Photoelectrochemical Water Splitting by SnO2/CuO Thin Film Heterostructure-Based Photocatalysts for Hydrogen Generation
Abstract
1. Introduction
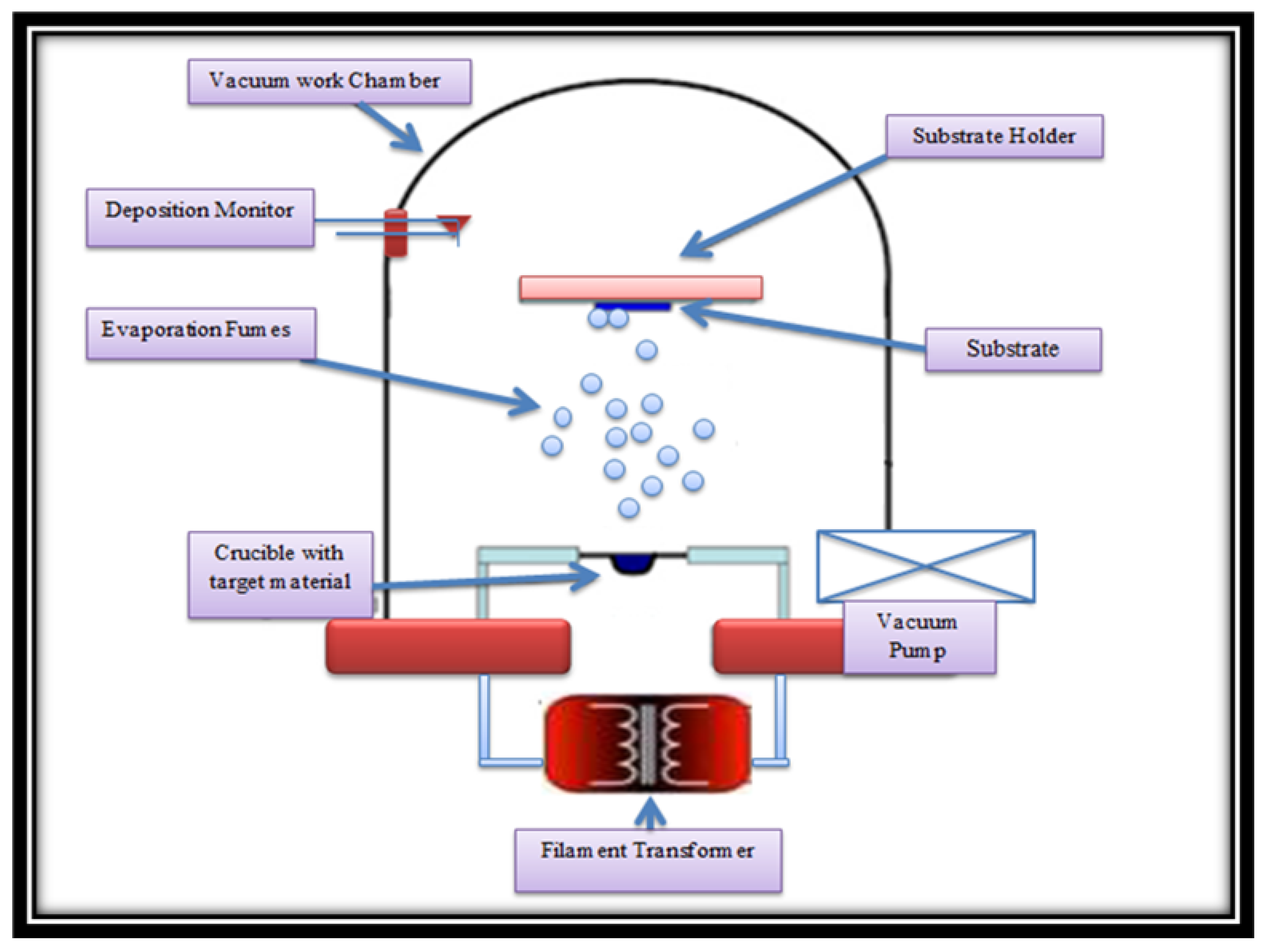
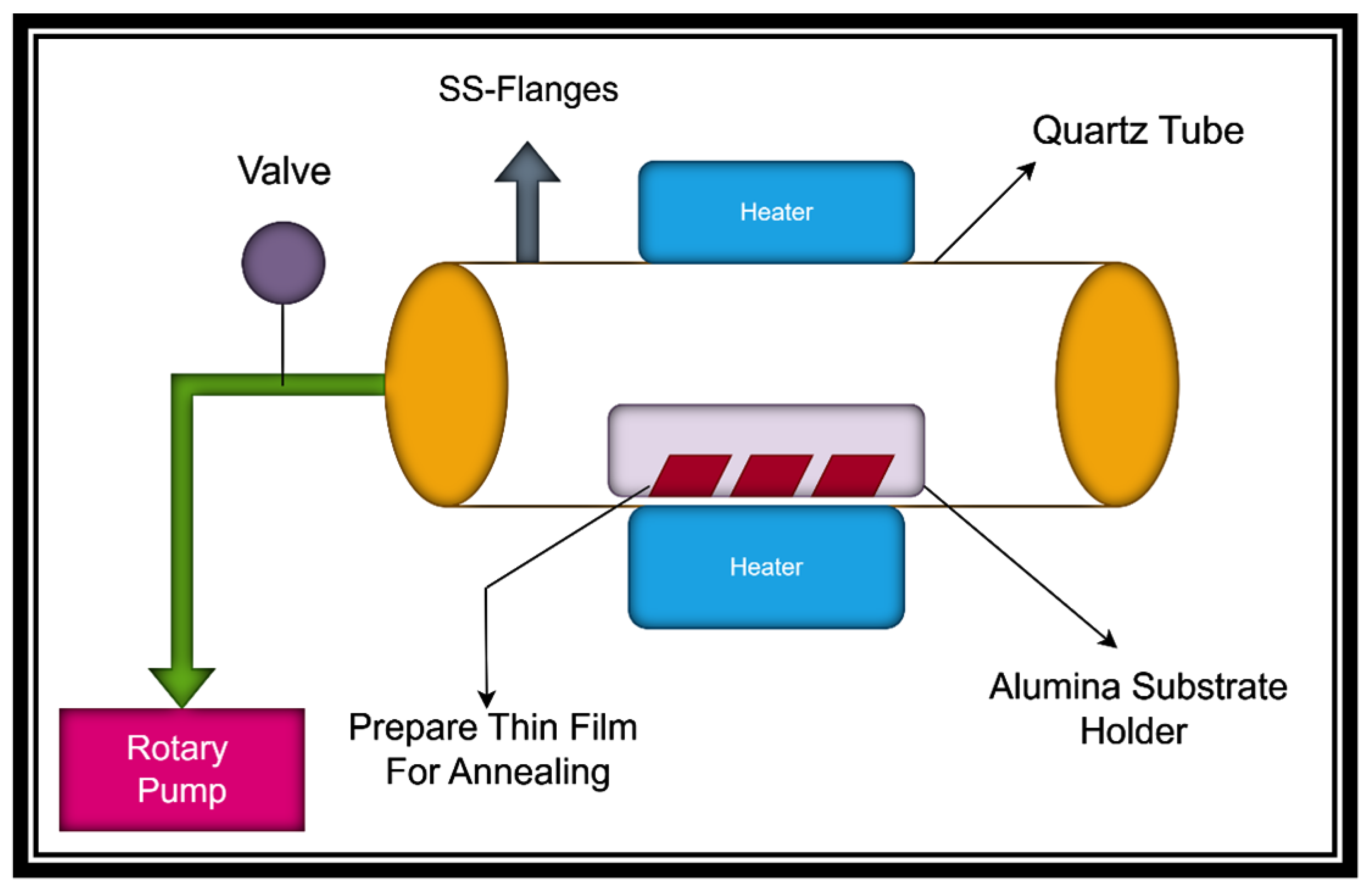
2. Experimental Procedure
3. Results and Discussion
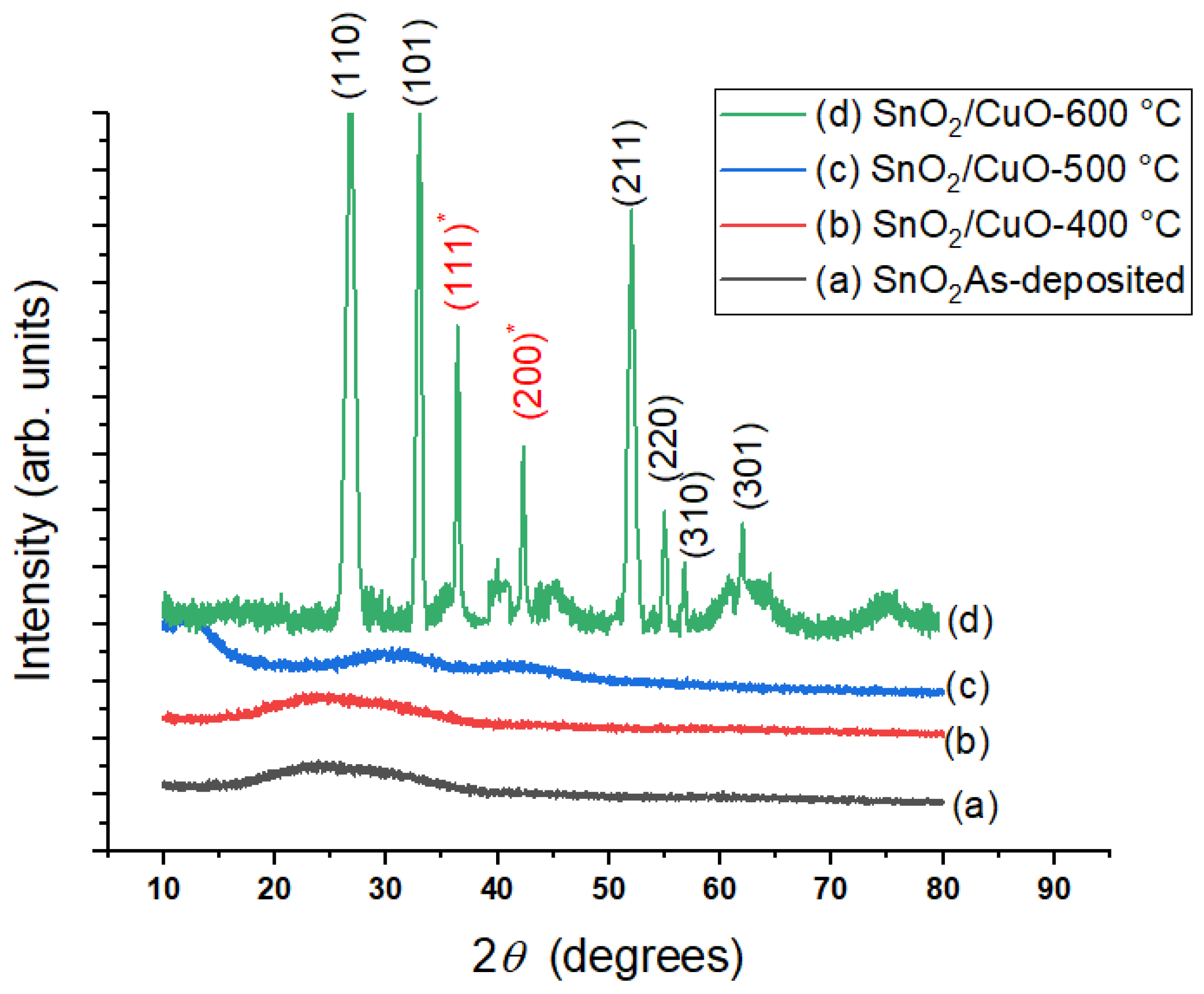
3.1. Structural Analysis
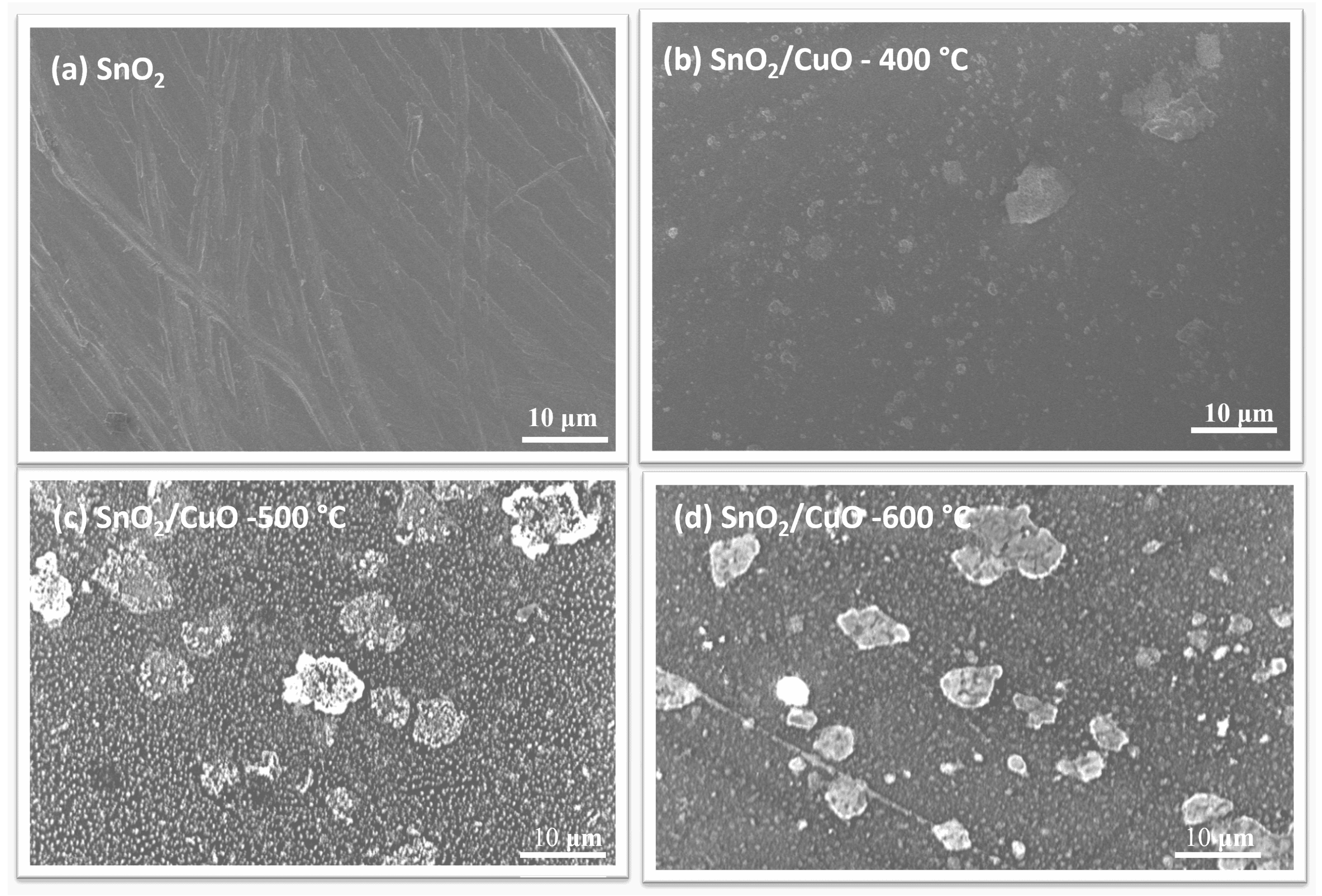
3.2. Morphological Analysis
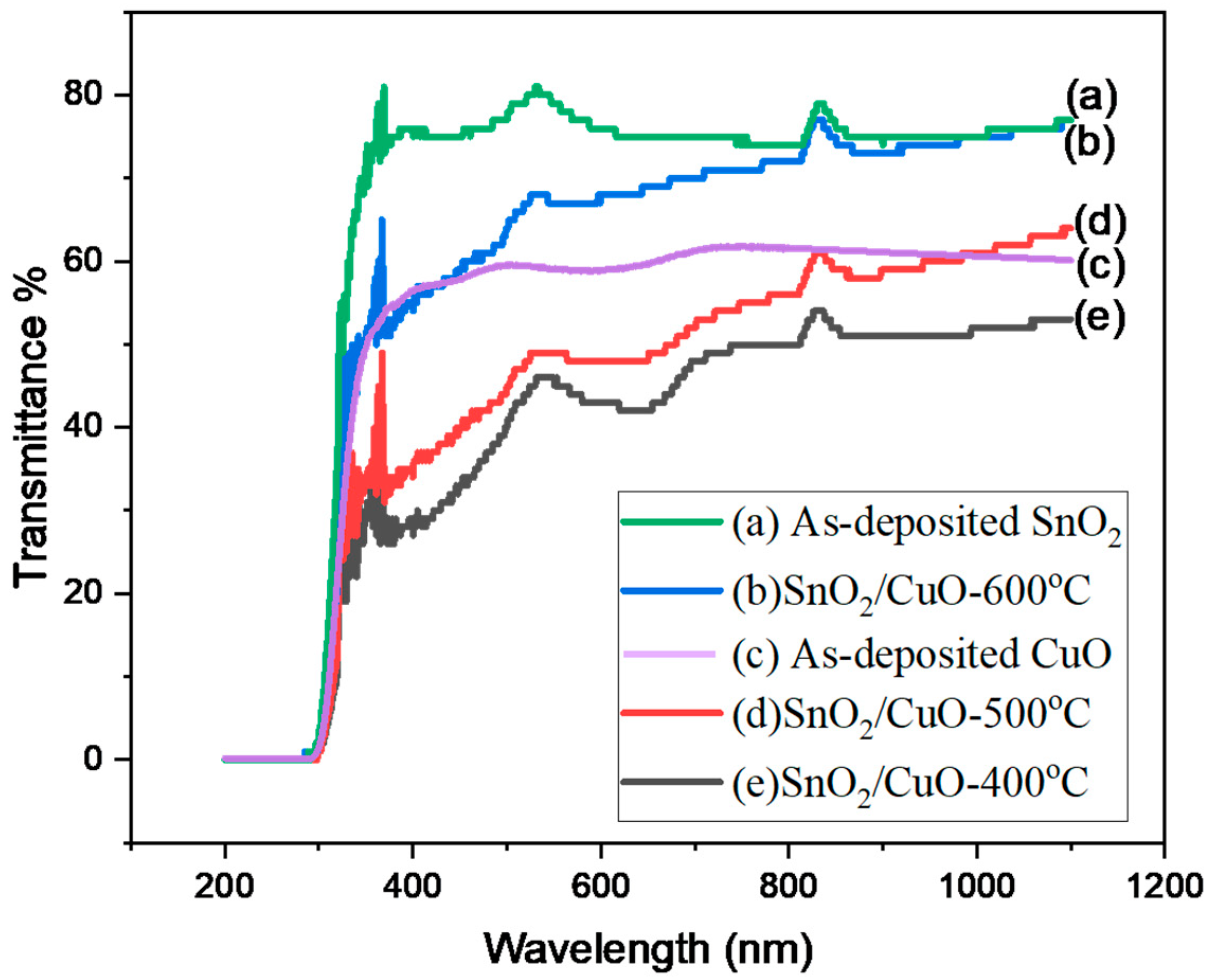
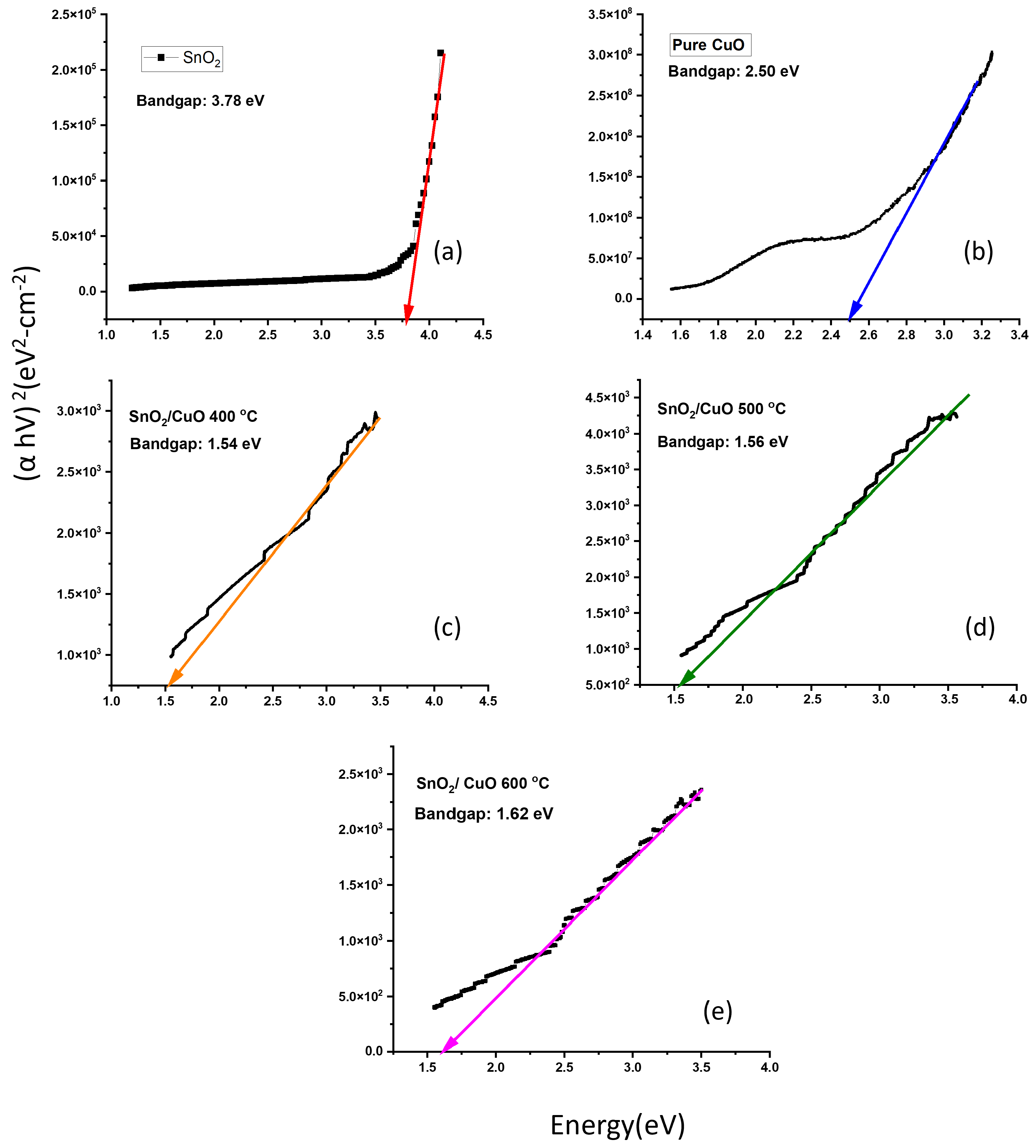
3.3. Bandgap Determination
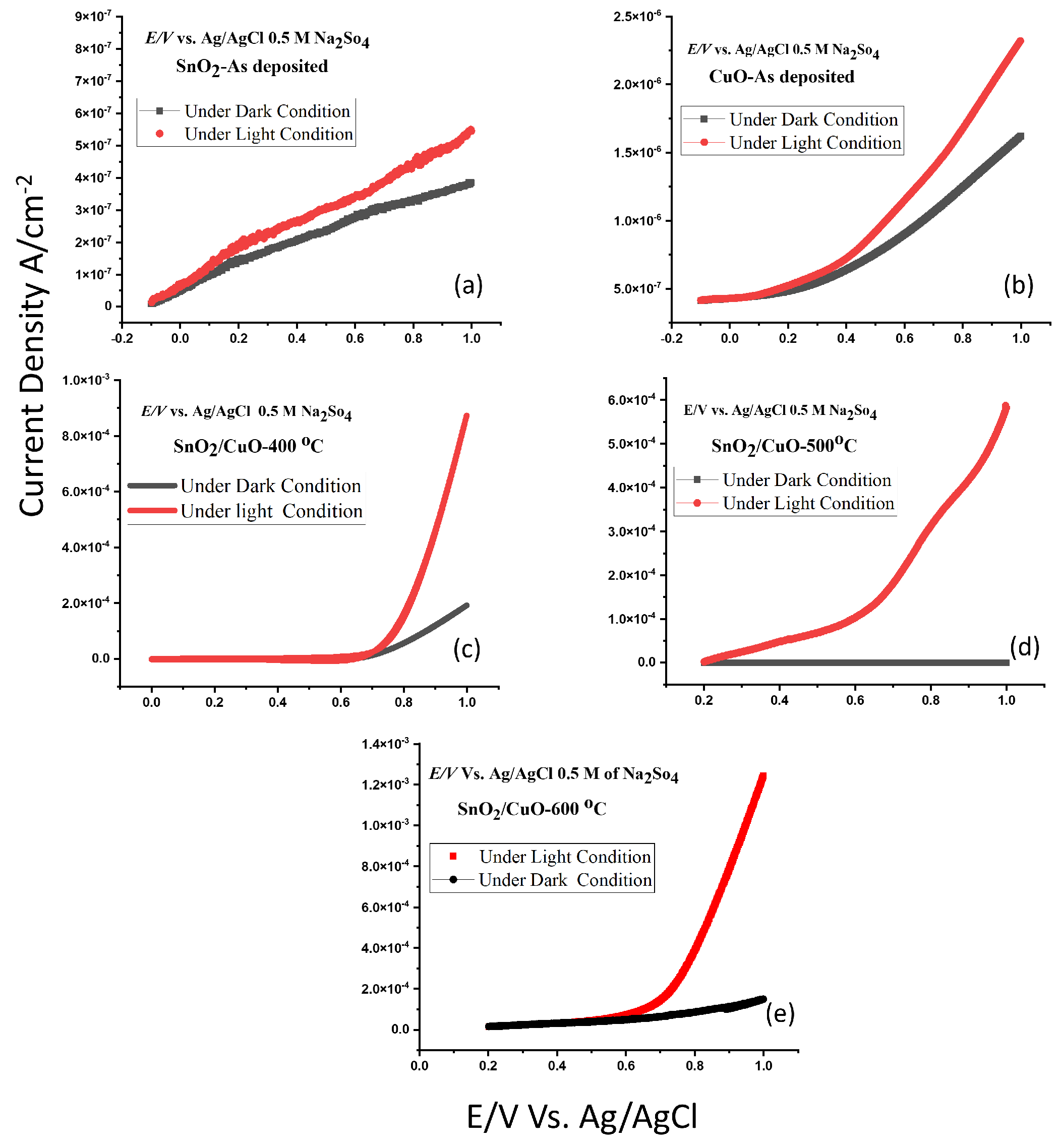
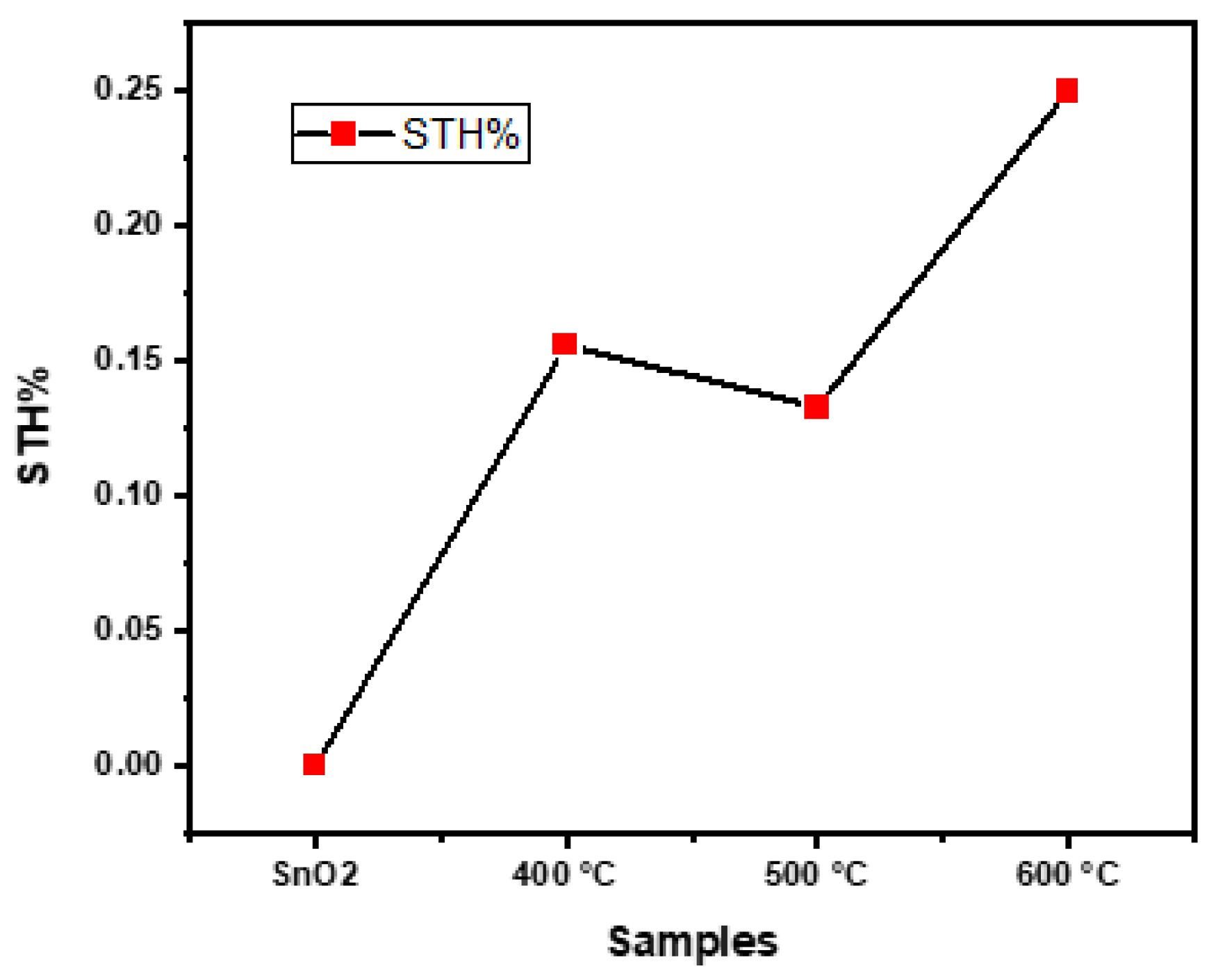
3.4. Photoelectrochemical Water Splitting
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, J.; Azam, W. Natural resource scarcity, fossil fuel energy consumption, and total greenhouse gas emissions in top emitting countries. Geo. Front. 2024, 15, 101757. [Google Scholar] [CrossRef]
- Luque-Urrutia, J.A.; Ortiz-García, T.; Solà, M.; Poater, A. Green energy by hydrogen production from water splitting, water oxidation catalysis and acceptorless dehydrogenative coupling. Inorganics 2023, 11, 88. [Google Scholar] [CrossRef]
- Dutta, T.; Chaturvedi, P.; Thakur, A.; Mishra, S.K. Review and Outlook of Graphene-Based Catalysis: Revolutionizing Water Splitting for Sustainable Hydrogen Production. Energy Fuels 2025, 39, 8827–8870. [Google Scholar] [CrossRef]
