The Devil Lies in the Details: Small Structural and Chemical Changes in Iron Oxide Pigments Largely Alter the Biological Outcomes in Macrophages
Abstract
1. Introduction
2. Material and Methods
2.1. Pigment Particles Preparation and Characterization
2.2. Cell Culture and Treatment
2.2.1. Determination of Sublethal Dose (LD20)
2.2.2. Recovery Exposure Settings
2.3. Cellular Quantification of Metals by Inductively Coupled Plasma–Mass Spectrometry (ICP-MS)
2.4. Assessment of Cellular Functional Parameters
2.5. Measurement of Cytokines Secretion
3. Results
3.1. Physicochemical Characterization of Iron-Based Pigments
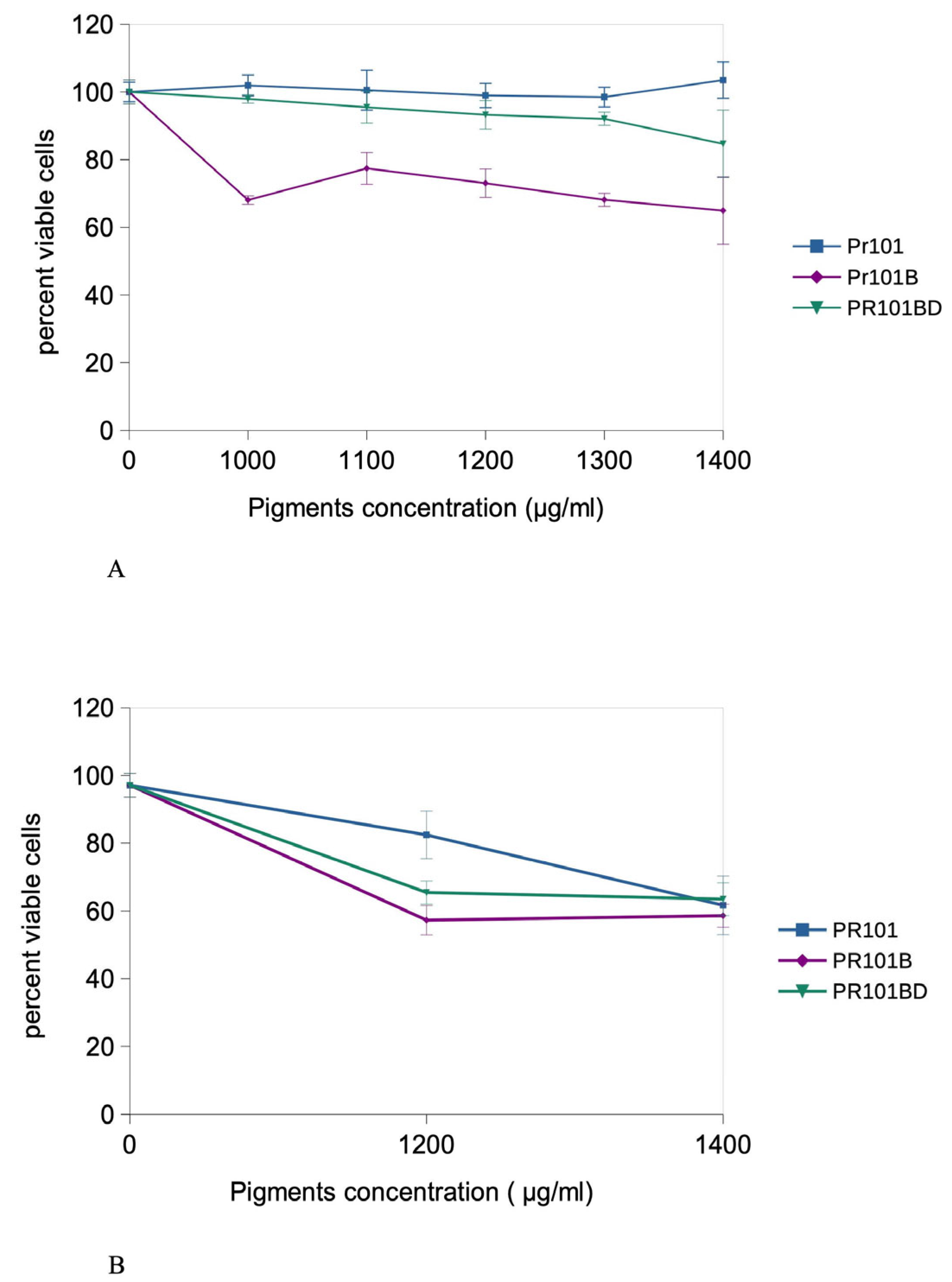
3.2. Cell Exposure and Dose Selection
3.3. Initial Experiments in Acute Exposure Mode
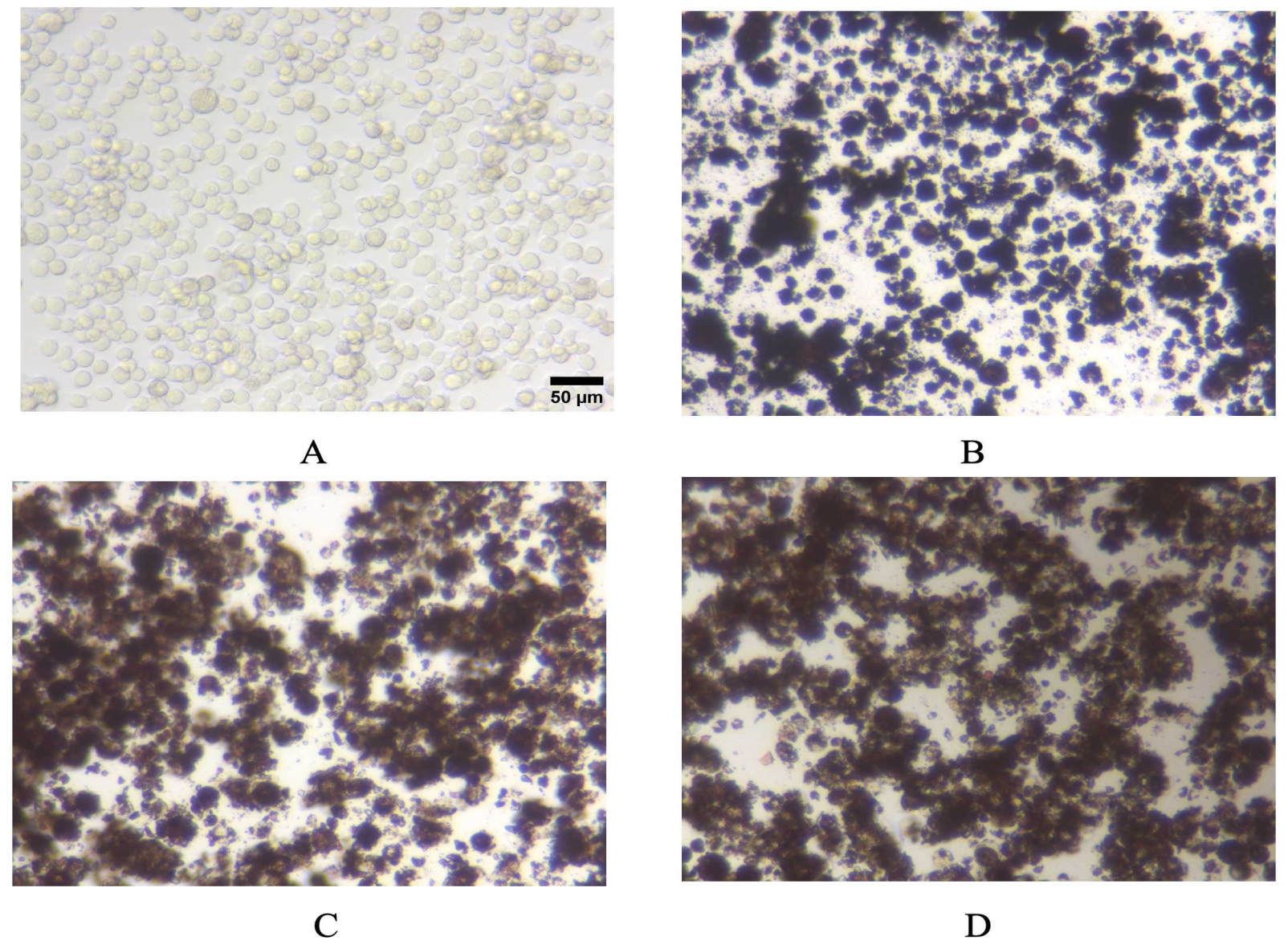
3.4. Pigments Uptake and Dissolution by Cells
3.5. Mitochondria
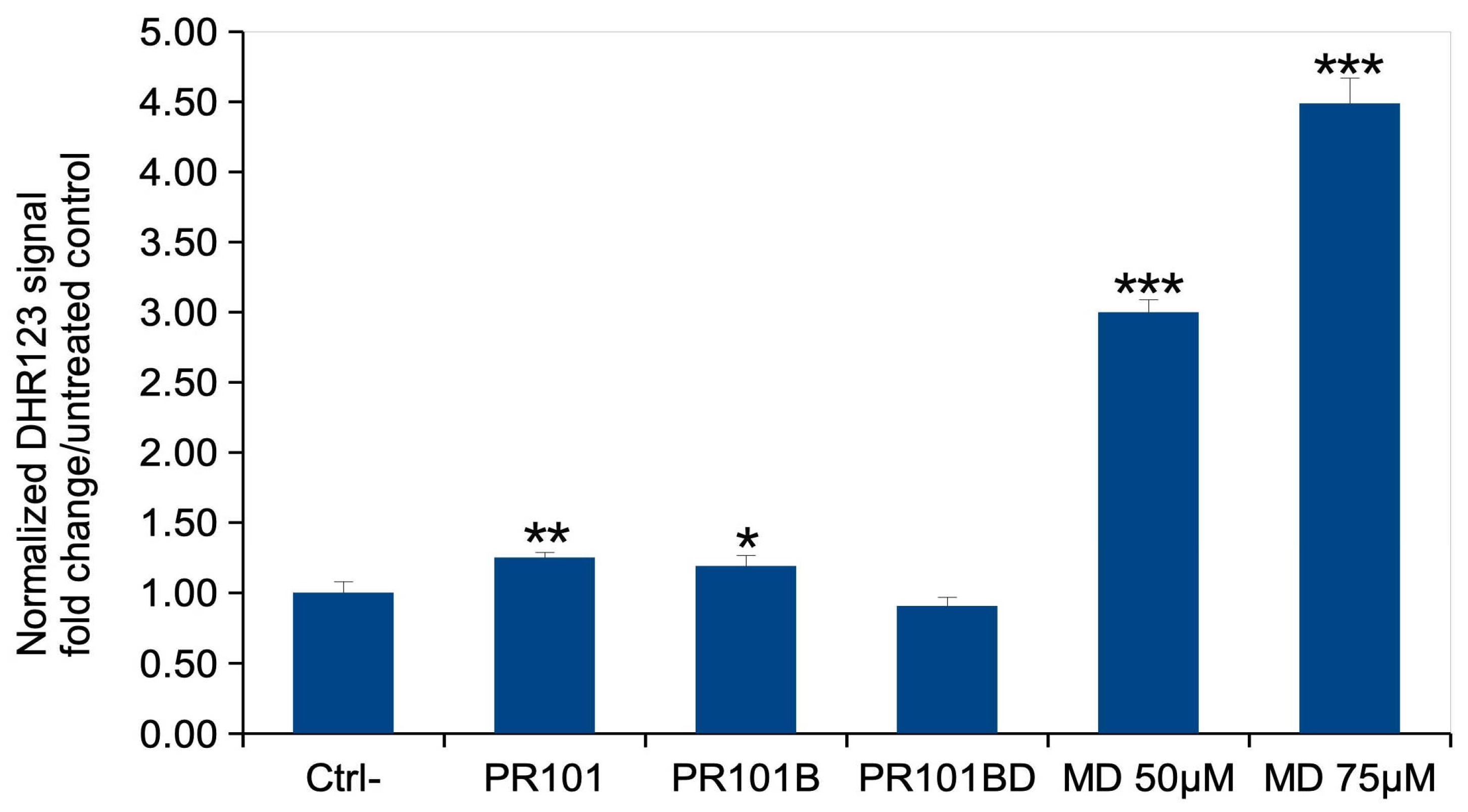
3.6. Oxidative Stress
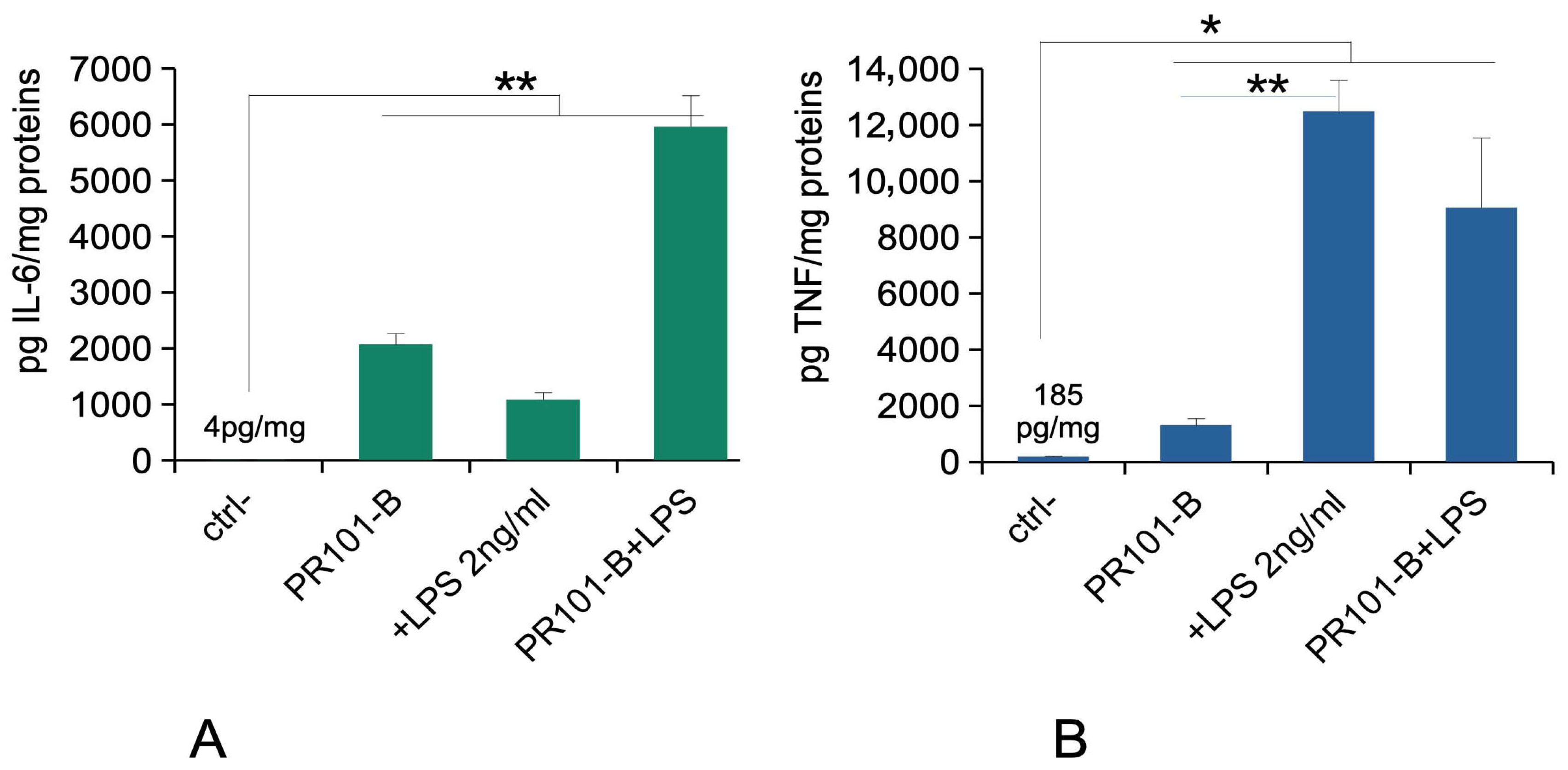
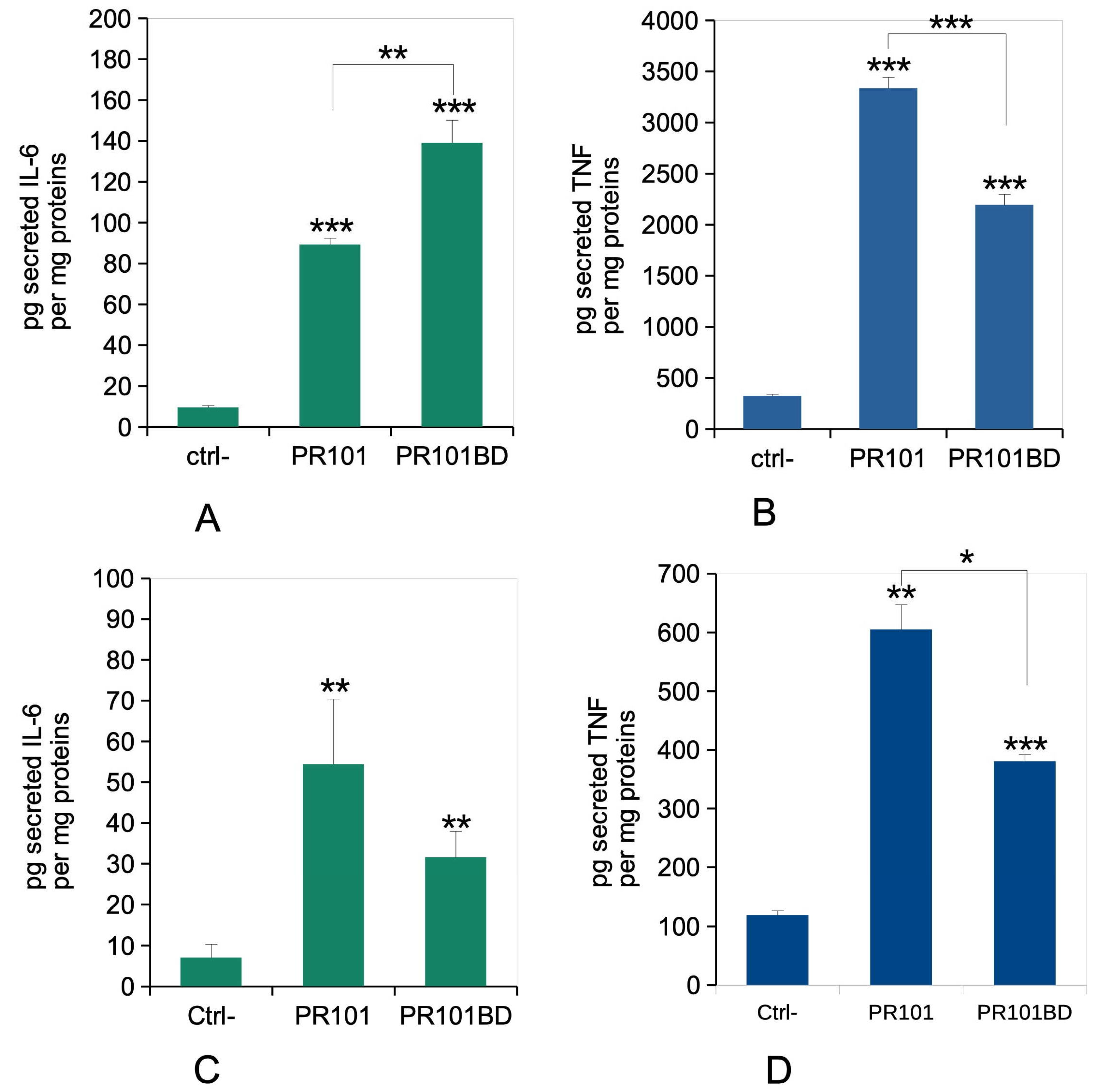
3.7. Immune Functions
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aloupi, E.; Karydas, A.G.; Paradellis, T. Pigment Analysis of Wall Paintings and Ceramics from Greece and Cyprus. The Optimum Use of x-Ray Spectrometry on Specific Archaeological Issues. X-Ray Spectrom. 2000, 29, 18–24. [Google Scholar] [CrossRef]
- Cianchetta, I.; Trentelman, K.; Walton, M.S.; Maish, J.; Mehta, A.; Foran, B. Reverse Engineering Ancient Greek Ceramics: Morphological and Spectral Characterization of Replicates. J. Am. Ceram. Soc. 2016, 99, 1792–1801. [Google Scholar] [CrossRef]
- Rodler-Rørbo, A.; Baragona, A.J.; Verbeemen, E.J.; Sørensen, L.V.; Çakmakoğlu, B.; Helvaci, C.; Bolea-Fernandez, E.; Rua-Ibarz, A.; Vanhaecke, F.; Becker, H.; et al. Cinnabar for Roman Ephesus: Material Quality, Processing and Provenance. J. Archaeol. Sci. 2025, 173, 106122. [Google Scholar] [CrossRef]
- Sepúlveda, M.; Rousseliere, H.; Van Elslande, E.; Arriaza, B.; Standen, V.; Santoro, C.M.; Walter, P. Study of Color Pigments Associated to Archaic Chinchorro Mummies and Grave Goods in Northern Chile (7000–3500 B.P.). Herit. Sci. 2014, 2, 7. [Google Scholar] [CrossRef]
