Upcycling Coffee Waste into Sustainable Nano Zerovalent Iron for Environmental Contaminant Remediation: Characterization, Applicability and Cytotoxicity
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of SCG Extracts
2.3. Phenolic Content and Antioxidant Activity
2.4. High-Performance Liquid Chromatography with Diode-Array Detection (HPLC-DAD) Analysis
2.5. nZVI Synthesis
2.6. nZVI Characterization
2.7. In Vitro Cytotoxic Activity
2.8. Environmental Remediation Assays
2.9. Statistical Analysis
3. Results and Discussion
3.1. HPLC-DAD Analysis of the SCG Extracts
3.2. Evaluation of Phenolic Compounds and Their Antioxidant Properties
3.3. FTIR Analysis
3.4. UV-Vis Spectroscopy and Thermogravimetric Analysis
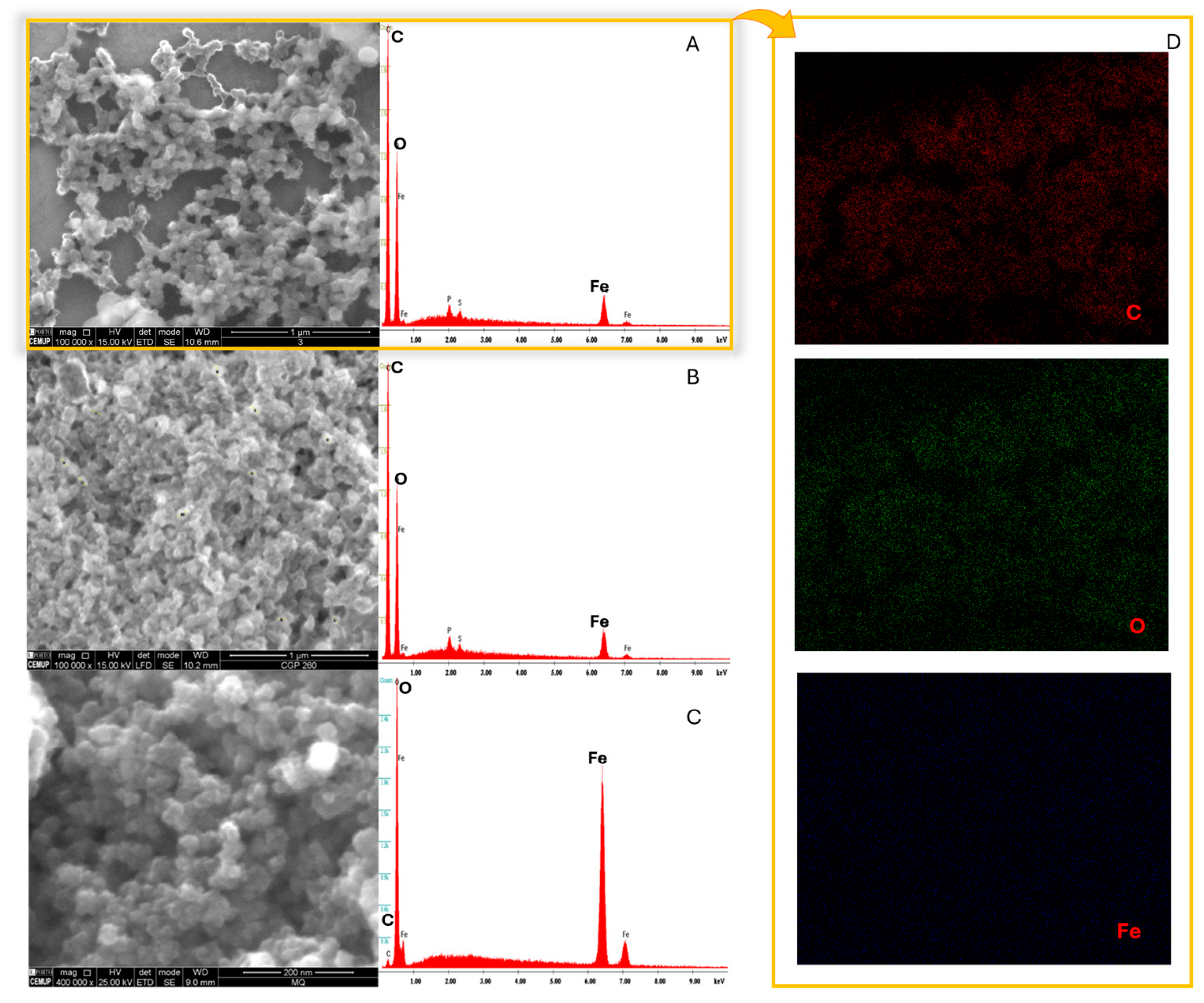
3.5. SEM/EDS Analysis
3.6. Dynamic Light Scattering Analysis
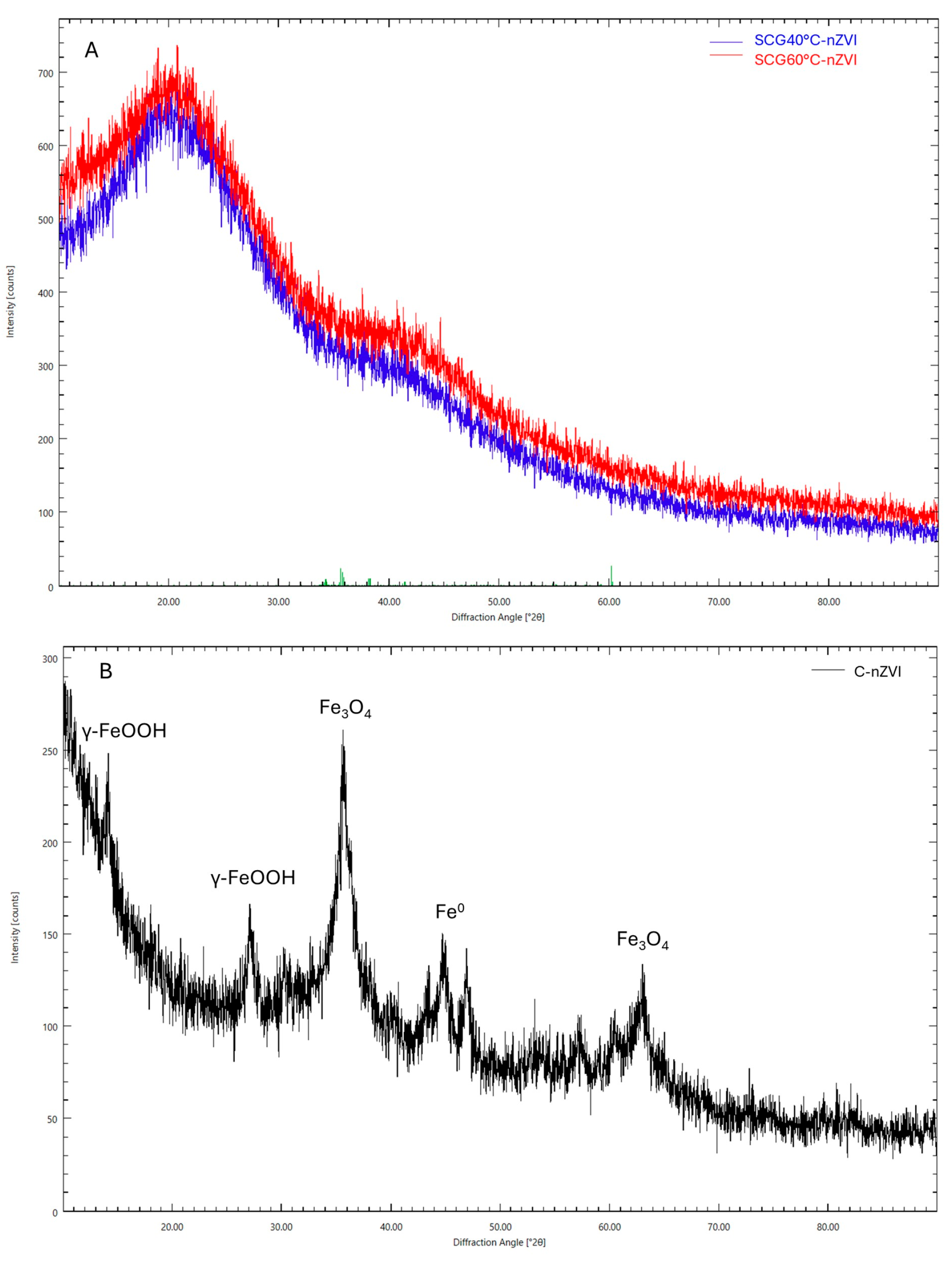
3.7. X-Ray Diffraction Analysis
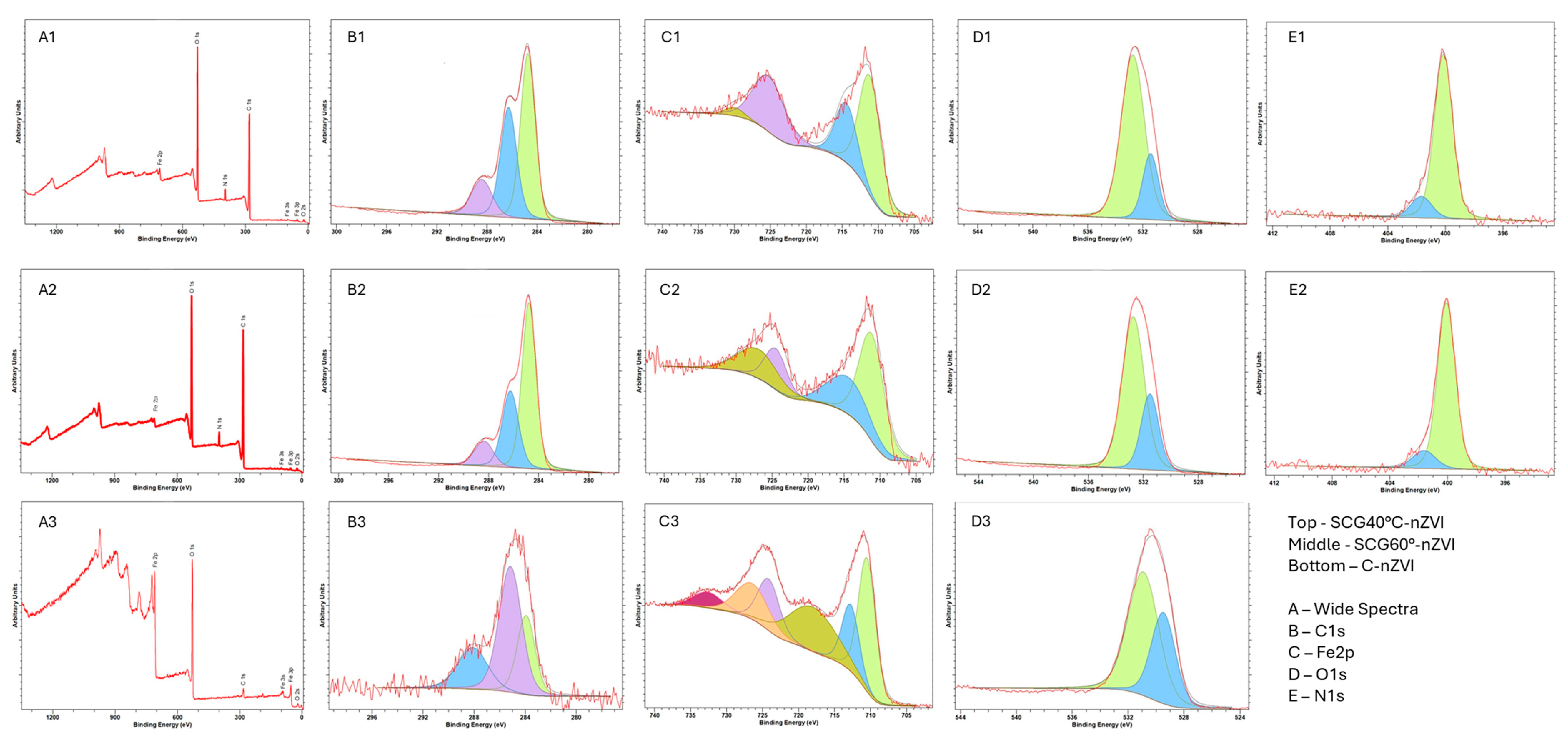
3.8. X-Ray Photoelectron Spectroscopy Analysis
3.9. Magnetic Properties
3.10. Stability
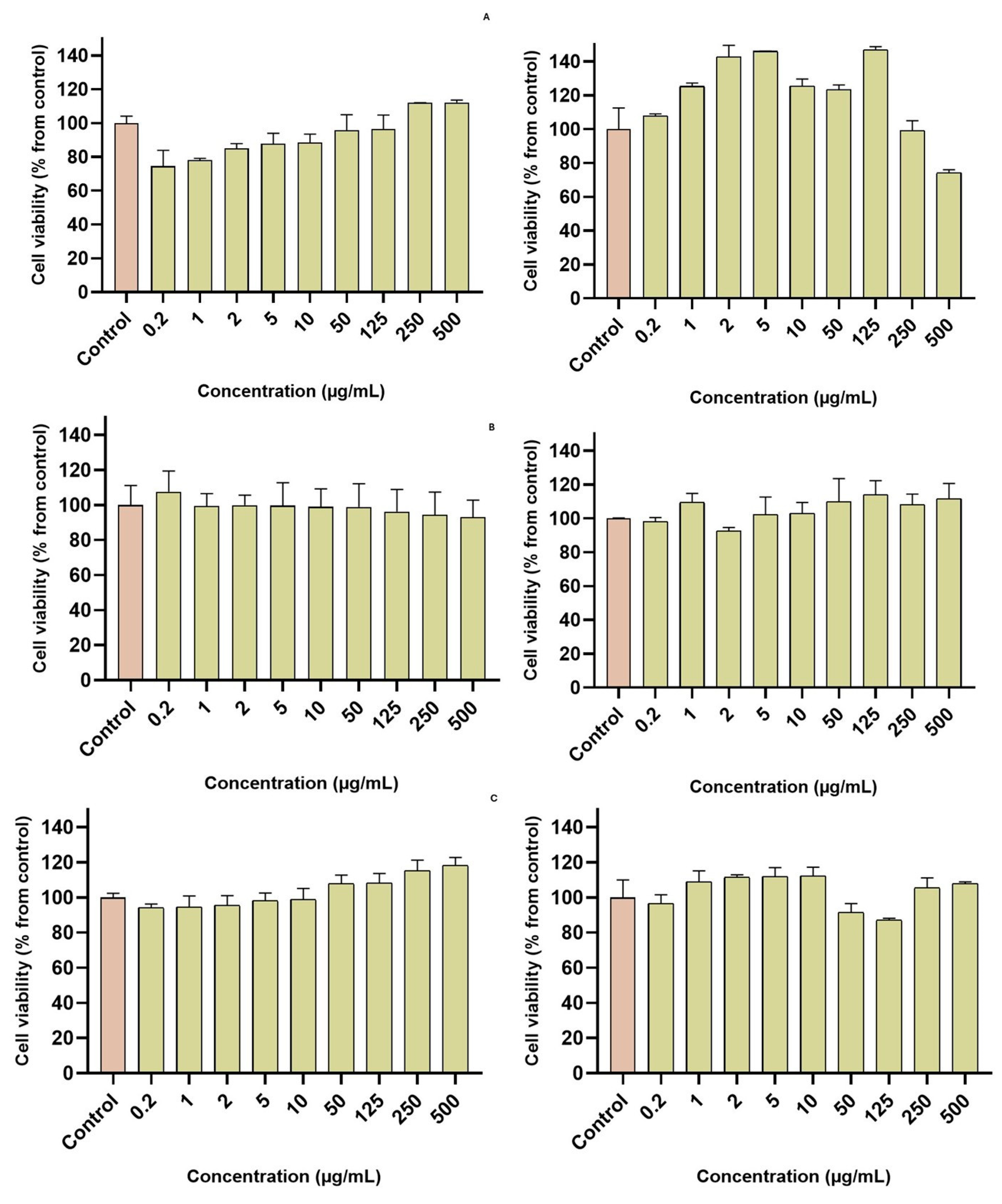
3.11. Cell Viability
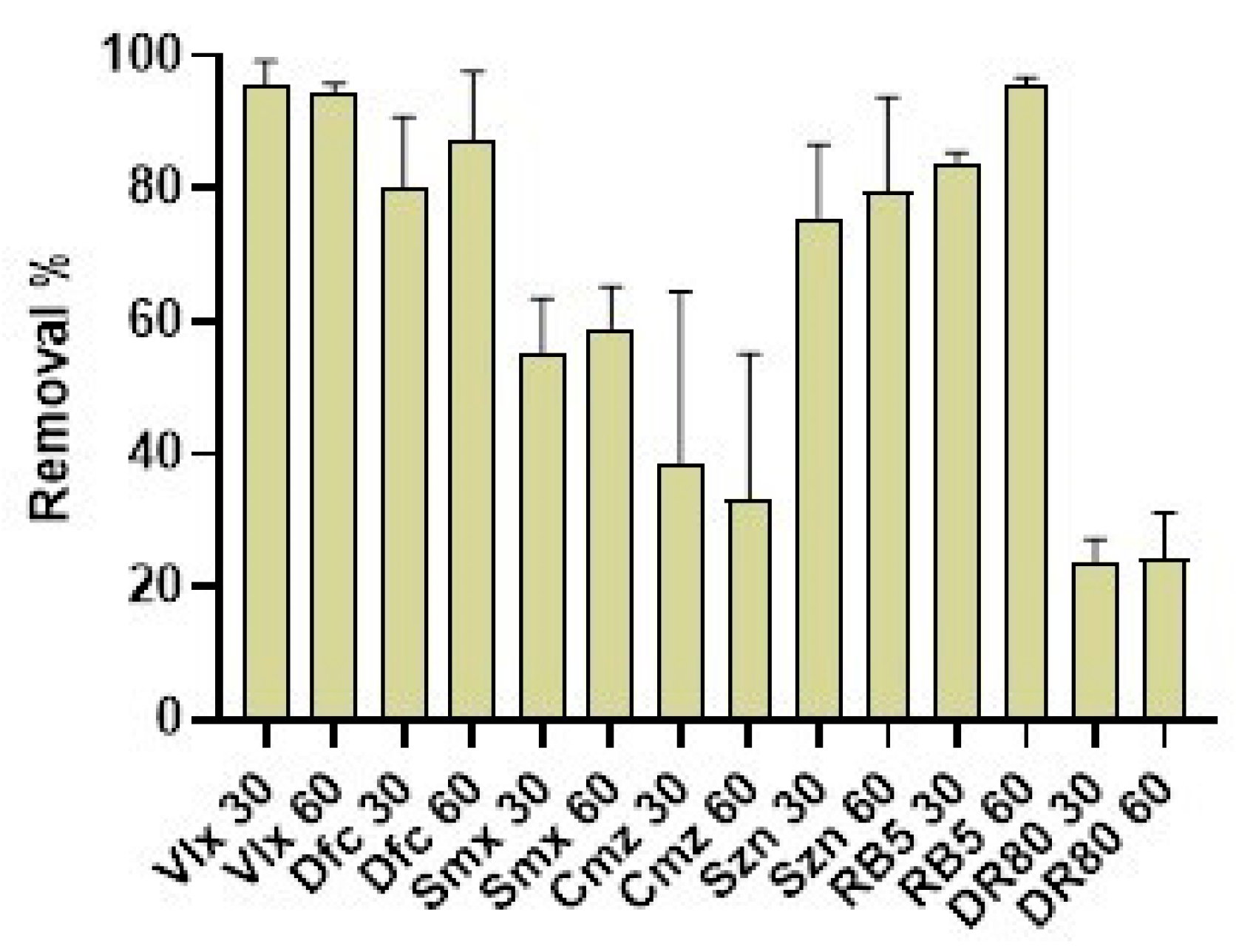
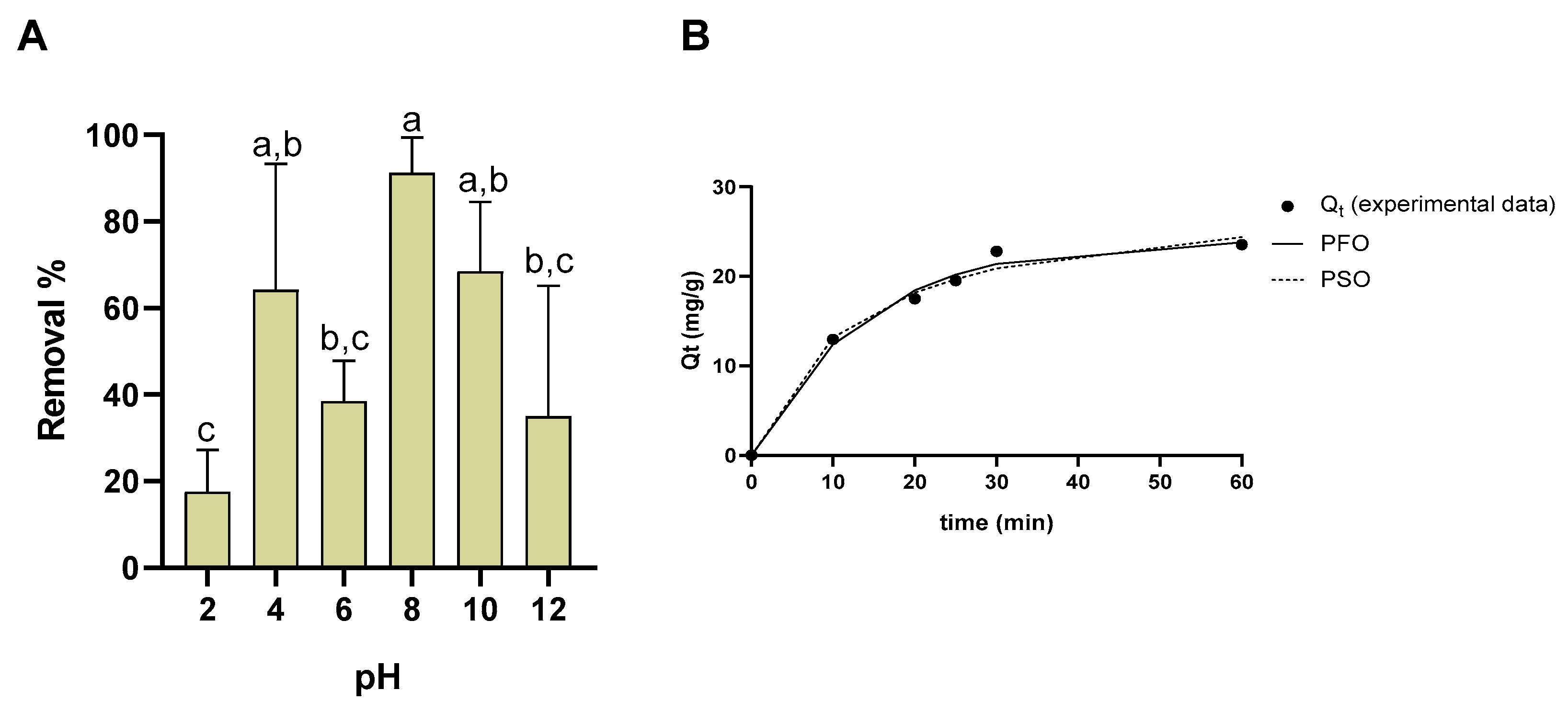
3.12. Remediation Assays
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Franca, A.S.; Oliveira, L.S. Potential Uses of Spent Coffee Grounds in the Food Industry. Foods 2022, 11, 2064. [Google Scholar] [CrossRef]
- Park, M.H.; Lee, J.; Kim, J.Y. Oxidation Resistance of Nanoscale Zero-Valent Iron Supported on Exhausted Coffee Grounds. Chemosphere 2019, 234, 179–186. [Google Scholar] [CrossRef]
- Arya, S.S.; Venkatram, R.; More, P.R.; Vijayan, P. The Wastes of Coffee Bean Processing for Utilization in Food: A Review. J. Food Sci. Technol. 2022, 59, 429–444. [Google Scholar] [CrossRef]
- Pauli, G. The Blue Economy 3.0: The Marriage of Science, Innovation and Entrepreneurship Creates a New Business Model That Transforms Society; Xlibris: Gordon, Auatralia; Bloomington, IN, USA, 2017; ISBN 9781524521059. [Google Scholar]
- Forcina, A.; Petrillo, A.; Travaglioni, M.; di Chiara, S.; De Felice, F. A Comparative Life Cycle Assessment of Different Spent Coffee Ground Reuse Strategies and a Sensitivity Analysis for Verifying the Environmental Convenience Based on the Location of Sites. J. Clean. Prod. 2023, 385, 135727. [Google Scholar] [CrossRef]
- Afriliana, A.; Hidayat, E.; Mitoma, Y.; Masuda, T.; Harada, H. Studies on Composting Spent Coffee Grounds by Aspergillus sp and Penicillium sp in Aerobic Static Batch Temperature Control. J. Agric. Chem. Environ. 2021, 10, 91–112. [Google Scholar] [CrossRef]
- Johnson, K.; Liu, Y.; Lu, M. A Review of Recent Advances in Spent Coffee Grounds Upcycle Technologies and Practices. Front. Chem. Eng. 2022, 4, 838605. [Google Scholar] [CrossRef]