- Hassan, Q.; Algburi, S.; Sameen, A.Z.; Jaszczur, M.; Salman, H.M. Hydrogen as an energy carrier: Properties, storage methods, challenges, and future implications. Envir. Syst. Decis. 2024, 44, 327–350. [Google Scholar] [CrossRef]
- Vilanova, A.; Dias, P.; Lopes, T.; Mendes, A. The route for commercial photoelectrochemical water splitting: A review of large-area devices and key upscaling challenges. Chem. Soc. Rev. 2024, 53, 2388–2434. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Chen, A.; Chen, Y.; Qiao, F.; Wang, J.; Yang, N.; Wen, Z. Advancements in electrochemical synthesis: Expanding from water electrolysis to dual-value-added products. EScience 2024, 5, 100333. [Google Scholar] [CrossRef]
- Peng, Y.; Rabeah, J.; Junge, H.; Beller, M. A Protocol for Unveiling the Nature of Photocatalytic Hydrogen Evolution Reactions: True Water Splitting or Sacrificial Reagent Acceptorless Dehydrogenation? Angew. Chem. Int. Ed. 2024, 63, e202408626. [Google Scholar] [CrossRef]
- Son, M.K. Key strategies on Cu2O photocathodes toward practical photoelectrochemical water splitting. Nanomaterials 2023, 13, 3142. [Google Scholar] [CrossRef]
- Sun, H.; Wang, Z.; Meng, Q.; White, S. Advancements in hydrogen storage technologies: Enhancing efficiency, safety, and economic viability for sustainable energy transition. Int. J. Hydrog. Energy 2025, 105, 10–22. [Google Scholar] [CrossRef]
