1. Introduction
Nanomedicine, an innovative field which combines nanotechnology with medical sciences, has recently achieved powerful results in improving humans’ lives [
1,
2]. Offering a wide range of applications, nanomaterials are known for their small dimensions and improved properties. Their high surface-area to volume, enhanced biocompatibility, and versatility have led to an increase in the amount of research on various materials used in nanomedicine [
3]. Nanoparticles are nanoscale structures with dimensions typically under 100 nm which possess high optical, electrical, or chemical activity [
1,
4]. Metallic nanoparticles, a complex category of nanomaterials used in biomedical applications, have shown promising results in healthcare, drug delivery, biosensing or tissue engineering applications [
5].
Silver nanoparticles (AgNPs) are metallic inorganic nanoparticles with superior optical, electric and antibacterial properties [
6]. In nanomedicine, AgNPs are well known for their ability to inhibit bacterial proliferation and to offer a strong antibacterial effect [
7,
8]. Since they are an interesting alternative to antibiotics, most of the literature studies that focus on them have investigated the conditions in which AgNPs exhibit this antimicrobial effect on Gram-positive or Gram-negative bacteria depending on the synthesis method performed [
9,
10,
11]. In contrast, AgNPs have also shown great results in nanoplasmonic sensing and detection applications. In biosensors, AgNPs are mainly used due to their localized surface plasmon resonance (LSPR) property [
12], which is caused by the free movements of electrons once the sample is excited with visible light [
13]. Depending on the size, shape or surface, LSPR can change, leading to the identification of more in-depth information about the suspensions tested or their interactions with various other small molecules [
14,
15]. These main factors are strongly correlated with the synthesis method, so the controlling the synthesis parameters is essential to achieve the highest yield of sensing with respect to the employed detection technique. Therefore, based on possible shifts in plasmonic bands, biosensors fabricated with AgNPs can detect modifications in absorbance once the target is attached to their surface [
13]. Nowadays, various possibilities have been exposed for AgNPs biosensors’ fabrication, depending on the detection technique. In biomedical applications, electrochemical [
16] and optical [
17] approaches showed the most promising results, offering specific detection at low concentration and improved biosensor performance [
13]. For instance, Mandal et al. [
18] developed an electrochemical biosensor by modifying a glassy carbon electrode with AgNPs for the early detection of prostate cancer. The results of the study proved the AgNPs’ nanosize dimensions and their proper surface, while a very small detection limit of 1 pg/mL compared to other existent biosensors for prostate cancer identification was assessed. Demonstrating an incredible sensitivity of about 0.38 µA/pg mL
−1 and a detection of very low concentrations of antigen, the proposed biosensor proved to be a cost-effective intelligent tool for cancer therapy [
18]. Jia et al. [
19] proposed a biosensor based on a AgNP embedded polymer–zirconium-based metal–organic framework (polyUiO-66@AgNPs) for the detection of respiratory virus SARS-CoV2. The AgNPs were prepared through a chemical hydrothermal method, while the ligand agent in the biosensor’s fabrication was a small molecule of carboxylic acid. Based on the high porosity and improved surface properties of AgNPs, the integration of biological molecules such as aptamers and antibodies was achieved with high precision. The system proved to have an extremely low detection limit of 23.4 fg mL
−1, with good stability and excellent reproducibility [
19].
The main aspect to consider in biosensing and biological applications is the synthesis method chosen for AgNPs, since their properties are strongly correlated with the fabrication techniques and the parameters used. Generally, AgNPs can be obtained through physical, chemical, or biological synthesis, depending on the reagents used [
20,
21]. Chemical routes are most frequently used in the literature because they can be easily obtained in the laboratory, and they are cost-effective and reproductible [
22]. Between the chemical strategies, chemical reduction is often preferred, since the reaction time is low, and the method can be easily adjusted. In chemical reduction, a metal salt is introduced in the reaction as a precursor, a reducing agent is used to reduce the metal ions, and in the final step, a stabilizing agent is added to increase the stability of the particles obtained [
23]. Over time, various reagents were proposed depending on the application that was targeted. For example, Khatoon et al. [
24] mediated the synthesis of AgNPs using NaBH
4 as both reducing and stabilizing agents. The physical–chemical characterization proved able to obtain nanosized AgNPs in a range of 10 nm–50 nm with spherical shape and crystalline structure [
24]. In a recent study, Sreelekha et al. [
25] proposed a comparison between chemical reduction using trisodium citrate and green chemistry. The chemical synthesis performed in the article showed dimensions between 9 and 14 nm compared to the green synthesis, for which higher diameters were identified. AgNPs exhibited enhanced efficiency in antioxidant activity for both synthesis methods, confirming their positive effect in biomedical applications [
25]. The choice of the proper parameters is extremely important, since AgNPs suspensions are sensitive to the concentration of solution adjusted. Also, the reagents used can influence the final surface of AgNPs, leading to inappropriate detections in case of biosensors or an insufficient effect in case of antibacterial applications.
In most of the literature studies, the final purpose of the synthesized AgNPs is for antimicrobial applications. Their implementation in sensing applications is underinvestigated compared to other type of nanoparticles; hence, less studies on surface chemistry have been reported for AgNPs. The size and the shape are extremely important, since they can affect the final properties of the material. The optical properties, the plasmonic features, and even the toxicity level depend on the synthesis process. Lately, several authors have described the chemical AgNPs synthesis and their impact in stabilizing AgNP-based colloidal suspensions [
26,
27,
28]. However, most of the existing studies rely on the investigation of multiple chemical reagents in similar concentrations or the investigation of the influence of one specific agent tested. There are a few studies in which both the reducing agents and the stabilizing agents are investigated in such variations in concentration in order to find the best condition to obtain nanosized AgNPs. Furthermore, the efficacy of AgNPs in biosensing applications is strongly dependent on their ability to efficiently graft target molecules onto their surface; thus, their surface chemistry is of the utmost importance in the development of versatile and sensitive biosensors. Nanoparticles’ surface reactivity is influenced by the synthesis agents and their interaction; hence, an in-depth investigation of the synthesis parameters and their effect on the AgNPs’ surface chemistry is required to establish the biosensor fabrication approach and corresponding detection analytical tool, as even a small amount of reagent can change the final properties of the particles and, implicitly, their sensing abilities. Moreover, most studies rely on biosensors’ fabrication using gold nanoparticles, which have been studied for many years now. There are fewer publications describing the implementation of AgNPs in sensing applications and surface chemistry; therefore, this work could offer promising results and in-depth information for future fabrication of biosensors using AgNPs.
