Advancements in Green Synthesis of Silver-Based Nanoparticles: Antimicrobial and Antifungal Properties in Various Films
Abstract
1. Introduction
2. Synthesis of Silver Nanoparticles
2.1. Chemical Methods
2.2. Physical Methods
2.3. Challenges Associated with Physical and Chemical Methods of Nanoparticle Synthesis
2.4. Biological Methods
2.4.1. Bacteria
2.4.2. Fungi
| Species | Scientific Names | Substrate | Substrate Conc. | Shape | Size (nm) | Wavelength (nm) | Technique Used | Applications | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Bacteria | Bacillus brevis | AgNO3 | 1 mM | Spherical | 41–68 | 420 | UV–Vis, TLC, FTIR, AFM, SEM | Antibacterial activity | [126] |
| Fungus | Fusarium scirpi | AgNO3 | - | Quasi-spherical | 2–20 | 200 and 900 | UV–Vis, XRD, STEM, HRTEM, EDX, TEM | Antimicrobial | [127] |
| Bacteria | Phenerochaete chrysosporium | AgNO3 | 1 mM | Spherical and oval shapes | 34–90 | 430 | UV–Vis, TEM, AFM, FTIR | Antibacterial activity | [128] |
| Algae | Enteromorpha fexuosa | AgNO3 | 1 mM | Circular | 2–32 | 430 | S, EDS, XRD, TEM | Antimicrobial activity | [129] |
| Algae | Botryococcus braunii | AgNO3 | 1 mM | Cubical and spherical | 40–90 | 490 | UV, FTIR, SEM, XRD | Antimicrobial | [130] |
| Fungus | Setosphaeria rostrata | AgNO3 | - | Spherical | 2–20 | 400 | UV–Vis, FTIR, SEM, TEM, EDAX, | Antibacterial | [131] |
| Bacteria | Bacillus siamensis | AgNO3 | 3 mM | Spherical | 25–50 | 400 to 450 | UV–Vis, FTIR, SEM, XRD, TEM | Antibacterial activity | [132] |
| Bacteria | Pseudoduganella eburnean | AgNO3 | 1 mM | Spherical | 8–24 | 448 | UV–Vis, XRD, FTIR, TEM | Antimicrobial activity | [133] |
| Algae | Noctiluca scintillans | AgNO3 | 0.1 M | Spherical | 4.5 | 436 | UV–Vis, SEM, DLS, HRTEM | Antibacterial | [134] |
| Fungus | Penicillium oxalicum | AgNO3 | 1 mm | Spherical | 60–80 | 600 | UV–Vis XRD SEM | Antibacterial | [135] |
| Bacteria | Bacillus sp. | AgNO3 | 1 mM | Spherical | 22–41 | 447 | UV–Vis, FTIR, XRD, SEM, TEM, EDX | Antifungal activity | [136] |
| Fungus | Aspergillus brunneoviolaceus | AgNO3 | 10 mM | Spherical | 0.72–15.21 | 411 | UV–Vis, FTIR, TEM, XRD | Antibacterial and antioxidative activity | [137] |
| Algae | Enteromorpha compressa | AgNO3 | 1 mM | Spherical | 4–24 | 421 | S, HR-TEM, EDS | Cytotoxic, antifungal, and antibacterial properties Biomedical characteristics | [138] |
| Fungus | Penicillium verrucosum | AgNO3 | - | Spherical | 10–12 | 420 | UV–Vis, TEM, SEM, XRD | Antifungal | [139] |
| Fungus | Trichoderma harzianum | AgNO3 | 1 mM | Spherical | 31.13 | 430 | UV–Vis, TEM | Antifungal | [140] |
| Bacteria | Streptomyces sp. | AgNO3 | - | Spherical | 10–30 | - | - | Antibacterial activity | [141] |
| Algae | Chlorella vulgaris | AgNO3 | 1 mM | Spherical | 55 | 410–450 | UV–Vis FTIR, XRD | Photocatalytic dye degradation activity | [142] |
| Fungus | Talaromyces purpureogenus | AgNO3 | - | Spherical | 5–70 | 450 | UV–Vis, FTIR, FEG-SEM, HRTEM, XRD | Antifungal | [143] |
| Bacteria | Bacillus cereus | AgNO3 | - | Spherical | 20–40 | 425 | UV–Vis, XRD, FTIR, SEM, | Antibacterial activity and Antioxidant | [144] |
| Algae | Hypnea musciformis | AgNO3 | 1 mM | Spherical | 40–65 | 420 | S, FTIR, SEM, EDX, XRD | Larvicidal activity | [145] |
| Bacteria | Lactobacillus acidophilus | AgNO3 | Spherical | 10–20 | UV–Vis, XRD, SEM, TEM | Antimicrobial activity and antioxidant | [146] | ||
| Fungus | Arthroderma fulvum | AgNO3 | 1.5 mM | Spherical | 15.5 ± 2.5 | 420 | UV–Vis, XRD, TEM | Antifungal against Candida, Fusarium spp., and Aspergillus spp. | [147] |
| Fungus | Penicillium citrinum | AgNO3 | 1 mM | Spherical | 109 | 400–420 | FTIR, photon correlation spectroscopy (PCS), SEM, fluorescence spectroscopy, UV–Vis | Amide linkage groups were present in the fungal extract | [148] |
| Fungus | Trichoderma asperellum | AgNO3 | 1 mM | - | 13–18 | 410 | UV–Vis, FTIR, TEM, XRD, SERS | For six months, the AgNPs that were produced were quite stable | [149] |
| Fungus | Aspergillus clavatus | AgNO3 | 1 mM | Spherical or hexagonal | 10 to 25 | 415 | UV–Vis, FTIR, XRD, TEM, AFM | Antimicrobial against Escherichia coli, Pseudomonas fluorescens, and Candida albicans | [150] |
| Fungus | Aspergillus terreus | AgNO3 | 10 mM | Spherical | 1 to 20 | 440 | XRD, TEM, UV–Vis | Antifungal and antibacterial | [151] |
| Bacteria | Psychrophilic | AgNO3 | 1 mM | Spherical | 6 to 13 | 400–430 | UV–Vis spectroscopy, transmission electron microscopy, atomic force microscopy | Stable for 8 months in the dark | [152] |
| Bacteria | Pantoea ananatis | AgNO3 | 0.1 mM | Spherical | 8.06 to 91.32 | 421 | UV–Vis, TEM, SEM, FTIR, zeta potential | Antimicrobial for microorganisms that are resistant to multiple drugs | [153] |
| Bacteria | Klebsiella pneumonia | AgNO3 | 1 mM | - | 3 | - | XRD, UV–Vis, TEM, EDS | [154] | |
| Fungus | Candida glabrata | AgNO3 | 1 mM | Spherical | 2 to 15 | 460.64 | FTIR, UV–Vis, TEM | Antimicrobial activity against bacterial and fungal clinical strains | [155] |
| Fungus | Trichoderma viride | AgNO3 | 10 mM | Globular | 1 to 50 | 350–450 | UV–Vis, TEM, SEM | Antibacterial activity against human pathogenic bacteria | [156] |
| Fungus | Aspergillus niger | AgNO3 | 10 mM | Spherical | 1 to 20 | 440 | UV–Vis, XRD, TEM | Antimicrobial activity | [157] |
| Algae | Chaetomorpha ligustica | AgNO3 | 5 mM | Spherical | 2–12 | 420 | FTIR, GC-MS, UV–Vis, TEM, | Anticancer | [158] |
| Algae | Sargassum muticum | AgNO3 | 1 mM | Spherical | 43–79 | 420 | S, FTIR, SEM, EDS, XRD | Antibacterial and insecticidal activity | [159,160] |
2.4.3. Plants
3. Characterization of Silver Nanoparticles
3.1. Ultraviolet–Visible (UV–Vis) Spectroscopy
3.2. Fourier Transform Infrared (FT-IR)
3.3. Scanning Electron Microscopy
3.4. Transmission Electron Microscopy
3.5. X-Ray Diffraction (XRD)
3.6. DPPH Radical Scavenging Activity
3.7. Dynamic Light Scattering (DLS)
3.8. X-Ray Photoelectron Spectroscopy (XPS)
3.9. Atomic Force Microscopy (AFM)
3.10. Surface Plasmon Resonance (SPR)
3.11. Zeta Potential Analysis
4. Applications of AgNPs in Food Packaging
4.1. Antimicrobial Packaging Materials
4.2. Edible Coatings
4.3. Polymer Matrices
5. Release of AgNPs from Film to Food
6. Cytotoxicity of AgNPs
7. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hashmi, S.S.; Ibrahim, M.; Adnan, M.; Ullah, A.; Khan, M.N.; Kamal, A.; Zaman, W. Green synthesis of silver nanoparticles from Olea europaea L. extracted polysaccharides, characterization, and its assessment as an antimicrobial agent against multiple pathogenic microbes. Open Chem. 2024, 22, 20240016. [Google Scholar] [CrossRef]
- Ullah, R.; Bibi, S.; Khan, M.N.; Al Mohaimeed, A.M.; Naz, Q.; Kamal, A. Application of bio-inspired gold nanoparticles as advanced nanomaterial in halting nociceptive pathways and hepatotoxicity via triggering the antioxidation system. Catalysts 2023, 13, 786. [Google Scholar] [CrossRef]
- Ul Haq, T.; Ullah, R.; Khan, M.N.; Nazish, M.; Almutairi, S.M.; Rasheed, R.A. Seed priming with glutamic-acid-functionalized iron nanoparticles modulates response of Vigna radiata (L.) R. Wilczek (Mung bean) to osmotic stress. Micromachines 2023, 14, 736. [Google Scholar] [CrossRef] [PubMed]
- Parikh, R.Y.; Singh, S.; Prasad, B.L.V.; Patole, M.S.; Sastry, M.; Shouche, Y.S. Extracellular synthesis of crystalline silver nanoparticles and molecular evidence of silver resistance from Morganella sp.: Towards understanding biochemical synthesis mechanism. ChemBioChem 2008, 9, 1415–1422. [Google Scholar] [CrossRef]
- Maaz, K. (Ed.) Silver Nanoparticles: Fabrication, Characterization, and Applications; IntechOpen: London, UK, 2023. [Google Scholar]
- Goswami, L.; Kim, K.H.; Deep, A.; Das, P.; Bhattacharya, S.S.; Kumar, S.; Adelodun, A.A. Engineered nanoparticles: Nature, behavior, and effects on the environment. J. Environ. Manag. 2017, 196, 297–315. [Google Scholar] [CrossRef]
- Ahamed, M.; AlSalhi, M.S.; Siddiqui, M.K.J. Silver nanoparticle applications and human health. Clin. Chim. Acta 2010, 411, 1841–1848. [Google Scholar] [CrossRef]
- Fahimirad, S.; Ajalloueian, F.; Ghorbanpour, M. Synthesis and therapeutic potential of silver nanomaterials derived from plant extracts. Ecotoxicol. Environ. Saf. 2019, 168, 260–278. [Google Scholar] [CrossRef]
- Menazea, A.A. Femtosecond laser ablation-assisted synthesis of silver nanoparticles in organic and inorganic liquid media and their antibacterial efficiency. Radiat. Phys. Chem. 2020, 168, 108616. [Google Scholar] [CrossRef]
- Salleh, A.; Naomi, R.; Utami, N.D.; Mohammad, A.W.; Mahmoudi, E.; Mustafa, N.; Fauzi, M.B. The potential of silver nanoparticles for antiviral and antibacterial applications: A mechanism of action. Nanomaterials 2020, 10, 1566. [Google Scholar] [CrossRef]
- Zhang, P.; Wyman, I.; Hu, J.; Lin, S.; Zhong, Z.; Tu, Y. Silver nanowires: Synthesis technologies, growth mechanism, and multifunctional applications. Mater. Sci. Eng. 2017, 1, 1–23. [Google Scholar] [CrossRef]
- Mcgillicuddy, E.; Murray, I.; Kavanagh, S.; Morrison, L.; Fogarty, A.; Cormican, M.; Dockery, P.; Prendergast, M.; Rowan, N.; Morris, D. Silver nanoparticles in the environment: Sources, detection, and ecotoxicology. Sci. Total Environ. 2017, 575, 231–246. [Google Scholar] [CrossRef]
- Duran, N.; Marcato, D.P.; Alves, L.O.; De Souza, G.; Esposito, E. Mechanical aspects of biosynthesis of silver nanoparticles by several Fusarium oxysporum strains. J. Nanobiotechnol. 2005, 3, 8. [Google Scholar] [CrossRef]
- Marcato, P.D.; Conti, M.R.; Alves, O.L.; Costa, F.T.M.; Brocchi, M.; Durán, N. Potential use of silver nanoparticles on pathogenic bacteria, their toxicity, and possible mechanisms of action. J. Braz. Chem. Soc. 2010, 21, 6949–6959. [Google Scholar]
- Zahra, Q.; Fraz, A.; Anwar, A.; Awais, M.; Abbas, M.A. Mini review on the synthesis of Ag-nanoparticles by chemical reduction method and their biomedical applications. NUST J. Eng. Sci. 2016, 9, 1–7. [Google Scholar]
- Arole, V.M.; Munde, S.V. Fabrication of nanomaterials by top-down and bottom-up approaches—An overview. J. Mater. Sci. 2014, 1, 89–93. [Google Scholar]
- Iqbal, P.; Preece, J.A.; Mendes, P.M. Nanotechnology: The “top-down” and “bottom-up” approaches. Supramol. Chem. Mol. Nanomater. 2012, 1, 195. [Google Scholar]
- Fu, X.; Cai, J.; Zhang, X.; Li, W.D.; Ge, H.; Hu, Y. Top-down fabrication of shape-controlled, monodisperse nanoparticles for biomedical applications. Adv. Drug Deliv. Rev. 2018, 132, 169–187. [Google Scholar] [CrossRef] [PubMed]
- Abid, N.; Khan, A.M.; Shujait, S.; Chaudhary, K.; Ikram, M.; Imran, M.; Maqbool, M. Synthesis of nanomaterials using various top-down and bottom-up approaches, influencing factors, advantages, and disadvantages: A review. Adv. Colloid Interface Sci. 2022, 300, 102597. [Google Scholar] [CrossRef]
- Parashar, M.; Shukla, V.K.; Singh, R. Metal oxides nanoparticles via sol–gel method: A review on synthesis, characterization, and applications. J. Mater. Sci. Mater. Electron. 2020, 31, 3729–3749. [Google Scholar] [CrossRef]
- Quintero-Quiroz, C.; Acevedo, N.; Zapata-Giraldo, J.; Botero, L.E.; Quintero, J.; Zárate-Triviño, D.; Pérez, V.Z. Optimization of silver nanoparticle synthesis by chemical reduction and evaluation of its antimicrobial and toxic activity. Biomater. Res. 2019, 23, 27. [Google Scholar] [CrossRef]
