Therapeutic Potential of Nanoscale Metal–Organic Frameworks in Hepatocellular Carcinoma
Abstract
1. Introduction
2. Metal–Organic Frameworks-Based Therapies for Hepatocellular Carcinoma
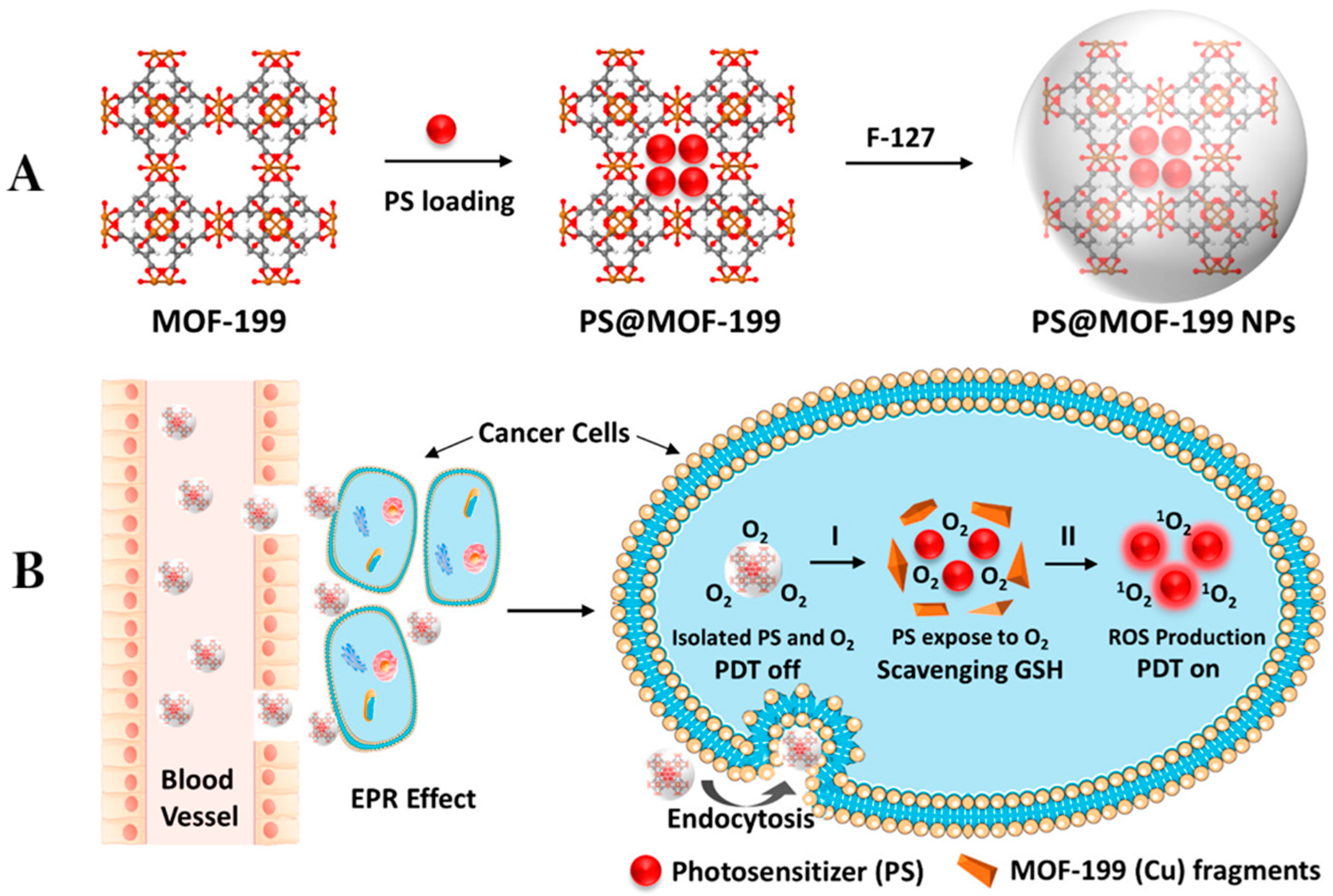
2.1. Metal–Organic Frameworks for Photodynamic Therapy of Cancer
2.2. Metal–Organic Frameworks for Photothermal Therapy of Cancer
2.3. Metal–Organic Frameworks for Chemodynamic Therapy of Cancer
2.4. Metal–Organic Frameworks for Sonodynamic Therapy of Cancer
3. Conclusions and Future Directions
- (1)
- Currently available phototherapeutic agents face notable limitations, such as restricted tissue penetration of light used in phototherapy, dependence on oxygen for ROS generation in PDT, and non-uniform heat distribution in PTT. Although strategies like upconversion NPs or two-photon-activated photosensitizers can enhance light penetration, targeting deep organs like the liver remains difficult. As a result, imaging-guided approaches have become a critical component in phototherapy. Minimally invasive laparoscopic techniques allow optical fibers to access deeper tissues, activating photosensitizers for effective treatment. Such methods have already advanced to clinical applications and show great promise for precision cancer theranostics. Additionally, light-independent modalities, including chemiluminescence and bioluminescence, offer alternative means to overcome the limitations associated with light penetration [50,93,94].