- Machado, S.; Pacheco, J.G.; Nouws, H.P.A.; Albergaria, J.T.; Delerue-Matos, C. Characterization of Green Zero-Valent Iron Nanoparticles Produced with Tree Leaf Extracts. Sci. Total Environ. 2015, 533, 76–81. [Google Scholar] [CrossRef]
- Machado, S.; Stawiński, W.; Slonina, P.; Pinto, A.R.; Grosso, J.P.; Nouws, H.P.A.; Albergaria, J.T.; Delerue-Matos, C. Application of Green Zero-Valent Iron Nanoparticles to the Remediation of Soils Contaminated with Ibuprofen. Sci. Total Environ. 2013, 461–462, 323–329. [Google Scholar] [CrossRef]
- Galdames, A.; Ruiz-Rubio, L.; Orueta, M.; Sánchez-Arzalluz, M.; Vilas-Vilela, J.L. Zero-Valent Iron Nanoparticles for Soil and Groundwater Remediation. Int. J. Environ. Res. Public Health 2020, 17, 5817. [Google Scholar] [CrossRef]
- Martins, F.; Machado, S.; Albergaria, T.; Delerue-Matos, C. LCA Applied to Nano Scale Zero Valent Iron Synthesis. Int. J. Life Cycle Assess. 2017, 22, 707–714. [Google Scholar] [CrossRef]
- Zhan, G.; Zhang, Z. Nano Zero-Valent Iron—Rubber Seed Shell Biochar (NZVI-RSSB) Enhances Removal of Cadmium from Water. Appl. Sci. (Switzerland) 2025, 15, 9807. [Google Scholar] [CrossRef]
- Mandal, S.; Pu, S.; Shangguan, L.; Liu, S.; Ma, H.; Adhikari, S.; Hou, D. Synergistic Construction of Green Tea Biochar Supported NZVI for Immobilization of Lead in Soil: A Mechanistic Investigation. Environ. Int. 2020, 135, 105374. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.S.; Maamoun, I.; Falyouna, O.; Eljamal, O.; Saha, B.B. Arsenic Removal from Contaminated Water Utilizing Novel Green Composite Chlorella vulgaris and Nano Zero-Valent Iron. J. Mol. Liq. 2023, 370, 121005. [Google Scholar] [CrossRef]
- Chen, W.; Phillips, E.; De Vera, J.; Lollar, B.S.; Garcia, A.N.; Passeport, E.; Sleep, B.; Passeport, E.; O’Carroll, D.M.; Lollar, B.S. Quantifying Remediation of Chlorinated Volatile Compounds by Sulfidated Nano Zerovalent Iron Treatment Using Numerical Modeling and CSIA. Water Res. 2024, 263, 122149. [Google Scholar] [CrossRef]
- Lai, C.; Wang, N.; Xu, F.; Zhang, M.; Huang, D.; Ma, D.; Zhou, X.; Xu, M.; Li, L.; Yan, H.; et al. Pomelo Peel Biochar Supported NZVI@Bi0 as a Persulfate Activator for the Degradation of Acetaminophen: Enhanced Performance and Degradation Mechanism. Sep. Purif. Technol. 2024, 350, 127966. [Google Scholar] [CrossRef]
- Abd El-Monaem, E.M.; Omer, A.M.; El-Subruiti, G.M.; Mohy-Eldin, M.S.; Eltaweil, A.S. Zero-Valent Iron Supported-Lemon Derived Biochar for Ultra-Fast Adsorption of Methylene Blue. Biomass Convers. Biorefin. 2024, 14, 1697–1709. [Google Scholar] [CrossRef]
