Enhanced Persistent Luminescence from Cr3+-Doped ZnGa2O4 Nanoparticles upon Immersion in Simulated Physiological Media
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Preparation of PBS Solution
2.3. Synthesis of CZGO NPs
2.4. Surface Hydroxylation of CZGO NPs
2.5. Immersion of CZGO-0 in the PBS Solution
2.6. Characterization
3. Results and Discussion
3.1. Morphology
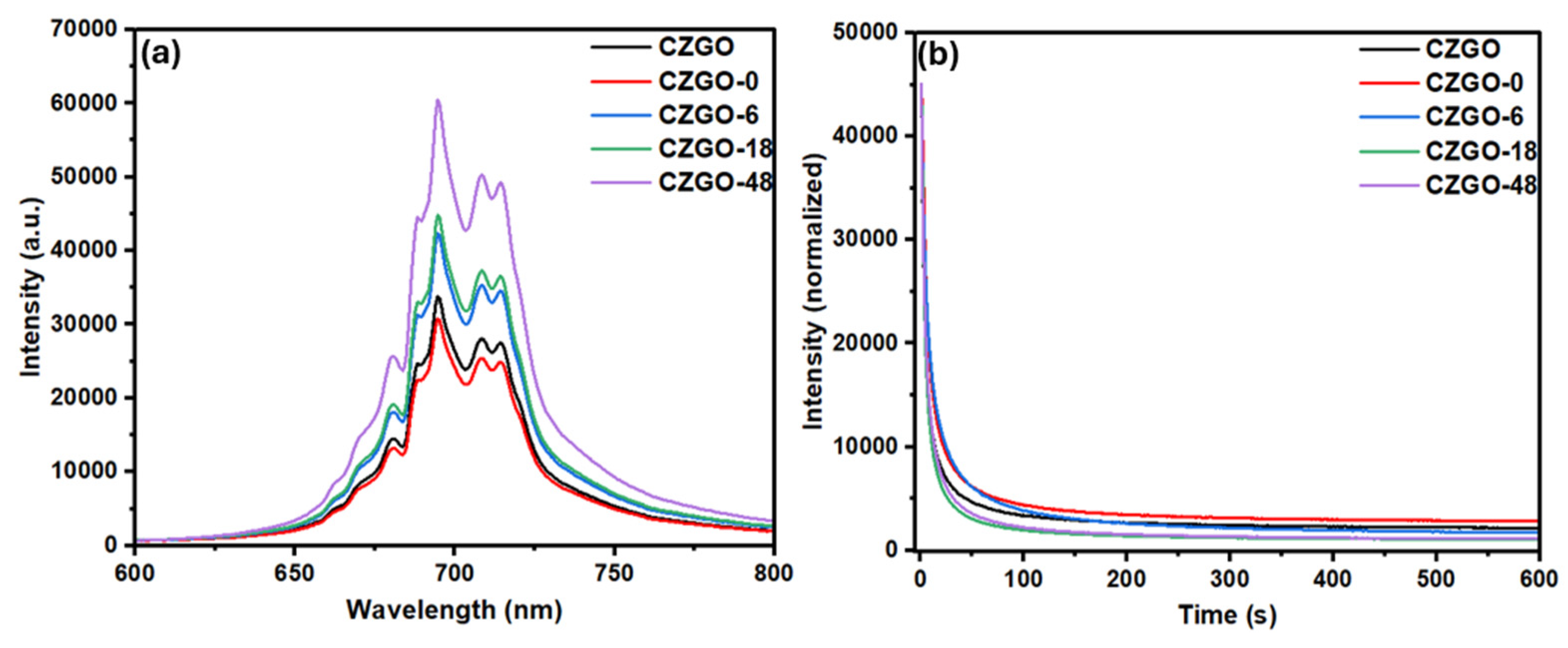
3.2. Luminescence Properties
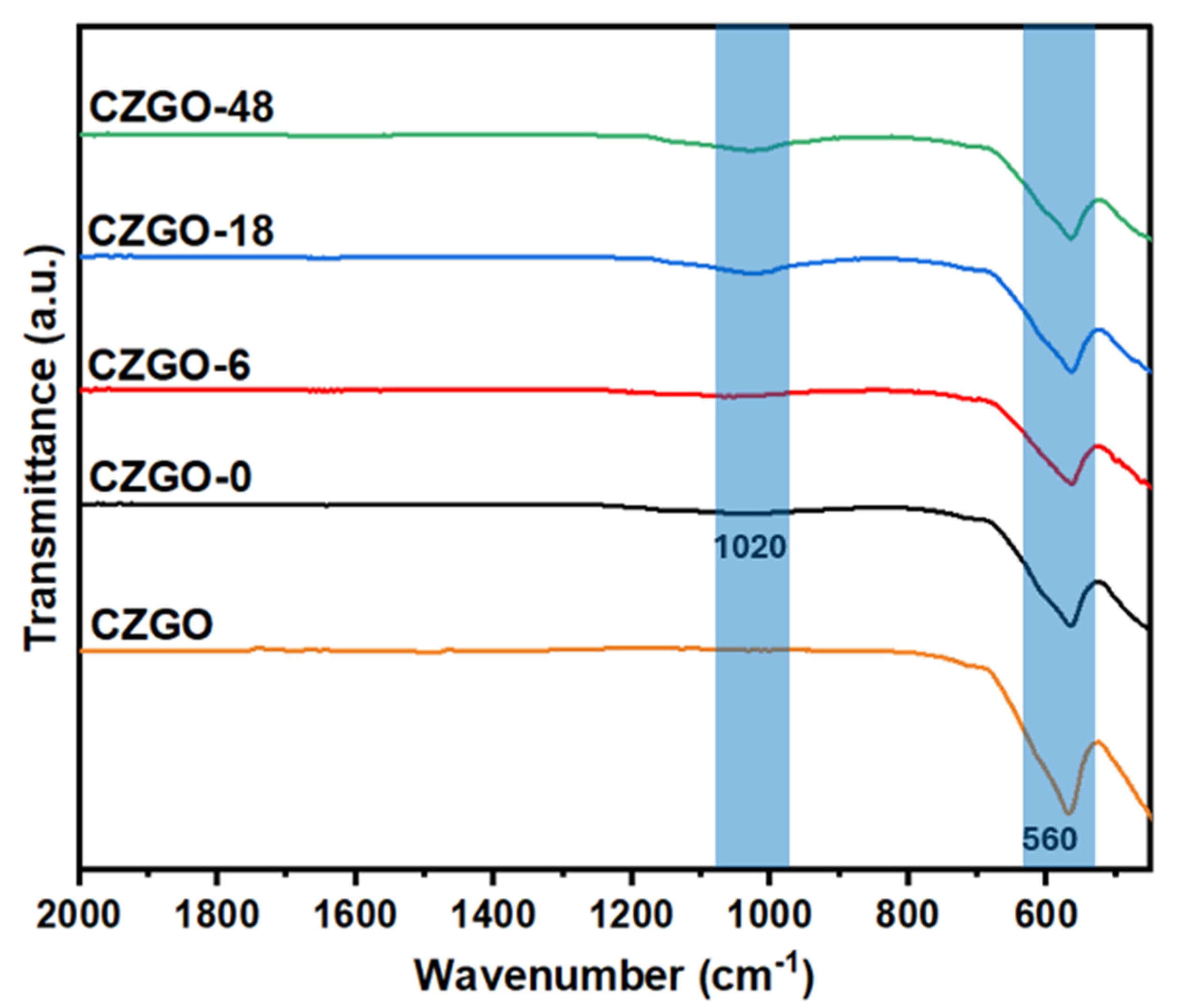
3.3. Surface Chemistry and Crystal Structure
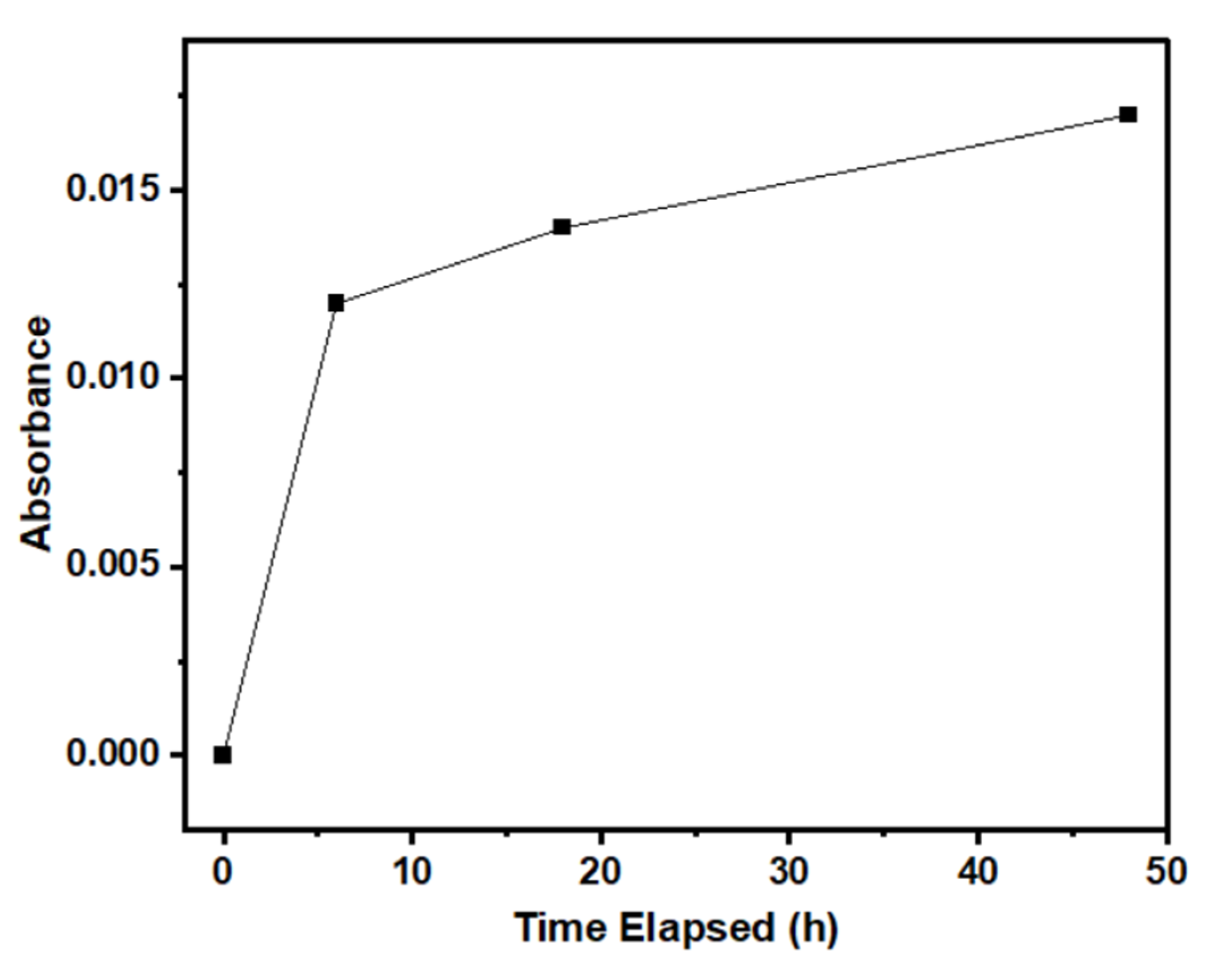
3.4. Proposed Formation Mechanism of ZnO
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yang, F.; Zhang, Q.; Huang, S.; Ma, D. Recent Advances of near Infrared Inorganic Fluorescent Probes for Biomedical Applications. J. Mater. Chem. B 2020, 8, 7856–7879. [Google Scholar] [CrossRef] [PubMed]
- Parchur, A.K.; Sharma, G.; Jagtap, J.M.; Gogineni, V.R.; LaViolette, P.S.; Flister, M.J.; White, S.B.; Joshi, A. Vascular Interventional Radiology-Guided Photothermal Therapy of Colorectal Cancer Liver Metastasis with Theranostic Gold Nanorods. ACS Nano 2018, 12, 6597–6611. [Google Scholar] [CrossRef]
- Shirahata, N. Nanoparticle Biomarkers Adapted for Near-Infrared Fluorescence Imaging. In System-Materials Nanoarchitectonics; NIMS Monographs; Wakayama, Y., Ariga, K., Eds.; Springer: Tokyo, Japan, 2022; pp. 27–50. [Google Scholar] [CrossRef]
- Zhou, Y.; Sun, X.; Zhou, L.; Zhang, X. pH-Sensitive and Long-Circulation Nanoparticles for Near-Infrared Fluorescence Imaging-Monitored and Chemo-Photothermal Synergistic Treatment Against Gastric Cancer. Front. Pharmacol. 2020, 11, 610883. [Google Scholar] [CrossRef]
- Lifante, J.; Shen, Y.; Ximendes, E.; Martín Rodríguez, E.; Ortgies, D.H. The Role of Tissue Fluorescence in in Vivo Optical Bioimaging. J. Appl. Phys. 2020, 128, 171101. [Google Scholar] [CrossRef]
- Kuchimaru, T.; Iwano, S.; Kiyama, M.; Mitsumata, S.; Kadonosono, T.; Niwa, H.; Maki, S.; Kizaka-Kondoh, S. A Luciferin Analogue Generating Near-Infrared Bioluminescence Achieves Highly Sensitive Deep-Tissue Imaging. Nat. Commun. 2016, 7, 11856. [Google Scholar] [CrossRef] [PubMed]
- Miao, Q.; Xie, C.; Zhen, X.; Lyu, Y.; Duan, H.; Liu, X.; Jokerst, J.V.; Pu, K. Molecular Afterglow Imaging with Bright, Biodegradable Polymer Nanoparticles. Nat. Biotechnol. 2017, 35, 1102–1110. [Google Scholar] [CrossRef] [PubMed]