- Dou, Y.; Sun, L.; Ren, J.; Dong, L. Opportunities and future challenges in hydrogen economy for sustainable development. Hydrogen Econ. 2023, 537–569. [Google Scholar]
- Shaban, M.; Almohammedi, A.; Saad, R.; Sayed, A.N.E. Design of SnO2: Ni, Ir nanoparticulate photoelectrodes for efficient photoelectrochemical water splitting. Nanomaterials 2022, 12, 453. [Google Scholar] [CrossRef] [PubMed]
- Alasali, F.; Abuashour, M.I.; Hammad, W.; Almomani, D.; Obeidat, A.M.; Holderbaum, W. A review of hydrogen production and storage materials for efficient integrated hydrogen energy systems. Energy Sci. Eng. 2024, 12, 1934–1968. [Google Scholar] [CrossRef]
- Faraji, M.; Yousefi, M.; Yousefzadeh, S.; Zirak, M.; Naseri, N.; Jeon, T.H.; Moshfegh, A.Z. Two-dimensional materials in semiconductor photoelectrocatalytic systems for water splitting. Ener. Environ. Sci. 2019, 12, 59–95. [Google Scholar] [CrossRef]
- Rizzi, G.A.; Girardi, L. Metal oxides for photoelectrochemical fuel production. In Tailored Functional Oxide Nanomaterials: From Design to Multi-Purpose Applications; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2022; pp. 339–377. [Google Scholar]
- Prasad, A.; Verma, J.; Suresh, S.; Arisutha, S. Recent advancements in the applicability of SnO2-based photo-catalysts for hydrogen production: Challenges and solutions. Waste Dispos. Sustain. Energy 2022, 4, 179–192. [Google Scholar] [CrossRef]
