The Ubiquitous Use of Polyethylene Glycol in Pharmaceutical Design and Development: Technological Aspects and Future Perspectives
Abstract
1. Introduction
- Liposomes: Vesicular structures made of lipids with hydrophobic tails on the inside and hydrophilic heads on the outside, which allows them to capture both hydrophobic and hydrophilic APIs inside their aqueous core [8]. PEG manages to bind onto liposomes by forming PEG–lipid conjugates that get integrated into the liposome bilayer using linkages like amide bonds [9,10].
- Micelles: Spherical or non-spherical structures made of amphiphilic low- or high-molecular-mass molecules with hydrophilic heads and hydrophobic tails. Their nonpolar interior allows them to entrap only hydrophobic APIs [11]. PEG is successfully attached to micelles through amphiphilic block copolymers and surfactants via covalent bonds or lipid-based conjugation [12,13].
- Polymersomes: A class of artificial vesicles formed from synthetic amphiphilic block copolymers. They are able to enclose hydrophilic APIs inside their aqueous core, whereas hydrophobic APIs are enclosed inside the hydrophobic block copolymer’s outer bilayers [14]. PEG requires covalent conjugation to amphiphilic block copolymers during synthesis in order to form morphologically strong structures [15,16].
- Inorganic nanoparticles: A wide variety of nanomaterials that induce conjugation with both hydrophilic and hydrophobic APIs through chemical covalent bonds [17]. PEG utilizes its electrostatic stability to connect with inorganic nanoparticles. The type of bond depends on the type of material used and is correlated to the nanoparticle’s surface topography [18,19].
- Niosomes: Non-ionic surfactants and neutrally charged lipid components. They possess the ability to entrap both hydrophilic and hydrophobic APIs inside their core structures [20]. PEG gets embedded into the niosome bilayer during the formation of the latter via hydrophilic interactions with the surfactants [21,22].
2. Methods
3. Results and Discussion
3.1. Solid Dosage Forms—Tablets
3.1.1. Self-Nanoemulsifying Tablets
3.1.2. Orodispersible Tablets
3.1.3. Tablets with Fast Dissolution
3.1.4. Coated Tablets
3.1.5. Osmotic Pump Tablets
3.1.6. Liquisolid Compact Tablets
3.1.7. Formulations with Modified Release
3.1.8. Fused Deposition Method
3.1.9. Tablets Formulated with 3D-Printing Technology
3.1.10. Other Examples of Tablets
3.2. Solid Dosage Forms—Capsules
3.2.1. Capsules with Special Characteristics
3.2.2. Targeted Administration
3.2.3. Coated Enteric Capsules
3.2.4. Solid Dispersions
3.3. Nanocarriers
3.3.1. Liposomes
3.3.2. Micelles
3.3.3. Polymersomes
3.3.4. Inorganic Nanoparticles
3.3.5. Niosomes
3.3.6. Nanoparticles
4. Overview of PEG Functionality
5. Conclusions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| PEG | Polyethylene Glycol |
| API | Active pharmaceutical ingredient |
| PRISMA | Preferred Reporting Items for Systematic Reviews |
| Cmax | Maximum Concentration |
| AUC | Area Under Curve |
| DCT | Directly compressed tablets |
| 3D | Three-dimensional |
| DSC | Differential Scanning Calorimetry |
| Type 2DM | Type 2 Diabetes Mellitus |
| PVP | Polyvinylpyrrolidone |
| HPMC | Hydroxypropyl Methylcellulose |
| PVA | Polyvinyl Alcohol |
| Tmax | Time to Maximum Concentration |
| P-gp | P-glycoprotein |
| PEG-DA | Polyethylene glycol diacrylate |
| UV | Ultraviolet radiation |
| HIV | Human Immunodeficiency Virus |
| Tg | Glass Transition Temperature |
| MW | Molecular weight |
| DTZ | Diltiazem Hydrochloride |
| PEO | Polyethylene Oxide |
| NMR | Nuclear Magnetic Resonance |
| GIT | Gastrointestinal tract |
| POL188/P188 | Poloxamer 188 |
| ALA | Alpha Lipoic Acid |
| mRNA | Messenger Ribonucleic Acid |
| PLA | Polylactic Acid |
| SO2 | Sulfur Dioxide |
| SN-38 | 7-Ethyl-10-hydroxycamptothecin |
| PCL | Polycaprolactone |
| PLGA | Poly(lactic-co-glycolic acid) |
| ABZ-SO | Albendazole Sulfoxide |
| DSPE | 1,2-Distearoyl-sn-glycero-3-phosphoethanolamine |
| DPPC | Dipalmitoylphosphatidylcholine |
| PDI | Polydispersity index |
| DMF | Dimethyl Formamide |
| IC50 | Half-maximal Inhibitory Concentration |
| PdLLA | Poly-D,L-Lactic Acid |
| NP | Nanoparticle |
| Span 60 | Sorbitan Monostearate |
| Tween 60 | Polyoxyethylene Sorbitan Monostearate |
| HPMA | Polymer N-(2-hydroxypropyl) Methacrylamide |
| GRGDS | Glycyl-arginyl-glycyl-aspartyl-serine |
| PMMA | Poly(methyl methacrylate) |
| ZnO | Zinc Oxide |
References
- Beach, M.A.; Nayanathara, U.; Gao, Y.; Zhang, C.; Xiong, Y.; Wang, Y.; Such, G.K. Polymeric Nanoparticles for Drug Delivery. Chem. Rev. 2024, 124, 5505–5616. [Google Scholar] [CrossRef] [PubMed]
- Senturk, F.; Cakmak, S. Fabrication of curcumin-loaded magnetic PEGylated-PLGA nanocarriers tagged with GRGDS peptide for improving anticancer activity. MethodsX 2023, 10, 102229. [Google Scholar] [CrossRef] [PubMed]
- D’souza, A.A.; Shegokar, R. Polyethylene glycol (PEG): A versatile polymer for pharmaceutical applications. Expert Opin. Drug. Deliv. 2016, 139, 1257–1275. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wu, Z.; Liu, Y.; Zhan, Z.; Yang, L.; Wang, C.; Jiang, Q.; Ran, H.; Li, P.; Wang, Z. ROS-responsive liposomes as an inhaled drug delivery nanoplatform for idiopathic pulmonary fibrosis treatment via Nrf2 signaling. J. Nanobiotechnol. 2022, 20, 213. [Google Scholar] [CrossRef]
- Santhanakrishnan, K.R.; Koilpillai, J.; Narayanasamy, D. PEGylation in Pharmaceutical Development: Current Status and Emerging Trends in Macromolecular and Immunotherapeutic Drugs. Cureus 2024, 16, e66669. [Google Scholar] [CrossRef]
- Alqahtani, M.S.; Kazi, M.; Alsenaidy, M.A.; Ahmad, M.Z. Advances in Oral Drug Delivery. Front. Pharmacol. 2021, 12, 618411. [Google Scholar] [CrossRef]
- Shah, P.; Desai, H.; Vyas, B.; Lalan, M.; Kulkarni, M. Quality-by-Design-Based Development of Rivaroxaban-Loaded Liquisolid Compact Tablets with Improved Biopharmaceutical Attributes. AAPS PharmSciTech 2023, 24, 176. [Google Scholar] [CrossRef]
- Shubhra, Q.T.H. Multi-compartment liposomes forge new paths in drug delivery. Nat. Chem. 2024, 16, 1578–1579. [Google Scholar] [CrossRef]
- Albertsen, C.H.; Kulkarni, J.A.; Witzigmann, D.; Lind, M.; Petersson, K.; Simonsen, J.B. The role of lipid components in lipid nanoparticles for vaccines and gene therapy. Adv. Drug Deliv. Rev. 2022, 188, 114416. [Google Scholar] [CrossRef]
