized cellulose nanofibers-Cd2+ derived hierarchically porous carbon aerogel for oxygen reduction electrocatalysis. J. Electroanal. Chem. 2022, 910, 116168. [Google Scholar] [CrossRef]
- Ma, N.; Zhang, Y.; Wang, Y.; Zhao, J.; Liang, B.; Xiong, Y.; Luo, S.; Huang, C.; Fan, J. Curvature effects regulate the catalytic activity of Co@N4-doped carbon nanotubes as bifunctional ORR/OER catalysts. J. Colloid Interface Sci. 2024, 654, 1458–1468. [Google Scholar] [CrossRef]
- Li, Z.; Cao, L.; Yang, T.; He, J.; Wang, Z.; He, J.; Zhao, Y.; Chai, Z. Janus structural TaON/Graphene-like carbon dual-supported Pt electrocatalyst enables efficient oxygen reduction reaction. J. Colloid Interface Sci. 2025, 677, 677–686. [Google Scholar] [CrossRef]
- Choo, T.-F.; Mat Zali, N.; Ubaidah Saidin, N.; Kok, K.-Y.; Azhar, N. Gamma irradiation synthesis and modification of palladium supported on graphene oxide as oxygen reduction reaction electrocatalyst. Chem. Phys. Lett. 2023, 830, 140822. [Google Scholar] [CrossRef]
