One-Pot Synthesis for Doped Amorphous Carbon-Based Compounds: Influence of ZnO Dopant on the Charge Transfer Efficiency
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Synthesis of Amorphous Carbon
2.3. Synthesis of ZnO-Impurified Amorphous Carbon
2.4. Physical and Chemical Characterization
2.5. Charge Transfer Evaluation
3. Results and Discussion
3.1. Carbon Synthesis Based on Glucose
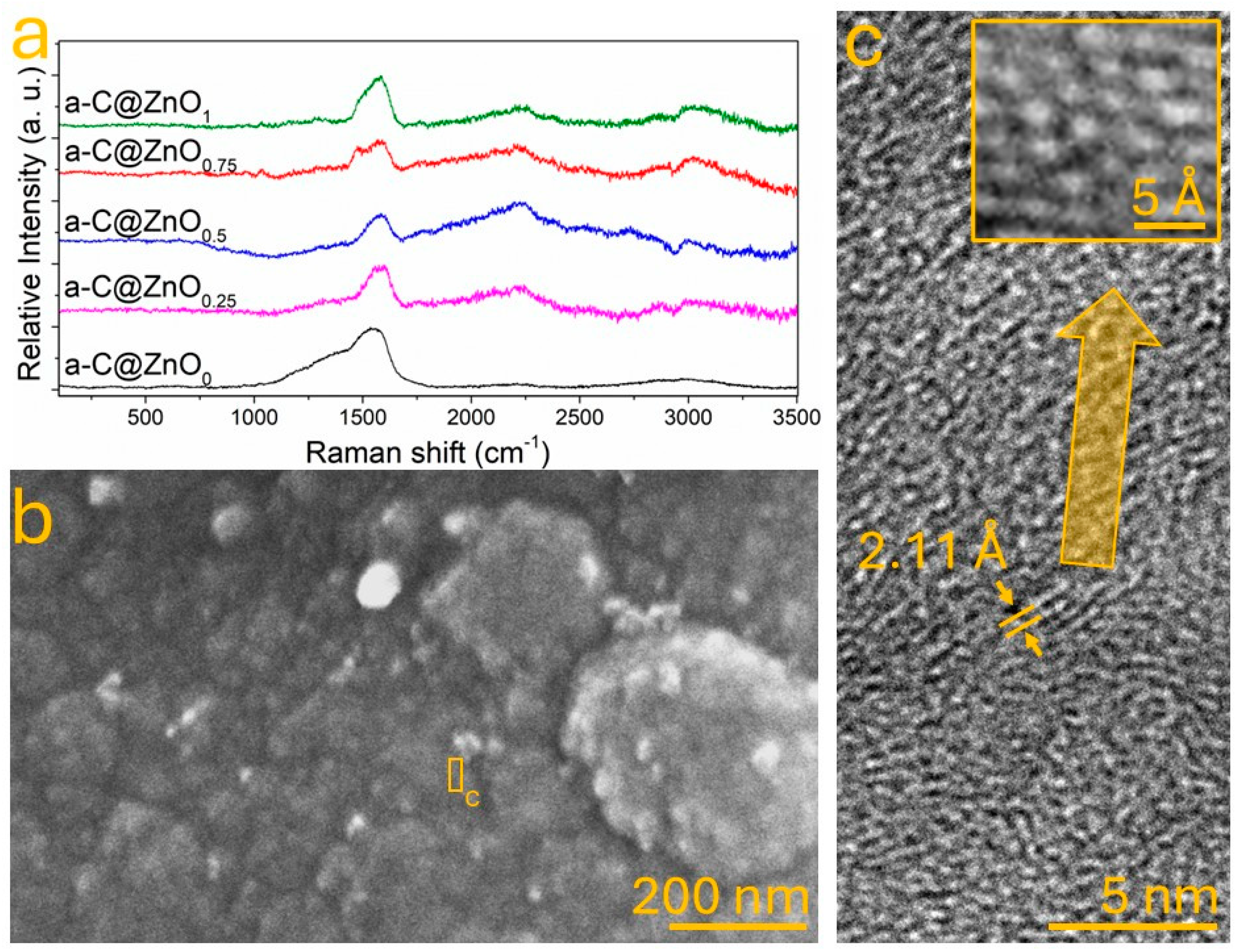
3.2. ZnO Incorporation on the Amorphous Carbon
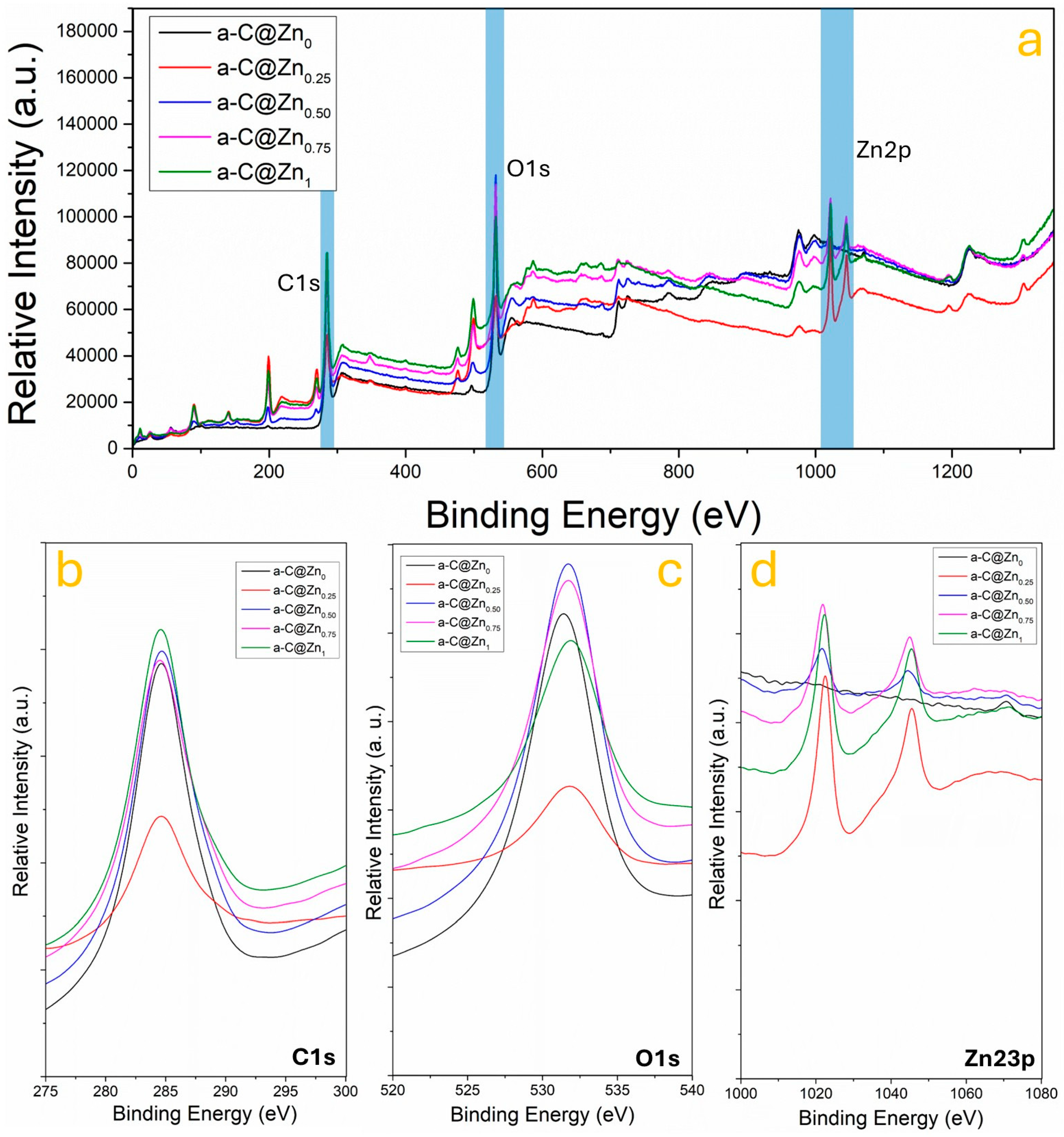
3.3. Chemical Bonding Between ZnO and Amorphous Carbon
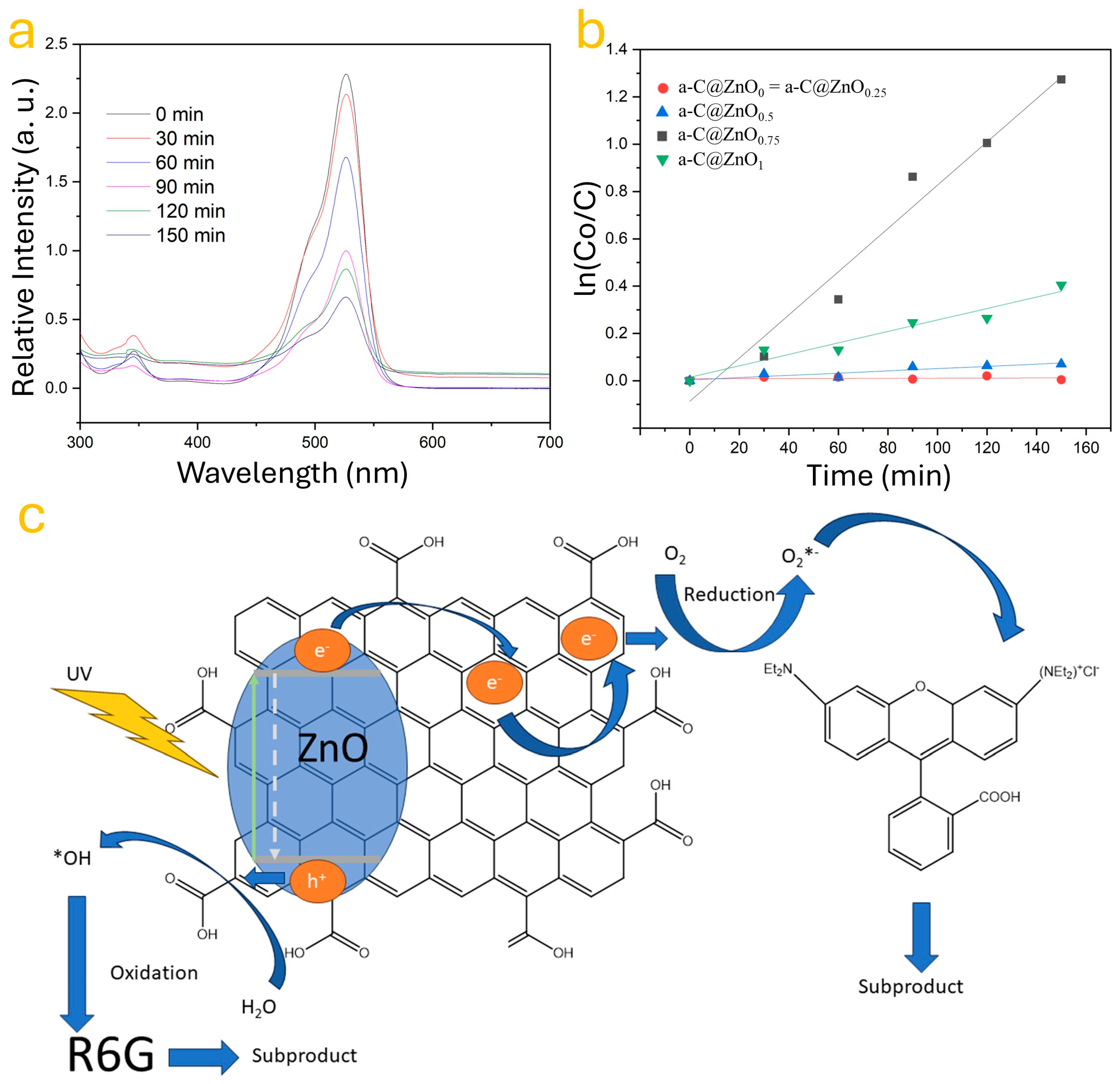
3.4. Influence of ZnO on the Photocatalytic Activity
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Patil, S.; Pise, S.; Dey, N. Tuneable charge-transfer probes for environmental monitoring: Phthalimide-linked pyrene-dione probes for ratiometric sensing of Cu2+. J. Mol. Struct. 2025, 1337, 142103. [Google Scholar] [CrossRef]
- Saeed, A.; Alghamdi, A.M.; Alenizi, M.A.; Alzahrani, E.; Althobiti, R.A.; Al-Ghamdi, S.; Alwafi, R.; Asnag, G.; Al-Hakimi, A.N.; Salem, A.; et al. Synergistic enhancement of electrical and ionic conductivity in polyvinyl alcohol/polyvinylpyrrolidone-copper/lithium titanate oxide electrolyte nanocomposite films for Li-ion battery applications. J. Energy Storage 2024, 104, 114534. [Google Scholar] [CrossRef]
- Sengar, M.S.; Kumari, P.; Sengar, N.; Satsangee, S.P.; Jain, R. Co3O4/fluoro-copolymer nanocomposite modified boron-doped diamond electrode non-enzymatic sensor for the determination of skeletal muscle relaxant drug cyclobenzaprine in biological fluids. Talanta 2025, 287, 127636. [Google Scholar] [CrossRef]
- Su, D.; Zhan, C.; Xiang, Y.; Wang, W.; Li, C.; Zhou, Q.; Dong, D.; Xiao, S. N-doped semiconductive polymer using methoxy-modified JLBI for optimized miscibility and Coulomb interactions towards high thermoelectric performance. J. Mater. Chem. A 2024, 13, 3903–3912. [Google Scholar] [CrossRef]