- Tenchov, R.; Sasso, J.M.; Zhou, Q.A. PEGylated Lipid Nanoparticle Formulations: Immunological Safety and Efficiency Perspective. Bioconjugate Chem. 2023, 34, 941–960. [Google Scholar] [CrossRef]
- Assiri, A.A.; Glover, K.; Mishra, D.; Waite, D.; Vora, L.K.; Thakur, R.R.S. Block copolymer micelles as ocular drug delivery systems. Drug Discov. Today 2024, 29, 104098. [Google Scholar] [CrossRef] [PubMed]
- Sinha, S.; Tripathi, A.K.; Pandey, A.; Pandey, A.; Naik, P.; Verma, V.S. Self-assembled PEGylated micelles for precise and targeted drug delivery: Current challenges and future directions. Biocatal. Agric. Biotechnol. 2024, 60, 103296. [Google Scholar] [CrossRef]
- Shiraishi, K.; Yokoyama, M. Toxicity and immunogenicity concerns related to PEGylated-micelle carrier systems: A review. Sci. Technol. Adv. Mater. 2019, 20, 324–336. [Google Scholar] [CrossRef]
- Fonseca, M.; Jarak, I.; Victor, F.; Domingues, C.; Veiga, F.; Figueiras, A. Polymersomes as the Next Attractive Generation of Drug Delivery Systems: Definition, Synthesis and Applications. Materials 2024, 17, 319. [Google Scholar] [CrossRef] [PubMed]
- Wauters, A.C.; Pijpers, I.A.B.; Mason, A.F.; Williams, D.S.; Tel, J.; Abdelmohsen, L.K.E.A.; van Hest, J.C.M. Development of Morphologically Discrete PEG−PDLLA Nanotubes for Precision Nanomedicine. Biomacromolecules 2019, 20, 177–183. [Google Scholar] [CrossRef]
- Schmidt, A.C.; Hebels, E.R.; Weitzel, C.; Kletzmayr, A.; Bao, Y.; Steuer, C.; Leroux, J.C. Engineered Polymersomes for the Treatment of Fish Odor Syndrome: A First Randomized Double Blind Olfactory Study. Adv. Sci. 2020, 7, 1903697. [Google Scholar] [CrossRef]
- Unnikrishnan, G.; Joy, A.; Megha, M.; Kolanthai, E.; Senthilkumar, M. Exploration of inorganic nanoparticles for revolutionary drug delivery applications: A critical review. Discov. Nano 2023, 18, 157. [Google Scholar] [CrossRef]
- Donadoni, E.; Siani, P.; Gambari, S.; Campi, D.; Frigerio, G.; Valentin, C. Optimizing Polyethylene Glycol Coating for Stealth Nanodiamonds. ACS Appl. Mater. Interfaces 2025, 17, 19304–19316. [Google Scholar] [CrossRef]
- Xue, Y.; Gao, H.M.; Yu, L.; Zhang, N.N.; Kang, J.; Wang, C.Y.; Lu, Z.Y.; Whittaker, A.; Liu, K. Physisorption of Poly(ethylene glycol) on Inorganic Nanoparticles. ACS Nanomed. 2022, 16, 6634–6645. [Google Scholar] [CrossRef]
- Witika, B.A.; Bassey, K.E.; Demana, P.H.; Siwe-Noundou, X.; Poka, M.S. Current Advances in Specialised Niosomal Drug Delivery: Manufacture, Characterization and Drug Delivery Applications. Int. J. Mol. Sci. 2022, 23, 9668. [Google Scholar] [CrossRef]
- Shahbazi, R.; Mirjafary, Z.; Zarghami, N.; Saeidian, H. Efficient PEGylated magnetic nanoniosomes for co-delivery of artemisinin and metformin: A new frontier in chemotherapeutic efficacy and cancer therapy. Sci. Rep. 2024, 14, 27380. [Google Scholar] [CrossRef]
- Baldino, L.; Riccardi, D.; Reverchon, E. Production of PEGylated Vancomycin-Loaded Niosomes by a Continuous Supercritical CO2 Assisted Process. Nanomaterials 2024, 14, 846. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Zhang, J.; Zhao, M.; Tang, S.; Cheng, X.; Zhang, W.; Li, W.; Liu, X.; Peng, H.; Wang, Q. Effects of polyethylene glycol on the surface of nanoparticles for targeted drug delivery. Nanoscale 2021, 13, 10748–10764. [Google Scholar] [CrossRef] [PubMed]
- Cahn, D.; Duncan, G.A. High-Density Branched PEGylation for Nanoparticle Drug Delivery. Cel. Mol. Bioeng. 2022, 15, 355–366. [Google Scholar] [CrossRef]
- Popescu, R.C.; Vasile, B.Ş.; Savu, D.I.; Mogoşanu, G.D.; Bejenaru, L.E.; Andronescu, E.; Grumezescu, A.M.; Mogoantă, L. Influence of Polymer Shell Molecular Weight on Functionalized Iron Oxide Nanoparticles Morphology and In Vivo Biodistribution. Pharmaceutics 2022, 14, 1877. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Li, J.; Zhao, D.; Yan, N.; Zhang, H.; Liu, M.; Tang, X.; Hu, Y.; Ding, J.; Zhang, N.; et al. Branched PEG-modification: A new strategy for nanocarriers to evade of the accelerated blood clearance phenomenon and enhance anti-tumor efficacy. Biomaterials 2022, 283, 121415. [Google Scholar] [CrossRef]
- Jeong, H.; Lim, H.; Lee, D.Y.; Song, Y.S.; Kim, B.Y. Preparation and Drug Release Behavior of Nifedipine-Loaded Poly(lactic acid)/Polyethylene Glycol Microcapsules. J. Nanosci. Nanotechnol. 2021, 21, 3735–3741. [Google Scholar] [CrossRef]
- Jovičić-Bata, J.; Todorović, N.; Krstonošić, V.; Ristić, I.; Kovačević, Z.; Vuković, M.; Lalić-Popović, M. Liquid- and Semisolid-Filled Hard Gelatin Capsules Containing Alpha-Lipoic Acid as a Suitable Dosage Form for Compounding Medicines and Dietary Supplements. Pharmaceutics 2024, 16, 892. [Google Scholar] [CrossRef]
- Yadav, M.; Sarolia, J.; Vyas, B.; Lalan, M.; Mangrulkar, S.; Shah, P. Amalgamation of Solid Dispersion and Melt Adsorption Technique: Improved In Vitro and In Vivo Performance of Ticagrelor Tablets. AAPS PharmSciTech 2021, 22, 257. [Google Scholar] [CrossRef]
- Nair, A.B.; Singh, B.; Shah, J.; Jacob, S.; Aldhubiab, B.; Sreeharsha, N.; Morsy, M.A.; Venugopala, K.N.; Attimarad, M.; Shinu, P. Formulation and Evaluation of Self-Nanoemulsifying Drug Delivery System Derived Tablet Containing Sertraline. Pharmaceutics 2022, 14, 336. [Google Scholar] [CrossRef]
- Dalal, L.; Allaf, A.W.; El-Zein, H. Formulation and in vitro evaluation of self-nanoemulsifying liquisolid tablets of furosemide. Sci. Rep. 2021, 11, 1315. [Google Scholar] [CrossRef]
- Kestur, U.; Desai, D.; Zong, Z.; Abraham, A.; Fiske, J. Effect of coating excipients on chemical stability of active coated tablets. Pharm. Dev. Technol. 2020, 26, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Ali, H.S.M.; Ahmed, S.A.; Alqurshi, A.A.; Alalawi, A.M.; Shehata, A.M.; Alahmadi, Y.M. Tadalafil-Loaded Self-Nanoemulsifying Chewable Tablets for Improved Bioavailability: Design, In Vitro, and In Vivo Testing. Pharmaceutics 2022, 14, 1927. [Google Scholar] [CrossRef] [PubMed]
- Algahtani, M.S.; Mohammed, A.A.; Ahmad, J.; Abdullah, M.M.; Saleh, E. 3D Printing of Dapagliflozin Containing Self-Nanoemulsifying Tablets: Formulation Design and In Vitro Characterization. Pharmaceutics 2021, 13, 993. [Google Scholar] [CrossRef] [PubMed]
- Eisa, A.M.; El-Megrab, N.A.; El-Nahas, H.M. Formulation and evaluation of fast dissolving tablets of haloperidol solid dispersion. Saudi Pharm. J. 2022, 30, 1589–1602. [Google Scholar] [CrossRef]
- El Mabrouki, H.; Kaukhova, I.E. Formulation and Development of Aqueous Film Coating for Moisture Protection of Hygroscopic Herniaria glabra L. Tablets. Turk. J. Pharm. Sci. 2022, 19, 153–160. [Google Scholar] [CrossRef]