- Qin, B.; Ma, H.; Hossain, M.; Zhong, M.; Xia, Q.; Li, B.; Duan, X. Substrates in the synthesis of two-dimensional materials via chemical vapor deposition. Chem. Mater. 2020, 32, 10321–10347. [Google Scholar] [CrossRef]
- Ma, X.; Chen, Y.; Qian, J.; Yuan, Y.; Liu, C. Controllable synthesis of spherical hydroxyapatite nanoparticles using inverse microemulsion method. Mater. Chem. Phys. 2016, 183, 220–229. [Google Scholar] [CrossRef]
- Peng, Z.; Yu, Z.; Wang, L.; Hou, Y.; Shi, Y.; Wu, L.; Li, Z. Facile synthesis of Pd–Fe nanoparticles modified Ni foam electrode and its behaviors in electrochemical reduction of tetrabromobisphenol A. Mater. Lett. 2016, 166, 300–303. [Google Scholar] [CrossRef]
- Chen, Z.; Balankura, T.; Fichthorn, K.A.; Rioux, R.M. Revisiting the polyol synthesis of silver nanostructures: Role of chloride in nanocube formation. ACS Nano 2019, 13, 1849–1860. [Google Scholar] [CrossRef]
- Lee, S.H.; Jun, B.H. Silver nanoparticles: Synthesis and application for nanomedicine. Int. J. Mol. Sci. 2019, 20, 865. [Google Scholar] [CrossRef]
- Zahoor, M.; Nazir, N.; Iftikhar, M.; Naz, S.; Zekker, I.; Burlakovs, J.; Ali Khan, F. A review on silver nanoparticles: Classification, various methods of synthesis, and their potential roles in biomedical applications and water treatment. Water 2021, 13, 2216. [Google Scholar] [CrossRef]
- Abbasi, E.; Milani, M.; Fekri Aval, S.; Kouhi, M.; Akbarzadeh, A.; Tayefi Nasrabadi, H.; Samiei, M. Silver nanoparticles: Synthesis methods, bio-applications and properties. Crit. Rev. Microbiol. 2016, 42, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Chugh, D.; Viswamalya, V.S.; Das, B. Green synthesis of silver nanoparticles with algae and the importance of capping agents in the process. J. Genet. Eng. Biotechnol. 2021, 19, 126. [Google Scholar] [CrossRef] [PubMed]
- Iravani, S.; Korbekandi, H.; Mirmohammadi, S.V.; Zolfaghari, B. Synthesis of Silver Nanoparticles: Chemical, Physical and Biological Methods. Res. Pharm. Sci. 2014, 9, 385–406. [Google Scholar] [PubMed]
- Alahmad, A.; Eleoui, M.; Falah, A.; Alghoraibi, I. Preparation of Colloidal Silver Nanoparticles and Structural Characterization. Phys. Sci. Res. Int. 2013, 1, 89–96. [Google Scholar]
- Shahjahan, M.; Rahman, M.H.; Hossain, M.S.; Khatun, M.A.; Islam, A.; Begum, M.A. Synthesis and Characterization of Silver Nanoparticles by Sol-Gel Technique. Nanosci. Nanometrol. 2017, 3, 34–39. [Google Scholar] [CrossRef]
- Nakano, M.; Fujiwara, T.; Koga, N. Thermal Decomposition of Silver Acetate: Physico-Geometrical Kinetic Features and Formation of Silver Nanoparticles. J. Phys. Chem. C 2016, 120, 8841–8854. [Google Scholar] [CrossRef]
- Verma, P.; Maheshwari, S.K. Applications of silver nanoparticles in diverse sectors. Int. J. Nano Dimens. 2019, 10, 18–36. [Google Scholar]
- Zehra, S.M.; Bibi, M.; Mahmood, A.; Khattak, A.; Asad, M.Z.; Zehra, S.H. Phenol–Furfural Resin/Graphite/Ag-Based Electrically Conductive Adhesive Composites from Waste Bagasse with Enhanced Thermo-Electric Properties. Polymers 2023, 15, 3283. [Google Scholar] [CrossRef]
- Yaqoob, A.A.; Umar, K.; Ibrahim, M.N.M. Silver nanoparticles: Various methods of synthesis, size affecting factors and their potential applications—A review. Appl. Nanosci. 2020, 10, 1369–1378. [Google Scholar] [CrossRef]
- Xu, L.; Wang, Y.Y.; Huang, J.; Chen, C.Y.; Wang, Z.X.; Xie, H. Silver nanoparticles: Synthesis, medical applications and biosafety. Theranostics 2020, 10, 8996. [Google Scholar] [CrossRef]
- Ismail, R.A.; Sulaiman, G.M.; Mohsin, M.H.; Saadoon, A.H. Preparation of silver iodide nanoparticles using laser ablation in liquid for antibacterial applications. IET Nanobiotechnol. 2018, 12, 781–786. [Google Scholar] [CrossRef] [PubMed]
- Scuderi, M.; Esposito, M.; Todisco, F.; Simeone, D.; Tarantini, I.; De Marco, L.; Cuscuna, M. Nanoscale study of the tarnishing process in electron beam lithography-fabricated silver nanoparticles for plasmonic applications. J. Phys. Chem. C 2016, 120, 24314–24323. [Google Scholar] [CrossRef]
- Amiruddin, E.; Prayitno, A. The synthesis of magnetic nanoparticles from natural iron sand of Kata beach Pariaman West Sumatera using ball milling method as environmental material. MATEC Web Conf. 2019, 276, 06014. [Google Scholar] [CrossRef]
- Wang, F.; Hong, R. Continuous preparation of structure-controlled carbon nanoparticle via arc plasma and the reinforcement of polymeric composites. Chem. Eng. J. 2017, 328, 1098–1111. [Google Scholar] [CrossRef]
- Liu, Y.C.; Chang, Y.H.; Lin, Y.H.; Liou, C.C.; Kuo, T.R. High-performance sample substrate of gold nanoparticle multilayers for surface-assisted laser desorption/ionization mass spectrometry. Nanomaterials 2019, 9, 1078. [Google Scholar] [CrossRef] [PubMed]
- Phakatkar, A.H.; Saray, M.T.; Rasul, M.G.; Sorokina, L.V.; Ritter, T.G.; Shokuhfar, T.; Shahbazian-Yassar, R. Ultrafast synthesis of high entropy oxide nanoparticles by flame spray pyrolysis. Langmuir 2021, 37, 9059–9068. [Google Scholar] [CrossRef]
- Freitas de Freitas, L.; Varca, G.H.C.; dos Santos Batista, J.G.; Benévolo Lugão, A. An overview of the synthesis of gold nanoparticles using radiation technologies. Nanomaterials 2018, 8, 939. [Google Scholar] [CrossRef] [PubMed]
- Zakaria, M.A.; Menazea, A.A.; Mostafa, A.M.; Al-Ashkar, E.A. Ultra-Thin Silver Nanoparticles Film Prepared via Pulsed Laser Deposition: Synthesis, Characterization, and Its Catalytic Activity on Reduction of 4-Nitrophenol. Surf. Interfaces 2020, 19, 100438. [Google Scholar] [CrossRef]
- Deng, L.; Nguyen, M.T.; Mei, S.; Tokunaga, T.; Kudo, M.; Matsumura, S.; Yonezawa, T. Preparation and Growth Mechanism of Pt/Cu Alloy Nanoparticles by Sputter Deposition onto a Liquid Polymer. Langmuir 2019, 35, 8418–8427. [Google Scholar] [CrossRef] [PubMed]
- Al-Hossainy, A.F.; Ibrahim, A.; Zoromba, M.S. Synthesis and Characterization of Mixed Metal Oxide Nanoparticles Derived from Co–Cr Layered Double Hydroxides and Their Thin Films. J. Mater. Sci. Mater. Electron. 2019, 30, 11627–11642. [Google Scholar] [CrossRef]
- Nagasawa, T.; Matsumoto, K.; Minegishi, N.; Kosaka, H. Structural Characterization of Ceria-Supported Pt Nanoparticles by Flame-Assisted Spray Pyrolysis Using a Burner Diffusion Flame. Energy Fuels 2021, 35, 12380–12391. [Google Scholar] [CrossRef]
- Benzaoui, K.; Ales, A.; Mekki, A.; Zaoui, A.; Bouhemadou, A.; Bouaouina, B.; Benyoubi, F. Study of the Substrate Surface Treatment of Flexible Polypyrrole-Silver Composite Films on EMI Shielding Effectiveness: Theoretical and Experimental Investigation. Frequenz 2022, 76, 479–494. [Google Scholar] [CrossRef]
- Amendola, V.; Meneghetti, M. Laser Ablation Synthesis in Solution and Size Manipulation of Noble Metal Nanoparticles. Phys. Chem. Chem. Phys. 2009, 11, 3805–3821. [Google Scholar] [CrossRef] [PubMed]
- Khayati, G.R.; Janghorban, K. The Nanostructure Evolution of Ag Powder Synthesized by High Energy Ball Milling. Adv. Powder Technol. 2021, 23, 393–397. [Google Scholar] [CrossRef]
- Stagon, S.P.; Huang, H. Syntheses and Applications of Small Metallic Nanorods from Solution and Physical Vapor Deposition. Nanotechnol. Rev. 2013, 2, 259–267. [Google Scholar] [CrossRef]
- Dhand, C.; Dwivedi, N.; Loh, X.J.; Ying, A.N.J.; Verma, N.K.; Beuerman, R.W.; Ramakrishna, S. Methods and Strategies for the Synthesis of Diverse Nanoparticles and Their Applications: A Comprehensive Overview. RSC Adv. 2015, 5, 105003–105037. [Google Scholar] [CrossRef]
- Tien, D.C.; Liao, C.Y.; Huang, J.C.; Tseng, K.H.; Lung, J.K.; Tsung, T.T.; Stobinski, L. Novel Technique for Preparing a Nano-Silver Water Suspension by the Arc-Discharge Method. Rev. Adv. Mater. Sci. 2008, 18, 752–758. [Google Scholar]
- Zhaleh, M.; Zangeneh, A.; Goorani, S.; Seydi, N.; Zangeneh, M.M.; Tahvilian, R.; Pirabbasi, E. In vitro and in vivo evaluation of cytotoxicity, antioxidant, antibacterial, antifungal, and cutaneous wound healing properties of gold nanoparticles produced via a green chemistry synthesis using Gundelia tournefortii L. as a capping and reducing agent. Appl. Organomet. Chem. 2019, 33, e5015. [Google Scholar] [CrossRef]
- Javed, B.; Ikram, M.; Farooq, F.; Sultana, T.; Mashwani, Z.U.R.; Raja, N.I. Biogenesis of silver nanoparticles to treat cancer, diabetes, and microbial infections: A mechanistic overview. Appl. Microbiol. Biotechnol. 2021, 105, 2261–2275. [Google Scholar] [CrossRef]
- Islam, M.A.; Jacob, M.V.; Antunes, E. A Critical Review on Silver Nanoparticles: From Synthesis and Applications to Its Mitigation Through Low-Cost Adsorption by Biochar. J. Environ. Manag. 2021, 281, 111918. [Google Scholar] [CrossRef]
- Punjabi, K.; Choudhary, P.; Samant, L.; Mukherjee, S.; Vaidya, S.; Chowdhary, A. Biosynthesis of Nanoparticles: A Review. Int. J. Pharm. Sci. Rev. Res. 2015, 30, 219–226. [Google Scholar]
- Gudikandula, K.; Charya Maringanti, S. Synthesis of Silver Nanoparticles by Chemical and Biological Methods and Their Antimicrobial Properties. J. Exp. Nanosci. 2016, 11, 714–721. [Google Scholar] [CrossRef]
- Alabdallah, N.M.; Hasan, M.M. Plant-Based Green Synthesis of Silver Nanoparticles and Its Effective Role in Abiotic Stress Tolerance in Crop Plants. Saudi J. Biol. Sci. 2021, 28, 5631–5639. [Google Scholar] [CrossRef]
- Tariq, M.; Mohammad, K.N.; Ahmed, B.; Siddiqui, M.A.; Lee, J. Biological Synthesis of Silver Nanoparticles and Prospects in Plant Disease Management. Molecules 2022, 27, 4754. [Google Scholar] [CrossRef]
- Martínez-Castañon, G.A.; Nino-Martinez, N.; Martinez-Gutierrez, F.; Martínez-Mendoza, J.R.; Ruiz, F. Synthesis and Antibacterial Activity of Silver Nanoparticles with Different Sizes. J. Nanopart. Res. 2008, 10, 1343–1348. [Google Scholar] [CrossRef]
- Panáček, A.; Kvítek, L.; Prucek, R.; Kolář, M.; Večeřová, R.; Pizúrová, N.; Zbořil, R. Silver Colloid Nanoparticles: Synthesis, Characterization, and Their Antibacterial Activity. J. Phys. Chem. B 2006, 110, 16248–16253. [Google Scholar] [CrossRef] [PubMed]
- Pal, S.; Tak, Y.K.; Song, J.M. Does the Antibacterial Activity of Silver Nanoparticles Depend on the Shape of the Nanoparticle? A Study of the Gram-Negative Bacterium Escherichia coli. Appl. Environ. Microbiol. 2007, 73, 1712–1720. [Google Scholar] [CrossRef]
- Bhattacharya, D.; Gupta, R.K. Nanotechnology and potential of microorganisms. Crit. Rev. Biotechnol. 2005, 25, 199–204. [Google Scholar] [CrossRef]
- Mandal, D.; Bolander, M.E.; Mukhopadhyay, D.; Sarkar, G.; Mukherjee, P. The use of microorganisms for the formation of metal nanoparticles and their application. Appl. Microbiol. Biotechnol. 2006, 69, 485–492. [Google Scholar] [CrossRef]
- Mohanpuria, P.; Rana, N.K.; Yadav, S.K. Biosynthesis of nanoparticles: Technological concepts and future applications. J. Nanopart. Res. 2008, 10, 507–517. [Google Scholar] [CrossRef]
- Gericke, M.; Pinches, A. Microbial production of gold nanoparticles. Gold Bull. 2006, 39, 22–28. [Google Scholar] [CrossRef]
- Harris, A.T.; Bali, R. On the formation and extent of uptake of silver nanoparticles by live plants. J. Nanopart. Res. 2008, 10, 691–695. [Google Scholar] [CrossRef]
- Sastry, M.; Ahmad, A.; Khan, M.I.; Kumar, R. Biosynthesis of metal nanoparticles using fungi and actinomycete. Curr. Sci. 2003, 85, 162–170. [Google Scholar]