- Zhang, B.; Wu, L.; Yang, X.; Liu, Y.; Li, J.; Chen, R.; Ma, F.; Hu, Y. Interface engineering-induced built-in electric field enhances charge-transfer kinetics in centimeter-sized silicon anodes for lithium-ion batteries. J. Mater. Sci. Technol. 2025, 237, 1–9. [Google Scholar] [CrossRef]
- Sun, H.; Chen, Y.; Yang, Y.; Liu, Y.; Chen, J.; Chen, M.; Cao, D.; Li, F. Optimizing charge transfer and paradox reaction to enhance the performance of Fe3O4 anodes for aqueous energy storage. J. Power Sources 2024, 623, 235502. [Google Scholar] [CrossRef]
- Wang, Q.; Hu, C.; Zhang, L.; Lei, Y.; Hu, Z.; Wang, Z.; Qiao, Y.; Lv, B. A highly efficient 3D/0D CdIn2S4/Cu2O photoanode with a p–n type heterojunction for boosted photoelectrochemical water splitting under visible light irradiation. J. Mater. Chem. C 2024, 13, 2790–2803. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, J.; Liu, C.; Huo, P. Core-shell CuO@In2O3 with close contact interface enhances interfacial charge transfer for highly selective CO2 photoreduction in pure water. Sep. Purif. Technol. 2025, 366, 132817. [Google Scholar] [CrossRef]
- Ma, X.; Li, C.; Zhang, X.; Gao, M.; Wang, Y.; Li, G. Interface Optimisation of the Fe2O3/C3N4 Heterojunction with Metal Nanoparticles and Their Negative and Positive Photoelectric Responses in a Broadband Light Spectrum Range. Coatings 2024, 14, 1595. [Google Scholar] [CrossRef]
- Xie, M.; Li, T.; Zhang, X.; Wan, K.; Yan, W.; Wei, Y.; Xu, J. Interfacial W-S bond and sulfur vacancy-enhanced W/Sv-CdS photocatalysts for efficient hydrogen evolution. J. Environ. Chem. Eng. 2024, 12, 114932. [Google Scholar] [CrossRef]
- Abid, M.H.; Alharbi, F.; Gassoumi, A.; Aslam, M. FeSnO3/rGO nanocomposite electrode development for supercapacitor application. J. Alloy. Compd. 2024, 1008, 176632. [Google Scholar] [CrossRef]
- Pujari, S.S.; Bobade, R.G.; Shaikh, S.F.; Al-Enizi, A.M.; Ambare, R.C.; Lokhande, B.J. A binderless Ru:α-Fe2O3 binary nanocomposite electrode for supercapacitor applications. J. Mater. Sci. Mater. Electron. 2024, 35, 2162. [Google Scholar] [CrossRef]