- Hao, F.; Yan, Z.-Y.; Wang, Z.; Yan, X.-P. Persistent Luminescence Nanoplatform for Autofluorescence-Free Tracking of Submicrometer Plastic Particles in Plant. Anal. Chem. 2024, 96, 10662–10668. [Google Scholar] [CrossRef]
- Wang, R.; Shi, J.; Zhang, Q.; Peng, Q.; Sun, X.; Song, L.; Zhang, Y. Dual-Triggered Near-Infrared Persistent Luminescence Nanoprobe for Autofluorescence-Free Imaging-Guided Precise Therapy of Rheumatoid Arthritis. Adv. Sci. 2023, 10, 2205320. [Google Scholar] [CrossRef] [PubMed]
- Dai, W.; Qi, B.; Li, Z.; Wang, J. Bimodal Persistent Luminescence for Autofluorescence-Free Ratiometric Biosensing. Anal. Bioanal. Chem. 2023, 415, 6723–6731. [Google Scholar] [CrossRef]
- Brito, H.F.; Hölsä, J.; Laamanen, T.; Lastusaari, M.; Malkamäki, M.; Rodrigues, L.C.V. Persistent Luminescence Mechanisms: Human Imagination at Work. Opt. Mater. Express 2012, 2, 371. [Google Scholar] [CrossRef]
- Zeng, C.; Hu, T.; Hou, N.; Liu, S.; Gao, W.; Cong, R.; Yang, T. Photocatalytic Pure Water Splitting Activities for ZnGa2O4 Synthesized by Various Methods. Mater. Res. Bull. 2015, 61, 481–485. [Google Scholar] [CrossRef]
- Yang, W.; Li, J.; Liu, B.; Zhang, X.; Zhang, C.; Niu, P.; Jiang, X. Multi-Wavelength Tailoring of a ZnGa2O4 Nanosheet Phosphor via Defect Engineering. Nanoscale 2018, 10, 19039–19045. [Google Scholar] [CrossRef] [PubMed]
- Pan, Z.; Lu, Y.-Y.; Liu, F. Sunlight-Activated Long-Persistent Luminescence in the near-Infrared from Cr3+-Doped Zinc Gallogermanates. Nat. Mater. 2012, 11, 58–63. [Google Scholar] [CrossRef]
- Luchechko, A.; Zhydachevskyy, Y.; Ubizskii, S.; Kravets, O.; Popov, A.I.; Rogulis, U.; Elsts, E.; Bulur, E.; Suchocki, A. Afterglow, TL and OSL Properties of Mn2+-Doped ZnGa2O4 Phosphor. Sci. Rep. 2019, 9, 9544. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.; Mao, X.; Liu, Q.; Zhu, D.; Chen, H.; Xie, T.; Xu, J.; Hreniak, D.; Nikl, M.; Li, J. Effect of Dopant Concentration on the Optical Characteristics of Cr3+:ZnGa2O4 Transparent Ceramics Exhibiting Persistent Luminescence. Opt. Mater. 2022, 125, 112127. [Google Scholar] [CrossRef]
- Viana, B.; Sharma, S.K.; Gourier, D.; Maldiney, T.; Teston, E.; Scherman, D.; Richard, C. Long Term in Vivo Imaging with Cr3+ Doped Spinel Nanoparticles Exhibiting Persistent Luminescence. J. Lumin. 2016, 170, 879–887. [Google Scholar] [CrossRef]
