Nanotoxicity of Porous Silica Nanoparticles: Physicochemical Properties and Mechanistic Cellular Endpoints
Abstract
1. Introduction
1.1. Applications and Clinical Translation of PSNs
1.2. Double-Edged PSNs: Potential Toxicity Risks
2. Safe-by-Design: Modulating Nanoparticle Physicochemical Properties to Control and Minimize PSN Toxicity
2.1. Safe-by-Design Principles of PSNs
2.2. Standardised Characterization
2.2.1. Particle Size
2.2.2. Particle Shape
2.2.3. Pore Size
2.2.4. Surface Charge
2.2.5. Surface Functionalisation
2.2.6. Agglomeration
2.2.7. Crystallinity State
2.3. Assessment of Biocompatibility
2.3.1. Cytotoxicity of PSNs
Mechanism of Cytotoxicity-Oxidative Stress
2.3.2. Genotoxicity of PSNs
Physicochemical Dependent Properties Affecting Genotoxicity
Mechanisms of Genotoxicity
2.3.3. Gene Expression Profiles
2.3.4. Protein Corona
2.3.5. Immunogenicity of PSNs
2.4. Facilitation of Toxicity: Exposure Routes and Relevant Models
2.4.1. Inhalation
2.4.2. Dermal
2.4.3. Oral
2.4.4. Systemic
2.4.5. Mucosal
2.4.6. Biodegradation and Clearance
2.5. Evaluation of Clinical Translation
3. Summary
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| α | Alpha |
| β | Beta |
| OH | Hydroxyl radical |
| 16HBE14o | Human bronchial epithelial cells |
| 3T3 | Mouse fibroblast cells |
| A2780 | Human ovarian cancer cells |
| A549 | Human lung cancer cells |
| AgNPs | Silver nanoparticles |
| AKT | Protein kinase B |
| AMPK | Adenosine monophosphate-activated protein kinase |
| APC | Antigen presenting cells |
| Atg | Autophagy-related genes |
| ATM | Ataxia telangiectasia mutated |
| ATR | Ataxia telangiectasia and Rad3-related |
| AuNP | Gold nanoparticle |
| BAD | Bcl2-associated agonist of cell death |
| BAK | B-cell lymphoma 2 (BCL-2) antagonist |
| BAL | Human bronchioalveolar cells |
| BAX | BCL-2-associated X protein |
| BBB | Blood–brain barrier |
| Bcl-2 | B-cell lymphoma 2 |
| Bcl-XL | B-cell lymphoma 2-extra-large |
| BEAS-2B | Human bronchial epithelial cells |
| BSA | Bovine serum albumin |
| C3 | Complement protein 3 |
| C3b | Complement protein 3b |
| C5 | Complement 5 protein |
| C6 | Complement 6 protein |
| C7 | Complement 7 protein |
| C8 | Complement 8 protein |
| C9 | Complement 9 protein |
| Caco-2 | Human colon cancer cells |
| Calu-3 | Human lung adenocarcinoma cells |
| CBMN | Cytokinesis block micronucleus |
| CDK | Cyclin-dependent kinase |
| CDK2 | Cyclin-dependent kinase 2 |
| DCFDA | 2′,7′-dichlorofluorescein diacetate |
| DLS | Dynamic light scattering |
| DRAM1 | DNA Damage-Regulated Autophagy Modulator 1 |
| e.g., | Exempli gratia, for example |
| EPR | Enhanced permeability and retention |
| ERK1 | Extracellular-signal-regulating kinase 1 |
| ERK2 | Extracellular-signal-regulating kinase 2 |
| et al. | Et alii |
| FDA | Food and Drug Administration |
| GIT | Gastrointestinal tract |
| GPx | Glutathione peroxidase |
| GRO-α | Growth-regulated oncogene alpha |
| H2O2 | Hydrogen peroxide |
| HeLa | Human cervical cancer cells |
| HepG2 | Human hepatocellular carcinoma cells |
| h | hours |
| HT-29 | Human colorectal adenocarcinoma-derived cells |
| HUVEC | Human umbilical vein endothelial cells |
| IL-1β | Interleukin 1β |
| IL-6 | Interleukin 6 |
| IONP | Iron oxide nanoparticle |
| ISO | International Organisation for Standardisation |
| JNK | c-Jun-terminal kinase |
| MAC | Membrane attack complex |
| MAPK | Mitogen-activated protein kinase |
| MASP | Mannose-binding lectin associated serine proteases |
| MBL | Mannose binding lectin |
| MCF-7 | Human breast cancer cells |
| MDA-MB 231 | Human breast cancer cells |
| MDM2 | Murine double minute 2 |
| MOMP | Mitochondrial outer membrane permeabilisation |
| MPS | Mononuclear phagocyte system |
| MSN | Mesoporous silica nanoparticle |
| mTOR | Mammalian Target of Rapamycin |
| MTT | 3- (4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| NF-kB | Nuclear factor kappa B |
| NINP | Niobium oxide nanoparticle |
| NiNP | Nickel nanoparticle |
| NLRP3 | NOD-like receptor protein 3 |
| NOXA | Phorbal-12-myristate-13-acetate-induced protein 1 |
| NP | Nanoparticle |
| O2•− | Superoxide anion |
| OECD | Organisation for Economic Co-Operation and Development |
| p-NF-kB | Phosphorylated nuclear factor kappa B |
| P13K | Phosphatidylinositol-4,5-bisphosphate 3-kinase |
| p21 | Cyclin-dependent kinase inhibitor 1A (CKDN1A) |
| p53 | Tumour suppressor protein |
| PEG | Polyethylene glycol |
| PEI | Polyethylenimine |
| PDGF-AA | Platelet-derived growth factor-AA |
| PI | Propidium Iodide |
| PSN | Porous silica nanoparticle |
| PUMA | p53 upregulated modulator of apoptosis |
| ROS | Reactive oxygen species |
| SbD | Safe-by-Design |
| SDS-PAGE | Sodium dodecyl sulphate-polyacrylamide gel electrophoresis |
| Si | Silicon |
| SiNP | Silica nanoparticle |
| SiO2NP | Silicon dioxide nanoparticle |
| SOD | Superoxide dismutase |
| SWCNT | Single-walled carbon nanotube |
| THP-1 | Human monocyte cells |
| TGF-1 | Transforming growth factor beta 1 |
| TiO2 | Titanium dioxide |
| TK6 | Human lymphocyte cells |
| TNF-α | Tumour necrotic factor-α |
| TP53 | Tumour suppressor gene |
| TSC1 | Hamartin |
| TSC2 | Tuberin |
| ZnO | Zinc oxide |
| µg | Microgram |
References
- Kazemzadeh, P.; Sayadi, K.; Toolabi, A.; Sayadi, J.; Zeraati, M.; Chauhan, N.P.S.; Sargazi, G. Structure-Property Relationship for Different Mesoporous Silica Nanoparticles and Its Drug Delivery Applications: A Review. Front. Chem. 2022, 10, 823785. [Google Scholar] [CrossRef]
- Fang, L.; Zhou, H.; Cheng, L.; Wang, Y.; Liu, F.; Wang, S. The Application of Mesoporous Silica Nanoparticles as a Drug Delivery Vehicle in Oral Disease Treatment. Front. Cell. Infect. Microbiol. 2023, 13, 1124411. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Ge, X.; Ke, D.M.; Tang, H.; Zhang, J.Z.; Calvaresi, M.; Gao, B.; Sun, L.; Su, Q.; Wang, H. The Bioavailability, Biodistribution, and Toxic Effects of Silica-Coated Upconversion Nanoparticles in Vivo. Front. Chem. 2019, 7, 218. [Google Scholar] [CrossRef]
- Yu, J.; Dan, N.; Eslami, S.M.; Lu, X. State of the Art of Silica Nanoparticles: An Overview on Biodistribution and Preclinical Toxicity Studies. AAPS J. 2024, 26, 35. [Google Scholar] [CrossRef]
- Lérida-Viso, A.; Estepa-Fernández, A.; García-Fernández, A.; Martí-Centelles, V.; Martínez-Máñez, R. Biosafety of Mesoporous Silica Nanoparticles; towards Clinical Translation. Adv. Drug Deliv. Rev. 2023, 201, 115049. [Google Scholar] [CrossRef] [PubMed]
- Fatima, R.; Katiyar, P.; Kushwaha, K. Recent Advances in Mesoporous Silica Nanoparticle: Synthesis, Drug Loading, Release Mechanisms, and Diverse Applications. Front. Nanotechnol. 2025, 7, 1564188. [Google Scholar] [CrossRef]
- Kumarasamy, R.V.; Natarajan, P.M.; Umapathy, V.R.; Roy, J.R.; Mironescu, M.; Palanisamy, C.P. Clinical Applications and Therapeutic Potentials of Advanced Nanoparticles: A Comprehensive Review on Completed Human Clinical Trials. Front. Nanotechnol. 2024, 6, 1479993. [Google Scholar] [CrossRef]
- Janjua, T.I.; Cao, Y.; Yu, C.; Popat, A. Clinical Translation of Silica Nanoparticles. Nat. Rev. Mater. 2021, 6, 1072–1074. [Google Scholar] [CrossRef] [PubMed]
- Janjua, T.I.; Cao, Y.; Kleitz, F.; Linden, M.; Yu, C.; Popat, A. Silica Nanoparticles: A Review of Their Safety and Current Strategies to Overcome Biological Barriers. Adv. Drug Deliv. Rev. 2023, 203, 115115. [Google Scholar] [CrossRef]
- National Library of Medicine Safety Evaluation of Porous Silica in Men. Available online: https://www.clinicaltrials.gov/study/NCT03667430 (accessed on 13 October 2025).
- National Library of Medicine Effect of Different NanoScaffolds on Pulp Regeneration in Non-Vital Immature Permanent Teeth. Available online: https://clinicaltrials.gov/study/NCT07121348 (accessed on 13 October 2025).
- Zanoni, D.K.; Stambuk, H.E.; Madajewski, B.; Montero, P.H.; Matsuura, D.; Busam, K.J.; Ma, K.; Turker, M.Z.; Sequeira, S.; Gonen, M.; et al. Use of Ultrasmall Core-Shell Fluorescent Silica Nanoparticles for Image-Guided Sentinel Lymph Node Biopsy in Head and Neck Melanoma. JAMA Netw. Open 2021, 4, e211936. [Google Scholar] [CrossRef]
- National Library of Medicine Targeted Silica Nanoparticles for Real-Time Image-Guided Intraoperative Mapping of Nodal Metastases. Available online: https://www.clinicaltrials.gov/study/NCT02106598 (accessed on 13 October 2025).
- Baek, J.; Robert-Nicoud, G.; Herrera Hidalgo, C.; Borg, M.L.; Iqbal, M.N.; Berlin, R.; Lindgren, M.; Waara, E.; Uddén, A.; Pietiläinen, K.; et al. Engineered Mesoporous Silica Reduces Long-Term Blood Glucose, HbA1c, and Improves Metabolic Parameters in Prediabetics. Nanomedicine 2022, 17, 9–22. [Google Scholar] [CrossRef] [PubMed]
- National Library of Medicine STAR Study Investigating Performance and Safety of the Medical Device SiPore15 TM. Available online: https://clinicaltrials.gov/study/NCT03823027?a=3 (accessed on 13 October 2025).
- Benkő, F.; Kristó, K.; Sovány, T. Mesoporous Silica Nanoparticles as Drug Delivery Systems. Pharmaceuticals 2025, 18, 1392. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, H.; Qiu, X.; Zuo, F.; Wang, B. Mesoporous Silica Nanoparticles as a Drug Delivery Mechanism. Open Life Sci. 2024, 19, 20220867. [Google Scholar] [CrossRef]
- Porrang, S.; Davaran, S.; Rahemi, N.; Allahyari, S.; Mostafavi, E. How Advancing Are Mesoporous Silica Nanoparticles? A Comprehensive Review of the Literature. Int. J. Nanomed. 2022, 17, 1803–1827. [Google Scholar] [CrossRef]
- Sayed, E.; Karavasili, C.; Ruparelia, K.; Haj-Ahmad, R.; Charalambopoulou, G.; Steriotis, T.; Giasafaki, D.; Cox, P.; Singh, N.; Giassafaki, L.P.N.; et al. Electrosprayed Mesoporous Particles for Improved Aqueous Solubility of a Poorly Water Soluble Anticancer Agent: In Vitro and Ex Vivo Evaluation. J. Control. Release 2018, 278, 142–155. [Google Scholar] [CrossRef]
- Alyassin, Y.; Sayed, E.G.; Mehta, P.; Ruparelia, K.; Arshad, M.S.; Rasekh, M.; Shepherd, J.; Kucuk, I.; Wilson, P.B.; Singh, N.; et al. Application of Mesoporous Silica Nanoparticles as Drug Delivery Carriers for Chemotherapeutic Agents. Drug Discov. Today 2020, 25, 1513–1520. [Google Scholar] [CrossRef]
- Sayed, E.; Ruparelia, K.; Zafar, S.; Faheem, A.; Fatouros, D.; Arshad, M.S.; Singh, N.; Ahmad, Z. Novel Electrosprayed Core-Shell Polyethyleneimine and Phospholipid Coated MSNs for Co-Delivery of KAZ3 and MDR-1 SiRNA for Efficient Chemotherapy in Multidrug-Resistant Colon Cancer. Eur. J. Pharm. Biopharm. 2025, 215, 114838. [Google Scholar] [CrossRef]
- Yan, J.; Siwakoti, P.; Shaw, S.; Bose, S.; Kokil, G.; Kumeria, T. Porous Silicon and Silica Carriers for Delivery of Peptide Therapeutics. Drug Deliv. Transl. Res. 2024, 14, 3549–3567. [Google Scholar] [CrossRef] [PubMed]
- Pamshong, S.R.; Bhatane, D.; Sarnaik, S.; Alexander, A. Mesoporous Silica Nanoparticles: An Emerging Approach in Overcoming the Challenges with Oral Delivery of Proteins and Peptides. Colloids Surf. B Biointerfaces 2023, 232, 113613. [Google Scholar] [CrossRef]
- Wei, J.; Tan, Y.; Bai, Y.; He, J.; Cao, H.; Guo, J.; Su, Z. Mesoporous Silicon Nanoparticles with Liver-Targeting and PH-Response-Release Function Are Used for Targeted Drug Delivery in Liver Cancer Treatment. Int. J. Mol. Sci. 2024, 25, 2525. [Google Scholar] [CrossRef]
- Castillo, R.R.; Lozano, D.; Vallet-Regí, M. Mesoporous Silica Nanoparticles as Carriers for Therapeutic Biomolecules. Pharmaceutics 2020, 12, 432. [Google Scholar] [CrossRef]
- Martins, J.P.; Figueiredo, P.; Wang, S.; Espo, E.; Celi, E.; Martins, B.; Kemell, M.; Moslova, K.; Mäkilä, E.; Salonen, J.; et al. Neonatal Fc Receptor-Targeted Lignin-Encapsulated Porous Silicon Nanoparticles for Enhanced Cellular Interactions and Insulin Permeation across the Intestinal Epithelium. Bioact. Mater. 2022, 9, 299–315. [Google Scholar] [CrossRef]
- Valdés-Sánchez, L.; Borrego-González, S.; Montero-Sánchez, A.; Massalini, S.; de la Cerda, B.; Díaz-Cuenca, A.; Díaz-Corrales, F.J. Mesoporous Silica-Based Nanoparticles as Non-Viral Gene Delivery Platform for Treating Retinitis Pigmentosa. J. Clin. Med. 2022, 11, 2170. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.J.; Xu, P. Smart Mesoporous Silica Nanoparticles for Protein Delivery. Nanomaterials 2019, 9, 511. [Google Scholar] [CrossRef]
- Jänicke, P.; Lennicke, C.; Meister, A.; Seliger, B.; Wessjohann, L.A.; Kaluđerović, G.N. Fluorescent Spherical Mesoporous Silica Nanoparticles Loaded with Emodin: Synthesis, Cellular Uptake and Anticancer Activity. Mater. Sci. Eng. C 2021, 119, 111619. [Google Scholar] [CrossRef] [PubMed]
- Yuan, D.; Ellis, C.M.; Davis, J.J. Mesoporous Silica Nanoparticles in Bioimaging. Materials 2020, 13, 3795. [Google Scholar] [CrossRef]
- Li, Z.; Guo, J.; Zhang, M.; Li, G.; Hao, L. Gadolinium-Coated Mesoporous Silica Nanoparticle for Magnetic Resonance Imaging. Front. Chem. 2022, 10, 837032. [Google Scholar] [CrossRef]
- Kankala, R.K.; Han, Y.H.; Xia, H.Y.; Wang, S.B.; Chen, A.Z. Nanoarchitectured Prototypes of Mesoporous Silica Nanoparticles for Innovative Biomedical Applications. J. Nanobiotechnol. 2022, 20, 126. [Google Scholar] [CrossRef] [PubMed]
- Moschini, E.; Colombo, G.; Chirico, G.; Capitani, G.; Dalle-Donne, I.; Mantecca, P. Biological Mechanism of Cell Oxidative Stress and Death during Short-Term Exposure to Nano CuO. Sci. Rep. 2023, 13, 2326. [Google Scholar] [CrossRef]
- Yu, Z.; Li, Q.; Wang, J.; Yu, Y.; Wang, Y.; Zhou, Q.; Li, P. Reactive Oxygen Species-Related Nanoparticle Toxicity in the Biomedical Field. Nanoscale Res. Lett. 2020, 15, 115. [Google Scholar] [CrossRef]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering Precision Nanoparticles for Drug Delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef]
- Zhang, Y.; Lin, X.; Chen, X.; Fang, W.; Yu, K.; Gu, W.; Wei, Y.; Zheng, H.; Piao, J.; Li, F. Strategies to Regulate the Degradation and Clearance of Mesoporous Silica Nanoparticles: A Review. Int. J. Nanomed. 2024, 19, 5859–5878. [Google Scholar] [CrossRef]