- Han, C.; Chen, C.; Shi, H.; Chen, W.; Sun, W.; Li, B. Advances in single-molecule electrical transport studies of peptides. Phys. Chem. Chem. Phys. 2025, 27, 8026–8038. [Google Scholar] [CrossRef]
- Ramos-Álvarez, D.; Hernández-Rodríguez, Y.; Vega-Gómez, J.; Cigarroa-Mayorga, O. Influence of copper support on the charge transfer enhancement of zinc oxide nanoflakes. Mater. Lett. 2023, 349, 134875. [Google Scholar] [CrossRef]
- Chen, J.; Zhong, Q.; Sirotti, E.; Zhou, G.; Wolz, L.; Streibel, V.; Dittloff, J.; Eichhorn, J.; Ji, Y.; Zhao, L.; et al. Ligand-Tuned AgBiS2 Planar Heterojunctions Enable Efficient Ultrathin Solar Cells. ACS Nano 2024, 18, 33348–33358. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, K.; Yasuda, T.; Minari, T.; Yoshio, M.; Kuwabara, J.; Takeuchi, M. Overestimation of Operational Stability in Polymer-Based Organic Field-Effect Transistors Caused by Contact Resistance. ACS Appl. Mater. Interfaces 2024, 16, 68081–68090. [Google Scholar] [CrossRef]
- Tian, J.; Yang, H.; Hou, Y.; Pan, J.H.; Huang, Z. Charge transfer and interfacial binding strategy: Enhancing photocatalytic CO2 reduction efficiency in graphene oxide-modified Cs3BiSbBr9 halide perovskites. Sep. Purif. Technol. 2024, 359, 130527. [Google Scholar] [CrossRef]
- Zhang, Z.; Hu, H.; Yang, J.; He, Z.; Zhu, G.; Wen, C. The Application of Porous Carbon Derived from Furfural Residue as the Electrode Material in Supercapacitors. Polymers 2024, 16, 3421. [Google Scholar] [CrossRef]
- Jiang, X.; Guan, S.; Chen, L.; Deng, F.; Yan, H.; Liu, F.; Zhai, X.; Martínez-Huitle, C.A.; Ding, J. Designing carbon-based catalysts for enhanced sulfite activation: Strategies for pollutant degradation. J. Environ. Chem. Eng. 2024, 12, 114719. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Z. Sugar gourd-like amorphous carbon coated CoS/Co9S8 nanoparticles anchored on carbon nanotubes for potassium-ion batteries. J. Energy Storage 2024, 104, 114641. [Google Scholar] [CrossRef]
- Shi, Y.; Li, X.; Yang, J.; Jin, K.; Guo, W.; Tian, K.; Wang, C.; Wang, Y.; Wang, H. Controlling of pseudo-graphite proportion in amorphous carbon to enhance dipole polarization for microwave absorption. J. Colloid Interface Sci. 2025, 691, 137422. [Google Scholar] [CrossRef]
