Green Synthesis of Multi-Walled Carbon Nanotube-Reinforced Hydroxyapatite Doped with Silver and Silver-Core Selenium-Shell Nanoparticles: Synthesis, Characterization, and Biological Activity
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
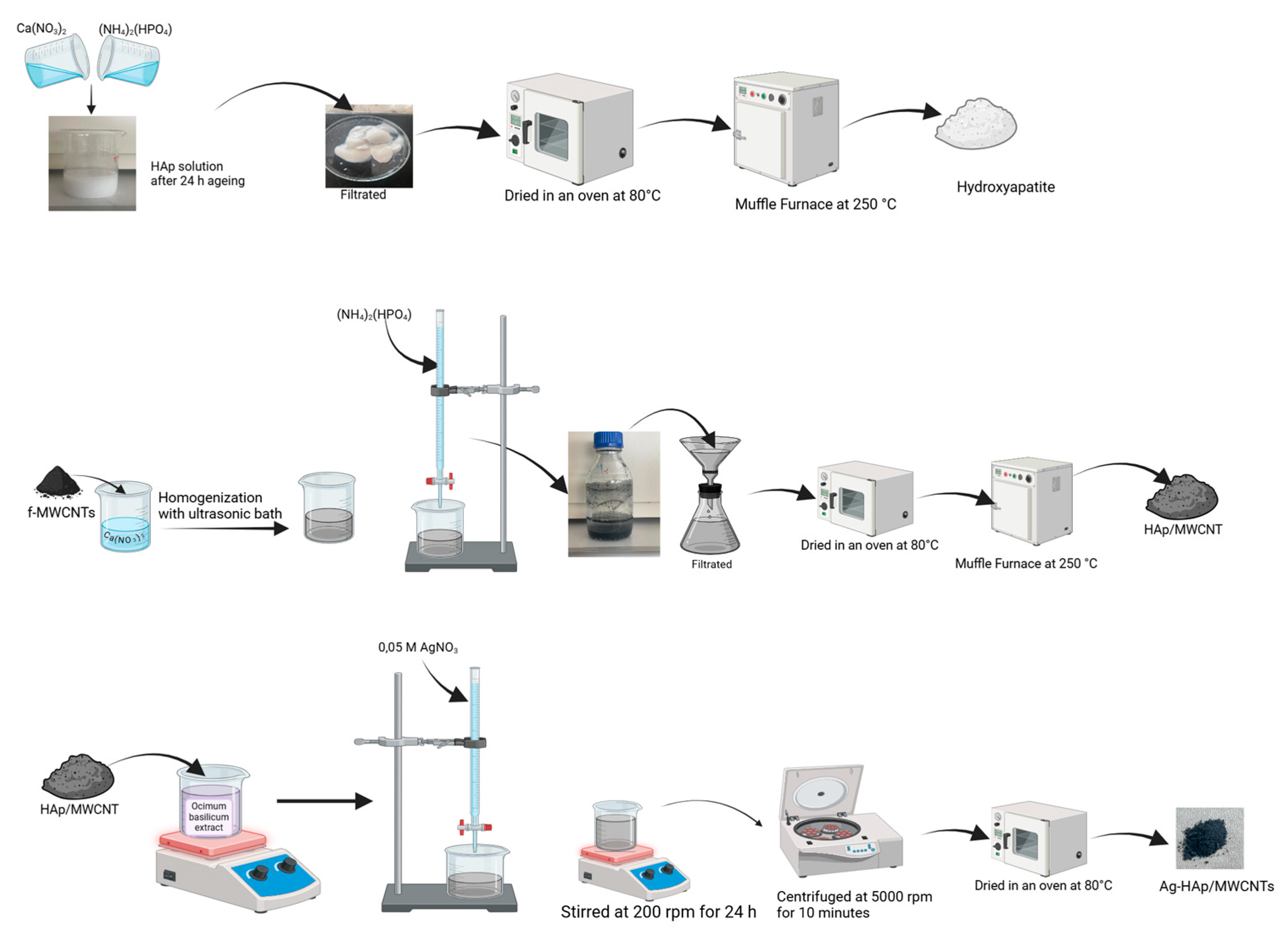
2.2.1. Preparation of Functional MWCNTs
2.2.2. Synthesis Hydroxyapatite
2.2.3. Synthesis of MWCNT-Reinforced Hydroxyapatite
2.2.4. Preparation of Plant Extract
2.2.5. Biosynthesis of AgNP- and Ag@SeNP-Doped HAp/MWCNT Nanocomposite
2.3. Characterization
2.4. Antimicrobial Test
3. Results
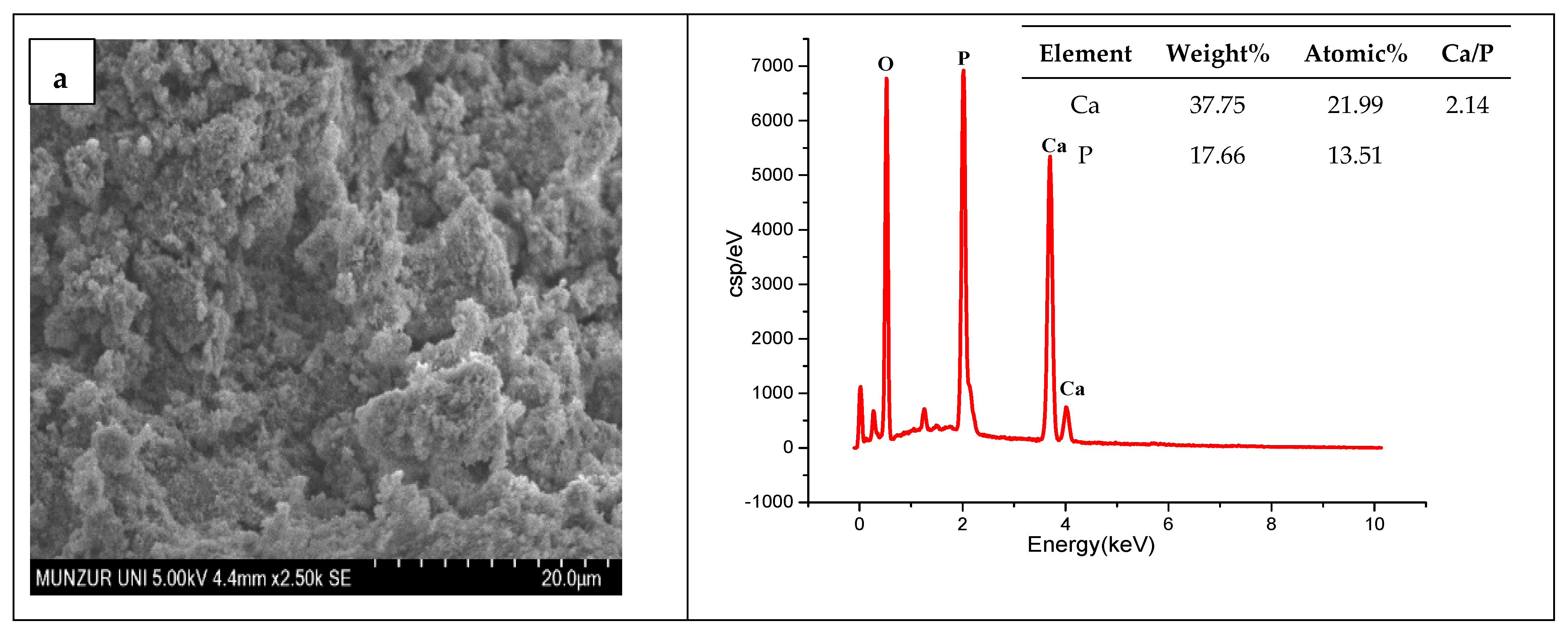
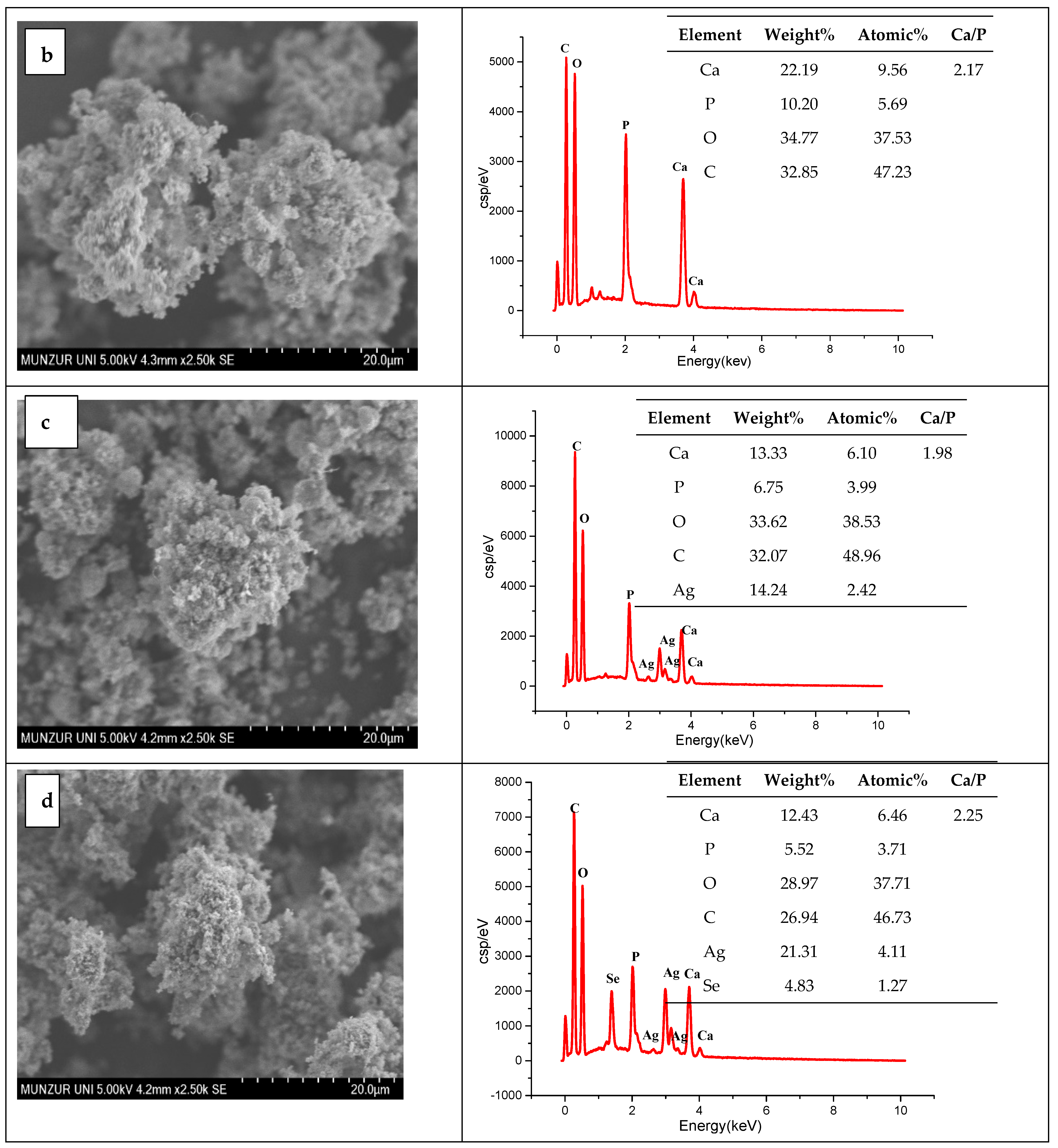
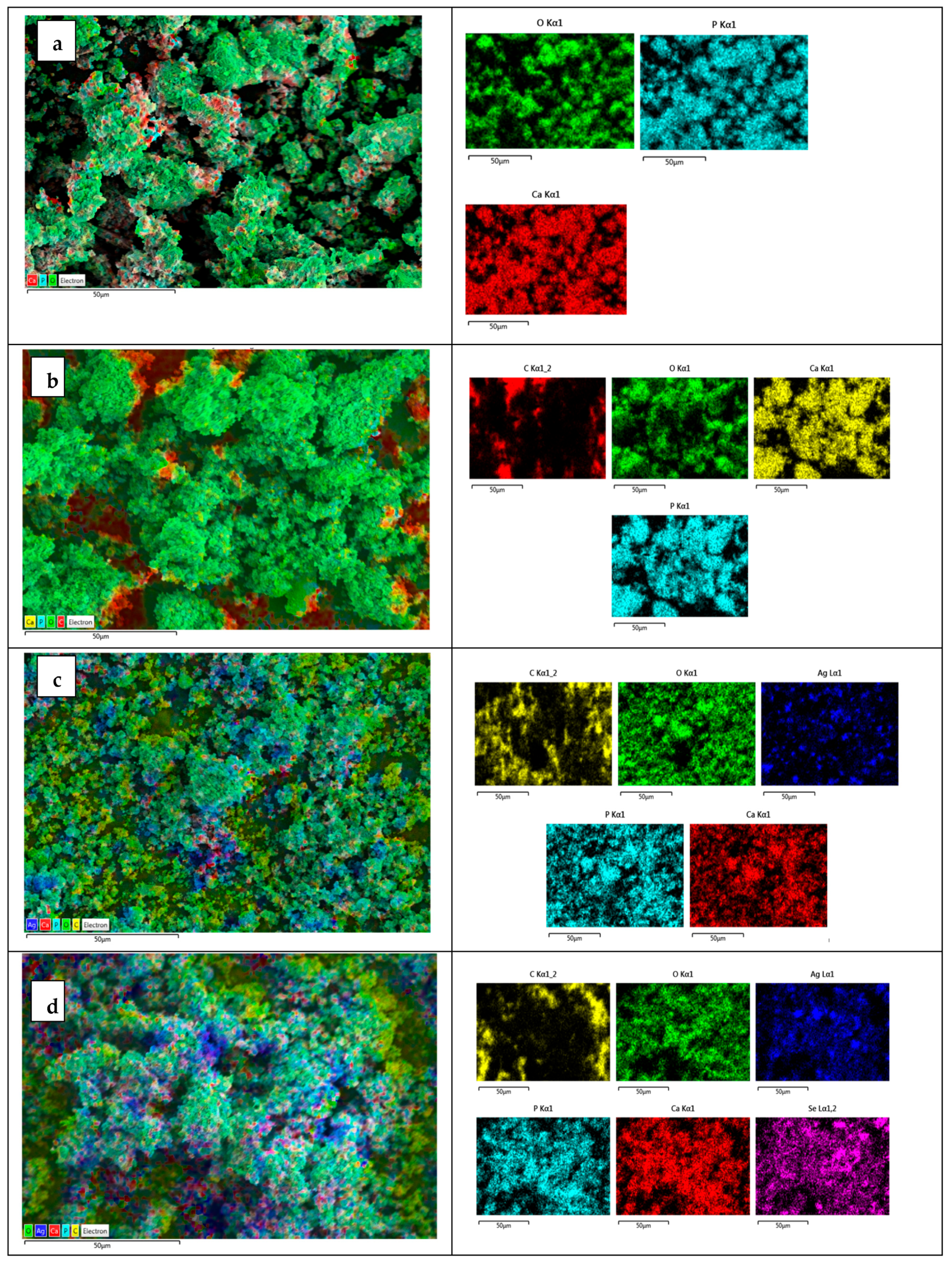
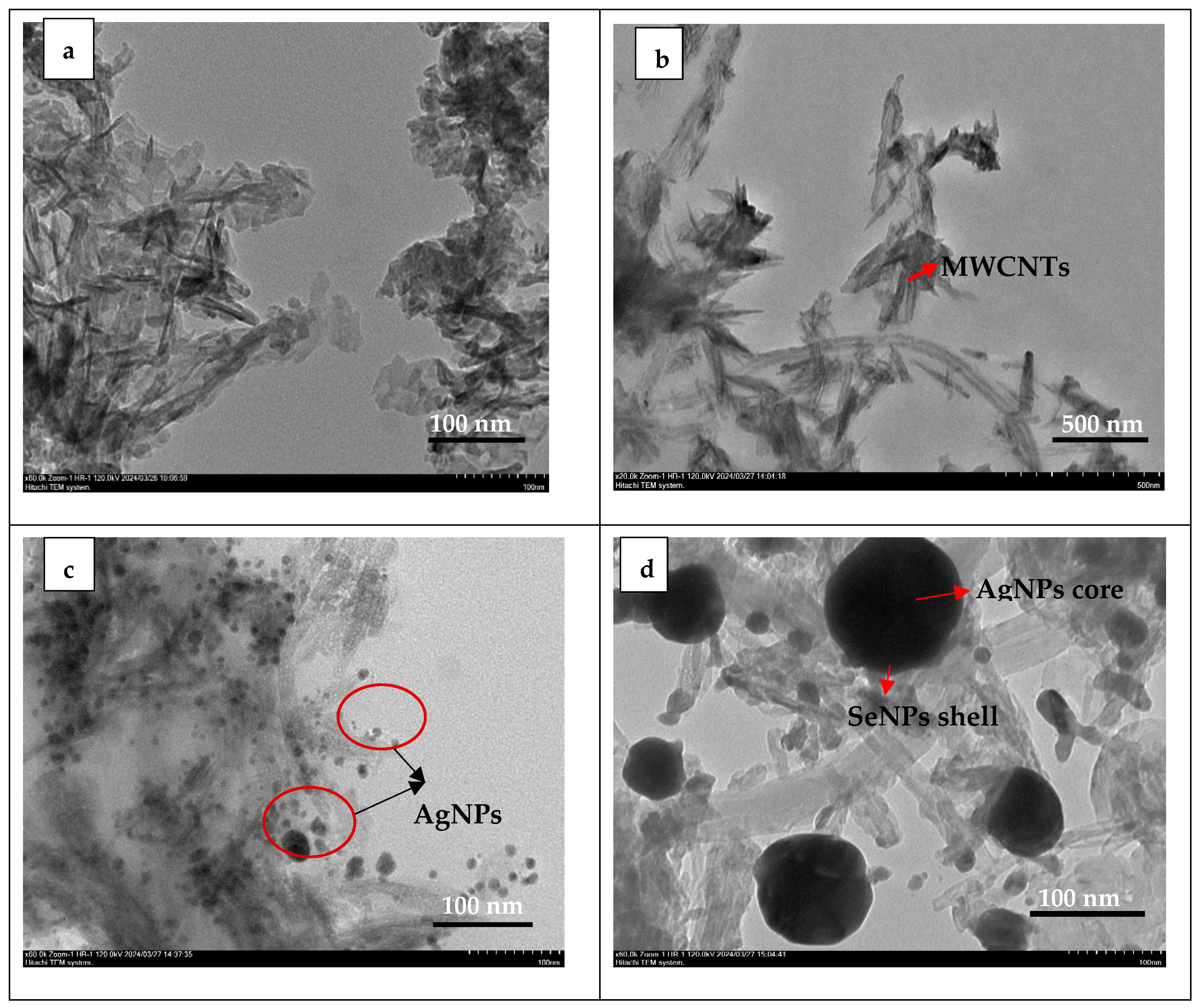
3.1. SEM-EDX Results
3.2. TEM Results
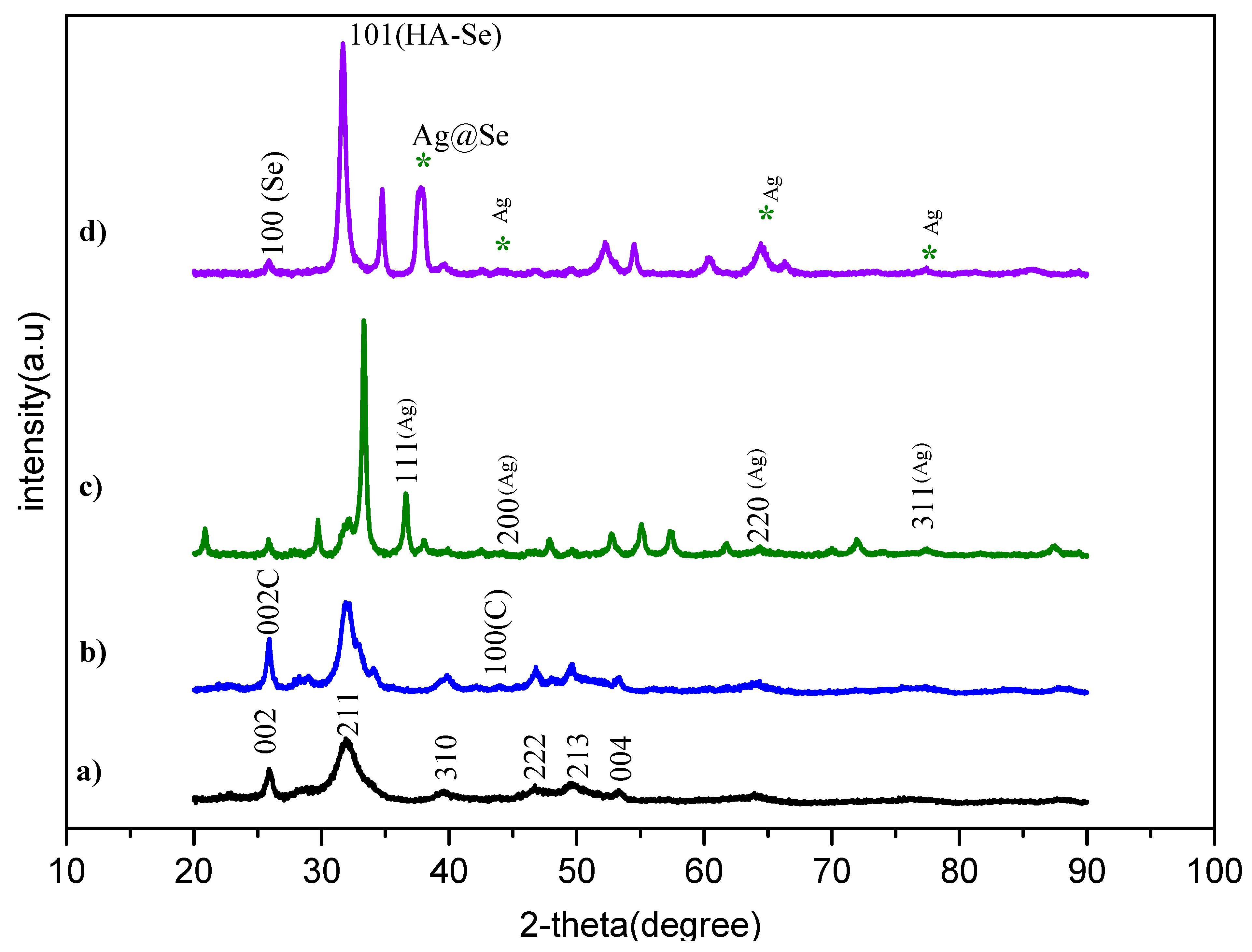
3.3. XRD Results
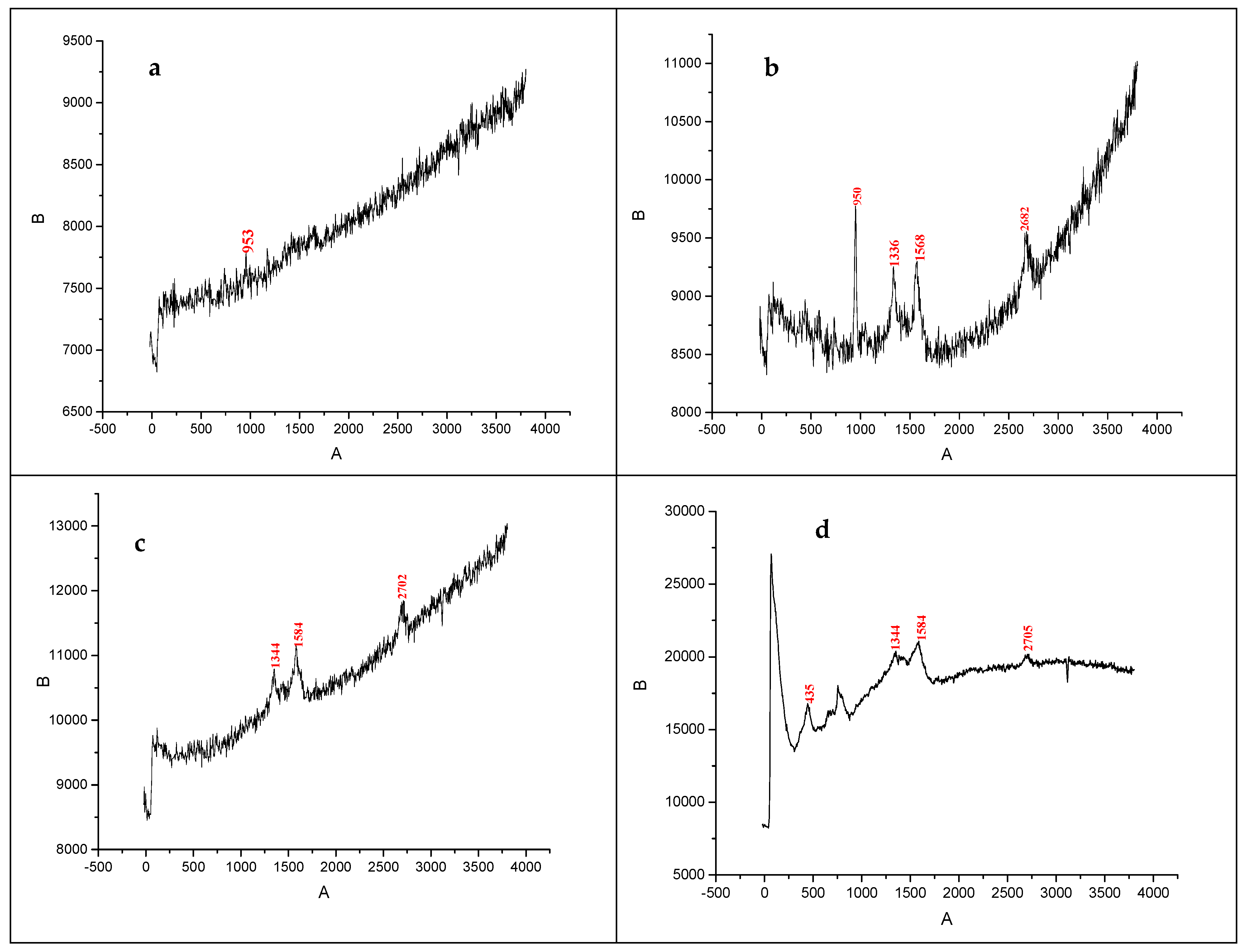
3.4. Raman Spectroscopy Results
3.5. BET Analysis Results
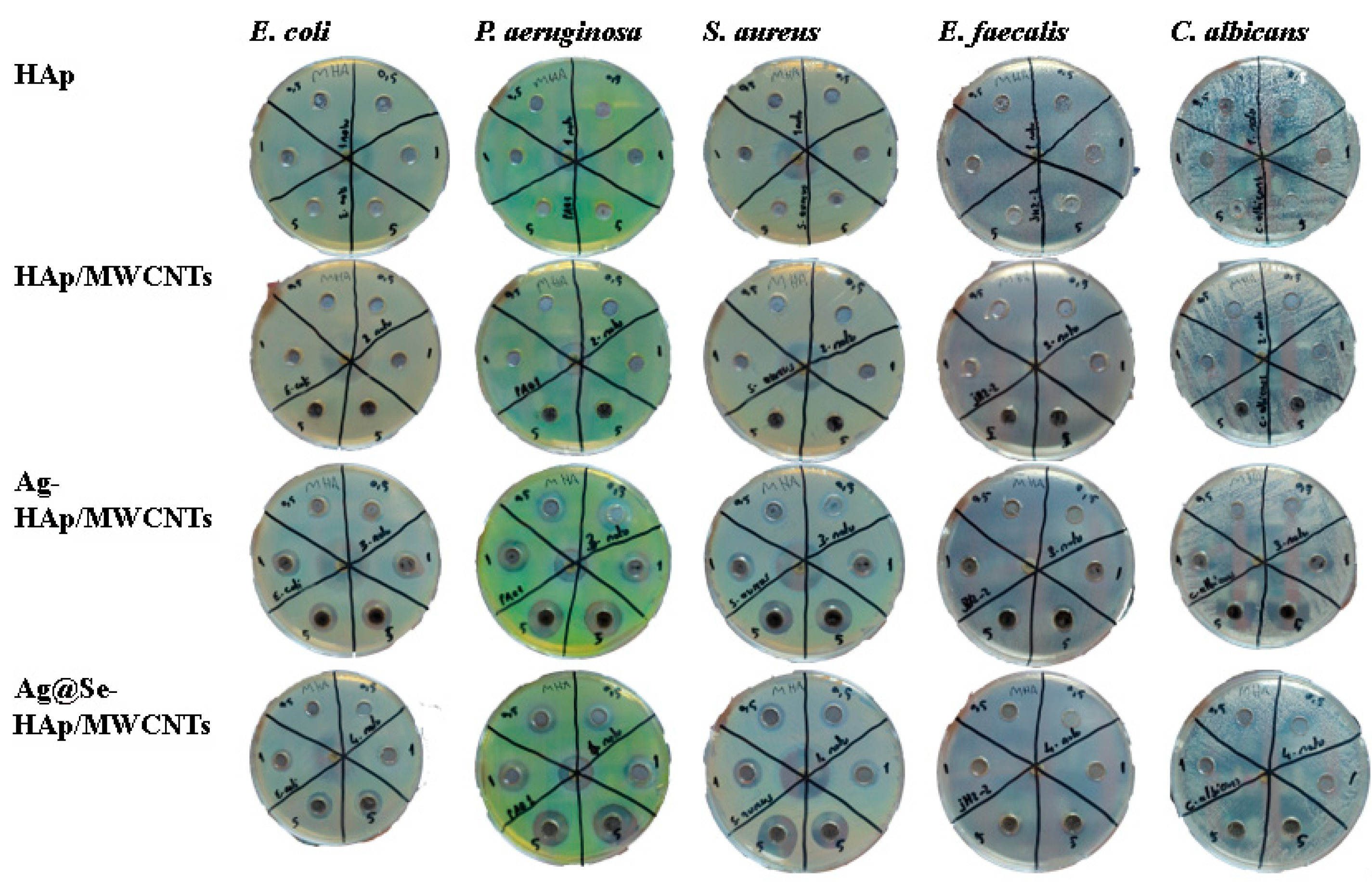
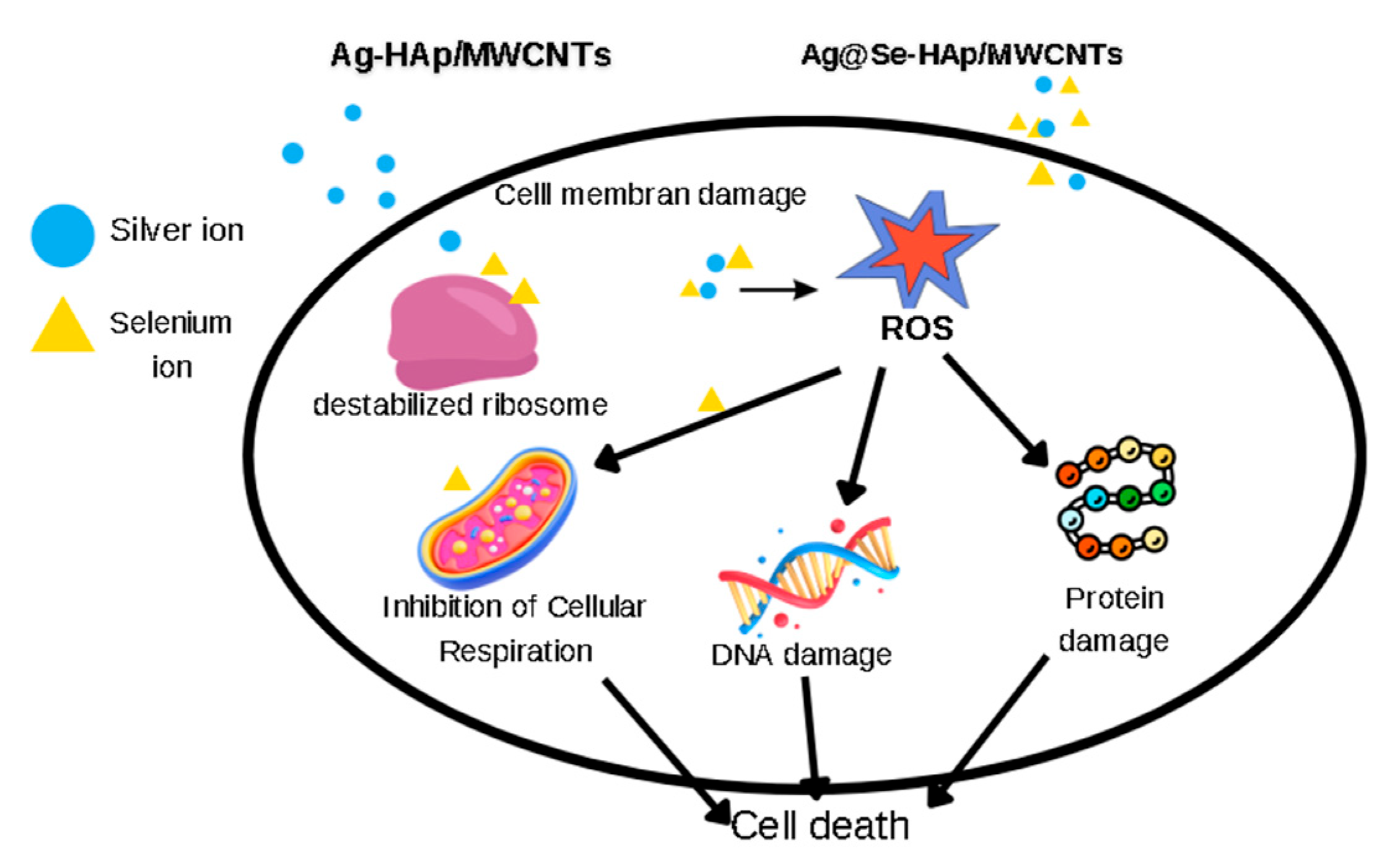
3.6. Antimicrobial Inhibition Zone
4. Conclusions
Funding
Data Availability Statement
Conflicts of Interest
References
- Szcześ, A.; Hołysz, L.; Chibowski, E. Synthesis of hydroxyapatite for biomedical applications. Adv. Colloid Interface Sci. 2017, 249, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Cursaru, L.M.; Iota, M.; Piticescu, R.M.; Tarnita, D.; Savu, S.V.; Savu, I.D.; Dumitrescu, G.; Popescu, D.; Hertzog, R.-G.; Calin, M. Hydroxyapatite from Natural Sources for Medical Applications. Materials 2022, 15, 5091. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Zhou, Z.; Li, W.; Fan, Y.; Li, Z.; Wei, J. Hydroxyapatite-based materials for bone tissue engineering: A brief and comprehensive introduction. Crystals 2021, 11, 149. [Google Scholar] [CrossRef]
