Curcumin-Loaded Polysaccharide Nanoparticles Enhance Aqueous Dispersibility and In Vitro Cytotoxicity in Breast Cancer Cell Lines
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characterization of Prepared NPs
2.3. EE% and Drug Loading (DL%) of the Prepared NPs
2.4. Cell Culture
2.5. Cell Viability Assay
2.6. Fluorescein CUR-NP Conjugates
2.7. Fluorescent Microscopy
2.8. Flow Cytometric Quantification
2.9. Statistical Analysis
3. Results
3.1. Characterization of CUR-NPs
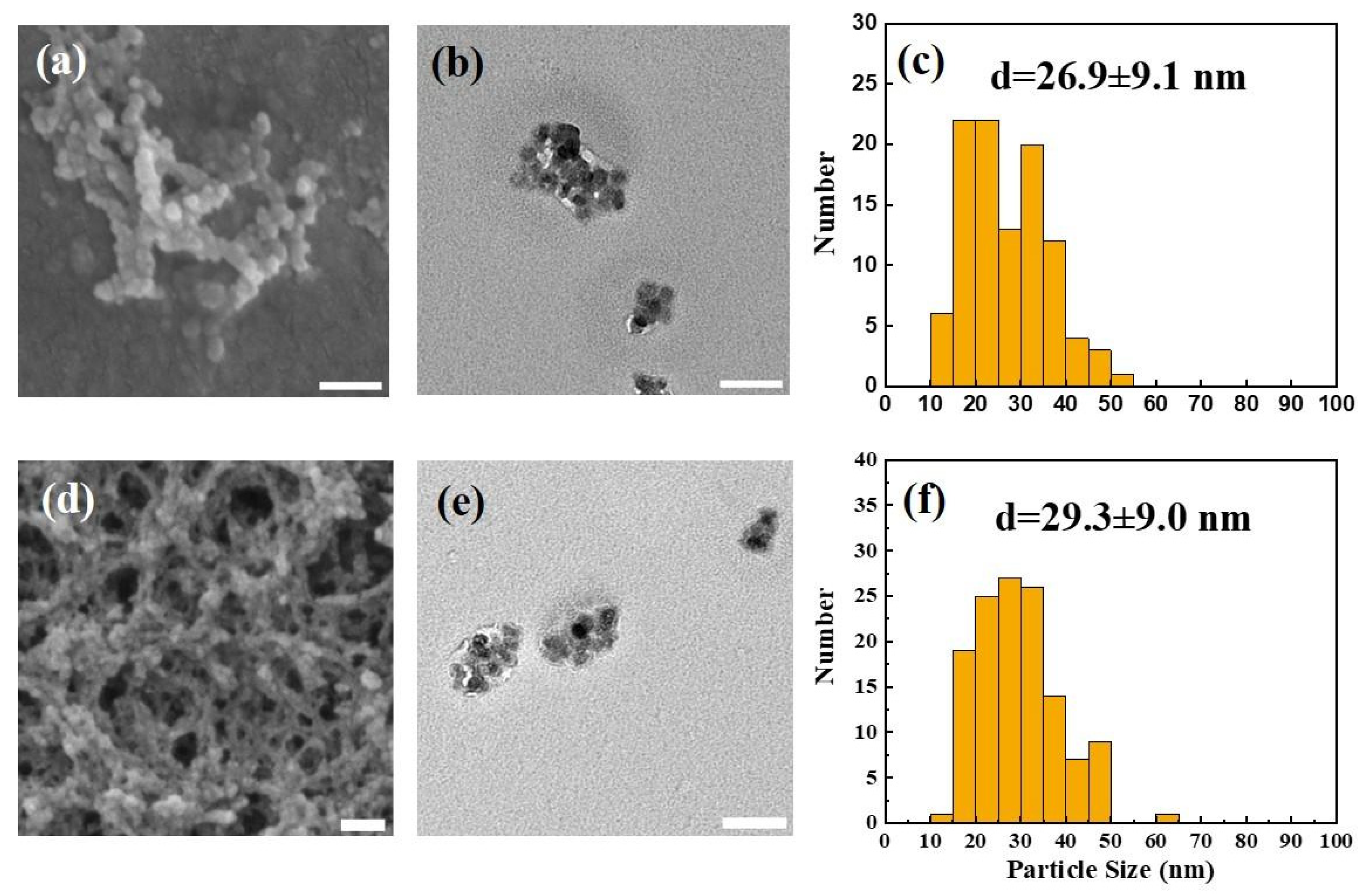
3.1.1. Surface Morphology and Particle Size of Synthesized CUR-NPs
| Sample | SEM/TEM Diameter (nm) | DLS Hydrodynamic Size (nm) | PDI | Zeta Potential (mV) |
|---|---|---|---|---|
| UL-NPs | 26.9 ± 9.1 | 39.8 ± 7.1 | 0.43 | +36.2 |
| CUR-NPs | 29.3 ± 9.0 | 46.1 ± 8.1 | 0.49 | +35.3 |
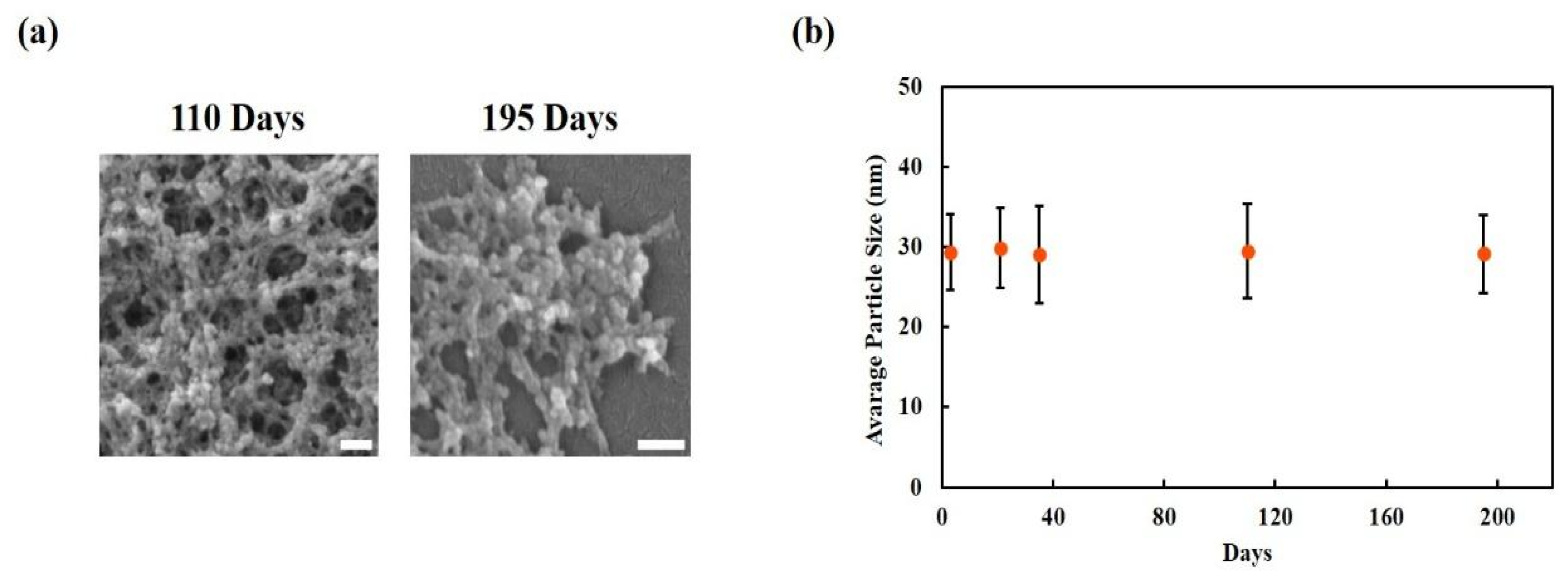
3.1.2. Long-Term Morphological Stability of CUR-NPs
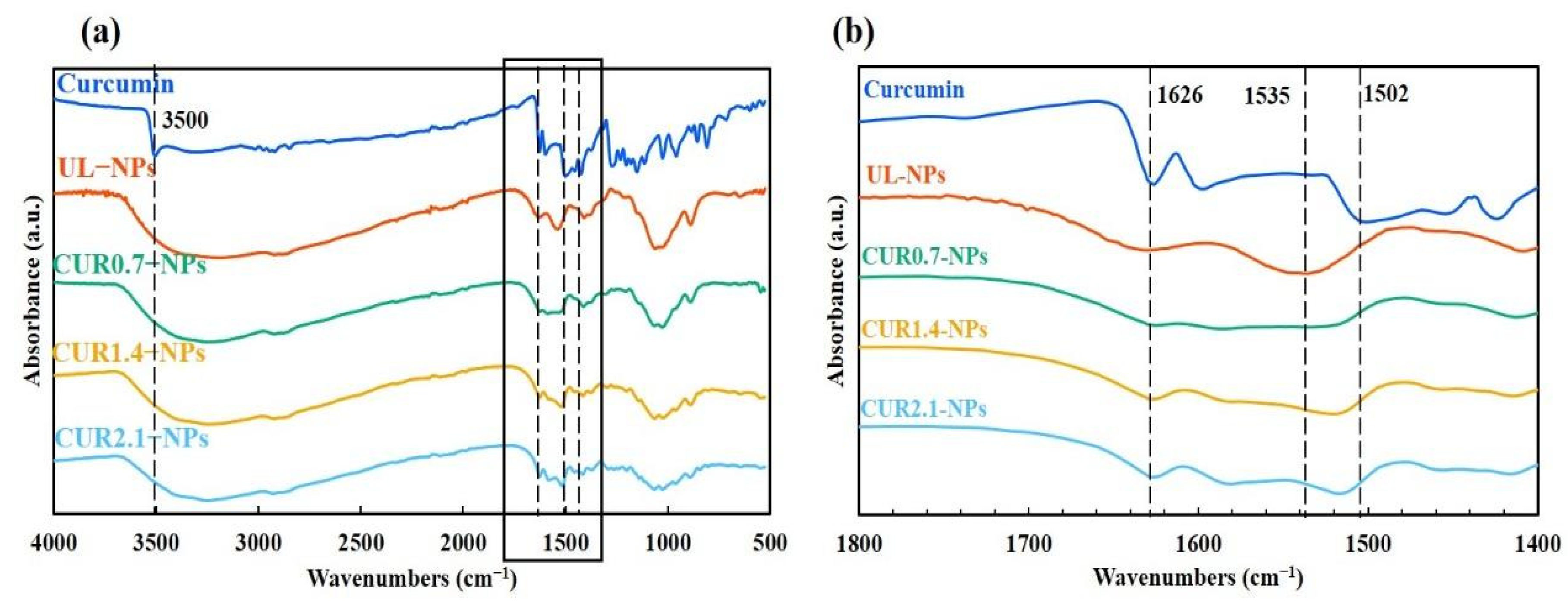
3.1.3. Structural Characterization of CUR-NPs Using FTIR Analysis
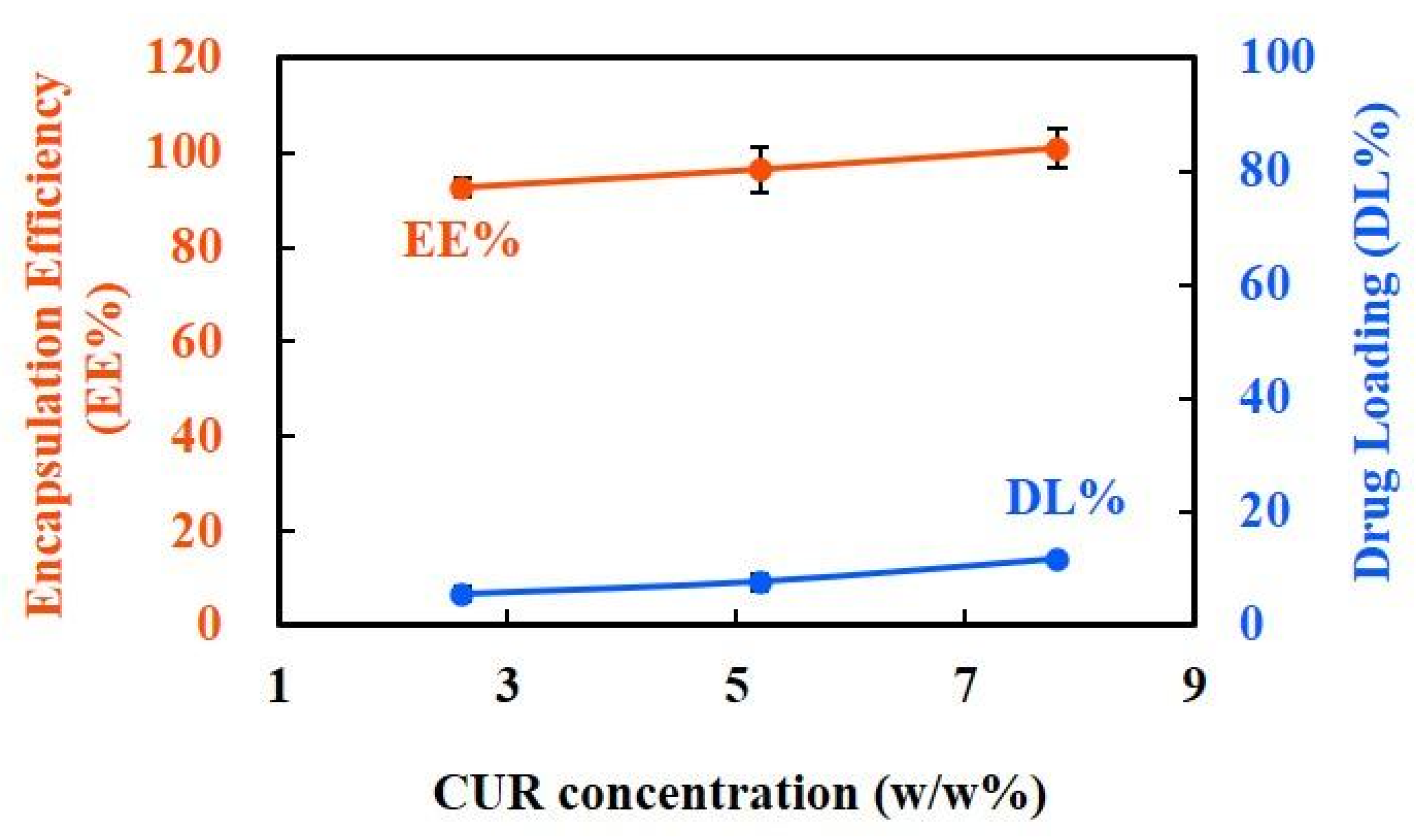