- van Dalen Luna, P.; Majchrzak, L.; Malek, K.; Kuncewicz, J.; Miskowiec, P. The Multimodal Chemical Study of Pre-Columbian Peruvian Mummies. Analyst 2020, 145, 5670–5681. [Google Scholar] [CrossRef]
- Mangiapane, G.; Di Francia, E.; Gerst, R.; Radelet, T.; Aceto, M.; Agostino, A.; Boano, R. Rare Tattoos Shape and Composition on a South American Mummy. J. Cult. Herit. 2025, 73, 561–570. [Google Scholar] [CrossRef]
- Medley, S.S. Childhood Lead Toxicity: A Paradox of Modern Technology. Ann. Am. Acad. Pol. Soc. Sci. 1982, 461, 63–73. [Google Scholar] [CrossRef]
- Marino, P.E.; Landrigan, P.J.; Graef, J.; Nussbaum, A.; Bayan, G.; Boch, K.; Boch, S. A Case Report of Lead Paint Poisoning during Renovation of a Victorian Farmhouse. Am. J. Public. Health 1990, 80, 1183–1185. [Google Scholar] [CrossRef]
- Davis, R.G. Hazards of Tattooing: Report of Two Cases of Dermatitis Caused by Sensitization to Mercury (Cinnabar). U. S. Armed Forces Med. J. 1960, 11, 261–280. [Google Scholar]
- Biro, L.; Klein, W.P. Unusual Complications of Mercurial (Cinnabar) Tattoo. Generalized Eczematous Eruption Following Laceration of a Tattoo. Arch. Dermatol. 1967, 96, 165–167. [Google Scholar] [CrossRef]
- Young, Y.-H.; Chuu, J.-J.; Liu, S.-H.; Lin-Shiau, S.-Y. Neurotoxic Mechanism of Cinnabar and Mercuric Sulfide on the Vestibulo-Ocular Reflex System of Guinea Pigs. Toxicol. Sci. 2002, 67, 256–263. [Google Scholar] [CrossRef]
- Huang, C.-F.; Liu, S.-H.; Lin-Shiau, S.-Y. Neurotoxicological Effects of Cinnabar (a Chinese Mineral Medicine, HgS) in Mice. Toxicol. Appl. Pharmacol. 2007, 224, 192–201. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, L.M.; Permin, H. Rheumatic Disease, Heavy-Metal Pigments, and the Great Masters. Lancet 1988, 1, 1267–1269. [Google Scholar] [CrossRef] [PubMed]
- Chalmin, E.; Schmitt, B.; Chanteraud, C.; De Kergommeaux, A.C.; Soufi, F.; Salomon, H. How to Distinguish Red Coloring Matter Used in Prehistoric Time? The Contribution of Visible Near-infrared Diffuse Reflectance Spectroscopy. Color. Res. Appl. 2021, 46, 653–673. [Google Scholar] [CrossRef]
- Pomiés, M.-P.; Menu, M.; Vignaud, C. Red Palaeolithic Pigments: Natural Hematite or Heated Goethite? Archaeometry 1999, 41, 275–285. [Google Scholar] [CrossRef]
