Comparative Carcinogenicity of Double-Walled Carbon Nanotubes of Different Lengths Administered by Intratracheal Installation into Rat Lungs
Abstract
1. Introduction
2. Materials and Methods
2.1. DWCNTs and MWCNT-7
2.2. CNT Suspensions
2.3. Animals
2.4. Experimental Design
2.5. Tissue Sampling and Histopathology
2.6. Electron Microscopy
2.7. CCL2, CCL3, and HO-1 ELISA
2.8. Measurement of DWCNT and MWCNT in the Lung
2.9. Statistical Analysis
3. Results
3.1. Characterization of Test CNTs
3.2. Lung Lesions at Six Weeks (Table 2)
3.3. Survival Time
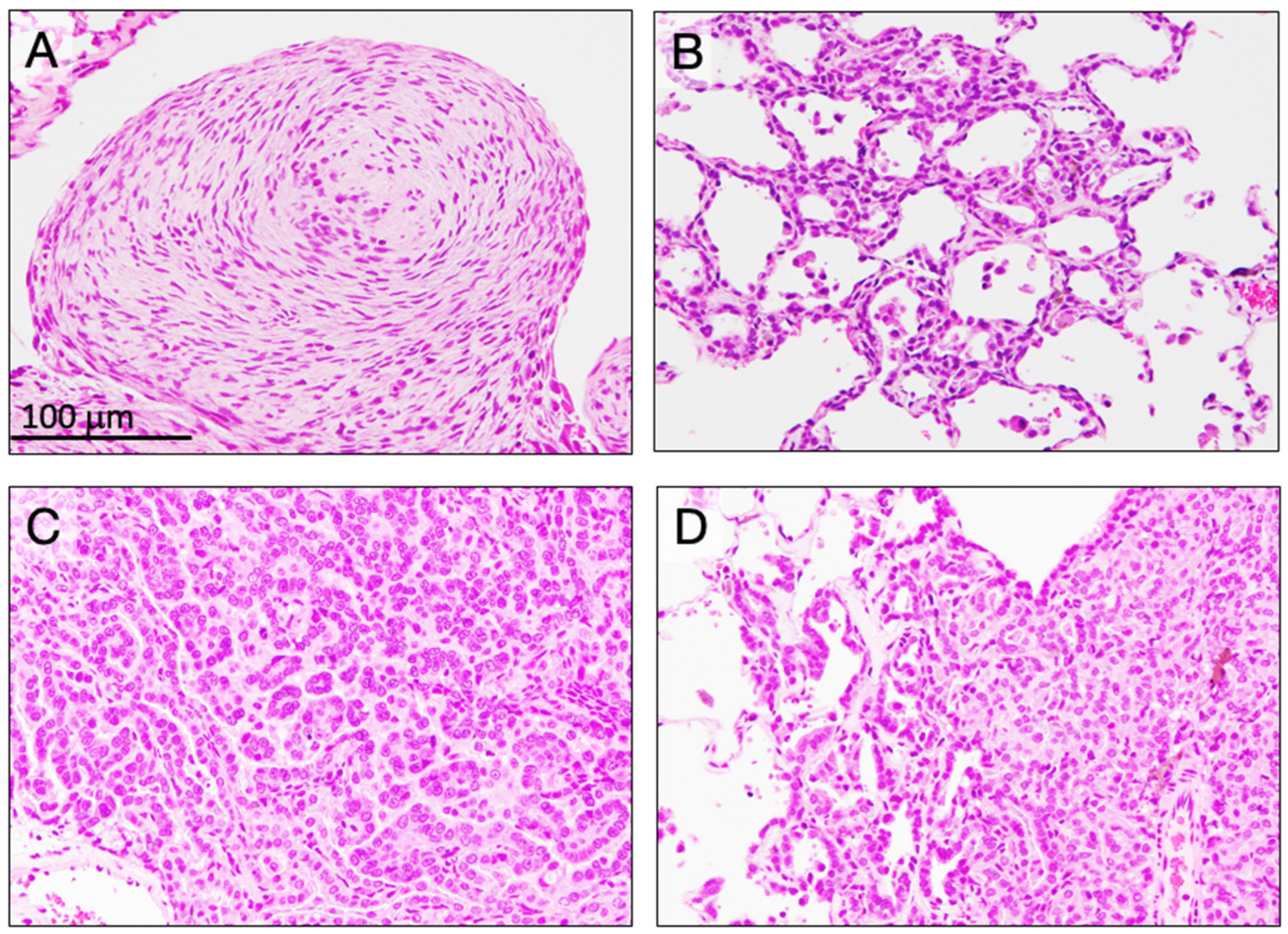
3.4. Incidence of Mesothelioma (Table 3)
3.5. Incidence of Preneoplastic and Neoplastic Lesions in the Lung (Table 4)
3.6. Fiber-Induced Toxicity Not Related to Pleural and Lung Toxicities
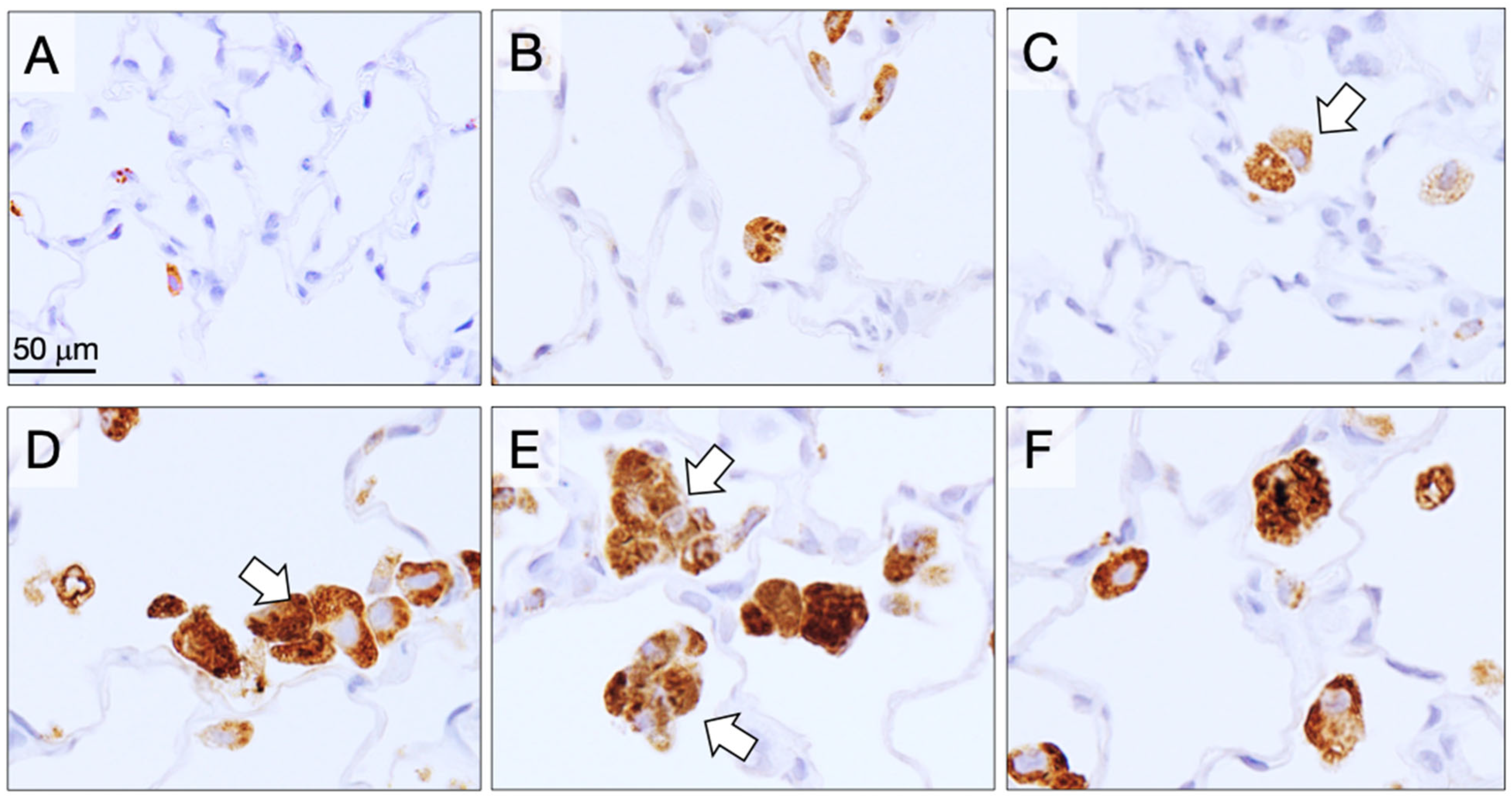
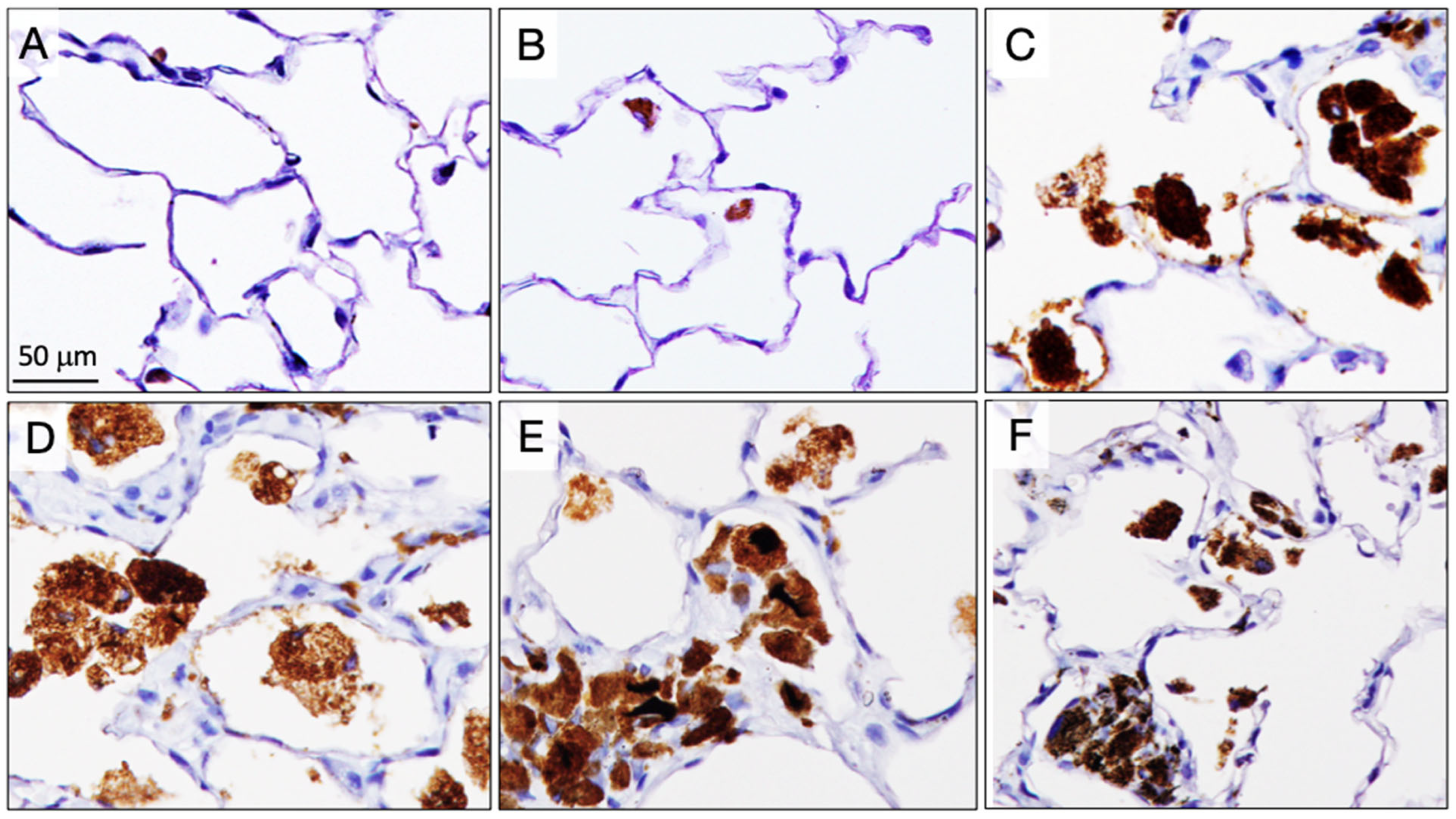
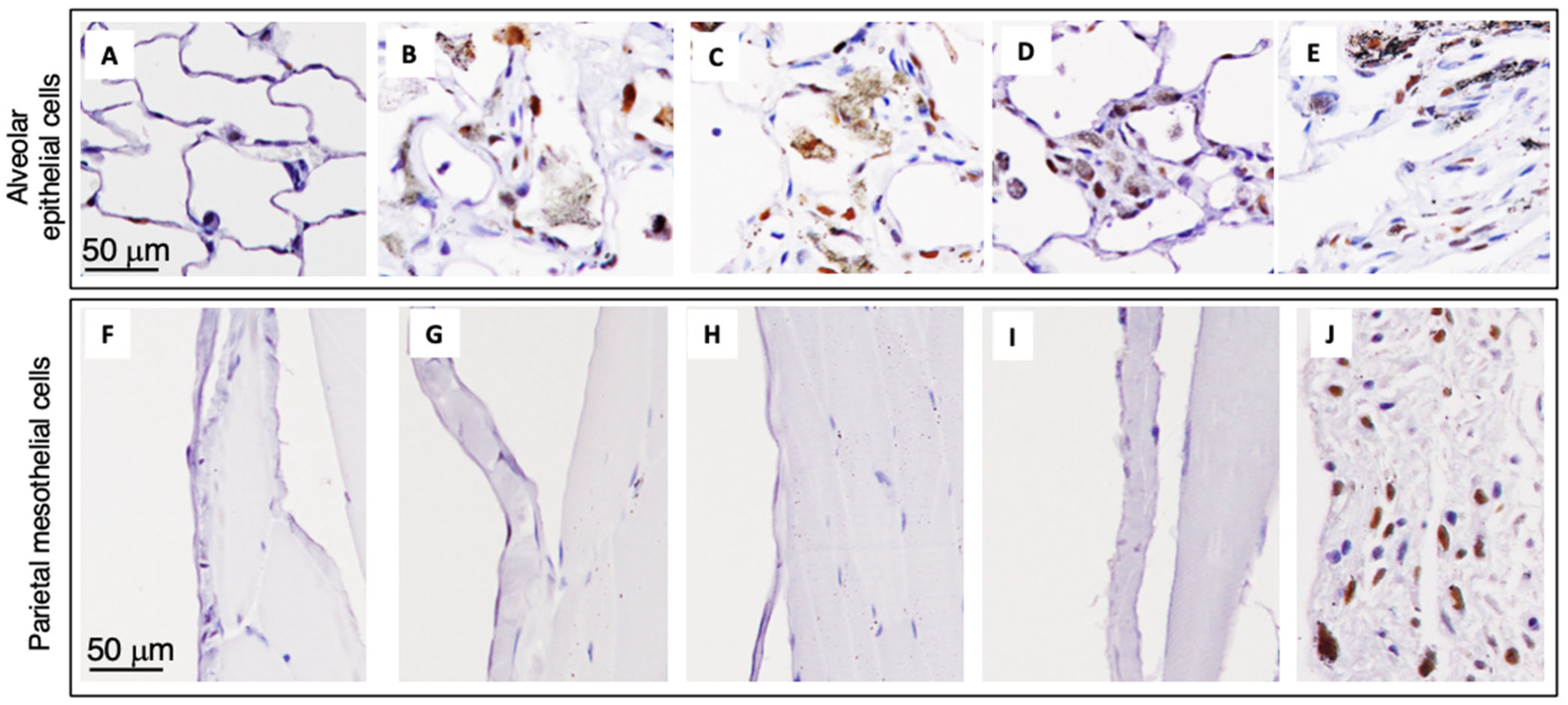
3.7. Alveolar Granulomas, CD68-Positive Macrophages, and PCNA-Positive Lung Alveolar Epithelial and Pleural Cell Counts at the Final Sacrifice (Table 5)
3.8. CCL2, CCL3, and HO-1 in Non-Tumor Lung Tissue (Table 6)
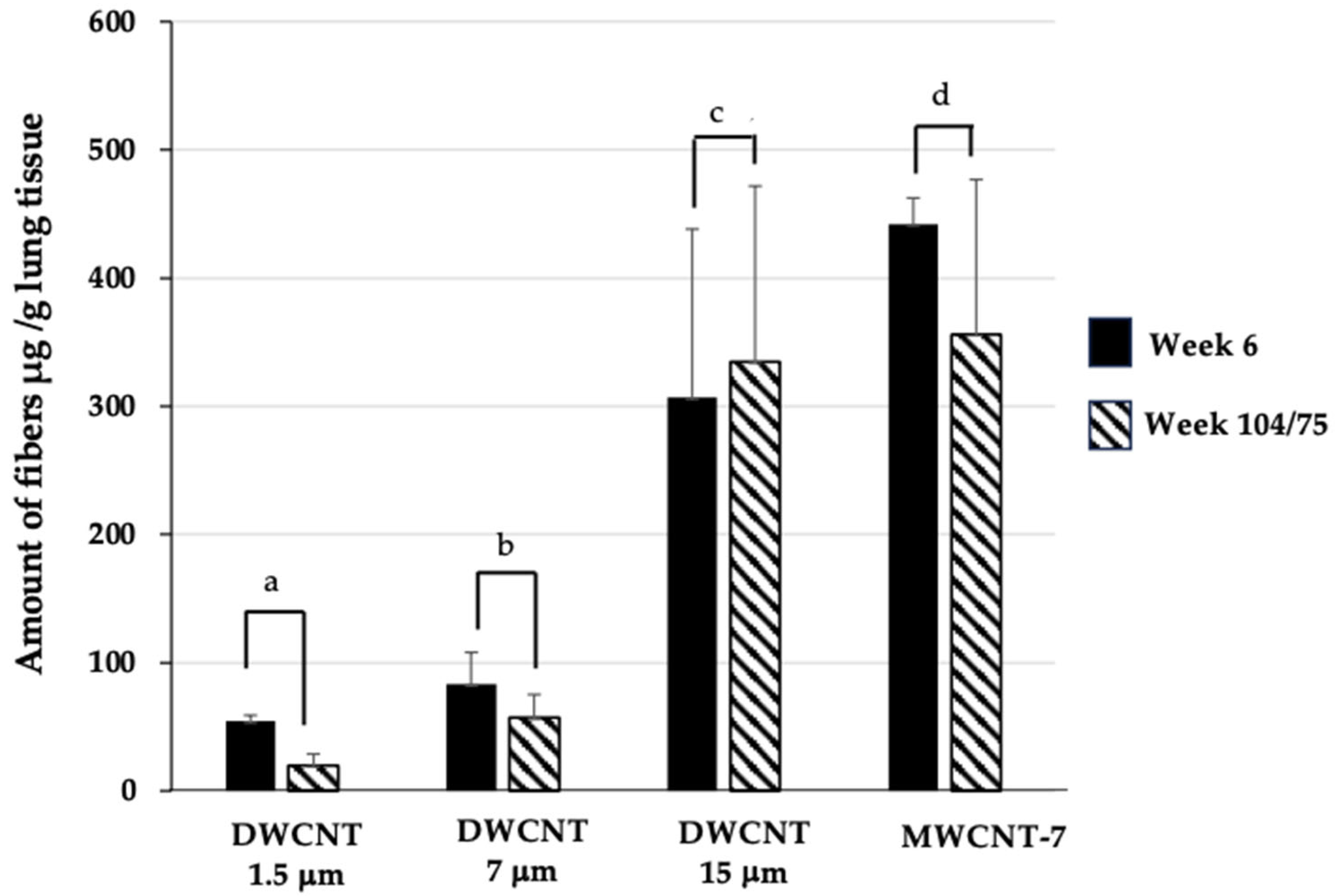
3.9. Fiber Retention in the Lungs
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DWCNT | Double-walled carbon nanotube |
| MWCNT | Multi-walled carbon nanotube |
| TIPS | Trans-tracheal intra-pulmonary spraying |
| CNT | Carbon nanotube |
| HMGB1 | High mobility group box protein 1 |
| PCNA | Proliferating cell nuclear antigen |
| SEM | Scanning electron microscope |
| TEM | Transmission electron microscope |
References
- Sargent, L.M.; Porter, D.W.; Staska, L.M.; Hubbs, A.F.; Lowry, D.T.; Battelli, L.; Siegrist, K.J.; Kashon, M.L.; Mercer, R.R.; Bauer, A.K.; et al. Promotion of lung adenocarcinoma following inhalation exposure to multi-walled carbon nanotubes. Part. Fibre Toxicol. 2014, 11, 3. [Google Scholar] [CrossRef]