- Conde-Cid, M.; Paíga, P.; Moreira, M.M.; Albergaria, J.T.; Álvarez-Rodríguez, E.; Arias-Estévez, M.; Delerue-Matos, C. Sulfadiazine Removal Using Green Zero-Valent Iron Nanoparticles: A Low-Cost and Eco-Friendly Alternative Technology for Water Remediation. Environ. Res. 2021, 198, 110451. [Google Scholar] [CrossRef]
- Liu, M.; Chen, G.; Xu, L.; He, Z.; Ye, Y. Environmental Remediation Approaches by Nanoscale Zero Valent Iron (NZVI) Based on Its Reductivity: A Review. RSC Adv. 2024, 14, 21118–21138. [Google Scholar] [CrossRef]
- Zafar, A.M.; Javed, M.A.; Hassan, A.A.; Mohamed, M.M. Groundwater Remediation Using Zero-Valent Iron Nanoparticles (NZVI). Groundw. Sustain. Dev. 2021, 15, 100694. [Google Scholar] [CrossRef]
- Schiefler, A.A.; Tuxen, N.; Mayanna, S.; Benning, L.G.; Tobler, D.J. Bacterial Toxicity of Sulfidated Nanoscale Zerovalent Iron in Aerobic and Anaerobic Systems: Implications for Chlorinated Solvent Clean-up Strategies. Geo-Bio Interfaces 2024, 1, e2. [Google Scholar] [CrossRef]
- Hou, J.; Yang, M.; Wu, X.; Chen, Q.; Lu, Y.; Zhang, J.; Lin, D. Epidermal Microorganisms Contributed to the Toxic Mechanism of NZVI and TCEP in Earthworms by Robbing Metal Elements and Nutrients. Eco-Environ. Health 2024, 3, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Aparicio, J.D.; Lacalle, R.G.; Artetxe, U.; Urionabarrenetxea, E.; Becerril, J.M.; Polti, M.A.; Garbisu, C.; Soto, M. Successful Remediation of Soils with Mixed Contamination of Chromium and Lindane: Integration of Biological and Physico-Chemical Strategies. Environ. Res. 2021, 194, 110666. [Google Scholar] [CrossRef] [PubMed]
- Kirthi, A.V.; Kumar, G.; Pant, G.; Pant, M.; Hossain, K.; Ahmad, A.; Alshammari, M.B. Toxicity of Nanoscaled Zero-Valent Iron Particles on Tilapia, Oreochromis mossambicus. ACS Omega 2022, 7, 47869–47879. [Google Scholar] [CrossRef] [PubMed]
- Goren, A.Y.; Genisoglu, M.; Okten, H.E. Use of Nano Zero-Valent Iron Coated Coffee Grounds for Removal of Zn(II) and Ni(II) from Aqueous Solutions. Desalin. Water Treat. 2019, 172, 177–183. [Google Scholar] [CrossRef]
- Fernandes, F.; Gorissen, K.; Delerue-Matos, C.; Grosso, C. Valorisation of Agro-Food By-Products for the Extraction of Phenolic Compounds. Biol. Life Sci. Forum 2022, 18, 61. [Google Scholar] [CrossRef]
- Macedo, C.; Silva, A.M.; Ferreira, A.S.; Moreira, M.M.; Delerue-matos, C.; Rodrigues, F. Microwave- and Ultrasound-Assisted Extraction of Cucurbita pepo Seeds: A Comparison Study of Antioxidant Activity, Phenolic Profile, and In-Vitro Cells Effects. Appl. Sci. 2022, 12, 1763. [Google Scholar] [CrossRef]