- Šupová, M. Substituted hydroxyapatites for biomedical applications: A review. Ceram. Int. 2015, 41, 9203–9231. [Google Scholar] [CrossRef]
- Say, Y. The Effects of Hydroxyapatite on the Corrosion Behaviour of AZ Series Mg Alloys. Turkish J. Sci. Technol. 2023, 18, 45–57. [Google Scholar] [CrossRef]
- Ratha, I.; Datta, P.; Balla, V.K.; Nandi, S.K.; Kundu, B. Effect of doping in hydroxyapatite as a coating material on biomedical implants by plasma spraying method: A review. Ceram. Int. 2021, 47, 4426–4445. [Google Scholar] [CrossRef]
- Fu, Z.; Cui, J.; Zhao, B.; Shen, S.G.; Lin, K. An overview of polyester/hydroxyapatite composites for bone tissue repairing. J. Orthop. Transl. 2021, 28, 118–130. [Google Scholar] [CrossRef]
- Chacon, E.L.; Bertolo, M.R.V.; de Guzzi Plepis, A.M.; da Conceição Amaro Martins, V.; dos Santos, G.R.; Pinto, C.A.L.; Pelegrine, A.A.; Teixeira, M.L.; Buchaim, D.V.; Nazari, F.M.; et al. Collagen-chitosan-hydroxyapatite composite scaffolds for bone repair in ovariectomized rats. Sci. Rep. 2023, 13, 28. [Google Scholar] [CrossRef]
- Bauer, L.; Rogina, A.; Ivanković, M.; Ivanković, H. Medical-Grade Poly(Lactic Acid)/Hydroxyapatite Composite Films: Thermal and In Vitro Degradation Properties. Polymers 2023, 15, 1512. [Google Scholar] [CrossRef]
- Li, M.; Liu, Q.; Jia, Z.; Xu, X.; Cheng, Y.; Zheng, Y.; Xi, T.; Wei, S. Graphene oxide/hydroxyapatite composite coatings fabricated by electrophoretic nanotechnology for biological applications. Carbon 2014, 67, 185–197. [Google Scholar] [CrossRef]
- Janković, A.; Eraković, S.; Mitrić, M.; Matić, I.Z.; Juranić, Z.D.; Tsui, G.C.P.; Tang, C.-Y.; Mišković-Stanković, V.; Rhee, K.Y.; Park, S.J. Bioactive hydroxyapatite/graphene composite coating and its corrosion stability in simulated body fluid. J. Alloys Compd. 2015, 624, 148–157. [Google Scholar] [CrossRef]