To respond to the above-mentioned requirements, the aim of this paper is to compare and to optimize the chemical reduction of AgNPs for further potential (bio)sensing applications. In this regard, various ratios of reducing (NaBH4) and stabilizing (TSC) agents were proposed to identify their impact on nanoparticles’ shape, dimension, and sensitivity. Through this approach, we aim to achieve a balance between performance and nanosize dimensions, leading to an improvement in the surface reactivity of the AgNPs suspensions. To obtain as much information as possible, two different volumes of 400 μL and 1 mL of NaBH4 were tested, while for TSC, three variations of 10, 20, and 50 μL were proposed. The influence of both reagents was determined from an AgNPs dimensional perspective, while the sensitivity properties were assessed for all synthesis variations. Surface chemistry studies were performed to evaluate the AgNPs’ capabilities to efficiently graft target molecules onto their surface and, implicitly, to assess their ability to be implemented as signal transducers for different detection techniques in order to achieve the best conditions for further potential biosensing applications.
3. Results and Discussions
3.1. Optical and Morphological Characterization: UV-VIS Analysis and TEM Microscopy
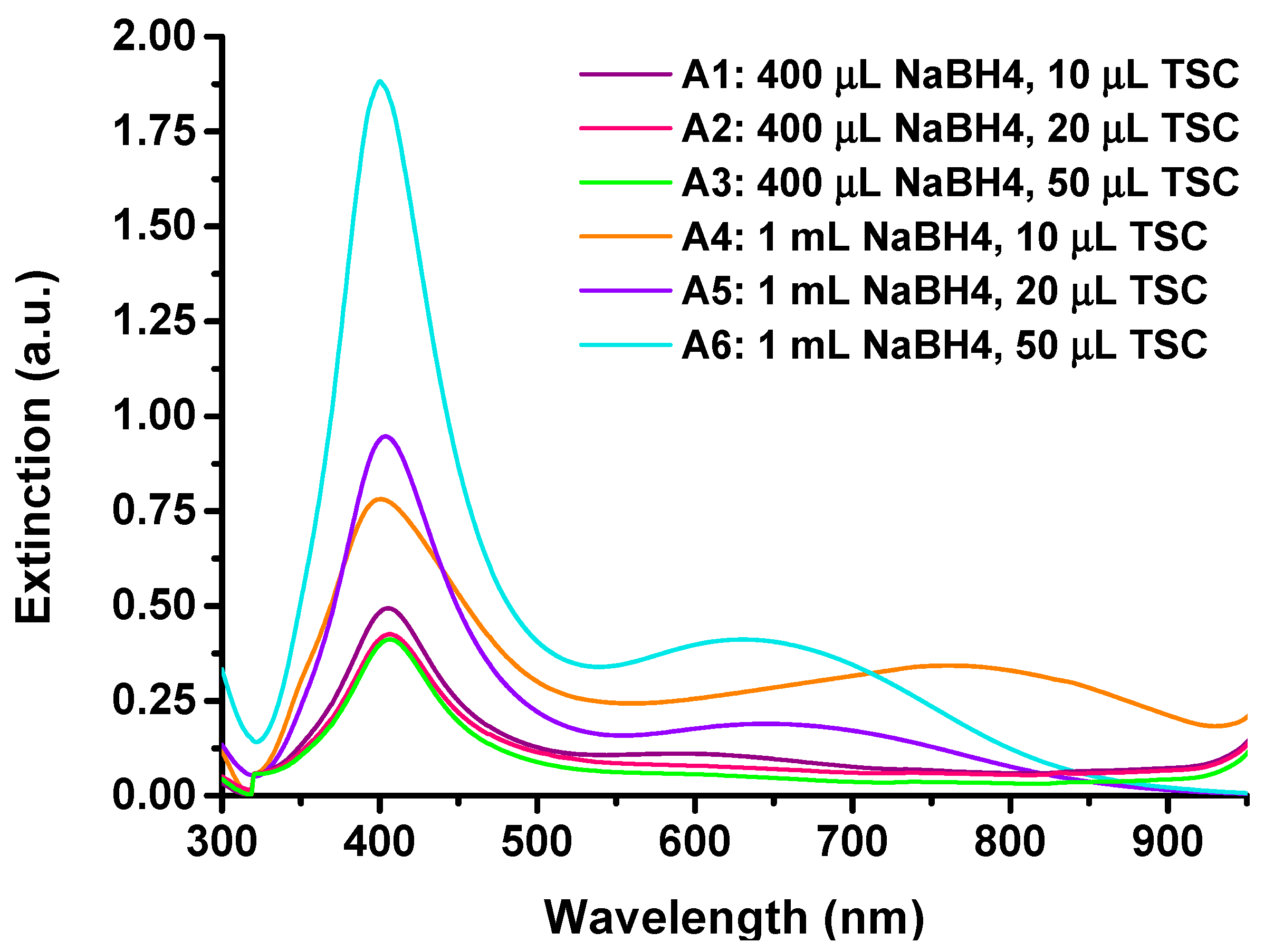
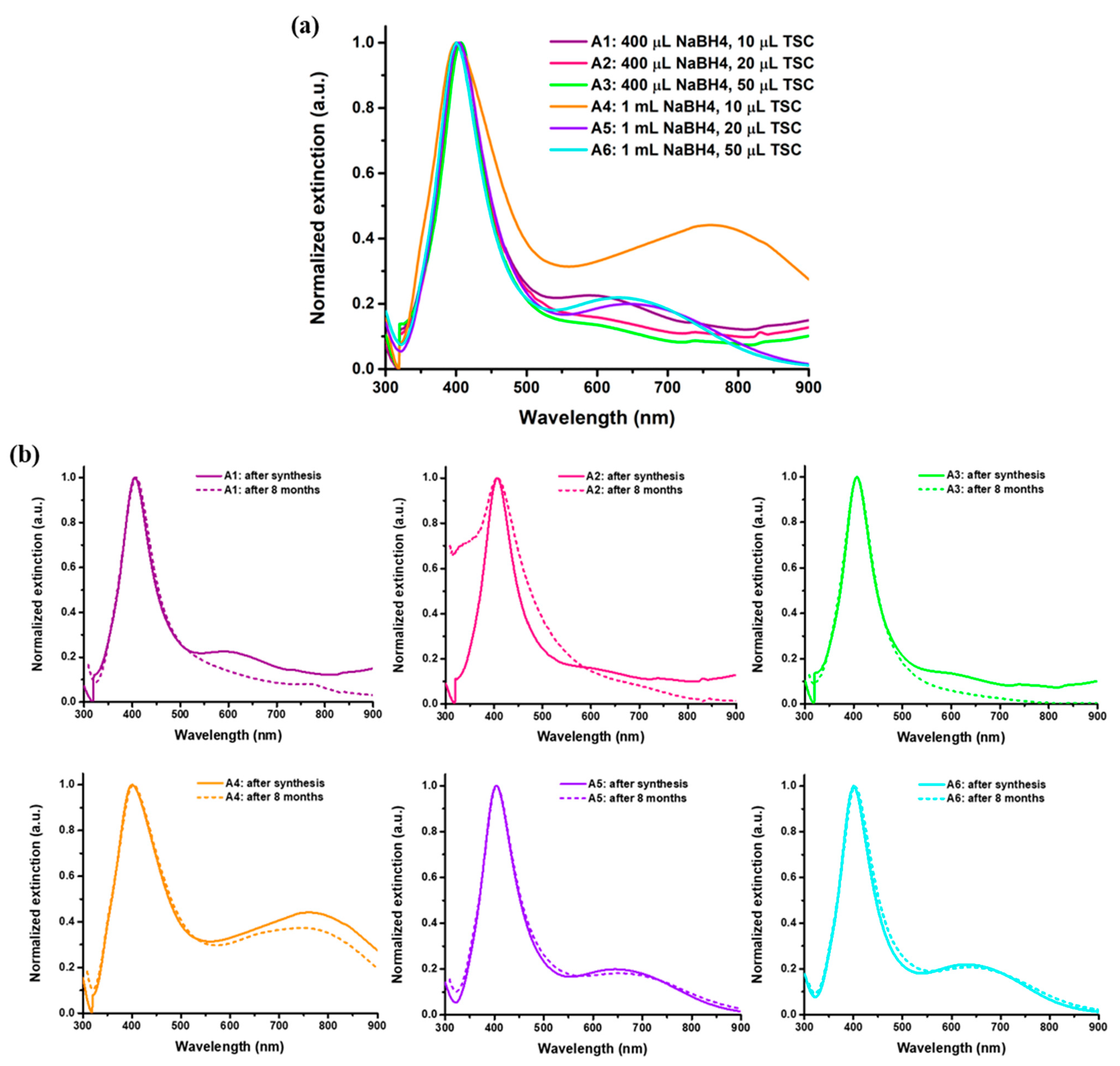
Firstly, the as-synthesized AgNPs in aqueous solution were thoroughly optically and morphologically characterized. UV-VIS absorptions of AgNPs synthesized using various ratios of reducing and stabilizing agents are shown in
Figure 2a. The raw extinction spectra of all colloidal AgNPs samples are found in
Appendix A,
Figure A1.
The LSPR spectra exhibit two extinction bands. Concretely, the formation of AgNPs is confirmed by the well-defined first extinction band for all six samples. The high intensity of the main peak recorded in the range of 400 nm to 405 nm suggests the formation of nanosized AgNPs, confirming that, for all tested variations in the reducing and stabilizing agents, the syntheses were successfully realized. The identification of the AgNPs characteristic LSPR peak sustain that both TSC and NaBH