- Shi, L.; Zhang, J.; Zhao, M.; Tang, S.; Cheng, X.; Zhang, W.; Li, W.; Liu, X.; Peng, H.; Wang, Q. Effects of Polyethylene Glycol on the Surface of Nanoparticles for Targeted Drug Delivery. Nanoscale 2021, 13, 10748–10764. [Google Scholar] [CrossRef] [PubMed]
- Chi, M.; Li, N.; Cui, J.; Karlin, S.; Rohr, N.; Sharma, N.; Thieringer, F.M. Biomimetic, Mussel-Inspired Surface Modification of 3D-Printed Biodegradable Polylactic Acid Scaffolds with Nano-Hydroxyapatite for Bone Tissue Engineering. Front. Bioeng. Biotechnol. 2022, 10, 989729. [Google Scholar] [CrossRef]
- Zhao, C.; Zhou, B. Polyethyleneimine-Based Drug Delivery Systems for Cancer Theranostics. J. Funct. Biomater. 2023, 14, 12. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, X.; Liu, Y.; Zheng, K.; Pang, Z.; Peng, S. Recent Advances in Zwitterionic Nanoscale Drug Delivery Systems to Overcome Biological Barriers. Asian J. Pharm. Sci. 2024, 19, 100883. [Google Scholar] [CrossRef]
- Colli, C.; Masi, I.; Jacchetti, E.; Santoni, S.; Sponchioni, M.; Colosimo, B.M.; Rosanò, L.; Raimondi, M.T.; Mauri, E.; Moscatelli, D. Zwitterionic Nanoparticles for Thermally Activated Drug Delivery in Hyperthermia Cancer Treatment. Nanoscale 2024, 16, 12635–12649. [Google Scholar] [CrossRef]
- Mohammadi Dargah, M.; Pedram, P.; Cabrera-Barjas, G.; Delattre, C.; Nesic, A.; Santagata, G.; Cerruti, P.; Moeini, A. Biomimetic Synthesis of Nanoparticles: A Comprehensive Review on Green Synthesis of Nanoparticles with a Focus on Prosopis Farcta Plant Extracts and Biomedical Applications. Adv. Colloid Interface Sci. 2024, 332, 103277. [Google Scholar] [CrossRef]
- Tikhonov, A.; Kachanov, A.; Yudaeva, A.; Danilik, O.; Ponomareva, N.; Karandashov, I.; Kostyusheva, A.; Zamyatnin, A.A.; Parodi, A.; Chulanov, V.; et al. Biomimetic Nanoparticles for Basic Drug Delivery. Pharmaceutics 2024, 16, 1306. [Google Scholar] [CrossRef]
- Dekkers, S.; Wijnhoven, S.W.P.; Braakhuis, H.M.; Soeteman-Hernandez, L.G.; Sips, A.J.A.M.; Tavernaro, I.; Kraegeloh, A.; Noorlander, C.W. Safe-by-Design Part I: Proposal for Nanospecific Human Health Safety Aspects Needed along the Innovation Process. NanoImpact 2020, 18, 100227. [Google Scholar] [CrossRef]
- Mech, A.; Gottardo, S.; Amenta, V.; Amodio, A.; Belz, S.; Bøwadt, S.; Drbohlavová, J.; Farcal, L.; Jantunen, P.; Małyska, A.; et al. Safe- and Sustainable-by-Design: The Case of Smart Nanomaterials. A Perspective Based on a European Workshop. In Regulatory Toxicology and Pharmacology; Academic Press Inc.: Cambridge, MA, USA, 2022; Volume 128. [Google Scholar]
- Schmutz, M.; Borges, O.; Jesus, S.; Borchard, G.; Perale, G.; Zinn, M.; Sips, Ä.A.J.A.M.; Soeteman-Hernandez, L.G.; Wick, P.; Som, C. A Methodological Safe-by-Design Approach for the Development of Nanomedicines. Front. Bioeng. Biotechnol. 2020, 8, 258. [Google Scholar] [CrossRef]
- Sabourian, P.; Yazdani, G.; Ashraf, S.S.; Frounchi, M.; Mashayekhan, S.; Kiani, S.; Kakkar, A. Effect of Physico-Chemical Properties of Nanoparticles on Their Intracellular Uptake. Int. J. Mol. Sci. 2020, 21, 8019. [Google Scholar] [CrossRef]
- Sanità, G.; Carrese, B.; Lamberti, A. Nanoparticle Surface Functionalization: How to Improve Biocompatibility and Cellular Internalization. Front. Mol. Biosci. 2020, 7, 587012. [Google Scholar] [CrossRef]
- Yuan, T.; Gao, L.; Zhan, W.; Dini, D. Effect of Particle Size and Surface Charge on Nanoparticles Diffusion in the Brain White Matter. Pharm. Res. 2022, 39, 767–781. [Google Scholar] [CrossRef]
- Ahmadi, A.; Sokunbi, M.; Patel, T.; Chang, M.W.; Ahmad, Z.; Singh, N. Influence of Critical Parameters on Cytotoxicity Induced by Mesoporous Silica Nanoparticles. Nanomaterials 2022, 12, 2016. [Google Scholar] [CrossRef]
- Grunberger, J.W.; Newton, H.S.; Donohue, D.; Dobrovolskaia, M.A.; Ghandehari, H. Role of Physicochemical Properties in Silica Nanoparticle-Mediated Immunostimulation. Nanotoxicology 2024, 18, 599–617. [Google Scholar] [CrossRef]
- Herdiana, Y.; Wathoni, N.; Shamsuddin, S.; Muchtaridi, M. Scale-up Polymeric-Based Nanoparticles Drug Delivery Systems: Development and Challenges. OpenNano 2022, 7, 100048. [Google Scholar] [CrossRef]
- Perrigue, P.M.; Henschke, A.; Grześkowiak, B.F.; Przysiecka, Ł.; Jaskot, K.; Mielcarek, A.; Coy, E.; Moya, S.E. Cellular Uptake and Retention Studies of Silica Nanoparticles Utilizing Senescent Fibroblasts. Sci. Rep. 2023, 13, 475. [Google Scholar] [CrossRef] [PubMed]
- Sousa De Almeida, M.; Susnik, E.; Drasler, B.; Taladriz-Blanco, P.; Petri-Fink, A.; Rothen-Rutishauser, B. Understanding Nanoparticle Endocytosis to Improve Targeting Strategies in Nanomedicine. Chem. Soc. Rev. 2021, 50, 5397–5434. [Google Scholar] [CrossRef]
- Rancan, F.; Gao, Q.; Graf, C.; Troppens, S.; Hadam, S.; Hackbarth, S.; Kembuan, C.; Blume-Peytavi, U.; Rühl, E.; Lademann, J.; et al. Skin Penetration and Cellular Uptake of Amorphous Silica Nanoparticles with Variable Size, Surface Functionalization, and Colloidal Stability. ACS Nano 2012, 6, 6829–6842. [Google Scholar] [CrossRef] [PubMed]
- Zha, S.; Liu, H.; Li, H.; Li, H.; Wong, K.L.; All, A.H. Functionalized Nanomaterials Capable of Crossing the Blood-Brain Barrier. ACS Nano 2024, 18, 1820–1845. [Google Scholar] [CrossRef]
- Niroumand, U.; Firouzabadi, N.; Goshtasbi, G.; Hassani, B.; Ghasemiyeh, P.; Mohammadi-Samani, S. The Effect of Size, Morphology and Surface Properties of Mesoporous Silica Nanoparticles on Pharmacokinetic Aspects and Potential Toxicity Concerns. Front. Mater. 2023, 10, 1189463. [Google Scholar] [CrossRef]
- Ohta, S.; Kikuchi, E.; Ishijima, A.; Azuma, T.; Sakuma, I.; Ito, T. Investigating the Optimum Size of Nanoparticles for Their Delivery into the Brain Assisted by Focused Ultrasound-Induced Blood–Brain Barrier Opening. Sci. Rep. 2020, 10, 18220. [Google Scholar] [CrossRef]
- Colaço, M.; Marques, A.P.; Jesus, S.; Duarte, A.; Borges, O. Safe-by-Design of Glucan Nanoparticles: Size Matters When Assessing the Immunotoxicity. Chem. Res. Toxicol. 2020, 33, 915–932. [Google Scholar] [CrossRef]
- Chen, L.; Liu, J.; Zhang, Y.; Zhang, G.; Kang, Y.; Chen, A.; Feng, X.; Shao, L. The Toxicity of Silica Nanoparticles to the Immune System. Nanomedicine 2018, 13, 1939–1962. [Google Scholar] [CrossRef]
- Nabeshi, H.; Yoshikawa, T.; Akase, T.; Yoshida, T.; Tochigi, S.; Hirai, T.; Uji, M.; Ichihashi, K.-i.; Yamashita, T.; Higashisaka, K.; et al. Effect of Amorphous Silica Nanoparticles on in Vitro Rankl-Induced Osteoclast Differentiation in Murine Macrophages. Nanoscale Res. Lett. 2011, 6, 464. [Google Scholar] [CrossRef]
- He, Y.; Wang, Y.; Wang, L.; Jiang, W.; Wilhelm, S. Understanding Nanoparticle-Liver Interactions in Nanomedicine. Expert. Opin. Drug Deliv. 2024, 21, 829–843. [Google Scholar] [CrossRef] [PubMed]
- Sen Karaman, D.; Desai, D.; Senthilkumar, R.; Johansson, E.M.; Råtts, N.; Odén, M.; Eriksson, J.E.; Sahlgren, C.; Toivola, D.M.; Rosenholm, J.M. Shape Engineering vs Organic Modification of Inorganic Nanoparticles as a Tool for Enhancing Cellular Internalization. Nanoscale Res. Lett. 2012, 7, 358. [Google Scholar] [CrossRef] [PubMed]
- Bartneck, M.; Keul, H.A.; Singh, S.; Czaja, K.; Bornemann, J.; Bockstaller, M.; Moeller, M.; Zwadlo-Klarwasser, G.; Groll, J. Rapid Uptake of Gold Nanorods by Primary Human Blood Phagocytes and Immunomodulatory Effects of Surface Chemistry. ACS Nano 2010, 4, 3073–3086. [Google Scholar] [CrossRef]
- Zhang, X.; Ma, G.; Wei, W. Simulation of Nanoparticles Interacting with a Cell Membrane: Probing the Structural Basis and Potential Biomedical Application. NPG Asia Mater. 2021, 13, 52. [Google Scholar] [CrossRef]
- Zelepukin, I.V.; Shevchenko, K.G.; Deyev, S.M. Rediscovery of Mononuclear Phagocyte System Blockade for Nanoparticle Drug Delivery. Nat. Commun. 2024, 15, 4366. [Google Scholar] [CrossRef]
- Lu, J.; Gao, X.; Wang, S.; He, Y.; Ma, X.; Zhang, T.; Liu, X. Advanced Strategies to Evade the Mononuclear Phagocyte System Clearance of Nanomaterials. Exploration 2023, 3, 20220045. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Z.; Liu, Y.; Zhu, G.; Jacobson, O.; Fu, X.; Bai, R.; Lin, X.; Lu, N.; Yang, X.; et al. Suppressing Nanoparticle-Mononuclear Phagocyte System Interactions of Two-Dimensional Gold Nanorings for Improved Tumor Accumulation and Photothermal Ablation of Tumors. ACS Nano 2017, 11, 10539–10548. [Google Scholar] [CrossRef] [PubMed]
- Izci, M.; Maksoudian, C.; Manshian, B.B.; Soenen, S.J. The Use of Alternative Strategies for Enhanced Nanoparticle Delivery to Solid Tumors. Chem. Rev. 2021, 121, 1746–1803. [Google Scholar] [CrossRef]
- Nowak-Jary, J.; Machnicka, B. Pharmacokinetics of Magnetic Iron Oxide Nanoparticles for Medical Applications. J. Nanobiotechnol. 2022, 20, 305. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Shen, S.; Kong, F.; Jiang, T.; Tang, C.; Yin, C. Effects of Pore Size on in Vitro and in Vivo Anticancer Efficacies of Mesoporous Silica Nanoparticles. RSC Adv. 2018, 8, 24633–24640. [Google Scholar] [CrossRef] [PubMed]
- Giri, K.; Kuschnerus, I.; Lau, M.; Ruan, J.; Garcia-Bennett, A. Pore Structure and Particle Shape Modulates the Protein Corona of Mesoporous Silica Particles. Mater. Adv. 2020, 1, 599–603. [Google Scholar] [CrossRef]
- Ma, Z.; Bai, J.; Wang, Y.; Jiang, X. Impact of Shape and Pore Size of Mesoporous Silica Nanoparticles on Serum Protein Adsorption and RBCs Hemolysis. ACS Appl. Mater. Interfaces 2014, 6, 2431–2438. [Google Scholar] [CrossRef]
- Hong, X.; Zhong, X.; Du, G.; Hou, Y.; Zhang, Y.; Zhang, Z.; Gong, T.; Zhang, L.; Sun, X. The Pore Size of Mesoporous Silica Nanoparticles Regulates Their Antigen Delivery Efficiency. Sci. Adv. 2020, 6, eaaz4462. [Google Scholar] [CrossRef]
- Awashra, M.; Młynarz, P. The Toxicity of Nanoparticles and Their Interaction with Cells: An in Vitro Metabolomic Perspective. Nanoscale Adv. 2023, 5, 2674–2723. [Google Scholar] [CrossRef]
- Sood, V.; Katti, D.S. Physicochemical Changes in Plasma Membrane Mirror Nanoparticle-Mediated Cytotoxicity. bioRxiv 2019. [Google Scholar] [CrossRef]
- Prabhakar, N.; Merisaari, J.; Le Joncour, V.; Peurla, M.; Karaman, D.Ş.; Casals, E.; Laakkonen, P.; Westermarck, J.; Rosenholm, J.M. Circumventing Drug Treatment? Intrinsic Lethal Effects of Polyethyleneimine (Pei)-Functionalized Nanoparticles on Glioblastoma Cells Cultured in Stem Cell Conditions. Cancers 2021, 13, 2631. [Google Scholar] [CrossRef]
- Monteiro-Riviere, N.A.; Samberg, M.E.; Oldenburg, S.J.; Riviere, J.E. Protein Binding Modulates the Cellular Uptake of Silver Nanoparticles into Human Cells: Implications for in Vitro to in Vivo Extrapolations? Toxicol. Lett. 2013, 220, 286–293. [Google Scholar] [CrossRef]
- Zhu, Y.; Yue, M.; Guo, T.; Li, F.; Li, Z.; Yang, D.; Lin, M. PEI-PEG-Coated Mesoporous Silica Nanoparticles Enhance the Antitumor Activity of Tanshinone IIA and Serve as a Gene Transfer Vector. Evid. Based Complement. Altern. Med. 2021, 2021, 6756763. [Google Scholar] [CrossRef]
- Xu, B.; Li, S.; Shi, R.; Liu, H. Multifunctional Mesoporous Silica Nanoparticles for Biomedical Applications. Signal Transduct. Target. Ther. 2023, 8, 435. [Google Scholar] [CrossRef]
- Ly, P.D.; Ly, K.N.; Phan, H.L.; Nguyen, H.H.T.; Duong, V.A.; Nguyen, H.V. Recent Advances in Surface Decoration of Nanoparticles in Drug Delivery. Front. Nanotechnol. 2024, 6, 1456939. [Google Scholar] [CrossRef]
- Xuan, L.; Ju, Z.; Skonieczna, M.; Zhou, P.K.; Huang, R. Nanoparticles-Induced Potential Toxicity on Human Health: Applications, Toxicity Mechanisms, and Evaluation Models. MedComm 2023, 4, e327. [Google Scholar] [CrossRef] [PubMed]
- Ebrahiminezhad, A.; Rasoul-Amini, S.; Kouhpayeh, A.; Davaran, S.; Barar, J.; Ghasemi, Y. Impacts of Amine Functionalized Iron Oxide Nanoparticles on HepG2 Cell Line. Curr. Nanosci. 2014, 11, 113–119. [Google Scholar] [CrossRef]
- Matadamas-Ortiz, A.; Pérez-Robles, J.F.; Reynoso-Camacho, R.; Amaya-Llano, S.L.; Amaro-Reyes, A.; Di Pierro, P.; Regalado-González, C. Effect of Amine, Carboxyl, or Thiol Functionalization of Mesoporous Silica Particles on Their Efficiency as a Quercetin Delivery System in Simulated Gastrointestinal Conditions. Foods 2024, 13, 1208. [Google Scholar] [CrossRef] [PubMed]
- Cahill, H.F.; Scott, B.S.; Peter, O.O.; Stapleton, G.V.L.; MacCormack, T.J.; Meli, M.-V.; Rourke, J.L. Multiparametric Cytotoxicity Profiling Reveals Cell-Line and Ligand-Dependent Toxicity for Pegylated Gold Nanoparticles (AuNP-PEG). Can. J. Chem. 2024, 102, 134–143. [Google Scholar] [CrossRef]
- Pierre, V.C.; Pasek-Allen, J.L.; Wilharm, R.K.; Bischof, J.C. NMR Characterization of Polyethylene Glycol Conjugates for Nanoparticle Functionalization. ACS Omega 2023, 8, 4331–4336. [Google Scholar] [CrossRef]
- Suk, J.S.; Xu, Q.; Kim, N.; Hanes, J.; Ensign, L.M. PEGylation as a Strategy for Improving Nanoparticle-Based Drug and Gene Delivery. Adv. Drug Deliv. Rev. 2016, 99, 28–51. [Google Scholar] [CrossRef]
- He, Q.; Zhang, J.; Shi, J.; Zhu, Z.; Zhang, L.; Bu, W.; Guo, L.; Chen, Y. The Effect of PEGylation of Mesoporous Silica Nanoparticles on Nonspecific Binding of Serum Proteins and Cellular Responses. Biomaterials 2010, 31, 1085–1092. [Google Scholar] [CrossRef]
- Salmaso, S.; Caliceti, P. Stealth Properties to Improve Therapeutic Efficacy of Drug Nanocarriers. J. Drug Deliv. 2013, 2013, 374252. [Google Scholar] [CrossRef]
- Marques, A.C.; Costa, P.J.; Velho, S.; Amaral, M.H. Functionalizing Nanoparticles with Cancer-Targeting Antibodies: A Comparison of Strategies. J. Control. Release 2020, 320, 180–200. [Google Scholar] [CrossRef]
- Kumari, M.; Acharya, A.; Krishnamurthy, P.T. Antibody-Conjugated Nanoparticles for Target-Specific Drug Delivery of Chemotherapeutics. Beilstein J. Nanotechnol. 2023, 14, 912–926. [Google Scholar] [CrossRef]
- McDaid, W.J.; Lissin, N.; Pollheimer, E.; Greene, M.; Leach, A.; Smyth, P.; Bossi, G.; Longley, D.; Cole, D.K.; Scott, C.J. Enhanced Target-Specific Delivery of Docetaxel-Loaded Nanoparticles Using Engineered T Cell Receptors. Nanoscale 2021, 13, 15010–15020. [Google Scholar] [CrossRef]
- Papini, E.; Tavano, R.; Mancin, F. Opsonins and Dysopsonins of Nanoparticles: Facts, Concepts, and Methodological Guidelines. Front. Immunol. 2020, 11, 7365. [Google Scholar] [CrossRef] [PubMed]