- Mendoza-Sánchez, A.R.; Hernández-Rodríguez, Y.; Casas-Espínola, J.; Cigarroa-Mayorga, O. Nanostructural modulation of Schottky barrier in Au/α-MoO3 heterojunction via Au nanoparticle size control. Appl. Surf. Sci. 2024, 670, 160624. [Google Scholar] [CrossRef]
- Gamiño-Barocio, I.; Vázquez-Vázquez, E.F.; Hernández-Rodríguez, Y.M.; Cigarroa-Mayorga, O.E. Tuning the Charge Transfer in MWCNTs via the Incorporation of ZnONPs and AgNPs: The Role of Carbon Binding with ZnO/Ag Heterostructures in Reactive Species Formation. Nanomaterials 2024, 14, 1517. [Google Scholar] [CrossRef]
- Xie, W.; Weng, L.-T.; Ng, K.M.; Chan, C.K.; Chan, C.-M. Defects of clean graphene and sputtered graphite surfaces characterized by time-of-flight secondary ion mass spectrometry and X-ray photoelectron spectroscopy. Carbon 2017, 112, 192–200. [Google Scholar] [CrossRef]
- Ali, M.D.; Starczewska, A.; Das, T.K.; Jesionek, M. Exploration of Sp-Sp2 Carbon Networks: Advances in Graphyne Research and Its Role in Next-Generation Technologies. Int. J. Mol. Sci. 2025, 26, 5140. [Google Scholar] [CrossRef]
- Moseenkov, S.I.; Kuznetsov, V.L.; Zolotarev, N.A.; Kolesov, B.A.; Prosvirin, I.P.; Ishchenko, A.V.; Zavorin, A.V. Investigation of Amorphous Carbon in Nanostructured Carbon Materials (A Comparative Study by TEM, XPS, Raman Spectroscopy and XRD). Materials 2023, 16, 1112. [Google Scholar] [CrossRef]
- Vicarelli, L.; Heerema, S.J.; Dekker, C.; Zandbergen, H.W. Controlling Defects in Graphene for Optimizing the Electrical Properties of Graphene Nanodevices. ACS Nano 2015, 9, 3428–3435. [Google Scholar] [CrossRef]
- Schmies, H.; Bengen, N.; Müller-Hülstede, J.; Ibitowa, O.A.; Wagner, P.; Wark, M. How Effective Is Graphitization of Biomasses for the Carbon Stability of Pt/C ORR Catalysts? Catalysts 2023, 13, 343. [Google Scholar] [CrossRef]