4 used in the reactions create a proper environment for the reduction of the Ag ions and further stabilization of AgNPs. The second LSPR band exhibited by the synthesized AgNPs can be assigned to larger nanostructures, as the LSPR is highly dependent on the size of the nanoparticles. According to the Mie theory, an increase in size can be observed as a translation of the LSPR to higher wavelengths [
30]. The presence of both LSPR bands indicates the presence of both smaller and larger nanoparticles with respect to the differences between the reducing and stabilizing agents’ volumes. Regarding the AgNPs shape, LSPR bands suggest the formation of predominantly spherical particles due to the main peak observed around 405 nm. The second shoulder observed at higher wavelengths suggests the formation of other shapes and morphologies [
31]; however, its small intensity exposes the theory in which only small parts of the particles are found in different shapes. Furthermore, the second LSPR peak is identified mostly for samples A4–A6, leading to the assumption that an increase in the reducing agent can lead to a variation in AgNPs’ shape. The results are confirmed by TEM images, in which slightly larger nanoparticles are also observed. For the samples using a higher amount of reducing agent (NaBH4), a higher polydispersity is suggested. Samples A1–A3 present a smoother extinction shoulder, while for samples A4–A6 this shoulder is more defined and shifted at even higher wavelengths. These results are in good correlation with literature studies where a range of 400–450 nm is well known to be specific for AgNPs synthesis [
32,
33].
To further characterize the AgNPs colloidal solutions, the full-width at half maximum (FWHM) and the AgNPs molar concentration were determined (
Table 3). For the calculation of the concentration, for samples A1, A2, A3, and A5, the extinction coefficient was 1.45 × 10
10 L/mol·cm, while, for samples A4 and A6, the value for the extinction coefficient was 4.18 × 10
9 L/mol·cm, according to literature reports [
34]. The FWHM values range between 61 and 100 nm, confirming the narrow LSPR response and, implicitly, the presence of prevalently individual AgNPs with similar sizes. Thus, the homogeneity in terms of size is confirmed. The molar concentrations determinations show that the use of higher amounts of reducing agent leads to a higher AgNPs synthesis yield.
Furthermore, the stability of the AgNPs over time was evaluated by recording their extinction spectra after 8 months of storage at 4 °C in the dark.