- Wu, J.; Wu, Y.; Li, J.; Guo, C.; Wu, T.; Deng, H.; Zeng, K.; Yang, R. In-situ construction of ionic liquid salt-derived graphene-like dual-heteroatoms doping engineering as efficient metal-free electrocatalyst for oxygen reduction reaction. J. Alloys Compd. 2024, 1003, 175688. [Google Scholar] [CrossRef]
- Dhanda, A.; Sathe, S.M.; Dubey, B.K.; Ghangrekar, M.M. CuO-SnO2/N-doped reduced graphene oxide as superior oxygen reduction electrocatalyst for microbial fuel cell. Biomass Convers. Biorefin. 2024. [Google Scholar] [CrossRef]

| Synthesis Method | Usage | Advantages | Disadvantages |
|---|---|---|---|
| Chemical Vapor Deposition (CVD) | 42% |
|
|
| Liquid Phase Methods (Solvothermal, Liquid-PhaseReduction) | 28% |
|
|
| Laser-Enhanced Chemica Vapor Deposition (LCVD) | 10% |
|
|
| Laser Ablation | 5% |
|
|
| Arc Discharge | 5% |
|
|
| Microwave lrradiation | 5% |
|
|
| Self-Assembly | 5% |
|
|
| Electrocatalyst | E10 (V vs. RHE) | Tafel Slope (mV dec−1) | Electrolyte | Reference |
|---|---|---|---|---|
| OER | OER | |||
| FeNi3@GCDs-10 | 238 mV | 48.7 | 0.1 M KOH | [30] |
| NiO-Mn2O3-CDs | 298 mV | 141.1 | 0.1 M KOH | [34] |
| Co/N-CNT | 390 mV | 78.0 | 0.1 M KOH | [64] |
| CNT-550-NS | 1610 mV | 105.0 | 0.1 M KOH | [69] |
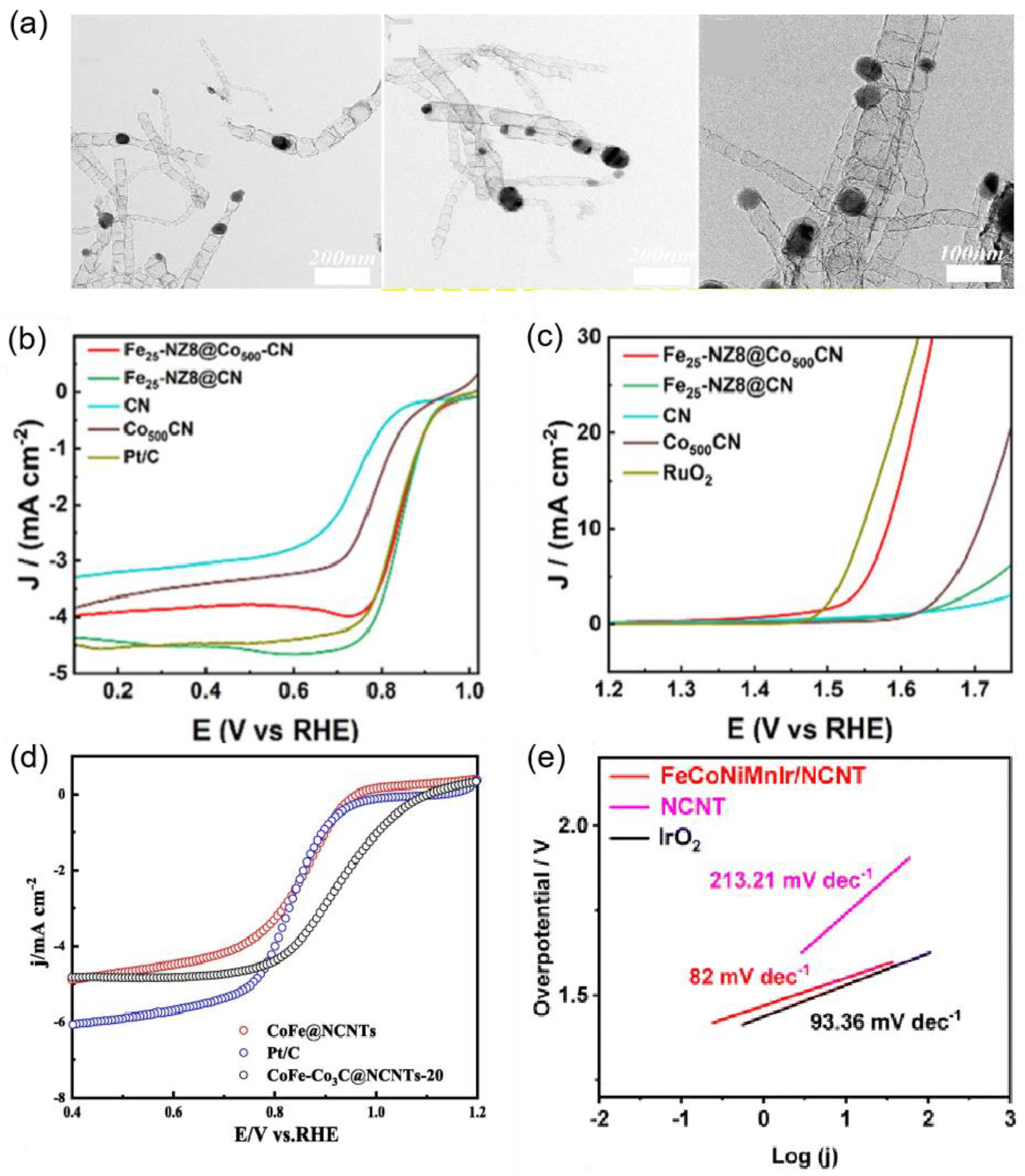
| Fe25−NZ8@Co500-CN | 350 mV | 82.2 | 0.1 M KOH | [70] |
| CoFe-Co3C@NCNTs-20 | 320 mV | 121.5 | 0.1 M KOH | [71] |
| Co@H-NCNT | 580 mV | 57.6 | 0.1 M KOH | [73] |
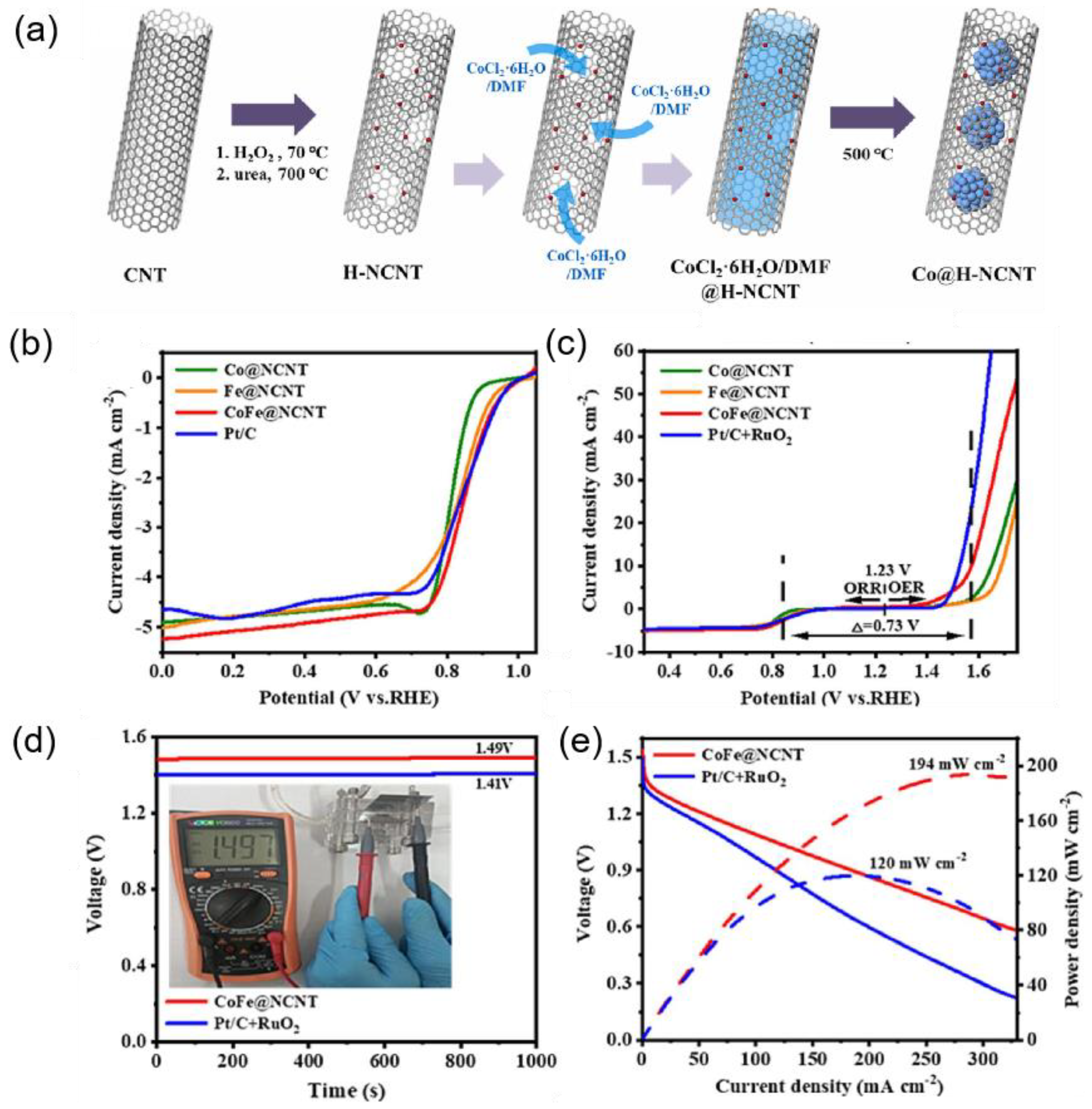
| CoFe@NCNT | 340 mV | 146.3 | 0.1 M KOH | [74] |
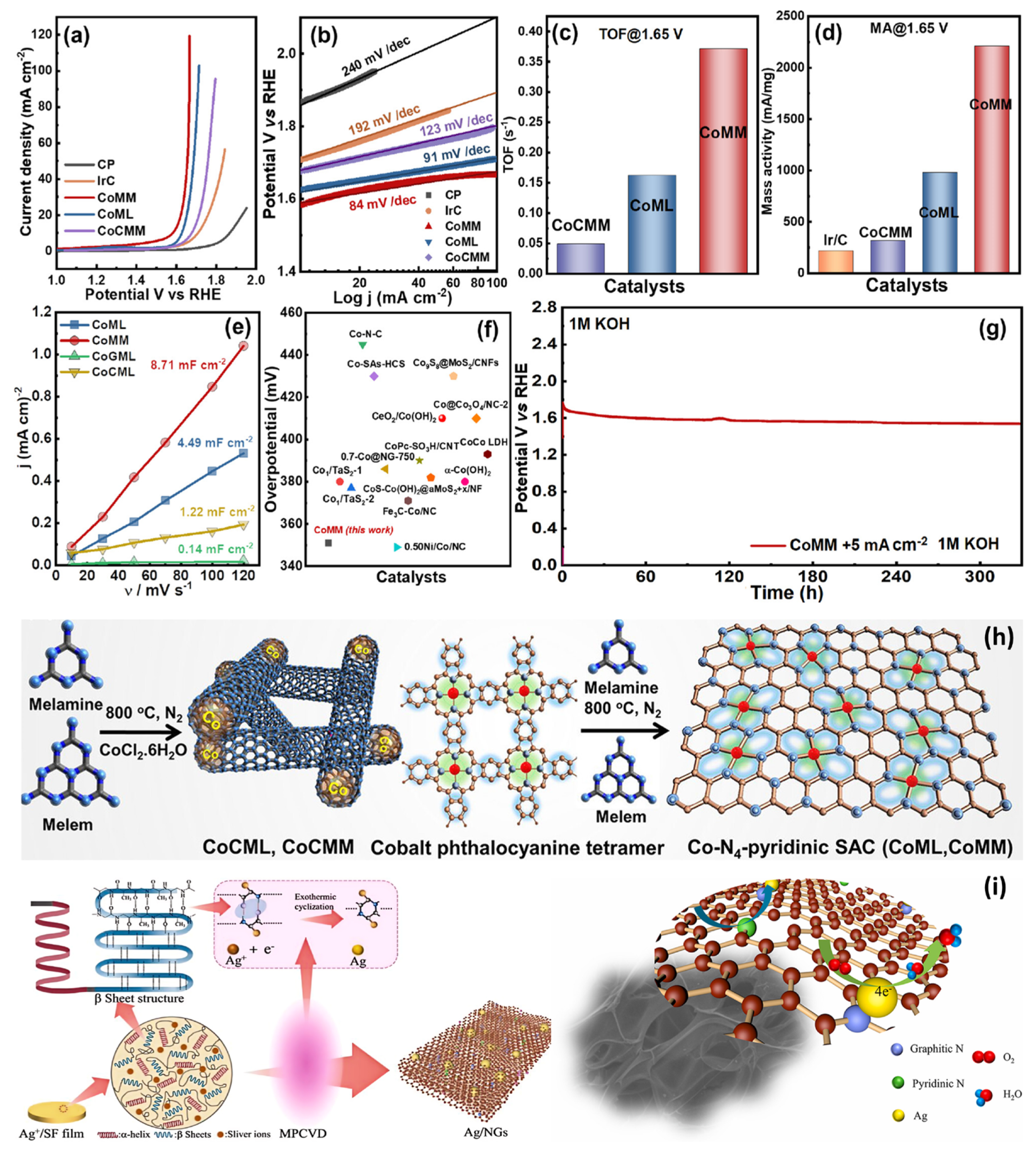
| CoMM | 351 mV | 1 M KOH | [96] | |
| NiFe NNG | 292 mV | 48.0 | 1 M KOH | [103] |
| Cd-MOF 1 | 279 mV | 85.1 | 0.1 M KOH | [108] |
| BIF-90 | 460 mV | 0.1 M KOH | [109] | |
| MOFs 4 | 381 mV | 75.1 | 0.1 M KOH | [110] |
| MOFs 5 | 397 mV | 74.4 | 0.1 M KOH | [110] |