- (2)
- To improve the biosafety of MOFs as drug carriers, it is important to use biocompatible ligands and metal ions, such as Fe, Ca, and Zn. Surface modifications using peptides, antibodies, or small molecules have proven effective for enhancing tumor targeting and accumulation. Despite these advances, the pharmacokinetics of many MOF systems remain inadequately studied. Once MOFs reach the tumor site, they should degrade efficiently into small molecules or ions that can be metabolized safely, minimizing adverse effects. Although various stimuli—including pH, biothiols, ATP, and hypoxic conditions—have been investigated to trigger MOF degradation, their effectiveness requires further optimization [95,96,97,98,99,100,101].
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global Cancer Statistics 2022: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Tutunchi, H.; Naeini, F.; Mobasseri, M.; Ostadrahimi, A. Triglyceride Glucose (TyG) Index and the Progression of Liver Fibrosis: A Cross-Sectional Study. Clin. Nutr. ESPEN 2021, 44, 483–487. [Google Scholar] [CrossRef] [PubMed]
- Tutunchi, H.; Arefhosseini, S.; Nomi-Golzar, S.; Ebrahimi-Mameghani, M. Effects of Hydroxycitric Acid Supplementation on Body Composition, Obesity Indices, Appetite, Leptin, and Adiponectin of Women with NAFLD on a Calorie-Restricted Diet. Int. J. Clin. Pract. 2023, 2023, 6492478. [Google Scholar] [CrossRef] [PubMed]
- Venook, A.P.; Papandreou, C.; Furuse, J.; de Guevara, L.L. The Incidence and Epidemiology of Hepatocellular Carcinoma: A Global and Regional Perspective. Oncologist 2010, 15 (Suppl. 4), 5–13. [Google Scholar] [CrossRef]
- Zafar, A.; Khatoon, S.; Khan, M.J.; Abu, J.; Naeem, A. Advancements and Limitations in Traditional Anti-Cancer Therapies: A Comprehensive Review of Surgery, Chemotherapy, Radiation Therapy, and Hormonal Therapy. Discov. Oncol. 2025, 16, 607. [Google Scholar] [CrossRef]
- Wang, K.; Tepper, J.E. Radiation Therapy-Associated Toxicity: Etiology, Management, and Prevention. CA Cancer J. Clin. 2021, 71, 437–454. [Google Scholar] [CrossRef]
- Wang, W.; Wei, C. Advances in the Early Diagnosis of Hepatocellular Carcinoma. Genes Dis. 2020, 7, 308–319. [Google Scholar] [CrossRef]
- Feng, Y.; Wu, W.; Li, M. Metal–Organic Frameworks for Hepatocellular Carcinoma Therapy and Mechanism. Front. Pharmacol. 2022, 13, 1025780. [Google Scholar] [CrossRef]
- Tutunchi, H.; Ebrahimi-Mameghani, M.; Hosseinzadeh-Attar, M.J.; Roshanravan, N.; Mobasseri, M.; Najafipour, F.; Naeini, F.; Naghshi, S.; Asghari, S.; Akbarzadeh, M.; et al. Effects of Oleoylethanolamide Supplementation on the Expression of Lipid Metabolism-Related Genes and Serum NRG4 Levels in Patients with Non-Alcoholic Fatty Liver Disease: A Randomized Controlled Trial. Clin. Nutr. ESPEN 2023, 58, 311–319. [Google Scholar] [CrossRef] [PubMed]
- Kong, J.; Cai, M.; Zhu, R.; Zhang, Y.; Du, Y.; Jing, X.; Sun, Y.; Chang, R.; Qu, C.; Dong, X.; et al. The Utilization of Metal–Organic Frameworks in Tumor-Targeted Drug Delivery Systems. J. Sci. Adv. Mater. 2024, 9, 100770. [Google Scholar] [CrossRef]
- Linnane, E.; Haddad, S.; Melle, F.; Mei, Z.; Fairen-Jimenez, D. The Uptake of Metal–Organic Frameworks: A Journey into the Cell. Chem. Soc. Rev. 2022, 51, 6065–6086. [Google Scholar] [CrossRef]
- Wang, Y.; Yan, J.; Wen, N.; Xiong, H.; Cai, S.; He, Q.; Hu, Y.; Peng, D.; Liu, Z.; Liu, Y. Metal–Organic Frameworks for Stimuli-Responsive Drug Delivery. Biomaterials 2020, 230, 119619. [Google Scholar] [CrossRef]
- Pacheco, C.; Baião, A.; Ding, T.; Cui, W.; Sarmento, B. Recent Advances in Long-Acting Drug Delivery Systems for Anticancer Drug. Adv. Drug Deliv. Rev. 2023, 194, 114724. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Guo, B.; Gu, J.; Ta, N.; Yu, H.; Sun, M.; Han, T. Emerging Advances in Drug Delivery Systems (DDSs) for Optimizing Cancer Complications. Mater. Today Bio 2024, 30, 101375. [Google Scholar] [CrossRef]