3.1.4. EE% and DL% Capacity of CUR-NPs
3.2. In Vitro Biological Evaluation of CUR-NPs
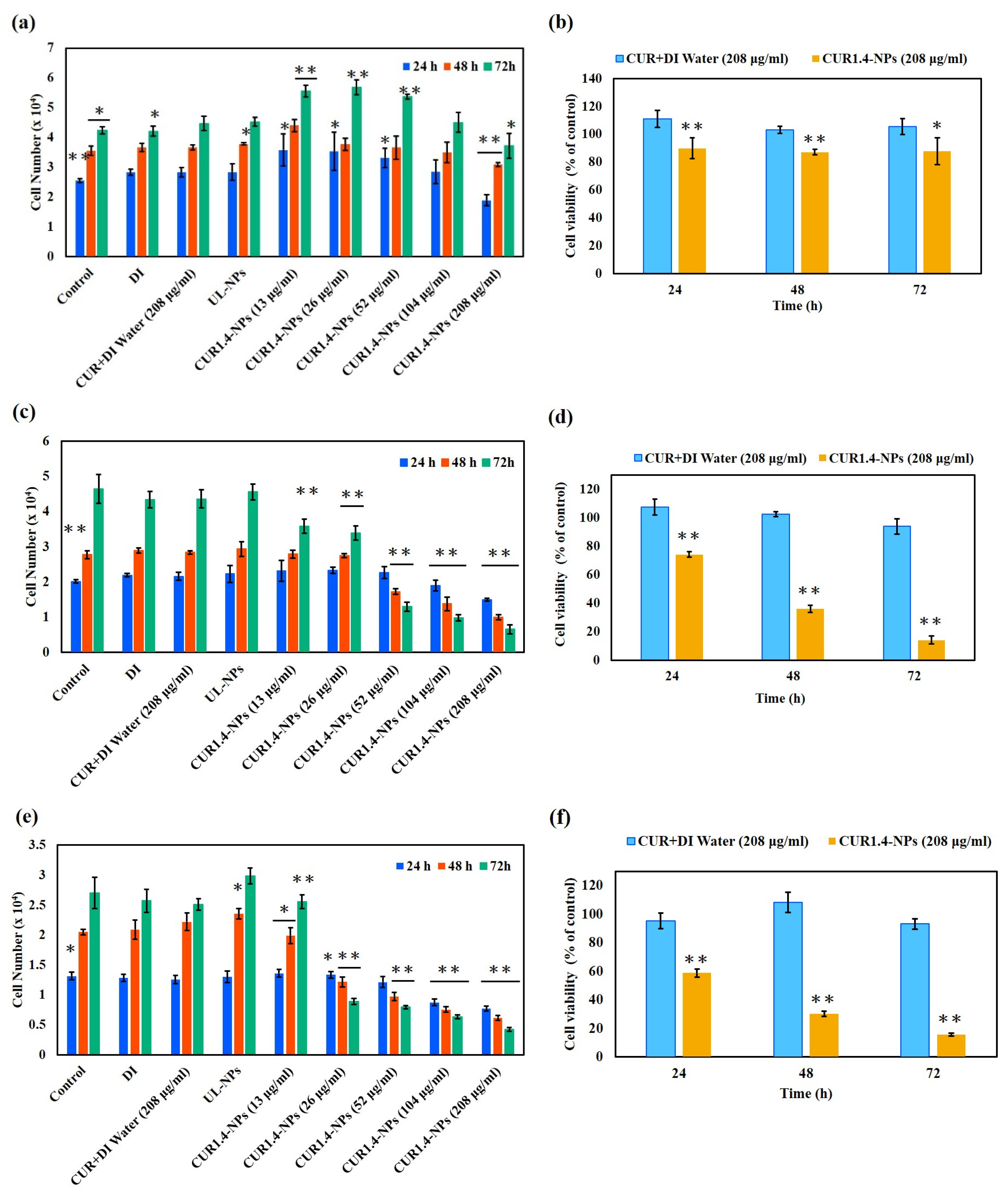
3.2.1. Cytotoxicity
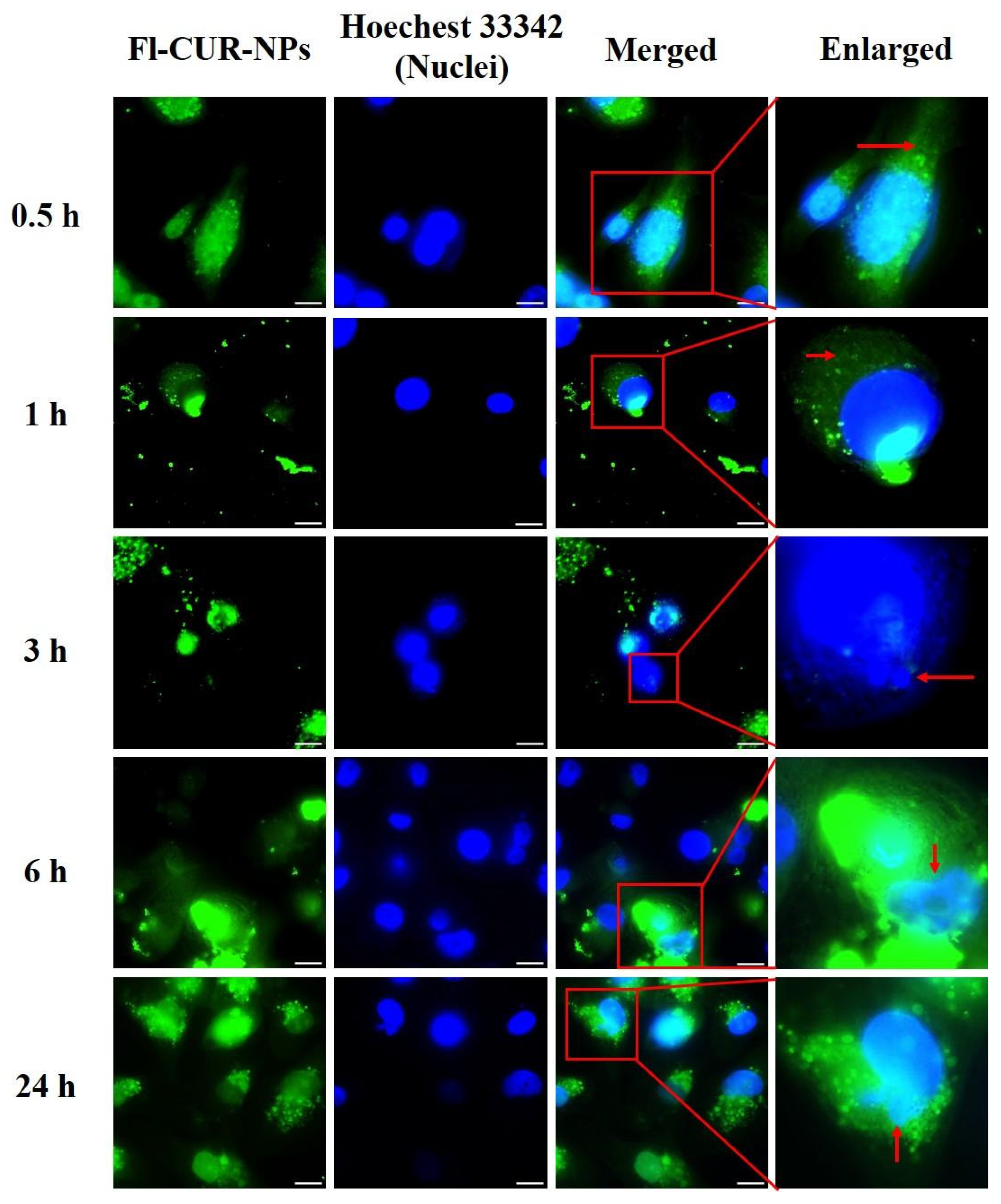
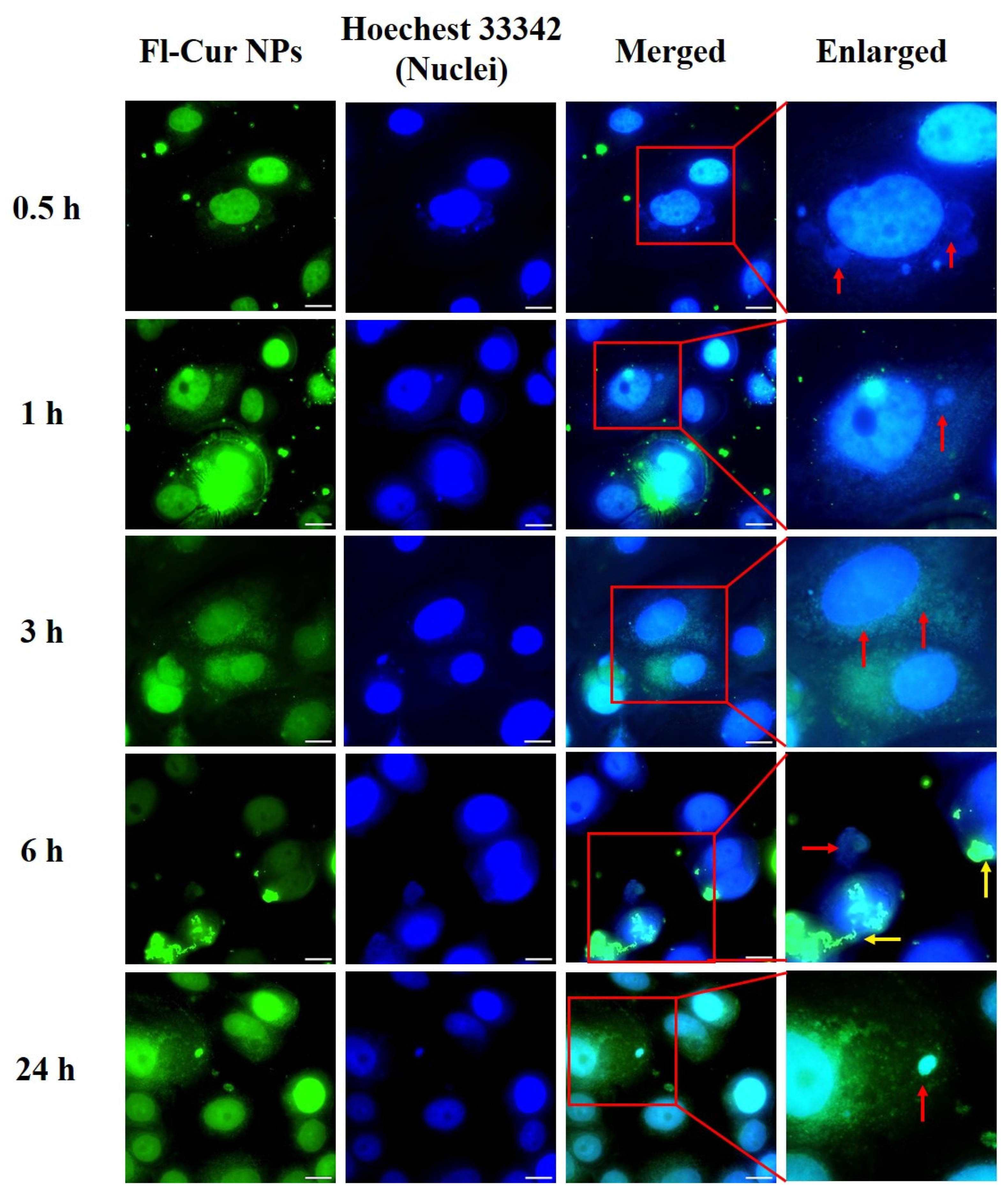
3.2.2. Cellular Uptake Behavior and Morphological Alterations of CUR-NPs
Visualization of CUR-NP Uptake and Morphological Changes Using Fluorescence Microscopy
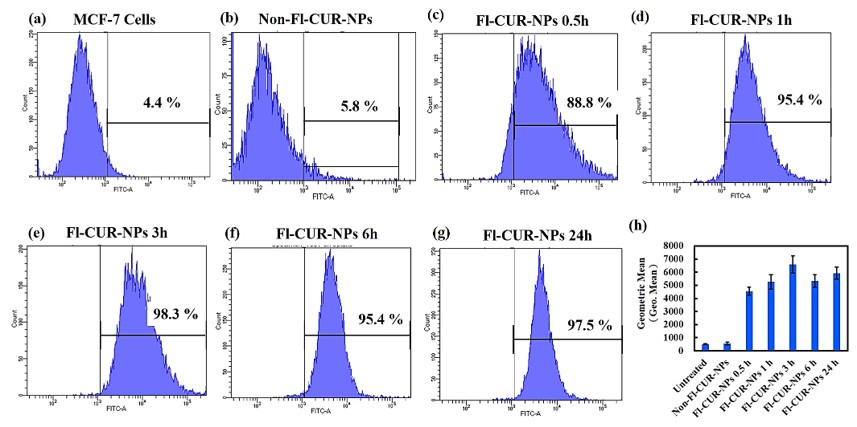
Quantitative Analysis of Cellular Uptake Using Flow Cytometry
4. Discussion
4.1. Improved Solubility and Cellular Uptake of CUR from CS/ALG/HA NPs
4.2. Controlled Particle Size and Fabrication Advantages of CS/ALG/HA NPs
4.3. Evaluating the Stability of CUR-NPs
4.4. Confirmation of CUR Encapsulation and Intermolecular Interaction
4.5. High EE% and Concentration-Dependent DL% of CUR-NPs
4.6. In Vitro Cytotoxicity of CUR-NPs
4.7. Confocal and Flow Cytometric Evaluation of NP Uptake Across Cell Types
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| ALG | Alginate |
| CS | Chitosan |
| CUR | Curcumin |