- Kupka, K.; Leino, A.; Ren, W.; Vázquez, H.; Åhlgren, E.; Nordlund, K.; Tomut, M.; Trautmann, C.; Kluth, P.; Toulemonde, M.; et al. Graphitization of amorphous carbon by swift heavy ion impacts: Molecular dynamics simulation. Diam. Relat. Mater. 2018, 83, 134–140. [Google Scholar] [CrossRef]
- Katagiri, G.; Ishida, H.; Ishitani, A. Raman spectra of graphite edge planes. Carbon 1988, 26, 565–571. [Google Scholar] [CrossRef]
- Solati, N.; Mobassem, S.; Kahraman, A.; Ogasawara, H.; Kaya, S. A comprehensive study on the characteristic spectroscopic features of nitrogen doped graphene. Appl. Surf. Sci. 2019, 495, 143518. [Google Scholar] [CrossRef]
- Islam, A.E.; Susner, M.A.; Carpena-Núñez, J.; Back, T.C.; Rao, R.; Jiang, J.; Pachter, R.; Tenney, S.A.; Boeckl, J.J.; Maruyama, B. Defect engineering of graphene using electron-beam chemistry with radiolyzed water. Carbon 2020, 166, 446–455. [Google Scholar] [CrossRef]
- Flores-Lasluisa, J.X.; Cazorla-Amorós, D.; Morallón, E. Deepening the Understanding of Carbon Active Sites for ORR Using Electrochemical and Spectrochemical Techniques. Nanomaterials 2024, 14, 1381. [Google Scholar] [CrossRef]
- Poonia, K.; Soni, V.; Sonu; Singh, P.; Chaudhary, V.; Thakur, S.; Nguyen, V.-H.; Van Le, Q.; Raizada, P. Metal-free defects in 2D Nanocarbons toward photocatalytic processes: Revolutionizing solar-to-energy conversion. Appl. Energy 2024, 382, 125195. [Google Scholar] [CrossRef]
- Gao, Y.; Zhang, X.; Wang, P.; Lei, Y.; Yang, X.; Fa, W.; Niu, H.; Zheng, Z. A novel π–conjugated Zn⟵S→Zn unit interface in the ZnS/Zn(S)2L inorganic/orgainc hybrids for significant photoelectric response. Appl. Surf. Sci. 2017, 402, 336–343. [Google Scholar] [CrossRef]
- Ding, Y.; Niu, L.; Chen, Y.; Wang, M. Study on the Defect Structure of Carbon-Doped ZnO Materials. Cryst. Res. Technol. 2023, 58, 2300015. [Google Scholar] [CrossRef]
- Baruah, S.; Dutta, J. Hydrothermal growth of ZnO nanostructures. Sci. Technol. Adv. Mater. 2009, 10, 013001. [Google Scholar] [CrossRef]
- Rojas-Chávez, H.; Miralrio, A.; Hernández-Rodríguez, Y.; Cruz-Martínez, H.; Pérez-Pérez, R.; Cigarroa-Mayorga, O. Needle- and cross-linked ZnO microstructures and their photocatalytic activity using experimental and DFT approach. Mater. Lett. 2021, 291, 129474. [Google Scholar] [CrossRef]