- Cavallo, G.; Fontana, F.; Gialanella, S.; Gonzato, F.; Riccardi, M.P.; Zorzin, R.; Peresani, M. Heat Treatment of Mineral Pigment During the Upper Palaeolithic in North-East Italy. Archaeometry 2018, 60, 1045–1061. [Google Scholar] [CrossRef]
- Leskelä, T.; Leskelä, M. Preparation of Yellow and Red Iron Oxide Pigments from Iron(II) Sulfate by Alkali Precipitation. Thermochim. Acta 1984, 77, 177–184. [Google Scholar] [CrossRef]
- Stefenon, F.L.; Jaerger, S.; Schneider, R.; Anaissi, F.J.; González-Borrero, P.P. Synthesis and Characterisation of Iron Oxides: Effect of Calcination Temperature and Their Application as Inorganic Pigment. Color. Technol. 2025, cote.12826. [Google Scholar] [CrossRef]
- Leskelä, T.; Leskelä, M.; Niinistö, L. Preparation of Red Iron Oxide Pigments by Thermal Treatment of Iron(II) Sulfate. Thermochim. Acta 1984, 72, 229–237. [Google Scholar] [CrossRef]
- Touzi, N.; Horchani-Naifer, K. A Study on the Preparation and Characterization of Pigment Quality from Mill Scale Steel Wastes. Environ. Sci. Pollut. Res. 2023, 31, 40538–40553. [Google Scholar] [CrossRef]
- Uhlmann, N.R.; Martins, M.M.; Piato, S. 3D Areola Dermopigmentation (Nipple-areola Complex). Breast J. 2019, 25, 1214–1221. [Google Scholar] [CrossRef] [PubMed]
- Kuruvilla, A.S.; Gopman, J.M.; Cham, S.; Henderson, P.W. Nipple-Areolar Tattoo: Comprehensive Review of History, Theory, Technique, and Outcomes. J. Plast. Reconstr. Aesthetic Surg. 2022, 75, 544–549. [Google Scholar] [CrossRef] [PubMed]
- Gabby, J.L. Toxicity of Cadmium Sulfide and Cadmium Sulfoselenide Pigments. Arch. Ind. Hyg. Occup. Med. 1950, 1, 677–684. [Google Scholar]
- Luengo, Y.; Nardecchia, S.; Morales, M.P.; Serrano, M.C. Different Cell Responses Induced by Exposure to Maghemite Nanoparticles. Nanoscale 2013, 5, 11428–11437. [Google Scholar] [CrossRef]
- Park, E.-J.; Umh, H.N.; Choi, D.-H.; Cho, M.H.; Choi, W.; Kim, S.-W.; Kim, Y.; Kim, J.-H. Magnetite- and Maghemite-Induced Different Toxicity in Murine Alveolar Macrophage Cells. Arch. Toxicol. 2014, 88, 1607–1618. [Google Scholar] [CrossRef]