- Delerue, T.; Fátima Barroso, M.; Dias-Teixeira, M.; Figueiredo-González, M.; Delerue-Matos, C.; Grosso, C. Interactions between Ginkgo biloba L. and Scutellaria baicalensis georgi in Multicomponent Mixtures towards Cholinesterase Inhibition and ROS Scavenging. Food Res. Int. 2021, 140, 109857. [Google Scholar] [CrossRef]
- Lima, C.F.; Pereira-Wilson, C.; Rattan, S.I.S. Curcumin Induces Heme Oxygenase-1 in Normal Human Skin Fibroblasts through Redox Signaling: Relevance for Anti-Aging Intervention. Mol. Nutr. Food Res. 2011, 55, 430–442. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid Colorimetric Assay for Cellular Growth and Survival: Application to Proliferation and Cytotoxicity Assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Wang, J.; Guo, X. Adsorption Kinetic Models: Physical Meanings, Applications, and Solving Methods. J. Hazard. Mater. 2020, 390, 122156. [Google Scholar] [CrossRef]
- Simonin, J.P. On the Comparison of Pseudo-First Order and Pseudo-Second Order Rate Laws in the Modeling of Adsorption Kinetics. Chem. Eng. J. 2016, 300, 254–263. [Google Scholar] [CrossRef]
- Angeloni, S.; Freschi, M.; Marrazzo, P.; Hrelia, S.; Beghelli, D.; Juan-García, A.; Juan, C.; Caprioli, G.; Sagratini, G.; Angeloni, C. Antioxidant and Anti-Inflammatory Profiles of Spent Coffee Ground Extracts for the Treatment of Neurodegeneration. Oxid. Med. Cell Longev. 2021, 2021, 6620913. [Google Scholar] [CrossRef] [PubMed]
- Bravo, J.; Juániz, I.; Monente, C.; Caemmerer, B.; Kroh, L.W.; De Peña, M.P.; Cid, C. Evaluation of Spent Coffee Obtained from the Most Common Coffeemakers as a Source of Hydrophilic Bioactive Compounds. J. Agric. Food Chem. 2012, 60, 12565–12573. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Wang, W.; Liu, X.; Yuan, F.; Gao, Y. Antioxidative Phenolics Obtained from Spent Coffee Grounds (Coffea arabica L.) by Subcritical Water Extraction. Ind. Crops Prod. 2015, 76, 946–954. [Google Scholar] [CrossRef]
- Ballesteros, L.F.; Ramirez, M.J.; Orrego, C.E.; Teixeira, J.A.; Mussatto, S.I. Optimization of Autohydrolysis Conditions to Extract Antioxidant Phenolic Compounds from Spent Coffee Grounds. J. Food Eng. 2017, 199, 1–8. [Google Scholar] [CrossRef]
- Solomakou, N.; Loukri, A.; Tsafrakidou, P.; Michaelidou, A.M.; Mourtzinos, I.; Goula, A.M. Recovery of Phenolic Compounds from Spent Coffee Grounds through Optimized Extraction Processes. Sustain. Chem. Pharm. 2022, 25, 100592. [Google Scholar] [CrossRef]
- Abdeltaif, S.A.; Sirelkhatim, K.A.; Hassan, A.B. Estimation of Phenolic and Flavonoid Compounds and Antioxidant Activity of Spent Coffee and Black Tea (Processing) Waste for Potential Recovery and Reuse in Sudan. Recycling 2018, 3, 27. [Google Scholar] [CrossRef]