- Chen, Q.; Yang, Z.; Liu, H.; Man, J.; Oladejo, A.O.; Ibrahim, S.; Wang, S.; Hao, B. Novel Drug Delivery Systems: An Important Direction for Drug Innovation Research and Development. Pharmaceutics 2024, 16, 674. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.; Singhal, S.; Pahwa, S.; Arora Sethi, V.; Sharma, S.; Singh, P.; Kale, R.D.; Wazed Ali, S.; Sagadevan, S. Nanomedicine and Drug Delivery: A Comprehensive Review of Applications and Challenges. Nano-Struct. Nano-Objects 2024, 40, 101403. [Google Scholar] [CrossRef]
- Malik, S.; Muhammad, K.; Waheed, Y. Emerging Applications of Nanotechnology in Healthcare and Medicine. Molecules 2023, 28, 6624. [Google Scholar] [CrossRef]
- Xu, M.; Han, X.; Xiong, H.; Gao, Y.; Xu, B.; Zhu, G.; Li, J. Cancer Nanomedicine: Emerging Strategies and Therapeutic Potentials. Molecules 2023, 28, 5145. [Google Scholar] [CrossRef]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering Precision Nanoparticles for Drug Delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef]
- Basu, S.; Biswas, P.; Anto, M.; Singh, N.; Mukherjee, K. Nanomaterial-Enabled Drug Transport Systems: A Comprehensive Exploration of Current Developments and Future Avenues in Therapeutic Delivery. 3 Biotech 2024, 14, 289. [Google Scholar] [CrossRef]
- Egwu, C.O.; Aloke, C.; Onwe, K.T.; Umoke, C.I.; Nwafor, J.; Eyo, R.A.; Chukwu, J.A.; Ufebe, G.O.; Ladokun, J.; Audu, D.T.; et al. Nanomaterials in Drug Delivery: Strengths and Opportunities in Medicine. Molecules 2024, 29, 2584. [Google Scholar] [CrossRef] [PubMed]
- Davodabadi, F.; Sargazi, S.; Baino, F. Recent Advances in Hydrogel-Based Drug Delivery Systems for Enhanced Cancer Therapy: A Review. Mater. Today Commun. 2025, 48, 113615. [Google Scholar] [CrossRef]
- Baweja, R.; Ravi, R.; Baweja, R.; Gupta, S.; Sachan, A.; Pratyusha, V.; Warsi, M.K.; Purohit, S.D.; Mishra, A.; Ahmad, R. A Comprehensive Review of Hydrogels as Potential Drug Carriers for Anticancer Therapies: Properties, Development and Future Prospects. Next Mater. 2025, 8, 100913. [Google Scholar] [CrossRef]
- Omer, A.M.; Abd El-Monaem, E.M.; Eltaweil, A.S.; Tamer, T.M.; Mohy Eldin, M.S.; Ouyang, X.-K.; Heydari, A. Advances in Stimuli-Responsive Polymeric Hydrogels for Anticancer Drug Delivery: A Review. J. Drug Deliv. Sci. Technol. 2024, 102, 106394. [Google Scholar] [CrossRef]
- Delgado-Pujol, E.J.; Martínez, G.; Casado-Jurado, D.; Vázquez, J.; León-Barberena, J.; Rodríguez-Lucena, D.; Torres, Y.; Alcudia, A.; Begines, B. Hydrogels and Nanogels: Pioneering the Future of Advanced Drug Delivery Systems. Pharmaceutics 2025, 17, 215. [Google Scholar] [CrossRef]
- Segneanu, A.-E.; Bejenaru, L.E.; Bejenaru, C.; Blendea, A.; Mogoșanu, G.D.; Biță, A.; Boia, E.R. Advancements in Hydrogels: A Comprehensive Review of Natural and Synthetic Innovations for Biomedical Applications. Polymers 2025, 17, 2026. [Google Scholar] [CrossRef]
- Pandya, I.; Kumar, S.; Aswal, V.K.; El Seoud, O.; Assiri, M.A.; Malek, N. Metal–Organic Framework-Based Polymeric Hydrogel: A Promising Drug Delivery Vehicle for the Treatment of Breast Cancer. Int. J. Pharm. 2024, 658, 124206. [Google Scholar] [CrossRef]
- Guo, A.; Cao, Q.; Fang, H.; Tian, H. Recent Advances and Challenges of Injectable Hydrogels in Drug Delivery. J. Control. Release 2025, 385, 114021. [Google Scholar] [CrossRef]
- Narayanaswamy, R.; Torchilin, V.P. Hydrogels and Their Applications in Targeted Drug Delivery. Molecules 2019, 24, 603. [Google Scholar] [CrossRef] [PubMed]
- Gressler, S.; Hipfinger, C.; Part, F.; Pavlicek, A.; Zafiu, C.; Giese, B. A Systematic Review of Nanocarriers Used in Medicine and Beyond: Definition and Categorization Framework. J. Nanobiotechnol. 2025, 23, 90. [Google Scholar] [CrossRef]
- Fan, Y.; Han, Q.; Li, H.; Cai, X.; Dyett, B.; Qiao, R.; Drummond, C.J.; Thang, S.H.; Zhai, J. Recent Developments in Nanoparticle–Hydrogel Hybrid Materials for Controlled Release. Adv. Sci. 2025, 12, e07209. [Google Scholar] [CrossRef]