- Rudolf, M.M.; Bortel, G.; Márkus, B.G.; Jegenyes, N.; Verkhovlyuk, V.; Kamarás, K.; Simon, F.; Gali, A.; Beke, D. Optimization of Chromium-Doped Zinc Gallate Nanocrystals for Strong Near-Infrared Emission by Annealing. ACS Appl. Nano Mater. 2022, 5, 8950–8961. [Google Scholar] [CrossRef]
- Song, T.; Zhang, M.; Liu, Y.; Yang, J.; Gong, Z.; Yan, H.; Zhu, H.; Yan, D.; Liu, C.; Xu, C. Enhanced near Infrared Persistent Luminescence of Zn2Ga2.98Ge0.75O8:Cr0.023+ Nanoparticles by Partial Substitution of Ge4+ by Sn4+. RSC Adv. 2018, 8, 10954–10963. [Google Scholar] [CrossRef]
- Sun, X.; Shi, J.; Fu, X.; Yang, Y.; Zhang, H. Long-Term in Vivo Biodistribution and Toxicity Study of Functionalized near-Infrared Persistent Luminescence Nanoparticles. Sci. Rep. 2018, 8, 10595. [Google Scholar] [CrossRef]
- Liu, H.; Ren, F.; Zhang, H.; Han, Y.; Qin, H.; Zeng, J.; Wang, Y.; Sun, Q.; Li, Z.; Gao, M. Oral Administration of Highly Bright Cr3+ Doped ZnGa2O4 Nanocrystals for in Vivo Targeted Imaging of Orthotopic Breast Cancer. J. Mater. Chem. B 2018, 6, 1508–1518. [Google Scholar] [CrossRef]
- Cai, G.; Seguin, J.; Naillon, T.; Chanéac, C.; Corvis, Y.; Scherman, D.; Mignet, N.; Viana, B.; Richard, C. Chromium-Doped Zinc Gallate: Impact of Sn4+ Co-Doping on the Persistent Luminescence Properties at the Nanoscale Applied to Bio-Imaging. Chem. Eng. J. 2024, 490, 151643. [Google Scholar] [CrossRef]
- Hussen, M.K.; Dejene, F.B. Influence of Annealing Temperature on Material Properties of Red Emitting ZnGa2O4:Cr3+ Nanostructures. J. Sol-Gel Sci. Technol. 2018, 88, 454–464. [Google Scholar] [CrossRef]
- Wang, J.; Ma, Q.; Hu, X.-X.; Liu, H.; Zheng, W.; Chen, X.; Yuan, Q.; Tan, W. Autofluorescence-Free Targeted Tumor Imaging Based on Luminous Nanoparticles with Composition-Dependent Size and Persistent Luminescence. ACS Nano 2017, 11, 8010–8017. [Google Scholar] [CrossRef] [PubMed]
- Abdukayum, A.; Chen, J.-T.; Zhao, Q.; Yan, X.-P. Functional Near Infrared-Emitting Cr3+/Pr3+ Co-Doped Zinc Gallogermanate Persistent Luminescent Nanoparticles with Superlong Afterglow for in Vivo Targeted Bioimaging. J. Am. Chem. Soc. 2013, 135, 14125–14133. [Google Scholar] [CrossRef]