- Andrade, K.S.; Gonalvez, R.T.; Maraschin, M.; Ribeiro-Do-Valle, R.M.; Martínez, J.; Ferreira, S.R.S. Supercritical Fluid Extraction from Spent Coffee Grounds and Coffee Husks: Antioxidant Activity and Effect of Operational Variables on Extract Composition. Talanta 2012, 88, 544–552. [Google Scholar] [CrossRef]
- Tesnim, D.; Hédi, B.A.; Ridha, D.; Cid-Samamed, A. Green Low-Cost Synthesis of Zero-Valent Iron Nanoparticles from Palm Petiole Extract for Cr(VI) Removal from Water. Environ. Sci. Pollut. Res. 2024, 31, 44272–44288. [Google Scholar] [CrossRef]
- Abdel-Aziz, H.M.; Farag, R.S.; Abdel-Gawad, S.A. Carbamazepine Removal from Aqueous Solution by Green Synthesis Zero-Valent Iron/Cu Nanoparticles with Ficus benjamina Leaves’ Extract. Int. J. Environ. Res. 2019, 13, 843–852. [Google Scholar] [CrossRef]
- Desalegn, B.; Megharaj, M.; Chen, Z.; Naidu, R. Green Synthesis of Zero Valent Iron Nanoparticle Using Mango Peel Extract and Surface Characterization Using XPS and GC-MS. Heliyon 2019, 5, e01750. [Google Scholar] [CrossRef] [PubMed]
- Abdelfatah, A.M.; Fawzy, M.; Eltaweil, A.S.; El-Khouly, M.E. Green Synthesis of Nano-Zero-Valent Iron Using Ricinus Communis Seeds Extract: Characterization and Application in the Treatment of Methylene Blue-Polluted Water. ACS Omega 2021, 6, 25397–25411. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.; Ma, S.; Liu, T.; Deng, X. Green Synthesis of Nano Zero-Valent Iron/Cu by Green Tea to Remove Hexavalent Chromium from Groundwater. J. Clean. Prod. 2018, 174, 184–190. [Google Scholar] [CrossRef]
- Mohamed, A.; Atta, R.R.; Kotp, A.A.; Abo El-Ela, F.I.; Abd El-Raheem, H.; Farghali, A.; Alkhalifah, D.H.M.; Hozzein, W.N.; Mahmoud, R. Green Synthesis and Characterization of Iron Oxide Nanoparticles for the Removal of Heavy Metals (Cd2+ and Ni2+) from Aqueous Solutions with Antimicrobial Investigation. Sci. Rep. 2023, 13, 7227. [Google Scholar] [CrossRef]
- Jara, Y.S.; Mekiso, T.T.; Washe, A.P. Highly Efficient Catalytic Degradation of Organic Dyes Using Iron Nanoparticles Synthesized with Vernonia amygdalina Leaf Extract. Sci. Rep. 2024, 14, 6997. [Google Scholar] [CrossRef]
- Zhang, Y.; Tang, Y.; Yan, R.; Liang, S.; Liu, Z.; Yang, Y. Green-Synthesized, Biochar-Supported NZVI from Mango Kernel Residue for Aqueous Hexavalent Chromium Removal: Performance, Mechanism and Regeneration. Chin. J. Chem. Eng. 2024, 71, 91–101. [Google Scholar] [CrossRef]
- Li, S.; Tang, J.; Liu, Q.; Liu, X.; Gao, B. A Novel Stabilized Carbon-Coated NZVI as Heterogeneous Persulfate Catalyst for Enhanced Degradation of 4-Chlorophenol. Environ. Int. 2020, 138, 105639. [Google Scholar] [CrossRef]
- Lem, O.; Yoon, S.; Bae, S.; Lee, W. The Enhanced Reduction of Bromate by Highly Reactive and Dispersive Green Nano-Zerovalent Iron (G-NZVI) Synthesized with Onion Peel Extract. RSC Adv. 2021, 11, 5008–5018. [Google Scholar] [CrossRef]