Figure 2b shows a comparison between the extinction spectrum of the AgNPs after synthesis and after 8 months of storage. No significant modifications in the LSPR response are identified—the extinction spectra recorded after 8 months overlap with the ones acquired after synthesis, thus proving the high stability of the colloidal AgNPs solutions in time and, implicitly, their long shelf-life.
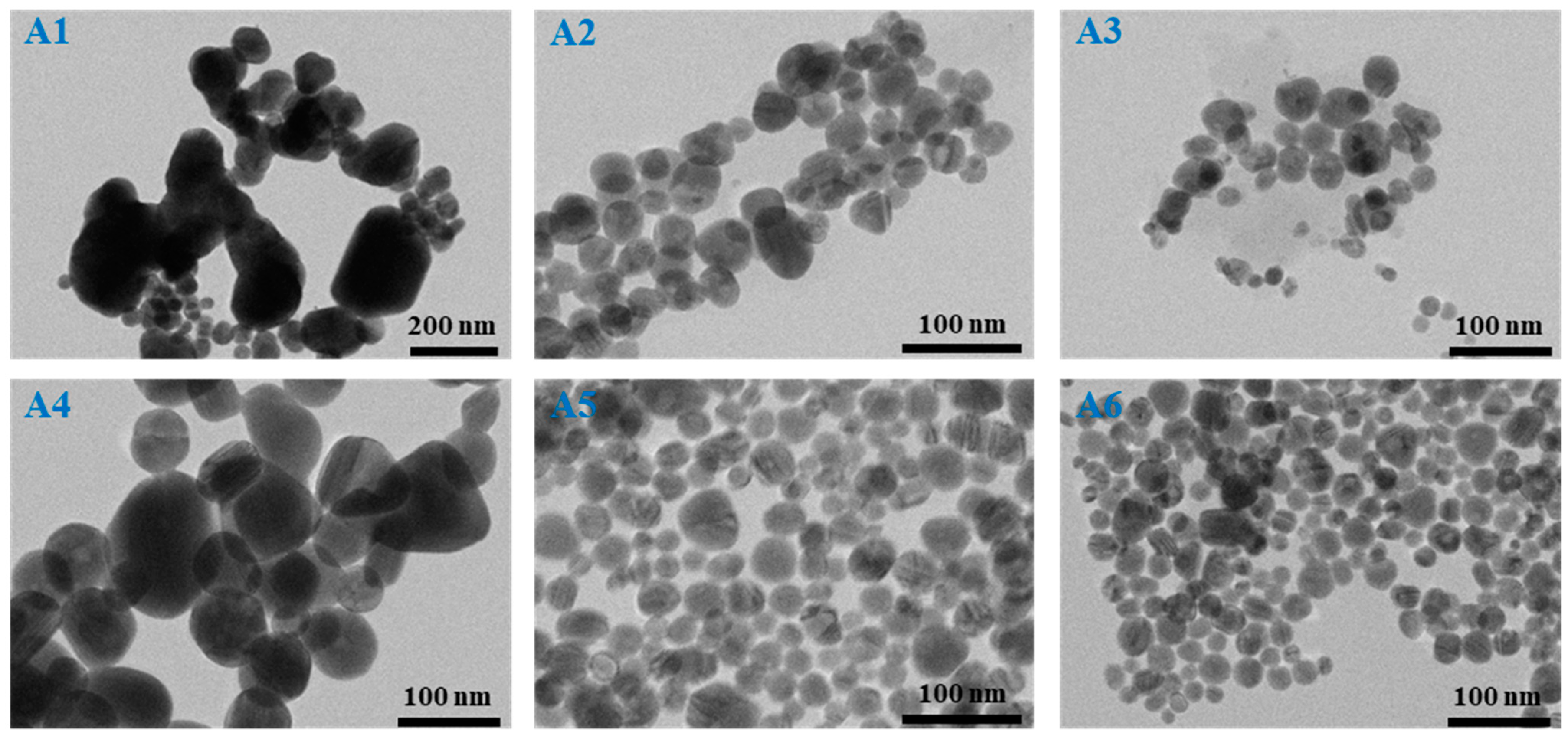
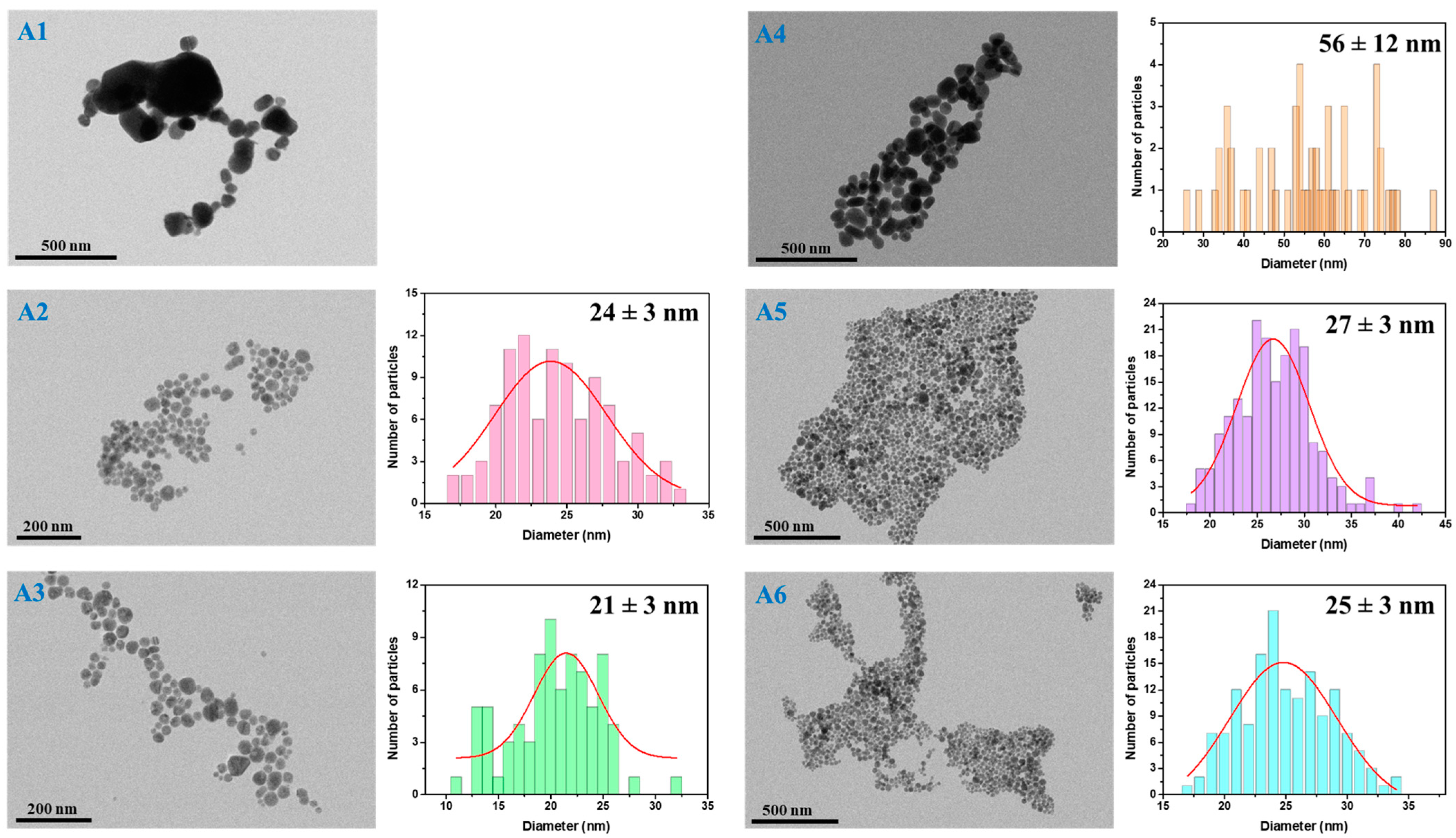
The next step was to evaluate the as-synthesized AgNPs in terms of their morphology and size; thus, TEM microscopy was employed for all samples. Microscopic TEM images were recorded and analyzed using the commercially available ImageJ toolkit. For the determination of the average AgNPs diameter, sets of 100 to 200 nanostructures were measured and represented as particle size distribution histograms.
Figure 3 shows representative TEM microscopic images of all AgNPs with their corresponding size distribution histograms. Inserted into the histograms are the determined average diameter sizes with the standard deviations. Additional TEM microscopic images at higher magnification are found in the
Appendix A,
Figure A2.
As indicated by UV-VIS observations, all samples exhibit the formation of AgNPs with differences according to the reducing–stabilizing agents’ ratio, with the TEM analysis being in good agreement with the optical determinations. Specifically, the proposed synthesis strategies lead to the formation of a rather high yield of spherical nanostructures and neglectable amounts of byproducts, proving to have high homogeneity in terms of shape.
Moreover, samples A5–A6, which were obtained with a higher amount of reducing agent, show slightly larger nanoparticles than their counterparts (A2–A3), where less NaBH4 was added. However, the polydispersity in terms of size is lower in the case of the A2–A3 samples. Additionally, a higher amount of TSC stabilizing agent induces a decrease in the AgNPs’ diameter for both NaBH4 concentrations. In the case of A1 and A4 samples, the polydispersity is highest, showing the formation of both small and considerably larger nanostructures, making the realization of the particle size distribution histograms rather difficult. For the synthesis of these samples, 10 μL of TSC was used, leading to the assumption that the stabilizing agent concentration was too low