- Nassar, M.H.; El-Nowihy, G.H. CdS–SnO2–ZnO–Doped TiO2 Nanocomposite: A High-Efficiency Photoanode for Dye-Sensitized Solar Cells. ACS Appl. Energy Mater. 2025, 8, 16066–16074. [Google Scholar] [CrossRef]
- Uma, H.B.; Kumar, M.S.V.; Ananda, S. Semiconductor-assisted photodegradation of textile dye, photo-voltaic and antibacterial property of electrochemically synthesized Sr-doped CuO nano photocatalysts. J. Mol. Struct. 2022, 1264, 133110. [Google Scholar] [CrossRef]
- Liu, D.; Kuang, Y. Particle-based photoelectrodes for PEC water splitting: Concepts and perspectives. Adv. Mater. 2024, 36, 2311692. [Google Scholar] [CrossRef] [PubMed]
- Riffat, M.; Ali, H.; Qayyum, H.A.; Bilal, M.; Hussain, T. Enhanced solar-driven water splitting by ZnO/CdTe heterostructure thin films-based photocatalysts. Int. J. Hydrogen Energy 2023, 48, 22069–22078. [Google Scholar] [CrossRef]
- Islam, M.S.; Rahman, M.K.; Hossain, M.S.; Howlader, A.S.; Mim, J.J.; Islam, S.; Hossain, N. Advantages of Narrow Bandgap Nanoparticles in Semiconductor Development and their Applications. Semiconductors 2024, 58, 849–873. [Google Scholar] [CrossRef]
- Pinto, A.H.; Nogueira, A.E.; Dalmaschio, C.J.; Frigini, I.N.; de Almeida, J.C.; Ferrer, M.M.; de Mendonça, V.R. Doped tin dioxide (d-SnO2) and its nanostructures: Review of the theoretical aspects, photocatalytic and biomedical applications. Solids 2022, 3, 327–360. [Google Scholar] [CrossRef]