- Chakraborty, R.; Seesala, V.S.; Sen, M.; Sengupta, S.; Dhara, S.; Saha, P.; Das, K.; Das, S. MWCNT reinforced bone-like calcium phosphate—Hydroxyapatite composite coating developed through pulsed electrodeposition with varying amounts of apatite phase and crystallinity to promote superior osteoconduction, cytocompatibility, and corrosion protection. Surf. Coatings Technol. 2017, 325, 496–514. [Google Scholar] [CrossRef]
- Sivaraj, D.; Vijayalakshmi, K.; Ganeshkumar, A.; Rajaram, R. Tailoring Cu substituted hydroxyapatite/functionalized multiwalled carbon nanotube composite coating on 316L SS implant for enhanced corrosion resistance, antibacterial and bioactive properties. Int. J. Pharm. 2020, 590, 119946. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Li, K.-Z.; Si, Z.-S.; Song, Q.; Li, H.-J.; Lu, J.-H.; Guo, L.-J. Compressive and interlaminar shear properties of carbon/carbon composite laminates reinforced with carbon nanotube-grafted carbon fibers produced by injection chemical vapor deposition. Mater. Sci. Eng. A 2015, 626, 449–457. [Google Scholar] [CrossRef]
- Lahiri, D.; Ghosh, S.; Agarwal, A. Carbon nanotube reinforced hydroxyapatite composite for orthopedic application: A review. Mater. Sci. Eng. C 2012, 32, 1727–1758. [Google Scholar] [CrossRef]
- De Volder, M.F.; Tawfick, S.H.; Baughman, R.H.; Hart, A.J. Carbon Nanotubes: Present and Future Commercial Applications. Science 2013, 339, 535–539. Available online: http://science.sciencemag.org/content/339/6119/535?sid=599bece0-48b5-4fcc-9d21-5e29be89422c (accessed on 8 June 2024). [CrossRef]
- Vahdati, M.; Tohidi Moghadam, T. Synthesis and Characterization of Selenium Nanoparticles-Lysozyme Nanohybrid System with Synergistic Antibacterial Properties. Sci. Rep. 2020, 10, 510. [Google Scholar] [CrossRef]