| BMMOF11 | 400 mV | 85.0 | 0.1 M KOH | [111] |
| Co@C1N3C | 625 mV | 95.8 | 0.1 M KOH | [112] |
| Co/Co3Fe7@NCNTs-800 | 280 mV | 86.0 | 0.1 M KOH | [113] |
| CoFe/S-N-C | 1588 mV | 259.0 | 0.1 M KOH | [114] |
| Co/SP-NC | 85.0 | [115] | ||
| CNT@Co2-Fe1/FePc | 43.6 | 0.1 M KOH | [116] | |
| FeNi-rGO/FeNi/Ni foam | 234 mV | 76.0 | 0.1 M KOH | [117] |
| NiFL | 292 mV | 45.4 | 1 M KOH | [118] |
| GQDs-Co3O4 | 321 mV | 76.0 | 1 M KOH | [119] |
| ZFO-NG | 240 mV | 63.5 | 1 M KOH | [120] |
| Electrocatalyst | E1/2 (V vs. RHE) | Tafel Slope (mV dec−1) | Electrolyte | Reference |
|---|---|---|---|---|
| ORR | ORR | |||
| S,N-GQD/TiO2/C-800 | 820 mV | 61 | 0.1 M KOH | [28] |
| GP high N | 685 mV | 0.1 M KOH | [29] | |
| BN-CDs@CNT | 800 mV | 72 | 0.1 M KOH | [32] |
| 3%-N,P-PCNFs-900 | 720 mV | 91 | 0.1 M KOH | [33] |
| NiO-Mn2O3-CDs | 840 mV | 126 | 0.1 M KOH | [34] |
| Co/N-CNT | 850 mV | 72 | 0.1 M KOH | [64] |