| DL | Drug loading |
| DMEM | Dulbecco’s modified Eagle’s medium |
| EE | Encapsulation efficiency |
| ER | Estrogen receptor |
| FBS | Fetal bovine serum |
| FTIR | Fourier-transform infrared |
| HA | Hyaluronic acid |
| NPs | Nanoparticles |
| PBS | Phosphate-buffered saline |
| SD | Standard deviation |
| SEM | Scanning electron microscopy |
| TEM | Transmission electron microscopy |
| TNBC | Triple-negative breast cancer |
References
- Kim, J.; Harper, A.; McCormack, V.; Sung, H.; Houssami, N.; Morgan, E.; Mutebi, M.; Garvey, G.; Soerjomataram, I.; Fidler-Benaoudia, M.M. Global patterns and trends in breast cancer incidence and mortality across 185 countries. Nat. Med. 2025, 31, 1154–1162. [Google Scholar] [CrossRef]
- Siegel, R.L.; Kratzer, T.B.; Giaquinto, A.N.; Sung, H.; Jemal, A. Cancer statistics, 2025. CA Cancer J. Clin. 2025, 75, 10–45. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Chaffer, C.L.; Weinberg, R.A. A perspective on cancer cell metastasis. Science 2011, 331, 1559–1564. [Google Scholar] [CrossRef]