- Afzal, M.A.F.; Kalmodia, S.; Kesarwani, P.; Basu, B.; Balani, K. Bactericidal effect of silver-reinforced carbon nanotube and hydroxyapatite composites. J. Biomater. Appl. 2013, 27, 967–978. [Google Scholar] [CrossRef]
- Padmanabhan, V.P.; Sivashanmugam, P.; Kulandaivelu, R.; Sagadevan, S.; Sridevi, B.; Govindasamy, R.; Thiruvengadam, M. Biosynthesised Silver Nanoparticles Loading onto Biphasic Calcium Phosphate for Antibacterial and Bone Tissue Engineering Applications. Antibiotics 2022, 11, 1780. [Google Scholar] [CrossRef]
- Unal, I.; Aydogdu, B.; Aytar, M. Plant extract-based biosynthesis of silver nanoparticle and silver–zinc nanocomposites-doped hydroxyapatite and its antimicrobial activity. Adv. Appl. Ceram. 2024, 123, 22–29. [Google Scholar] [CrossRef]
- Barbanente, A.; Palazzo, B.; Esposti, L.D.; Adamiano, A.; Iafisco, M.; Ditaranto, N.; Migoni, D.; Gervaso, F.; Nadar, R.; Ivanchenko, P.; et al. Selenium-doped hydroxyapatite nanoparticles for potential application in bone tumor therapy. J. Inorg. Biochem. 2021, 215, 111334. [Google Scholar] [CrossRef] [PubMed]
- Olawale, F.; Ariatti, M.; Singh, M. Biogenic synthesis of silver-core selenium-shell nanoparticles using Ocimum tenuiflorum L.: Response surface methodology-based optimization and biological activity. Nanomaterials 2021, 11, 2516. [Google Scholar] [CrossRef] [PubMed]
- Mostafa, H.Y.; El-Sayyad, G.S.; Nada, H.G.; Ellethy, R.A.; Zaki, E.G. Promising antimicrobial and antibiofilm activities of Orobanche aegyptiaca extract-mediated bimetallic silver-selenium nanoparticles synthesis: Effect of UV-exposure, bacterial membrane leakage reaction mechanism, and kinetic study. Arch. Biochem. Biophys. 2023, 736, 109539. [Google Scholar] [CrossRef] [PubMed]
- Swamiappan, S.; Sekar, V.; Devaraj, S.; Dx, A.; Preethi, K. Evaluation of antibacterial and antibiofilm activity of synthesized zinc-hydroxyapatite composites from Labeo rohita fish scale waste. Mater. Res. Express 2018, 5, 025407. [Google Scholar]