- Fujita, K.; Fukuda, M.; Fukui, H.; Horie, M.; Endoh, S.; Uchida, K.; Shichiri, M.; Morimoto, Y.; Ogami, A.; Iwahashi, H. Intratracheal instillation of single-wall carbon nanotubes in the rat lung induces time-dependent changes in gene expression. Nanotoxicology 2015, 9, 290–301. [Google Scholar] [CrossRef] [PubMed]
- Suzui, M.; Futakuchi, M.; Fukamachi, K.; Numano, T.; Abdelgied, M.; Takahashi, S.; Ohnishi, M.; Omori, T.; Tsuruoka, S.; Hirose, A.; et al. Multiwalled carbon nanotubes intratracheally instilled into the rat lung induce development of pleural malignant mesothelioma and lung tumors. Cancer Sci. 2016, 107, 924–935. [Google Scholar] [CrossRef] [PubMed]
- Kasai, T.; Umeda, Y.; Ohnishi, M.; Mine, T.; Kondo, H.; Takeuchi, T.; Matsumoto, M.; Fukushima, S. Lung carcinogenicity of inhaled multi-walled carbon nanotube in rats. Part. Fibre Toxicol. 2016, 13, 53. [Google Scholar] [CrossRef]
- Honda, K.; Naya, M.; Takehara, H.; Kataura, H.; Fujita, K.; Ema, M. A 104-week pulmonary toxicity assessment of long and short single-wall carbon nanotubes after a single intratracheal instillation in rats. Inhal. Toxicol. 2017, 29, 471–482. [Google Scholar] [CrossRef]
- Numano, T.; Higuchi, H.; Alexander, D.B.; Alexander, W.T.; Abdelgied, M.; El-Gazzar, A.M.; Saleh, D.; Takase, H.; Hirose, A.; Naiki-Ito, A.; et al. MWCNT-7 administered to the lung by intratracheal instillation induces development of pleural mesothelioma in F344 rats. Cancer Sci. 2019, 110, 2485–2492. [Google Scholar] [CrossRef] [PubMed]
- Saleh, D.M.; Alexander, W.T.; Numano, T.; Ahmed, O.H.M.; Gunasekaran, S.; Alexander, D.B.; Abdelgied, M.; El-Gazzar, A.M.; Takase, H.; Xu, J.; et al. Comparative carcinogenicity study of a thick, straight-type and a thin, tangled-type multi-walled carbon nanotube administered by intra-tracheal instillation in the rat. Part. Fibre Toxicol. 2020, 17, 48. [Google Scholar] [CrossRef]