- Narayanan, K.B.; Sakthivel, N. Biological synthesis of metal nanoparticles by microbes. Adv. Colloid Interface Sci. 2010, 156, 1–13. [Google Scholar] [CrossRef]
- Kaviya, S.; Santhanalakshmi, J.; Viswanathan, B.; Muthumary, J.; Srinivasan, K. Bio-synthesis of silver nanoparticles using Citrus sinensis peel extract and its antibacterial activity. Spectrochim. Acta A 2011, 79, 594–598. [Google Scholar] [CrossRef]
- Senapati, S.; Mandal, D.; Ahmad, A.; Khan, M.I.; Sastry, M.; Kumar, R. Fungus-mediated synthesis of silver nanoparticles: A novel biological approach. Indian J. Phys. 2004, 78, 101–105. [Google Scholar]
- Rai, M.; Yadav, A.; Gade, A. Current trends in phytosynthesis of metal nanoparticles. Crit. Rev. Biotechnol. 2008, 28, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Jain, D.; Daima, H.K.; Kachhwaha, S.; Kothari, S.L. Synthesis of plant-mediated silver nanoparticles using papaya fruit extract and evaluation of their antimicrobial activities. Dig. J. Nanomater. Bios. 2009, 4, 557–563. [Google Scholar]
- Saifuddin, N.; Wong, C.W.; Yasumira, A.N. Rapid biosynthesis of silver nanoparticles using culture supernatant of bacteria with microwave irradiation. J. Chem. 2009, 6, 61–70. [Google Scholar] [CrossRef]
- Verma, V.C.; Kharwar, R.N.; Gange, A.C. Biosynthesis of antimicrobial silver nanoparticles by the endophytic fungus Aspergillus clavatus. Nanomedicine 2010, 5, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Willner, I.; Basnar, B.; Willner, B. Nanoparticle–enzyme hybrid systems for nanobiotechnology. FEBS J. 2007, 274, 302–309. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Jain, D.; Upadhyay, M.K.; Khandelwal, N.; Verma, H.N. Green synthesis of silver nanoparticles using Argemone mexicana leaf extract and evaluation of their antimicrobial activities. Dig. J. Nanomater. Bios. 2010, 5, 483–489. [Google Scholar]
- Kumar, V.; Yadav, S.K. Plant-mediated synthesis of silver and gold nanoparticles and their applications. J. Chem. Technol. Biotechnol. 2009, 84, 151–157. [Google Scholar] [CrossRef]
- Sathyavathi, R.; Krishna, M.B.; Rao, S.V.; Saritha, R.; Rao, D.N. Biosynthesis of silver nanoparticles using Coriandrum sativum leaf extract and their application in nonlinear optics. Adv. Sci. Lett. 2010, 3, 138–143. [Google Scholar] [CrossRef]
- Bar, H.; Bhui, D.K.; Sahoo, G.P.; Sarkar, P.; De, S.P.; Misra, A. Green synthesis of silver nanoparticles using latex of Jatropha curcas. Colloids Surf. A 2009, 339, 134–139. [Google Scholar] [CrossRef]
- Jha, A.K.; Prasad, K. Green synthesis of silver nanoparticles using Cycas leaf. Int. J. Green Nanotechnol. Phys. Chem. 2010, 1, P110–P117. [Google Scholar] [CrossRef]
- Vinodhini, S.; Vithiya, B.S.M.; Prasad, T.A.A. Green synthesis of silver nanoparticles by employing Allium fistulosum, Tabernaemontana divaricata, and Basella alba leaf extracts for antimicrobial applications. J. King Saud Univ. Sci. 2022, 34, 101939. [Google Scholar] [CrossRef]
- Raj, S.; Mali, S.C.; Trivedi, R. Green synthesis and characterization of silver nanoparticles using Enicostemma axillare (Lam.) leaf extract. Biochem. Biophys. Res. Commun. 2018, 503, 2814–2819. [Google Scholar] [CrossRef] [PubMed]
- Githala, C.K.; Raj, S.; Dhaka, A.; Mali, S.C.; Trivedi, R. Phyto-fabrication of silver nanoparticles and their catalytic dye degradation and antifungal efficacy. Front. Chem. 2022, 10, 994721. [Google Scholar] [CrossRef]
- Dhaka, A.; Raj, S.; Githala, C.K.; Chand Mali, S.; Trivedi, R. Balanites aegyptiaca leaf extract-mediated synthesis of silver nanoparticles and their catalytic dye degradation and antifungal efficacy. Front. Bioeng. Biotechnol. 2022, 10, 977101. [Google Scholar] [CrossRef] [PubMed]
- Pungle, R.; Nile, S.H.; Makwana, N.; Singh, R.; Singh, R.P.; Kharat, A.S. Green synthesis of silver nanoparticles using the Tridax procumbens plant extract and screening of their antimicrobial and anticancer activities. Oxidative Med. Cell. Longev. 2022, 2022, 9671594. [Google Scholar] [CrossRef]
- Hawar, S.N.; Al-Shmgani, H.S.; Al-Kubaisi, Z.A.; Sulaiman, G.M.; Dewir, Y.H.; Rikisahedew, J.J. Green synthesis of silver nanoparticles from Alhagi graecorum leaf extract and evaluation of their cytotoxicity and antifungal activity. J. Nanomater. 2022, 2022, 1058119. [Google Scholar] [CrossRef]
- Melkamu, W.W.; Bitew, L.T. Green synthesis of silver nanoparticles using Hagenia abyssinica (Bruce) J.F. Gmel plant leaf extract and their antibacterial and antioxidant activities. Heliyon 2021, 7, e08153. [Google Scholar] [CrossRef]
- Mali, S.C.; Raj, S.; Trivedi, R. Nanotechnology: A novel approach to enhance crop productivity. Biochem. Biophys. Rep. 2020, 24, 100821. [Google Scholar]
- Mali, S.C.; Dhaka, A.; Sharma, S.; Trivedi, R. Review on biogenic synthesis of copper nanoparticles and its potential applications. Inorg. Chem. Commun. 2023, 149, 110448. [Google Scholar] [CrossRef]
- Kalimuthu, K.; Babu, R.S.; Venkataraman, D.; Bilal, M.; Gurunathan, S. Biosynthesis of silver nanocrystals by Bacillus licheniformis. Colloids Surf. B 2008, 65, 150–153. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X. Application of microorganisms in biosynthesis nanomaterials: A review. Wei Sheng Wu Xue Bao 2011, 51, 297–304. [Google Scholar] [PubMed]
- Yang, Y.; Waterhouse, G.I.; Chen, Y.; Sun-Waterhouse, D.; Li, D. Microbial-Enabled Green Biosynthesis of Nanomaterials: Current Status and Future Prospects. Biotechnol. Adv. 2022, 55, 107914. [Google Scholar]
- Shahverdi, A.R.; Minaeian, S.; Shahverdi, H.R.; Jamalifar, H.; Nohi, A.A. Rapid synthesis of silver nanoparticles using culture supernatants of Enterobacteria: A novel biological approach. Process Biochem. 2007, 42, 919–923. [Google Scholar] [CrossRef]
- Liu, C.; Yang, D.; Wang, Y.; Shi, J.; Jiang, Z. Fabrication of antimicrobial bacterial cellulose—Ag/AgCl nanocomposite using bacteria as versatile biofactory. J. Nanopart. Res. 2012, 14, 1084–1095. [Google Scholar] [CrossRef]
- Gopinathan, P.; Ashok, A.M.; Selvakumar, R. Bacterial flagella as biotemplate for the synthesis of silver nanoparticle impregnated bionanomaterial. Appl. Surf. Sci. 2013, 276, 717–722. [Google Scholar] [CrossRef]
- Hosseini-Abari, A.; Emtiazi, G.; Ghasemi, S.M. Development of an eco-friendly approach for biogenesis of silver nanoparticles using spores of Bacillus atrophaeus. World J. Microbiol. Biotechnol. 2013, 29, 2359–2364. [Google Scholar] [CrossRef] [PubMed]
- Kanmani, P.; Lim, S.T. Synthesis and structural characterization of silver nanoparticles using bacterial exopolysaccharide and its antimicrobial activity against food and multidrug-resistant pathogens. Process Biochem. 2013, 48, 1099–1106. [Google Scholar] [CrossRef]
- Morsy, F.M.; Nafady, N.A.; Abd-Alla, M.H.; Elhady, D.A. Green synthesis of silver nanoparticles by water-soluble fraction of the extracellular polysaccharides/matrix of the cyanobacterium Nostoc commune and its application as a potent fungal surface sterilizing agent of seed crops. Univ. J. Microbiol. Res. 2014, 2, 36–43. [Google Scholar] [CrossRef]
- Nanda, A.; Saravanan, M. Biosynthesis of silver nanoparticles from Staphylococcus aureus and its antimicrobial activity against MRSA and MRSE. Nanomedicine 2009, 5, 452–456. [Google Scholar] [CrossRef]
- Reddy, A.S.; Chen, C.Y.; Chen, C.C.; Jean, J.S.; Chen, H.R.; Tseng, M.J.; Fan, C.W.; Wang, J.C. Biological synthesis of gold and silver nanoparticles mediated by the bacteria Bacillus subtilis. J. Nanosci. Nanotechnol. 2010, 10, 6567–6574. [Google Scholar] [CrossRef] [PubMed]
- Shivaji, S.; Madhu, S.; Singh, S. Extracellular synthesis of antibacterial silver nanoparticles using psychrophilic bacteria. Process Biochem. 2011, 49, 830–837. [Google Scholar] [CrossRef]
- Wei, X.; Luo, M.; Li, W.; Yang, L.; Liang, X.; Xu, L.; Kong, P.; Liu, H. Synthesis of silver nanoparticles by solar irradiation of cell-free Bacillus amyloliquefaciens extracts and AgNO3. Bioresour. Technol. 2012, 103, 273–278. [Google Scholar] [CrossRef]
- Saravanan, M.; Vemu, A.K.; Barik, S.K. Rapid biosynthesis of silver nanoparticles from Bacillus megaterium (NCIM 2326) and their antibacterial activity on multidrug-resistant clinical pathogens. Colloids Surf. B 2011, 88, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Priyadarshini, S.; Gopinath, V.; Meera Priyadharsshini, N.; Mubarak Ali, D.; Velusamy, P. Synthesis of anisotropic silver nanoparticles using novel strain Bacillus flexus and its biomedical application. Colloids Surf. B Biointerfaces 2013, 102, 232–237. [Google Scholar] [CrossRef] [PubMed]
- Das, V.L.; Thomas, R.; Varghese, R.T.; Soniya, E.V.; Mathew, J.; Radhakrishnan, E.K. Extracellular synthesis of silver nanoparticles by the Bacillus strain CS 11 isolated from industrialized area. 3 Biotech 2014, 4, 121–126. [Google Scholar] [CrossRef]
- Kalishwaralal, K.; Deepak, V.; Ramkumarpandian, S.; Nellaiah, H.; Sangiliyandi, G. Extracellular biosynthesis of silver nanoparticles by the culture supernatant of Bacillus licheniformis. Mater. Lett. 2008, 62, 4411–4413. [Google Scholar] [CrossRef]
- Firdhouse, M.J.; Lalitha, P. Biosynthesis of silver nanoparticles and its applications. J. Nanotechnol. 2015, 2015, 829526. [Google Scholar] [CrossRef]
- Alharbi, F.A.; Alarfaj, A.A. Green synthesis of silver nanoparticles from Neurada procumbens and its antibacterial activity against multi-drug resistant microbial pathogens. J. King Saud Univ. Sci. 2020, 32, 1346–1352. [Google Scholar] [CrossRef]
- Krishnaraj, C.; Jagan, E.G.; Rajasekar, S.; Selvakumar, P.; Kalaichelvan, P.T.; Mohan, N. Optimization for rapid synthesis of silver nanoparticles and its effect on phytopathogenic fungi. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2012, 93, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Das, R.; Nath, S.S.; Chakdar, D.; Gope, G.; Bhattacharjee, R. Synthesis of silver nanoparticles and their optical properties. J. Exp. Nanosci. 2010, 5, 357–362. [Google Scholar] [CrossRef]
- Siddiqi, K.S.; Husen, A. Fabrication of metal nanoparticles from fungi and metal salts: Scope and application. Nanoscale Res. Lett. 2016, 11, 98. [Google Scholar] [CrossRef]
- Vigneshwaran, N.; Ashtaputre, N.M.; Varadarajan, P.V.; Nachane, R.P.; Paralikar, K.M.; Balasubramanya, R.H. Biological synthesis of silver nanoparticles using the fungus Aspergillus flavus. Mater. Lett. 2007, 61, 1413–1418. [Google Scholar] [CrossRef]
- Ingle, A.; Gade, A.; Pierrat, S.; Sönnichsen, C.; Rai, M. Mycosynthesis of silver nanoparticles using the fungus Fusarium acuminatum and its activity against some human pathogenic bacteria. Curr. Nanosci. 2008, 4, 141–144. [Google Scholar] [CrossRef]
- Ingle, A.; Rai, M.; Gade, A.; Bawaskar, M. Fusarium solani: A novel biological agent for the extracellular synthesis of silver nanoparticles. J. Nanopart. Res. 2009, 11, 2079–2085. [Google Scholar] [CrossRef]
- Kathiresan, K.; Manivannan, S.; Nabeal, M.A.; Dhivya, B. Studies on silver nanoparticles synthesized by a marine fungus, Penicillium fellutanum, isolated from coastal mangrove sediment. Colloids Surf. B Biointerfaces 2009, 71, 133–137. [Google Scholar] [CrossRef] [PubMed]
- Durán, N.; Marcato, P.D.; De Souza, G.I.H.; Alves, O.L.; Esposito, E. Antibacterial effect of silver nanoparticles produced by fungal process on textile fabrics and their effluent treatment. J. Biomed. Nanotechnol. 2007, 3, 203–208. [Google Scholar] [CrossRef]