- Pondman, K.; Le Gac, S.; Kishore, U. Nanoparticle-Induced Immune Response: Health Risk versus Treatment Opportunity? Immunobiology 2023, 228, 152317. [Google Scholar] [CrossRef] [PubMed]
- Vu, V.P.; Gifford, G.B.; Chen, F.; Benasutti, H.; Wang, G.; Groman, E.V.; Scheinman, R.; Saba, L.; Moghimi, S.M.; Simberg, D. Immunoglobulin Deposition on Biomolecule Corona Determines Complement Opsonization Efficiency of Preclinical and Clinical Nanoparticles. Nat. Nanotechnol. 2019, 14, 260–268. [Google Scholar] [CrossRef] [PubMed]
- Schorr, K.; Beck, S.; Zimmer, O.; Baumann, F.; Keller, M.; Witzgall, R.; Goepferich, A. The Quantity of Ligand–Receptor Interactions between Nanoparticles and Target Cells. Nanoscale Horiz. 2025, 10, 803–823. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Du, X.; Zheng, N.; Xu, L.; Xu, J.; Li, S. Contribution of Carboxyl Modified Chiral Mesoporous Silica Nanoparticles in Delivering Doxorubicin Hydrochloride in Vitro: PH-Response Controlled Release, Enhanced Drug Cellular Uptake and Cytotoxicity. Colloids Surf. B Biointerfaces 2016, 141, 374–381. [Google Scholar] [CrossRef]
- Zhang, C.; Xie, H.; Zhang, Z.; Wen, B.; Cao, H.; Bai, Y.; Che, Q.; Guo, J.; Su, Z. Applications and Biocompatibility of Mesoporous Silica Nanocarriers in the Field of Medicine. Front. Pharmacol. 2022, 13, 829796. [Google Scholar] [CrossRef]
- Ajit, J. Nanoparticles in Drug Delivery. Prem. J. Sci. 2025, 1, 100048. [Google Scholar] [CrossRef]
- Maccuaig, W.M.; Samykutty, A.; Foote, J.; Luo, W.; Filatenkov, A.; Li, M.; Houchen, C.; Grizzle, W.E.; McNally, L.R. Toxicity Assessment of Mesoporous Silica Nanoparticles upon Intravenous Injection in Mice: Implications for Drug Delivery. Pharmaceutics 2022, 14, 969. [Google Scholar] [CrossRef]
- Murugadoss, S.; Godderis, L.; Ghosh, M.; Hoet, P.H. Assessing the Toxicological Relevance of Nanomaterial Agglomerates and Aggregates Using Realistic Exposure in Vitro. Nanomaterials 2021, 11, 1793. [Google Scholar] [CrossRef]
- Cabellos, J.; Gimeno-Benito, I.; Catalán, J.; Lindberg, H.K.; Vales, G.; Fernandez-Rosas, E.; Ghemis, R.; Jensen, K.A.; Atluri, R.; Vázquez-Campos, S.; et al. Short-Term Oral Administration of Non-Porous and Mesoporous Silica Did Not Induce Local or Systemic Toxicity in Mice. Nanotoxicology 2020, 14, 1324–1341. [Google Scholar] [CrossRef]
- Voicu, S.N.P.; Dinu, D.; Sima, C.; Hermenean, A.; Ardelean, A.; Codrici, E.; Stan, M.S.; Zărnescu, O.; Dinischiotu, A. Silica Nanoparticles Induce Oxidative Stress and Autophagy but Not Apoptosis in the MRC-5 Cell Line. Int. J. Mol. Sci. 2015, 16, 29398–29416. [Google Scholar] [CrossRef]
- Proquin, H.; Rodríguez-Ibarra, C.; Moonen, C.G.J.; Urrutia Ortega, I.M.; Briedé, J.J.; de Kok, T.M.; van Loveren, H.; Chirino, Y.I. Titanium Dioxide Food Additive (E171) Induces ROS Formation and Genotoxicity: Contribution of Micro and Nano-Sized Fractions. Mutagenesis 2017, 32, 139–149. [Google Scholar] [CrossRef]
- Krug, H.F. A Systematic Review on the Hazard Assessment of Amorphous Silica Based on the Literature from 2013 to 2018. Front. Public Health 2022, 10, 902893. [Google Scholar] [CrossRef] [PubMed]
- Sukhanova, A.; Bozrova, S.; Sokolov, P.; Berestovoy, M.; Karaulov, A.; Nabiev, I. Dependence of Nanoparticle Toxicity on Their Physical and Chemical Properties. Nanoscale Res. Lett. 2018, 13, 44. [Google Scholar] [CrossRef]
- Dong, X.; Wu, Z.; Li, X.; Xiao, L.; Yang, M.; Li, Y.; Duan, J.; Sun, Z. The Size-Dependent Cytotoxicity of Amorphous Silica Nanoparticles: A Systematic Review of in Vitro Studies. Int. J. Nanomed. 2020, 15, 9089–9113. [Google Scholar] [CrossRef]
- Keller, J.G.; Graham, U.M.; Koltermann-Jülly, J.; Gelein, R.; Ma-Hock, L.; Landsiedel, R.; Wiemann, M.; Oberdörster, G.; Elder, A.; Wohlleben, W. Predicting Dissolution and Transformation of Inhaled Nanoparticles in the Lung Using Abiotic Flow Cells: The Case of Barium Sulfate. Sci. Rep. 2020, 10, 458. [Google Scholar] [CrossRef]
- Murugadoss, S.; Van Den Brule, S.; Brassinne, F.; Sebaihi, N.; Mejia, J.; Lucas, S.; Petry, J.; Godderis, L.; Mast, J.; Lison, D.; et al. Is Aggregated Synthetic Amorphous Silica Toxicologically Relevant? Part Fibre Toxicol. 2020, 17, 1. [Google Scholar] [CrossRef]
- Croissant, J.G.; Butler, K.S.; Zink, J.I.; Brinker, C.J. Synthetic Amorphous Silica Nanoparticles: Toxicity, Biomedical and Environmental Implications. Nat. Rev. Mater. 2020, 5, 886–909. [Google Scholar] [CrossRef]
- Martin; Watanabe, R.; Hashimoto, K.; Higashisaka, K.; Haga, Y.; Tsutsumi, Y.; Mizuguchi, K. Evidence-Based Prediction of Cellular Toxicity for Amorphous Silica Nanoparticles. ACS Nano 2023, 17, 9987–9999. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Li, P.; Zhao, R.; Zhao, L.; Liu, J.; Peng, S.; Fu, X.; Wang, X.; Luo, R.; Wang, R.; et al. Silica Nanoparticles: Biomedical Applications and Toxicity. Biomed. Pharmacother. 2022, 151, 113053. [Google Scholar] [CrossRef]
- Wang, J.; Ni, R.; Jiang, T.; Peng, D.; Ming, Y.; Cui, H.; Liu, Y. The Applications of Functional Materials-Based Nano-Formulations in the Prevention, Diagnosis and Treatment of Chronic Inflammation-Related Diseases. Front. Pharmacol. 2023, 14, 1222642. [Google Scholar] [CrossRef]
- Mohammadpour, R.; Yazdimamaghani, M.; Cheney, D.L.; Jedrzkiewicz, J.; Ghandehari, H. Subchronic Toxicity of Silica Nanoparticles as a Function of Size and Porosity. J. Control. Release 2019, 304, 216–232. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, S.; de Haan, L.H.J.; Evers, N.M.; Jiang, X.; Marcelis, A.T.M.; Zuilhof, H.; Rietjens, I.M.C.M.; Alink, G.M. Role of Surface Charge and Oxidative Stress in Cytotoxicity of Organic Monolayer-Coated Silicon Nanoparticles towards Macrophage NR8383 Cells. Part. Fibre Toxicol. 2010, 7, 25. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.; Singh, R.K.; Tyagi, P.K.; Gore, D. Assessment of Toxicity and Safety Profiles of Nanoparticles. Lett. Appl. NanoBioScience 2021, 10, 1877–1888. [Google Scholar] [CrossRef]
- Sun, H.; Jiang, C.; Wu, L.; Bai, X.; Zhai, S. Cytotoxicity-Related Bioeffects Induced by Nanoparticles: The Role of Surface Chemistry. Front. Bioeng. Biotechnol. 2019, 7, 414. [Google Scholar] [CrossRef]
- Andrée, L.; Joziasse, L.S.; Adjobo-Hermans, M.J.W.; Yang, F.; Wang, R.; Leeuwenburgh, S.C.G. Effect of Hydroxyapatite Nanoparticle Crystallinity and Colloidal Stability on Cytotoxicity. ACS Biomater. Sci. Eng. 2024, 10, 6964–6973. [Google Scholar] [CrossRef]
- Aplicada, B. An Approach to The Safety Assessment of Nanoparticles and Nanomaterials. Am. J. Biomed. Sci. Res. 2022, 16, 621–626. [Google Scholar] [CrossRef]
- Abbasi, R.; Shineh, G.; Mobaraki, M.; Doughty, S.; Tayebi, L. Structural Parameters of Nanoparticles Affecting Their Toxicity for Biomedical Applications: A Review. J. Nanoparticle Res. 2023, 25, 43. [Google Scholar] [CrossRef]
- Tirumala, M.G.; Anchi, P.; Raja, S.; Rachamalla, M.; Godugu, C. Novel Methods and Approaches for Safety Evaluation of Nanoparticle Formulations: A Focus Towards In Vitro Models and Adverse Outcome Pathways. Front. Pharmacol. 2021, 12, 612659. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, S.; Ershov, D.; Islam, M.A.; Kämpfer, A.M.; Maslowska, K.A.; Van Der Gucht, J.; Alink, G.M.; Marcelis, A.T.M.; Zuilhof, H.; Rietjens, I.M.C.M. Role of Membrane Disturbance and Oxidative Stress in the Mode of Action Underlying the Toxicity of Differently Charged Polystyrene Nanoparticles. RSC Adv. 2014, 4, 19321–19330. [Google Scholar] [CrossRef]
- Li, X.; Kang, B.; Eom, Y.; Zhong, J.; Lee, H.K.; Kim, H.M.; Song, J.S. Comparison of Cytotoxicity Effects Induced by Four Different Types of Nanoparticles in Human Corneal and Conjunctival Epithelial Cells. Sci. Rep. 2022, 12, 155. [Google Scholar] [CrossRef] [PubMed]
- Patrón-Romero, L.; Luque-Morales, P.A.; Loera-Castañeda, V.; Lares-Asseff, I.; Leal-Ávila, M.Á.; Alvelais-Palacios, J.A.; Plasencia-López, I.; Almanza-Reyes, H. Mitochondrial Dysfunction Induced by Zinc Oxide Nanoparticles. Crystals 2022, 12, 1089. [Google Scholar] [CrossRef]
- Chang, X.; Wang, X.; Li, J.; Shang, M.; Niu, S.; Zhang, W.; Li, Y.; Sun, Z.; Gan, J.; Li, W.; et al. Silver Nanoparticles Induced Cytotoxicity in HT22 Cells through Autophagy and Apoptosis via PI3K/AKT/MTOR Signaling Pathway. Ecotoxicol. Envrion. Saf. 2021, 208, 111696. [Google Scholar] [CrossRef]
- Yuan, X.; Nie, W.; He, Z.; Yang, J.; Shao, B.; Ma, X.; Zhang, X.; Bi, Z.; Sun, L.; Liang, X.; et al. Carbon Black Nanoparticles Induce Cell Necrosis through Lysosomal Membrane Permeabilization and Cause Subsequent Inflammatory Response. Theranostics 2020, 10, 4589–4605. [Google Scholar] [CrossRef]
- Fard, J.K.; Jafari, S.; Eghbal, M.A. A Review of Molecular Mechanisms Involved in Toxicity of Nanoparticles. Adv. Pharm. Bull. 2015, 5, 447–454. [Google Scholar] [CrossRef]
- Sahu, D.; Kannan, G.M.; Tailang, M.; Vijayaraghavan, R. In Vitro Cytotoxicity of Nanoparticles: A Comparison between Particle Size and Cell Type. J. Nanosci. 2016, 2016, 4023852. [Google Scholar] [CrossRef]
- Lanone, S.; Rogerieux, F.; Geys, J.; Dupont, A.; Maillot-Marechal, E.; Boczkowski, J.; Lacroix, G.; Hoet, P. Comparative Toxicity of 24 Manufactured Nanoparticles in Human Alveolar Epithelial and Macrophage Cell Lines. Part. Fibre Toxicol. 2009, 6, 14. [Google Scholar] [CrossRef]
- Zhang, X.F.; Shen, W.; Gurunathan, S. Silver Nanoparticle-Mediated Cellular Responses in Various Cell Lines: An in Vitro Model. Int. J. Mol. Sci. 2016, 17, 1603. [Google Scholar] [CrossRef] [PubMed]
- Popova, T.; Tzankov, B.; Voycheva, C.; Spassova, I.; Kovacheva, D.; Tzankov, S.; Aluani, D.; Tzankova, V.; Lambov, N. Mesoporous Silica MCM-41 and HMS as Advanced Drug Delivery Carriers for Bicalutamide. J. Drug Deliv. Sci. Technol. 2021, 62, 102340. [Google Scholar] [CrossRef]
- Heikkilä, T.; Santos, H.A.; Kumar, N.; Murzin, D.Y.; Salonen, J.; Laaksonen, T.; Peltonen, L.; Hirvonen, J.; Lehto, V.P. Cytotoxicity Study of Ordered Mesoporous Silica MCM-41 and SBA-15 Microparticles on Caco-2 Cells. Eur. J. Pharm. Biopharm. 2010, 74, 483–494. [Google Scholar] [CrossRef]
- Fuentes, C.; Ruiz-Rico, M.; Fuentes, A.; Barat, J.M.; Ruiz, M.J. Comparative Cytotoxic Study of Silica Materials Functionalised with Essential Oil Components in HepG2 Cells. Food Chem. Toxicol. 2021, 147, 111858. [Google Scholar] [CrossRef]
- Andrés, C.M.C.; Pérez de la Lastra, J.M.; Andrés Juan, C.; Plou, F.J.; Pérez-Lebeña, E. Superoxide Anion Chemistry—Its Role at the Core of the Innate Immunity. Int. J. Mol. Sci. 2023, 24, 1841. [Google Scholar] [CrossRef] [PubMed]
- Manke, A.; Wang, L.; Rojanasakul, Y. Mechanisms of Nanoparticle-Induced Oxidative Stress and Toxicity. Biomed. Res. Int. 2013, 2013, 94291. [Google Scholar] [CrossRef]
- Daré, R.G.; Lautenschlager, S.O.S. Nanoparticles with Antioxidant Activity. Antioxidants 2025, 14, 221. [Google Scholar] [CrossRef]
- Snezhkina, A.V.; Kudryavtseva, A.V.; Kardymon, O.L.; Savvateeva, M.V.; Melnikova, N.V.; Krasnov, G.S.; Dmitriev, A.A. ROS Generation and Antioxidant Defense Systems in Normal and Malignant Cells. Oxid. Med. Cell. Longev. 2020, 2019, 6175804. [Google Scholar] [CrossRef] [PubMed]
- Endale, H.T.; Tesfaye, W.; Mengstie, T.A. ROS Induced Lipid Peroxidation and Their Role in Ferroptosis. Front. Cell Dev. Biol. 2023, 11, 1226044. [Google Scholar] [CrossRef]
- Wang, B.; Wang, Y.; Zhang, J.; Hu, C.; Jiang, J.; Li, Y.; Peng, Z. ROS-Induced Lipid Peroxidation Modulates Cell Death Outcome: Mechanisms behind Apoptosis, Autophagy, and Ferroptosis. Arch. Toxicol. 2023, 97, 1439–1451. [Google Scholar] [CrossRef] [PubMed]
- Poetsch, A.R. The Genomics of Oxidative DNA Damage, Repair, and Resulting Mutagenesis. Comput. Struct. Biotechnol. J. 2020, 18, 207–219. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.; Collins, L.B.; Chen, T.; Herr, N.; Takeda, S.; Sun, W.; Swenberg, J.A.; Nakamura, J. Oxidative Stress at Low Levels Can Induce Clustered DNA Lesions Leading to NHEJ Mediated Mutations. Oncotarget 2016, 7, 25377–25390. [Google Scholar] [CrossRef]
- Yang, H.; Xie, Y.; Yang, D.; Ren, D. Oxidative Stress-Induced Apoptosis in Granulosa Cells Involves JNK, P53 and Puma. Oncotarget 2017, 8, 25310–25322. [Google Scholar] [CrossRef]
- Jomova, K.; Raptova, R.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Valko, M. Reactive Oxygen Species, Toxicity, Oxidative Stress, and Antioxidants: Chronic Diseases and Aging. Arch. Toxicol. 2023, 97, 2499–2574. [Google Scholar] [CrossRef]
- Dhanasekaran, D.N.; Reddy, E.P. JNK Signaling in Apoptosis. Oncogene 2008, 27, 6245–6251. [Google Scholar] [CrossRef]
- Martins, S.G.; Zilhão, R.; Thorsteinsdóttir, S.; Carlos, A.R. Linking Oxidative Stress and DNA Damage to Changes in the Expression of Extracellular Matrix Components. Front. Genet. 2021, 12, 673002. [Google Scholar] [CrossRef]
- Ighodaro, O.M.; Akinloye, O.A. First Line Defence Antioxidants-Superoxide Dismutase (SOD), Catalase (CAT) and Glutathione Peroxidase (GPX): Their Fundamental Role in the Entire Antioxidant Defence Grid. Alex. J. Med. 2018, 54, 287–293. [Google Scholar] [CrossRef]
- Radovanovic, J.; Banjac, K.; Obradovic, M.; Isenovic, E.R. Antioxidant Enzymes and Vascular Diseases. Explor. Med. 2021, 2, 544–555. [Google Scholar] [CrossRef]
- Sharma, P.; Jha, A.B.; Dubey, R.S.; Pessarakli, M. Reactive Oxygen Species, Oxidative Damage, and Antioxidative Defense Mechanism in Plants under Stressful Conditions. J. Bot. 2012, 2012, 217037. [Google Scholar] [CrossRef]
- Pei, J.; Pan, X.; Wei, G.; Hua, Y. Research Progress of Glutathione Peroxidase Family (GPX) in Redoxidation. Front. Pharmacol. 2023, 14, 1147414. [Google Scholar] [CrossRef]
- Ursini, F.; Maiorino, M. Lipid Peroxidation and Ferroptosis: The Role of GSH and GPx4. Free Radic. Biol. Med. 2020, 152, 175–185. [Google Scholar] [CrossRef]
- Dumanović, J.; Nepovimova, E.; Natić, M.; Kuča, K.; Jaćević, V. The Significance of Reactive Oxygen Species and Antioxidant Defense System in Plants: A Concise Overview. Front. Plant Sci. 2021, 11, 552969. [Google Scholar] [CrossRef] [PubMed]
- An, X.; Yu, W.; Liu, J.; Tang, D.; Yang, L.; Chen, X. Oxidative Cell Death in Cancer: Mechanisms and Therapeutic Opportunities. Cell Death Dis. 2024, 15, 556. [Google Scholar] [CrossRef]