- Hojo, M.; Maeno, A.; Sakamoto, Y.; Ohnuki, A.; Tada, Y.; Yamamoto, Y.; Ikushima, K.; Inaba, R.; Suzuki, J.; Taquahashi, Y.; et al. Two-year intermittent exposure of a multiwalled carbon nanotube by intratracheal instillation induces lung tumors and pleural mesotheliomas in F344 rats. Part. Fibre Toxicol. 2022, 19, 38. [Google Scholar] [CrossRef]
- Saleh, D.M.; Luo, S.; Ahmed, O.H.M.; Alexander, D.B.; Alexander, W.T.; Gunasekaran, S.; El-Gazzar, A.M.; Abdelgied, M.; Numano, T.; Takase, H.; et al. Assessment of the toxicity and carcinogenicity of double-walled carbon nanotubes in the rat lung after intratracheal instillation: A two-year study. Part. Fibre Toxicol. 2022, 19, 30. [Google Scholar] [CrossRef]
- Sheema, A.N.; Naiki-Ito, A.; Kakehashi, A.; Ahmed, O.H.M.; Alexander, D.B.; Alexander, W.T.; Numano, T.; Kato, H.; Goto, Y.; Takase, H.; et al. Fullerene and fullerene whisker are not carcinogenic to the lungs and pleura in rat long-term study after 2-week intra-tracheal intrapulmonary administration. Arch. Toxicol. 2024, 98, 4143–4158. [Google Scholar] [CrossRef]
- Ahmed, O.H.M.; Naiki-Ito, A.; Takahashi, S.; Alexander, W.T.; Alexander, D.B.; Tsuda, H. A Review of the Carcinogenic Potential of Thick Rigid and Thin Flexible Multi-Walled Carbon Nanotubes in the Lung. Nanomaterials 2025, 15, 168. [Google Scholar] [CrossRef]
- Qi, F.; Okimoto, G.; Jube, S.; Napolitano, A.; Pass, H.I.; Laczko, R.; Demay, R.M.; Khan, G.; Tiirikainen, M.; Rinaudo, C.; et al. Continuous exposure to chrysotile asbestos can cause transformation of human mesothelial cells via HMGB1 and TNF-alpha signaling. Am. J. Pathol. 2013, 183, 1654–1666. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Rivera, Z.; Jube, S.; Nasu, M.; Bertino, P.; Goparaju, C.; Franzoso, G.; Lotze, M.T.; Krausz, T.; Pass, H.I.; et al. Programmed necrosis induced by asbestos in human mesothelial cells causes high-mobility group box 1 protein release and resultant inflammation. Proc. Natl. Acad. Sci. USA 2010, 107, 12611–12616. [Google Scholar] [CrossRef]
- Xue, J.; Patergnani, S.; Giorgi, C.; Suarez, J.; Goto, K.; Bononi, A.; Tanji, M.; Novelli, F.; Pastorino, S.; Xu, R.; et al. Asbestos induces mesothelial cell transformation via HMGB1-driven autophagy. Proc. Natl. Acad. Sci. USA 2020, 117, 25543–25552. [Google Scholar] [CrossRef]
- Nagai, H.; Okazaki, Y.; Chew, S.H.; Misawa, N.; Yamashita, Y.; Akatsuka, S.; Ishihara, T.; Yamashita, K.; Yoshikawa, Y.; Yasui, H.; et al. Diameter and rigidity of multiwalled carbon nanotubes are critical factors in mesothelial injury and carcinogenesis. Proc. Natl. Acad. Sci. USA 2011, 108, E1330–E1338. [Google Scholar] [CrossRef]
- Sakamoto, Y.; Hojo, M.; Kosugi, Y.; Watanabe, K.; Hirose, A.; Inomata, A.; Suzuki, T.; Nakae, D. Comparative study for carcinogenicity of 7 different multi-wall carbon nanotubes with different physicochemical characteristics by a single intraperitoneal injection in male Fischer 344 rats. J. Toxicol. Sci. 2018, 43, 587–600. [Google Scholar] [CrossRef]
- Renne, R.; Brix, A.; Harkema, J.; Herbert, R.; Kittel, B.; Lewis, D.; March, T.; Nagano, K.; Pino, M.; Rittinghausen, S.; et al. Proliferative and Nonproliferative Lesions of the Rat and Mouse Respiratory Tract. Toxicol. Pathol. 2009, 37, 5S–73S. [Google Scholar] [CrossRef] [PubMed]