- Babu, M.H.; Podder, J.; Dev, B.C.; Sharmin, M. p to n-type transition with wide blue shift optical band gap of spray synthesized Cd doped CuO thin films for optoelectronic device applications. Surfaces Interfaces 2020, 19, 100459. [Google Scholar] [CrossRef]
- Zhang, Y.M.; Zhao, Z.Y.; Tang, W.; Feng, J.Y.; Zhang, J.; Liu, Q.J.; Zou, Z.G. CuCoO2/CuO heterostructure: Understanding the role of band alignment in selective catalysis for overall water splitting. J. Mater. Chem. A 2023, 11, 20151–20164. [Google Scholar] [CrossRef]
- Selleswari, D.; Meena, P.; Mangalaraj, D. Design of CuO/SnO2 heterojunction photocatalyst with enhanced UV light-driven photocatalytic activity on congo-red and malachite green dyes. J. Iran. Chem. Soc. 2019, 16, 1291–1300. [Google Scholar] [CrossRef]
- Animasahun, L.O.; Taleatu, B.A.; Adewinbi, S.A.; Bolarinwa, H.S.; Fasasi, A.Y. Synthesis of SnO2/CuO/SnO2 multi-layered structure for photoabsorption: Compositional and some interfacial structural studies. J. Niger. Soc. Phys. Sci. 2021, 3, 74–81. [Google Scholar] [CrossRef]
- Salmanizadeh, M.; Sadeghzadeh-Attar, A. Anchoring TiO2 and CuO nanoparticles on SnO2 nanotube arrays to construct ternary heterostructure for visible-light-driven photocatalytic degradation of green malachite dye. J. Mol. Liq. 2024, 414, 126271. [Google Scholar] [CrossRef]