- Trayes, K.P.; Cokenakes, S.E.H. Breast cancer treatment. Am. Fam. Physician 2021, 104, 171–178. [Google Scholar] [PubMed]
- Simpson, J.; Scholefield, J.H. Treatment of colorectal cancer: Surgery, chemotherapy and radiotherapy. Surgery (Oxford) 2008, 26, 329–333. [Google Scholar] [CrossRef]
- Orrantia-Borunda, E.; Anchondo-Nuñez, P.; Acuña-Aguilar, L.E.; Gómez-Valles, F.O.; Ramírez-Valdespino, C.A. Subtypes of breast cancer. In Breast; Mayrovitz, H.N., Ed.; Chapter 3; Exon Publications: Brisbane, Australia, 2022; pp. 31–42. [Google Scholar] [CrossRef]
- Carvalho, E.; Canberk, S.; Schmitt, F.; Vale, N. Molecular subtypes and mechanisms of breast cancer: Precision medicine approaches for targeted therapies. Cancers 2025, 17, 1102. [Google Scholar] [CrossRef]
- Wu, J.; Shaidani, S.; Theodossiou, S.K.; Hartzell, E.J.; Kaplan, D.L. Localized, on-demand, sustained drug delivery from biopolymer-based materials. Expert Opin. Drug Deliv. 2022, 19, 1317–1335. [Google Scholar] [CrossRef]