- Dalzon, B.; Torres, A.; Reymond, S.; Gallet, B.; Saint-Antonin, F.; Collin-Faure, V.; Moriscot, C.; Fenel, D.; Schoehn, G.; Aude-Garcia, C.; et al. Influences of Nanoparticles Characteristics on the Cellular Responses: The Example of Iron Oxide and Macrophages. Nanomaterials 2020, 10, 266. [Google Scholar] [CrossRef]
- Chrishtop, V.V.; Mironov, V.A.; Prilepskii, A.Y.; Nikonorova, V.G.; Vinogradov, V.V. Organ-Specific Toxicity of Magnetic Iron Oxide-Based Nanoparticles. Nanotoxicology 2021, 15, 167–204. [Google Scholar] [CrossRef]
- Matsuzaki, H.; Maeda, M.; Lee, S.; Nishimura, Y.; Kumagai-Takei, N.; Hayashi, H.; Yamamoto, S.; Hatayama, T.; Kojima, Y.; Tabata, R.; et al. Asbestos-Induced Cellular and Molecular Alteration of Immunocompetent Cells and Their Relationship with Chronic Inflammation and Carcinogenesis. J. Biomed. Biotechnol. 2012, 2012, 492608. [Google Scholar] [CrossRef]
- Pollard, K.M. Silica, Silicosis, and Autoimmunity. Front. Immunol. 2016, 7, 97. [Google Scholar] [CrossRef]
- Vitipon, M.; Akingbagbohun, E.; Devime, F.; Diemer, H.; Hirschler, A.; Fenel, D.; Ravanel, S.; Carapito, C.; Rabilloud, T. Beyond the Ink: Cellular and Molecular Effects of Iron-Based Pigments on Macrophages. NanoImpact 2025, 39, 100578. [Google Scholar] [CrossRef]
- Tanaka, Y.; Nishikawa, M.; Mizukami, Y.; Kusamori, K.; Ogino, Y.; Nishimura, S.; Shimizu, K.; Konishi, S.; Takahashi, Y.; Takakura, Y. Control of Polarization and Tumoricidal Activity of Macrophages by Multicellular Spheroid Formation. J. Control. Release 2018, 270, 177–183. [Google Scholar] [CrossRef]
- Tsuji, K.; Harrison, S.J. Dry-Heat Destruction of Lipopolysaccharide: Dry-Heat Destruction Kinetics. Appl. Environ. Microbiol. 1978, 36, 710–714. [Google Scholar] [CrossRef] [PubMed]
- Vitipon, M.; Akingbagbohun, E.; Rabilloud, T. The VVBlue Assay: A Plate-Readable, Dye Exclusion-Based Cell Viability Assay for the Toxicological Testing of Chemicals. RSC Adv. 2025, 15, 17885–17896. [Google Scholar] [CrossRef] [PubMed]
- Dalzon, B.; Torres, A.; Devcic, J.; Fenel, D.; Sergent, J.-A.; Rabilloud, T. A Low-Serum Culture System for Prolonged in Vitro Toxicology Experiments on a Macrophage System. Front. Toxicol. 2021, 3, 780778. [Google Scholar] [CrossRef] [PubMed]