- Giri, A.K.; Jena, B.; Biswal, B.; Pradhan, A.K.; Arakha, M.; Acharya, S.; Acharya, L. Green synthesis and characterization of silver nanoparticles using Eugenia roxburghii DC. extract and activity against biofilm-producing bacteria. Sci. Rep. 2022, 12, 8383. [Google Scholar] [CrossRef]
- Degefa, A.; Bekele, B.; Jule, L.T.; Fikadu, B.; Ramaswamy, S.; Dwarampudi, L.P.; Nagaprasad, N.; Ramaswamy, K. Green synthesis, characterization of zinc oxide nanoparticles, and examination of properties for dye-sensitive solar cells using various vegetable extracts. J. Nanomater. 2021, 2021, 3941923. [Google Scholar] [CrossRef]
- Hussain, I.; Singh, N.B.; Singh, A.; Singh, H.; Singh, S.C. Green synthesis of nanoparticles and its potential application. Biotechnol. Lett. 2016, 38, 545–560. [Google Scholar] [CrossRef]
- Unal, I.; Egri, S.; Ates, M. Green Synthesis (Paeonia kesrouanensis) of Silver Nanoparticles and Toxicity Studies in Artemia salina. Bull. Environ. Contam. Toxicol. 2022, 109, 1150–1154. [Google Scholar] [CrossRef]
- Thi Mai Hoa, L. Characterization of multi-walled carbon nanotubes functionalized by a mixture of HNO3/H2SO4. Diam. Relat. Mater. 2018, 89, 43–51. [Google Scholar] [CrossRef]
- David, M.E.; Grigorescu, R.M.; Iancu, L.; Andrei, E.R.; Somoghi, R.; Frone, A.N.; Atkinson, I.; Predoana, L.; Nicoara, A.-I.; Ion, R.-M. Synthesis and characterization of multi-walled carbon nanotubes decorated with hydroxyapatite. Fuller. Nanotub. Carbon Nanostructures 2021, 29, 423–430. [Google Scholar] [CrossRef]
- Zhao, S.; Cui, W.; Rajendran, N.K.; Su, F.; Rajan, M. Investigations of gold nanoparticles-mediated carbon nanotube reinforced hydroxyapatite composite for bone regenerations. J. Saudi Chem. Soc. 2021, 25, 101261. [Google Scholar] [CrossRef]