- Feng, Y.; Zhang, L.; Liu, R.; Lv, Y. Modulating Near-Infrared Persistent Luminescence of Core-Shell Nanoplatform for Imaging of Glutathione in Tumor Mouse Model. Biosens. Bioelectron. 2019, 144, 111671. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, Y.; Jiang, K.; Sun, S.; Qian, S.; Wu, Q.; Lin, H. A Persistent Luminescence-Based Label-Free Probe for the Ultrasensitive Detection of Hemoglobin in Human Serum. Talanta 2020, 206, 120206. [Google Scholar] [CrossRef]
- Zhou, Z.; Zheng, W.; Kong, J.; Liu, Y.; Huang, P.; Zhou, S.; Chen, Z.; Shi, J.; Chen, X. Rechargeable and LED-Activated ZnGa2O4:Cr 3+ near-Infrared Persistent Luminescence Nanoprobes for Background-Free Biodetection. Nanoscale 2017, 9, 6846–6853. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, J.-M.; Zhang, D.; Ge, K.; Wang, P.; Liu, H.; Fang, G.; Wang, S. Persistent Luminescence Nanophosphor Involved Near-Infrared Optical Bioimaging for Investigation of Foodborne Probiotics Biodistribution in Vivo: A Proof-of-Concept Study. J. Agric. Food Chem. 2017, 65, 8229–8240. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.-M.; Yuan, X.-Y.; Liu, H.-L.; Cheng, D.; Wang, S. Fabrication of an Activatable Hybrid Persistent Luminescence Nanoprobe for Background-Free Bioimaging-Guided Investigation of Food-Borne Aflatoxin in Vivo. RSC Adv. 2018, 8, 28414–28420. [Google Scholar] [CrossRef] [PubMed]
- Ding, D.; Zhang, Y.; Sykes, E.A.; Chen, L.; Chen, Z.; Tan, W. The Influence of Physiological Environment on the Targeting Effect of Aptamer-Guided Gold Nanoparticles. Nano Res. 2019, 12, 129–135. [Google Scholar] [CrossRef]
- Amézaga González, M.F.; Acosta Bezada, J.; Gómez Flores, V.; Chapa González, C.; Farias Mancilla, J.R.; Castillo, S.J.; Avila Orta, C.; García-Casillas, P.E. Effect of Physiological Fluid on the Photothermal Properties of Gold Nanostructured. Int. J. Mol. Sci. 2023, 24, 8339. [Google Scholar] [CrossRef] [PubMed]
- Lécuyer, T.; Durand, M.-A.; Volatron, J.; Desmau, M.; Lai-Kuen, R.; Corvis, Y.; Seguin, J.; Wang, G.; Alloyeau, D.; Scherman, D.; et al. Degradation of ZnGa2O4:Cr3+ Luminescent Nanoparticles in Lysosomal-like Medium. Nanoscale 2020, 12, 1967–1974. [Google Scholar] [CrossRef] [PubMed]