and, therefore, the AgNPs tended to aggregate and form larger nanostructures. The reducing agent concentration also plays an important role, as a higher NaBH4 and lower TSC concentration induce growth of up to around 90 nm AgNPs with an average diameter of 56 ± 12 nm. The reduction reaction in the presence of both lower NaBH4 and TSC concentrations produces a higher number of Ag nanostructures with diameters over 100 nm.
Furthermore, samples A2–A3 and A5–A6 exhibit similar LSPR band positions located at around 400–405 nm, suggesting the presence of nanosized nanoparticles with comparable diameters. These determinations are supported and confirmed by the particle size distribution histograms, as the diameter size varies between 27 and 21 nm with 3 nm standard deviations. A 30 μL increase in the TSC amount slightly decreases their size; however, the increase in the reducing agent leads to a higher yield of AgNPs. Therefore, according to TEM microscopy, synthesis A2 and A5, in which 20 μL of TSC were used, seems to be the most promising choice for further applications.
3.2. FT-IR Analysis
FT-IR analysis was performed for all six syntheses in order to identify the main chemical groups found in AgNPs and to observe the influence of the reducing and stabilizing agents on the variations in the compounds found on their surfaces and inside the particles’ suspensions. The results of FT-IR spectroscopy, observed in
Figure 4, suggest a real difference between samples A1–A3 and A4–A6.
FT-IR spectroscopy reveals the vibrational bands for various chemical molecules involved in the reducing and stabilizing process. The peak identified at 3120 cm
−1 is associated with the vibrational O-H groups from the citrate, while the peaks from 2910 and 2855 cm
−1 are correlated with C-H stretching from the chemicals used in the reaction [
35]. The functionalization with TSC for all synthesized AgNPs is confirmed by the peak recorded at 1680 cm
−1 and 1580 cm
−1, which is associated with C=O and C-C stretching, indicating the presence of a -COO- chemical group on the surface of each nanoparticle [
36]. Samples A1–A3 presents higher intensities for these peaks compared to their counterparts A4–A6; therefore, a bigger quantity of TSC could be found on their surface. The last peaks identified around 755 and 690 cm
−1 suggest the presence of C=C bending or a symmetric stretching band of B-O [
37].