- Chen, J.; Zhu, Y.; Kaskel, S. Porphyrin-Based Metal–Organic Frameworks for Biomedical Applications. Angew. Chem. Int. Ed. 2020, 60, 5010–5035. [Google Scholar] [CrossRef]
- Cai, M.; Yang, Y.; Kong, J.; Zhang, Y.; Li, S.; Zhang, H.; Xv, X.; Ni, J.; Yin, X. Nanoscale Metal–Organic Frameworks as a Versatile Platform for Synergistic Combination Tumor Therapy. J. Nanobiotechnol. 2025, 23, 601. [Google Scholar] [CrossRef]
- Zhou, T.; Liang, X.; Wang, P.; Hu, Y.; Qi, Y.; Jin, Y.; Du, Y.; Fang, C.; Tian, J. A Hepatocellular Carcinoma Targeting Nanostrategy with Hypoxia-Ameliorating and Photothermal Abilities That, Combined with Immunotherapy, Inhibits Metastasis and Recurrence. ACS Nano 2020, 14, 12679–12696. [Google Scholar] [CrossRef] [PubMed]
- Hawthorne, D.; Pannala, A.; Sandeman, S.; Lloyd, A. Sustained and Targeted Delivery of Hydrophilic Drug Compounds: A Review of Existing and Novel Technologies from Bench to Bedside. J. Drug Deliv. Sci. Technol. 2022, 78, 103936. [Google Scholar] [CrossRef]
- Wang, X.; Zhong, X.; Liu, Z.; Cheng, L. Recent Progress of Chemodynamic Therapy-Induced Combination Cancer Therapy. Nano Today 2020, 35, 100946. [Google Scholar] [CrossRef]
- Li, D.; Yadav, A.; Zhou, H.; Roy, K.; Thanasekaran, P.; Lee, C. Advances and Applications of Metal–Organic Frameworks (MOFs) in Emerging Technologies: A Comprehensive Review. Glob. Chall. 2023, 8, 2300244. [Google Scholar] [CrossRef]
- Nabipour, H.; Rohani, S. Metal–Organic Frameworks for Overcoming the Blood–Brain Barrier in the Treatment of Brain Diseases: A Review. Nanomaterials 2024, 14, 1379. [Google Scholar] [CrossRef]
- Qiu, M.; Singh, A.K.; Fan, Q.; Wu, Z.; He, C.; Zhao, Y.; He, S.; Prasad, P.N. Biocompatible and Biodegradable Inorganic Nanostructures for Nanomedicine: Silicon and Black Phosphorus. Nano Today 2019, 25, 135–155. [Google Scholar] [CrossRef]
- Yan, X.; Zhao, X.; Fan, M.; Zheng, W.; Zhu, G.; Li, B.; Wang, L. Acidic Environment-Responsive Metal–Organic Framework-Mediated Dihydroartemisinin Delivery for Triggering Production of Reactive Oxygen Species in Drug-Resistant Lung Cancer. Int. J. Nanomed. 2024, 19, 3847–3859. [Google Scholar] [CrossRef]
- Cai, M.; Chen, G.; Qin, L.; Qu, C.; Dong, X.; Ni, J.; Yin, X. Metal–Organic Frameworks as Drug Targeting Delivery Vehicles in the Treatment of Cancer. Pharmaceutics 2020, 12, 232. [Google Scholar] [CrossRef] [PubMed]
- Benny, A.; Rajendra Pai, S.D.; Pinheiro, D.; Chundattu, S.J. Metal–Organic Frameworks in Biomedicine: Innovations in Drug Delivery. Results Chem. 2024, 7, 101414. [Google Scholar] [CrossRef]
- Khulood, M.T.; Jijith, U.S.; Naseef, P.; Kallungal, S.; Geetha, V.; Pramod, K. Advances in Metal–Organic Framework-Based Drug Delivery Systems. Int. J. Pharm. 2025, 673, 125380. [Google Scholar] [CrossRef]
- Filiz, A.; Bayazit, Ş.; Barlas, F. Metal–Organic Frameworks (MOFs) in Drug Delivery: Emerging Trends, Functional Enhancements, and Biocompatibility Challenges. J. Drug Deliv. Sci. Technol. 2025, 12, 107284. [Google Scholar] [CrossRef]
- Singh, S.; Sivaram, N.; Nath, B.; Khan, N.A.; Singh, J.; Ramamurthy, P.C. Metal–Organic Frameworks for Wastewater Treatment, Renewable Energy and Circular Economy Contributions. NPJ Clean Water 2024, 7, 124. [Google Scholar] [CrossRef]
- Fu, X.; Yang, Z.; Deng, T.; Chen, J.; Wen, Y.; Fu, X.; Zhou, L.; Zhu, Z.; Yu, C. A Natural Polysaccharide-Mediated MOF-Based Ce6 Delivery System with Improved Biological Properties for Photodynamic Therapy. J. Mater. Chem. B 2020, 8, 1481–1488. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Wu, W.; Qin, Y.; Liu, C.; Wei, P.; Hu, J.; Seeberger, P.H.; Yin, J. Fabrication of Glyco–Metal–Organic Frameworks for Targeted Interventional Photodynamic/Chemotherapy for Hepatocellular Carcinoma through Percutaneous Transperitoneal Puncture. Adv. Funct. Mater. 2020, 30, 1910084. [Google Scholar] [CrossRef]