- Ohnishi, M.; Yajima, H.; Kasai, T.; Umeda, Y.; Yamamoto, M.; Yamamoto, S.; Okuda, H.; Suzuki, M.; Nishizawa, T.; Fukushima, S. Novel method using hybrid markers: Development of an approach for pulmonary measurement of multi-walled carbon nanotubes. J. Occup. Med. Toxicol. 2013, 8, 30. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Walter, D. Primary particles–agglomerates–aggregates. Nanomaterials 2013, 20, 9–24. [Google Scholar][Green Version]
- Williams, K.; Schwartz, A.; Corey, S.; Orandle, M.; Kennedy, W.; Thompson, B.; Alvarez, X.; Brown, C.; Gartner, S.; Lackner, A. Proliferating cellular nuclear antigen expression as a marker of perivascular macrophages in simian immunodeficiency virus encephalitis. Am. J. Pathol. 2002, 161, 575–585. [Google Scholar] [CrossRef][Green Version]
- Campbell, N.K.; Fitzgerald, H.K.; Dunne, A. Regulation of inflammation by the antioxidant haem oxygenase 1. Nat. Rev. Immunol. 2021, 21, 411–425. [Google Scholar] [CrossRef] [PubMed]
- Tokarz, D.A.; Gruebbel, M.M.; Willson, G.A.; Hardisty, J.F.; Pearse, G.; Cesta, M.F. Spontaneous Primary Pleural Mesothelioma in Fischer 344 (F344) and Other Rat Strains: A Retrospective Review. Toxicol. Pathol. 2022, 50, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Rittinghausen, S.; Hackbarth, A.; Creutzenberg, O.; Ernst, H.; Heinrich, U.; Leonhardt, A.; Schaudien, D. The carcinogenic effect of various multi-walled carbon nanotubes (MWCNTs) after intraperitoneal injection in rats. Part. Fibre Toxicol. 2014, 11, 59. [Google Scholar] [CrossRef]
- Jube, S.; Rivera, Z.S.; Bianchi, M.E.; Powers, A.; Wang, E.; Pagano, I.; Pass, H.I.; Gaudino, G.; Carbone, M.; Yang, H. Cancer cell secretion of the DAMP protein HMGB1 supports progression in malignant mesothelioma. Cancer Res. 2012, 72, 3290–3301. [Google Scholar] [CrossRef]
- Wang, Y.; Jiang, Z.; Yan, J.; Ying, S. HMGB1 as a Potential Biomarker and Therapeutic Target for Malignant Mesothelioma. Dis. Markers 2019, 2019, 4183157. [Google Scholar] [CrossRef]