- Zheng, S.; Li, Y.; Hao, J.; Fang, H.; Yuan, Y.; Tsai, H.-S.; Sun, Q.; Wan, P.; Zhang, X.; Wang, Y. Hierarchical assembly of graphene-bridged SnO2-rGO/SnS2 heterostructure with interfacial charge transfer highway for high-performance NO2 detection. Appl. Surf. Sci. 2021, 568, 150926. [Google Scholar] [CrossRef]
- Claros, M.; Setka, M.; Jimenez, Y.P.; Vallejos, S. AACVD Synthesis and Characterization of Iron and Copper Oxides Modified ZnO Structured Films. Nanomaterials 2020, 10, 471. [Google Scholar] [CrossRef]
- Dementjev, A.; Maslakov, K. Possibilities of C 1s XPS and N(E) C KVV Auger spectroscopy for identification of inherent peculiarities of diamond growth. Appl. Surf. Sci. 2006, 253, 1095–1100. [Google Scholar] [CrossRef]
- Saw, K.; du Plessis, J. The X-ray photoelectron spectroscopy C 1s diamond peak of chemical vapour deposition diamond from a sharp interfacial structure. Mater. Lett. 2004, 58, 1344–1348. [Google Scholar] [CrossRef]
- Grey, L.H.; Nie, H.-Y.; Biesinger, M.C. Defining the nature of adventitious carbon and improving its merit as a charge correction reference for XPS. Appl. Surf. Sci. 2024, 653, 159319. [Google Scholar] [CrossRef]
- Cigarroa-Mayorga, O. Enhancement of photocatalytic activity in ZnO NWs array due to Fe2O3 NPs electrodeposited on the nanowires surface: The role of ZnO-Fe2O3 interface. Mater. Today Commun. 2022, 33, 104879. [Google Scholar] [CrossRef]
- Sivkov, D.V.; Petrova, O.V.; Nekipelov, S.V.; Vinogradov, A.S.; Skandakov, R.N.; Bakina, K.A.; Isaenko, S.I.; Ob’edkov, A.M.; Kaverin, B.S.; Vilkov, I.V.; et al. Quantitative Characterization of Oxygen-Containing Groups on the Surface of Carbon Materials: XPS and NEXAFS Study. Appl. Sci. 2022, 12, 7744. [Google Scholar] [CrossRef]
- Nagpal, K.; Rauwel, E.; Estephan, E.; Soares, M.R.; Rauwel, P. Significance of Hydroxyl Groups on the Optical Properties of ZnO Nanoparticles Combined with CNT and PEDOT:PSS. Nanomaterials 2022, 12, 3546. [Google Scholar] [CrossRef]