- Pan, M.-M.; Li, P.; Yu, Y.-P.; Jiang, M.; Yang, X.; Zhang, P.; Nie, J.; Hu, J.; Yu, X.; Xu, L. Bimetallic Ions Functionalized Metal–Organic-Framework Nanozyme for Tumor Microenvironment Regulating and Enhanced Photodynamic Therapy for Hypoxic Tumor. Adv. Healthc. Mater. 2023, 12, 2300821. [Google Scholar] [CrossRef]
- Shang, Y.; Yi, X.; Xiang, D.; Zhou, L. Nanoagent-Mediated Photothermal Therapy: From Delivery System Design to Synergistic Theranostic Applications. Int. J. Nanomed. 2025, 20, 6891–6927. [Google Scholar] [CrossRef]
- Tong, P.-H.; Yang, J.-J.; Zhou, Y.-F.; Tang, Y.-F.; Tang, M.-T.; Zang, Y.; Pan, Y.-F.; Dong, L.-W.; Tan, Y.-X.; Nam, K.T.; et al. Metal–Organic Frameworks (MOFs) for Phototherapy and Synergistic Phototherapy of Cancer. Coord. Chem. Rev. 2025, 526, 216381. [Google Scholar] [CrossRef]
- Xu, R.; Wang, S.; Guo, Q.; Zhong, R.; Chen, X.; Xia, X. Anti-Tumor Strategies of Photothermal Therapy Combined with Other Therapies Using Nanoplatforms. Pharmaceutics 2025, 17, 306. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Liu, Q.; Zhu, J.; Tong, Y.; Huang, D.; Zhou, D.; Long, S.; Wang, L.; Li, M.; Chen, X.; et al. Dimerized Pentamethine Cyanines for Synergistically Boosting Photodynamic/Photothermal Therapy. Aggregate 2025, 6, e706. [Google Scholar] [CrossRef]
- Han, H.S.; Choi, K.Y. Advances in Nanomaterial-Mediated Photothermal Cancer Therapies: Toward Clinical Applications. Biomedicines 2021, 9, 305. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Chu, C.; Zhang, Y.; Su, Z.; Lin, H.; Pang, X.; Wang, X.; Liu, G.; Li, W. Self-Assembled Metal–Organic Nanoparticles for Multimodal Imaging-Guided Photothermal Therapy of Hepatocellular Carcinoma. J. Biomed. Nanotechnol. 2018, 14, 1934–1943. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Hu, S.; Teng, Y.; Chen, J.; Wang, H.; Xu, Y.; Wang, K.; Xu, J.; Cheng, Y.; Gao, X. Current Advance of Nanotechnology in Diagnosis and Treatment for Malignant Tumors. Signal Transduct. Target. Ther. 2024, 9, 200. [Google Scholar] [CrossRef]
- Lafi, Z.; Matalqah, S.; Abu-Saleem, E.; Asha, N.; Mhaidat, H.; Asha, S.; Al-Nashash, L.; Janabi, H.S. Metal–Organic Frameworks as Nanoplatforms for Combination Therapy in Cancer Treatment. Med. Oncol. 2025, 42, 26. [Google Scholar] [CrossRef]
- Sampaio, P.A.; Pereira, E.C.V.; Sá, P.G.S.; Alencar Filho, J.M.T.; Ferraz, L.R.M.; Nishimura, R.H.V.; Ferreira, A.S.; Rolim Neto, P.J.; Araújo, E.S.; Rolim, L.A. A Review on Metal–Organic Frameworks as Technological Excipients: Synthesis, Characterization, Toxicity, and Application in Drug Delivery Systems. Compounds 2025, 5, 1. [Google Scholar] [CrossRef]
- Lawson, H.D.; Walton, S.P.; Chan, C. Metal–Organic Frameworks for Drug Delivery: A Design Perspective. ACS Appl. Mater. Interfaces 2021, 13, 7004–7020. [Google Scholar] [CrossRef]
- Kush, P.; Singh, R.; Kumar, P. Recent Advances in Metal–Organic Framework-Based Anticancer Hydrogels. Gels 2025, 11, 76. [Google Scholar] [CrossRef]
- Xie, Y.; Liu, M.; Cai, C.; Ye, C.; Guo, T.; Yang, K.; Xiao, H.; Tang, X.; Liu, H. Recent Progress of Hydrogel-Based Local Drug Delivery Systems for Postoperative Radiotherapy. Front. Oncol. 2023, 13, 1027254. [Google Scholar] [CrossRef]
- Tran, V.A.; Le, V.T.; Doan, V.D.; Vo, G.N.L. Utilization of Functionalized Metal–Organic Framework Nanoparticle as Targeted Drug Delivery System for Cancer Therapy. Pharmaceutics 2023, 15, 931. [Google Scholar] [CrossRef]
- Khan, M.U.; Alissa, M.; Inam, M.; Alsuwat, M.A.; Abdulaziz, O.; Mostafa, Y.S.; Hussain, T.; ur Rehman, K.; Zaman, U.; Khan, D. Comprehensive Overview of Utilizing Metal–Organic Frameworks (MOFs) for Precise Cancer Drug Delivery. Microchem. J. 2024, 204, 111056. [Google Scholar]
- Yang, N.; He, Z.; Lang, T. Drug Delivery Systems Based on Metal–Organic Frameworks for Tumor Immunotherapy. Pharmaceutics 2025, 17, 225. [Google Scholar] [CrossRef] [PubMed]
- Soman, S.; Kulkarni, S.; Kulkarni, J.; Dhas, N.; Roy, A.A.; Pokale, R.; Mukharya, A.; Mutalik, S. Metal–Organic Frameworks: A Biomimetic Odyssey in Cancer Theranostics. Nanoscale 2025, 17, 12620–12647. [Google Scholar] [CrossRef]