From the results obtained, it is suggested that NaBH4 influences the final surface properties of the nanoparticles. Samples A1–A3, which contain less reducing agent, show a larger quantity of more intense bands compared to samples A4–A6, in which the intensity of the peaks is decreasing. Furthermore, TSC’s influence is also confirmed, since the samples with more stabilizing agent show less absorbance in IR. These results could be explained by a possible mechanism between the two reagents used. Adding a higher quantity of NaBH4 to the reaction can lead to a faster reduction. Therefore, a higher number of synthesized particles can lead to a larger surface which could be further covered by TSC. Since the TSC is added in very small amounts, each particle will receive less TSC on their surface, leading therefore to smaller intensities in FT-IR spectra for the samples where NaBH4 was added in larger quantities. These assumptions are sustained by the differences between syntheses A1 and A3, for example, where the same amount of TSC was added. The sample with more NaBH4 shows stronger absorption in IR. Also, it could be the intercalation of TSC between two or more particles when more NaBH4 is used, leading to a decrease in the vibrations of the molecules. This would lead to less identification of TSC on the surface using FT-IR spectroscopy, since it will also be trapped between the particles. However, for this hypothesis to be sustained, higher intensities in further SERS analysis should be identified. The SERS results proved that a bigger amount of reducing agent will lead to higher densities of TSC on AgNPs surfaces; therefore, this mechanism is highly likely to happen.
3.3. SERS Analysis of the AgNPs Surface
In order to assess the AgNPs’ capabilities to efficiently detect modifications in their close vicinity and, thus, operate as signal transducers in biosensing applications, their surface was characterized using SERS spectroscopy. Concretely, the next step was to record the SERS response of the as-synthesized AgNPs using a Raman spectrometer. For this purpose, the colloidal AgNPs in aqueous solutions were exposed to a laser with an excitation wavelength of 532 nm with a set power at 10% and an integration time of 10 s.
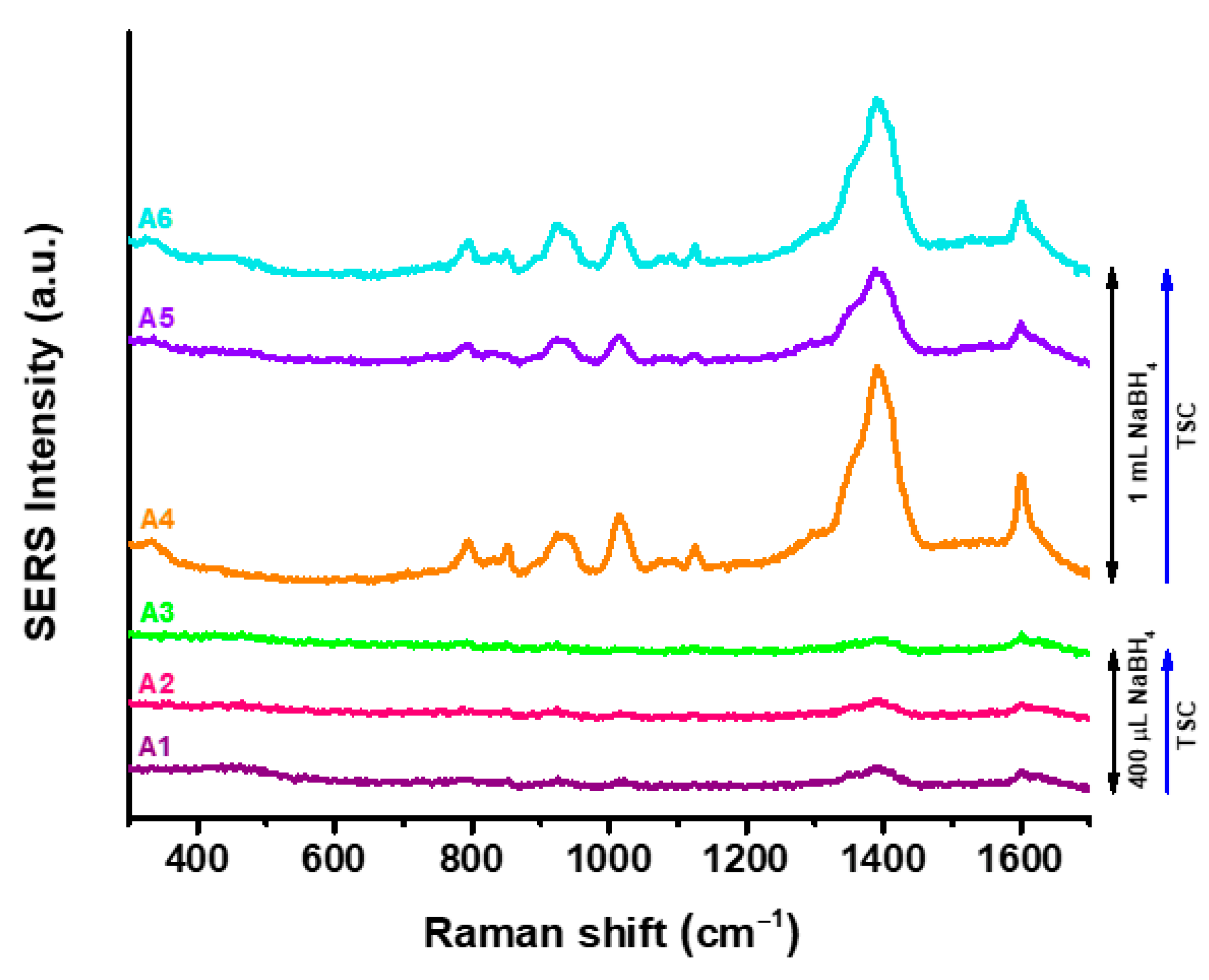
From the SERS spectra recorded for each of the synthesized AgNPs (
Figure 5), it can be observed that AgNPs obtained using 400 µL of NaBH
4 have a weak SERS response irrespective to the TSC concentration, in agreement with the literature [
38]. For a higher concentration of reducing agent, characteristic TSC bands appear in the SERS spectra, indicating the presence of a large amount of stabilizing agent on the nanoparticles’ surface. Specifically, in the SERS spectra of A4–A6, the following characteristic vibrational modes can be identified: the bands located at 830–852 cm
−1 are assigned to C-C stretching vibrations; those at 925–943 cm
−1 are indicative of C-COO stretching modes; at 1125 cm
−1, stretching vibrations of C-OH are identified; the band at 1391 cm
−1 corresponds to symmetrical stretching vibrations of COO; while the COO asymmetric vibrational modes are indicated by the Raman band located at 1600 cm
−1 [
39]. Thus, the presence of TSC on the surface of the AgNPs is specifically identified and confirmed. However, the reducing agent concentration influences the coverage of the metallic surface with TSC, as the characteristic Raman bands of the stabilizing agent are considerably enhanced when higher amounts of NaBH
4 are used to reduce the silver ions. Thus, for SERS biosensing applications, the AgNPs with lower amounts of NaBH
4 are better candidates of choice, as the TSC signal does not overlap with or cover the Raman vibrational bands of the target analytes.