- Ibrahim, H.M.; Awad, M.; Al-farraj, A.S.; Al-turki, A.M. Stability and Dynamic Aggregation of Bare and Stabilized Zero-valent Iron Nanoparticles under Variable Solution Chemistry. Nanomaterials 2020, 10, 192. [Google Scholar] [CrossRef]
- Bhattacharjee, S. DLS and Zeta Potential—What They Are and What They Are Not? J. Control Release 2016, 235, 337–351. [Google Scholar] [CrossRef]
- Mahl, D.; Diendorf, J.; Meyer-Zaika, W.; Epple, M. Possibilities and Limitations of Different Analytical Methods for the Size Determination of a Bimodal Dispersion of Metallic Nanoparticles. Colloids Surf. A Physicochem. Eng. Asp. 2011, 377, 386–392. [Google Scholar] [CrossRef]
- Hinterwirth, H.; Wiedmer, S.K.; Moilanen, M.; Lehner, A.; Allmaier, G.; Waitz, T.; Lindner, W.; Lämmerhofer, M. Comparative Method Evaluation for Size and Size-Distribution Analysis of Gold Nanoparticles. J. Sep. Sci. 2013, 36, 2952–2961. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, R.; Kotp, A.A.; Abo El-Ela, F.I.; Farghali, A.A.; Moaty, S.A.A.; Zahran, H.Y.; Amin, R. Green Synthesis of Iron Nanoparticles of Clove and Green Coffee Origin with an in Vivo Hepatoprotective Investigation. J. Environ. Chem. Eng. 2021, 9, 106320. [Google Scholar] [CrossRef]
- Ruiz-Torres, C.A.; Araujo-Martínez, R.F.; Martínez-Castañón, G.A.; Morales-Sánchez, J.E.; Lee, T.J.; Shin, H.S.; Hwang, Y.; Hurtado-Macías, A.; Ruiz, F. A Cost-Effective Method to Prepare Size-Controlled Nanoscale Zero-Valent Iron for Nitrate Reduction. Environ. Eng. Res. 2019, 24, 463–473. [Google Scholar] [CrossRef]
- Liu, A.; Liu, J.; Han, J.; Zhang, W.X. Evolution of Nanoscale Zero-Valent Iron (NZVI) in Water: Microscopic and Spectroscopic Evidence on the Formation of Nano- and Micro-Structured Iron Oxides. J. Hazard. Mater. 2017, 322, 129–135. [Google Scholar] [CrossRef]
- Somchaidee, P.; Tedsree, K. Green Synthesis of High Dispersion and Narrow Size Distribution of Zero-Valent Iron Nanoparticles Using Guava Leaf (Psidium guajava L) Extract. Adv. Nat. Sci. Nanosci. Nanotechnol. 2018, 9, 035006. [Google Scholar] [CrossRef]
- Fan, M.; Li, T.; Hu, J.; Cao, R.; Wu, Q.; Wei, X.; Li, L.; Shi, X.; Ruan, W. Synthesis and Characterization of Reduced Graphene Oxide-Supported Nanoscale Zero-Valent Iron (NZVI/RGO) Composites Used for Pb(II) Removal. Materials 2016, 9, 687. [Google Scholar] [CrossRef]
- Jia, Z.; Hao, L.; Zhao, X.; Liu, X. Nano-Zero-Valent Iron-Incorporated Acidified Activated Carbon Coupled with Persulfate Catalyst for High-Efficient Degradation of Phenol from Water. J. Mater. Sci. 2025, 60, 12722–12738. [Google Scholar] [CrossRef]