- Saboorizadeh, B.; Zare-Dorabei, R.; Safavi, M.; Safarifard, V. Applications of Metal–Organic Frameworks (MOFs) in Drug Delivery, Biosensing, and Therapy: A Comprehensive Review. Langmuir 2024, 40, 22477–22503. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Zhao, Y.; Sun, Y.; Cao, J. Recent Progress of Metal–Organic Framework-Based Photodynamic Therapy for Cancer Treatment. Int. J. Nanomed. 2022, 17, 2367–2395. [Google Scholar] [CrossRef]
- Abodunrin, T.; Egharevba, G.; Owoeye, F.; Omojola, J.; Adeyemi, O. Metal–Organic Frameworks in Photodynamic Therapy of Cancer: Functional Roles of Active Metal Centres and Integrated and Loaded Photosensitizers in the Framework. Mater. Adv. 2025, 6, 1554–1577. [Google Scholar] [CrossRef]
- Correia, J.H.; Rodrigues, J.A.; Pimenta, S.; Dong, T.; Yang, Z. Photodynamic Therapy Review: Principles, Photosensitizers, Applications, and Future Directions. Pharmaceutics 2021, 13, 1332. [Google Scholar] [CrossRef]
- Al-Jamal, A.N.; Al-Hussainy, A.F.; Abdulrazzaq Mohammed, B.; Hussein Abbas, H.; Mohammed Kadhim, I.; Hassan Ward, Z.; Kar Mahapatra, D.; Muringayil Joseph, T.; Kianfar, E.; Thomas, S. Photodynamic Therapy (PDT) in Drug Delivery: Nano-Innovations Enhancing Treatment Outcomes. Health Sci. Rev. 2025, 14, 100218. [Google Scholar] [CrossRef]
- Liu, W.; Wang, Y.; Li, Y.; Cai, S.; Yin, X.; He, X.; Zhang, Y. Fluorescent Imaging-Guided Chemotherapy-and-Photodynamic Dual Therapy with Nanoscale Porphyrin Metal–Organic Framework. Small 2017, 13, 1603459. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, W.; Liu, J.; Manghnani, P.N.; Hu, F.; Ma, D.; Teh, C.; Wang, B.; Liu, B. Cancer-Cell-Activated Photodynamic Therapy Assisted by Cu(II)-Based Metal–Organic Framework. ACS Nano 2019, 13, 6879–6890. [Google Scholar] [CrossRef]
- Cai, Z.; Xin, F.; Wei, Z.; Wu, M.; Lin, X.; Du, X.; Chen, G.; Zhang, D.; Zhang, Z.; Liu, X.; et al. Photodynamic Therapy Combined with Antihypoxic Signaling and CpG Adjuvant as an In Situ Tumor Vaccine Based on Metal–Organic Framework Nanoparticles to Boost Cancer Immunotherapy. Adv. Healthc. Mater. 2020, 9, e1900996. [Google Scholar] [CrossRef] [PubMed]
- Di, X.; Pei, Z.; Pei, Y.; James, T. Tumor Microenvironment-Oriented MOFs for Chemodynamic Therapy. Coord. Chem. Rev. 2023, 484, 215098. [Google Scholar] [CrossRef]
- Li, M.; Zhang, W.; Xu, X.; Liu, G.; Dong, M.; Sun, K.; Zhang, P. Nanosystems for Chemodynamic-Based Combination Therapy: Strategies and Recent Advances. Front. Pharmacol. 2022, 13, 1065438. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Sun, Z.; Chu, H.; Wang, Y. Self-assembled Nanoplatforms for Chemodynamic Therapy. Chem. Eng. J. 2024, 479, 147702. [Google Scholar] [CrossRef]
- Ding, Y.; Xu, H.; Xu, C.; Tong, Z.; Zhang, S.; Bai, Y.; Chen, Y.; Xu, Q.; Zhou, L.; Ding, H.; et al. A Nanomedicine Fabricated from Gold Nanoparticles-Decorated Metal–Organic Framework for Cascade Chemo/Chemodynamic Cancer Therapy. Adv. Sci. 2020, 7, 2001060. [Google Scholar] [CrossRef]
- Nowak, K.M.; Schwartz, M.R.; Breza, V.R.; Price, R.J. Sonodynamic Therapy: Rapid Progress and New Opportunities for Non-Invasive Tumor Cell Killing with Sound. Cancer Lett. 2022, 532, 215592. [Google Scholar] [CrossRef]
- Pan, X.; Wang, H.; Wang, S.; Sun, X.; Wang, L.; Wang, W.; Shen, H.; Liu, H. Sonodynamic Therapy (SDT): A Novel Strategy for Cancer Nanotheranostics. Sci. China Life Sci. 2018, 61, 415–426. [Google Scholar] [CrossRef]
- Bai, Y.; Wang, J.; Yan, R.; Zhao, Y.; Han, S.; Zhang, Z.; Zhao, Y. Progress of Metal–Organic Frameworks in Improving the Effect of Sonodynamic Therapy. Biomed. Technol. 2025, 9, 100070. [Google Scholar] [CrossRef]
- Yang, F.; Dong, J.; Li, Z.; Wang, Z. Metal–Organic Frameworks (MOF)-Assisted Sonodynamic Therapy in Anticancer Applications. ACS Nano 2023, 17, 4102–4133. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wang, N.; Yan, F.; Shi, Z.; Feng, S. Metal–Organic Frameworks as Candidates for Tumor Sonodynamic Therapy: Designable Structures for Targeted Multifunctional Transformation. Acta Biomater. 2024, 181, 67–97. [Google Scholar] [CrossRef]