- Opriș, O.; Mormile, C.; Lung, I.; Stegarescu, A.; Soran, M.-L.; Soran, A. An overview of Biopolymers for drug delivery applications. Appl. Sci. 2024, 14, 1383. [Google Scholar] [CrossRef]
- Raafat, D.; Sahl, H.-G. Chitosan and its antimicrobial potential—A critical literature survey. Microb. Biotechnol. 2009, 2, 186–201. [Google Scholar] [CrossRef] [PubMed]
- Wathoni, N.; Herdiana, Y.; Suhandi, C.; Mohammed, A.F.A.; El-Rayyes, A.; Narsa, A.C. Chitosan/alginate-based nanoparticles for antibacterial agents delivery. Int. J. Nanomed. 2024, 19, 5021–5044. [Google Scholar] [CrossRef]
- Xie, W.M.; Xu, P.X.; Liu, Q. Antioxidant activity of water-soluble chitosan derivatives. Bioorg. Med. Chem. Lett. 2001, 11, 1699–1701. [Google Scholar] [CrossRef]
- Lee, H.W.; Park, Y.S.; Choi, J.W.; Yi, S.Y.; Shin, W.S. Antidiabetic effects of chitosan oligosaccharides in neonatal streptozotocin-induced noninsulin-dependent diabetes mellitus in rats. Biol. Pharm. Bull. 2003, 26, 1100–1103. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, M.; Yagi, K.; Iwakawa, S.; Hirai, M. Chitosan induces apoptosis via caspase-3 activation in bladder tumor cells. Jpn. J. Cancer Res. 2001, 92, 459–466. [Google Scholar] [CrossRef] [PubMed]