- Mosley-Kellum, K.; Bagde, A.; Spencer, S.; Dev, S.; Singh, M. Development of 3D DLP Printed Sustained Release Ibuprofen Tablets and Their Pharmacokinetic Evaluation in Rats. AAPS PharmSciTech 2024, 24, 88. [Google Scholar] [CrossRef]
- Adhikari, S.; Budhathoki, U.; Thapa, P. Development and Characterization of Hygroscopicity-Controlled Sustain Release Formulation of Divalproex Sodium. Turk. J. Pharm. Sci. 2022, 19, 422–430. [Google Scholar] [CrossRef]
- Fița, A.C.; Secăreanu, A.A.; Musuc, A.M.; Ozon, E.A.; Sarbu, I.; Atkinson, I.; Rusu, A.; Mati, E.; Anuta, V.; Pop, A.L. The Influence of the Polymer Type on the Quality of Newly Developed Oral Immediate-Release Tablets Containing Amiodarone Solid Dispersions Obtained by Hot-Melt Extrusion. Molecules 2022, 27, 6600. [Google Scholar] [CrossRef]
- Zhang, X.; Xiao, Y.; Huang, Z.; Chen, J.; Cui, Y.; Niu, B.; Huang, Y.; Pan, X.; Wu, C. Smart phase transformation system based on lyotropic liquid crystalline@hard capsules for sustained release of hydrophilic and hydrophobic drugs. Drug. Deliv. 2020, 27, 449–459. [Google Scholar] [CrossRef]
- Zhang, N.; Li, X.; Ye, J.; Yang, Y.; Huang, Y.; Zhang, X.; Xiao, M. Effect of Gellan Gum and Xanthan Gum Synergistic Interactions and Plasticizers on Physical Properties of Plant-Based Enteric Polymer Films. Polymers 2020, 12, 121. [Google Scholar] [CrossRef]
- Milián-Guimerá, C.; De Vittorio, L.; McCabe, R.; Göncü, N.; Krishnan, S.; Thamdrup, L.H.E.; Boisen, A.; Ghavami, M. Flexible Coatings Facilitate pH-Targeted Drug Release via Self-Unfolding Foils: Applications for Oral Drug Delivery. Pharmaceutics 2024, 16, 81. [Google Scholar] [CrossRef] [PubMed]
- Dong, C.L.; Zheng, S.D.; Liu, Y.Y.; Cui, W.Q.; Hao, M.Q.; God’spower, B.O.; Chen, X.Y.; Li, Y.H. Albendazole solid dispersions prepared using PEG6000 and Poloxamer188: Formulation, characterization and in vivo evaluation. Pharm. Dev. Technol. 2020, 25, 1043–1052. [Google Scholar] [CrossRef] [PubMed]
- Alavi, S.E.; Esfahani, M.K.M.; Raza, A.; Adelnia, H.; Shahmabadi, H.E. PEG-grafted liposomes for enhanced antibacterial and antibiotic activities: An in vivo study. J. Imp. 2022, 25, 100384. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chen, J.; He, Z.; Luo, H.; Sun, Y.; Ge, D.; Liu, X.; Shi, W. Ferrocene-liposome-PEG: A robust OH/lipid peroxide nano-converter for inducing tumor ferroptosis. Biomater. Sci. 2023, 11, 542–553. [Google Scholar] [CrossRef]
- Wang, S.; Wang, Z.; Xu, C.; Cui, L.; Meng, G.; Yang, S.; Wu, J.; Liu, Z.; Guo, X. PEG-α-CD/AM/liposome @amoxicillin double network hydrogel wound dressing—Multiple barriers for long-term drug release. J. Biomater. Appl. 2021, 35, 1085–1095. [Google Scholar] [CrossRef]
- Minamisakamoto, T.; Nishiguchi, S.; Hashimoto, K.; Ogawara, K.I.; Maruyama, M.; Higaki, K. Sequential administration of PEG-Span 80 niosome enhances anti-tumor effect of doxorubicin-containing PEG liposome. Eur. J. Pharm. Biopharm. 2021, 169, 20–28. [Google Scholar] [CrossRef]
- Maghsoudi, S.; Hosseini, S.A.; Soraya, H.; Roosta, Y.; Mohammadzadeh, A. Development of doxorubicin-encapsulated magnetic liposome@PEG for treatment of breast cancer in BALB/c mice. Drug Deliv. Transl. Res. 2023, 13, 2589–2603. [Google Scholar] [CrossRef]
- Tang, W.; Zhang, Z.; Li, C.; Chu, Y.; Qian, J.; Ying, T.; Lu, W.; Zhan, C. Facile Separation of PEGylated Liposomes Enabled by Anti-PEG scFv. Nano Lett. 2021, 21, 9835–10148. [Google Scholar] [CrossRef]
- Rasoulianboroujeni, M.; Repp, L.; Lee, H.J.; Kwon, G.S. Production of paclitaxel-loaded PEG-b-PLA micelles using PEG for drug loading and freeze-drying. J. Con. Rel. 2022, 350, 350–359. [Google Scholar] [CrossRef]
- Wang, M.; Zhan, J.; Xu, L.; Wang, Y.; Lu, D.; Li, Z.; Li, J.; Luo, F.; Tan, H. Synthesis and characterization of PLGA-PEG-PLGA based thermosensitive polyurethane micelles for potential drug delivery. J. Bio. Sci. Polym. Ed. 2021, 32, 613–634. [Google Scholar] [CrossRef]
- Feng, Y.; Wang, J.; Zhang, S.; Li, Y.; Wang, B.; Zhang, J.; Qiu, Y.; Zhang, Y.; Zhang, Y. Preparation of amentoflavone-loaded DSPE PEG2000 micelles with improved bioavailability and in vitro antitumor efficacy. Biomed. Chromatogr. 2023, 37, e5690. [Google Scholar] [CrossRef]
- Brandt, J.V.; Piazza, R.D.; Carvalho Dos Santos, C.; Vega-Chacón, J.; Amantéa, B.E.; Pinto, G.C.; Magnani, M.; Piva, H.L.; Tedesco, A.C.; Primo, F.L.; et al. Synthesis and colloidal characterization of folic acid-modified PEG-b-PCL Micelles for methotrexate delivery. J. Col. Surf. B Biointerfaces 2019, 177, 228–234. [Google Scholar] [CrossRef]
- Sun, S.; Du, X.; Fu, M.; Khan, A.R.; Ji, J.; Liu, W.; Zhai, G. Galactosamine-modified PEG-PLA/TPGS micelles for the oral delivery of curcumin. Int. J. Pharm. 2021, 595, 120227. [Google Scholar] [CrossRef]
- Du, J.; Wu, Q.; Li, Y.; Liu, P.; Han, X.; Wang, L.; Yuan, J.; Meng, X.; Xiao, Y. Preparation and characterization of Keratin-PEG conjugate-based micelles as a tumor microenvironment-responsive drug delivery system. J. Bio. Sci. Polym. Ed. 2020, 31, 1163–1178. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, Z.; Song, X.; Zhu, J.; Sng, J.A.; Li, J.; Wen, Y. Synthesis and Characterization of Palmitoyl-blockpoly (methacryloyloxyethyl phosphorylcholine) Polymer Micelles for Anticancer Drug Delivery. Biomacromolecules 2022, 23, 4586–4596. [Google Scholar] [CrossRef] [PubMed]
- Pan, Q.; Zhang, J.; Li, X.; Han, X.; Zou, Q.; Zhang, P.; Luo, Y.; Jin, Y. Preparation and pharmacokinetics of bifunctional epirubicin-loaded micelles. Pharmazie 2019, 74, 577–582. [Google Scholar] [CrossRef] [PubMed]
- Jiaying, Y.; Bo, S.; Xiaolu, W.; Yanyan, Z.; Hongjie, W.; Nan, S.; Bo, G.; Linna, W.; Yan, Z.; Wenya, G.; et al. Arenobufagin-loaded PEG-PLA nanoparticles for reducing toxicity and enhancing cancer therapy. Drug Deliv. 2023, 30, 2177362. [Google Scholar] [CrossRef] [PubMed]
- Singam, A.; Killi, N.; Patel, P.R.; Gundloori, R.V.N. PEGylated ethyl cellulose micelles as a nanocarrier for drug delivery. RSC Adv. 2021, 11, 30532–30543. [Google Scholar] [CrossRef]
- Köthe, T.; Martin, S.; Reich, G.; Fricker, G. Dual asymmetric centrifugation as a novel method to prepare highly concentrated dispersions of PEG-b-PCL polymersomes as drug carriers. Int. J. Pharm. 2020, 579, 119087. [Google Scholar] [CrossRef]