- Korshunov, S.S.; Skulachev, V.P.; Starkov, A.A. High Protonic Potential Actuates a Mechanism of Production of Reactive Oxygen Species in Mitochondria. FEBS Lett. 1997, 416, 15–18. [Google Scholar] [CrossRef]
- Starkov, A.A.; Fiskum, G. Regulation of Brain Mitochondrial H2O2 Production by Membrane Potential and NAD(P)H Redox State. J. Neurochem. 2003, 86, 1101–1107. [Google Scholar] [CrossRef]
- Kattumuri, V.; Katti, K.; Bhaskaran, S.; Boote, E.J.; Casteel, S.W.; Fent, G.M.; Robertson, D.J.; Chandrasekhar, M.; Kannan, R.; Katti, K.V. Gum Arabic as a Phytochemical Construct for the Stabilization of Gold Nanoparticles: In Vivo Pharmacokinetics and X-Ray-Contrast-Imaging Studies. Small 2007, 3, 333–341. [Google Scholar] [CrossRef]
- Simon-Deckers, A.; Loo, S.; Mayne-L’hermite, M.; Herlin-Boime, N.; Menguy, N.; Reynaud, C.; Gouget, B.; Carrière, M. Size-, Composition- and Shape-Dependent Toxicological Impact of Metal Oxide Nanoparticles and Carbon Nanotubes toward Bacteria. Environ. Sci. Technol. 2009, 43, 8423–8429. [Google Scholar] [CrossRef]
- EFSA Panel on Contaminants in the Food Chain (CONTAM). Presence of Microplastics and Nanoplastics in Food, with Particular Focus on Seafood. EFSA J. 2016, 14, e04501. [Google Scholar] [CrossRef]
- Huang, C.; Sun, M.; Yang, Y.; Wang, F.; Ma, X.; Li, J.; Wang, Y.; Ding, Q.; Ying, H.; Song, H.; et al. Titanium Dioxide Nanoparticles Prime a Specific Activation State of Macrophages. Nanotoxicology 2017, 11, 737–750. [Google Scholar] [CrossRef]
- Palomäki, J.; Karisola, P.; Pylkkänen, L.; Savolainen, K.; Alenius, H. Engineered Nanomaterials Cause Cytotoxicity and Activation on Mouse Antigen Presenting Cells. Toxicology 2010, 267, 125–131. [Google Scholar] [CrossRef]
- Giovanni, M.; Yue, J.; Zhang, L.; Xie, J.; Ong, C.N.; Leong, D.T. Pro-Inflammatory Responses of RAW264.7 Macrophages When Treated with Ultralow Concentrations of Silver, Titanium Dioxide, and Zinc Oxide Nanoparticles. J. Hazard. Mater. 2015, 297, 146–152. [Google Scholar] [CrossRef] [PubMed]