- Mi, F.L.; Wu, Y.B.; Shyu, S.S.; Schoung, J.Y.; Huang, Y.B.; Tsai, Y.H.; Hao, J.Y. Control of wound infections using a bilayer chitosan wound dressing with sustainable antibiotic delivery. J. Biomed. Mater. Res. 2002, 59, 438–449. [Google Scholar] [CrossRef]
- Haider, A.; Khan, S.; Iqbal, D.N.; Shrahili, M.; Haider, S.; Mohammad, K.; Mohammad, A.; Rizwan, M.; Kanwal, Q.; Mustafa, G. Advances in chitosan-based drug delivery systems: A comprehensive review for therapeutic applications. Eur. Polym. J. 2024, 210, 112983. [Google Scholar] [CrossRef]
- Szymańska, E.; Winnicka, K. Stability of chitosan—A challenge for pharmaceutical and biomedical applications. Mar. Drugs 2015, 13, 1819–1846. [Google Scholar] [CrossRef]
- Sorasitthiyanukarn, F.N.; Ratnatilaka Na Bhuket, P.R.N.; Muangnoi, C.; Rojsitthisak, P.; Rojsitthisak, P. Chitosan/alginate nanoparticles as a promising carrier of novel curcumin diethyl diglutarate. Int. J. Biol. Macromol. 2019, 131, 1125–1136. [Google Scholar] [CrossRef]