The differences between FT-IR and SERS appear due to the basic principles of the technique. In FT-IR, the total amount of compounds is recorded, yet for SERS only the surface of the particle is analyzed. Therefore, in the case of samples A4–A6, where the peak intensity is lower in FT-IR, but higher in SERS, TSC is most likely to be distributed between two or more particles, not only on their surface. FT-IR spectroscopy and SERS analysis correlation confirmed the functionalization and stabilization with TSC on AgNPs surface, a process which could be the main reason for the optimal stability of all AgNPs samples. For further analysis, the samples A2 and A5 were chosen in order to evaluate the optical sensing capabilities of both AgNPs categories.
3.4. Study of the AgNPs Bulk Refractive Index Sensitivity (RIS)
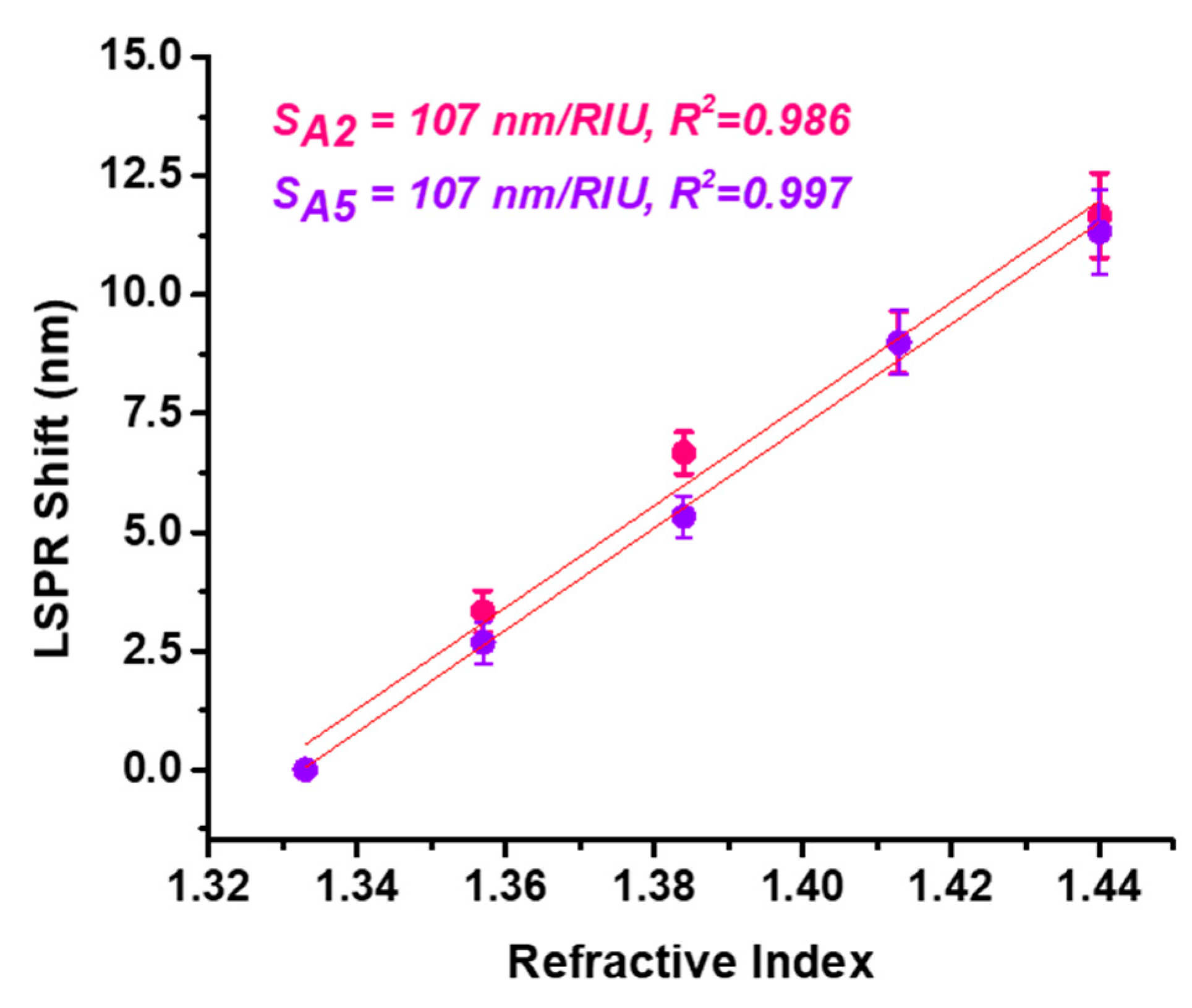
Lastly, the
bulk refractive index sensitivity (RIS) of samples A2 and A5 was studied to determine their optical biosensing capabilities. Specifically, the sensitivity of the nanoparticles to changes in the microenvironment in the nanoparticles’ close vicinity was evaluated. For this aim, the dielectric medium of the nanoparticles previously in aqueous solution was changed with water–glycerol mixtures of different concentrations, i.e., 0, 20, 40, 60 and 80% glycerol. The LSPR property of metallic nanoparticles is dependent on the refractive index of the medium in which the nanoparticles are located; thus, an increase in the refractive index leads to a red-shift in the LSPR band. By employing different water–glycerol mixtures, the refractive index was changed from 1.333 corresponding to water to 1.44 corresponding to an 80% glycerol concentration. The LSPR response was monitored for each glycerol concentration, followed by the extraction of their spectral positions. With an increasing refractive index, the LSPR of the A2 sample red-shifted up to 8 nm for the 80% water–glycerol mixture, whereas for A5 the extinction band was translated to higher wavelengths with 12 nm, exhibiting higher sensitivity to refractive index changes in the microenvironment in the AgNPs’ proximity. Further, the LSPR red-shift with respect to 0% glycerol concentration was plotted as a function of the refractive index (
Figure 6). From the obtained linear regressions, the RIS values for AgNPs were extracted as the slope of the fitted lines resulting in RIS of 107 nm/RIU for both samples. The R
2 coefficients show the good correlation of the fit. These results prove the strong influence of the synthesis parameters and the need to optimize the methods applied in order to achieve the best results for further biosensing applications.