- Schoenenberger, A.D.; Schipanski, A.; Malheiro, V.; Kucki, M.; Snedeker, J.G.; Wick, P.; Maniura-Weber, K. Macrophage Polarization by Titanium Dioxide (TiO2) Particles: Size Matters. ACS Biomater. Sci. Eng. 2016, 2, 908–919. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Zhao, F.; Fan, M.; He, C.; Yang, X.; Huang, Z.; Fu, Z. The Influence of Titanium Dioxide Nanoparticles on Their Cellular Response to Macrophage Cells. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2019, 223, 42–52. [Google Scholar] [CrossRef] [PubMed]
- Young, N.S.; Levin, J.; Prendergast, R.A. An Invertebrate Coagulation System Activated by Endotoxin: Evidence for Enzymatic Mediation. J. Clin. Investig. 1972, 51, 1790–1797. [Google Scholar] [CrossRef]
- Val, S.; Hussain, S.; Boland, S.; Hamel, R.; Baeza-Squiban, A.; Marano, F. Carbon Black and Titanium Dioxide Nanoparticles Induce Pro-Inflammatory Responses in Bronchial Epithelial Cells: Need for Multiparametric Evaluation Due to Adsorption Artifacts. Inhal. Toxicol. 2009, 21 (Suppl. S1), 115–122. [Google Scholar] [CrossRef]
- Padhy, I.; Dwibedy, S.K.; Mohapatra, S.S. A Molecular Overview of the Polymyxin-LPS Interaction in the Context of Its Mode of Action and Resistance Development. Microbiol. Res. 2024, 283, 127679. [Google Scholar] [CrossRef]
- Nikolova, M.; Pasheva, M.; Tasinov, O.; Kiselova-Kaneva, Y. Lps-Induced Response In J774a.1 Macrophage Cell Culture-Indicative Markers for Stimulated Antioxidant Defense, Inflammation and Phagocytosis. Scr. Sci. Pharm. 2019, 6, 31–36. [Google Scholar] [CrossRef]
- Schreiver, I.; Hesse, B.; Seim, C.; Castillo-Michel, H.; Villanova, J.; Laux, P.; Dreiack, N.; Penning, R.; Tucoulou, R.; Cotte, M.; et al. Synchrotron-Based ν-XRF Mapping and μ-FTIR Microscopy Enable to Look into the Fate and Effects of Tattoo Pigments in Human Skin. Sci. Rep. 2017, 7, 11395. [Google Scholar] [CrossRef]
- Nielsen, C.; Jerkeman, M.; Jöud, A.S. Tattoos as a Risk Factor for Malignant Lymphoma: A Population-Based Case–Control Study. eClinicalMedicine 2024, 72, 102649. [Google Scholar] [CrossRef]
- Brydon, N. Endotoxin Is Inactivated by Ethylene Oxide, Gamma, Electron Beam, and Steam Sterilization. Biomed. Instrum. Technol. 2023, 57, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Food and Drug Administration. Insanitary Conditions in the Preparation, Packing, and Holding of Tattoo Inks and the Risk of Microbial Contamination Guidance for Industry. 2024. Available online: https://www.fda.gov/media/183019/download (accessed on 20 November 2025).