- Lehman, S.E.; Morris, A.S.; Mueller, P.S.; Salem, A.K.; Grassian, V.H.; Larsen, S.C. Silica Nanoparticle-Generated ROS as a Predictor of Cellular Toxicity: Mechanistic Insights and Safety by Design. Envrion. Sci. Nano 2016, 3, 56–66. [Google Scholar] [CrossRef]
- Ahamed, M. Silica Nanoparticles-Induced Cytotoxicity, Oxidative Stress and Apoptosis in Cultured A431 and A549 Cells. Hum. Exp. Toxicol. 2013, 32, 186–195. [Google Scholar] [CrossRef]
- Duan, J.; Yu, Y.; Li, Y.; Yu, Y.; Li, Y.; Zhou, X.; Huang, P.; Sun, Z. Toxic Effect of Silica Nanoparticles on Endothelial Cells through DNA Damage Response via Chk1-Dependent G2/M Checkpoint. PLoS ONE 2013, 8, e62087. [Google Scholar] [CrossRef]
- Savi, M.; Bocchi, L.; Cacciani, F.; Vilella, R.; Buschini, A.; Perotti, A.; Galati, S.; Montalbano, S.; Pinelli, S.; Frati, C.; et al. Cobalt Oxide Nanoparticles Induce Oxidative Stress and Alter Electromechanical Function in Rat Ventricular Myocytes. Part. Fibre Toxicol. 2021, 18, 1. [Google Scholar] [CrossRef]
- Ghasemi, M.; Turnbull, T.; Sebastian, S.; Kempson, I. The MTT Assay: Utility, Limitations, Pitfalls, and Interpretation in Bulk and Single-Cell Analysis. Int. J. Mol. Sci. 2021, 22, 2827. [Google Scholar] [CrossRef] [PubMed]
- Apopa, P.L.; Qian, Y.; Shao, R.; Guo, N.L.; Schwegler-Berry, D.; Pacurari, M.; Porter, D.; Shi, X.; Vallyathan, V.; Castranova, V.; et al. Iron Oxide Nanoparticles Induce Human Microvascular Endothelial Cell Permeability through Reactive Oxygen Species Production and Microtubule Remodeling. Part. Fibre Toxicol. 2009, 6, 1. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Feng, G.; Yang, S.; Liu, B.; Niu, Y.; Fan, P.; Liu, Z.; Chen, J.; Cui, L.; Zhou, G.; et al. Polyethylenimine-Modified Mesoporous Silica Nanoparticles Induce a Survival Mechanism in Vascular Endothelial Cells via Microvesicle-Mediated Autophagosome Release. ACS Nano 2021, 15, 10640–10658. [Google Scholar] [CrossRef] [PubMed]
- Fröhlich, E.; Samberger, C.; Kueznik, T.; Absenger, M.; Roblegg, E.; Zimmer, A.; Pieber, T.R. Cytotoxicity of Nanoparticles Independent from Oxidative Stress. J. Toxicol. Sci. 2001, 34, 363–375. [Google Scholar] [CrossRef]
- Kohl, Y.; Rundén-Pran, E.; Mariussen, E.; Hesler, M.; El Yamani, N.; Longhin, E.M.; Dusinska, M. Genotoxicity of Nanomaterials: Advanced in Vitro Models and High Throughput Methods for Human Hazard Assessment—A Review. Nanomaterials 2020, 10, 1911. [Google Scholar] [CrossRef]
- Sicińska, P.; Mokra, K.; Wozniak, K.; Michałowicz, J.; Bukowska, B. Genotoxic Risk Assessment and Mechanism of DNA Damage Induced by Phthalates and Their Metabolites in Human Peripheral Blood Mononuclear Cells. Sci. Rep. 2021, 11, 1658. [Google Scholar] [CrossRef]
- Elespuru, R.K.; Doak, S.H.; Collins, A.R.; Dusinska, M.; Pfuhler, S.; Manjanatha, M.; Cardoso, R.; Chen, C.L. Common Considerations for Genotoxicity Assessment of Nanomaterials. Front. Toxicol. 2022, 4, 859122. [Google Scholar] [CrossRef]
- Menz, J.; Götz, M.E.; Gündel, U.; Gürtler, R.; Herrmann, K.; Hessel-Pras, S.; Kneuer, C.; Kolrep, F.; Nitzsche, D.; Pabel, U.; et al. Genotoxicity Assessment: Opportunities, Challenges and Perspectives for Quantitative Evaluations of Dose–Response Data. Arch. Toxicol. 2023, 97, 2303–2328. [Google Scholar] [CrossRef]
- Landsiedel, R.; Honarvar, N.; Seiffert, S.B.; Oesch, B.; Oesch, F. Genotoxicity Testing of Nanomaterials. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2022, 14, e1833. [Google Scholar] [CrossRef]
- Solorio-Rodriguez, S.A.; Wu, D.; Boyadzhiev, A.; Christ, C.; Williams, A.; Halappanavar, S. A Systematic Genotoxicity Assessment of a Suite of Metal Oxide Nanoparticles Reveals Their DNA Damaging and Clastogenic Potential. Nanomaterials 2024, 14, 743. [Google Scholar] [CrossRef]
- Lebedová, J.; Hedberg, Y.S.; Odnevall Wallinder, I.; Karlsson, H.L. Size-Dependent Genotoxicity of Silver, Gold and Platinum Nanoparticles Studied Using the Mini-Gel Comet Assay and Micronucleus Scoring with Flow Cytometry. Mutagenesis 2018, 33, 77–85. [Google Scholar] [CrossRef]
- Xia, Q.; Li, H.; Liu, Y.; Zhang, S.; Feng, Q.; Xiao, K. The Effect of Particle Size on the Genotoxicity of Gold Nanoparticles. J. Biomed. Mater. Res. A 2017, 105, 710–719. [Google Scholar] [CrossRef]
- Yazdimamaghani, M.; Moos, P.J.; Dobrovolskaia, M.A.; Ghandehari, H. Genotoxicity of Amorphous Silica Nanoparticles: Status and Prospects. Nanomedicine 2019, 16, 106–125. [Google Scholar] [CrossRef] [PubMed]
- Park, M.V.D.Z.; Verharen, H.W.; Zwart, E.; Hernandez, L.G.; van Benthem, J.; Elsaesser, A.; Barnes, C.; McKerr, G.; Howard, C.V.; Salvati, A.; et al. Genotoxicity Evaluation of Amorphous Silica Nanoparticles of Different Sizes Using the Micronucleus and the Plasmid LacZ Gene Mutation Assay. Nanotoxicology 2011, 5, 168–181. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Chen, Y.; Wang, Y.; Yin, Y.; Qu, G.; Song, M.; Wang, H. Ultra-Long Silver Nanowires Induced Mitotic Abnormalities and Cytokinetic Failure in A549 Cells. Nanotoxicology 2019, 13, 543–557. [Google Scholar] [CrossRef] [PubMed]
- García-Rodríguez, A.; Vila, L.; Cortés, C.; Hernández, A.; Marcos, R. Effects of Differently Shaped TiO2NPs (Nanospheres, Nanorods and Nanowires) on the in Vitro Model (Caco-2/HT29) of the Intestinal Barrier. Part. Fibre Toxicol. 2018, 15, 33. [Google Scholar] [CrossRef]
- Encinas-Gimenez, M.; Martin-Duque, P.; Martín-Pardillos, A. Cellular Alterations Due to Direct and Indirect Interaction of Nanomaterials with Nucleic Acids. Int. J. Mol. Sci. 2024, 25, 1983. [Google Scholar] [CrossRef]
- Green, J.J. 2011 Rita Schaffer Lecture: Nanoparticles for Intracellular Nucleic Acid Delivery. Ann. Biomed. Eng. 2012, 40, 1408–1418. [Google Scholar] [CrossRef]
- Schaeublin, N.M.; Braydich-Stolle, L.K.; Schrand, A.M.; Miller, J.M.; Hutchison, J.; Schlager, J.J.; Hussain, S.M. Surface Charge of Gold Nanoparticles Mediates Mechanism of Toxicity. Nanoscale 2011, 3, 410. [Google Scholar] [CrossRef]
- Platel, A.; Carpentier, R.; Becart, E.; Mordacq, G.; Betbeder, D.; Nesslany, F. Influence of the Surface Charge of PLGA Nanoparticles on Their in Vitro Genotoxicity, Cytotoxicity, ROS Production and Endocytosis. J. Appl. Toxicol. 2016, 36, 434–444. [Google Scholar] [CrossRef]
- Fresegna, A.M.; Ursini, C.L.; Ciervo, A.; Maiello, R.; Casciardi, S.; Iavicoli, S.; Cavallo, D. Assessment of the Influence of Crystalline Form on Cyto-Genotoxic and Inflammatory Effects Induced by Tio2 Nanoparticles on Human Bronchial and Alveolar Cells. Nanomaterials 2021, 11, 253. [Google Scholar] [CrossRef]
- Rim, K.T.; Song, S.W.; Kim, H.Y. Oxidative DNA Damage from Nanoparticle Exposure and Its Application to Workers’ Health: A Literature Review. Saf. Health Work 2013, 4, 177–186. [Google Scholar] [CrossRef]
- Carrière, M.; Arnal, M.-E.; Douki, T. TiO2 Genotoxicity: An Update of the Results Published over the Last Six Years. Mutat. Res./Genet. Toxicol. Environ. Mutagen. 2020, 854–855, 503198. [Google Scholar] [CrossRef] [PubMed]
- Demir, E.; Castranova, V. Genotoxic Effects of Synthetic Amorphous Silica Nanoparticles in the Mouse Lymphoma Assay. Toxicol. Rep. 2016, 3, 807–815. [Google Scholar] [CrossRef] [PubMed]
- Schardosim, R.F.D.C.; Cardozo, T.R.; de Souza, A.P.; Seeber, A.; Flores, W.H.; Lehmann, M.; Dihl, R.R. Cyto—Genotoxicity of Crystalline and Amorphous Niobium (V) Oxide Nanoparticles in CHO-K1 Cells. Toxicol. Res. 2022, 11, 765–773. [Google Scholar] [CrossRef] [PubMed]
- Vecchiotti, G.; Colafarina, S.; Aloisi, M.; Zarivi, O.; Di Carlo, P.; Poma, A. Genotoxicity and Oxidative Stress Induction by Polystyrene Nanoparticles in the Colorectal Cancer Cell Line HCT116. PLoS ONE 2021, 16, e0255120. [Google Scholar] [CrossRef]
- Rinna, A.; Magdolenova, Z.; Hudecova, A.; Kruszewski, M.; Refsnes, M.; Dusinska, M. Effect of Silver Nanoparticles on Mitogen-Activated Protein Kinases Activation: Role of Reactive Oxygen Species and Implication in DNA Damage. Mutagenesis 2015, 30, 59–66. [Google Scholar] [CrossRef]
- Shukla, R.K.; Badiye, A.; Vajpayee, K.; Kapoor, N. Genotoxic Potential of Nanoparticles: Structural and Functional Modifications in DNA. Front. Genet. 2021, 12, 728250. [Google Scholar] [CrossRef]
- Choudante, P.C.; Nethi, S.K.; Díaz-García, D.; Prashar, S.; Misra, S.; Gómez-Ruiz, S.; Patra, C.R. Tin-Loaded Mesoporous Silica Nanoparticles: Antineoplastic Properties and Genotoxicity Assessment. Biomater. Adv. 2022, 137, 212819. [Google Scholar] [CrossRef]
- Ansari, M.O.; Parveen, N.; Ahmad, M.F.; Wani, A.L.; Afrin, S.; Rahman, Y.; Jameel, S.; Khan, Y.A.; Siddique, H.R.; Tabish, M.; et al. Evaluation of DNA Interaction, Genotoxicity and Oxidative Stress Induced by Iron Oxide Nanoparticles Both in Vitro and in Vivo: Attenuation by Thymoquinone. Sci. Rep. 2019, 9, 6912. [Google Scholar] [CrossRef]
- Babonaitė, M.; Striogaitė, E.; Grigorianaitė, G.; Lazutka, J.R. In Vitro Evaluation of DNA Damage Induction by Silver (Ag), Gold (Au), Silica (SiO2), and Aluminum Oxide (Al2O3) Nanoparticles in Human Peripheral Blood Mononuclear Cells. Curr. Issues Mol. Biol. 2024, 46, 6986–7000. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, J.; Ren, L.; Wei, J.; Zhang, F.; Li, Y.; Guo, C.; Duan, J.; Sun, Z.; Zhou, X. Silica Nanoparticles Induce Abnormal Mitosis and Apoptosis via PKC-Δ Mediated Negative Signaling Pathway in GC-2 cells of Mice. Chemosphere 2018, 208, 942–950. [Google Scholar] [CrossRef]
- George, J.M.; Magogotya, M.; Vetten, M.A.; Buys, A.V.; Gulumian, M. An Investigation of the Genotoxicity and Interference of Gold Nanoparticles in Commonly Used in Vitro Mutagenicity and Genotoxicity Assays. Toxicol. Sci. 2017, 156, 149–166. [Google Scholar] [CrossRef]
- Zhang, W.; Cho, W.C.; Bloukh, S.H.; Edis, Z.; Du, W.; He, Y.; Hu, H.Y.; ten Hagen, T.L.M.; Falahati, M. An Overview on the Exploring the Interaction of Inorganic Nanoparticles with Microtubules for the Advancement of Cancer Therapeutics. Int. J. Biol. Macromol. 2022, 212, 358–369. [Google Scholar] [CrossRef]
- Guan, L.; Chen, J.; Tian, Z.; Zhu, M.; Bian, Y.; Zhu, Y. Mesoporous Organosilica Nanoparticles: Degradation Strategies and Application in Tumor Therapy. View 2021, 2, 20200117. [Google Scholar] [CrossRef]
- Sargent, L.M.; Hubbs, A.F.; Young, S.H.; Kashon, M.L.; Dinu, C.Z.; Salisbury, J.L.; Benkovic, S.A.; Lowry, D.T.; Murray, A.R.; Kisin, E.R.; et al. Single-Walled Carbon Nanotube-Induced Mitotic Disruption. Mutat. Res. Genet. Toxicol. Envrion. Mutagen. 2012, 745, 28–37. [Google Scholar] [CrossRef]
- Xu, X.; Xu, S.; Wan, J.; Wang, D.; Pang, X.; Gao, Y.; Ni, N.; Chen, D.; Sun, X. Disturbing Cytoskeleton by Engineered Nanomaterials for Enhanced Cancer Therapeutics. Bioact. Mater. 2023, 29, 50–71. [Google Scholar] [CrossRef]
- Bannister, A.; Dissanayake, D.; Kowalewski, A.; Cicon, L.; Bromma, K.; Chithrani, D.B. Modulation of the Microtubule Network for Optimization of Nanoparticle Dynamics for the Advancement of Cancer Nanomedicine. Bioengineering 2020, 7, 56. [Google Scholar] [CrossRef]
- Doak, S.H.; Andreoli, C.; Burgum, M.J.; Chaudhry, Q.; Bleeker, E.A.J.; Bossa, C.; Domenech, J.; Drobne, D.; Fessard, V.; Jeliazkova, N.; et al. Current Status and Future Challenges of Genotoxicity OECD Test Guidelines for Nanomaterials: A Workshop Report. Mutagenesis 2023, 38, 183–191. [Google Scholar] [CrossRef]
- Senapati, V.A.; Kumar, A.; Gupta, G.S.; Pandey, A.K.; Dhawan, A. ZnO Nanoparticles Induced Inflammatory Response and Genotoxicity in Human Blood Cells: A Mechanistic Approach. Food Chem. Toxicol. 2015, 85, 61–70. [Google Scholar] [CrossRef]
- Nakad, R.; Schumacher, B. DNA Damage Response and Immune Defense: Links and Mechanisms. Front. Genet. 2016, 7, 147. [Google Scholar] [CrossRef]
- Lin, R.; Zhang, C.; Zheng, J.; Tian, D.; Lei, Z.; Chen, D.; Xu, Z.; Su, M. Chronic Inflammation-Associated Genomic Instability Paves the Way for Human Esophageal Carcinogenesis. Oncotarget 2016, 7, 24564–24571. [Google Scholar] [CrossRef]
- Bourdon, J.A.; Williams, A.; Kuo, B.; Moffat, I.; White, P.A.; Halappanavar, S.; Vogel, U.; Wallin, H.; Yauk, C.L. Gene Expression Profiling to Identify Potentially Relevant Disease Outcomes and Support Human Health Risk Assessment for Carbon Black Nanoparticle Exposure. Toxicology 2013, 303, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Aubrey, B.J.; Kelly, G.L.; Janic, A.; Herold, M.J.; Strasser, A. How Does P53 Induce Apoptosis and How Does This Relate to P53-Mediated Tumour Suppression? Cell. Death Differ. 2018, 25, 104–113. [Google Scholar] [CrossRef]
- Chen, J. The Cell-Cycle Arrest and Apoptotic Functions of P53 in Tumor Initiation and Progression. Cold Spring Harb. Perspect. Med. 2016, 6, a026104. [Google Scholar] [CrossRef]
- Abuetabh, Y.; Wu, H.H.; Chai, C.; Al Yousef, H.; Persad, S.; Sergi, C.M.; Leng, R. DNA Damage Response Revisited: The P53 Family and Its Regulators Provide Endless Cancer Therapy Opportunities. Exp. Mol. Med. 2022, 54, 1658–1669. [Google Scholar] [CrossRef]
- Okazaki, R. Role of P53 in Regulating Radiation Responses. Life 2022, 12, 1099. [Google Scholar] [CrossRef]
- Wang, H.; Guo, M.; Wei, H.; Chen, Y. Targeting P53 Pathways: Mechanisms, Structures, and Advances in Therapy. Signal Transduct. Target. Ther. 2023, 8, 92. [Google Scholar] [CrossRef]
- Barr, A.R.; Cooper, S.; Heldt, F.S.; Butera, F.; Stoy, H.; Mansfeld, J.; Novák, B.; Bakal, C. DNA Damage during S-Phase Mediates the Proliferation-Quiescence Decision in the Subsequent G1 via P21 Expression. Nat. Commun. 2017, 8, 14728. [Google Scholar] [CrossRef]
- Engeland, K. Cell Cycle Arrest through Indirect Transcriptional Repression by P53: I Have a DREAM. Cell Death Differ. 2018, 25, 114–132. [Google Scholar] [CrossRef]
- Bowen, M.E.; Mulligan, A.S.; Sorayya, A.; Attardi, L.D. Puma- and Caspase9-Mediated Apoptosis Is Dispensable for P53-Driven Neural Crest-Based Developmental Defects. Cell Death Differ. 2021, 28, 2083–2094. [Google Scholar] [CrossRef]
- Hao, Q.; Chen, J.; Lu, H.; Zhou, X. The ARTS of P53-Dependent Mitochondrial Apoptosis. J. Mol. Cell. Biol. 2022, 14, mjac074. [Google Scholar] [CrossRef]