- Jaafaripour, M.; Nemati, A.; Salahi, E.; Amin, M. Effects of MWCNTs dispersion on the microstructure of sol-gel derived hydroxyapatite. Int. J. Nanosci. Nanotechnol. 2010, 6, 114–124. [Google Scholar]
- Nocerino, N.; Fulgione, A.; Iannaccone, M.; Tomasetta, L.; Ianniello, F.; Martora, F.; Roveri, N.; Capparelli, R.; Capuano, F. Biological activity of lactoferrin-functionalized biomimetic hydroxyapatite nanocrystals. Int. J. Nanomed. 2014, 9, 1175–1184. [Google Scholar]
- Bouropoulos, N.; Stampolakis, A.; Mouzakis, D.E. Dynamic mechanical properties of calcium alginate-hydroxyapatite nanocomposite hydrogels. Sci. Adv. Mater. 2010, 2, 239–242. [Google Scholar] [CrossRef]
- Atchudan, R.; Pandurangan, A.; Joo, J. Effects of nanofillers on the thermo-mechanical properties and chemical resistivity of epoxy nanocomposites. J. Nanosci. Nanotechnol. 2015, 15, 4255–4267. [Google Scholar] [CrossRef]
- Anandalakshmi, K.; Venugobal, J.; Ramasamy, V. Characterization of silver nanoparticles by green synthesis method using Pedalium murex leaf extract and their antibacterial activity. Appl. Nanosci. 2016, 6, 399–408. [Google Scholar] [CrossRef]
- Sharma, G.; Sharma, A.R.; Bhavesh, R.; Park, J.; Ganbold, B.; Nam, J.S.; Lee, S.-S. Biomolecule-mediated synthesis of selenium nanoparticles using dried Vitis vinifera (raisin) extract. Molecules 2014, 19, 2761–2770. [Google Scholar] [CrossRef]
- Mišković-Stanković, V.; Eraković, S.; Janković, A.; Vukašinović-Sekulić, M.; Mitrić, M.; Jung, Y.C.; Park, S.J.; Rhee, K.Y. Electrochemical synthesis of nanosized hydroxyapatite/graphene composite powder. Carbon Lett. 2015, 16, 233–240. [Google Scholar]
- Ji, D.; Wang, Y.; Liu, Y.; Hao, S.; Yang, J.; Yan, Y.; Lu, C.; Guan, S.; Gao, Q.; Wu, H. Efficient capture of uranium by a hydroxyapatite-modified polyethyleneimine@carbon nanotube composite from radioactive nuclear waste. Dalton Trans. 2023, 52, 10136–10144. [Google Scholar] [CrossRef]