- Syed, A.; Saraswati, S.; Kundu, G.C.; Ahmad, A. Biological synthesis of silver nanoparticles using the fungus Humicola sp. and evaluation of their cytotoxicity using normal and cancer cell lines. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2013, 114, 144–147. [Google Scholar] [CrossRef]
- Ahmad, A.; Mukherjee, P.; Senapati, S.; Mandal, D.; Khan, M.I.; Kumar, R.; Sastry, M. Extracellular biosynthesis of silver nanoparticles using the fungus Fusarium oxysporum. Colloids Surf. B Biointerfaces 2003, 28, 313–318. [Google Scholar] [CrossRef]
- Yassin, M.A.; Elgorban, A.M.; El-Samawaty, A.E.R.M.; Almunqedhi, B.M. Biosynthesis of Silver Nanoparticles Using Penicillium verrucosum and Analysis of Their Antifungal Activity. Saudi J. Biol. Sci. 2021, 28, 2123–2127. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Senapati, S.; Khan, M.I.; Kumar, R.; Ramani, R.; Srinivas, V.; Sastry, M. Intracellular synthesis of gold nanoparticles by a novel alkalotolerant actinomycete, Rhodococcus species. Nanotechnology 2003, 14, 824–828. [Google Scholar] [CrossRef]
- Kumar, S.A.; Kazemian, A.M.; Gosavi, S.W.; Sulabha, K.K.; Renu, P.; Absar, A.; Khan, M.I. Nitrate reductase-mediated synthesis of silver nanoparticles from AgNO3. Biotechnol. Lett. 2007, 29, 439–445. [Google Scholar] [CrossRef] [PubMed]
- Korbekandi, H.; Ashari, Z.; Iravani, S.; Abbasi, S. Optimization of biological synthesis of silver nanoparticles using Fusarium oxysporum. Iran. J. Pharm. Res. 2013, 12, 289–298. [Google Scholar]
- Saravanan, M.; Barik, S.K.; MubarakAli, D.; Prakash, P.; Pugazhendhi, A. Synthesis of silver nanoparticles from Bacillus brevis (NCIM 2533) and their antibacterial activity against pathogenic bacteria. Microb. Pathog. 2018, 116, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Serrano, C.; Guzmán-Moreno, J.; Ángeles-Chávez, C.; Rodríguez-González, V.; Ortega-Sigala, J.J.; Ramírez-Santoyo, R.M.; Vidales-Rodríguez, L.E. Biosynthesis of silver nanoparticles by Fusarium scirpi and its potential as antimicrobial agent against uropathogenic Escherichia coli biofilms. PLoS ONE 2020, 15, e0230275. [Google Scholar] [CrossRef]
- Saravanan, M.; Arokiyaraj, S.; Lakshmi, T.; Pugazhendhi, A. Synthesis of silver nanoparticles from Phenerochaete chrysosporium (MTCC-787) and their antibacterial activity against human pathogenic bacteria. Microb. Pathog. 2018, 117, 68–72. [Google Scholar] [CrossRef]
- Yousefzadi, M.; Rahimi, Z.; Ghafori, V. The green synthesis, characterization and antimicrobial activities of silver nanoparticles synthesized from green alga Enteromorpha flexuosa (Wulfen). J. Agardh. Mater. Lett. 2014, 137, 1–4. [Google Scholar] [CrossRef]
- Arya, A.; Mishra, V.; Chundawat, T.S. Green synthesis of silver nanoparticles from green algae (Botryococcus braunii) and its catalytic behavior for the synthesis of benzimidazoles. Chem. Data Collect. 2019, 20, 100190. [Google Scholar] [CrossRef]
- Akther, T.; Khan, M.S.; Hemalatha, S. Biosynthesis of silver nanoparticles via fungal cell filtrate and their anti-quorum sensing against Pseudomonas aeruginosa. J. Environ. Chem. Eng. 2020, 8, 104365. [Google Scholar] [CrossRef]
- Ibrahim, E.; Fouad, H.; Zhang, M.; Zhang, Y.; Qiu, W.; Yan, C.; Li, B.; Mo, J.; Chen, J. Biosynthesis of silver nanoparticles using endophytic bacteria and their role in inhibition of rice pathogenic bacteria and plant growth promotion. RSC Adv. 2019, 9, 29293–29299. [Google Scholar] [CrossRef]
- Huq, M.A. Green synthesis of silver nanoparticles using Pseudoduganella eburnea MAHUQ-39 and their antimicrobial mechanisms investigation against drug-resistant human pathogens. Int. J. Mol. Sci. 2020, 21, 1510. [Google Scholar] [CrossRef] [PubMed]
- Elgamouz, A.; Idriss, H.; Nassab, C.; Bihi, A.; Bajou, K.; Hasan, K.; Abu Haija, M.; Patole, S.P. Green synthesis, characterization, antimicrobial, anti-cancer, and optimization of colorimetric sensing of hydrogen peroxide of algae extract capped silver nanoparticles. Nanomaterials 2020, 10, 1861. [Google Scholar] [CrossRef]
- Feroze, N.; Arshad, B.; Younas, M.; Afridi, M.I.; Saqib, S.; Ayaz, A. Fungal mediated synthesis of silver nanoparticles and evaluation of antibacterial activity. Microsc. Res. Tech. 2020, 83, 72–80. [Google Scholar] [CrossRef]
- Ajaz, S.; Ahmed, T.; Shahid, M.; Noman, M.; Shah, A.A.; Mehmood, M.A.; Abbas, A.; Cheema, A.I.; Iqbal, M.Z.; Li, B. Bioinspired green synthesis of silver nanoparticles by using a native Bacillus sp. strain AW1-2: Characterization and antifungal activity against Colletotrichum falcatum Went. Enzyme Microb. Technol. 2021, 144, 109745. [Google Scholar] [CrossRef] [PubMed]
- Mistry, H.; Thakor, R.; Patil, C.; Trivedi, J.; Bariya, H. Biogenically proficient synthesis and characterization of silver nanoparticles employing marine procured fungi Aspergillus brunneoviolaceus along with their antibacterial and antioxidative potency. Biotechnol. Lett. 2021, 43, 307–316. [Google Scholar] [CrossRef]
- Guilger-Casagrande, M.; de Lima, R. Synthesis of silver nanoparticles mediated by fungi: A review. Front. Bioeng. Biotechnol. 2019, 7, 287. [Google Scholar] [CrossRef]
- El-Ashmony, R.M.; Zaghloul, N.S.; Milošević, M.; Mohany, M.; Al-Rejaie, S.S.; Abdallah, Y.; Galal, A.A. The biogenically efficient synthesis of silver nanoparticles using the fungus Trichoderma harzianum and their antifungal efficacy against Sclerotinia sclerotiorum and Sclerotium rolfsii. J. Fungi 2022, 8, 597. [Google Scholar] [CrossRef] [PubMed]
- Goel, N.; Ahmad, R.; Singh, R.; Sood, S.; Khare, S.K. Biologically synthesized silver nanoparticles by Streptomyces sp. EMB24 extracts used against the drug-resistant bacteria. Bioresour. Technol. Rep. 2021, 15, 100753. [Google Scholar] [CrossRef]
- Rajkumar, R.; Ezhumalai, G.; Gnanadesigan, M. A green approach for the synthesis of silver nanoparticles by Chlorella vulgaris and its application in photocatalytic dye degradation a ctivity. Environ. Technol. Innov. 2021, 21, 101282. [Google Scholar] [CrossRef]
- Sharma, A.; Sagar, A.; Rana, J.; Rani, R. Green synthesis of silver nanoparticles and its antibacterial activity using fungus Talaromyces purpureogenus isolated from Taxus baccata Linn. Micro Nano Syst. Lett. 2022, 10, 2. [Google Scholar] [CrossRef]
- Mujaddidi, N.; Nisa, S.; Al Ayoubi, S.; Bibi, Y.; Khan, S.; Sabir, M.; Zia, M.; Ahmad, S.; Qayyum, A. Pharmacological properties of biogenically synthesized silver nanoparticles using endophyte Bacillus cereus extract of Berberis lyceum against oxidative stress and pathogenic multidrug-resistant bacteria. Saudi J. Biol. Sci. 2021, 28, 6432–6440. [Google Scholar] [CrossRef] [PubMed]
- Abishad, P.; Vergis, J.; Unni, V.; Ram, V.P.; Niveditha, P.; Yasur, J.; Juliet, S.; John, L.; Byrappa, K.; Nambiar, P.; et al. Green synthesized silver nanoparticles using Lactobacillus acidophilus as an antioxidant, antimicrobial, and antibiofilm agent against multi-drug resistant enteroaggregative Escherichia coli. Probiotics Antimicrob. Proteins 2022, 14, 904–914. [Google Scholar] [CrossRef]
- Xue, B.; He, D.; Gao, S.; Wang, D.; Yokoyama, K.; Wang, L. Biosynthesis of silver nanoparticles by the fungus Arthroderma fulvum and its antifungal activity against genera of Candida, Aspergillus, and Fusarium. Int. J. Nanomed. 2016, 11, 1899–1906. [Google Scholar]
- Honary, S.; Barabadi, H.; Gharaei-Fathabad, E.; Naghibi, F. Green synthesis of silver nanoparticles induced by the fungus Penicillium citrinum. Trop. J. Pharm. Res. 2013, 12, 7–11. [Google Scholar] [CrossRef]
- Mukherjee, P.; Roy, M.; Mandal, B.P.; Dey, G.K.; Mukherjee, P.K.; Ghatak, J.; Tyagi, A.K.; Kale, S.P. Green synthesis of highly stabilized nanocrystalline silver particles by a non-pathogenic and agriculturally important fungus T. asperellum. Nanotechnology 2008, 19, 075103. [Google Scholar] [CrossRef] [PubMed]
- Jaidev, L.R.; Narasimha, G. Fungal-mediated biosynthesis of silver nanoparticles, characterization, and antimicrobial activity. Colloids Surf. B Biointerfaces 2010, 81, 430–433. [Google Scholar] [CrossRef]
- Li, G.; He, D.; Qian, Y.; Guan, B.; Gao, S.; Cui, Y.; Yokoyama, K.; Wang, L. Fungus-mediated green synthesis of silver nanoparticles using Aspergillus terreus. Int. J. Mol. Sci. 2011, 13, 466–476. [Google Scholar] [CrossRef]
- Guzman, M.; Dille, J.; Godet, S. Synthesis and antibacterial activity of silver nanoparticles against gram-positive and gram-negative bacteria. Nanomed. Nanotechnol. Biol. Med. 2012, 8, 37–45. [Google Scholar] [CrossRef]
- Monowar, T.; Rahman, M.S.; Bhore, S.J.; Raju, G.; Sathasivam, K.V. Silver nanoparticles synthesized by using the endophytic bacterium Pantoea ananatis are promising antimicrobial agents against multidrug-resistant bacteria. Molecules 2018, 23, 3220. [Google Scholar] [CrossRef] [PubMed]
- Mokhtari, N.; Daneshpajouh, S.; Seyedbagheri, S.; Atashdehghan, R.; Abdi, K.; Sarkar, S.; Minaian, S.; Shahverdi, H.R.; Shahverdi, A.R. Biological synthesis of very small silver nanoparticles by culture supernatant of Klebsiella pneumoniae: The effects of visible-light irradiation and the liquid mixing process. Mater. Res. Bull. 2009, 44, 1415–1421. [Google Scholar] [CrossRef]
- Jalal, M.; Ansari, M.A.; Alzohairy, M.A.; Ali, S.G.; Khan, H.M.; Almatroudi, A.; Raees, K. Biosynthesis of silver nanoparticles from oropharyngeal Candida glabrata isolates and their antimicrobial activity against clinical strains of bacteria and fungi. Nanomaterials 2018, 8, 586. [Google Scholar] [CrossRef] [PubMed]
- Eugenio, M.; Müller, N.; Frases, S.; Almeida-Paes, R.; Lima, L.M.T.; Lemgruber, L.; Sant’Anna, C. Yeast-derived biosynthesis of silver/silver chloride nanoparticles and their antiproliferative activity against bacteria. RSC Adv. 2016, 6, 9893–9904. [Google Scholar] [CrossRef]
- Sagar, G.; Ashok, B. Green synthesis of silver nanoparticles using Aspergillus niger and its efficacy against human pathogens. Eur. J. Exp. Biol. 2012, 2, 1654–1658. [Google Scholar]
- Al-Zahrani, S.A.; Bhat, R.S.; Al Rashed, S.A.; Mahmood, A.; Al Fahad, A.; Alamro, G.; Al Daihan, S. Green-synthesized silver nanoparticles with aqueous extract of green algae Chaetomorpha ligustica and its anticancer potential. Green Process. Synth. 2021, 10, 711–721. [Google Scholar] [CrossRef]
- Adebayo-Tayo, B.; Salaam, A.; Ajibade, A. Green Synthesis of Silver Nanoparticle Using Oscillatoria sp. Extract, Its Antibacterial, Antibiofilm Potential and Cytotoxicity Activity. Heliyon 2019, 5, e02602. [Google Scholar] [CrossRef]
- Ramkumar, V.S.; Pugazhendhi, A.; Gopalakrishnan, K.; Sivagurunathan, P.; Saratale, G.D.; Dung, T.N.B.; Kannapiran, E. Biofabrication and characterization of silver nanoparticles using aqueous extract of seaweed Enteromorpha compressa and its biomedical properties. Biotechnol. Rep. 2017, 14, 1–7. [Google Scholar] [CrossRef]
- Roni, M.; Murugan, K.; Panneerselvam, C.; Subramaniam, J.; Nicoletti, M.; Madhiyazhagan, P.; Benelli, G. Characterization and biotoxicity of Hypnea musciformis-synthesized silver nanoparticles as a potential eco-friendly control tool against Aedes aegypti and Plutella xylostella. Ecotoxicol. Environ. Saf. 2015, 121, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Madhiyazhagan, P.; Murugan, K.; Kumar, A.N.; Nataraj, T.; Dinesh, D.; Panneerselvam, C.; Benelli, G. Sargassum muticum-synthesized silver nanoparticles: An effective control tool against mosquito vectors and bacterial pathogens. Parasitol. Res. 2015, 114, 4305–4317. [Google Scholar] [CrossRef]