- Lécuyer, T.; Seguin, J.; Balfourier, A.; Delagrange, M.; Burckel, P.; Lai-Kuen, R.; Mignon, V.; Ducos, B.; Tharaud, M.; Saubaméa, B.; et al. Fate and Biological Impact of Persistent Luminescence Nanoparticles after Injection in Mice: A One-Year Follow-Up. Nanoscale 2022, 14, 15760–15771. [Google Scholar] [CrossRef] [PubMed]
- Sanità, G.; Carrese, B.; Lamberti, A. Nanoparticle Surface Functionalization: How to Improve Biocompatibility and Cellular Internalization. Front. Mol. Biosci. 2020, 7, 587012. [Google Scholar] [CrossRef]
- Nie, S. Understanding and Overcoming Major Barriers in Cancer Nanomedicine. Nanomedicine 2010, 5, 523–528. [Google Scholar] [CrossRef]
- Tang, L.; Yang, X.; Yin, Q.; Cai, K.; Wang, H.; Chaudhury, I.; Yao, C.; Zhou, Q.; Kwon, M.; Hartman, J.A.; et al. Investigating the Optimal Size of Anticancer Nanomedicine. Proc. Natl. Acad. Sci. USA 2014, 111, 15344–15349. [Google Scholar] [CrossRef] [PubMed]
- Phosphate-Buffered Saline (PBS); Cold Spring Harbor Protocols; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA. [CrossRef]
- Chen, H.; Zhang, H.; Wang, J.; Zhu, X. An Injectable Persistent Luminescence Hydrogel for Repeated Photodynamic Therapy of Hypoxic Tumors. J. Mater. Res. 2024, 39, 636–648. [Google Scholar] [CrossRef]
- Mishra, P.; Singh, U.; Pandey, C.; Mishra, P.; Pandey, G. Application of Student’s t-Test, Analysis of Variance, and Covariance. Ann. Card. Anaesth. 2019, 22, 407. [Google Scholar] [CrossRef] [PubMed]
- Basavaraju, N.; Priolkar, K.R.; Gourier, D.; Sharma, S.K.; Bessière, A.; Viana, B. The Importance of Inversion Disorder in the Visible Light Induced Persistent Luminescence in Cr3+ Doped AB2O4 (A = Zn or Mg and B = Ga or Al). Phys. Chem. Chem. Phys. 2015, 17, 1790–1799. [Google Scholar] [CrossRef]
- Zhou, C.; Long, M.; Qin, Y.; Sun, X.; Zheng, J. Luminescent Gold Nanoparticles with Efficient Renal Clearance. Angew. Chem. Int. Ed. Engl. 2011, 50, 3168–3172. [Google Scholar] [CrossRef] [PubMed]
- Calderón-Olvera, R.M.; Arroyo, E.; Jankelow, A.M.; Bashir, R.; Valera, E.; Ocaña, M.; Becerro, A.I. Persistent Luminescence Zn2GeO4:Mn2+ Nanoparticles Functionalized with Polyacrylic Acid: One-Pot Synthesis and Biosensing Applications. ACS Appl. Mater. Interfaces 2023, 15, 20613–20624. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Wang, J.; Chen, N.; Ma, Q.; Lu, D.; Yuan, Q. Enhancement of Long-Lived Luminescence in Nanophosphors by Surface Defect Passivation. Chem. Commun. 