- Leong, W.F.; Chau, J.F.L.; Li, B. P53 Deficiency Leads to Compensatory Up-Regulation of P16INK4a. Mol. Cancer Res. 2009, 7, 354–360. [Google Scholar] [CrossRef] [PubMed]
- Qian, Y.; Chen, X. Senescence Regulation by the P53 Protein Family. Methods Mol. Biol. 2013, 965, 37–61. [Google Scholar] [CrossRef] [PubMed]
- Szewczyk-Roszczenko, O.; Barlev, N.A. The Role of P53 in Nanoparticle-Based Therapy for Cancer. Cells 2023, 12, 2803. [Google Scholar] [CrossRef] [PubMed]
- Wierzbicki, M.; Sawosz, E.; Strojny, B.; Jaworski, S.; Grodzik, M.; Chwalibog, A. NF-ΚB-Related Decrease of Glioma Angiogenic Potential by Graphite Nanoparticles and Graphene Oxide Nanoplatelets. Sci. Rep. 2018, 8, 14733. [Google Scholar] [CrossRef]
- Alswady-hoff, M.; Erdem, J.S.; Phuyal, S.; Knittelfelder, O.; Sharma, A.; Fonseca, D.d.M.; Skare, Ø.; Slupphaug, G.; Zienolddiny, S. Long-term Exposure to Nanosized Tio2 Triggers Stress Responses and Cell Death Pathways in Pulmonary Epithelial Cells. Int. J. Mol. Sci. 2021, 22, 5349. [Google Scholar] [CrossRef]
- Tavakoli, S.; Motavalizadehkakhky, A.; Homayouni Tabrizi, M.; Mehrzad, J.; Zhiani, R. Study of the Anti-Cancer Activity of a Mesoporous Silica Nanoparticle Surface Coated with Polydopamine Loaded with Umbelliprenin. Sci. Rep. 2024, 14, 11450. [Google Scholar] [CrossRef]
- Chen, J.; Stark, L.A. Insights into the Relationship between Nucleolar Stress and the NF-ΚB Pathway. Trends Genet. 2019, 35, 768–780. [Google Scholar] [CrossRef]
- Chen, J.; Lobb, I.T.; Morin, P.; Novo, S.M.; Simpson, J.; Kennerknecht, K.; Von Kriegsheim, A.; Batchelor, E.E.; Oakley, F.; Stark, L.A. Identification of a Novel TIF-IA-NF-ΚB Nucleolar Stress Response Pathway. Nucleic Acids Res. 2018, 46, 6188–6205. [Google Scholar] [CrossRef]
- Lowe, J.M.; Menendez, D.; Bushel, P.R.; Shatz, M.; Kirk, E.L.; Troester, M.A.; Garantziotis, S.; Fessler, M.B.; Resnick, M.A. P53 and NF-ΚB Coregulate Proinflammatory Gene Responses in Human Macrophages. Cancer Res. 2014, 74, 2182–2192. [Google Scholar] [CrossRef]
- Carrà, G.; Lingua, M.F.; Maffeo, B.; Taulli, R.; Morotti, A. P53 vs NF-ΚB: The Role of Nuclear Factor-Kappa B in the Regulation of P53 Activity and Vice Versa. Cell. Mol. Life Sci. 2020, 77, 4449–4458. [Google Scholar] [CrossRef]
- Konrath, F.; Mittermeier, A.; Cristiano, E.; Wolf, J.; Loewer, A. A Systematic Approach to Decipher Crosstalk in the P53 Signaling Pathway Using Single Cell Dynamics. PLoS Comput. Biol. 2020, 16, e1007901. [Google Scholar] [CrossRef]
- Milani, D.; Caruso, L.; Zauli, E.; Al Owaifeer, A.M.; Secchiero, P.; Zauli, G.; Gemmati, D.; Tisato, V. P53/NF-KB Balance in SARS-CoV-2 Infection: From OMICs, Genomics and Pharmacogenomics Insights to Tailored Therapeutic Perspectives (COVIDomics). Front. Pharmacol. 2022, 13, 871583. [Google Scholar] [CrossRef]
- Liu, X.; Sun, J. Endothelial Cells Dysfunction Induced by Silica Nanoparticles through Oxidative Stress via JNK/P53 and NF-ΚB Pathways. Biomaterials 2010, 31, 8198–8209. [Google Scholar] [CrossRef]
- Hanson, R.L.; Batchelor, E. Coordination of MAPK and P53 Dynamics in the Cellular Responses to DNA Damage and Oxidative Stress. Mol. Syst. Biol. 2022, 18, e11401. [Google Scholar] [CrossRef] [PubMed]
- De, S.; Campbell, C.; Venkitaraman, A.R.; Esposito, A. Pulsatile MAPK Signaling Modulates P53 Activity to Control Cell Fate Decisions at the G2 Checkpoint for DNA Damage. Cell Rep. 2020, 30, 2083–2093.e5. [Google Scholar] [CrossRef] [PubMed]
- Lafarga, V.; Cuadrado, A.; Lopez de Silanes, I.; Bengoechea, R.; Fernandez-Capetillo, O.; Nebreda, A.R. P38 Mitogen-Activated Protein Kinase- and HuR-Dependent Stabilization of P21 Cip1 MRNA Mediates the G 1/S Checkpoint. Mol. Cell Biol. 2009, 29, 4341–4351. [Google Scholar] [CrossRef]
- Sugiura, R.; Satoh, R.; Takasaki, T. ERK: A Double-Edged Sword in Cancer. ERK-Dependent Apoptosis as a Potential Therapeutic Strategy for Cancer. Cells 2021, 10, 2509. [Google Scholar] [CrossRef]
- Deschênes-Simard, X.; Gaumont-Leclerc, M.F.; Bourdeau, V.; Lessard, F.; Moiseeva, O.; Forest, V.; Igelmann, S.; Mallette, F.A.; Saba-El-Leil, M.K.; Meloche, S.; et al. Tumor Suppressor Activity of the ERK/MAPK Pathway by Promoting Selective Protein Degradation. Genes. Dev. 2013, 27, 900–915. [Google Scholar] [CrossRef]
- Zuo, J.; Ma, S. Resveratrol-Laden Mesoporous Silica Nanoparticles Regulate the Autophagy and Apoptosis via ROS-Mediated P38-MAPK/HIF-1a/P53 Signaling in Hypertrophic Scar Fibroblasts. Heliyon 2024, 10, e24985. [Google Scholar] [CrossRef]
- Zhao, X.; Wu, Y.; Li, J.; Li, D.; Jin, Y.; Zhu, P.; Liu, Y.; Zhuang, Y.; Yu, S.; Cao, W.; et al. JNK Activation-Mediated Nuclear SIRT1 Protein Suppression Contributes to Silica Nanoparticle-Induced Pulmonary Damage via P53 Acetylation and Cytoplasmic Localisation. Toxicology 2019, 423, 42–53. [Google Scholar] [CrossRef]
- Rahman, M.A.; Park, M.N.; Rahman, M.H.; Rashid, M.M.; Islam, R.; Uddin, M.J.; Hannan, M.A.; Kim, B. P53 Modulation of Autophagy Signaling in Cancer Therapies: Perspectives Mechanism and Therapeutic Targets. Front. Cell Dev. Biol. 2022, 10, 761080. [Google Scholar] [CrossRef] [PubMed]
- Feroz, W.; Sheikh, A.M.A. Exploring the Multiple Roles of Guardian of the Genome: P53. Egypt. J. Med. Hum. Genet. 2020, 21, 49. [Google Scholar] [CrossRef]
- Shim, D.; Duan, L.; Maki, C.G. P53-Regulated Autophagy and Its Impact on Drug Resistance and Cell Fate. Cancer Drug Resistance 2021, 4, 85–95. [Google Scholar] [CrossRef]
- Shi, Y.; Norberg, E.; Vakifahmetoglu-Norberg, H. Mutant P53 as a Regulator and Target of Autophagy. Front. Oncol. 2021, 10, 607149. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Zuo, W.; Shen, X.; Liu, Y.; Zhao, Y.; Xiong, Y.; Cao, H.; Wang, Y.; Liang, Z. NF-ΚB Is Involved in the Regulation of Autophagy in Mutant P53 Cells in Response to Ionizing Radiation. Cell Death Discov. 2021, 7, 159. [Google Scholar] [CrossRef]
- Verzella, D.; Pescatore, A.; Capece, D.; Vecchiotti, D.; Ursini, M.V.; Franzoso, G.; Alesse, E.; Zazzeroni, F. Life, Death, and Autophagy in Cancer: NF-ΚB Turns up Everywhere. Cell Death Dis. 2020, 11, 210. [Google Scholar] [CrossRef]
- Rahman, M.A.; Jalouli, M.; Bhajan, S.K.; Al-Zharani, M.; Harrath, A.H. A Comprehensive Review of Nanoparticle-Based Drug Delivery for Modulating PI3K/AKT/MTOR-Mediated Autophagy in Cancer. Int. J. Mol. Sci. 2025, 26, 1868. [Google Scholar] [CrossRef]
- Coronel, L.; Häckes, D.; Schwab, K.; Riege, K.; Hoffmann, S.; Fischer, M. P53-Mediated AKT and MTOR Inhibition Requires RFX7 and DDIT4 and Depends on Nutrient Abundance. Oncogene 2022, 41, 1063–1069. [Google Scholar] [CrossRef]
- Tavakol, S.; Ashrafizadeh, M.; Deng, S.; Azarian, M.; Abdoli, A.; Motavaf, M.; Poormoghadam, D.; Khanbabaei, H.; Ghasemipour Afshar, E.; Mandegary, A.; et al. Autophagy Modulators: Mechanistic Aspects and Drug Delivery Systems. Biomolecules 2019, 9, 530. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Meng, H.; Deng, G.; Lin, H. Sustainable Release Selenium Laden with SiO2 Restoring Peripheral Nerve Injury via Modulating PI3K/AKT Pathway Signaling Pathway. Int. J. Nanomed. 2024, 19, 7851–7870. [Google Scholar] [CrossRef]
- Yang, B.Y.; Zhou, Z.Y.; Liu, S.Y.; Shi, M.J.; Liu, X.J.; Cheng, T.M.; Deng, G.Y.; Tian, Y.; Song, J.; Li, X.H. Porous Se@SiO2 Nanoparticles Enhance Wound Healing by ROS-PI3K/Akt Pathway in Dermal Fibroblasts and Reduce Scar Formation. Front. Bioeng. Biotechnol. 2022, 10, 852482. [Google Scholar] [CrossRef]
- Liao, X.; Liu, Y.; Zheng, J.; Zhao, X.; Cui, L.; Hu, S.; Xia, T.; Si, S. Diverse Pathways of Engineered Nanoparticle-Induced NLRP3 Inflammasome Activation. Nanomaterials 2022, 12, 3908. [Google Scholar] [CrossRef]
- Rampado, R.; Crotti, S.; Caliceti, P.; Pucciarelli, S.; Agostini, M. Recent Advances in Understanding the Protein Corona of Nanoparticles and in the Formulation of “Stealthy” Nanomaterials. Front. Bioeng. Biotechnol. 2020, 8, 166. [Google Scholar] [CrossRef]
- Akhter, M.H.; Khalilullah, H.; Gupta, M.; Alfaleh, M.A.; Alhakamy, N.A.; Riadi, Y.; Md, S. Impact of Protein Corona on the Biological Identity of Nanomedicine: Understanding the Fate of Nanomaterials in the Biological Milieu. Biomedicines 2021, 9, 1496. [Google Scholar] [CrossRef]
- Bai, X.; Wang, J.; Mu, Q.; Su, G. In Vivo Protein Corona Formation: Characterizations, Effects on Engineered Nanoparticles’ Biobehaviors, and Applications. Front. Bioeng. Biotechnol. 2021, 9, 646708. [Google Scholar] [CrossRef]
- Baimanov, D.; Wang, J.; Zhang, J.; Liu, K.; Cong, Y.; Shi, X.; Zhang, X.; Li, Y.; Li, X.; Qiao, R.; et al. In Situ Analysis of Nanoparticle Soft Corona and Dynamic Evolution. Nat. Commun. 2022, 13, 5389. [Google Scholar] [CrossRef]
- Bashiri, G.; Padilla, M.S.; Swingle, K.L.; Shepherd, S.J.; Mitchell, M.J.; Wang, K. Nanoparticle Protein Corona: From Structure and Function to Therapeutic Targeting. Lab Chip 2023, 23, 1432–1466. [Google Scholar] [CrossRef] [PubMed]
- García-álvarez, R.; Vallet-Regí, M. Hard and Soft Protein Corona of Nanomaterials: Analysis and Relevance. Nanomaterials 2021, 11, 888. [Google Scholar] [CrossRef]
- Vilanova, O.; Martinez-Serra, A.; Monopoli, M.P.; Franzese, G. Characterizing the Hard and Soft Nanoparticle-Protein Corona with Multilayer Adsorption. Front. Nanotechnol. 2025, 6, 1531039. [Google Scholar] [CrossRef]
- Mohammad-Beigi, H.; Hayashi, Y.; Zeuthen, C.M.; Eskandari, H.; Scavenius, C.; Juul-Madsen, K.; Vorup-Jensen, T.; Enghild, J.J.; Sutherland, D.S. Mapping and Identification of Soft Corona Proteins at Nanoparticles and Their Impact on Cellular Association. Nat. Commun. 2020, 11, 4535. [Google Scholar] [CrossRef] [PubMed]
- Marichal, L.; Klein, G.; Armengaud, J.; Boulard, Y.; Chédin, S.; Labarre, J.; Pin, S.; Renault, J.P.; Aude, J.C. Protein Corona Composition of Silica Nanoparticles in Complex Media: Nanoparticle Size Does Not Matter. Nanomaterials 2020, 10, 240. [Google Scholar] [CrossRef]
- Xu, W.; Xu, M.; Xiao, Y.; Yu, L.; Xie, H.; Jiang, X.; Chen, M.; Gao, H.; Wang, L. Changes in Target Ability of Nanoparticles Due to Protein Corona Composition and Disease State. Asian J. Pharm. Sci. 2022, 17, 401–411. [Google Scholar] [CrossRef]
- Nierenberg, D.; Khaled, A.R.; Flores, O. Formation of a Protein Corona Influences the Biological Identity of Nanomaterials. Rep. Pract. Oncol. Radiother. 2018, 23, 300–308. [Google Scholar] [CrossRef]
- Salvati, A.; Pitek, A.S.; Monopoli, M.P.; Prapainop, K.; Bombelli, F.B.; Hristov, D.R.; Kelly, P.M.; Åberg, C.; Mahon, E.; Dawson, K.A. Transferrin-Functionalized Nanoparticles Lose Their Targeting Capabilities When a Biomolecule Corona Adsorbs on the Surface. Nat. Nanotechnol. 2013, 8, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Bertrand, N.; Grenier, P.; Mahmoudi, M.; Lima, E.M.; Appel, E.A.; Dormont, F.; Lim, J.M.; Karnik, R.; Langer, R.; Farokhzad, O.C. Mechanistic Understanding of in Vivo Protein Corona Formation on Polymeric Nanoparticles and Impact on Pharmacokinetics. Nat. Commun. 2017, 8, 777. [Google Scholar] [CrossRef]
- Palmieri, V.; Caracciolo, G. Tuning the Immune System by Nanoparticle-Biomolecular Corona. Nanoscale Adv. 2022, 4, 3300–3308. [Google Scholar] [CrossRef]
- Miao, Y.; Li, L.; Wang, Y.; Wang, J.; Zhou, Y.; Guo, L.; Zhao, Y.; Nie, D.; Zhang, Y.; Zhang, X.; et al. Regulating Protein Corona on Nanovesicles by Glycosylated Polyhydroxy Polymer Modification for Efficient Drug Delivery. Nat. Commun. 2024, 15, 1159. [Google Scholar] [CrossRef]
- Lee, Y.K.; Choi, E.J.; Webster, T.J.; Kim, S.H.; Khang, D. Effect of the Protein Corona on Nanoparticles for Modulating Cytotoxicity and Immunotoxicity. Int. J. Nanomed. 2014, 10, 97–113. [Google Scholar] [CrossRef]
- Lima, T.; Bernfur, K.; Vilanova, M.; Cedervall, T. Understanding the Lipid and Protein Corona Formation on Different Sized Polymeric Nanoparticles. Sci. Rep. 2020, 10, 1129. [Google Scholar] [CrossRef]
- Panico, S.; Capolla, S.; Bozzer, S.; Toffoli, G.; Dal Bo, M.; Macor, P. Biological Features of Nanoparticles: Protein Corona Formation and Interaction with the Immune System. Pharmaceutics 2022, 14, 2605. [Google Scholar] [CrossRef]
- Yang, C.; Zhao, H.; Sun, Y.; Wang, C.; Geng, X.; Wang, R.; Tang, L.; Han, D.; Liu, J.; Tan, W. Programmable Manipulation of Oligonucleotide-Albumin Interaction for Elongated Circulation Time. Nucleic Acids Res. 2022, 50, 3083–3095. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.; Park, J.Y.; Kim, K.; Yoo, R.J.; Suh, M.; Gu, G.J.; Kim, J.S.; Choi, T.H.; Byun, J.W.; Ju, Y.W.; et al. Circulation Time-Optimized Albumin Nanoplatform for Quantitative Visualization of Lung Metastasis via Targeting of Macrophages. ACS Nano 2022, 16, 12262–12275. [Google Scholar] [CrossRef]
- Das, A.; Chakrabarti, A.; Das, P.K. Suppression of Protein Aggregation by Gold Nanoparticles: A New Way to Store and Transport Proteins. RSC Adv. 2015, 5, 38558–38570. [Google Scholar] [CrossRef]
- Chen, F.; Wang, G.; Griffin, J.I.; Brenneman, B.; Banda, N.K.; Holers, V.M.; Backos, D.S.; Wu, L.; Moghimi, S.M.; Simberg, D. Complement Proteins Bind to Nanoparticle Protein Corona and Undergo Dynamic Exchange in Vivo. Nat. Nanotechnol. 2017, 12, 387–393. [Google Scholar] [CrossRef]
- Strojan, K.; Leonardi, A.; Bregar, V.B.; Križaj, I.; Svete, J.; Pavlin, M. Dispersion of Nanoparticles in Different Media Importantly Determines the Composition of Their Protein Corona. PLoS ONE 2017, 12, e0169552. [Google Scholar] [CrossRef] [PubMed]
- Derakhshankhah, H.; Hosseini, A.; Taghavi, F.; Jafari, S.; Lotfabadi, A.; Ejtehadi, M.R.; Shahbazi, S.; Fattahi, A.; Ghasemi, A.; Barzegari, E.; et al. Molecular Interaction of Fibrinogen with Zeolite Nanoparticles. Sci. Rep. 2019, 9, 1558. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Guan, J.; Jiang, Z.; Yang, Y.; Liu, J.; Hua, W.; Mao, Y.; Li, C.; Lu, W.; Qian, J.; et al. Brain-Targeted Drug Delivery by Manipulating Protein Corona Functions. Nat. Commun. 2019, 10, 3561. [Google Scholar] [CrossRef]