- Liu, F. Safety Assessment of Endotoxin Related to Drug Products and Medical Devices: A Review. Toxicol. Lett. 2025, 412, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Wallace, R.J.; Gropp, J.; Dierick, N.; Costa, L.G.; Martelli, G.; Brantom, P.G.; Bampidis, V.; Renshaw, D.W.; Leng, L. Risks Associated with Endotoxins in Feed Additives Produced by Fermentation. Environ. Health 2016, 15, 5. [Google Scholar] [CrossRef] [PubMed]
- Høgsberg, T.; Saunte, D.M.; Frimodt-Møller, N.; Serup, J. Microbial Status and Product Labelling of 58 Original Tattoo Inks. Acad. Dermatol. Venereol. 2013, 27, 73–80. [Google Scholar] [CrossRef]
- Negi, S.; Bala, L.; Shukla, S.; Chopra, D. Tattoo Inks Are Toxicological Risks to Human Health: A Systematic Review of Their Ingredients, Fate inside Skin, Toxicity Due to Polycyclic Aromatic Hydrocarbons, Primary Aromatic Amines, Metals, and Overview of Regulatory Frameworks. Toxicol. Ind. Health 2022, 38, 417–434. [Google Scholar] [CrossRef]
- Yoon, S.; Kondakala, S.; Foley, S.L.; Moon, M.S.; Huang, M.-C.J.; Periz, G.; Zang, J.; Katz, L.M.; Kim, S.-J.; Kweon, O. Detection of Anaerobic and Aerobic Bacteria from Commercial Tattoo and Permanent Makeup Inks. Appl. Environ. Microbiol. 2024, 90, e00276-24. [Google Scholar] [CrossRef]
- Da Guarda Souza, M.O.; Dos Santos, M.V.R.; Castro, L.M.F.; Da Silva, C.P. Production and in Situ Transformation of Hematite into Magnetite from the Thermal Decomposition of Iron Nitrate or Goethite Mixed with Biomass. J. Therm. Anal. Calorim. 2020, 139, 1731–1739. [Google Scholar] [CrossRef]
- Pineau, A.; Kanari, N.; Gaballah, I. Kinetics of Reduction of Iron Oxides by H2. Thermochim. Acta 2006, 447, 89–100. [Google Scholar] [CrossRef]
- Ponomar, V.P. Synthesis and Magnetic Properties of Magnetite Prepared by Chemical Reduction from Hematite of Various Particle Sizes. J. Alloys Compd. 2018, 741, 28–34. [Google Scholar] [CrossRef]