- Ma, Y.; Lu, N.; Yan, S.; Wang, H.; Cao, X.; Feike, T.; Guan, J. Hydrochar Supported Strategy for NZVI to Remove Bisphenol A and Cr(VI): Performance, Synergetic Mechanism, and Life Cycle Assessment. Sep. Purif. Technol. 2025, 358, 130423. [Google Scholar] [CrossRef]
- Sun, P.; Xu, H.; Xu, L. Serine Grafted Silica Coated Nanoscale Zero-Valent Iron with Enhanced Fenton-Like Degradation of Mixed Organic Solvents of Tributyl Phosphate and N-dodecane. Adv. Sci. 2025, 12, e09319. [Google Scholar] [CrossRef]
- Huang, T.; Zhang, G.; Zhang, N.; Ye, J.; Lu, P. Fe0-H2O2 for Advanced Treatment of Citric Acid Wastewater: Detailed Study of Catalyst after Several Times Use. Chem. Eng. J. 2018, 336, 233–240. [Google Scholar] [CrossRef]
- Ashraf, H.; Anjum, T.; Riaz, S.; Batool, T.; Naseem, S.; Li, G. Sustainable Synthesis of Microwave-Assisted IONPs Using Spinacia Oleracea L. for Control of Fungal Wilt by Modulating the Defense System in Tomato Plants. J. Nanobiotechnol. 2022, 20, 8. [Google Scholar] [CrossRef] [PubMed]
- Nadagouda, M.N.; Castle, A.B.; Murdock, R.C.; Hussain, S.M.; Varma, R.S. In Vitro Biocompatibility of Nanoscale Zerovalent Iron Particles (NZVI) Synthesized Using Tea Polyphenols. Green Chem. 2010, 12, 114–122. [Google Scholar] [CrossRef]
- Yu, H.H.; Lin, C.H.; Chen, Y.C.; Chen, H.H.; Lin, Y.J.; Lin, K.Y.A. Dopamine-Modified Zero-Valent Iron Nanoparticles for Dual-Modality Photothermal and Photodynamic Breast Cancer Therapy. ChemMedChem 2020, 15, 1645–1651. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, Z.; Bai, D. Biogenic Preparation of Zero-Valent Iron Nanoparticles and Evaluation of Anti-Cancer Effect and Heavy Metal Removal. Alex. Eng. J. 2024, 93, 44–50. [Google Scholar] [CrossRef]
- Masud, A.; Chavez Soria, N.G.; Aga, D.S.; Aich, N. Adsorption and Advanced Oxidation of Diverse Pharmaceuticals and Personal Care Products (PPCPs) from Water Using Highly Efficient RGO-NZVI Nanohybrids. Environ. Sci. 2020, 6, 2223–2238. [Google Scholar] [CrossRef]
- Guo, W.; Zhao, Q.; Du, J.; Wang, H.; Li, X.; Ren, N. Enhanced Removal of Sulfadiazine by Sulfidated ZVI Activated Persulfate Process: Performance, Mechanisms and Degradation Pathways. Chem. Eng. J. 2020, 388, 124303. [Google Scholar] [CrossRef]
- Shanableh, A.; Bhattacharjee, S.; Alani, S.; Darwish, N.; Abdallah, M.; Mousa, M.; Semreen, M. Assessment of Sulfamethoxazole Removal by Nanoscale Zerovalent Iron. Sci. Total Environ. 2021, 761, 143307. [Google Scholar] [CrossRef]




| Compound | RT (min) | SCG40 °C | SCG60 °C | p-Value | |
|---|---|---|---|---|---|
| 1 | Trigonelline | 5.40 | 11.59 ± 0.20 | 10.60 ± 0.07 | 0.0012 |
| 2 | 3-O-CQA | 10.85 | 7.49 ± 0.05 | 6.15 ± 0.10 | <0.0001 |
| 3 | 4-O-CQA | 15.60 | 11.90 ± 0.12 | 9.93 ± 0.04 | <0.0001 |