- Wagner, S.; Zensi, A.; Wien, S.L.; Tschickardt, S.E.; Maier, W.; Vogel, T.; Worek, F.; Pietrzik, C.U.; Kreuter, J.; von Briesen, H. Uptake Mechanism of ApoE-Modified Nanoparticles on Brain Capillary Endothelial Cells as a Blood-Brain Barrier Model. PLoS ONE 2012, 7, e32568. [Google Scholar] [CrossRef]
- Hartl, N.; Adams, F.; Merkel, O.M. From Adsorption to Covalent Bonding: Apolipoprotein E Functionalization of Polymeric Nanoparticles for Drug Delivery Across the Blood–Brain Barrier. Adv. Ther. 2021, 4, 2000092. [Google Scholar] [CrossRef]
- Vidaurre-Agut, C.; Rivero-Buceta, E.; Romaní-Cubells, E.; Clemments, A.M.; Vera-Donoso, C.D.; Landry, C.C.; Botella, P. Protein Corona over Mesoporous Silica Nanoparticles: Influence of the Pore Diameter on Competitive Adsorption and Application to Prostate Cancer Diagnostics. ACS Omega 2019, 4, 8852–8861. [Google Scholar] [CrossRef]
- Choi, J.K.; Park, J.Y.; Lee, S.; Choi, Y.A.; Kwon, S.; Shin, M.J.; Yun, H.S.; Jang, Y.H.; Kang, J.; Kim, N.; et al. Greater Plasma Protein Adsorption on Mesoporous Silica Nanoparticles Aggravates Atopic Dermatitis. Int. J. Nanomed. 2022, 17, 4599–4617. [Google Scholar] [CrossRef] [PubMed]
- Partikel, K.; Korte, R.; Stein, N.C.; Mulac, D.; Herrmann, F.C.; Humpf, H.U.; Langer, K. Effect of Nanoparticle Size and PEGylation on the Protein Corona of PLGA Nanoparticles. Eur. J. Pharm. Biopharm. 2019, 141, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Kyriakides, T.R.; Raj, A.; Tseng, T.H.; Xiao, H.; Nguyen, R.; Mohammed, F.S.; Halder, S.; Xu, M.; Wu, M.J.; Bao, S.; et al. Biocompatibility of Nanomaterials and Their Immunological Properties. Biomed. Mater. 2021, 16, 042005. [Google Scholar] [CrossRef]
- Sercombe, L.; Veerati, T.; Moheimani, F.; Wu, S.Y.; Sood, A.K.; Hua, S. Advances and Challenges of Liposome Assisted Drug Delivery. Front. Pharmacol. 2015, 6, 286. [Google Scholar] [CrossRef]
- Juhairiyah, F.; de Lange, E.C.M. Understanding Drug Delivery to the Brain Using Liposome-Based Strategies: Studies That Provide Mechanistic Insights Are Essential. AAPS J. 2021, 23, 114. [Google Scholar] [CrossRef] [PubMed]
- Giulimondi, F.; Digiacomo, L.; Pozzi, D.; Palchetti, S.; Vulpis, E.; Capriotti, A.L.; Chiozzi, R.Z.; Laganà, A.; Amenitsch, H.; Masuelli, L.; et al. Interplay of Protein Corona and Immune Cells Controls Blood Residency of Liposomes. Nat. Commun. 2019, 10, 3686. [Google Scholar] [CrossRef]
- Liu, X.Q.; Tang, R.Z. Biological Responses to Nanomaterials: Understanding Nano-Bio Effects on Cell Behaviors. Drug Deliv. 2017, 24, 1–15. [Google Scholar] [CrossRef]
- Ashkarran, A.A.; Gharibi, H.; Modaresi, S.M.; Saei, A.A.; Mahmoudi, M. Standardizing Protein Corona Characterization in Nanomedicine: A Multicenter Study to Enhance Reproducibility and Data Homogeneity. Nano Lett. 2024, 24, 9874–9881. [Google Scholar] [CrossRef]
- Ashkarran, A.A.; Gharibi, H.; Sadeghi, S.A.; Modaresi, S.M.; Wang, Q.; Lin, T.J.; Yerima, G.; Tamadon, A.; Sayadi, M.; Jafari, M.; et al. Small Molecule Modulation of Protein Corona for Deep Plasma Proteome Profiling. Nat. Commun. 2024, 15, 9638. [Google Scholar] [CrossRef]
- Pourali, P.; Neuhöferová, E.; Dzmitruk, V.; Benson, V. Investigation of Protein Corona Formed around Biologically Produced Gold Nanoparticles. Materials 2022, 15, 4615. [Google Scholar] [CrossRef]
- Bae, Y.; Liu, X. Unveiling the Effects of Protein Corona Formation on the Aggregation Kinetics of Gold Nanoparticles in Monovalent and Divalent Electrolytes. Environ. Pollut. 2024, 346, 123552. [Google Scholar] [CrossRef]
- Cedervall, T.; Lynch, I.; Lindman, S.; Berggård, T.; Thulin, E.; Nilsson, H.; Dawson, K.A.; Linse, S. Understanding the Nanoparticle-Protein Corona Using Methods to Quantify Exchange Rates and Affinities of Proteins for Nanoparticles. Proc. Natl. Acad. Sci. USA 2007, 104, 2050–2055. [Google Scholar]
- Arezki, Y.; Delalande, F.; Schaeffer-Reiss, C.; Cianférani, S.; Rapp, M.; Lebeau, L.; Pons, F.; Ronzani, C. Surface Charge Influences Protein Corona, Cell Uptake and Biological Effects of Carbon Dots. Nanoscale 2022, 14, 14695–14710. [Google Scholar] [CrossRef]
- Sakulkhu, U.; Mahmoudi, M.; Maurizi, L.; Coullerez, G.; Hofmann-Amtenbrink, M.; Vries, M.; Motazacker, M.; Rezaee, F.; Hofmann, H. Significance of Surface Charge and Shell Material of Superparamagnetic Iron Oxide Nanoparticle (SPION) Based Core/Shell Nanoparticles on the Composition of the Protein Corona. R. Soc. Chem. 2013, 3, 265–278. [Google Scholar] [CrossRef]
- El-Baz, N.; Nunn, B.M.; Bates, P.J.; O’Toole, M.G. The Impact of PEGylation on Cellular Uptake and In Vivo Biodistribution of Gold Nanoparticle MRI Contrast Agents. Bioengineering 2022, 9, 766. [Google Scholar] [CrossRef]
- Gutiérrez-Cruz, S.G.; Muñoz-Diosdado, A.; Gutiérrez-Calleja, R.A.; Rodríguez-Cortés, O.; Ortiz-Reyez, A.E.; Flores-Mejía, R. Influence of Physicochemical Factors on the Interaction of Metallic Nanoparticles with Immune System Cells. Front. Nanotechnol. 2024, 6, 1496230. [Google Scholar] [CrossRef]
- Neun, B.W.; Ilinskaya, A.N.; Dobrovolskaia, M.A. Analysis of Complement Activation by Nanoparticles. In Characterization of Nanoparticles Intended for Drug Delivery; Springer: New York, NY, USA, 2018; pp. 149–160. [Google Scholar]
- Haroon, H.B.; Dhillon, E.; Farhangrazi, Z.S.; Trohopoulos, P.N.; Simberg, D.; Moghimi, S.M. Activation of the Complement System by Nanoparticles and Strategies for Complement Inhibition. Eur. J. Pharm. Biopharm. 2023, 193, 227–240. [Google Scholar] [CrossRef]
- Wang, G.; Chen, F.; Banda, N.K.; Holers, V.M.; Wu, L.P.; Moghimi, S.M.; Simberg, D. Activation of Human Complement System by Dextran-Coated Iron Oxide Nanoparticles Is Not Affected by Dextran/Fe Ratio, Hydroxyl Modifications, and Crosslinking. Front. Immunol. 2016, 7, 418. [Google Scholar] [CrossRef]
- Shao, F.; Gao, Y.; Wang, W.; He, H.; Xiao, L.; Geng, X.; Xia, Y.; Guo, D.; Fang, J.; He, J.; et al. Silencing EGFR-Upregulated Expression of CD55 and CD59 Activates the Complement System and Sensitizes Lung Cancer to Checkpoint Blockade. Nat. Cancer 2022, 3, 1192–1210. [Google Scholar] [CrossRef]
- Moghimi, S.M.; Haroon, H.B.; Yaghmur, A.; Hunter, A.C.; Papini, E.; Farhangrazi, Z.S.; Simberg, D.; Trohopoulos, P.N. Perspectives on Complement and Phagocytic Cell Responses to Nanoparticles: From Fundamentals to Adverse Reactions. J. Control. Release 2023, 356, 115–129. [Google Scholar] [CrossRef]
- Coty, J.-B.; Eleamen Oliveira, E.; Vauthier, C. Tuning Complement Activation and Pathway through Controlled Molecular Architecture of Dextran Chains in Nanoparticle Corona. Int. J. Pharm. 2017, 532, 769–778. [Google Scholar] [CrossRef]
- Li, Y.; Wang, G.; Griffin, L.; Banda, N.K.; Saba, L.M.; Groman, E.V.; Scheinman, R.; Moghimi, S.M.; Simberg, D. Complement Opsonization of Nanoparticles: Differences between Humans and Preclinical Species. J. Control. Release 2021, 338, 548–556. [Google Scholar] [CrossRef]
- Zaleski, M.H.; Chase, L.S.; Hood, E.D.; Wang, Z.; Nong, J.; Espy, C.L.; Zamora, M.E.; Wu, J.; Morrell, L.J.; Muzykantov, V.R.; et al. Conjugation Chemistry Markedly Impacts Toxicity and Biodistribution of Targeted Nanoparticles, Mediated by Complement Activation. Adv. Mater. 2024, 37, e2409945. [Google Scholar] [CrossRef]
- La-Beck, N.M.; Islam, M.R.; Markiewski, M.M. Nanoparticle-Induced Complement Activation: Implications for Cancer Nanomedicine. Front. Immunol. 2021, 11, 603039. [Google Scholar] [CrossRef]
- Khan, H.A.; Kishore, U.; Alrokayan, S.H.; Ibrahim, K.E. Activation of the Complement Lectin Pathway by Iron Oxide Nanoparticles and Induction of Pro-Inflammatory Immune Response by Macrophages. Curr. Nanosci. 2025, 21, 82–91. [Google Scholar] [CrossRef]
- Dobó, J.; Kocsis, A.; Gál, P. Be on Target: Strategies of Targeting Alternative and Lectin Pathway Components in Complement-Mediated Diseases. Front. Immunol. 2018, 9, 1851. [Google Scholar] [CrossRef]
- Moghimi, S.M.; Wibroe, P.P.; Wu, L.; Farhangrazi, Z.S. Insidious Pathogen-Mimicking Properties of Nanoparticles in Triggering the Lectin Pathway of the Complement System. Eur. J. Nanomed. 2015, 7, 263–268. [Google Scholar] [CrossRef]
- Quach, Q.H.; Kah, J.C. Complement Activation by Gold Nanoparticles Passivated with Polyelectrolyte Ligands. RSC Adv. 2018, 8, 6616–6619. [Google Scholar] [CrossRef]
- Roumenina, L.T. Terminal Complement without C5 Convertase? Blood 2021, 137, 431–432. [Google Scholar] [CrossRef]
- Li, Y.; Wu, W.; Liu, Q.; Wu, Q.; Ren, P.; Xi, X.; Liu, H.; Zhao, J.; Zhang, W.; Wang, Z.; et al. Specific Surface-Modified Iron Oxide Nanoparticles Trigger Complement-Dependent Innate and Adaptive Antileukaemia Immunity. Nat. Commun. 2024, 15, 10400. [Google Scholar] [CrossRef]
- Barbey, C.; Wolf, H.; Wagner, R.; Pauly, D.; Breunig, M. A Shift of Paradigm: From Avoiding Nanoparticular Complement Activation in the Field of Nanomedicines to Its Exploitation in the Context of Vaccine Development. Eur. J. Pharm. Biopharm. 2023, 193, 119–128. [Google Scholar] [CrossRef]
- Padín-González, E.; Lancaster, P.; Bottini, M.; Gasco, P.; Tran, L.; Fadeel, B.; Wilkins, T.; Monopoli, M.P. Understanding the Role and Impact of Poly (Ethylene Glycol) (PEG) on Nanoparticle Formulation: Implications for COVID-19 Vaccines. Front. Bioeng. Biotechnol. 2022, 10, 882363. [Google Scholar] [CrossRef]
- Yang, Q.; Lai, S.K. Anti-PEG Immunity: Emergence, Characteristics, and Unaddressed Questions. Nanomed. Nanobiotechnol. 2015, 7, 655–677. [Google Scholar] [CrossRef]
- Overby, C.; Park, S.; Summers, A.; Benoit, D.S.W. Zwitterionic Peptides: Tunable next-Generation Stealth Nanoparticle Modifications. Bioact. Mater. 2023, 27, 113–124. [Google Scholar] [CrossRef]
- Liao, H.; Niu, C. Role of CD47-SIRPα Checkpoint in Nanomedicine-Based Anti-Cancer Treatment. Front. Bioeng. Biotechnol. 2022, 10, 887463. [Google Scholar] [CrossRef]
- Zhang, W.; Huang, Q.; Xiao, W.; Zhao, Y.; Pi, J.; Xu, H.; Zhao, H.; Xu, J.; Evans, C.E.; Jin, H. Advances in Anti-Tumor Treatments Targeting the CD47/SIRPα Axis. Front. Immunol. 2020, 11, 18. [Google Scholar] [CrossRef]
- Peng, Q.; Zhang, S.; Yang, Q.; Zhang, T.; Wei, X.Q.; Jiang, L.; Zhang, C.L.; Chen, Q.M.; Zhang, Z.R.; Lin, Y.F. Preformed Albumin Corona, a Protective Coating for Nanoparticles Based Drug Delivery System. Biomaterials 2013, 34, 8521–8530. [Google Scholar] [CrossRef]
- Mariam, J.; Sivakami, S.; Dongre, P.M. Albumin Corona on Nanoparticles—A Strategic Approach in Drug Delivery. Drug Deliv. 2016, 23, 2668–2676. [Google Scholar] [CrossRef]
- Chubarov, A.S. Serum Albumin for Magnetic Nanoparticles Coating. Magnetochemistry 2022, 8, 13. [Google Scholar] [CrossRef]
- Muhammad, Q.; Jang, Y.; Kang, S.H.; Moon, J.; Kim, W.J.; Park, H. Modulation of Immune Responses with Nanoparticles and Reduction of Their Immunotoxicity. Biomater. Sci. 2020, 8, 1490–1501. [Google Scholar] [CrossRef]
- Chao, C.J.; Zhang, E.; Trinh, D.N.; Udofa, E.; Lin, H.; Silvers, C.; Huo, J.; He, S.; Zheng, J.; Cai, X.; et al. Integrating Antigen Capturing Nanoparticles and Type 1 Conventional Dendritic Cell Therapy for in Situ Cancer Immunization. Nat. Commun. 2025, 16, 4578. [Google Scholar] [CrossRef]
- Baron, L.; Gombault, A.; Fanny, M.; Villeret, B.; Savigny, F.; Guillou, N.; Panek, C.; Le Bert, M.; Lagente, V.; Rassendren, F.; et al. The NLRP3 Inflammasome Is Activated by Nanoparticles through ATP, ADP and Adenosine. Cell Death Dis. 2015, 6, e1629. [Google Scholar] [CrossRef]
- Mellors, J.; Tipton, T.; Longet, S.; Carroll, M. Viral Evasion of the Complement System and Its Importance for Vaccines and Therapeutics. Front. Immunol. 2020, 11, 1450. [Google Scholar] [CrossRef]
- Ray, P.; Haideri, N.; Haque, I.; Mohammed, O.; Chakraborty, S.; Banerjee, S.; Quadir, M.; Brinker, A.E.; Banerjee, S.K. The Impact of Nanoparticles on the Immune System: A Gray Zone of Nanomedicine. J. Immunol. Sci. 2021, 5, 19–33. [Google Scholar] [CrossRef]
- Sushnitha, M.; Evangelopoulos, M.; Tasciotti, E.; Taraballi, F. Cell Membrane-Based Biomimetic Nanoparticles and the Immune System: Immunomodulatory Interactions to Therapeutic Applications. Front. Bioeng. Biotechnol. 2020, 8, 627. [Google Scholar] [CrossRef]
- Poon, W.; Zhang, Y.-N.; Ouyang, B.; Kingston, B.R.; Wu, J.L.Y.; Wilhelm, S.; Chan, W.C.W. Elimination Pathways of Nanoparticles. ACS Nano 2019, 13, 5785–5798. [Google Scholar] [CrossRef]
- Yah, C.S.; Iyuke, S.E.; Simate, G.S. A Review of Nanoparticles Toxicity and Their Routes of Exposures. Iran. J. Pharm. Sci. Winter 2011, 8, 299–314. [Google Scholar]
- Nazarenko, Y.; Han, T.W.; Lioy, P.J.; Mainelis, G. Potential for Exposure to Engineered Nanoparticles from Nanotechnology-Based Consumer Spray Products. J. Expo. Sci. Envrion. Epidemiol. 2011, 21, 515–528. [Google Scholar] [CrossRef]
- Wan, Q.; Zhang, X.; Zhou, D.; Xie, R.; Cai, Y.; Zhang, K.; Sun, X. Inhaled Nano-Based Therapeutics for Pulmonary Fibrosis: Recent Advances and Future Prospects. J. Nanobiotechnol. 2023, 21, 215. [Google Scholar] [CrossRef]
- Wang, M.; Lai, X.; Shao, L.; Li, L. Evaluation of Immunoresponses and Cytotoxicity from Skin Exposure to Metallic Nanoparticles. Int. J. Nanomed. 2018, 13, 4445–4459. [Google Scholar] [CrossRef]
- Date, A.A.; Hanes, J.; Ensign, L.M. Nanoparticles for Oral Delivery: Design, Evaluation and State-of-the-Art. J. Control. Release 2016, 240, 504–526. [Google Scholar] [CrossRef]
- Gaillet, S.; Rouanet, J.M. Silver Nanoparticles: Their Potential Toxic Effects after Oral Exposure and Underlying Mechanisms—A Review. Food Chem. Toxicol. 2015, 77, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Han, S.; Zhou, D.; Zhou, S.; Jia, G. Effects of Oral Exposure to Titanium Dioxide Nanoparticles on Gut Microbiota and Gut-Associated Metabolism in Vivo. Nanoscale 2019, 11, 22398–22412. [Google Scholar] [CrossRef]
- Lotfipour, F.; Shahi, S.; Farjami, A.; Salatin, S.; Mahmoudian, M.; Dizaj, S.M. Safety and Toxicity Issues of Therapeutically Used Nanoparticles from the Oral Route. Biomed. Res. Int. 2021, 2021, 9322282. [Google Scholar] [CrossRef]
- Huang, X.; Kaneda-Nakashima, K.; Kadonaga, Y.; Kabayama, K.; Shimoyama, A.; Ooe, K.; Kato, H.; Toyoshima, A.; Shinohara, A.; Haba, H.; et al. Astatine-211-Labeled Gold Nanoparticles for Targeted Alpha-Particle Therapy via Intravenous Injection. Pharmaceutics 2022, 14, 2705. [Google Scholar] [CrossRef]