- Shukla, A.K.; Alam, J.; Ansari, M.A.; Alhoshan, M.; Alam, M.; Kaushik, A. Selective ion removal and antibacterial activity of silver-doped multi-walled carbon nanotube/polyphenylsulfone nanocomposite membranes. Mater. Chem. Phys. 2019, 233, 102–112. [Google Scholar] [CrossRef]
- Cremonini, E.; Zonaro, E.; Donini, M.; Lampis, S.; Boaretti, M.; Dusi, S.; Vallini, G. Biogenic selenium nanoparticles: Characterization, antimicrobial activity and effects on human dendritic cells and fibroblasts. Microb. Biotechnol. 2016, 9, 758–771. [Google Scholar] [CrossRef] [PubMed]
- Alghofaily, M.; Alfraih, J.; Alsaud, A.; Almazrua, N.; Sumague, T.S.; Auda, S.H.; Alsalleeh, F. The Effectiveness of Silver Nanoparticles Mixed with Calcium Hydroxide against Candida albicans: An Ex Vivo Analysis. Microorganisms 2024, 12, 289. [Google Scholar] [CrossRef] [PubMed]
- Utama, G.L.; Oktaviani, L.; Balia, R.L.; Rialita, T. Potential application of yeast cell wall biopolymers as probiotic encapsulants. Polymers 2023, 15, 3481. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Hu, C. The antimicrobial activity of nanoparticles: Present situation and prospects for the future. Front. Microbiol. 2017, 12, 1227–1249. [Google Scholar] [CrossRef]
- Vasiliev, G.; Kubo, A.L.; Vija, H.; Kahru, A.; Bondar, D.; Karpichev, Y.; Bondarenko, O. Synergistic antibacterial effect of copper and silver nanoparticles and their mechanism of action. Sci. Rep. 2023, 13, 9202. [Google Scholar] [CrossRef]
| Samples | Surface Area (m2/g) | Pore Size (nm) | Pore Volume (cm3/g) |
|---|---|---|---|
| HAp | 109.4 | 14.2 | 0.39 |
| MWCNTs/HAp | 71.4 | 10.8 | 0.19 |
| Ag-MWCNTs/HAp | 47.5 | 10.3 | 0.12 |
| Ag@Se-MWCNTs/HAp | 35.3 | 12.3 | 0.10 |