- Wu, Y.-Z.; Tseng, W.J. Preparation of ZnO@ZnS core-shell nanorod arrays with enhanced photocurrent for removal of methylene blue dyes in wastewater. Open Ceram. 2025, 21, 100756. [Google Scholar] [CrossRef]
- Schorr, S.; Gurieva, G.; Guc, M.; Dimitrievska, M.; Pérez-Rodríguez, A.; Izquierdo-Roca, V.; Schnohr, C.S.; Kim, J.; Jo, W.; Merino, J.M. Point defects, compositional fluctuations, and secondary phases in non-stoichiometric kesterites. J. Physics: Energy 2020, 2, 012002. [Google Scholar] [CrossRef]
- Lee, H.J.; Moon, T.; Hyun, S.D.; Kang, S.; Hwang, C.S. Characterization of a 2D Electron Gas at the Interface of Atomic-Layer Deposited Al2O3/ZnO Thin Films for a Field-Effect Transistor. Adv. Electron. Mater. 2021, 7, 2000876. [Google Scholar] [CrossRef]
- Arbeloa, F.L.; Martínez, V.M.; Arbeloa, T.; Arbeloa, I.L. Photoresponse and anisotropy of rhodamine dye intercalated in ordered clay layered films. J. Photochem. Photobiol. C Photochem. Rev. 2007, 8, 85–108. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, J.; Zhou, G.; Liu, F.; Zhu, X.; Zhang, F. Electric Field Facilitating Hole Transfer in Non-Fullerene Organic Solar Cells with a Negative HOMO Offset. J. Phys. Chem. C 2020, 124, 15132–15139. [Google Scholar] [CrossRef]
- Chinaglia, S.; Tosin, M.; Degli-Innocenti, F. Biodegradation rate of biodegradable plastics at molecular level. Polym. Degrad. Stab. 2018, 147, 237–244. [Google Scholar] [CrossRef]
- Guan, Z.; Chen, Y.; Ding, Y.; Lin, J.; Zhao, Y.; Jiao, Y.; Tian, G. Efficient charge transfer and CO2 photoreduction of hierarchical CeO2@SnS2 heterostructured hollow spheres with spatially separated active sites. Appl. Surf. Sci. 2022, 592, 153192. [Google Scholar] [CrossRef]
- Muhmood, T.; Xia, M.; Lei, W.; Wang, F.; Mahmood, A. Fe-ZrO2 imbedded graphene like carbon nitride for acarbose (ACB) photo-degradation intermediate study. Adv. Powder Technol. 2018, 29, 3233–3240. [Google Scholar] [CrossRef]