- Barua, S.; Mitragotri, S. Challenges Associated with Penetration of Nanoparticles across Cell and Tissue Barriers: A Review of Current Status and Future Prospects. Nano Today 2014, 9, 223–243. [Google Scholar] [CrossRef]
- Liu, C.; Jiang, X.; Gan, Y.; Yu, M. Engineering Nanoparticles to Overcome the Mucus Barrier for Drug Delivery: Design, Evaluation and State-of-the-Art. Med. Drug Discov. 2021, 12, 100110. [Google Scholar] [CrossRef]
- Tian, X.; Chong, Y.; Ge, C. Understanding the Nano–Bio Interactions and the Corresponding Biological Responses. Front. Chem. 2020, 8, 446. [Google Scholar] [CrossRef]
- Sonwani, S.; Madaan, S.; Arora, J.; Suryanarayan, S.; Rangra, D.; Mongia, N.; Vats, T.; Saxena, P. Inhalation Exposure to Atmospheric Nanoparticles and Its Associated Impacts on Human Health: A Review. Front. Sustain. Cities 2021, 3, 690444. [Google Scholar] [CrossRef]
- McCormick, S.; Niang, M.; Dahm, M.M. Occupational Exposures to Engineered Nanomaterials: A Review of Workplace Exposure Assessment Methods. Curr. Envrion. Health Rep. 2021, 8, 223–234. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Jin, W.; Ma, J. Lung Inflammation Perturbation by Engineered Nanoparticles. Front. Bioeng. Biotechnol. 2023, 11, 1199230. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.E.; Rezaee, F. Nanoparticles and Airway Epithelial Cells: Exploring the Impacts and Methodologies in Toxicity Assessment. Int. J. Mol. Sci. 2024, 25, 7885. [Google Scholar] [CrossRef]
- Zhu, Y.; Chidekel, A.; Shaffer, T.H. Cultured Human Airway Epithelial Cells (Calu-3): A Model of Human Respiratory Function, Structure, and Inflammatory Responses. Crit. Care Res. Pract. 2010, 2010, 394578. [Google Scholar] [CrossRef]
- Meindl, C.; Öhlinger, K.; Zrim, V.; Steinkogler, T.; Fröhlich, E. Screening for Effects of Inhaled Nanoparticles in Cell Culture Models for Prolonged Exposure. Nanomaterials 2021, 11, 606. [Google Scholar] [CrossRef]
- Rabiei, M.; Kashanian, S.; Samavati, S.S.; Jamasb, S.; McInnes, S.J.P. Nanomaterial and Advanced Technologies in Transdermal Drug Delivery. J. Drug Target. 2020, 28, 356–367. [Google Scholar] [CrossRef]
- Piluk, T.; Faccio, G.; Letsiou, S.; Liang, R.; Freire-Gormaly, M. A Critical Review Investigating the Use of Nanoparticles in Cosmetic Skin Products. Envrion. Sci. Nano 2024, 11, 3674–3692. [Google Scholar] [CrossRef]
- Tak, Y.K.; Pal, S.; Naoghare, P.K.; Rangasamy, S.; Song, J.M. Shape-Dependent Skin Penetration of Silver Nanoparticles: Does It Really Matter? Sci. Rep. 2015, 5, 16908. [Google Scholar] [CrossRef]
- Liang, X.; Xu, Z.; Grice, J.; Zvyagin, A.; Roberts, M.; Liu, X. Penetration of Nanoparticles into Human Skin. Curr. Pharm. Des. 2013, 19, 6353–6366. [Google Scholar] [CrossRef]
- Liang, Y.; Simaiti, A.; Xu, M.; Lv, S.; Jiang, H.; He, X.; Fan, Y.; Zhu, S.; Du, B.; Yang, W.; et al. Antagonistic Skin Toxicity of Co-Exposure to Physical Sunscreen Ingredients Zinc Oxide and Titanium Dioxide Nanoparticles. Nanomaterials 2022, 12, 2769. [Google Scholar] [CrossRef]
- Ailawadi, S.; Talreja, R.; Panstingel, N.; Sulentic, C. Titanium Dioxide and Zinc Oxide Nanoparticles in Sunscreen: Potential Impact on Cytokine Expression in Human Skin Pre- and Post-UVB Exposure. Arch. Microbiol. Immunol. 2023, 7, 443–455. [Google Scholar] [CrossRef]
- Singh, G.; Thakur, N.; Kumar, R. Nanoparticles in Drinking Water: Assessing Health Risks and Regulatory Challenges. Sci. Total Environ. 2024, 949, 174940. [Google Scholar] [CrossRef]
- Vitulo, M.; Gnodi, E.; Meneveri, R.; Barisani, D. Interactions between Nanoparticles and Intestine. Int. J. Mol. Sci. 2022, 23, 4339. [Google Scholar] [CrossRef]
- Karavolos, M.; Holban, A. Nanosized Drug Delivery Systems in Gastrointestinal Targeting: Interactions with Microbiota. Pharmaceuticals 2016, 9, 62. [Google Scholar] [CrossRef]
- Jakic, K.; Selc, M.; Razga, F.; Nemethova, V.; Mazancova, P.; Havel, F.; Sramek, M.; Zarska, M.; Proska, J.; Masanova, V.; et al. Long-Term Accumulation, Biological Effects and Toxicity of BSA-Coated Gold Nanoparticles in the Mouse Liver, Spleen, and Kidneys. Int. J. Nanomed. 2024, 19, 4103–4120. [Google Scholar] [CrossRef]
- Tsoi, K.M.; Macparland, S.A.; Ma, X.Z.; Spetzler, V.N.; Echeverri, J.; Ouyang, B.; Fadel, S.M.; Sykes, E.A.; Goldaracena, N.; Kaths, J.M.; et al. Mechanism of Hard-Nanomaterial Clearance by the Liver. Nat. Mater. 2016, 15, 1212–1221. [Google Scholar] [CrossRef] [PubMed]
- Elje, E.; Mariussen, E.; Moriones, O.H.; Bastús, N.G.; Puntes, V.; Kohl, Y.; Dusinska, M.; Rundén-Pran, E. Hepato(Geno)Toxicity Assessment of Nanoparticles in a Hepg2 Liver Spheroid Model. Nanomaterials 2020, 10, 545. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Poon, W.; Tavares, A.J.; McGilvray, I.D.; Chan, W.C.W. Nanoparticle–Liver Interactions: Cellular Uptake and Hepatobiliary Elimination. J. Control. Release 2016, 240, 332–348. [Google Scholar] [CrossRef]
- Bannunah, A.; Cavanagh, R.; Shubber, S.; Vllasaliu, D.; Stolnik, S. Difference in Endocytosis Pathways Used by Differentiated Versus Nondifferentiated Epithelial Caco-2 Cells to Internalize Nanosized Particles. Mol. Pharm. 2024, 21, 3603–3612. [Google Scholar] [CrossRef]
- Mahlert, L.; Anderski, J.; Mulac, D.; Langer, K. The Impact of Gastrointestinal Mucus on Nanoparticle Penetration—In Vitro Evaluation of Mucus-Penetrating Nanoparticles for Photodynamic Therapy. Eur. J. Pharm. Sci. 2019, 133, 28–39. [Google Scholar] [CrossRef] [PubMed]
- Vincentini, O.; Prota, V.; Cecchetti, S.; Bertuccini, L.; Tinari, A.; Iosi, F.; De Angelis, I. Towards the Standardization of Intestinal In Vitro Advanced Barrier Model for Nanoparticles Uptake and Crossing: The SiO2 Case Study. Cells 2022, 11, 3357. [Google Scholar] [CrossRef] [PubMed]
- Trac, N.; Chung, E.J. Overcoming Physiological Barriers by Nanoparticles for Intravenous Drug Delivery to the Lymph Nodes. Exp. Biol. Med. 2021, 246, 2358–2371. [Google Scholar] [CrossRef] [PubMed]
- Ernst, L.M.; Casals, E.; Italiani, P.; Boraschi, D.; Puntes, V. The Interactions between Nanoparticles and the Innate Immune System from a Nanotechnologist Perspective. Nanomaterials 2021, 11, 2991. [Google Scholar] [CrossRef]
- Omo-Lamai, S.; Zamora, M.E.; Patel, M.N.; Wu, J.; Nong, J.; Wang, Z.; Peshkova, A.; Chase, L.S.; Essien, E.-O.; Muzykantov, V.; et al. Physicochemical Targeting of Lipid Nanoparticles to the Lungs Induces Clotting: Mechanisms and Solutions. Adv. Mater. 2023, 36, e2312026. [Google Scholar] [CrossRef]
- Huang, Y.; Guo, X.; Wu, Y.; Chen, X.; Feng, L.; Xie, N.; Shen, G. Nanotechnology’s Frontier in Combatting Infectious and Inflammatory Diseases: Prevention and Treatment. Signal Transduct. Target. Ther. 2024, 9, 34. [Google Scholar] [CrossRef]
- Al Khafaji, A.S.; Donovan, M.D. Endocytic Uptake of Solid Lipid Nanoparticles by the Nasal Mucosa. Pharmaceutics 2021, 13, 761. [Google Scholar] [CrossRef]
- Gandhi, S.; Shastri, D.H.; Shah, J.; Nair, A.B.; Jacob, S. Nasal Delivery to the Brain: Harnessing Nanoparticles for Effective Drug Transport. Pharmaceutics 2024, 16, 481. [Google Scholar] [CrossRef]
- Zhu, Q.; Chen, Z.; Paul, P.K.; Lu, Y.; Wu, W.; Qi, J. Oral Delivery of Proteins and Peptides: Challenges, Status Quo and Future Perspectives. Acta Pharm. Sin. B 2021, 11, 2416–2448. [Google Scholar] [CrossRef]
- Koo, J.; Lim, C.; Oh, K.T. Recent Advances in Intranasal Administration for Brain-Targeting Delivery: A Comprehensive Review of Lipid-Based Nanoparticles and Stimuli-Responsive Gel Formulations. Int. J. Nanomed. 2024, 19, 1767–1807. [Google Scholar] [CrossRef]
- Huang, Q.; Chen, X.; Yu, S.; Gong, G.; Shu, H. Research Progress in Brain-Targeted Nasal Drug Delivery. Front. Aging Neurosci. 2023, 15, 1341295. [Google Scholar] [CrossRef]
- Alhowyan, A.A.; Kalam, M.A.; Iqbal, M.; Raish, M.; El-Toni, A.M.; Alkholief, M.; Almomen, A.A.; Alshamsan, A. Mesoporous Silica Nanoparticles Coated with Carboxymethyl Chitosan for 5-Fluorouracil Ocular Delivery: Characterization, In Vitro and In Vivo Studies. Molecules 2023, 28, 1260. [Google Scholar] [CrossRef]
- Zhu, S.; Gong, L.; Li, Y.; Xu, H.; Gu, Z.; Zhao, Y. Safety Assessment of Nanomaterials to Eyes: An Important but Neglected Issue. Adv. Sci. 2019, 6, 1802289. [Google Scholar] [CrossRef]
- Clementino, A.R.; Pellegrini, G.; Banella, S.; Colombo, G.; Cantù, L.; Sonvico, F.; Del Favero, E. Structure and Fate of Nanoparticles Designed for the Nasal Delivery of Poorly Soluble Drugs. Mol. Pharm. 2021, 18, 3132–3146. [Google Scholar] [CrossRef]
- Yang, C.; Yang, J.; Lu, A.; Gong, J.; Yang, Y.; Lin, X.; Li, M.; Xu, H. Nanoparticles in Ocular Applications and Their Potential Toxicity. Front. Mol. Biosci. 2022, 9, 931759. [Google Scholar] [CrossRef] [PubMed]
- Sundaram, S.; Roy, S.K.; Ambati, B.K.; Kompella, U.B. Surface-functionalized Nanoparticles for Targeted Gene Delivery across Nasal Respiratory Epithelium. FASEB J. 2009, 23, 3752–3765. [Google Scholar] [CrossRef] [PubMed]
- Croissant, J.G.; Fatieiev, Y.; Khashab, N.M. Degradability and Clearance of Silicon, Organosilica, Silsesquioxane, Silica Mixed Oxide, and Mesoporous Silica Nanoparticles. Adv. Mater. 2017, 29, 1604634. [Google Scholar] [CrossRef] [PubMed]
- Kraegeloh, A.; Suarez-Merino, B.; Sluijters, T.; Micheletti, C. Implementation of Safe-by-Design for Nanomaterial Development and Safe Innovation: Why We Need a Comprehensive Approach. Nanomaterials 2018, 8, 239. [Google Scholar] [CrossRef]
- Đorđević, S.; Gonzalez, M.M.; Conejos-Sánchez, I.; Carreira, B.; Pozzi, S.; Acúrcio, R.C.; Satchi-Fainaro, R.; Florindo, H.F.; Vicent, M.J. Current Hurdles to the Translation of Nanomedicines from Bench to the Clinic. Drug Deliv. Transl. Res. 2022, 12, 500–525. [Google Scholar] [CrossRef]
- Frickenstein, A.N.; Hagood, J.M.; Britten, C.N.; Abbott, B.S.; McNally, M.W.; Vopat, C.A.; Patterson, E.G.; Maccuaig, W.M.; Jain, A.; Walters, K.B.; et al. Mesoporous Silica Nanoparticles: Properties and Strategies for Enhancing Clinical Effect. Pharmaceutics 2021, 13, 570. [Google Scholar] [CrossRef]
- Rasmussen, K.; Rauscher, H.; Kearns, P.; González, M.; Riego Sintes, J. Developing OECD Test Guidelines for Regulatory Testing of Nanomaterials to Ensure Mutual Acceptance of Test Data. Regul. Toxicol. Pharmacol. 2019, 104, 74–83. [Google Scholar] [CrossRef]
- OECD. Building Trust and Enhancing Dialogue for Safe-and-Sustainable-by-Design (SSBD) Innovation: Developing Tools to Enhance Trusted Environments. In OECD Series on the Safety of Manufactured Nanomaterials and Other Advanced Materials; OECD Publishing: Paris, France, 2024. [Google Scholar] [CrossRef]
- OECD. Developments in Delegations on the Safety of Manufactured Nanomaterials and Advanced Materials Between July 2023 and June 2024—Tour de Table. In OECD Series on the Safety of Manufactured Nanomaterials and Other Advanced Materials; OECD Publishing: Paris, France, 2024; ISBN 9789264647909. [Google Scholar]
- Oualikene-Gonin, W.; Sautou, V.; Ezan, E.; Bastos, H.; Bellissant, E.; Belgodère, L.; Maison, P.; Ankri, J. Regulatory Assessment of Nano-Enabled Health Products in Public Health Interest. Position of the Scientific Advisory Board of the French National Agency for the Safety of Medicines and Health Products. Front. Public Health 2023, 11, 1125577. [Google Scholar] [CrossRef]
- Paradise, J. Regulating Nanomedicine at the Food and Drug Administration. AMA J. Ethics 2019, 21, E347–E355. [Google Scholar] [CrossRef]
- Clogston, J.D. The Importance of Nanoparticle Physicochemical Characterization for Immunology Research: What We Learned and What We Still Need to Understand. Adv. Drug Deliv. Rev. 2021, 176, 113897. [Google Scholar] [CrossRef]
- ISO/TR 10993–22:2017(en); Biological Evaluation of Medical Devices—Part 22: Guidance on Nanomaterials. International Organization for Standardization: Geneva, Switzerland, 2013. Available online: https://www.iso.org/obp/ui#iso:std:iso:tr:10993:-22:ed-1:v1:en (accessed on 10 October 2025).
- Rodríguez-Ibarra, C.; Déciga-Alcaraz, A.; Ispanixtlahuatl-Meráz, O.; Medina-Reyes, E.I.; Delgado-Buenrostro, N.L.; Chirino, Y.I. International Landscape of Limits and Recommendations for Occupational Exposure to Engineered Nanomaterials. Toxicol. Lett. 2020, 322, 111–119. [Google Scholar] [CrossRef]
- Sharmile, N.; Chowdhury, R.R.; Desai, S. A Comprehensive Review of Quality Control and Reliability Research in Micro–Nano Technology. Technologies 2025, 13, 94. [Google Scholar] [CrossRef]
- Khan, F.; Shariq, M.; Asif, M.; Siddiqui, M.A.; Malan, P.; Ahmad, F. Green Nanotechnology: Plant-Mediated Nanoparticle Synthesis and Application. Nanomaterials 2022, 12, 673. [Google Scholar] [CrossRef]
- Hano, C.; Abbasi, B.H. Plant-Based Green Synthesis of Nanoparticles: Production, Characterization and Applications. Biomolecules 2022, 12, 31. [Google Scholar] [CrossRef] [PubMed]
- Abuzeid, H.M.; Julien, C.M.; Zhu, L.; Hashem, A.M. Green Synthesis of Nanoparticles and Their Energy Storage, Environmental, and Biomedical Applications. Crystals 2023, 13, 1576. [Google Scholar] [CrossRef]
- Isigonis, P.; Afantitis, A.; Antunes, D.; Bartonova, A.; Beitollahi, A.; Bohmer, N.; Bouman, E.; Chaudhry, Q.; Cimpan, M.R.; Cimpan, E.; et al. Risk Governance of Emerging Technologies Demonstrated in Terms of Its Applicability to Nanomaterials. Small 2020, 16, e2003303. [Google Scholar] [CrossRef] [PubMed]
- Isigonis, P.; Bouman, E.A.; Varsou, D.D.; Jensen, K.A.; Fransman, W.; Drobne, D.; Rollon, B.P.; Ballesteros, A.; Rodríguez-Llopis, I.; Säämänen, A.; et al. A Nano Risk Governance Portal Supporting Risk Governance of Nanomaterials and Nano-Enabled Products. Comput. Struct. Biotechnol. J. 2025, 29, 177–185. [Google Scholar] [CrossRef]
- Johnston, L.J.; Du, X.; Zborowski, A.; Kennedy, D.C. Characterization and Cellular Toxicity Studies of Commercial Manganese Oxide Nanoparticles. Nanomaterials 2024, 14, 198. [Google Scholar] [CrossRef]
- Bruckmann, F.d.S.; Nunes, F.B.; Salles, T.d.R.; Franco, C.; Cadoná, F.C.; Bohn Rhoden, C.R. Biological Applications of Silica-Based Nanoparticles. Magnetochemistry 2022, 8, 131. [Google Scholar] [CrossRef]