- Salih, B.M.; Ramizy, A.; Salih, E.Y.; Afzal, N.; Eisa, M.H.; Mustapha, N. Preparation and Simulation of AlGaN/CdS/Si Double Heterojunction for Solar Cell Application. Plasmonics 2025, 20, 10117–10132. [Google Scholar] [CrossRef]
- Guo, X.; Yu, J.; Ren, S.; Gao, R.T.; Wu, L.; Wang, L. Controlled defective engineering on CuIr catalyst promotes nitrate selective reduction to ammonia. ACS Nano 2024, 18, 24252–24261. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wu, Y.; Li, J.; Jin, Z.; Li, Y.; Tsubaki, N. Amorphous/crystalline heterojunction interface driving the spatial separation of charge carriers for efficient photocatalytic hydrogen evolution. Mater. Today Phys. 2022, 27, 100767. [Google Scholar] [CrossRef]
- Liang, G.; Li, Z.; Ishaq, M.; Zheng, Z.; Su, Z.; Ma, H.; Chen, S. Charge separation enhancement enables record photocurrent density in Cu2ZnSn(S,Se)4 photocathodes for efficient solar hydrogen production. Adv. Energy Mater. 2023, 13, 2300215. [Google Scholar] [CrossRef]
- Huang, Y.S.; Liu, Y.T.; Perng, T.P.; Lu, M.Y.; Chueh, Y.L.; Chen, L.J. Enhancing photocatalytic properties of continuous few-layer MoS2 thin films for hydrogen production by water splitting through defect engineering with Ar plasma treatment. Nano Energy 2023, 109, 108295. [Google Scholar] [CrossRef]