- Raza, H.; John, A.; Shafarin, J. NAC Attenuates LPS-Induced Toxicity in Aspirin-Sensitized Mouse Macrophages via Suppression of Oxidative Stress and Mitochondrial Dysfunction. PLoS ONE 2014, 9, e103379. [Google Scholar] [CrossRef]
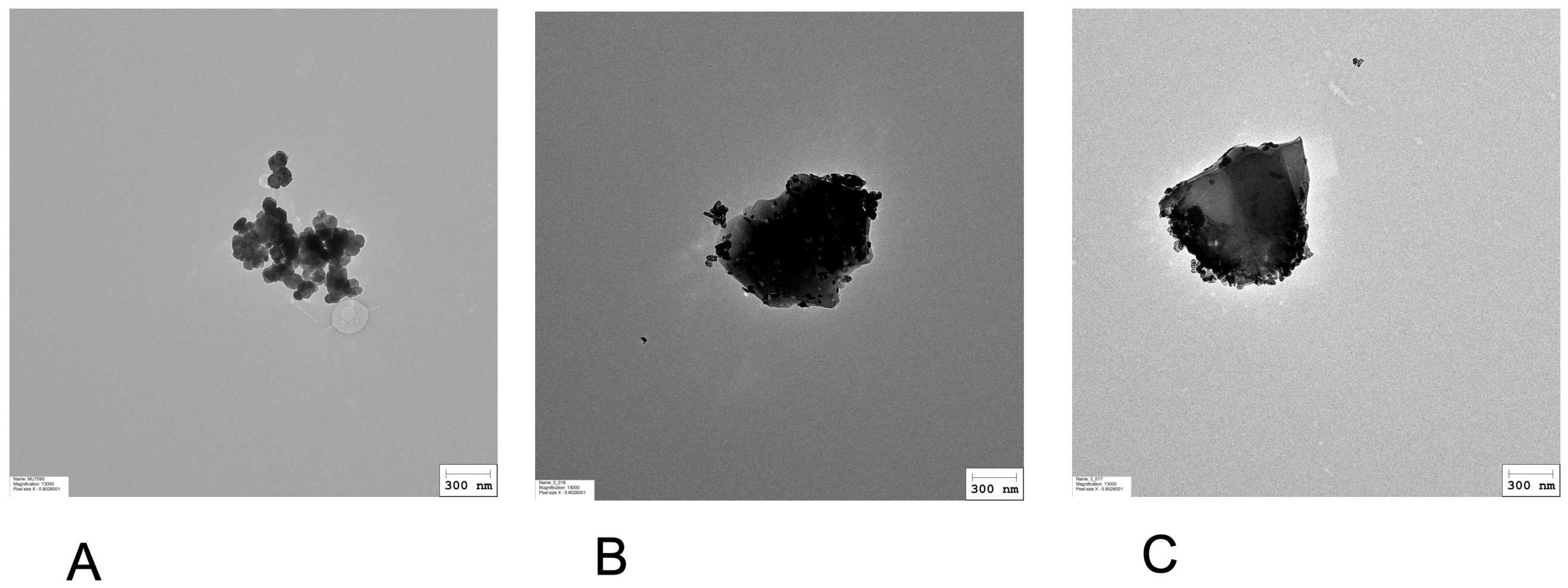

| PR101 | PR101B | PR101BD | |
|---|---|---|---|
| Mean Hydrodynamic diameter (nm) | 386.71 ± 3.44 | 417.06 ± 18.56 | 427.60 ± 22.57 |
| Polydispersity index (%) | 18.79 ± 3.07 | 24.95 ± 0.73 | 26.31 ± 0.92 |
| Zeta potential (mV) | −31.46 ± 0.08 | −30.13 ± 0.22 | −29.81 ± 0.06 |
| Control Cells | PR101BD-Exposed Cells | |
|---|---|---|
| Iron input (measured) | ND | 143.6 ± 19.1 |
| Total cell-associated iron | ND | 83.8 ± 12 |
| Non-sedimentable, cell-associated iron | ND | 0.106 ± 0.045 |
| Mineralization yield (%) | ND | 17.1 ± 2.2 |
| Percent iron internalized | ND | 58.4 ± 8.4 |
| Percent internalized iron dissolved | ND | 0.016 ± 0.009 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vitipon, M.; Akingbagbohun, E.; Devime, F.; Fenel, D.; Ravanel, S.; Rabilloud, T. The Devil Lies in the Details: Small Structural and Chemical Changes in Iron Oxide Pigments Largely Alter the Biological Outcomes in Macrophages. Nanomaterials 2025, 15, 1772. https://doi.org/10.3390/nano15231772
Vitipon M, Akingbagbohun E, Devime F, Fenel D, Ravanel S, Rabilloud T. The Devil Lies in the Details: Small Structural and Chemical Changes in Iron Oxide Pigments Largely Alter the Biological Outcomes in Macrophages. Nanomaterials. 2025; 15(23):1772. https://doi.org/10.3390/nano15231772
Chicago/Turabian StyleVitipon, Marianne, Esther Akingbagbohun, Fabienne Devime, Daphna Fenel, Stéphane Ravanel, and Thierry Rabilloud. 2025. "The Devil Lies in the Details: Small Structural and Chemical Changes in Iron Oxide Pigments Largely Alter the Biological Outcomes in Macrophages" Nanomaterials 15, no. 23: 1772. https://doi.org/10.3390/nano15231772
APA StyleVitipon, M., Akingbagbohun, E., Devime, F., Fenel, D., Ravanel, S., & Rabilloud, T. (2025). The Devil Lies in the Details: Small Structural and Chemical Changes in Iron Oxide Pigments Largely Alter the Biological Outcomes in Macrophages. Nanomaterials, 15(23), 1772. https://doi.org/10.3390/nano15231772