- Sweeney, S.; Berhanu, D.; Misra, S.K.; Thorley, A.J.; Valsami-Jones, E.; Tetley, T.D. Multi-walled carbon nanotube length as a critical determinant of bioreactivity with primary human pulmonary alveolar cells. Carbon. N. Y. 2014, 78, 26–37. [Google Scholar] [CrossRef]
- Sweeney, S.; Grandolfo, D.; Ruenraroengsak, P.; Tetley, T.D. Functional consequences for primary human alveolar macrophages following treatment with long, but not short, multiwalled carbon nanotubes. Int. J. Nanomed. 2015, 10, 3115–3129. [Google Scholar] [CrossRef] [PubMed]
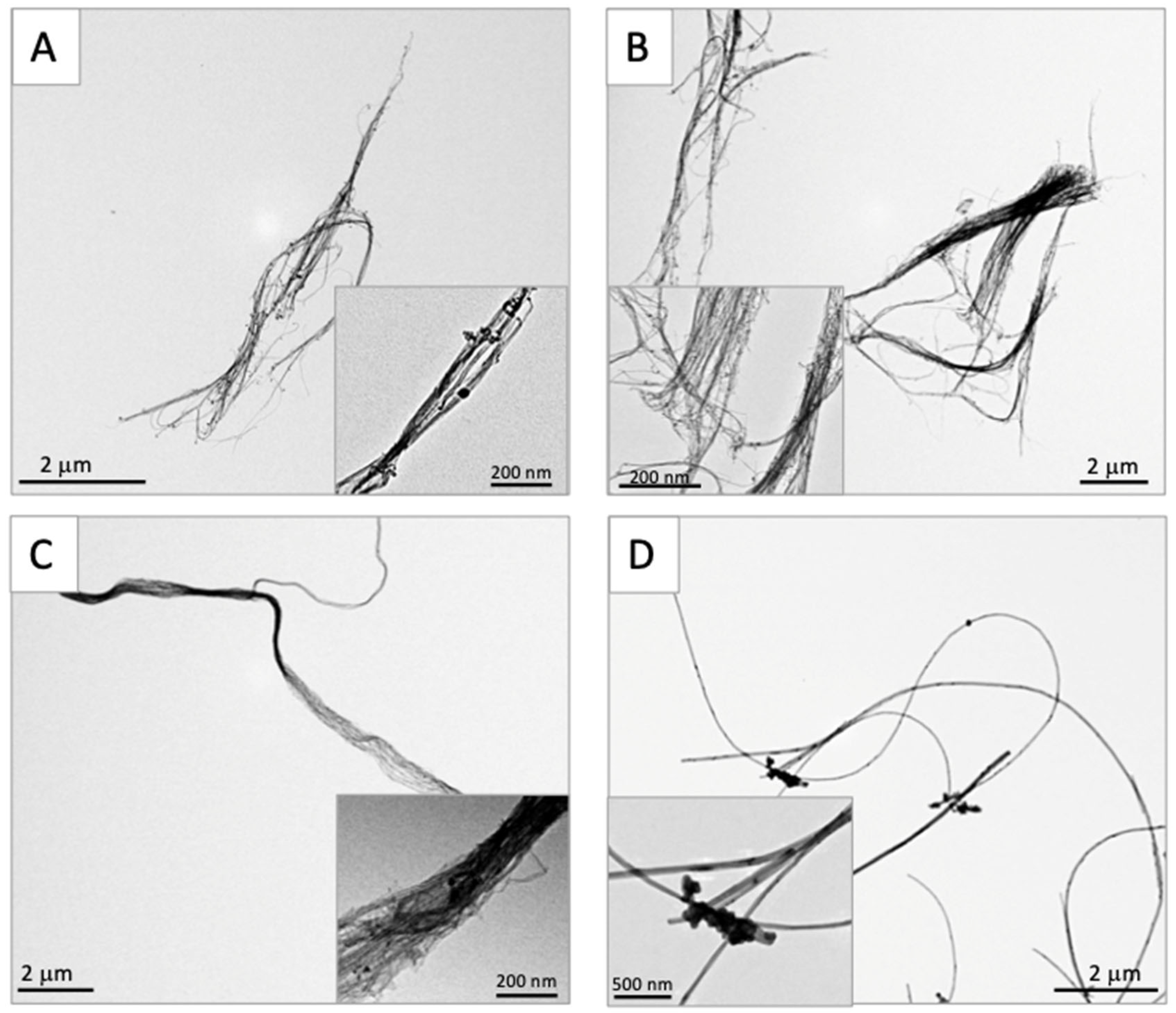



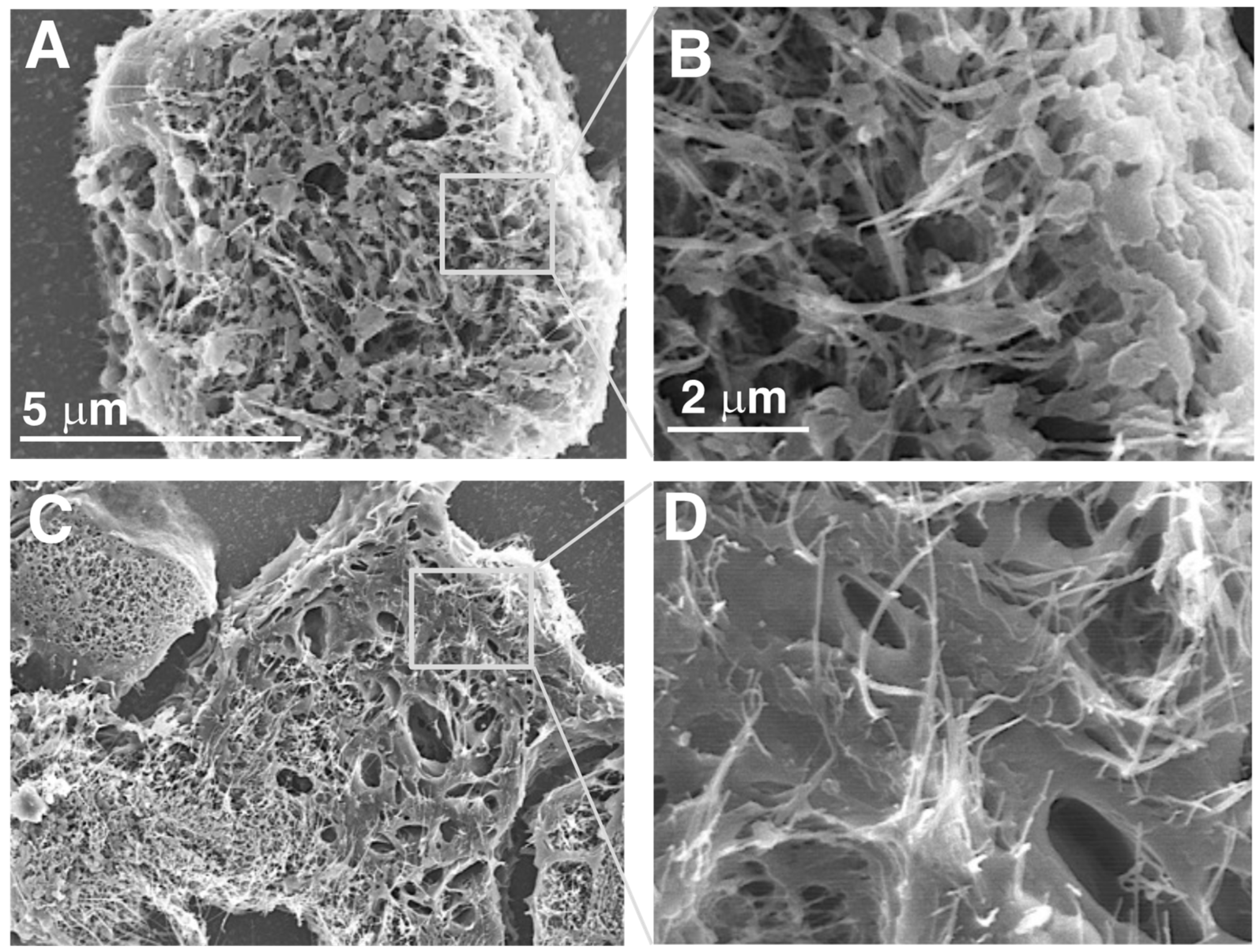

| DWCNT 1.5 μm | DWCNT 7 μm | DWCNT 15 μm | MWCNT-7 | |
|---|---|---|---|---|
| Length of the agglomerates | 4.5 ± 2.2 μm | 6.8 ± 8.2 μm | 10.2 ± 5.8 μm | 8.5 ± 4.5 μm |
| Diameter of the agglomerates | 143.7 ± 120.1 nm | 268.2 ± 416.1 nm | 227.6 ± 172.5 nm | 63.9 ± 20.0 nm |
| Group | Group 1 | Group 2 | Group 3 | Group 4 | Group 5 | Group 6 | |
|---|---|---|---|---|---|---|---|
| Tissue Parameter | Untreated | Vehicle | DWCNT | MWCNT-7 (0.5 #) | |||
| 1.5 μm (0.050 #) | 7 μm (0.232 #) | 15 μm (0.504 #) | |||||
| Granuloma count/cm2 Mean ± SD | 0 ± 0 | 0.6 ± 0.5 | 12.1 ± 9.5 *** | 50.5 ± 5.8 ***a | 194.7 ± 36.2 ***b | 49.8 ± 15.6 ***c | |
| Macrophage cell & count/cm2 Mean ± SD | 26.6 ± 19.4 | 124.3 ± 20.5 | 351.3 ± 26.6 *** | 313.6 ± 15.5 *** | 388.6 ± 29.3 ***d | 468.6 ± 15.3 ***e | |
| Alveolar epithelial cell PCNA index % | 1.24 ± 0.55 | 0.93 ± 0.25 | 1.96 ± 0.23 | 3.73 ± 2.19 * | 8.53 ± 3.63 *** | 3.74 ± 0.79 *** | |
| Group 1 | Group 2 | Group 3 | Group 4 | Group 5 | Group 6 | |
|---|---|---|---|---|---|---|
| Untreated | Vehicle | DWCNT | MWCNT-7 | |||
| 1.5 μm (0.050 #) | 7 μm (0.232 #) | 15 μm (0.504 #) | ||||