| Nanocomposite | Concentration (mg/mL) | E. coli | P. aeruginosa | S. aureus | E. faecalis | C. albicans |
|---|---|---|---|---|---|---|
| HAp | 0.1 | ND | ND | ND | ND | ND |
| 1 | ND | ND | ND | ND | ND | |
| 5 | ND | ND | ND | ND | ND | |
| HAp/MWCNTs | 0.1 | ND | ND | ND | ND | ND |
| 1 | ND | ND | ND | ND | ND | |
| 5 | ND | ND | ND | ND | ND | |
| Ag-HAp/MWCNTs | 0.1 | 10 | 14 | 12 | ND | ND |
| 1 | 13 | 14 | 14 | ND | 13 | |
| 5 | 14 | 18 | 16 | 11 | 17 | |
| Ag@Se-HAp/MWCNTs | 0.1 | 12 | 14 | 13 | ND | 10 |
| 1 | 12 | 15 | 13 | ND | 12 | |
| 5 | 14 | 18 | 18 | 12 | 20 | |
| Gentamicin | Control | 24 | 15 | 23 | 11 | |
| Fluconazole | Control | 30 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Unal, İ. Green Synthesis of Multi-Walled Carbon Nanotube-Reinforced Hydroxyapatite Doped with Silver and Silver-Core Selenium-Shell Nanoparticles: Synthesis, Characterization, and Biological Activity. Nanomaterials 2025, 15, 179. https://doi.org/10.3390/nano15030179
Unal İ. Green Synthesis of Multi-Walled Carbon Nanotube-Reinforced Hydroxyapatite Doped with Silver and Silver-Core Selenium-Shell Nanoparticles: Synthesis, Characterization, and Biological Activity. Nanomaterials. 2025; 15(3):179. https://doi.org/10.3390/nano15030179
Chicago/Turabian StyleUnal, İlkay. 2025. "Green Synthesis of Multi-Walled Carbon Nanotube-Reinforced Hydroxyapatite Doped with Silver and Silver-Core Selenium-Shell Nanoparticles: Synthesis, Characterization, and Biological Activity" Nanomaterials 15, no. 3: 179. https://doi.org/10.3390/nano15030179
APA StyleUnal, İ. (2025). Green Synthesis of Multi-Walled Carbon Nanotube-Reinforced Hydroxyapatite Doped with Silver and Silver-Core Selenium-Shell Nanoparticles: Synthesis, Characterization, and Biological Activity. Nanomaterials, 15(3), 179. https://doi.org/10.3390/nano15030179