- Chen, X.; Lv, S.; Liu, Y.; Gu, H.; Sun, X.; Hu, Q.; Guo, L. Antiferroelectric SnO2 network with amorphous surface for electrochemical N2 fixation. Ange. Chem. Inter. Edit. 2025, 64, 202515222. [Google Scholar] [CrossRef]
- Arjunan, K.; Ramesh Babu, R. Chemical spray deposited SnO2: MoO3 composite thin films for visible light photocatalytic dye degradation application. Ionics 2024, 30, 4227–4244. [Google Scholar] [CrossRef]
- Kaur, J.; Khanna, A.; Kumar, R.; Chandra, R. Growth and characterization of Cu2O and CuO thin films. J. Mater. Sci. Mater. Electron. 2022, 33, 16154–16166. [Google Scholar] [CrossRef]
- Qi, Z.; An, Z.; Huang, B.; Wu, M.; Wu, Q.; Jiang, D. Visible-light-catalyzed synthesis of 1, 3-benzoxazines via formal [4+2] cycloaddition of oximes with o-hydroxybenzyl alcohols. Organ. Biomol. Chem. 2023, 21, 6419–6423. [Google Scholar] [CrossRef]
- Srinivasan, S. Electrode/electrolyte interfaces: Structure and kinetics of charge transfer. In Fuel Cells: From Fundamentals to Applications; Springer: New York, NY, USA, 2006; pp. 27–92. [Google Scholar]
- Arora, I.; Chawla, H.; Chandra, A.; Sagadevan, S.; Garg, S. Bismuth-Based Photocatalytic Material for Clean Energy Production and CO2 Reduction. In Photocatalysis for Environmental Remediation and Energy Production: Recent Advances and Applications; Springer: Berlin/Heidelberg, Germany, 2023; pp. 363–394. [Google Scholar]