- Zavvar, T.; Babaei, M.; Abnous, K.; Taghdisi, S.M.; Nekooei, S.; Ramezani, M.; Alibolandi, M. Synthesis of multimodal polymersomes for targeted drug delivery and MR/fluorescence imaging in metastatic breast cancer model. Int. J. Pharm. 2020, 578, 119091. [Google Scholar] [CrossRef]
- Khodaverdi, E.; Tayarani-Najaran, Z.; Minbashi, E.; Alibolandi, M.; Hosseini, J.; Sepahi, S.; Kamali, H.; Hadizadeh, F. Docetaxel-Loaded Mixed Micelles and Polymersomes Composed of Poly (caprolactone)-Poly (ethylene glycol) (PEG-PCL) and Poly (lactic acid)-Poly (ethylene glycol) (PEG-PLA): Preparation and In-vitro Characterization. Iran. J. Pharm. Res. 2019, 18, 142–155. [Google Scholar] [PubMed]
- Manickavasagam, D.; Lin, L.; Oyewumi, M.O. Nose-to-brain co-delivery of repurposed simvastatin and BDNF synergistically attenuates LPS-induced neuroinflammation. J. Nano. 2020, 23, 102107. [Google Scholar] [CrossRef] [PubMed]
- Jain, J.P.; Kumar, N. Self assembly of amphiphilic (PEG)(3)-PLA copolymer as polymersomes: Preparation, characterization, and their evaluation as drug carrier. Biomacromolecules 2010, 11, 1027–1035. [Google Scholar] [CrossRef] [PubMed]
- Revdekar, A.; Shende, P. Block copolymers in Alzheimer’s disease therapy: A perceptive to revolutionize biomaterials. J. Con. Rel. 2021, 340, 271–281. [Google Scholar] [CrossRef]
- Khan, S.; McCabe, J.; Hill, K.; Beales, P.A. Biodegradable hybrid block copolymer–lipid vesicles as potential drug delivery systems. J. Col. Int. Sci. 2020, 562, 418–428. [Google Scholar] [CrossRef]
- Baghbanbashi, M.; Yong, H.W.; Zhang, I.; Lotocki, V.; Yuan, Z.; Pazuki, G.; Maysinger, D.; Kakkar, A. Stimuli-Responsive Miktoarm Polymer-Based Formulations for Fisetin Delivery and Regulatory Effects in Hyperactive Human Microglia. Macromol. Biosci. 2022, 22, 2200174. [Google Scholar] [CrossRef]
- Mamnoon, B.; Feng, L.; Froberg, J.; Choi, Y.; Sathish, V.; Taratula, O.; Taratula, O.; Mallik, S. Targeting Estrogen Receptor-Positive Breast Microtumors with Endoxifen Conjugated, Hypoxia-Sensitive Polymersomes. ACS Omega 2021, 6, 27654–27667. [Google Scholar] [CrossRef]
- Kim, M.; Kim, D.; Jang, Y.; Han, H.; Lee, S.; Moon, H.; Kim, J.; Kim, H. Development of a Polymersome-Based Nanomedicine for Chemotherapeutic and Sonodynamic Combination Therapy. Int. J. Mol. Sci. 2023, 24, 1194. [Google Scholar] [CrossRef]
- Mannu, R.; Karthikeyan, V.; Velu, N.; Arumugam, C.; Roy, V.A.L.; Gopalan, A.I.; Saianand, G.; Sonar, P.; Lee, K.P.; Kim, W.J.; et al. Polyethylene Glycol Coated Magnetic Nanoparticles: Hybrid Nanofluid Formulation, Properties and Drug Delivery Prospects. Nanomaterials 2021, 11, 440. [Google Scholar] [CrossRef]
- Bao, Y.; Li, H.; He, J.; Song, K.; Yu, H.; Tian, C.; Guo, J.; Zhou, X.; Liu, S. Polyethylene glycol modified graphene oxide-silver nanoparticles nanocomposite as a novel antibacterial material with high stability and activity. J. Col. Surf. B Biointerfaces 2023, 229, 113435. [Google Scholar] [CrossRef]
- Grundler, J.; Shin, K.; Suh, H.W.; Zhong, M.; Saltzman, W.M. Surface Topography of Polyethylene Glycol Shell Nanoparticles Formed from Bottlebrush Block Copolymers Controls Interactions with Proteins and Cells. ACS Nano 2021, 15, 16118–16129. [Google Scholar] [CrossRef]
- Guo, J.; Miao, Y.; Nie, F.; Gao, F.; Li, H.; Wang, Y.; Liu, Q.; Zhang, T.; Yang, X.; Liu, L.; et al. Zn-Shik-PEG nanoparticles alleviate inflammation and multi-organ damage in sepsis. J. Nano. 2023, 21, 448. [Google Scholar] [CrossRef]
- Caciandone, M.; Niculescu, A.G.; Roșu, A.R.; Grumezescu, V.; Negut, I.; Holban, A.M.; Oprea, O.; Vasile, B.Ș.; Bîrcă, A.C.; Grumezescu, A.M.; et al. PEG-Functionalized Magnetite Nanoparticles for Modulation of Microbial Biofilms on Voice Prosthesis. Antibiotics 2022, 11, 39. [Google Scholar] [CrossRef]
- Movileanu, C.; Anghelache, M.; Turtoi, M.; Voicu, G.; Neacsu, I.A.; Ficai, D.; Trusca, R.; Oprea, O.; Ficai, A.; Andronescu, E.; et al. Folic acid-decorated PEGylated magnetite nanoparticles as efficient drug carriers to tumor cells overexpressing folic acid receptor. Int. J. Pharm. 2022, 625, 122064. [Google Scholar] [CrossRef]
- Lupusoru, R.V.; Pricop, D.A.; Uritu, C.M.; Arvinte, A.; Coroaba, A.; Esanu, I.; Zaltariov, M.F.; Silion, M.; Stefanescu, C.; Pinteala, M. Effect of TAT-DOX-PEG irradiated gold nanoparticles conjugates on human osteosarcoma cells. Sci. Rep. 2020, 10, 6591. [Google Scholar] [CrossRef] [PubMed]
- Zygouri, E.; Bekiari, V.; Malis, G.; Karamanos, N.K.; Koutsakis, C.; Psomas, G.; Tangoulis, V. pH-Sensitive Gold Nanorods for Non-Steroidal Anti-Inflammatory Drugs (NSAIDs) Delivery and DNA-Binding Studies. Molecules 2023, 28, 3780. [Google Scholar] [CrossRef] [PubMed]
- Storjohann, R.; Gericke, B.; Reifenrath, J.; Herrmann, T.; Behrens, P.; Oltmanns, H.; Meißner, J. Influence of PEG Chain Length of Functionalized Magnetic Nanoparticles on the Cytocompatibility and Immune Competence of Primary Murine Macrophages and Dendritic Cells. Int. J. Mol. Sci. 2023, 24, 2565. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, S.; Baig, B.; Hisaindee, S.; Darwish, H.; Abdel-Ghany, A.; El-Maghraby, H.; Amin, A.; Greish, Y. Development and Evaluation of Crocetin-Functionalized Pegylated Magnetite Nanoparticles for Hepatocellular Carcinoma. Molecules 2023, 28, 2882. [Google Scholar] [CrossRef]
- Hosokawa, M.; Ito, S.; Noda, K.; Kono, Y.; Ogawara, K.I. Preparation and Evaluation of Paclitaxel-Loaded PEGylated Niosomes Composed of Sorbitan Esters. Bio. Pharm. Bull. 2023, 46, 1479–1483. [Google Scholar] [CrossRef]
- Saharkhiz, S.; Zarepour, A.; Zarrabi, A. Empowering Cancer Therapy: Comparing PEGylated and Non-PEGylated Niosomes Loaded with Curcumin and Doxorubicin on MCF-7 Cell Line. Bioengineering 2023, 10, 1159. [Google Scholar] [CrossRef] [PubMed]
- Riazi, H.; Goodarzi, M.T.; Tabrizi, M.H.; Mozaffari, M.; Neamati, A. Preparation of the Myricetin-Loaded PEGylated Niosomes and Evaluation of their in vitro Anti-Cancer Potentials. Chem. Biodivers. 2024, 21, e202301767. [Google Scholar] [CrossRef] [PubMed]
- Firozian, F.; Karami, S.; Ranjbar, A.; Azandaryani, M.T.; Nili-Ahmadabadi, A. Improvement of therapeutic potential N-acetylcysteine in acetaminophen hepatotoxicity by encapsulation in PEGylated nano-niosomes. Life Sci. 2020, 255, 117832. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Svirskis, D.; Proft, T.; Loh, J.; Huang, Y.; Wen, J. Cellular Uptake and Transport Mechanism Investigations of PEGylated Niosomes for Improving the Oral Delivery of Thymopentin. Pharmaceutics 2024, 16, 397. [Google Scholar] [CrossRef]