| No. of rats examined | 13 | 12 | 13 | 8 | 11 | 15 |
| Mean survival time (weeks) | 99.1 ± 10.1 | 101 ± 5.9 | 102 ± 7.9 | 104 | 100 ± 8.7 | 74.9 ± 10.3 |
| Pleural mesothelioma | 0 | 0 | 0 | 0 | 1 | 12 *** |
| Group 1 | Group 2 | Group 3 | Group 4 | Group 5 | |
|---|---|---|---|---|---|
| Untreated | Vehicle | DWCNT | |||
| 1.5 μm (0.050 #) | 7 μm (0.232 #) | 15 μm (0.504 #) | |||
| No. of rats examined | 13 | 12 | 12 | 8 | 10 |
| Mean survival time (weeks) | 99.1 ± 10.1 | 101 ± 5.9 | 102 ± 7.9 | 104 | 100 ± 8.7 |
| Bronchioloalveolar lesions Hyperplasia (BAH) | 0 | 0 | 0 | 5 ** | 5 ** |
| Adenoma (BAA) | 0 | 0 | 0 | 0 | 0 |
| Carcinoma (BAC) | 0 | 0 | 4 * | 3 * | 2 |
| Group | Group 1 | Group 2 | Group 3 | Group 4 | Group 5 | Group 6 | |
|---|---|---|---|---|---|---|---|
| Tissue Parameter | Untreated | Vehicle | DWCNT | MWCNT-7 | |||
| 1.5 μm (0.050 #) | 7 μm (0.232 #) | 15 μm (0.504 #) | |||||
| Granuloma count/cm2 Mean ± SD | 1.0 ± 0.8 | 1.0 ± 0.8 | 43.7 ± 14.4 *** | 133.7 ± 46.3 ***a | 188.5 ± 71.2 ***b | 83.8 ± 42.0 *** | |
| Macrophage cell & count/cm2 Mean ± SD | 78.5 ± 15.1 | 87.2 ± 16.2 | 250.4 ± 48.8 *** | 323.6 ± 36.5 ***c | 427.6 ± 96.8 ***d | 224.4 ± 82.3 ***e | |
| Alveolar epithelial cell PCNA index % | 2.1 ± 0.1 | 1.9 ± 0.6 | 12.4 ± 4.5 ** | 18.7 ± 4.2 ** | 29.2 ± 9.8 ***f | 24.5 ± 14.1 *** | |
| Pleural mesothelial cell PCNA index % | 0.4 ± 0.4 | 0.5 ± 0.2 | 0.8 ± 0.5 | 1.0 ± 0.6 | 0.9 ± 0.6 | 37.4 ± 12.5 ***g | |
| Group 1 | Group 2 | Group 3 | Group 4 | Group 5 | Group 6 | |
|---|---|---|---|---|---|---|
| Untreated | Vehicle | DWCNT | MWCNT-7 | |||
| 1.5 μm (0.050 #) | 7 μm (0.232 #) | 15 μm (0.504 #) | ||||
| CCL2 pg/mg protein lung tissue extract Mean ± SD | 0.06 ± 0.01 | 0.07 ± 0.01 | 0.09 ± 0.03 | 0.13 ± 0.04 ** | 0.21 ± 0.04 *** | 0.10 ± 0.03 |