- Xiao, C.; Zhou, Z.; Li, L.; Wu, S.; Li, X. Tin and oxygen-vacancy co-doping into hematite photoanode for improved photoelectrochemical performances. Nanoscale Res. Lett. 2020, 15, 54. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Faraz, J.A.; Hussain, T.; Bilal, M.; Ahmad, K.; Cotirla, L.-I. Photoelectrochemical Water Splitting by SnO2/CuO Thin Film Heterostructure-Based Photocatalysts for Hydrogen Generation. Nanomaterials 2025, 15, 1748. https://doi.org/10.3390/nano15221748
Faraz JA, Hussain T, Bilal M, Ahmad K, Cotirla L-I. Photoelectrochemical Water Splitting by SnO2/CuO Thin Film Heterostructure-Based Photocatalysts for Hydrogen Generation. Nanomaterials. 2025; 15(22):1748. https://doi.org/10.3390/nano15221748
Chicago/Turabian StyleFaraz, Joun Ali, Tanvir Hussain, Muhammad Bilal, Khaleel Ahmad, and Luminita-Ioana Cotirla. 2025. "Photoelectrochemical Water Splitting by SnO2/CuO Thin Film Heterostructure-Based Photocatalysts for Hydrogen Generation" Nanomaterials 15, no. 22: 1748. https://doi.org/10.3390/nano15221748
APA StyleFaraz, J. A., Hussain, T., Bilal, M., Ahmad, K., & Cotirla, L.-I. (2025). Photoelectrochemical Water Splitting by SnO2/CuO Thin Film Heterostructure-Based Photocatalysts for Hydrogen Generation. Nanomaterials, 15(22), 1748. https://doi.org/10.3390/nano15221748