- Nisha, R.; Kumar, P.; Gautam, A.K.; Bera, H.; Bhattacharya, B.; Parashar, P.; Saraf, S.A.; Saha, S. Assessments of in vitro and in vivo antineoplastic potentials of beta-sitosterol-loaded PEGylated niosomes against hepatocellular carcinoma. J. Liposome Res. 2021, 31, 304–315. [Google Scholar] [CrossRef]
- Alavi, S.E.; Raza, A.; Esfahani, M.K.M.; Akbarzadeh, A.; Abdollahi, S.H.; Shahmabadi, H.E. Carboplatin Niosomal Nanoplatform for Potentiated Chemotherapy. J. Pharm. Sci. 2022, 111, 3029–3037. [Google Scholar] [CrossRef]
- Megahed, M.A.; El-Sawy, H.S.; Reda, A.M.; Abd-Allah, F.I.; Elyazid, S.K.A.; Lila, A.E.; Ismael, H.R.; El-Say, K.M. Effect of nanovesicular surface-functionalization via chitosan and/or PEGylation on cytotoxicity of tamoxifen in induced-breast cancer model. Life Sci. 2022, 307, 120908. [Google Scholar] [CrossRef]
- Lalami, Z.A.; Tafvizi, F.; Naseh, V.; Salehipour, M. Fabrication, optimization, and characterization of pH-responsive PEGylated nanoniosomes containing gingerol for enhanced treatment of breast cancer. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2023, 396, 3867–3886. [Google Scholar] [CrossRef]
- Afereydoon, S.; Haghiralsadat, F.; Hamzian, N.; Shams, A.; Hemati, M.; Naghib, S.M.; Shabani, M.; Zandieh-Doulabi, B.; Tofighi, D. Multifunctional PEGylated Niosomal Nanoparticle-Loaded Herbal Drugs as a Novel Nano-Radiosensitizer and Stimuli-Sensitive Nanocarrier for Synergistic Cancer Therapy. Front. Bioeng. Biotechnol. 2022, 10, 917368. [Google Scholar] [CrossRef]
- Gajbhiye, K.R.; Pawar, A.; Mahadik, K.R.; Gajbhiye, V. PEGylated nanocarriers: A promising tool for targeted delivery to the brain. J. Col. Surf. B Biointerfaces 2020, 187, 110770. [Google Scholar] [CrossRef]
- Hashemy, S.I.; Amiri, H.; Hosseini, H.; Sadeghzadeh, F.; Jaseem, M.M.M.; Tabrizi, M.H. PEGylated Lecithin-Chitosan-Folic Acid Nanoparticles as Nanocarriers of Allicin for In Vitro Controlled Release and Anticancer Effects. Appl. Biochem. Biotechnol. 2023, 195, 4036–4052. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.J.; Chuang, K.H.; Lo, Y.J.; Chen, M.; Chen, Y.J.; Roffler, S.R.; Ho, H.O.; Lin, S.Y.; Sheu, M.T. Bispecific T-cell engagers non-covalently decorated drug-loaded PEGylated nanocarriers for cancer immunochemotherapy. J. Con. Rel. 2022, 344, 235–248. [Google Scholar] [CrossRef] [PubMed]
- Alhamhoom, Y.; Kumaraswamy, T.; Kumar, A.; Nanjappa, S.H.; Prakash, S.S.; Rahamathulla, M.; Thajudeen, K.Y.; Ahmed, M.M.; Shivanandappa, T.B. Formulation and Evaluation of pH-Modulated Amorphous Solid Dispersion-Based Orodispersible Tablets of Cefdinir. Pharmaceutics 2024, 16, 866. [Google Scholar] [CrossRef] [PubMed]
- Belayneh, A.; Molla, F.; Kahsay, G. Formulation and Optimization of Monolithic Fixed-Dose Combination of Metformin HCl and Glibenclamide Orodispersible Tablets. Adv. Pharmacol. Pharm. Sci. 2020, 2020, 3546597. [Google Scholar] [CrossRef]
- Kinani, A.A.Y.; Taghi, H.S. Formulation and characterization of orodispersible tablet of glimepiride. J. Adv. Pharm. Technol. Res. 2022, 13, 252–260. [Google Scholar] [CrossRef]
- Bao, W.; Zhang, X.; Wu, H.; Chen, R.; Guo, S. Synergistic Effect of Ultrasound and Polyethylene Glycol on the Mechanism of the Controlled Drug Release from Polylactide Matrices. Polymers 2019, 11, 880. [Google Scholar] [CrossRef]
- Moutaharrik, S.; Palugan, L.; Cerea, M.; Filippin, I.; Maroni, A.; Gazzaniga, A.; Foppoli, A. Cushion-coated pellets for tableting without external excipients. Int. J. Pharm. 2024, 653, 123874. [Google Scholar] [CrossRef]
- Li, Y.; Pan, H.; Duan, H.; Chen, J.; Zhu, Z.; Fan, J.; Li, P.; Yang, X.; Pan, W. Double-layered osmotic pump controlled release tablets of actarit: In vitro and in vivo evaluation. Asian J. Pharm. Sci. 2019, 14, 340–348. [Google Scholar] [CrossRef]
- Song, Q.; Jiang, C.; Wang, C.; Zhou, L.; Han, Z.; Sun, N.; Huang, P.; Wang, D. Preparation and in Vitro Evaluation of Osmotic-Pump Lorcaserin-hydrochloride Controlled-Release Tablets. Chem. Pharm. Bull. 2022, 70, 202–210. [Google Scholar] [CrossRef]
- Thakkar, H.P.; Vasava, D.; Patel, A.A.; Dhande, R.D. Formulation and evaluation of liquisolid compacts of itraconazole to enhance its oral bioavailability. Ther. Deliv. 2020, 11, 83–96. [Google Scholar] [CrossRef]
- Sánchez-Guirales, S.A.; Jurado, N.; Kara, A.; Lalatsa, A.; Serrano, D.R. Understanding Direct Powder Extrusion for Fabrication of 3D Printed Personalised Medicines: A Case Study for Nifedipine Minitablets. Pharmaceutics 2021, 13, 1583. [Google Scholar] [CrossRef] [PubMed]
- Krkobabić, M.; Medarević, D.; Pešić, N.; Vasiljević, D.; Ivković, B.; Ibrić, S. Digital Light Processing (DLP) 3D Printing of Atomoxetine Hydrochloride Tablets Using Photoreactive Suspensions. Pharmaceutics 2020, 12, 833. [Google Scholar] [CrossRef] [PubMed]
- Malebari, A.M.; Kara, A.; Khayyat, A.N.; Mohammad, K.A.; Serrano, D.R. Development of Advanced 3D-Printed Solid Dosage Pediatric Formulations for HIV Treatment. Polymers 2022, 15, 435. [Google Scholar] [CrossRef]
- Tsuji, T.; Ono, T.; Taguchi, H.; Leong, K.H.; Hayashi, Y.; Kumada, S.; Okada, K.; Onuki, Y. Continuous Monitoring of the Hydration Behavior of Hydrophilic Matrix Tablets Using Time-Domain NMR. Chem. Pharm. Bull. 2023, 71, 576–583. [Google Scholar] [CrossRef]
- Shi, K.; Salvage, J.P.; Maniruzzaman, M.; Nokhodchi, A. Role of release modifiers to modulate drug release from fused deposition modelling (FDM) 3D printed tablets. Int. J. Pharm. 2021, 597, 120315. [Google Scholar] [CrossRef]
- Lamichhane, S.; Park, J.B.; Sohn, D.H.; Lee, S. Customized Novel Design of 3D Printed Pregabalin Tablets for Intra-Gastric Floating and Controlled Release Using Fused Deposition Modeling. Pharmaceutics 2019, 11, 564. [Google Scholar] [CrossRef]
- Li, R.; Pan, Y.; Chen, D.; Xu, X.; Yan, G.; Fan, T. Design, Preparation and In Vitro Evaluation of Core-Shell Fused Deposition Modelling 3D-Printed Verapamil Hydrochloride Pulsatile Tablets. Pharmaceutics 2022, 14, 437. [Google Scholar] [CrossRef]
- Li, P.; Jia, H.; Zhang, S.; Yang, Y.; Sun, H.; Wang, H.; Pan, W.; Yin, F.; Yang, X. Thermal Extrusion 3D Printing for the Fabrication of Puerarin Immediate-Release Tablets. AAPS PharmSciTech 2019, 21, 20. [Google Scholar] [CrossRef]
- Rodríguez-Pombo, L.; Martínez-Castro, L.; Xu, X.; Ong, J.J.; Rial, C.; García, D.N.; González-Santos, A.; Flores-González, J.; Alvarez-Lorenzo, C.; Basit, A.W.; et al. Simultaneous fabrication of multiple tablets within seconds using tomographic volumetric 3D printing. Int. J. Pharm. X. 2023, 5, 100166. [Google Scholar] [CrossRef]