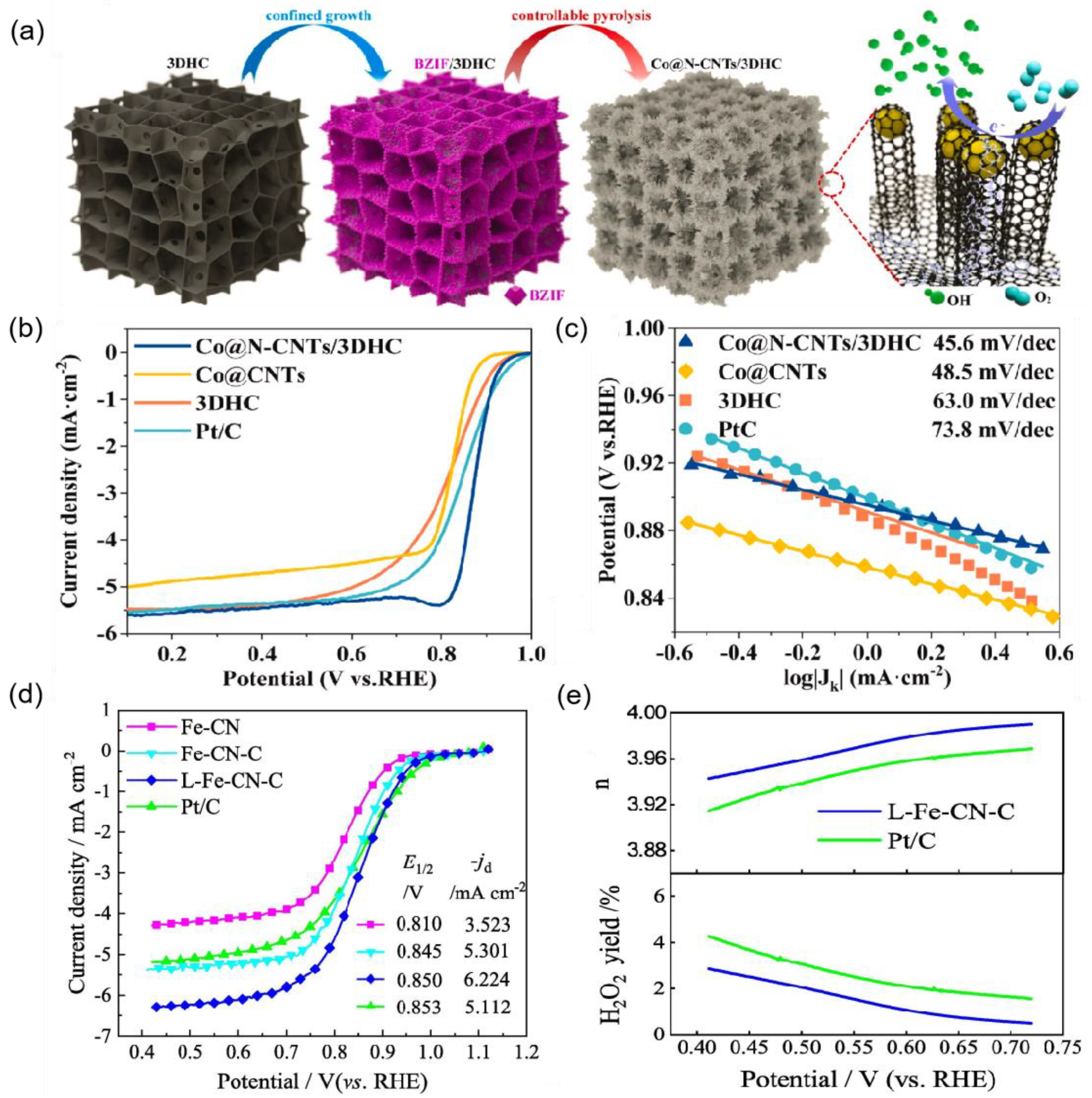
| Co@N-CNTs/3DHC | 880 mV | 45.6 | 0.1 M KOH | [67] |
| Fe25−NZ8@Co500-CN | 850 mV | 97.5 | 0.1 M KOH | [70] |
| CoFe-Co3C@NCNTs-20 | 934 mV | 66 | 1 M KOH | [71] |
| CoFe@NCNT | 840 mV | 90.3 | 1 M KOH | [74] |
| GFePc | 470 mV | pH=0 | [94] | |
| Vac-NiPt NPs/NG | 901mV | 58 | 0.1 M HClO4 | [95] |
| CoMM | 84 | 1 M KOH | [96] | |
| Ag-NGs | 820 mV | 75.15 | 0.1 M KOH | [97] |
| NT-3DFG | 830 mV | 55 | 0.1 M KOH | [99] |
| Ni-N4/GHSs/Fe-N4 | 830 mV | 81 | 0.1 M KOH | [102] |
| BIF-90 | 650 mV | 0.1 M KOH | [109] | |
| F, N-CD@Ag-0.1 | 900 mV | 69 | 0.1 M KOH | [121] |
| TOCNFs | 840 mV | 84 | 0.1 M KOH | [122] |
| Co@C1N3C | 840 mV | 0.1 M KOH | [112] | |
| Co/Co3Fe7@NCNTs-800 | 890 mV | 107 | 0.1 M KOH | [113] |
| Co@N4CNTs | 0.1 M KOH | [123] | ||
| CoFe/S-N-C | 855 mV | 102 | 0.1 M KOH | [114] |
| Co/SP-NC | 860.3 mV | 78 | 0.1 M KOH | [115] |
| Ni-N4/GHSs/Fe-N4 | 830 mV | 81 | 0.1 M KOH | [116] |