- Husen, A.; Siddiqi, K.S. Phytosynthesis of nanoparticles: Concept, controversy, and application. Nanoscale Res. Lett. 2014, 9, 229. [Google Scholar] [CrossRef] [PubMed]
- Husen, A. Gold nanoparticles from the plant system: Synthesis, characterization, and their application. In Nanoscience and Plant–Soil Systems; Springer: Berlin/Heidelberg, Germany, 2017; pp. 455–479. [Google Scholar]
- Beg, M.; Maji, A.; Mandal, A.K.; Das, S.; Aktara, M.N.; Jha, P.K.; Hossain, M. Green synthesis of silver nanoparticles using Pongamia pinnata seed: Characterization, antibacterial property, and spectroscopic investigation of interaction with human serum albumin. J. Mol. Recognit. 2017, 30, e2565. [Google Scholar] [CrossRef]
- Sampath, G.; Govarthanan, M.; Rameshkumar, N.; Vo, D.-V.N.; Krishnan, M.; Sivasankar, P.; Kayalvizhi, N. Eco-friendly biosynthesis metallic silver nanoparticles using Aegle marmelos(Indian bael) and its clinical and environmental applications. Appl. Nanosci. 2023, 13, 663–674. [Google Scholar] [CrossRef]
- Panneerselvam, C.; Murugan, K.; Roni, M.; Aziz, A.T.; Suresh, U.; Rajaganesh, R.; Benelli, G. Fern-synthesized nanoparticles in the fight against malaria: LC/MS analysis of Pteridium aquilinum leaf extract and biosynthesis of silver nanoparticles with high mosquitocidal and antiplasmodial activity. Parasitol. Res. 2016, 115, 997–1013. [Google Scholar] [PubMed]
- Murugan, K.; Labeeba, M.A.; Panneerselvam, C.; Dinesh, D.; Suresh, U.; Subramaniam, J.; Benelli, G. Aristolochia indica green-synthesized silver nanoparticles: A sustainable control tool against the malaria vector Anopheles stephensi. Res. Vet. Sci. 2015, 102, 127–135. [Google Scholar]
- Ghaffari-Moghaddam, M.; Hadi-Dabanlou, R.; Khajeh, M.; Rakhshanipour, M.; Kazemi, B. Green Synthesis of Silver Nanoparticles Using Plant Extracts. Korean J. Chem. Eng. 2014, 31, 548–557. [Google Scholar]
- Mohamad, N.A.N.; Arham, N.A.; Jai, J.; Hadi, A. Plant extract as a reducing agent in the synthesis of metallic nanoparticles: A review. Adv. Mater. Res. 2014, 832, 350–355. [Google Scholar] [CrossRef]
- Rajeshkumar, S.; Bharath, L.V. Mechanism of plant-mediated synthesis of silver nanoparticles: A review on biomolecules involved, characterization, and antibacterial activity. Chem.-Biol. Interact. 2017, 273, 219–227. [Google Scholar] [PubMed]
- Shukla, S.; Mehata, M.S. Selective picomolar detection of carcinogenic chromium ions using silver nanoparticles capped via biomolecules from flowers of Plumeria obtusa. J. Mol. Liq. 2023, 380, 121705. [Google Scholar] [CrossRef]
- Mehata, M.S. Green route synthesis of silver nanoparticles using plants/ginger extracts with enhanced surface plasmon resonance and degradation of textile dye. Mater. Sci. Eng. B 2021, 273, 115418. [Google Scholar] [CrossRef]
- Ovais, M.; Khalil, A.T.; Islam, N.U.; Ahmad, I.; Ayaz, M.; Saravanan, M.; Mukherjee, S. Role of plant phytochemicals and microbial enzymes in the biosynthesis of metallic nanoparticles. Appl. Microbiol. Biotechnol. 2018, 102, 6799–6814. [Google Scholar] [PubMed]
- Roy, A.; Bulut, O.; Some, S.; Mandal, A.K.; Yilmaz, M.D. Green synthesis of silver nanoparticles: Biomolecule–nanoparticle organizations targeting antimicrobial activity. RSC Adv. 2019, 9, 2673–2702. [Google Scholar] [CrossRef]
- Singh, R.; Hano, C.; Nath, G.; Sharma, B. Green biosynthesis of silver nanoparticles using leaf extract of Carissa carandas L. and their antioxidant and antimicrobial activity against human pathogenic bacteria. Biomolecules 2021, 11, 299. [Google Scholar] [CrossRef] [PubMed]
- Hassanisaadi, M.; Bonjar, A.H.S.; Rahdar, A.; Varma, R.S.; Ajalli, N.; Pandey, S. Eco-friendly biosynthesis of silver nanoparticles using Aloysia citrodora leaf extract and evaluations of their bioactivities. Mater. Today Commun. 2022, 33, 104183. [Google Scholar] [CrossRef]
- Dhaka, A.; Mali, S.C.; Sharma, S.; Trivedi, R. A review on biological synthesis of silver nanoparticles and their potential applications. Results Chem. 2023, 6, 101108. [Google Scholar] [CrossRef]
- Sadowski, Z.; Maliszewska, I.; Grochowalska, B.; Polowczyk, I.; Kozlecki, T. Synthesis of Silver Nanoparticles Using Microorganisms. Mater. Sci.-Pol. 2008, 26, 419–424. [Google Scholar]
- Pryshchepa, O.; Pomastowski, P.; Buszewski, B. Silver nanoparticles: Synthesis, investigation techniques, and properties. Adv. Colloid Interface Sci. 2020, 284, 102246. [Google Scholar] [CrossRef]
- Siddiqi, K.S.; Husen, A.; Rao, R.A.K. A review on biosynthesis of silver nanoparticles and their biocidal properties. J. Nanobiotechnol. 2018, 16, 14. [Google Scholar] [CrossRef] [PubMed]
- Manik, U.P.; Nande, A.; Raut, S.; Dhoble, S.J. Green synthesis of silver nanoparticles using plant leaf extraction of Artocarpus heterophyllus and Azadirachta indica. Results Mater. 2020, 6, 100086. [Google Scholar] [CrossRef]
- Hawadak, J.; Kojom Foko, L.P.; Pande, V.; Singh, V. In vitro antiplasmodial activity, hemocompatibility, and temporal stability of Azadirachta indica silver nanoparticles. Artif. Cells Nanomed. Biotechnol. 2022, 50, 286–300. [Google Scholar] [CrossRef] [PubMed]
- Moosa, A.A.; Ridha, A.M.; Al-Kaser, M. Process parameters for green synthesis of silver nanoparticles using leaves extract of Aloe vera plant. Int. J. Multi Curr. Res. 2015, 3, 966–975. [Google Scholar]
- Yadav, J.P.; Kumar, S.; Budhwar, L.; Yadav, A.; Yadav, M. Characterization and antibacterial activity of synthesized silver and iron nanoparticles using Aloe vera. J. Nanomed. Nanotechnol. 2016, 7, 1000384. [Google Scholar]
- Geethalakshmi, R.; Sarada, D.V.L. Characterization and antimicrobial activity of gold and silver nanoparticles synthesized using saponin isolated from Trianthema decandra L. Ind. Crops Prod. 2013, 51, 107–115. [Google Scholar]
- Geethalakshmi, R.; Sarada, D.V.L. Gold and silver nanoparticles from Trianthema decandra: Synthesis, characterization, and antimicrobial properties. Int. J. Nanomed. 2012, 7, 5375–5384. [Google Scholar] [CrossRef]
- Vidhu, V.K.; Aromal, S.A.; Philip, D. Green synthesis of silver nanoparticles using Macrotyloma uniflorum. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2011, 83, 392–397. [Google Scholar] [CrossRef] [PubMed]
- Dubey, S.P.; Lahtinen, M.; Sillanpää, M. Tansy fruit mediated greener synthesis of silver and gold nanoparticles. Process Biochem. 2010, 45, 1065–1071. [Google Scholar] [CrossRef]
- Tho, N.T.M.; An, T.N.M.; Tri, M.D.; Sreekanth, T.V.M.; Lee, J.S.; Nagajyothi, P.C.; Lee, K.D. Green synthesis of silver nanoparticles using Nelumbo nucifera seed extract and its antibacterial activity. Acta Chim. Slov. 2013, 60, 673–678. [Google Scholar] [PubMed]
- Yu, C.; Tang, J.; Liu, X.; Ren, X.; Zhen, M.; Wang, L. Green biosynthesis of silver nanoparticles using Eriobotrya japonica (Thunb.) leaf extract for reductive catalysis. Materials 2019, 12, 189. [Google Scholar] [CrossRef] [PubMed]
- Vishwasrao, C.; Momin, B.; Ananthanarayan, L. Green synthesis of silver nanoparticles using sapota fruit waste and evaluation of their antimicrobial activity. Waste Biomass Valorization 2019, 10, 2353–2363. [Google Scholar] [CrossRef]
- Shanmugavadivu, M.; Kuppusamy, S.; Ranjithkumar, R. Synthesis of pomegranate peel extract mediated silver nanoparticles and its antibacterial activity. Am. J. Adv. Drug Deliv. 2014, 2, 174–182. [Google Scholar]
- Nasiriboroumand, M.; Montazer, M.; Barani, H. Preparation and characterization of biocompatible silver nanoparticles using pomegranate peel extract. J. Photochem. Photobiol. B Biol. 2018, 179, 98–104. [Google Scholar] [CrossRef]
- Kouvaris, P.; Delimitis, A.; Zaspalis, V.; Papadopoulos, D.; Tsipas, S.A.; Michailidis, N. Green synthesis and characterization of silver nanoparticles produced using Arbutus unedo leaf extract. Mater. Lett. 2012, 76, 18–20. [Google Scholar] [CrossRef]
- Nisha, M.H.; Tamileswari, R.; Jesurani, S.S.; Kanagesan, S.; Hashim, M.; Alexander, S.C.P. Green synthesis of silver nanoparticles from pomegranate (Punica granatum) leaves and analysis of antibacterial activity. Int. J. Adv. Technol. Eng. Sci. 2015, 4, 1–8. [Google Scholar]
- Sarkar, S.; Kotteeswaran, V. Green synthesis of silver nanoparticles from aqueous leaf extract of pomegranate (Punica granatum) and their anticancer activity on human cervical cancer cells. Adv. Nat. Sci. Nanoscience Nanotechnol. 2018, 9, 025014. [Google Scholar] [CrossRef]
- Mehnath, S.; Sathishkumar, G.; Arivoli, A.; Rajan, M.; Praphakar, R.A.; Jeyaraj, M. Green synthesis of AgNPs by walnut seed extract and its role in photocatalytic degradation of a textile dye effluent. Trans. Eng. Sci. 2017, 5, 31–40. [Google Scholar]
- Huang, J.; Li, Q.; Sun, D.; Lu, Y.; Su, Y.; Yang, X.; Chen, C. Biosynthesis of silver and gold nanoparticles by novel sundried Cinnamomum camphora leaf. Nanotechnology 2007, 18, 105104. [Google Scholar] [CrossRef]
- Tongwanichniyom, S.; Phewrat, N.; Rangsarikorn, N.; Leasen, S.; Luangkamin, S.; Chumnanvej, N. Green synthesis of silver nanoparticles using mature-pseudostem extracts of Alpinia nigra and their bioactivities. Green Process Synth. 2024, 13, 20230226. [Google Scholar] [CrossRef]
- Behravan, M.; Panahi, A.H.; Naghizadeh, A.; Ziaee, M.; Mahdavi, R.; Mirzapour, A. Facile green synthesis of silver nanoparticles using Berberis vulgaris leaf and root aqueous extract and its antibacterial activity. Int. J. Biol. Macromol. 2019, 124, 148–154. [Google Scholar] [CrossRef]
- Vinay, S.P.; Chandrappa, C.P. Silver nanoparticles: Synthesized by leaves extract of avocado and their antibacterial activity. Int. J. Eng. Dev. Res. 2017, 5, 1608–1613. [Google Scholar]
- Joshi, P.; Joy, H.; Vyas, P. Green synthesis of silver nanoparticle using plant root extract of Croton sparsiflorus and their antimicrobial activity. Int. J. Sci. Res. 2016, 5, 12. [Google Scholar]
- Baskaran, C.; Ratha-bai, B. Green synthesis of silver nanoparticles using Coleus forskohlii roots extract and its antimicrobial activity against bacteria and fungus. Int. J. Drug Dev. Res. 2013, 5, 114–119. [Google Scholar]
- Vankar, P.S.; Shukla, D. Biosynthesis of silver nanoparticles using lemon leaves extract and its application for antimicrobial finish on fabric. Appl. Nanosci. 2012, 2, 163–168. [Google Scholar] [CrossRef]
- Serdar, G.; Albay, C.; Sökmen, M. Biosynthesis and characterization of silver nanoparticles from the lemon leaves extract. Cumhuriyet Sci. J. 2019, 40, 170–172. [Google Scholar] [CrossRef]
- Ibrahim, H.M. Green synthesis and characterization of silver nanoparticles using banana peel extract and their antimicrobial activity against representative microorganisms. J. Radiat. Res. Appl. Sci. 2015, 8, 265–275. [Google Scholar] [CrossRef]
- Rautela, A.; Rani, J. Green synthesis of silver nanoparticles from Tectona grandis seeds extract: Characterization and mechanism of antimicrobial action on different microorganisms. J. Anal. Sci. Technol. 2019, 10, 5. [Google Scholar] [CrossRef]
- Khalil, M.M.; Ismail, E.H.; El-Baghdady, K.Z.; Mohamed, D. Green synthesis of silver nanoparticles using olive leaf extract and its antibacterial activity. Arab. J. Chem. 2014, 7, 1131–1139. [Google Scholar] [CrossRef]