2020, 56, 6660–6663. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.-Y.; Lin, J.-W.; Huang, Y.-P.; Huang, Y.-C. Modeling and Assessment of Long Afterglow Decay Curves. Sci. World J. 2014, 2014, 102524. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.-Y.; Lin, J.-W.; Huang, Y.-P.; Huang, Y.-C. Effects of the Offset Term in Experimental Simulation on Afterglow Decay Curve. Sci. World J. 2014, 2014, 497270. [Google Scholar] [CrossRef]
- Kubo, H.; Aizawa, H.; Katsumata, T.; Komuro, S.; Morikawa, T. Characteristics of Long Afterglow Phosphorescent Calcium Aluminate Crystals for Fluorescence Thermometer Application. J. Cryst. Growth 2005, 275, e1767–e1771. [Google Scholar] [CrossRef]
- Wu, H.; Hu, Y.; Wang, X. Investigation of the Trap State of Sr2MgSi2O7: Eu2+, Dy3+ Phosphor and Decay Process. Radiat. Meas. 2011, 46, 591–594. [Google Scholar] [CrossRef]
- Zhu, Y.; Zeng, J.; Li, W.; Xu, L.; Guan, Q.; Liu, Y. Encapsulation of Strontium Aluminate Phosphors to Enhance Water Resistance and Luminescence. Appl. Surf. Sci. 2009, 255, 7580–7585. [Google Scholar] [CrossRef]
- Kumari, P.; Mondal, A.; Kumar Choudhary, A.; Ambast, D.K.S. Structural and Optical Properties of Chromium Doped Zinc Gallate Long Persistent Phosphor Prepared by Surfactant Assisted Hydrothermal Method. J. Phys. Conf. Ser. 2021, 1913, 012039. [Google Scholar] [CrossRef]
- Duarte, F.d.S.; Melo, A.L.M.d.S.; Ferro, A.d.B.; Zanta, C.L.d.P.e.S.; Duarte, J.L.d.S.; Oliveira, R.M.P.B. Magnetic Zinc Oxide/Manganese Ferrite Composite for Photodegradation of the Antibiotic Rifampicin. Materials 2022, 15, 8185. [Google Scholar] [CrossRef] [PubMed]
- Umasangari, T.; Anandhavelu, S.; Thambidurai, S. Eco-Friendly Preparation of Zinc Oxide Nanoparticles with Jackfruit Seed Flour Template and Microwave Assisted Heating. Adv. Sci. Eng. Med. 2013, 5, 841–845. [Google Scholar] [CrossRef]
- Dassonville, D.; Lécuyer, T.; Seguin, J.; Corvis, Y.; Liu, J.; Cai, G.; Mouton, J.; Scherman, D.; Mignet, N.; Richard, C. Zwitterionic Functionalization of Persistent Luminescence Nanoparticles: Physicochemical Characterizations and In Vivo Biodistribution in Mice. Coatings 2023, 13, 1913. [Google Scholar] [CrossRef]