- Lopez-Vidal, L.; Real, J.P.; Real, D.A.; Camacho, N.; Kogan, M.J.; Paredes, A.J.; Palma, S.D. Nanocrystal-based 3D-printed tablets: Semi-solid extrusion using melting solidification printing process (MESO-PP) for oral administration of poorly soluble drugs. Int. J. Pharm. 2022, 611, 121311. [Google Scholar] [CrossRef]
- Matijašić, G.; Gretić, M.; Kezerić, K.; Petanjek, J.; Vukelić, E. Preparation of Filaments and the 3D Printing of Dronedarone HCl Tablets for Treating Cardiac Arrhythmias. AAPS PharmSciTech 2019, 20, 310. [Google Scholar] [CrossRef]
- Lin, X.; Fu, H.; Hou, Z.; Si, Y.; Shan, W.; Yang, Y. Three-dimensional printing of gastro-floating tablets using polyethylene glycol diacrylate-based photocurable printing material. Int. J. Pharm. 2021, 603, 120674. [Google Scholar] [CrossRef] [PubMed]
- Liew, K.B.; Ming, L.C.; Goh, B.H.; Peh, K.K. Fast Melt Cocoa Butter Tablet: Effect of Waxes, Starch, and PEG 6000 on Physical Properties of the Preparation. Molecules 2022, 27, 3128. [Google Scholar] [CrossRef] [PubMed]
- Medarević, D.; Djuriš, J.; Krkobabić, M.; Ibrić, S. Improving Tableting Performance of Lactose Monohydrate by Fluid-Bed Melt Granulation Co-Processing. Pharmaceutics 2021, 13, 2165. [Google Scholar] [CrossRef]
- Pornpitchanarong, C.; Akkaramongkolporn, P.; Nattapulwat, N.; Opanasopit, P.; Patrojanasophon, P. Development and Optimization of Andrographis paniculata Extract-Loaded Self-Microemulsifying Drug Delivery System Using Experimental Design Model. Pharmaceutics 2024, 16, 166. [Google Scholar] [CrossRef] [PubMed]
- Michielin, G.; Maerkl, S.J. Direct encapsulation of biomolecules in semi-permeable microcapsules produced with double-emulsions. Sci. Rep. 2022, 12, 21391. [Google Scholar] [CrossRef]
- Kunte, N.; Westerfield, M.; McGraw, E.; Choi, J.; Akinsipe, T.; Whitaker, S.K.; Brannen, A.; Panizzi, P.; Tomich, J.M.; Avila, L.A. Evaluation of transfection efficacy, biodistribution, and toxicity of branched amphiphilic peptide capsules (BAPCs) associated with mRNA. Biomater. Sci. 2022, 10, 6980–6991. [Google Scholar] [CrossRef]
- Haimhoffer, Á.; Dossi, E.; Béresová, M.; Bácskay, I.; Váradi, J.; Afsar, A.; Rusznyák, Á.; Vasvári, G.; Fenyvesi, F. Preformulation Studies and Bioavailability Enhancement of Curcumin with a ‘Two in One’ PEG-β-Cyclodextrin Polymer. Pharmaceutics 2021, 13, 1710. [Google Scholar] [CrossRef]
- Li, R.; Huang, X.; Lu, G.; Feng, C. Sulfur dioxide signaling molecule-responsive polymeric nanoparticles. Biomater. Sci. 2020, 8, 2300–2307. [Google Scholar] [CrossRef]
- Yang, S.; Ding, F.; Gao, Z.; Guo, J.; Cui, J.; Zhang, P. Fabrication of Poly(ethylene glycol) Capsules via Emulsion Templating Method for Targeted Drug Delivery. Polymers 2020, 12, 1124. [Google Scholar] [CrossRef]
- Liparoti, S.; Franco, P.; Pantani, R.; De Marco, I. Polycaprolactone/polyethylene-glycol capsules made by injection molding: A drug release modeling. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 123, 112036. [Google Scholar] [CrossRef]
- Li, J.; Qiang, H.; Yang, W.; Xu, Y.; Feng, T.; Cai, H.; Wang, S.; Liu, Z.; Zhang, Z.; Zhang, J. Oral insulin delivery by epithelium microenvironment-adaptive nanoparticles. J. Control Release 2022, 341, 31–43. [Google Scholar] [CrossRef] [PubMed]
- Kalmer, R.R.; Haddadan, M.M.; Azizi, M.; Ghanbari, M.; Samandarian, D.; Sadjadinia, A.; Ramezanalizadeh, H.; Karimi, A.; Golizadeh, M. Industrial Manufacture of Enteric Hard Capsules Using Novel Formulations Based on Hypromellose Phthalate/Gelatin and Investigation of Pantoprazole Release. ACS Omega 2023, 8, 11293–11303. [Google Scholar] [CrossRef] [PubMed]
- Shaqour, B.; Natsheh, H.; Kittana, N.; Jaradat, N.; Abualhasan, M.; Eid, A.M.; Moqady, R.; AbuHijleh, A.; Abu Alsaleem, S.; Ratrout, S.; et al. Modified Release 3D-Printed Capsules Containing a Ketoprofen Self-Nanoemulsifying System for Personalized Medical Application. ACS Biomater. Sci. Eng. 2024, 10, 3833–3841. [Google Scholar] [CrossRef] [PubMed]
- Takata, H.; Shimizu, T.; Yamade, R.; Elsadek, N.E.; Emam, S.E.; Ando, H.; Ishima, Y.; Ishida, T. Anti-PEG IgM production induced by PEGylated liposomes as a function of administration route. J. Con. Rel. 2023, 360, 285–292. [Google Scholar] [CrossRef]
- Mehta, S.; Dumoga, S.; Malhotra, S.; Singh, N. Comparative analysis of PEG-liposomes and RBCs-derived nanovesicles for anti-tumor therapy. J. Col. Sur. 2022, 218, 112785. [Google Scholar] [CrossRef]
- Li, D.; Zhao, A.; Zhu, J.; Wang, C.; Shen, J.; Zheng, Z.; Pan, F.; Liu, Z.; Chen, Q.; Yang, Y. Inhaled Lipid Nanoparticles Alleviate Established Pulmonary Fibrosis. Small 2023, 19, 2300545. [Google Scholar] [CrossRef]
- Bobde, Y.; Patel, T.; Paul, M.; Biswas, S.; Ghosh, B. PEGylated N-(2 hydroxypropyl) methacrylamide polymeric micelles as nanocarriers for the delivery of doxorubicin in breast cancer. J. Col. Surf. B Biointerfaces 2021, 204, 111833. [Google Scholar] [CrossRef]
- Deng, X.; Zhao, J.; Liu, K.; Wu, C.; Liang, F. Stealth PEGylated chitosan polyelectrolyte complex nanoparticles as drug delivery carrier. J. Bio. Sci. Polym. Ed. 2021, 32, 1387–1405. [Google Scholar] [CrossRef]
- Hao, D.; Meng, Q.; Jiang, B.; Lu, S.; Xiang, X.; Pei, Q.; Yu, H.; Jing, X.; Xie, Z. Hypoxia-Activated PEGylated Paclitaxel Prodrug Nanoparticles for Potentiated Chemotherapy. ACS Nano 2022, 16, 14693–14702. [Google Scholar] [CrossRef]
- Li, Y.; Wang, G.; Wang, T.; Li, C.; Zhang, X.; Li, J.; Wang, Y.; Liu, N.; Chen, J.; Su, X. PEGylated Gambogic Acid Nanoparticles Enable Efficient Renal-Targeted Treatment of Acute Kidney Injury. Nano Lett. 2023, 23, 5641–5647. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.T.; Uddin, Z.; Javed, M.A.; Shah, N.; Bashir, H.; Shaikh, A.J.; Rajoka, M.S.R.; Amirzada, M.I.; Asad, M.H.H.B. PEGylated Protamine Letrozole Nanoparticles: A Promising Strategy to Combat Human Breast Cancer via MCF-7 Cell Lines. BioMed Res. Int. 2022, 2022, 4438518. [Google Scholar] [CrossRef]
- Shoukani, H.I.; Nisa, S.; Bibi, Y.; Zia, M.; Sajjad, A.; Ishfaq, A.; Ali, H. Ciprofloxacin loaded PEG coated ZnO nanoparticles with enhanced antibacterial and wound healing effects. Sci. Rep. 2024, 14, 4689. [Google Scholar] [CrossRef] [PubMed]