- Xiao, Z.; Chen, Q.; Yang, Y.; Tu, S.; Wang, B.; Qiu, Y.; Jiang, Y.; Huang, Q.; Ai, K. State of the Art Advancements in Sonodynamic Therapy (SDT): Metal–Organic Frameworks for SDT. Chem. Eng. J. 2022, 449, 137889. [Google Scholar] [CrossRef]
- Xu, Q.; Zhan, G.; Zhang, Z.; Yong, T.; Yang, X.; Gan, L. Manganese Porphyrin-Based Metal–Organic Framework for Synergistic Sonodynamic Therapy and Ferroptosis in Hypoxic Tumors. Theranostics 2021, 11, 1937–1952. [Google Scholar] [CrossRef] [PubMed]
- Duan, W.; Li, B.; Zhang, W.; Li, J.; Yao, X.; Tian, Y.; Zheng, J.; Li, D. Two-Photon Responsive Porphyrinic Metal–Organic Framework Involving Fenton-Like Reaction for Enhanced Photodynamic and Sonodynamic Therapy. J. Nanobiotechnol. 2022, 20, 217. [Google Scholar] [CrossRef]
- Liao, D.; Huang, J.; Jiang, C.; Zhou, L.; Zheng, M.; Nezamzadeh-Ejhieh, A.; Qi, N.; Lu, C.; Liu, J. A Novel Platform of MOF for Sonodynamic Therapy-Advanced Therapies. Pharmaceutics 2023, 15, 2071. [Google Scholar] [CrossRef] [PubMed]
- Zhong, L.; Yang, T.; Li, P.; Shi, L.; Lai, J.; Gu, L. Metal–Organic Framework-Based Nanotherapeutics with Tumor Hypoxia-Relieving Ability for Synergistic Sonodynamic/Chemo-Therapy. Front. Mater. 2022, 9, 841503. [Google Scholar] [CrossRef]
- Yang, H.; Li, R.; Jin, S.; Tian, Y.; Wang, C.; Sun, Y.; Dai, Z.; Cheng, W. Targeted Nanosensitizer-Augmented Sono-Immunotherapy with STING Agonist to Remodel the Immune Microenvironment in Hepatocellular Carcinoma. Acta Biomater. 2025, 199, 387–397. [Google Scholar] [CrossRef]
- Hu, C.; Hou, B.; Xie, S. Application of Nanosonosensitizer Materials in Cancer Sonodynamic Therapy. RSC Adv. 2022, 12, 22722–22747. [Google Scholar] [CrossRef]
- Chen, P.; Zhang, P.; Shah, N.H.; Cui, Y.; Wang, Y. A Comprehensive Review of Inorganic Sonosensitizers for Sonodynamic Therapy. Int. J. Mol. Sci. 2023, 24, 12001. [Google Scholar] [CrossRef] [PubMed]
- Jiao, L.; Seow, J.Y.R.; Skinner, W.S.; Wang, Z.U.; Jiang, H.-L. Metal–Organic Frameworks: Structures and Functional Applications. Mater. Today 2019, 27, 43–68. [Google Scholar] [CrossRef]
- Li, B.; Ashrafizadeh, M.; Jiao, T. Biomedical Application of Metal–Organic Frameworks (MOFs) in Cancer Therapy: Stimuli-Responsive and Biomimetic Nanocomposites in Targeted Delivery, Phototherapy and Diagnosis. Int. J. Biol. Macromol. 2024, 260 Pt 2, 129391. [Google Scholar] [CrossRef]
- Liu, J.; Huang, J.; Zhang, L.; Lei, J. Multifunctional Metal–Organic Framework Heterostructures for Enhanced Cancer Therapy. Chem. Soc. Rev. 2021, 50, 1188–1218. [Google Scholar] [CrossRef]
- Zou, H.; Wang, F.; Zhou, J.-J.; Liu, X.; He, Q.; Wang, C.; Zheng, Y.-W.; Wen, Y.; Xiong, L. Application of Photodynamic Therapy for Liver Malignancies. J. Gastrointest. Oncol. 2020, 11, 431–442. [Google Scholar] [CrossRef] [PubMed]
- Mallidi, S.; Anbil, S.; Bulin, A.-L.; Obaid, G.; Ichikawa, M.; Hasan, T. Beyond the Barriers of Light Penetration: Strategies, Perspectives, and Possibilities for Photodynamic Therapy. Theranostics 2016, 6, 2458–2487. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Xiao, Y.; Wu, W.; Zhe, M.; Yu, P.; Shakya, S.; Li, Z.; Xing, F. Metal–Organic Framework-Based Smart Stimuli-Responsive Drug Delivery Systems for Cancer Therapy: Advances, Challenges, and Future Perspectives. J. Nanobiotechnol. 2025, 23, 157. [Google Scholar] [CrossRef] [PubMed]
- Khafaga, D.S.R.; El-Morsy, M.T.; Faried, H.; Diab, A.H.; Shehab, S.; Saleh, A.M.; Al, G.A.M. Metal–Organic Frameworks in Drug Delivery: Engineering Versatile Platforms for Therapeutic Applications. RSC Adv. 2024, 14, 30201–30229. [Google Scholar] [CrossRef]
- Singh, N.; Qutuba, S.; Khashab, N.M. Biocompatibility and Biodegradability of Metal–Organic Frameworks for Biomedical Applications. J. Mater. Chem. B 2021, 9, 5925–5934. [Google Scholar] [CrossRef]