| Pt/TaON/GLC | 940 mV | 62 | 0.1 M KOH | [124] |
| Pd/GO | 810 mV | 56 | 0.1 M KOH | [125] |
| PNG | 800 mV | 0.1 M KOH | [126] | |
| N-rGO-CuSn | 0.1 M KOH | [127] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, Y.; Xin, Y.; Zhao, Q. Advanced Low-Dimensional Carbon Nanomaterials for Oxygen Electrocatalysis. Nanomaterials 2025, 15, 254. https://doi.org/10.3390/nano15040254
Yan Y, Xin Y, Zhao Q. Advanced Low-Dimensional Carbon Nanomaterials for Oxygen Electrocatalysis. Nanomaterials. 2025; 15(4):254. https://doi.org/10.3390/nano15040254
Chicago/Turabian StyleYan, Yue, Ying Xin, and Qingshan Zhao. 2025. "Advanced Low-Dimensional Carbon Nanomaterials for Oxygen Electrocatalysis" Nanomaterials 15, no. 4: 254. https://doi.org/10.3390/nano15040254
APA StyleYan, Y., Xin, Y., & Zhao, Q. (2025). Advanced Low-Dimensional Carbon Nanomaterials for Oxygen Electrocatalysis. Nanomaterials, 15(4), 254. https://doi.org/10.3390/nano15040254