- Ghosh, R.; Dutta, S.; Bhattacharyya, S. Green synthesis of silver nanoparticle by Acalypha indica and its antifungal effect against phytopathogen Colletotrichum capsici. Acta Sci. Agric. 2018, 2, 27–31. [Google Scholar]
- Krishnaraj, C.; Jagan, E.G.; Rajasekar, S.; Selvakumar, P.; Kalaichelvan, P.T.; Mohan, N.J.C.S.B.B. Synthesis of silver nanoparticles using Acalypha indica leaf extracts and its antibacterial activity against water borne pathogens. Colloids Surf. B Biointerfaces 2010, 76, 50–56. [Google Scholar] [CrossRef]
- Menon, S.; Agarwal, H.; Kumar, S.R.; Kumar, S.V. Green synthesis of silver nanoparticles using medicinal plant Acalypha indica leaf extracts and its application as an antioxidant and antimicrobial agent against foodborne pathogens. Int. J. Appl. Pharm. 2017, 9, 42–50. [Google Scholar] [CrossRef]
- Saxena, A.; Tripathi, R.M.; Zafar, F.; Singh, P. Green synthesis of silver nanoparticles using aqueous solution of Ficus benghalensis leaf extract and characterization of their antibacterial activity. Mater. Lett. 2012, 67, 91–94. [Google Scholar] [CrossRef]
- Murad, U.; Khan, S.A.; Ibrar, M.; Ullah, S.; Khattak, U. Synthesis of silver and gold nanoparticles from leaf of Litchi chinensis and its biological activities. Asian Pac. J. Trop. Biomed. 2018, 8, 142–149. [Google Scholar]
- Iqbal, M.J.; Ali, S.; Rashid, U.; Kamran, M.; Malik, M.F.; Sughra, K.; Zeeshan, N.; Afroz, A.; Saleem, J.; Saghir, M. Biosynthesis of silver nanoparticles from leaf extract of Litchi chinensis and its dynamic biological impact on microbial cells and human cancer cell lines. Cell. Mol. Biol. 2018, 64, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Baghayeri, M.; Mahdavi, B.; Hosseinpor-Mohsen Abadi, Z.; Farhadi, S. Green synthesis of silver nanoparticles using water extract of Salvia leriifolia: Antibacterial studies and applications as catalysts in the electrochemical detection of nitrite. Appl. Organomet. Chem. 2018, 32, e4057. [Google Scholar] [CrossRef]
- Huo, Y.; Singh, P.; Kim, Y.J.; Soshnikova, V.; Kang, J.; Markus, J.; Ahn, S.; Castro-Aceituno, V.; Mathiyalagan, R.; Chokkalingam, M.; et al. Biological synthesis of gold and silver chloride nanoparticles by Glycyrrhiza uralensis and in vitro applications. Artif. Cells Nanomed. Biotechnol. 2018, 46, 303–312. [Google Scholar] [CrossRef] [PubMed]
- De Barros, C.H.N.; Cruz, G.C.F.; Mayrink, W.; Tasic, L. Bio-based synthesis of silver nanoparticles from orange waste: Effects of distinct biomolecule coatings on size, morphology, and antimicrobial activity. Nanotechnol. Sci. Appl. 2018, 1, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Niluxsshun, M.C.D.; Masilamani, K.; Mathiventhan, U. Green synthesis of silver nanoparticles from the extracts of fruit peel of Citrus tangerina, Citrus sinensis, and Citrus limon for antibacterial activities. Bioinorg. Chem. Appl. 2021, 2021, 6695734. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.J.; Murtaza, G.; Mehmood, A.; Bhatti, T.M. Green synthesis of silver nanoparticles using leaves extract of Skimmia laureola: Characterization and antibacterial activity. Mater. Lett. 2015, 153, 10–13. [Google Scholar] [CrossRef]
- Krithiga, N.; Rajalakshmi, A.; Jayachitra, A. Green synthesis of silver nanoparticles using leaf extracts of Clitoria ternatea and Solanum nigrum and study of its antibacterial effect against common nosocomial pathogens. J. Nanosci. 2015, 2015, 928204. [Google Scholar] [CrossRef]
- Rao, N.H.; Lakshmidevi, N.; Pammi, S.V.N.; Kollu, P.; Ganapaty, S.; Lakshmi, P. Green synthesis of silver nanoparticles using methanolic root extracts of Diospyros paniculata and their antimicrobial activities. Mater. Sci. Eng. C 2016, 62, 553–557. [Google Scholar] [CrossRef] [PubMed]
- Anandalakshmi, K.; Venugobal, J.; Ramasamy, V.J.A.N. Characterization of silver nanoparticles by green synthesis method using Pedalium murex leaf extract and their antibacterial activity. Appl. Nanosci. 2016, 6, 399–408. [Google Scholar] [CrossRef]
- Allafchian, A.R.; Mirahmadi-Zare, S.Z.; Jalali, S.A.H.; Hashemi, S.S.; Vahabi, M.R. Green synthesis of silver nanoparticles using Phlomis leaf extract and investigation of their antibacterial activity. J. Nanostruct. Chem. 2016, 6, 129–135. [Google Scholar] [CrossRef]
- Ravichandran, V.; Vasanthi, S.; Shalini, S.; Shah, S.A.A.; Harish, R. Green synthesis of silver nanoparticles using Atrocarpus altilis leaf extract and the study of their antimicrobial and antioxidant activity. Mater. Lett. 2016, 180, 264–267. [Google Scholar] [CrossRef]
- Raja, S.; Ramesh, V.; Thivaharan, V. Green biosynthesis of silver nanoparticles using Calliandra haematocephala leaf extract, their antibacterial activity and hydrogen peroxide sensing capability. Arab. J. Chem. 2017, 10, 253–261. [Google Scholar] [CrossRef]
- Bharathi, D.; Diviya Josebin, M.; Vasantharaj, S.; Bhuvaneshwari, V. Biosynthesis of silver nanoparticles using stem bark extracts of Diospyros montana and their antioxidant and antibacterial activities. J. Nanostruct. Chem. 2018, 8, 83–92. [Google Scholar] [CrossRef]
- Moteriya, P.; Chanda, S. Biosynthesis of silver nanoparticles formation from Caesalpinia pulcherrima stem metabolites and their broad spectrum biological activities. J. Genet. Eng. Biotechnol. 2018, 16, 105–113. [Google Scholar] [CrossRef]
- Karthiga, P. Preparation of silver nanoparticles by Garcinia mangostana stem extract and investigation of the antimicrobial properties. Biotechnol. Res. Innov. 2018, 2, 30–36. [Google Scholar] [CrossRef]
- Oves, M.; Aslam, M.; Rauf, M.A.; Qayyum, S.; Qari, H.A.; Khan, M.S.; Alam, M.Z.; Tabrez, S.; Pugazhendhi, A.; Ismail, I.M. Antimicrobial and anticancer activities of silver nanoparticles synthesized from the root hair extract of Phoenix dactylifera. Mater. Sci. Eng. C 2018, 89, 429–443. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, A.V.; Devi, D.R.; Battu, G.; Basavaiah, K. A Facile plant mediated synthesis of silver nanoparticles using an aqueous leaf extract of Ficus hispida Linn. f. for catalytic, antioxidant and antibacterial applications. S. Afr. J. Chem. Eng. 2018, 26, 25–34. [Google Scholar] [CrossRef]
- Moodley, J.S.; Krishna, S.B.N.; Pillay, K.; Sershen, F.; Govender, P. Green synthesis of silver nanoparticles from Moringa oleifera leaf extracts and its antimicrobial potential. Adv. Nat. Sci. Nanosci. Nanotechnol. 2018, 9, 015011. [Google Scholar] [CrossRef]
- Balachandar, R.; Gurumoorthy, P.; Karmegam, N.; Barabadi, H.; Subbaiya, R.; Anand, K.; Boomi, P.; Saravanan, M. Plant-mediated synthesis, characterization and bactericidal potential of emerging silver nanoparticles using stem extract of Phyllanthus pinnatus: A recent advance in phytonanotechnology. J. Clust. Sci. 2019, 30, 1481–1488. [Google Scholar] [CrossRef]
- Dangi, S.; Gupta, A.; Gupta, D.K.; Singh, S.; Parajuli, N. Green synthesis of silver nanoparticles using aqueous root extract of Berberis asiatica and evaluation of their antibacterial activity. Chem. Data Collect. 2020, 28, 100411. [Google Scholar] [CrossRef]
- Sharifi-Rad, M.; Pohl, P.; Epifano, F.; Álvarez-Suarez, J.M. Green synthesis of silver nanoparticles using Astragalus tribuloides Delile. root extract: Characterization, antioxidant, antibacterial, and anti-inflammatory activities. Nanomaterials 2020, 10, 2383. [Google Scholar] [CrossRef]
- Olabemiwo, O.M.; Akintelu, S.A.; Waheed, A.A.; Okunlola, D.S.; Akinwale, D.R.; Adeyinka, G.C.; Adebisi, S.A. Green synthesis of silver nanoparticles using stem bark extract of Annona senegalensis: Characterization and its antibacterial potency. Curr. Res. Green Sustain. Chem. 2021, 4, 100219. [Google Scholar] [CrossRef]
- Devi, N.S.; Padma, Y.; Raju, R.V. Green synthesis of silver nanoparticles through reduction with Euphorbia nivulia Buch.-Ham., stem bark extract: Characterization and antimicrobial activity. J. Pharmacogn. Phytother. 2021, 13, 60–67. [Google Scholar]
- Nayak, S.; Sajankila, S.P.; Rao, C.V.; Hegde, A.R.; Mutalik, S. Biogenic synthesis of silver nanoparticles using Jatropha curcas seed cake extract and characterization: Evaluation of its antibacterial activity. Energy Sources Part A 2021, 43, 3415–3423. [Google Scholar] [CrossRef]
- Kanniah, P.; Chelliah, P.; Thangapandi, J.R.; Gnanadhas, G.; Mahendran, V.; Robert, M. Green synthesis of antibacterial and cytotoxic silver nanoparticles by Piper nigrum seed extract and development of antibacterial silver-based chitosan nanocomposite. Int. J. Biol. Macromol. 2021, 189, 18–33. [Google Scholar] [CrossRef] [PubMed]
- Ilahi, I.; Khuda, F.; Sahibzada, M.U.K.; Alghamdi, S.; Ullah, R.; Dablool, A.S.; Khalil, A.A.K. Synthesis of silver nanoparticles using root extract of Duchesnea indica and assessment of its biological activities. Arab. J. Chem. 2021, 14, 103110. [Google Scholar] [CrossRef]
- Awwad, A.M.; Salem, N.M. Green synthesis of silver nanoparticles by Mulberry Leaves extract. Nanosci. Nanotechnol. 2012, 2, 125–128. [Google Scholar] [CrossRef]
- Mehata, M.S. Green synthesis of silver nanoparticles using Kalanchoe pinnata leaves (life plant) and their antibacterial and photocatalytic activities. Chem. Phys. Lett. 2021, 778, 138760. [Google Scholar]
- Okaiyeto, K.; Hoppe, H.; Okoh, A.I. Plant-based synthesis of silver nanoparticles using aqueous leaf extract of Salvia officinalis: Characterization and its antiplasmodial activity. J. Clust. Sci. 2021, 32, 101–109. [Google Scholar] [CrossRef]
- Palithya, S.; Gaddam, S.A.; Kotakadi, V.S.; Penchalaneni, J.; Golla, N.; Krishna, S.B.N.; Naidu, C.V. Green synthesis of silver nanoparticles using flower extracts of Aerva lanata and their biomedical applications. Part. Sci. Technol. 2022, 40, 84–96. [Google Scholar] [CrossRef]
- Khanal, L.N.; Sharma, K.R.; Paudyal, H.; Parajuli, K.; Dahal, B.; Ganga, G.C.; Kalauni, S.K. Green synthesis of silver nanoparticles from root extracts of Rubus ellipticus Sm. and comparison of antioxidant and antibacterial activity. J. Nanomater. 2022, 2022, 1832587. [Google Scholar] [CrossRef]
- Kim, B.S.; Kwon, Y.J.; Kim, S.H.; Lee, J.H.; Park, C.Y. Biological Synthesis of Silver Nanoparticles Using Plant Leaf Extracts and Their Specific Antimicrobial Activity. New Biotechnol. 2014, 31, S173. [Google Scholar] [CrossRef]
- Franci, G.; Falanga, A.; Galdiero, S.; Palomba, L.; Rai, M.; Morelli, G.; Galdiero, M. Silver nanoparticles as potential antibacterial agents. Molecules 2015, 20, 8856–8874. [Google Scholar] [CrossRef] [PubMed]
- Rajeshkumar, S.; Menon, S.; Kumar, S.V.; Tambuwala, M.M.; Bakshi, H.A.; Mehta, M.; Dua, K. Antibacterial and antioxidant potential of biosynthesized copper nanoparticles mediated through Cissus arnotiana plant extract. J. Photochem. Photobiol. B 2019, 197, 111531. [Google Scholar] [CrossRef] [PubMed]
- Francis, S.; Joseph, S.; Koshy, E.P.; Mathew, B. Microwave assisted green synthesis of silver nanoparticles using leaf extract of Elephantopus scaber and its environmental and biological applications. Artif. Cells Nanomed. Biotechnol. 2018, 46, 795–804. [Google Scholar] [CrossRef] [PubMed]
- Yazdi, M.E.T.; Amiri, M.S.; Hosseini, H.A.; Oskuee, R.K.; Mosawee, H.; Pakravanan, K.; Darroudi, M. Plant-based synthesis of silver nanoparticles in Handelia trichophylla and their biological activities. Bull. Mater. Sci. 2019, 42, 155. [Google Scholar] [CrossRef]