- McSweeney, M.D.; Price, L.S.L.; Wessler, T.; Ciociola, E.C.; Herity, L.B.; Piscitelli, J.A.; DeWalle, A.C.; Harris, T.N.; Chan, A.K.P.; Saw, R.S.; et al. Overcoming anti-PEG antibody mediated accelerated blood clearance of PEGylated liposomes by pre-infusion with high molecular weight free PEG. J. Control. Release 2019, 311–312, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Shi, D.; Beasock, D.; Fessler, A.; Szebeni, J.; Ljubimova, J.Y.; Afonin, K.A.; Dobrovolskaia, M.A. To PEGylate or not to PEGylate: Immunological properties of nanomedicine’s most popular component, polyethylene glycol and its alternatives. Adv. Drug Deliv. Rev. 2022, 180, 114079. [Google Scholar] [CrossRef]
- Liu, Y.L.; Liao, T.Y.; Ho, K.W.; Liu, E.S.; Huang, B.C.; Hong, S.T.; Hsieh, Y.C.; Chang, M.S.; Wu, B.T.; Chen, F.M.; et al. Impact of Pre-existing Anti-polyethylene Glycol Antibodies on the Pharmacokinetics and Efficacy of a COVID-19 mRNA Vaccine (Comirnaty) In Vivo. Biomater. Res. 2024, 28, 0112. [Google Scholar] [CrossRef]
- Zahoranová, A.; Luxenhofer, R. Poly(2-oxazoline)- and Poly(2-oxazine)-Based Self-Assemblies, Polyplexes, and Drug Nanoformulations—An Update. Adv. Healthc. Mater. 2021, 10, e2001382. [Google Scholar] [CrossRef]
- Le Khanh, H.P.; Haimhoffer, Á.; Nemes, D.; Józsa, L.; Vasvári, G.; Budai, I.; Bényei, A.; Ujhelyi, Z.; Fehér, P.; Bácskay, I. Effect of Molecular Weight on the Dissolution Profiles of PEG Solid Dispersions Containing Ketoprofen. Polymers 2023, 15, 1758. [Google Scholar] [CrossRef]
- Ogawa, N.; Hiramatsu, T.; Suzuki, R.; Okamoto, R.; Shibagaki, K.; Fujita, K.; Takahashi, C.; Kawashima, Y.; Yamamoto, H. Improvement in the water solubility of drugs with a solid dispersion system by spray drying and hot-melt extrusion with using the amphiphilic polyvinyl caprolactam-polyvinyl acetate-polyethylene glycol graft copolymer and d-mannitol. Eur. J. Pharm. Sci. 2018, 111, 205–214. [Google Scholar] [CrossRef]
- Zhang, L.; Seow, B.Y.L.; Bae, K.H.; Zhang, Y.; Liao, K.C.; Wan, Y.; Yang, Y.Y. Role of PEGylated lipid in lipid nanoparticle formulation for in vitro and in vivo delivery of mRNA vaccines. J. Control Release 2025, 380, 108–124. [Google Scholar] [CrossRef]
- Zhang, Y.; Xia, Q.; Wu, T.; He, Z.; Li, Y.; Li, Z.; Hou, X.; He, Y.; Ruan, S.; Wang, Z.; et al. A novel multi-functionalized multicellular nanodelivery system for non-small cell lung cancer photochemotherapy. J. Nanobiotechnology 2021, 19, 245. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ruan, S.; Guo, J.; He, Z.; Xia, Q.; Wu, T.; Wang, Z.; Li, Z.; Hu, H.; Jing, Q.; et al. B16F10 Cell Membrane-Based Nanovesicles for Melanoma Therapy Are Superior to Hyaluronic Acid-Modified Nanocarriers. Mol. Pharm. 2022, 19, 2840–2853. [Google Scholar] [CrossRef]
- He, X.; Payne, T.J.; Takanashi, A.; Fang, Y.; Kerai, S.D.; Morrow, J.P.; Al-Wassiti, H.; Pouton, C.W.; Kempe, K. Tailored Monoacyl Poly(2-oxazoline)- and Poly(2-oxazine)-Lipids as PEG-Lipid Alternatives for Stabilization and Delivery of mRNA-Lipid Nanoparticles. Biomacromolecules 2024, 25, 4591–4603. [Google Scholar] [CrossRef]
- Holick, C.T.; Klein, T.; Mehnert, C.; Adermann, F.; Anufriev, I.; Streiber, M.; Harder, L.; Traeger, A.; Hoeppener, S.; Franke, C.; et al. Poly(2-ethyl-2-oxazoline) (POx) as Poly(ethylene glycol) (PEG)-Lipid Substitute for Lipid Nanoparticle Formulations. Small 2025, 21, 2411354. [Google Scholar] [CrossRef] [PubMed]
- Bleher, S.; Buck, J.; Muhl, C.; Sieber, S.; Barnert, S.; Witzigmann, D.; Huwyler, J.; Barz, M.; Süss, R. Poly(Sarcosine) Surface Modification Imparts Stealth-Like Properties to Liposomes. Small 2019, 15, 1904716. [Google Scholar] [CrossRef]
- Yao, X.; Sun, C.; Xiong, F.; Zhang, W.; Yao, W.; Xu, Y.; Fan, W.; Huo, F. Polysarcosine as PEG Alternative for Enhanced Camptothecin-Induced Cancer Immunogenic Cell Death. ACS Appl. Mater. Interfaces 2024, 16, 19472–19479. [Google Scholar] [CrossRef] [PubMed]
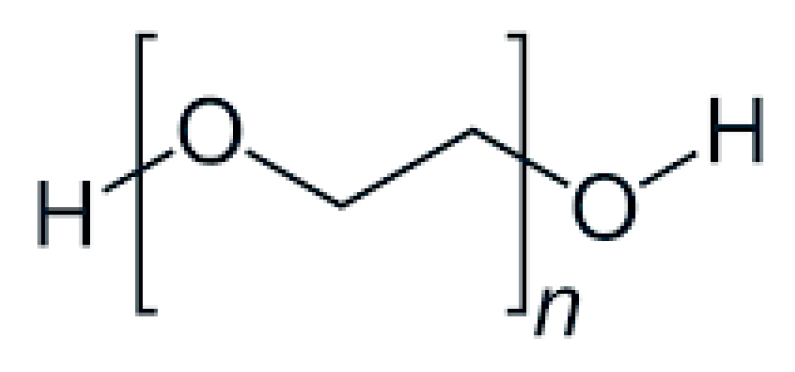
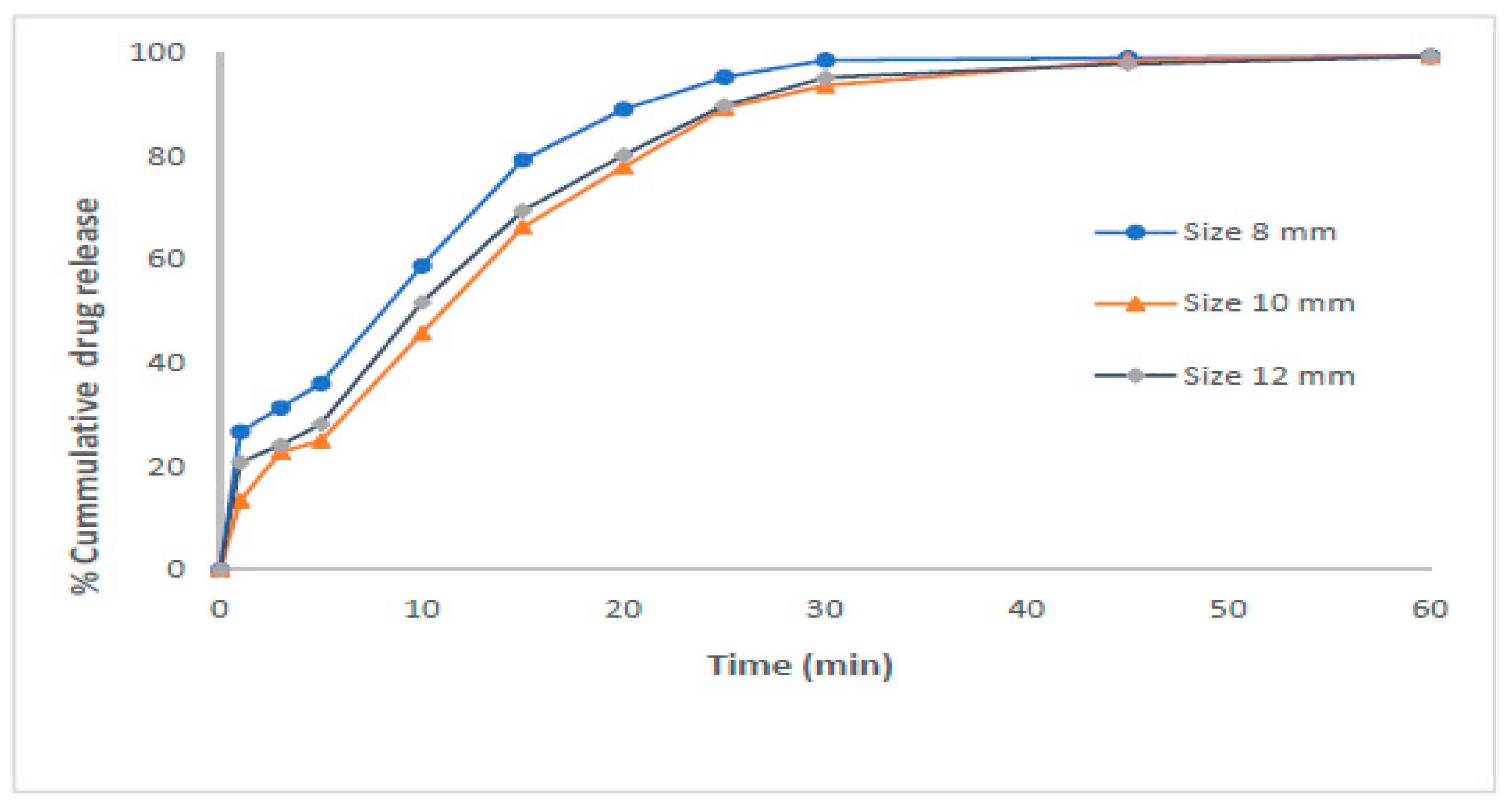
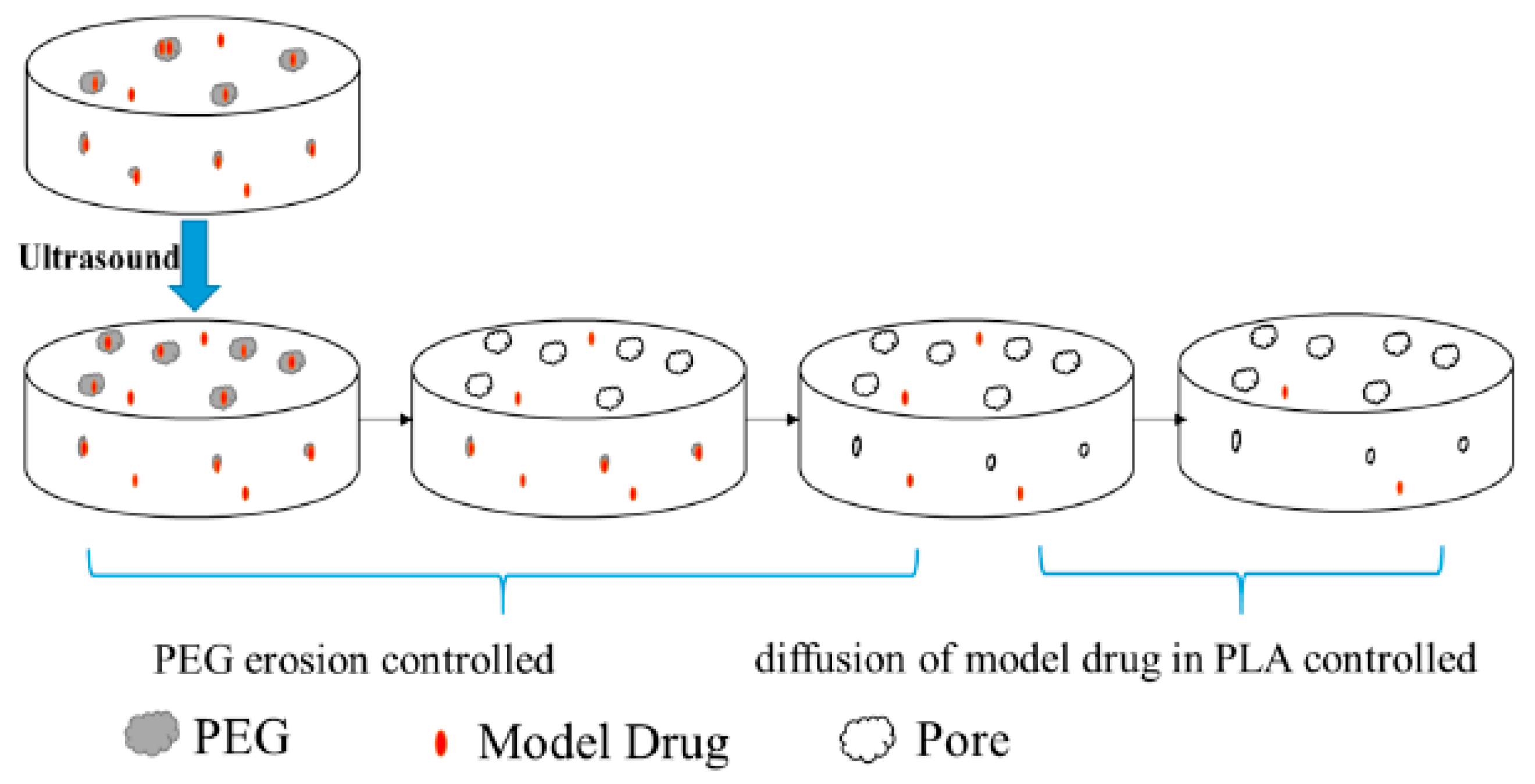

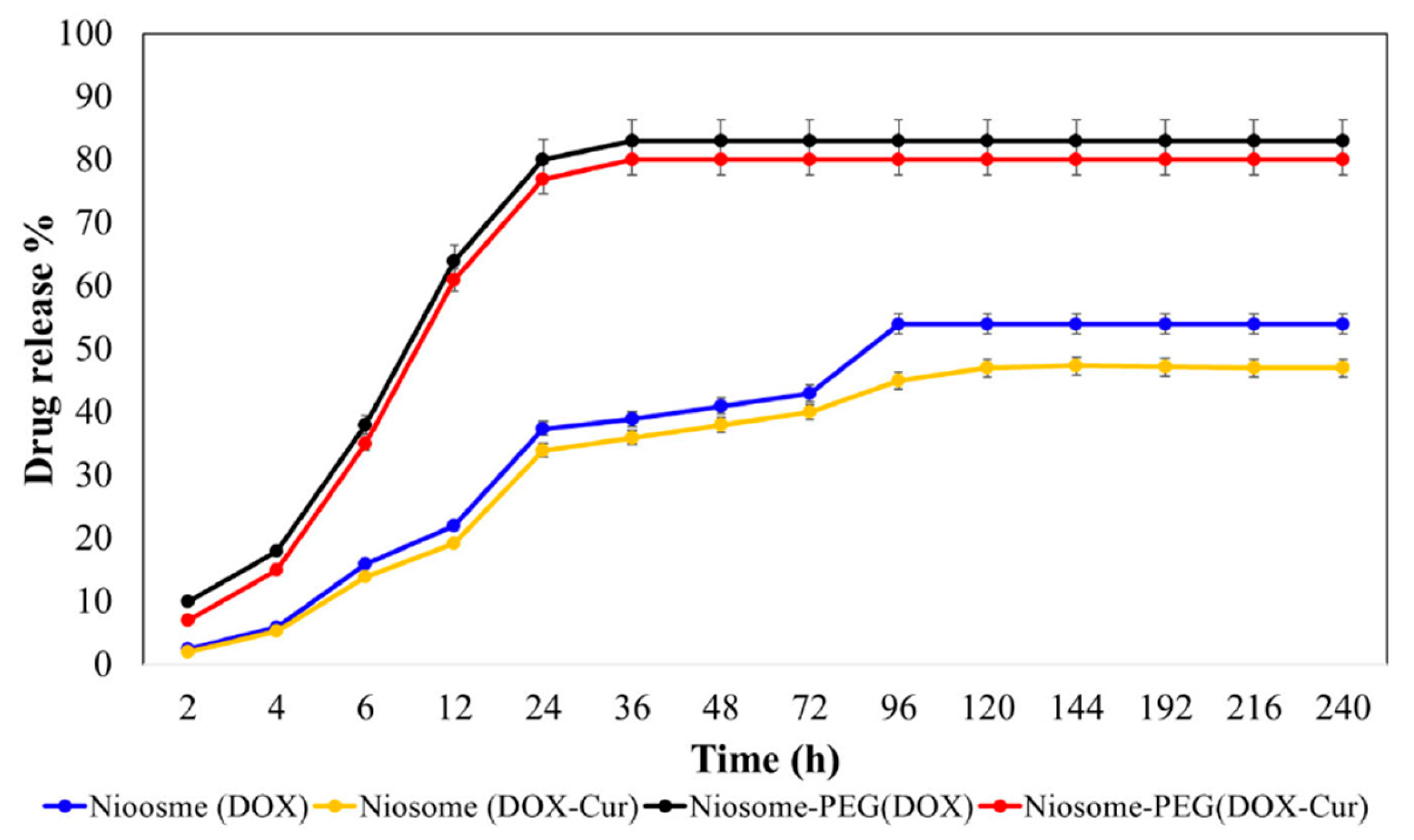
| Formulation | Characteristics of PEG Utilized | Characteristics of the Formulation | Added Value | Functionality of PEG | References |
|---|---|---|---|---|---|
| Self-nanoemulsifying tablets | Molecular weight: 40–400; high transmittance: 94–99% | Hardness: 4.23 kg/cm2; friability: 0.29%; disintegration time: 49 s; weight: 700 mg; drug content: 96.42%; dissolution: 84% (15 min) | Improved solubility and oral bioavailability, immediate release, reduced production cost, stability, simplified manufacturing, accurate dosing, and improved patient compliance | Surfactant, cosurfactant, and emulsifier | [29] |
| Orodispersible tablets | Molecular weight: 6000; Differential Scanning Calorimetry: exothermic peak at 63 °C | Drug solubility: 4.50 mg/mL; weight: 147–152 mg; hardness: 3.12–3.50 kg; thickness: 2.99–3.05 mm; DSC: thermograph at 230 °C; ΔHf: 239 J/g | Increased solubility at lower pH and increased drug release | Hydrophilic carrier | [33] |
| Orodispersible tablets | High hydrophilicity | Thickness: 3.47–3.89 mm; size: 9.95–10.03 mm; hardness: 4.1–10.2 kg/cm2; wetting time: 28–96 s; friability: 0.30%; disintegration time: 18.7 s | Adequate mechanical strength and faster disintegration time | Binding agent; enhances the dissolution of poorly soluble compounds, reduces pill burden, and overcomes swallowing problem | [34] |
| Coated tablets | Molecular weight: 3350, i.e., 30% w/w of the formulation | Coating polymer: PVA or HPMC plasticizer/detackifier, PEG or triacetin; API/coating material ratio: 1/6; HPMC-PEG: pH = 6.5; PVC-PEG: pH = 5.8; stability: triacetin better than PEG and HPMC than PVA | Enhanced solubility and reduced degradation | Plasticizer or detackifier | [35] |
| Cushion-coated pellet systems | Molecular weight: 1500; soft and soluble, with high solubility and low melting temperature (44–48 °C) | Thickness: 30 μm; disintegration time: 8–9.7 min | Administration of multiple-unit dosage forms | Improved mechanical strength, rapid disintegration, integrity of pellets, and compaction | [36] |
| Modified release, 3D-printed | Molecular weight: 700; photoreactive and hydrophilic | Size: 10 mm; thickness: 3 mm; weight change: 2.02%; friability: 0.96%; in vitro drug release after 24 h: 79% | Sustained release, increased bioavailability, and Cmax of 30.1 μg/mL | PEG-DA 700 photopolymer PEG 400 excipient; improvement in dissolution and release | [37] |