- Hosseinpour, S.; Walsh, L.J.; Xu, C. Biomedical Application of Mesoporous Silica Nanoparticles as Delivery Systems: A Biological Safety Perspective. J. Mater. Chem. B 2020, 8, 9863–9876. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, F.; Oo, M.K.; Chatterjee, B.; Alallam, B. Biocompatible Supramolecular Mesoporous Silica Nanoparticles as the Next-Generation Drug Delivery System. Front. Pharmacol. 2022, 13, 886981. [Google Scholar] [CrossRef] [PubMed]

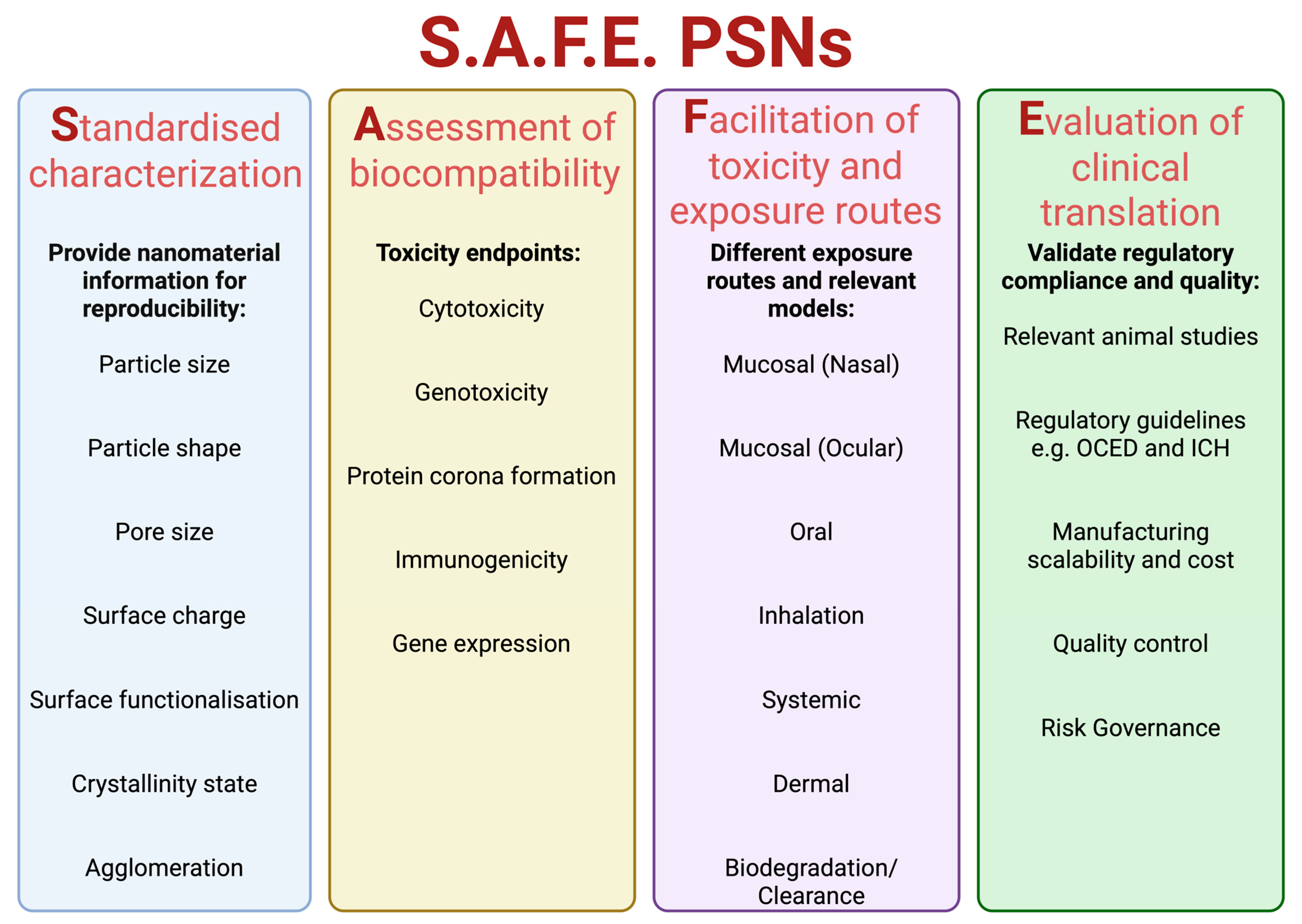
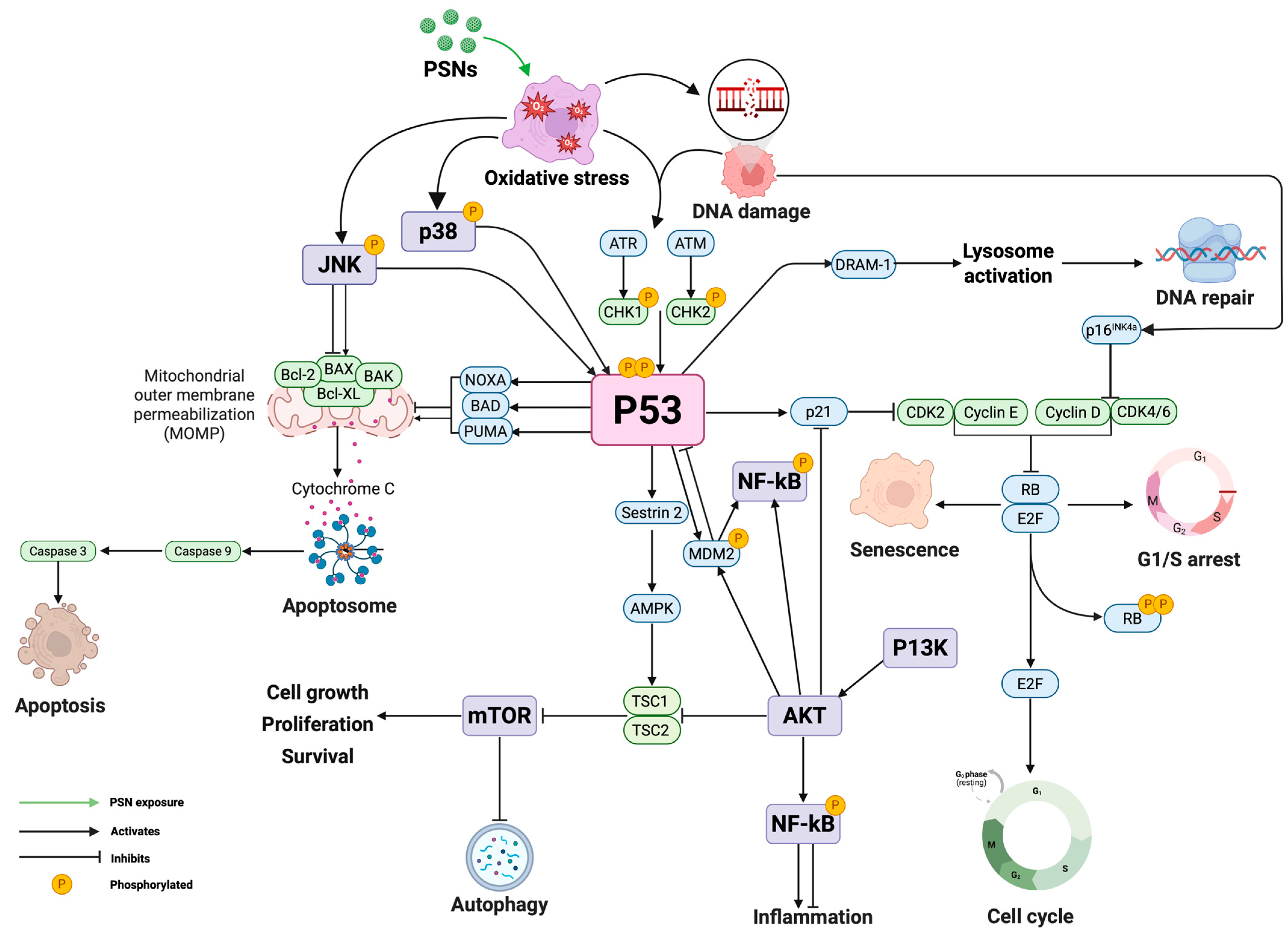

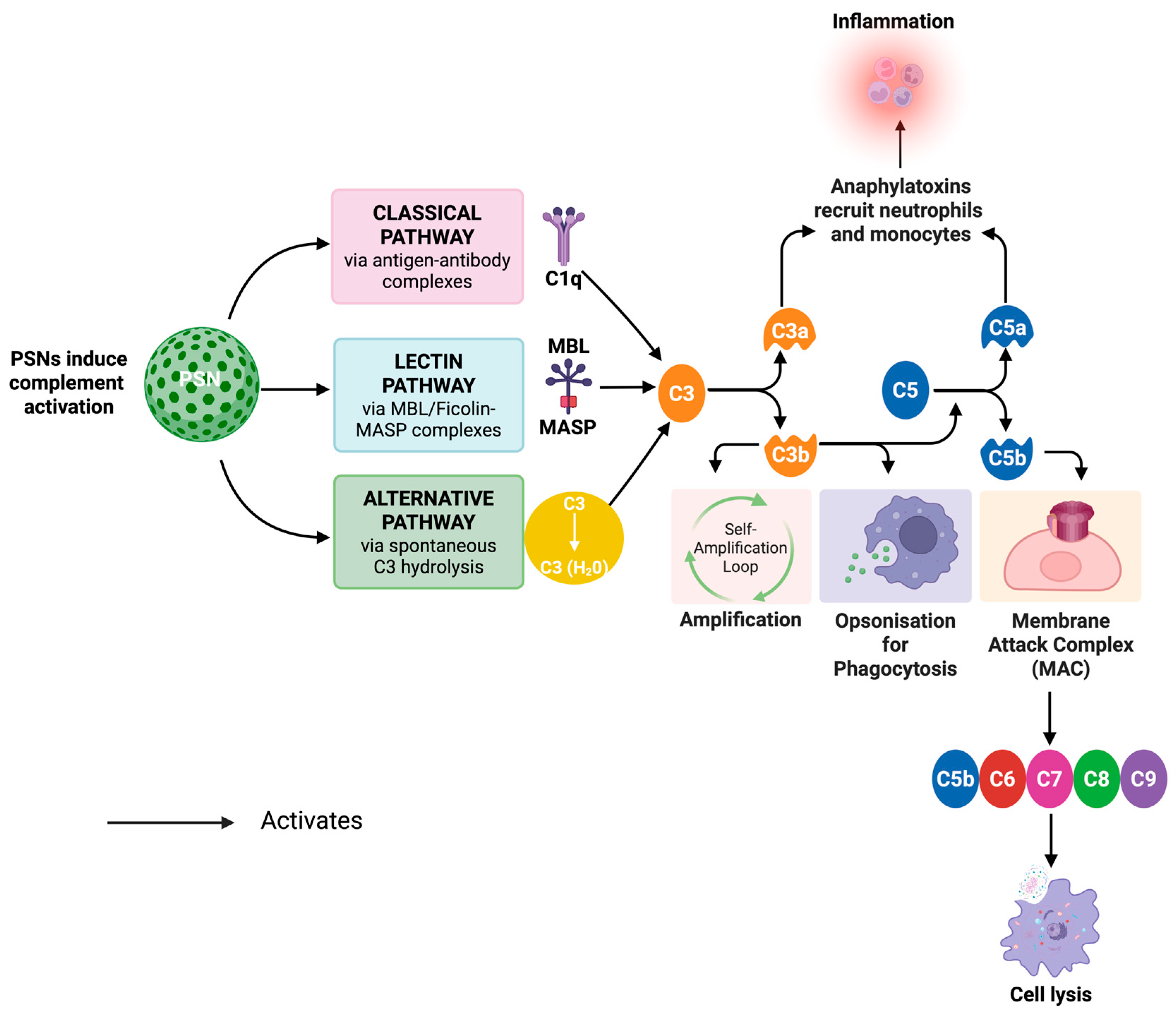
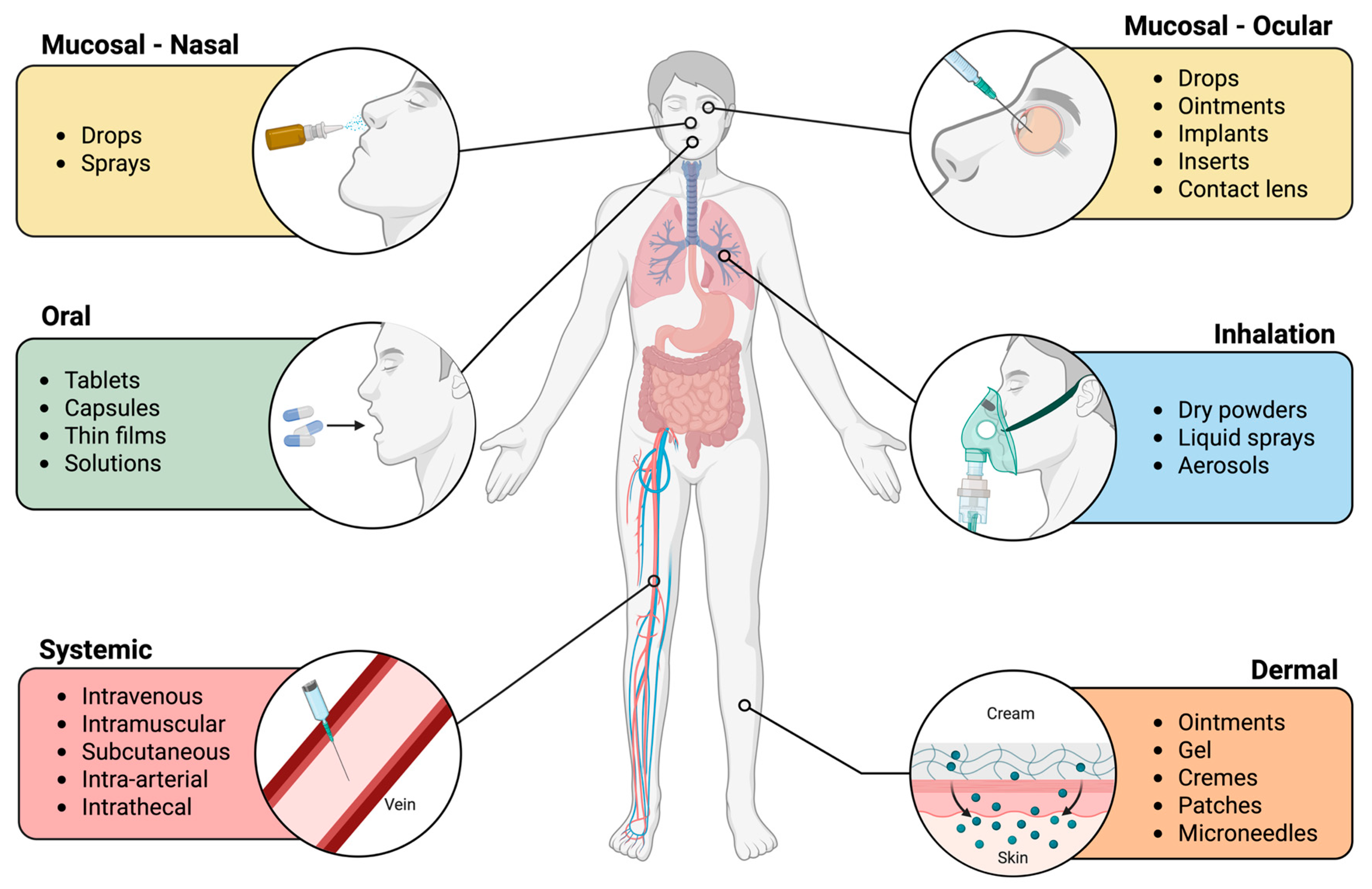
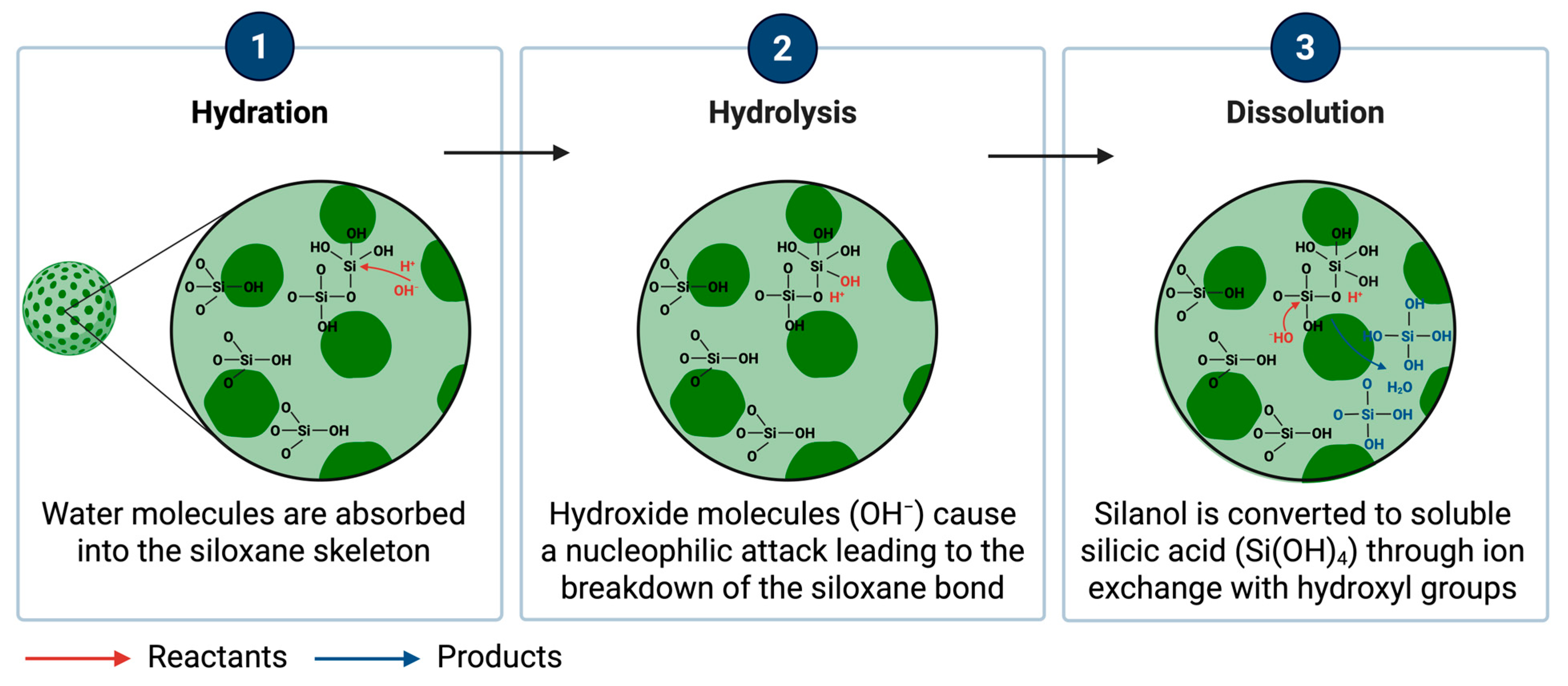

| Clinical Trial Study | Clinical Trial ID | Current Status | Study Type and Phase | Application | Purpose | Outcome |
|---|---|---|---|---|---|---|
| Safety Evaluation of Porous Silica in Men | NCT03667430 [9,10] | Completed in 2016 | Interventional and Not applicable | Weight Loss | To evaluate the safety and tolerability of oral administration of PSNs in healthy men, primarily for applications in weight management and metabolic health. | Completed: Oral intake up to 9 g/day of porous silica as a food additive was safe and well-tolerated, with only mild gastrointestinal side effects and no major adverse events reported. |
| Effect of Different NanoScaffolds on Pulp Regeneration in Non-Vital Immature Permanent Teeth | NCT07121348 [11] | Active, not recruiting | Interventional and Not applicable | Regenerative Medicine | To evaluate the clinical and radiographic outcomes of regenerative endodontic procedures in immature non-vital permanent teeth using different nanoscaffold materials, including MSNs. | Ongoing: Outcome measures include radiographic apical closure 12 months post-procedure. No published results yet. |
| Targeted Silica Nanoparticles for Real-Time Image-Guided Intraoperative Mapping of Nodal Metastases | NCT02106598 [12,13] | Active, not recruiting | Interventional and Phase 1, Phase 2 | Drug Delivery | To assess the feasibility and safety of using ultrasmall targeted core-shell silica nanoparticles for fluorescence-guided sentinel lymph node biopsy in patients with melanoma of the head and neck. | Ongoing: Nanoparticles enabled high-sensitivity lymph node mapping. No adverse events were observed. The approach was feasible and safe, offering improved intraoperative guidance. |
| STAR Study Investigating Performance and Safety of the Medical Device SiPore15TM | NCT03823027 [9,14,15] | Completed in 2019 | Interventional and Not applicable | Diabetes | To evaluate the performance and safety of engineered MSN (SiPore15TM) in reducing blood glucose (HbA1c) in overweight or obese subjects with prediabetes or newly diagnosed type 2 diabetes. | Completed: SiPore15 TM significantly reduced HbA1c without affecting body weight, showing no serious adverse effects. Demonstrating its potential in controlling glycaemia. |
| Assays | MTT Assay | LDH Assay | Trypan Blue Exclusion Assay | Caspase Activity Assay | Annexin V/PI Staining Assay | Alamar Blue Assay | Cytokinesis Block Proliferation Index and Relative Population Doubling | |
|---|---|---|---|---|---|---|---|---|
| Features | ||||||||
| Type of Cytotoxicity Measured | Metabolic Activity (Cell Viability) | Membrane Integrity (Cell Damage) | Membrane Integrity (Cell Damage) | Apoptosis (Programmed Cell Death) | Cell Death (Early Apoptosis, Late Apoptosis, Necrosis) | Metabolic Activity (Cell Viability) | Cytostasis (Reduction in growth rate) | |
| Test System | Mammalian cells e.g. human lymphocytes and human hepatocytes | Mammalian cells e.g. human lymphocytes and human hepatocytes | Mammalian cells in suspension e.g. human lymphocytes and human hepatocytes | Mammalian cells e.g. human lymphocytes and human hepatocytes | Mammalian cells in suspension e.g. human lymphocytes and human hepatocytes | Mammalian cells e.g. human lymphocytes and human hepatocytes | Mammalian cells e.g. human lymphocytes and human hepatocytes | |
| Principle | Yellow MTT tetrazolium salt is reduced to an insoluble purple formazan product by mitochondrial dehydrogenases in metabolically active cells. | LDH enzyme is released from damaged cells into the culture medium, which is measured as an indication of membrane integrity loss. | Living cells, which have an intact membrane, exclude the dye, whilst dead cells with damaged membranes take up the blue dye. | Measures the activation of caspase-3 and caspase-9, which are central drivers of apoptosis execution. | Annexin V labels early apoptosis (translocated phosphatidylserine). Propidium Iodide labels late apoptosis/necrosis (damaged membrane) | Blue resazurin is reduced to pink resorufin by metabolically active cells, producing a colourimetric or fluorescent signal. | To measure the average number of nuclear divisions a cell population has completed by scoring the frequency of cell with one, two or three nuclei after the addition of Cyto B to prevent cytokinesis. | |
| Advantages | Highly sensitive and reproducible. Measures the metabolic activity of living cells. Widely used and well-established. | Simple and quick. Detects subtle background membrane damage. | Simple, inexpensive, and rapid. Suitable for high-throughput applications. Direct visualisation of viable and non-viable cells. | Directly measures apoptosis-specific pathways. Sensitive detection of early apoptotic events. | Differentiates between early apoptosis, late apoptosis, and necrosis. Quantitative and qualitative analysis via flow cytometry. | Non-toxic and allows continuous monitoring. Does not produce insoluble products. High sensitivity; ideal for high-throughput screening. | Can analyse cytotoxicity along with genotoxicity. Provides a more accurate measure of the average number of cells than simple counting. | |
| Disadvantages | Insoluble formazan crystals require solubilisation. Metabolic activity may not always correlate with actual cell viability. | Cannot differentiate between necrosis and apoptosis. | Does not distinguish between apoptotic and necrotic cells. May overestimate dead cells due to transient membrane permeability. | Cannot detect necrosis or caspase-independent apoptosis. May miss late-stage cell death. | Requires precise flow cytometry gating. May generate false positives under non-apoptotic stress conditions. Transitional cell states may complicate interpretation (early and late apoptosis). | Metabolic activity may not directly equate to cell viability. Potential dye interactions with NPs; slower signal development. | Requires optimisation of concentration and exposure time of Cyto B. Labour intensive scoring. Only suitable for dividing cells. | |
| Relevance for PSNs/NPs | Easy and quick method for identifying NP cytotoxicity with various doses of test agents at different time points. | Assess the membrane integrity damage induced by NPs. | Quick and high-volume screening of NP cytotoxicity based on membrane damage. | It can be used to investigate the apoptotic pathways triggered by NPs and can determine the underlying mechanism of cell death when combined with ROS or ER stress markers. | Distinguishes apoptosis from necrosis and can help identify which pathway is triggered. | An alternative to the MTT assay for assessing cell viability, especially if NP interference occurs. | Applicable with sequential addition of Cyto B to ensure no interference with NP endocytosis | |
| Assays | Cytokinesis Block Micronucleus (CBMN) Assay | Comet Assay | Chromosome Aberration Test | γ-H2AX Assay | Ames Test | |
|---|---|---|---|---|---|---|
| Features | ||||||
| Type of Damage Detected | Clastogenicity (chromosome breakage) and aneugenicity (chromosome loss) | DNA single-strand breaks, double-strand breaks and oxidative lesions | Structural chromosomal changes (breaks, deletions and rearrangements) | DNA double-strand breaks | Gene mutations (point mutations). Specifically base- pair substitutions and frameshift mutations. | |
| Test System | Mammalian cells (e.g. human lymphocytes and human hepatocytes | Mammalian cells (e.g. human lymphocytes and human hepatocytes | Mammalian cells (e.g. human lymphocytes and human hepatocytes | Mammalian cells (e.g. human lymphocytes and human hepatocytes | Bacterial cells (e.g. Salmonella typhimurium and Escherichia coli) | |
| Principle | Detects chromosome breakage or loss by scoring micronuclei in binucleated cells after cytokinesis is blocked. | Measures DNA strand breakage by electrophoretic migration of DNA fragments, forming a ‘comet tail’. | Identifies structural or numerical chromosome changes in cells arrested at metaphase after exposure to test agent. | Detects DNA double-strand breaks by quantifying phosphorylated histone H2AX foci. | Assesses mutagenicity of chemicals by measuring reversion mutations in bacterial strains. | |
| Advantages | Simple and cost effective. High through-put analysis available to overcome manual scoring. | Single-cell analysis possible. Highly sensitive to low levels of DNA damage. | Structural and numerical aberrations are detected. Type of damage can be identified. | Allows early detection of DNA damage. Quantifiable using immunofluorescence or flow cytometry. Highly sensitive. | Quick and cost effective. Suitable for chemical mutagens. | |
| Disadvantages | Requires actively dividing cells. Time-consuming and subjective quantification of micronuclei. | Cannot differentiate between clastogenic and aneugenic events | Labour intensive. Less sensitive to low level or transient DNA alterations | May detect non-relevant background phosphorylation. Does not detect all types of DNA damage. | Not suitable for nanoparticle assessment due to poor nanoparticle penetration into the bacterial cells. May produce false negatives. Cannot be done on its own; needs another assay alongside. | |
| Relevance for PSNs/NPs | Measures DNA damage in daughter cells, which can help assess the long-term genomic instability from NP exposure | Useful for detecting early DNA damage by NPs as they can generate ROS or directly interact with DNA causing chromosomal damage | Detects chromosome changes caused by NPs, identifying carcinogenic and heritable effects of NP exposure | Can provide mechanistic insight into genotoxic stress pathways activated by NPs | Quick screening assay to identify mutagenicity alongside mammalian cell-based assays | |
| Category | Research Question | Current Knowledge and Gaps |
|---|---|---|
| Sample Preparation | What is the guidance for sample preparation with advanced nanomaterials which do not dissolve? Can a universal dissolution protocol be established for inter-study comparison? | Studies often use different buffers and pH. A single standardised dissolution assay/protocol could allow reproducibility across research and maintain stability. |
| Manufacturing | Can scalable, reproducible synthesis methods be developed? | Microemulsion, sol-gel and template methods exist; however, there is batch-to-batch variability. A method is needed to maintain consistent pore size, structure and surface chemistry. |
| Toxicity | Can the silica matrix of PSNs be safely excreted or metabolised? How does its stability influence degradation pathways in biological environments? | PSNs undergo biodegradation primarily through excretion via the kidneys. However, the structural stability of the silica matrix, the rate and completeness of degradation that depends on physicochemical properties and long-term bioaccumulation, remains insufficiently understood. |
| How do PSNs and their protein corona influence immunogenicity and potential immunotoxicity? | PSNs may activate the complement system or cytokine release depending on their physicochemical properties. However, comprehensive immunotoxicity studies of PSNs are limited. Standardised immune assays and long-term models are needed to clarify chronic immune effects and safety. | |
| What is the extent of biodistribution/accumulation of PSNs in major organs and tissues? | Short-term studies show accumulation in the liver, spleen and lungs with gradual clearance, but data on biodegradation rate and organ-specific accumulation after repeated dosing are limited. Comprehensive pharmacokinetic and chronic exposure studies are required. | |
| What are the long-term safety implications of PSNs in humans? | Preclinical studies show acute toxicity is low, but chronic toxicity has not been extensively studied. Standardised safety endpoints for PSNs must be established. | |
| Standardisation and Regulatory Challenges | Are regulatory and standardisation frameworks sufficient for PSN translation? How can PSNs be integrated into regulatory frameworks for medical devices or drug formulation? | Regulatory guidance exists for some advanced nanomaterials, but not specifically for PSNs. Standardised characterisation, dissolution testing, toxicity endpoints and manufacturing methods need to be established to meet clinical trial requirements. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patel, T.; Clipstone, C.; Girija, U.V.; Ahmad, Z.; Singh, N. Nanotoxicity of Porous Silica Nanoparticles: Physicochemical Properties and Mechanistic Cellular Endpoints. Nanomaterials 2025, 15, 1766. https://doi.org/10.3390/nano15231766
Patel T, Clipstone C, Girija UV, Ahmad Z, Singh N. Nanotoxicity of Porous Silica Nanoparticles: Physicochemical Properties and Mechanistic Cellular Endpoints. Nanomaterials. 2025; 15(23):1766. https://doi.org/10.3390/nano15231766
Chicago/Turabian StylePatel, Trisha, Callum Clipstone, Umakhanth Venkatraman Girija, Zeeshan Ahmad, and Neenu Singh. 2025. "Nanotoxicity of Porous Silica Nanoparticles: Physicochemical Properties and Mechanistic Cellular Endpoints" Nanomaterials 15, no. 23: 1766. https://doi.org/10.3390/nano15231766
APA StylePatel, T., Clipstone, C., Girija, U. V., Ahmad, Z., & Singh, N. (2025). Nanotoxicity of Porous Silica Nanoparticles: Physicochemical Properties and Mechanistic Cellular Endpoints. Nanomaterials, 15(23), 1766. https://doi.org/10.3390/nano15231766