- Liu, J.; Kotrchová, L.; Lécuyer, T.; Corvis, Y.; Seguin, J.; Mignet, N.; Etrych, T.; Scherman, D.; Randárová, E.; Richard, C. Coating Persistent Luminescence Nanoparticles With Hydrophilic Polymers for in Vivo Imaging. Front. Chem. 2020, 8, 584114. [Google Scholar] [CrossRef]
- Maldiney, T.; Richard, C.; Seguin, J.; Wattier, N.; Bessodes, M.; Scherman, D. Effect of Core Diameter, Surface Coating, and PEG Chain Length on the Biodistribution of Persistent Luminescence Nanoparticles in Mice. ACS Nano 2011, 5, 854–862. [Google Scholar] [CrossRef] [PubMed]
- Maldiney, T.; Bessière, A.; Seguin, J.; Teston, E.; Sharma, S.K.; Viana, B.; Bos, A.J.J.; Dorenbos, P.; Bessodes, M.; Gourier, D.; et al. The in Vivo Activation of Persistent Nanophosphors for Optical Imaging of Vascularization, Tumours and Grafted Cells. Nat. Mater. 2014, 13, 418–426. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, S.; Li, Z.; Zhu, Q. Near Infrared-Emitting Cr3+/Eu3+ Co-Doped Zinc Gallogermanate Persistence Luminescent Nanoparticles for Cell Imaging. Nanoscale Res. Lett. 2018, 13, 64. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Liu, Y.; Hulme, B.; Chang, L.; Ke, S.; Wang, E.; Wu, Y.; Lin, B.; Jiang, Y.; Liu, L. ZnGa2O4:Cr3+ @Calcium Phosphate Nanocomposite with Near-Infrared Persistent Luminescence and High Stability. ChemPhotoChem 2023, 7, e202300143. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Lau, L.W.M.; Gerson, A.R.; Smart, R.S.C. Resolving Surface Chemical States in XPS Analysis of First Row Transition Metals, Oxides and Hydroxides: Sc, Ti, V, Cu and Zn. Appl. Surf. Sci. 2010, 257, 887–898. [Google Scholar] [CrossRef]
- Wang, X.; Qiao, H.; Wang, X.; Xu, Y.; Liu, T.; Song, F.; An, Z.; Zhang, L.; Shi, F. NIR Photoluminescence of ZnGa2O4:Cr Nanoparticles Synthesized by Hydrothermal Process. J. Mater. Sci. Mater. Electron. 2022, 33, 19129–19137. [Google Scholar] [CrossRef]
- Miller, D.J.; Biesinger, M.C.; McIntyre, N.S. Interactions of CO2 and CO at Fractional Atmosphere Pressures with Iron and Iron Oxide Surfaces: One Possible Mechanism for Surface Contamination? Surf. Interface Anal. 2002, 33, 299–305. [Google Scholar] [CrossRef]
- Pinder, J.W.; Major, G.H.; Baer, D.R.; Terry, J.; Whitten, J.E.; Čechal, J.; Crossman, J.D.; Lizarbe, A.J.; Jafari, S.; Easton, C.D.; et al. Avoiding Common Errors in X-Ray Photoelectron Spectroscopy Data Collection and Analysis, and Properly Reporting Instrument Parameters. Appl. Surf. Sci. Adv. 2024, 19, 100534. [Google Scholar] [CrossRef]