| Tablets with modified release | Molecular weight: 8000; melting point: 60 °C | Size: 7 mm; oval tablets; weight: 615 mg; thickness: 6.8 mm; drug release kinetics: Korsmeyer–Peppas | Decreased frequency, better patient compliance, and fewer side effects | Controlled hygroscopicity and retarded drug release | [38] |
| Tablets with modified release | Molecular weight: 1500–8000; hygroscopic semi-crystalline character, with good plasticizer properties | Reduced drug crystallinity; thickness: 4.02–4.16 mm; size: 11.9–12.1 mm; weight: 492.1–506.3 mg; disintegration time: 10–160 s | Immediate release, optimal time of disintegration, and direct absorption by GIT | Carrier and copolymer | [39] |
| Capsules with special characteristics | Molecular weight: 20,000; hydrophilic, flexible, resistant to immunological recognition, and biocompatible | PLA-PEG ratio: 6/4; size: 6 μm; release ratio: 66% | Clinical safety and no cytotoxicity | Shell material and carrier | [40] |
| Capsules with targeted administration | PEG methyl ethyl acrylate; molecular weight: 480; PEG dimethyl acrylate for the fabrication of LPEG capsules; 8-arm PEG acrylate for the fabrication of MPEG capsules | Soft capsules, size of several micrometers, and negative zeta potential (presence of silicon oxide in the inner layer) | Biocompatibility, enhanced cellular uptake, low cell association, and improved delivery efficacy | Reduction in non-specific interactions, circumvention of biological carriers, and modification of nanocarriers | [41] |
| Enteric capsules | PEG Monomethyl Ether; molecular weight: 2000; part of copolymer PLGA-Hyd-PEG | Size: 130–140 nm; polydispersity index: <0.2 | Glucose reduction of up to 35% for a period of time of up to 10 h | Fabrication of pH-sensitive core and conversion of nanoparticle surface to hydrophobic | [42] |
| Enteric capsules | Molecular weight: 4000 | Moisture after the packaging process: 13.6%; gelation temperature: 34 °C; gelation time: 19 s; smooth surface; stable in the stomach for 120 min | Fabrication of enteric capsules without the coating step | Filler; reduces the moisture of capsules, eliminates porosity, and increases stability in the stomach | [43] |
| Formulation | Characteristics of PEG Utilized | Characteristics of the Nanocarrier | Added Value | Functionality of PEG | References |
|---|---|---|---|---|---|
| Liposomes | Hydrophilicity; non-ionic, with high density, cytocompatibility, non-immunogenicity, and high permeability; molecular weight: 2000–8000 | Unilamellar, electronically neutral, and uniform; size: 90–240 nm; zeta potential: −0.1–(−61) mV; polydispersity index: 0.14–0.26; EE: 43–98%; LE: 8.5–8.8% | T-cell independence, larger protein adoption, improved in vivo stability, hemocompatibility, and insignificant macrophage uptake | Increased stability, decreased toxicity, and increased biodistribution, antigenicity, and biocompatibility | [4,44,45,46,47,48,49,50,51,52] |
| Micelles | Hydrophilicity, molar mass dispersity, flexible structure, and low cytotoxicity; molecular weight: 1000–5000 | Anionic charge, amphiphilicity, and feasible manufacturing; size: 17–350 nm; zeta potential: −27–(−4.5) mV; polydispersity index: 0.11–0.47; EE: 41–99%; LE: 3–20% | Targeting regulation, prolonged circulation, efficient accumulation at the tumor site, reduced toxicity, and avoidance of macrophage clearance | Biocompatibility, biodegradation, temperature sensitivity, high water solubility, decreased opsonization by the RES system, and IC50 of 7 ± 14 μg/mL | [53,54,55,56,57,58,59,60,61,62] |
| Polymersomes | Hydrophilicity, high surface density, and large chain length; molecular weight: 750–5000 | Amphiphilicity, core–shell structure, thicker membranes, and high loading capacity; size: 33–265 nm; zeta potential: −20–(−0.3) mV; polydispersity index: 0.13–0.26; EE: 68–98%; LE: 0.9–52% | Higher retention time, deterrence of membrane opsonization, and enhanced systemic half-life in blood | Biocompatibility, decreased toxicity, reduced plasma protein adsorption, and resistance to cellular adhesion | [63,64,65,66,67,68,69,70,71,72] |
| Inorganic nanoparticles | High surface density, hydrophilicity, low cytotoxicity, and non-immunogenicity; molecular weight: 400–8000 | Superparamagnetism, colloidal stability, and reduced inflammatory damage; size: 6.8–650 nm; zeta potential: −36–0.6 mV; polydispersity index: 0.09–0.16; EE: 68–90% | Enhanced blood circulation time, and reduced opsonization and subsequent clearance by the phagocytosis system | Reduced toxicity, high water solubility, moisture retention, filtration and sterilization effects, biosafety, and IC50 of 46 ± 50 μg/mL | [73,74,75,76,77,78,79,80,81,82] |
| Niosomes | Hydrophilicity, cytotoxicity on cancer cells, and flexibility; molecular weight: 600–6000 | Non-ionic surfactant, spherical; size: 117–273 nm; zeta potential: −43–(−1.7) mV; polydispersity index: 0.08–0.54; EE: 72–96%; LE: 1.2–17% | Enhanced cellular uptake, improved curative properties, limited RES system capture, increased half-life, improved storage stability, and reduced side effects | Biocompatibility, protective coating agent, protection against degradation, increased cell viability, and IC50 of 0.43 ± 88 μg/mL | [83,84,85,86,87,88,89,90,91,92] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Christoforou, I.; Kalatzis, A.; Siamidi, A.; Vlachou, M.; Pispas, S.; Pippa, N. The Ubiquitous Use of Polyethylene Glycol in Pharmaceutical Design and Development: Technological Aspects and Future Perspectives. Nanomaterials 2025, 15, 1762. https://doi.org/10.3390/nano15231762
Christoforou I, Kalatzis A, Siamidi A, Vlachou M, Pispas S, Pippa N. The Ubiquitous Use of Polyethylene Glycol in Pharmaceutical Design and Development: Technological Aspects and Future Perspectives. Nanomaterials. 2025; 15(23):1762. https://doi.org/10.3390/nano15231762
Chicago/Turabian StyleChristoforou, Iliana, Anastasios Kalatzis, Angeliki Siamidi, Marilena Vlachou, Stergios Pispas, and Natassa Pippa. 2025. "The Ubiquitous Use of Polyethylene Glycol in Pharmaceutical Design and Development: Technological Aspects and Future Perspectives" Nanomaterials 15, no. 23: 1762. https://doi.org/10.3390/nano15231762
APA StyleChristoforou, I., Kalatzis, A., Siamidi, A., Vlachou, M., Pispas, S., & Pippa, N. (2025). The Ubiquitous Use of Polyethylene Glycol in Pharmaceutical Design and Development: Technological Aspects and Future Perspectives. Nanomaterials, 15(23), 1762. https://doi.org/10.3390/nano15231762