| 4 | 5-O-CQA | 17.10 | 11.29 ± 0.30 | 9.61 ± 0.30 | 0.0009 |
| 5 | 4-O-FQA | 21.65 | 12.58 ± 0.20 | 10.99 ± 0.27 | 0.0013 |
| 6 | Caffeine | 24.60 | 47.50 ± 0.76 | 41.68 ± 0.18 | 0.0002 |
| 7 | 5-O-FQA | 24.76 | 12.97 ± 0.39 | 11.73 ± 0.26 | 0.0103 |
| 8 | 3,4-di-O-CQA | 33.88 | 0.61 ± 0.02 | 0.49 ± 0.01 | 0.0005 |
| 9 | 3,5-di-O-CQA | 37.05 | 0.32 ± 0.03 | 0.28 ± 0.02 | n.s. |
| 10 | 4,5-di-O-CQA | 44.14 | 2.77 ± 0.04 | 2.53 ± 0.06 | 0.0041 |
| Total | 119.02 | 103.99 |
| Sample | TPC (mg GAE/g dw) | DPPH• (mg TE/g dw) | ABTS•+ (mg TE/g dw) | FRAP (mg AAE/g dw) |
|---|---|---|---|---|
| SCG40 °C | 134.64 ± 14.73 a | 142.09 ± 40.59 a | 219.44 ± 26.61 a | 87.79 ± 6.65 a |
| SCG60 °C | 104.30 ± 14.56 b | 120.16 ± 23.80 b | 193.32 ± 59.10 a | 80.02 ± 15.55 a |
| Sample | Size (nm) | PDI | ZP (mV) |
|---|---|---|---|
| SCG40 °C-nZVI w | 565.60 ± 80.84 b | 0.56 ± 0.08 a | −19.57 ± 0.95 a |
| SCG40 °C-nZVI w T | 14.64 ± 0.76 c | 0.24 ± 0.07 b,c | −5.99 ± 1.71 b |
| SCG-40 °C-nZVI met | 514.30 ± 135.39 b | 0.43 ± 0.08 a,b | −6.72 ± 2.76 b |
| SCG-40 °C-nZVI met T | 2112.33 ± 483.02 a | 0.52 ± 0.14 a | −4.23 ± 0.19 b |
| SCG-60 °C-nZVI w T | 22.68 ± 6.79 c | 0.24 ± 0.08 c | −6.97 ± 1.15 b |
| C-nZVI w | 868.16 ± 142.12 b | 0.57 ± 0.02 a | −18.36 ± 1.34 a |
| C-nZVI w T | 466.86 ± 24.52 b,c | 0.48 ± 0.12 a | −1.13 ± 0.24 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernandes, F.; Freitas, M.; Pinho, C.; Oliveira, A.I.; Delerue-Matos, C.; Grosso, C. Upcycling Coffee Waste into Sustainable Nano Zerovalent Iron for Environmental Contaminant Remediation: Characterization, Applicability and Cytotoxicity. Nanomaterials 2025, 15, 1788. https://doi.org/10.3390/nano15231788
Fernandes F, Freitas M, Pinho C, Oliveira AI, Delerue-Matos C, Grosso C. Upcycling Coffee Waste into Sustainable Nano Zerovalent Iron for Environmental Contaminant Remediation: Characterization, Applicability and Cytotoxicity. Nanomaterials. 2025; 15(23):1788. https://doi.org/10.3390/nano15231788
Chicago/Turabian StyleFernandes, Filipe, Maria Freitas, Cláudia Pinho, Ana Isabel Oliveira, Cristina Delerue-Matos, and Clara Grosso. 2025. "Upcycling Coffee Waste into Sustainable Nano Zerovalent Iron for Environmental Contaminant Remediation: Characterization, Applicability and Cytotoxicity" Nanomaterials 15, no. 23: 1788. https://doi.org/10.3390/nano15231788
APA StyleFernandes, F., Freitas, M., Pinho, C., Oliveira, A. I., Delerue-Matos, C., & Grosso, C. (2025). Upcycling Coffee Waste into Sustainable Nano Zerovalent Iron for Environmental Contaminant Remediation: Characterization, Applicability and Cytotoxicity. Nanomaterials, 15(23), 1788. https://doi.org/10.3390/nano15231788