- Mhettar, P.; Kale, N.; Pantwalawalkar, J.; Nangare, S.; Jadhav, N. Metal–Organic Frameworks: Drug Delivery Applications and Future Prospects. ADMET DMPK 2023, 12, 27–62. [Google Scholar] [CrossRef]
- Hou, Y.; Zhu, C.; Ban, G.; Shen, Z.; Liang, Y.; Chen, K.; Wang, C.; Shi, H. Advancements and Challenges in the Application of Metal–Organic Framework (MOF) Nanocomposites for Tumor Diagnosis and Treatment. Int. J. Nanomed. 2024, 19, 6295–6317. [Google Scholar] [CrossRef]
- Zhang, J.; Li, M.; Liu, M.; Yu, Q.; Ge, D.; Zhang, J. Metal–Organic Framework Nanomaterials as a Medicine for Catalytic Tumor Therapy: Recent Advances. Nanomaterials 2024, 14, 797. [Google Scholar] [CrossRef] [PubMed]
- Abánades Lázaro, I.; Chen, X.; Ding, M.; Eskandari, A.; Fairen-Jimenez, D.; Giménez-Marqués, M.; Gref, R.; Lin, W.; Luo, T.; Forgan, R.S. Metal–Organic Frameworks for Biological Applications. Nat. Rev. Methods Primers 2024, 4, 42. [Google Scholar] [CrossRef]
- Chai, W.; Chen, X.; Liu, J.; Zhang, L.; Liu, C.; Li, L.; Honiball, J.R.; Pan, H.; Cui, X.; Wang, D. Recent progress in functional metal–organic frameworks for biomedical applications. Regen. Biomater. 2024, 11, rbad115. [Google Scholar] [CrossRef] [PubMed]
| Study (Ref) | MOF Type/NP | Payload | Therapy Type | In Vitro IC50/Efficacy | In Vivo Model | Main Outcomes |
|---|---|---|---|---|---|---|
| Liu et al. [70] | Zr-TCPP NPMOF | DOX | PDT + Chemo | 67.72 µg mL−1; 90% cell lethality | HepG2 tumor-bearing mice | Two tumors disappeared, two shrank from 62.5 → 2 mm3; no skin/tissue damage |
| Wang et al. [71] | MOF-199 | Ce6 | PDT | High phototoxicity to HepG2 | Zebrafish larvae | GSH-responsive PS release; tumor volume reduced; improved imaging-guided PDT |
| Cai et al. [72] | PCN-224@ACF-CpG@HA | Ce6 + ACF + CpG | PDT + Immunotherapy | — | Tumor-bearing mice | Eliminated residual tumor cells; activated host anti-tumor immune response |
| Fu et al. [46] | ZIF-8@Ce6–HA | Ce6 | PDT | 88.4% HepG2 cell death | HepG2 cells | Enhanced Ce6 solubility, efficient uptake, mitigated aggregation; improved PDT efficacy |
| Hu et al. [47] | Gal-PCN-224 | DOX + Ce6 | PDT + Chemo | — | Orthotopic HCC mouse model | Tumor growth inhibited by 98%; ASGPR-targeted delivery; imaging-guided therapy |
| Pan et al. [48] | ZMRPC@HA | Ce6 | PDT | — | H22 tumor xenograft, BALB/c mice | Synergistic ROS and O2 production; effective inhibition under 660 nm laser |
| Shi et al. [54] | Mn2+–ICG MINPs | ICG | PTT | Significant HepG2 inhibition under 808 nm laser | Subcutaneous HepG2 tumors in mice | Tumor necrosis; enhanced MRI and PA imaging; time-dependent accumulation |
| Zhou et al. [34] | SP94-PB-SF-Cy5.5 NPs | Sorafenib | PTT + Targeted therapy | Effective tumor ablation | HepG2 and Hepa1–6 mouse models | High HCC accumulation; triple-modality imaging; hypoxia relief; immune activation; prevented recurrence |
| Ding et al. [76] | PEG-Au/FeMOF@CPT | CPT | CDT + Chemo | 0.31 ± 0.04 µg mL−1 | HepG2 cells | Strong synergistic tumor suppression: CDT enhanced CPT cytotoxicity |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tutunchi, H.; Nabipour, H.; Rohani, S. Therapeutic Potential of Nanoscale Metal–Organic Frameworks in Hepatocellular Carcinoma. Nanomaterials 2025, 15, 1771. https://doi.org/10.3390/nano15231771
Tutunchi H, Nabipour H, Rohani S. Therapeutic Potential of Nanoscale Metal–Organic Frameworks in Hepatocellular Carcinoma. Nanomaterials. 2025; 15(23):1771. https://doi.org/10.3390/nano15231771
Chicago/Turabian StyleTutunchi, Helda, Hafezeh Nabipour, and Sohrab Rohani. 2025. "Therapeutic Potential of Nanoscale Metal–Organic Frameworks in Hepatocellular Carcinoma" Nanomaterials 15, no. 23: 1771. https://doi.org/10.3390/nano15231771
APA StyleTutunchi, H., Nabipour, H., & Rohani, S. (2025). Therapeutic Potential of Nanoscale Metal–Organic Frameworks in Hepatocellular Carcinoma. Nanomaterials, 15(23), 1771. https://doi.org/10.3390/nano15231771