- Muhmood, T.; Xia, M.; Lei, W.; Wang, F.; Khan, M.A. Efficient and stable ZrO2/Fe modified hollow-C3N4 for photodegradation of the herbicide MTSM. RSC Adv. 2017, 7, 3966–3974. [Google Scholar] [CrossRef]
- Muhmood, T.; Ahmad, I.; Haider, Z.; Haider, S.K.; Shahzadi, N.; Aftab, A.; Ahmed, S.; Ahmad, F. Graphene-like graphitic carbon nitride (g-C3N4) as a semiconductor photocatalyst: Properties, classification, and defects engineering approaches. Mater. Today Sustain. 2024, 25, 100633. [Google Scholar] [CrossRef]
- Bibi, Z.; Ali, M.; Abohashrh, M.; Ahmad, I.; Khan, H.; Ali, M.; Akbar, F.; Ahmad, N.; Iqbal, A.; Ullah, F.; et al. Biologically Synthesized Silver Nanoparticles Efficiently Control Plant Pathogenic Bacteria-Erwinia carotovora and Ralstonia solanacearum. Inorganics 2023, 11, 309. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vargas-Vidal, B.A.; Baños-López, E.; Munguía-Fuentes, M.d.R.; Hernández-Rodríguez, Y.M.; Cigarroa-Mayorga, O.E. One-Pot Synthesis for Doped Amorphous Carbon-Based Compounds: Influence of ZnO Dopant on the Charge Transfer Efficiency. Nanomaterials 2025, 15, 1486. https://doi.org/10.3390/nano15191486
Vargas-Vidal BA, Baños-López E, Munguía-Fuentes MdR, Hernández-Rodríguez YM, Cigarroa-Mayorga OE. One-Pot Synthesis for Doped Amorphous Carbon-Based Compounds: Influence of ZnO Dopant on the Charge Transfer Efficiency. Nanomaterials. 2025; 15(19):1486. https://doi.org/10.3390/nano15191486
Chicago/Turabian StyleVargas-Vidal, Bernardo Alberto, Esperanza Baños-López, María del Rosario Munguía-Fuentes, Yazmín Mariela Hernández-Rodríguez, and Oscar Eduardo Cigarroa-Mayorga. 2025. "One-Pot Synthesis for Doped Amorphous Carbon-Based Compounds: Influence of ZnO Dopant on the Charge Transfer Efficiency" Nanomaterials 15, no. 19: 1486. https://doi.org/10.3390/nano15191486
APA StyleVargas-Vidal, B. A., Baños-López, E., Munguía-Fuentes, M. d. R., Hernández-Rodríguez, Y. M., & Cigarroa-Mayorga, O. E. (2025). One-Pot Synthesis for Doped Amorphous Carbon-Based Compounds: Influence of ZnO Dopant on the Charge Transfer Efficiency. Nanomaterials, 15(19), 1486. https://doi.org/10.3390/nano15191486