- Ahn, E.Y.; Jin, H.; Park, Y. Green synthesis and biological activities of silver nanoparticles prepared by Carpesium cernuum extract. Arch. Pharm. Res. 2019, 42, 926–934. [Google Scholar] [CrossRef] [PubMed]
- Seifipour, R.; Nozari, M.; Pishkar, L. Green synthesis of silver nanoparticles using Tragopogon collinus leaf extract and study of their antibacterial effects. J. Inorg. Organomet. Polym. Mater. 2020, 30, 2926–2936. [Google Scholar] [CrossRef]
- Lakshmanan, G.; Ramasamy, R.; Sundaram, R.; Siva, R.; Pandian, S.; Alharbi, N.S. Plant-Mediated Synthesis of Silver Nanoparticles Using Fruit Extract of Cleome viscosa L.: Assessment of Their Antibacterial and Anticancer Activity. Karbala Int. J. Mod. Sci. 2018, 4, 61–68. [Google Scholar]
- Falsafi, S.R.; Rostamabadi, H.; Assadpour, E.; Jafari, S.M. Morphology and microstructural analysis of bioactive-loaded micro/nanocarriers via microscopy techniques; CLSM/SEM/TEM/AFM. Adv. Colloid Interface Sci. 2020, 280, 102166. [Google Scholar] [CrossRef] [PubMed]
- Velgosova, O.; Dolinská, S.; Podolská, H.; Mačák, L.; Čižmárová, E. Impact of Plant Extract Phytochemicals on the Synthesis of Silver Nanoparticles. Materials 2024, 17, 2252. [Google Scholar] [CrossRef] [PubMed]
- Sriramulu, M.; Sumathi, S. Photocatalytic, Antioxidant, Antibacterial and Anti-Inflammatory Activity of Silver Nanoparticles Synthesised Using Forest and Edible Mushroom. Adv. Nat. Sci. Nanosci. Nanotechnol. 2017, 8, 045012. [Google Scholar] [CrossRef]
- Geoprincy, G.; Srri, B.V.; Poonguzhali, U.; Gandhi, N.N.; Renganathan, S. A review on green synthesis of silver nanoparticles. Asian J. Pharm. Clin. Res. 2013, 6, 8–12. [Google Scholar]
- Banasiuk, R.; Lewandowska, K.; Kowalski, P.; Adamus, G.; Kaczmarek, D.; Witkowska, N. Synthesis of Antimicrobial Silver Nanoparticles through a Photomediated Reaction in an Aqueous Environment. Int. J. Nanomed. 2016, 11, 315–324. [Google Scholar]
- Khan, M.; Khan, M.; Adil, S.F.; Tahir, M.N.; Tremel, W.; Alkhathlan, H.Z.; Siddiqui, M.R.H. Green synthesis of silver nanoparticles mediated by Pulicaria glutinosa extract. Int. J. Nanomed. 2013, 8, 1507–1516. [Google Scholar]
- Kasthuri, J.; Kathiravan, K.; Rajendiran, N.J. Phyllanthin-Assisted Biosynthesis of Silver and Gold Nanoparticles: A Novel Biological Approach. J. Nanopart. Res. 2009, 11, 1075–1085. [Google Scholar] [CrossRef]
- Tippayawat, P.; Phromviyo, N.; Boueroy, P.; Chompoosor, A. Green synthesis of silver nanoparticles in aloe vera plant extract prepared by a hydrothermal method and their synergistic antibacterial activity. PeerJ 2016, 4, e2589. [Google Scholar] [CrossRef]
- Alavi, M.; Karimi, N. Hemoglobin Self-Assembly and Antibacterial Activities of Bio-Modified Ag-MgO Nanocomposites by Different Concentrations of Artemisia haussknechtii and Protoparmeliopsis muralis Extracts. Int. J. Biol. Macromol. 2020, 152, 1174–1185. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, N.T.T.; Nguyen, L.M.; Nguyen, T.T.T.; Nguyen, T.T.; Nguyen, D.T.C.; Tran, T.V. Formation, antimicrobial activity, and biomedical performance of plant-based nanoparticles: A review. Environ. Chem. Lett. 2022, 20, 2531–2571. [Google Scholar] [CrossRef] [PubMed]
- Habouti, S.; Solterbeck, C.-H.; Es-Souni, M. Synthesis of silver nano-fir-twigs and application to single molecules detection. J. Mater. Chem. 2010, 20, 5215–5219. [Google Scholar] [CrossRef]
- Hu, X.H.; Chan, C.T. Photonic crystals with silver nanowires as a near-infrared superlens. Appl. Phys. Lett. 2004, 85, 1520–1522. [Google Scholar] [CrossRef]
- El Badawy, A.M.; Scheckel, K.G.; Suidan, M.; Tolaymat, T. The Impact of Stabilization Mechanism on the Aggregation Kinetics of Silver Nanoparticles. Sci. Total Environ. 2012, 429, 325–331. [Google Scholar] [CrossRef]
- Tomaszewska, E.; Soliwoda, K.; Kadziola, K.; Tkacz-Szczesna, B.; Celichowski, G.; Cichomski, M.; Grobelny, J. Detection Limits of DLS and UV-Vis Spectroscopy in Characterization of Polydisperse Nanoparticle Colloids. J. Nanomater. 2013, 2013, 313081. [Google Scholar] [CrossRef]
- Nastulyavichus, A.A.; Kudryashov, S.I.; Tolordava, E.R.; Khaertdinova, L.F.; Yushina, Y.K.; Borodina, T.N.; Ionin, A.A. Dynamic Light Scattering Detection of Silver Nanoparticles, Food Pathogen Bacteria, and Their Bactericidal Interactions. Laser Phys. Lett. 2021, 18, 086002. [Google Scholar] [CrossRef]
- Verma, P.; Maheshwari, S.K. Preparation of Silver and Selenium Nanoparticles and Their Characterization by Dynamic Light Scattering and Scanning Electron Microscopy. J. Microsc. Ultrastruct. 2018, 6, 182–187. [Google Scholar] [PubMed]
- Cascio, C.; Gilliland, D.; Rossi, F.; Calzolai, L.; Contado, C. Critical Experimental Evaluation of Key Methods to Detect, Size, and Quantify Nanoparticulate Silver. Anal. Chem. 2014, 86, 12143–12151. [Google Scholar] [CrossRef]
- Verma, P. A Review on Synthesis and Their Antibacterial Activity of Silver and Selenium Nanoparticles Against Biofilm Forming Staphylococcus aureus. World J. Pharm. Pharmaceut. Sci. 2015, 4, 652–677. [Google Scholar]
- Desimoni, E.; Brunetti, B. X-ray Photoelectron Spectroscopic Characterization of Chemically Modified Electrodes Used as Chemical Sensors and Biosensors: A Review. Chemosensors 2015, 3, 70. [Google Scholar] [CrossRef]
- Gautam, S.P.; Gupta, A.K.; Agraw, S.; Sureka, S. Spectroscopic Characterization of Dendrimers. Int. J. Pharm. Pharm. Sci. 2012, 4, 77–80. [Google Scholar]
- Joshi, M.; Bhattacharyya, A. Characterization Techniques for Nanotechnology Applications in Textiles. Indian J. Fiber Text. Res. 2008, 33, 304–317. [Google Scholar]
- Picas, L.; Milhiet, P.E.; Hernandez-Borrell, J. Atomic Force Microscopy: A Versatile Tool to Probe the Physical and Chemical Properties of Supported Membranes at the Nanoscale. Chem. Phys. Lipids 2012, 165, 845–860. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Kim, H.; Jang, Y.; Jang, J. Enhanced Antibacterial Activity of Silver/Polyrhodanine-Composite-Decorated Silica Nanoparticles. ACS Appl. Mater. Interfaces 2013, 5, 11563–11568. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.C.; Lin, S.; Wang, P.C.; Sridhar, R. Techniques for Physicochemical Characterization of Nanomaterials. Biotechnol. Adv. 2014, 32, 711–726. [Google Scholar] [CrossRef]
- Zhang, X.F.; Liu, Z.G.; Shen, W.; Gurunathan, S. Silver nanoparticles: Synthesis, characterization, properties, applications, and therapeutic approaches. Int. J. Mol. Sci. 2016, 17, 1534. [Google Scholar] [CrossRef]
- Ider, M.; Abderrafi, K.; Eddahbi, A.; Ouaskit, S.; Kassiba, A. Silver Metallic Nanoparticles with Surface Plasmon Resonance: Synthesis and Characterizations. J. Clust. Sci. 2017, 28, 1051–1069. [Google Scholar] [CrossRef]
- de Matos, R.A.; da Silva Cordeiro, T.; Samad, R.E.; Sicchieri, L.B.; Júnior, N.D.V.; Courrol, L.C. Synthesis of Silver Nanoparticles Using Agar–Agar Water Solution and Femtosecond Pulse Laser Irradiation. Colloids Surf. A Physicochem. Eng. Asp. 2013, 423, 58–62. [Google Scholar] [CrossRef]
- Mahmudin, L.; Suharyadi, E.; Utomo, A.B.S.; Abraha, K. Optical Properties of Silver Nanoparticles for Surface Plasmon Resonance (SPR)-Based Biosensor Applications. J. Mod. Phys. 2015, 6, 1071. [Google Scholar] [CrossRef]
- Rebe Raz, S.; Leontaridou, M.; Bremer, M.G.; Peters, R.; Weigel, S. Development of Surface Plasmon Resonance-Based Sensor for Detection of Silver Nanoparticles in Food and the Environment. Anal. Bioanal. Chem. 2012, 403, 2843–2850. [Google Scholar] [CrossRef] [PubMed]
- Taher, M.A.; Khojah, E.; Darwish, M.S.; Elsherbiny, E.A.; Elawady, A.A.; Dawood, D.H. Biosynthesis of Silver Nanoparticles by Polysaccharide of Leucaena leucocephala Seeds and Their Anticancer, Antifungal Properties and as Preservative of Composite Milk Sample. J. Nanomater. 2022, 2022, 7490221. [Google Scholar] [CrossRef]
- Erdogan, O.; Abbak, M.; Demirbolat, G.M.; Birtekocak, F.; Aksel, M.; Pasa, S.; Cevik, O. Green Synthesis of Silver Nanoparticles via Cynara scolymus Leaf Extracts: The Characterization, Anticancer Potential with Photodynamic Therapy in MCF7 Cells. PLoS ONE 2019, 14, e0216496. [Google Scholar] [CrossRef]
- Alshehri, A.H.; Jakubowska, M.; Młożniak, A.; Horaczek, M.; Rudka, D.; Free, C.; Carey, J.D. Enhanced electrical conductivity of silver nanoparticles for high-frequency electronic applications. ACS Appl. Mater. Interfaces 2012, 4, 7007–7010. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Lu, J.; Lam, C.; Yu, Y. A novel green synthesis approach for polymer nanocomposites decorated with silver nanoparticles and their antibacterial activity. Analyst 2014, 139, 5793–5799. [Google Scholar] [CrossRef]
- Braun, G.B.; Friman, T.; Pang, H.-B.; Pallaoro, A.; de Mendoza, T.H.; Willmore, A.-M.A.; Kotamraju, V.R.; Mann, A.P.; She, Z.-G.; Sugahara, K.N.; et al. Etchable plasmonic nanoparticle probes to image and quantify cellular internalization. Nat. Mater. 2014, 13, 904–911. [Google Scholar] [CrossRef]
- Goodman, A.M.; Cao, Y.; Urban, C.; Neumann, O.; Ayala-Orozco, C.; Knight, M.W.; Joshi, A.; Nordlander, P.; Halas, N.J. The surprising in vivo instability of near-IR-absorbing hollow Au–Ag nanoshells. ACS Nano 2014, 8, 3222–3231. [Google Scholar] [CrossRef]
- Mei, L.; Wang, Q. Advances in using nanotechnology structuring approaches for improving food packaging. Annu. Rev. Food Sci. Technol. 2020, 11, 339–364. [Google Scholar] [CrossRef] [PubMed]
- Sganzerla, W.G.; Longo, M.; de Oliveira, J.L.; da Rosa, C.G.; de Lima Veeck, A.P.; de Aquino, R.S.; Masiero, A.V.; Bertoldi, F.C.; Barreto, P.L.M.; Nunes, M.R. Nanocomposite poly(ethylene oxide) films functionalized with silver nanoparticles synthesized with Acca sellowiana extracts. Colloids Surf. A Physicochem. Eng. Aspects 2020, 602, 125125. [Google Scholar] [CrossRef]
- Nunes, M.R.; de Souza Maguerroski Castilho, M.D.S.M.; de Lima Veeck, A.P.D.L.; da Rosa, C.G.; Noronha, C.M.; Maciel, M.V.O.B.; Barreto, P.M. Antioxidant and antimicrobial methylcellulose films containing Lippia alba extract and silver nanoparticles. Carbohydr. Polym. 2018, 192, 37–43. [Google Scholar] [CrossRef]
- Costa, C.; Conte, A.; Buonocore, G.G.; Lavorgna, M.; Del Nobile, M.A. Calcium-alginate coating loaded with silver-montmorillonite nanoparticles to prolong the shelf-life of fresh-cut carrots. Food Res. Int. 2012, 48, 164–169. [Google Scholar] [CrossRef]
- Dakal, T.C.; Kumar, A.; Majumdar, R.S.; Yadav, V. Mechanistic basis of antimicrobial actions of silver nanoparticles. Front. Microbiol. 2016, 7, 1831. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.W.; Jahangir, M.; Qaisar, M.; Khan, S.A.; Mahmood, T.; Saeed, M.; Farid, A.; Liaquat, M. Storage stability of Kinnow fruit (Citrus reticulata) as affected by CMC and guar gum-based silver nanoparticle coatings. Molecules 2015, 20, 22645–22661. [Google Scholar] [CrossRef] [PubMed]
- Velgosova, O.; Mačák, L.; Múdra, E.; Vojtko, M.; Lisnichuk, M. Preparation, Structure, and Properties of PVA–AgNPs Nanocomposites. Polymers 2023, 15, 379. [Google Scholar] [CrossRef]
- Zhang, W.; Jiang, W. Antioxidant and Antibacterial Chitosan Film with Tea Polyphenols-Mediated Green Synthesis Silver Nanoparticle via a Novel One-Pot Method. Int. J. Biol. Macromol. 2020, 155, 1252–1261. [Google Scholar] [CrossRef]