- Moezzi, A.; Cortie, M.; McDonagh, A. Aqueous Pathways for the Formation of Zinc Oxide Nanoparticles. Dalton Trans. 2011, 40, 4871. [Google Scholar] [CrossRef]




| Sample | Mean Particle Size (nm) | Comparison of Time | p-Value |
|---|---|---|---|
| CZGO-0 | 97 ± 23 | - | - |
| CZGO-6 | 91 ± 19 | 0 ➔ 6 h | 0.09 |
| CZGO-18 | 87 ± 21 | 0 ➔ 18 h | 0.03 |
| CZGO-48 | 87 ± 22 | 0 ➔ 48 h | 0.02 |
| Sample | α1 | α2 | Lifetime (s) | |
|---|---|---|---|---|
| τ1 | τ2 | |||
| CZGO | 20,309.89 | 3783.79 | 3.76 | 45.04 |
| CZGO-0 | 14,001.08 | 2807.37 | 6.22 | 59.24 |
| CZGO-6 | 26,659.41 | 7738.26 | 6.15 | 60.02 |
| CZGO-18 | 47,945.92 | 8179.55 | 3.60 | 37.10 |
| CZGO-48 | 41,436.63 | 9147.23 | 4.02 | 38.93 |
| CZGO | CZGO-0 | CZGO-6 | CZGO-18 | CZGO-48 | |
|---|---|---|---|---|---|
| Ga/Zn | 2.16 ± 0.05 | 1.48 ± 0.17 | 1.60 ± 0.13 | 1.58 ± 0.14 | 1.76 ± 0.06 |
| Sample | B. E. (eV) | K. E. (eV) | Auger Parameter (eV) |
|---|---|---|---|
| CZGO-0 | 1021.73 | 988.5 | 2010.23 |
| CZGO-6 | 1021.66 | 989.0 | 2010.66 |
| CZGO-18 | 1021.49 | 989.0 | 2010.49 |
| CZGO-48 | 1021.18 | 989.4 | 2010.58 |
| CZGO | 1021.53 | 988.4 | 2009.93 |
| ZnO [59] | 1021.00 | 989.4 | 2010.40 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, C.; Park, D.; Shiu, W.-T.; Liu, Y.; Liu, L. Enhanced Persistent Luminescence from Cr3+-Doped ZnGa2O4 Nanoparticles upon Immersion in Simulated Physiological Media. Nanomaterials 2025, 15, 247. https://doi.org/10.3390/nano15030247
Lee C, Park D, Shiu W-T, Liu Y, Liu L. Enhanced Persistent Luminescence from Cr3+-Doped ZnGa2O4 Nanoparticles upon Immersion in Simulated Physiological Media. Nanomaterials. 2025; 15(3):247. https://doi.org/10.3390/nano15030247
Chicago/Turabian StyleLee, Clement, David Park, Wai-Tung Shiu, Yihong Liu, and Lijia Liu. 2025. "Enhanced Persistent Luminescence from Cr3+-Doped ZnGa2O4 Nanoparticles upon Immersion in Simulated Physiological Media" Nanomaterials 15, no. 3: 247. https://doi.org/10.3390/nano15030247
APA StyleLee, C., Park, D., Shiu, W.-T., Liu, Y., & Liu, L. (2025). Enhanced Persistent Luminescence from Cr3+-Doped ZnGa2O4 Nanoparticles upon Immersion in Simulated Physiological Media. Nanomaterials, 15(3), 247. https://doi.org/10.3390/nano15030247