3.5. “Proof-of-Concept” Biosensing
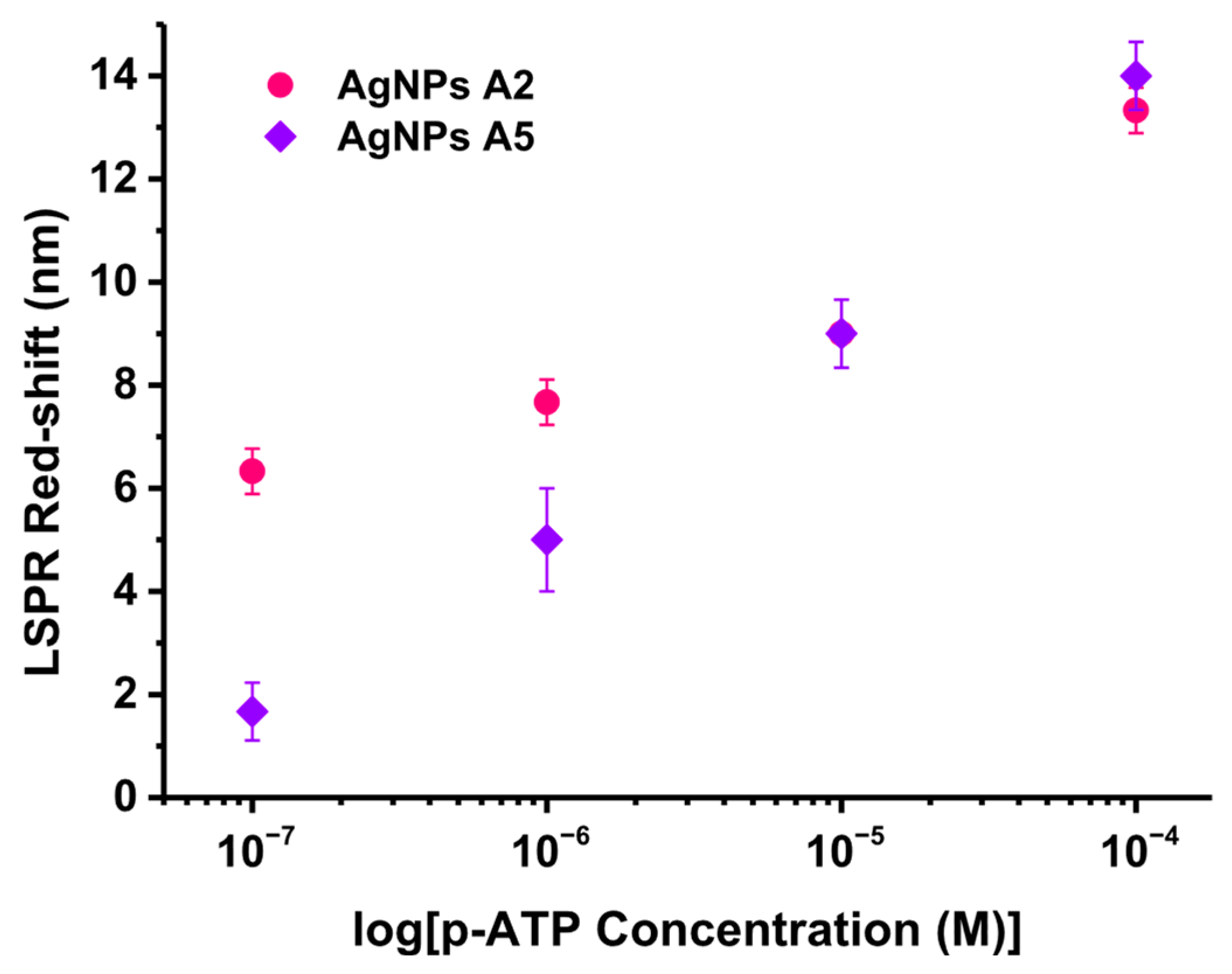
Finally, the biosensing capabilities of the A2 and A5 AgNPs samples were tested. p-ATP, a small well-known molecule, was chosen to be grafted onto the AgNPs due to its thiolated active functional group which binds to the metallic surface. Concretely, the AgNPs were exposed to various ethanolic p-ATP concentrations from 10
−4 to 10
−7 M, followed by the recording of their extinction spectra. The biosensing test was realized in triplicate. From the comparison to the LSPR responses of the unfunctionalized AgNPs, the LSPR red-shifts were extracted and graphically represented with respect to the p-ATP tested concentrations (
Figure 7). The functionalization with p-ATP induced red-shifts from 13 nm for the highest concentration to 6 nm for the lowest concentration in the case of AgNPs sample A2, while for sample A5 the red-shifts varied from 14 nm (highest concentration) to 2 nm (lowest concentration). Both samples showed LSPR biosensing capabilities; however, sample A2 shows better performances compared to A5, indicating that concentrations below 10
−7 M can be achieved.
Thus, the results presented in this work have proved that all six syntheses can be considered optimal for AgNPs fabrication. The UV-VIS spectra have shown specific LSPR peaks associated with nanometric dimensions and predominantly spherical shape, while the syntheses performance was confirmed by the high stability of the AgNPs in time (up to 8 months). The variation of TSC and NaBH4 suggested a real impact on the AgNPs surface, leading to the assumption that their size and shape can be influenced by the conditions in which the synthesis is performed. Even if all syntheses proved good results, samples A2 and A5, the ones with a moderate amount of TSC added (20 μL), exhibit better performances for further sensing applications. NaBH4 contribution has also been established for detection applications, as we demonstrated that sample A2, the one with less reducing agent (400 μL) and moderate stabilizing agent (20 μL), exhibits the best performance for biosensing applications.