- Mačák, L.; Velgosova, O.; Dolinská, S. Impact of Two Lavender Extracts on Silver Nanoparticle Synthesis, and the Study of Nanoparticles’ Antibiofilm Properties and Their Ability to Transfer Them into a Nontoxic Polymer. Micro 2023, 3, 879–891. [Google Scholar] [CrossRef]
- Yaqoob, N.; Zahira, A.; Kamal, S.; Almas, M.; Rehman, S. Development of Multifunctional Bioactive Food Packaging Based on Silver Nanoparticles/Grape Fruit Peel Extract Reinforced PVA Composites. Mater. Today Commun. 2023, 37, 107529. [Google Scholar] [CrossRef]
- Nafchi, A.M.; Alias, A.K.; Mahmud, S.; Robal, M. Antimicrobial, rheological, and physicochemical properties of sago starch films filled with nanorod-rich zinc oxide. J. Food Eng. 2012, 113, 511–519. [Google Scholar] [CrossRef]
- Gurunathan, S.; Qasim, M.; Park, C.; Yoo, H.; Choi, D.Y.; Song, H.; Hong, K. Cytotoxicity and Transcriptomic Analysis of Silver Nanoparticles in Mouse Embryonic Fibroblast Cells. Int. J. Mol. Sci. 2018, 19, 3618. [Google Scholar] [CrossRef]
- Zhang, T.; Wang, L.; Chen, Q.; Chen, C. Cytotoxic Potential of Silver Nanoparticles. Yonsei Med. J. 2014, 55, 283–291. [Google Scholar] [CrossRef]
- Darmadi, J.; Anwar, A. Cytotoxicity of Synthesized Silver Nanoparticles on Breast Cancer Cells. E3S Web Conf. 2024, 488, 03022. [Google Scholar] [CrossRef]
- Li, Y.; Bhalli, J.A.; Ding, W.; Yan, J.; Pearce, M.G.; Sadiq, R.; Chen, T. Cytotoxicity and Genotoxicity Assessment of Silver Nanoparticles in Mouse. Nanotoxicology 2014, 8 (Suppl. S1), 36–45. [Google Scholar] [CrossRef]
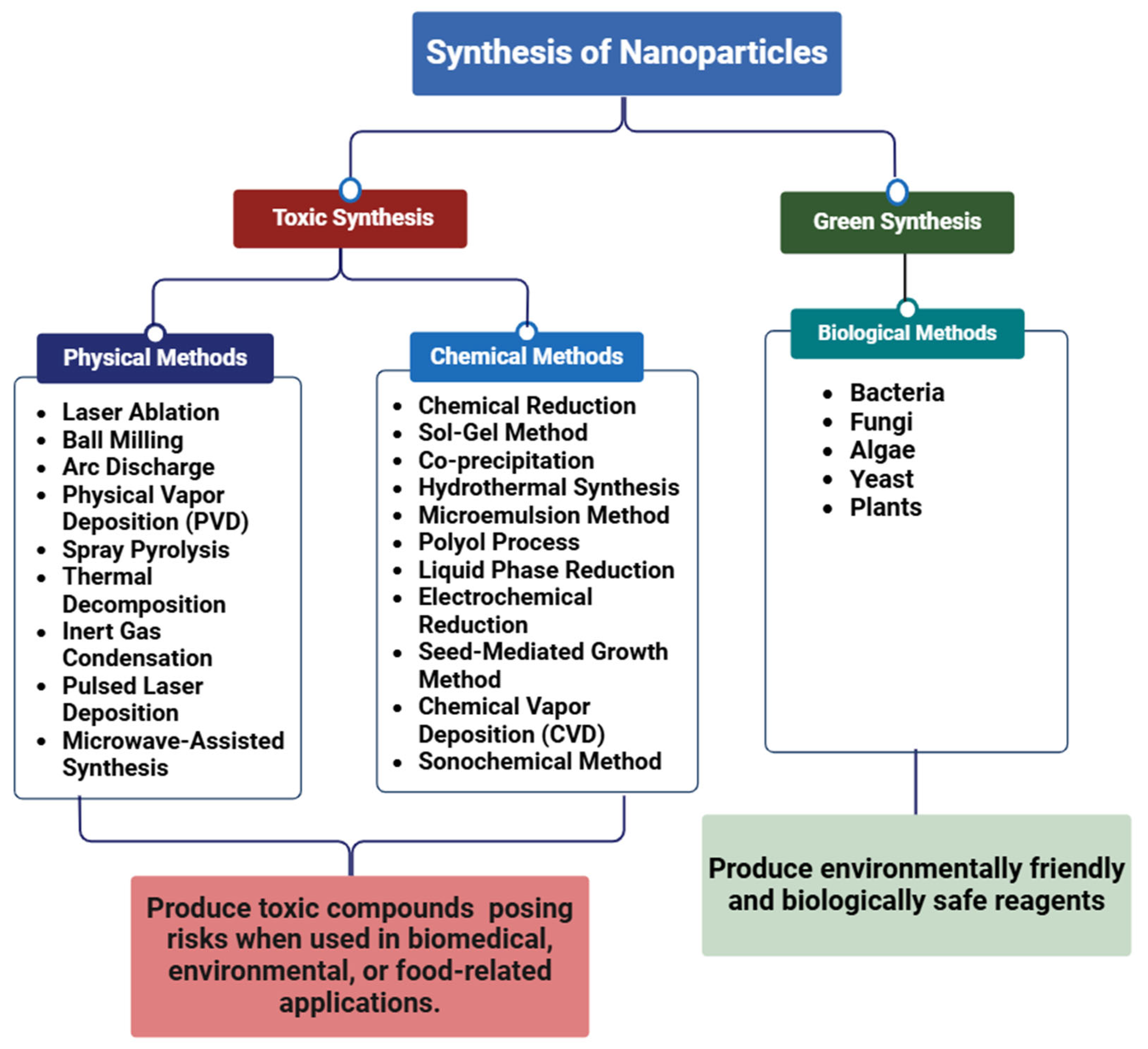
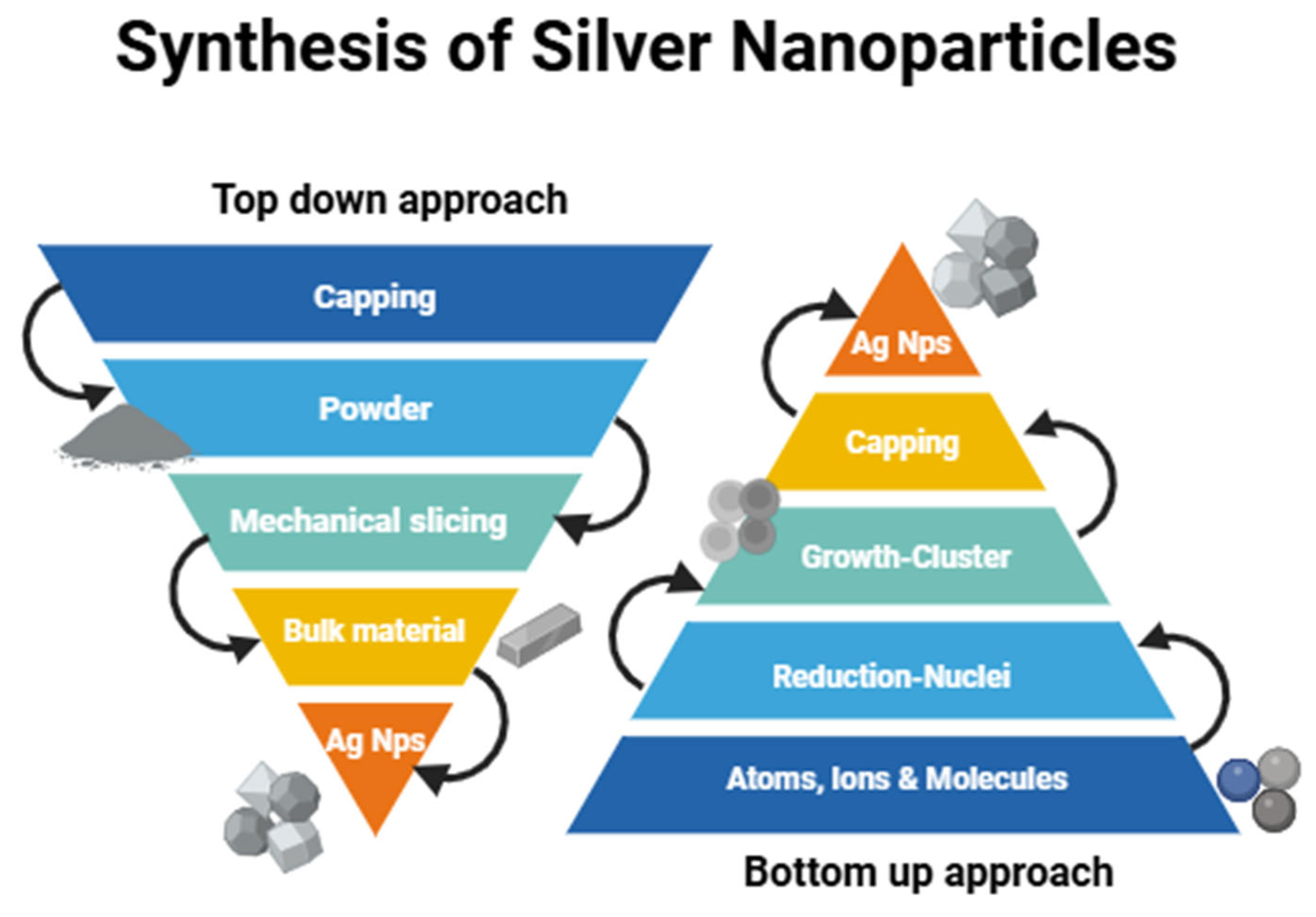
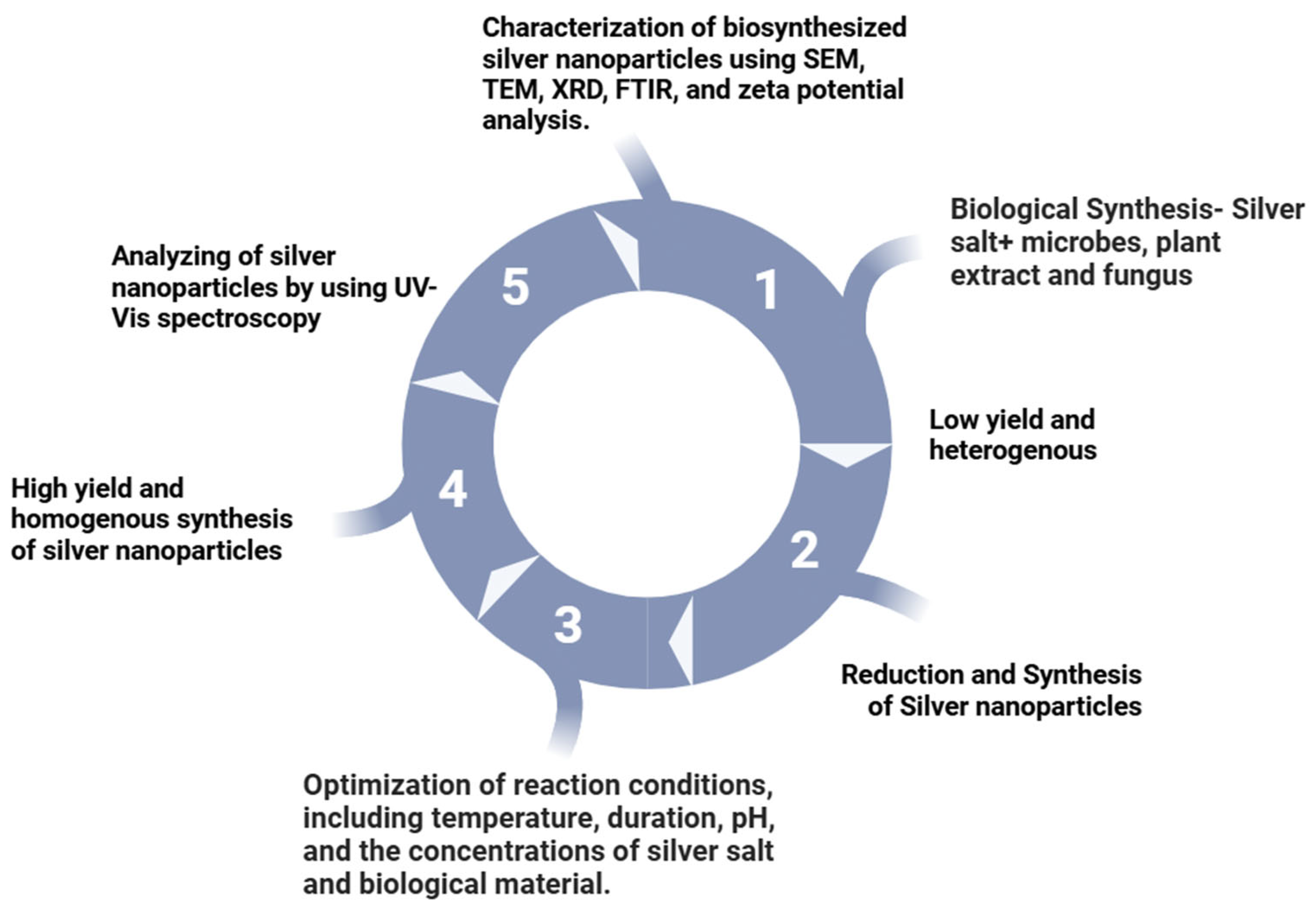
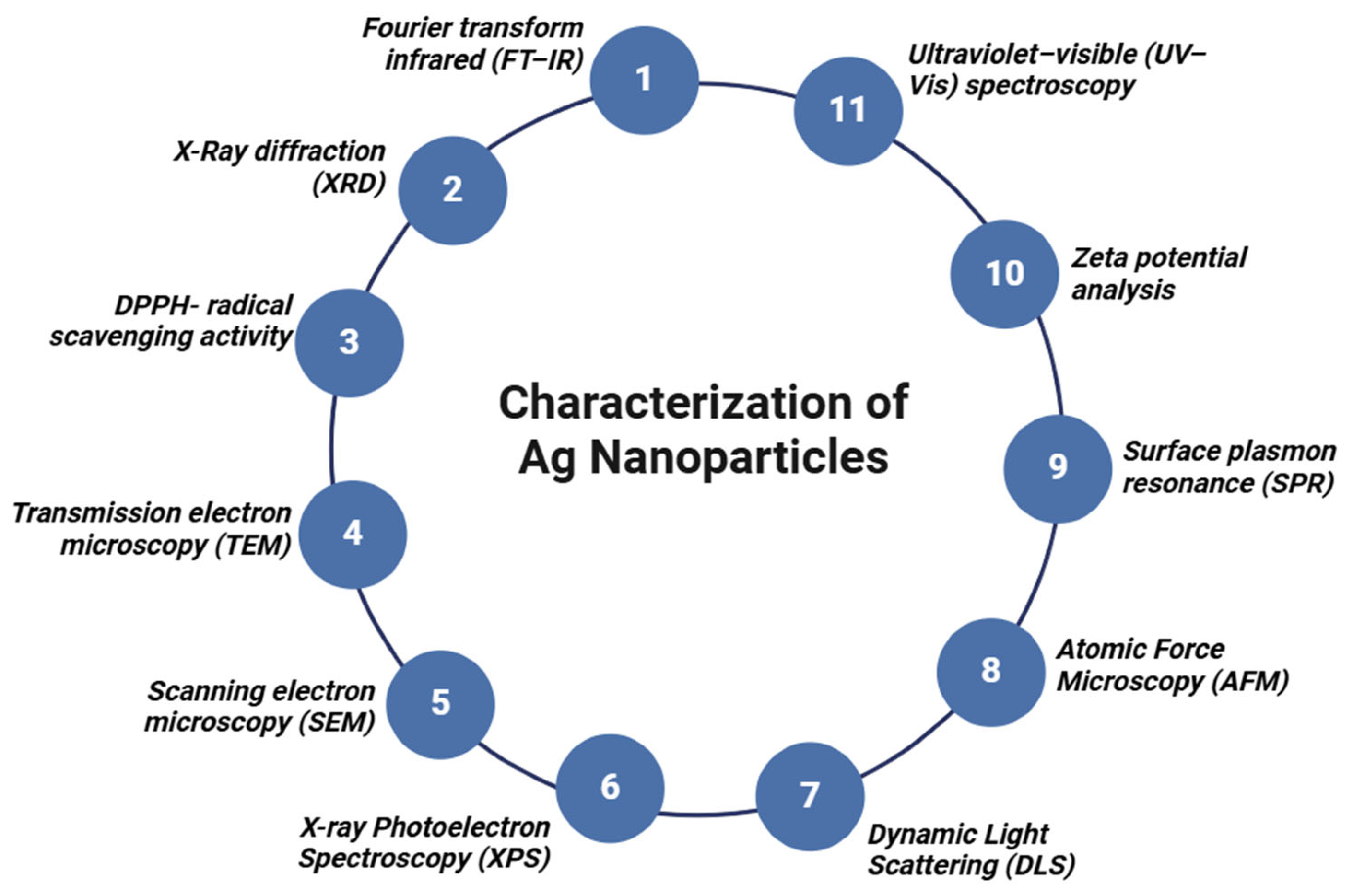


| Plant | Part | Shape | Size (nm) | Substrate | Substrate Conc | Wavelength (nm) | Technique Used | Application | Refs. |
|---|---|---|---|---|---|---|---|---|---|
| Azadirachta indica | Leaf | Spherical | 20–40 | AgNO3 | 1–5 mM | 441 | FTIR, UV–Vis, DLS, photoluminescence, TEM | Antimicrobial activity against Escherichia coli and Staphylococcus aureus | [180,181] |
| Aloe barbadensis miller | Leaf | Spherical | 34–55 | AgNO3 | 1 mM | 430 | UV, FTIR, XRD and TEM | Antibacterial activity | [182,183] |
| Trianthema decandra | Leaf | Spherical | 36–74 | AgNO3 | 1 mM | EDX, FTIR, UV–Vis, SEM | Antimicrobial activity | [184,185] | |
| Macrotyloma uniflorum | Seed | Spherical | 12 | AgNO3 | 0.59 mM | 433 | TEM, XRD, UV–Vis, FTIR | - | [186] |
| Rosa rugosa | Leaf | Spherical | 12 | AgNO3 | 1 mM | - | UV–Vis, XRD, EDX TEM, FTIR, zeta potential | - | [187] |
| Nelumbo nucifera | Seed | Spherical | 5.03–16.62 | AgNO3 | 1 mM | 400–450 | UV–Vis, TEM, FTIR, XRD, SEM | Antifungal and antibacterial | [188] |
| Eriobotrya japonica | Leaf | Triangular and hexagonal | 9.26 ± 2.72 at 20 °C | leaf extract and silver salt solution | 200–900 | UV–Vis, TEM, XRD, SEM, XRD, FTIR | Catalytic degradation of reactive dyes | [189] | |
| Manilkara zapota | Pomace | Spherical | 8–16 | AgNO3 | 7 mM | 300–800 | UV–Vis, DLS, XRD, FTIR, TEM, zeta potential | A strong ability to combat both Gram-negative and Gram-positive bacteria | [190] |
| Punica granatum | Peel | Spherical | 5–50 | AgNO3 | 1 mM | 371 | UV–Vis, FTIR, SEM | Antimicrobial action against infections caused by Escherichia coli, Pseudomonas aeruginosa, and Staphylococcus | [191] |
| Punica granatum | Leaf | - | 10–30 | AgNO3 | 1 mM | 300–700 | UV–Vis, FTIR, SEM, XRD, EDX | Antimicrobial and anticancer properties against human cervical cancer cells | [192,193] |
| Arbutus unedo | Leaf | Spherical | 2–20 | AgNO3 | 1 mM | 300–800 | UV–Vis, EDS, TEM, XRD | Cost effectiveness, and medical and pharmaceutical applications | [194] |
| Juglans regia | Seed | Spherical | 80–90 | AgNO3 | 1 mM | 420 | UV–Vis, TEM, FTIR, XRD, | Used in photocatalytic degradation of effluent dye | [195] |
| Cinnamomum camphora | Leaf | Spherical and triangular | 55–80 | AgNO3 | 1 mM | 440 | UV–Vis, AFM, XRD, TEM, SEM, FTIR | [196] | |
| Alpinia katsumadai | Seed | Spherical | 12.6 | AgNO3 | 10 mM | 300–700 | UV–Vis, EDX, FETEM, SAED, FTIR XRD | Free radical scavenging, antibacterial, and antioxidant | [197] |
| Berberis vulgaris | Leaf and root | Spherical | 30–70 | AgNO3 | 0.5, 1, 3, 10 mM | 450 | XRD, DLS, TEM, UV–Vis | Antimicrobial activity against Staphylococcus and Escherichia coli | [198] |
| Persea americana | Leaf | Spherical | 35.6 | AgNO3 | 5 mM | 432 | FTIR, XRD, SEM, UV–Vis | Antibacterial activity | [199] |
| Croton sparsiflorus | Root | Spherical | 30–50 | AgNO3 | 1 M | - | UV–Vis, SEM | Antimicrobial activity | [200] |
| Coleus forskohlii | Roots | Needle | 82.46 | AgNO3 | 1 mM | 420 | UV–Vis, SEM, EDS, FTIR, XRD | Antimicrobial activity | [201] |
| Lemon | Leaf | Multi-shaped | Smaller than 100 nm range | AgNO3 | 2 mM | 400–480 | FTIR, SEM, UV–Vis, TEM, AFM | Antimicrobial activity | [202,203] |
| Musaceae | Peel | Spherical | 23.7 | AgNO3 | 1.75 mM | 433 | UV–Vis, EDX, XRD, SEM | Antimicrobial activity | [204] |
| Tectona grandis | Seed | Spherical | 10–30 | AgNO3 | 1 mM | 300–600 | UV–Visible, TEM, XRD, FTIR, SEM/EDS, FESEM | Antimicrobial activity against microorganisms | [205] |
| Olea europaea | Leaf | Spherical | 20–25 | AgNO3 | 1 mM | 440–458 | TEM, UV–Vis, FTIR, TG, XRD | Antibacterial activity | [206] |
| Acalypha indica | Leaf | Shape | 20–30 | AgNO3 | 1 mM | 450 | UV–Vis, antifungal | Antifungal effect against Phytopathogen Colletotrichum capsica | [207,208,209] |
| Ficus benghalensis | Leaf | - | 16 | AgNO3 | - | - | UV–Vis, XRD, TEM-EDX, XRD | Antibacterial activity | [210] |
| Litchi chinensis | Leaf | Spherical | 41–55 | AgNO3 | 1 M | 300–500 | UV–Vis | Anti-inflammatory, analgesic, and powerful muscle-relaxant properties | [211,212] |
| Salvia leriifolia | Leaf | Spherical | 27 | AgNO3 | 1 mM | 200–800 | SEM, AFM, XRD, FTIR | Antibacterial activity against 9 bacteria | [213] |
| Glycyrrhiza uralensis | Root | Spherical | 5–15 | AgNO3 | 1 mM | 670 | UV–Vis, XRD, TEM, DLS, FTIR, SAED | Antimicrobial agent that inhibits Salmonella enterica, Pseudomonas aeruginosa, Staphylococcus aureus, and Escherichia coli | [214] |
| Citrus x sinensis (L.) | Peel | - | 48.1 ± 20.5 | AgNO3 | 1 mM | 300–700 | UV–Vis, DLS, FTIR, XRD, zeta potential, TEM | Antimicrobial activity | [215] |
| Citrus recticulata | Peel | 24 | AgNO3 | 1 mM | 460 | UV–Vis, SEM, FTIR, EDX, XRD | Antibacterial activity against Salmonella Paratyphi, Bacillus subtilis, Escherichia coli, Streptococcus pyogenes, Staphylococcus aureus, and Klebsiella pneumoniae | [216] | |
| Skimmia laureola | Leaf | Hexagonal and spherical | 46 | AgNO3 | 10 mM | 460 | UV–Vis, SEM, XRD, FTIR | Antibacterial | [217] |
| Clitoria ternatea and Solanum nigrum | Leaf | Spherical | 20–30 | AgNO3 | 0.1 M | 420–440 | UV–Vis, XRD, FTIR | Antibacterial | [218] |
| Diospyros paniculata | Root | Spherical | 17 | AgNO3 | 10 mM | 428 | UV–Vis, TEM, XRD | Antimicrobial | [219] |
| Pedalium murex | Leaf | Spherical | 20–50 | AgNO3 | 0.01 mM | 430 | UV–Vis, DLS, FTIR, XRD, EDAX, FE-SEM, TEM | Antibacterial | [220] |
| Phlomis | Leaf | Spherical | 25 | AgNO3 | 0.01 M | 450 | UV–Vis, FT-IR, TEM, XRD, SEM | Antibacterial | [221] |
| Atrocarpus altilis | Leaf | Spherical | 25–43 | AgNO3 | 0.01 M | 432 | UV–Vis, FT-IR, XRD | Antimicrobial and antioxidant activity | [222] |
| Calliandra haematocephala | Leaf | Spherical | 70 | AgNO3 | 1 mM | 414 | UV–Vis, EDS, FTIR, SEM, XRD | Antibacterial | [223] |
| Diospyros montana | Stem, bark | Spherical | 5–40 | AgNO3 | 1 mM | 200–600 | UV–Vis, SEM, XRD, FTIR | Antibacterial | [224] |
| Caesalpinia pulcherrima | Stem | Spherical | 3–15 | AgNO3 | 1 mM | 350–750 | UV–Vis, TEM, FTIR, XRD | Antimicrobial | [225] |
| Garcinia mangostana | Stem | Spherical | 30 | AgNO3 | 1 mM | 430 | UV–Vis, EDX, SEM, XRD | Antimicrobial | [226] |
| Malva sylvestris | Flower | Spherical | 20–40 | AgNO3 | 50 mM | 200–800 | UV–Vis, AFM, FTIR, TEM, EDX | Antibacterial | [227] |
| Phoenix dactylifera | Root | Spherical | 15–40 | AgNO3 | 0.1 mM | 420 | UV–Vis, XRD, FTIR | Antimicrobial and anticancer activities | [228] |
| Ficus hispida | Leaf | Spherical | 20 | AgNO3 | 4 mM | - | UV–Vis, SEM, FTIR, XRD, TEM | Catalytic, antioxidant, and antibacterial activities | [229] |
| Moringa oleifera | Leaf | Spherical | 57 | AgNO3 | 1 mM | 450 | UV–Vis, EDX, SEM, FTIR, TEM | Antimicrobial | [230] |
| Phyllanthus pinnatus | Stem | Cubical and spherical | - | AgNO3 | 1 mM | 490 | UV–Vis, FTIR, SEM, XRD | Antibacterial | [231] |
| Berberis asiatica | Root | Spherical | 9.8 | AgNO3 | 1 mM | 427 | UV–Vis, SPR, XRD, TEM | Antibacterial | [232] |
| Astragalus tribuloides | Root | Spherical | 16.2–51.5 | AgNO3 | 1 mM | 430 | UV–Vis, TEM, FTIR, XRD | Antioxidant and antibacterial activities | [233] |
| Annona senegalensis | Stem, bark | Spherical | 1–24 | AgNO3 | 5 mM | 431.19 | UV–Vis, TEM, SEM, FTIR, EDX | Antibacterial | [234] |
| Euphorbia nivulia | Stem, bark | Spherical | 20–90 | AgNO3 | 1 mM | 432 | UV–Vis, SEM, FTIR | Antimicrobial | [235] |
| Jatropha curcas | Seed | Spherical | 80–95 | AgNO3 | 1 mM | 400–460 | UV–Vis, SEM, FTIR | Antibacterial | [236] |
| Piper nigrum | Seed | Spherical | 15–38 | AgNO3 | 1 mM | UV–Vis, SPR, SEM, XRD, FTIR | Antibacterial | [237] | |
| Duchesnea indica | Root | Spherical | 20.49 | AgNO3 | 2 mM | 423 | UV–Vis, XRD, TEM, EDX, SEM, FTIR | Antimicrobial and anti-inflammatory activities | [238] |
| Hagenia abyssinica | Leaf | Spherical | 22.2 | AgNO3 | 5 mM | 430 | UV–Vis, XRD, FTIR | Antioxidant and antibacterial activities | [239] |
| Kalanchoe pinnata | Leaf | Spherical | 38 | AgNO3 | 1 mM | 430 | - | Photocatalytic and Antibacterial activities | [240] |
| Salvia officinalis | Leaf | Spherical | 41 | AgNO3 | 1 mM | 323 | UV–Vis, SEM, FTIR, TEM, XRD, EDX | Antiplasmodial activity | [241] |
| Aerva lanata | Flower | Spherical | 2–10 | AgNO3 | 0.002 M | 220–700 | UV–Vis, EDX, DLS, TEM | Catalytic and Antioxidant activity | [242] |
| Rubus ellipticus | Root | Spherical | 23 | AgNO3 | 1 mM | 416–420 | FTIR, XRD, TEM, TEM | Antibacterial | [243] |
| Alhagi graecorum | Leaf | Spherical | 22–36 | AgNO3 | 1 mM | 300–800 | UV–Vis, FTIR, SEM | Cytotoxicity and antifungal | [244] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zehra, S.H.; Ramzan, K.; Viskelis, J.; Viskelis, P.; Balciunaitiene, A. Advancements in Green Synthesis of Silver-Based Nanoparticles: Antimicrobial and Antifungal Properties in Various Films. Nanomaterials 2025, 15, 252. https://doi.org/10.3390/nano15040252
Zehra SH, Ramzan K, Viskelis J, Viskelis P, Balciunaitiene A. Advancements in Green Synthesis of Silver-Based Nanoparticles: Antimicrobial and Antifungal Properties in Various Films. Nanomaterials. 2025; 15(4):252. https://doi.org/10.3390/nano15040252
Chicago/Turabian StyleZehra, Syeda Hijab, Khadija Ramzan, Jonas Viskelis, Pranas Viskelis, and Aiste Balciunaitiene. 2025. "Advancements in Green Synthesis of Silver-Based Nanoparticles: Antimicrobial and Antifungal Properties in Various Films" Nanomaterials 15, no. 4: 252. https://doi.org/10.3390/nano15040252
APA StyleZehra, S. H., Ramzan, K., Viskelis, J., Viskelis, P., & Balciunaitiene, A. (2025). Advancements in Green Synthesis of Silver-Based Nanoparticles: Antimicrobial and Antifungal Properties in Various Films. Nanomaterials, 15(4), 252. https://doi.org/10.3390/nano15040252