| CCL3 pg/mg protein lung tissue extract Mean ± SD | 0.24 ± 0.05 | 0.21 ± 0.05 | 0.22 ± 0.03 | 0.21 ± 0.02 | 0.14 ± 0.03 | 0.15 ± 0.02 |
| HO-1 pg/mg protein lung tissue extract Mean ± SD | 0.25 ± 0.22 | 0.194 ± 0.11 | 0.17 ± 0.06 | 0.44 ± 0.03 * | 0.62 ± 0.27 * | 0.11 ± 0.06 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmed, O.H.M.; Saleh, D.M.; Alexander, W.T.; Takase, H.; Taquahashi, Y.; Hojo, M.; Maeno, A.; Fukamachi, K.; Gi, M.; Hirose, A.; et al. Comparative Carcinogenicity of Double-Walled Carbon Nanotubes of Different Lengths Administered by Intratracheal Installation into Rat Lungs. Nanomaterials 2025, 15, 1402. https://doi.org/10.3390/nano15181402
Ahmed OHM, Saleh DM, Alexander WT, Takase H, Taquahashi Y, Hojo M, Maeno A, Fukamachi K, Gi M, Hirose A, et al. Comparative Carcinogenicity of Double-Walled Carbon Nanotubes of Different Lengths Administered by Intratracheal Installation into Rat Lungs. Nanomaterials. 2025; 15(18):1402. https://doi.org/10.3390/nano15181402
Chicago/Turabian StyleAhmed, Omnia Hosny Mohamed, Dina Mourad Saleh, William T. Alexander, Hiroshi Takase, Yuhji Taquahashi, Motoki Hojo, Ai Maeno, Katsumi Fukamachi, Min Gi, Akihiko Hirose, and et al. 2025. "Comparative Carcinogenicity of Double-Walled Carbon Nanotubes of Different Lengths Administered by Intratracheal Installation into Rat Lungs" Nanomaterials 15, no. 18: 1402. https://doi.org/10.3390/nano15181402
APA StyleAhmed, O. H. M., Saleh, D. M., Alexander, W. T., Takase, H., Taquahashi, Y., Hojo, M., Maeno, A., Fukamachi, K., Gi, M., Hirose, A., Tsuruoka, S., Takahashi, S., Tsuda, H., & Naiki-Ito, A. (2025). Comparative Carcinogenicity of Double-Walled Carbon Nanotubes of Different Lengths Administered by Intratracheal Installation into Rat Lungs. Nanomaterials, 15(18), 1402. https://doi.org/10.3390/nano15181402