- Chen, T.; Li, S.; Zhu, W.; Liang, Z.; Zeng, Q. Self-assembly pH-sensitive chitosan/alginate coated polyelectrolyte complexes for oral delivery of insulin. J. Microencapsul. 2019, 36, 96–107. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Zhao, S.; Karnad, A.; Freeman, J.W. The biology and role of CD44 in cancer progression: Therapeutic implications. J. Hematol. Oncol. 2018, 11, 64. [Google Scholar] [CrossRef]
- Bhattacharya, D.; Svechkarev, D.; Souchek, J.J.; Hill, T.K.; Taylor, M.A.; Natarajan, A.; Mohs, A.M. Impact of structurally modifying hyaluronic acid on CD44 interaction. J. Mater. Chem. B 2017, 5, 8183–8192. [Google Scholar] [CrossRef] [PubMed]
- Bui, T.T.; Dong, T.N.; Nguyen, T.H.; Dang, K.T. Curcuma longa, the polyphenolic curcumin compound and pharmacological effects on liver. In Dietary Interventions in Liver Disease; Watson, R.R., Preedy, V.R., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 125–134. [Google Scholar]
- Yu, Y.; Shen, Q.; Lai, Y.; Park, S.Y.; Ou, X.; Lin, D.; Jin, M.; Zhang, W. Anti-inflammatory effects of curcumin in microglial cells. Front. Pharmacol. 2018, 9, 386. [Google Scholar] [CrossRef]
- Sonkaew, P.; Sane, A.; Suppakul, P. Antioxidant activities of curcumin and ascorbyl dipalmitate nanoparticles and their activities after incorporation into cellulose-based packaging films. J. Agric. Food Chem. 2012, 60, 5388–5399. [Google Scholar] [CrossRef]
- Cheng, Y.; Zhang, Y.; Deng, W.; Hu, J. Antibacterial and anticancer activities of asymmetric lollipop-like mesoporous silica nanoparticles loaded with curcumin and gentamicin sulfate. Colloids Surf. B Biointerfaces 2020, 186, 110744. [Google Scholar] [CrossRef]
- Mathew, D.; Hsu, W.L. Antiviral potential of curcumin. J. Funct. Foods 2018, 40, 692–699. [Google Scholar] [CrossRef]
- Zoi, V.; Galani, V.; Lianos, G.D.; Voulgaris, S.; Kyritsis, A.P.; Alexiou, G.A. The role of curcumin in cancer treatment. Biomedicines 2021, 9, 1086. [Google Scholar] [CrossRef]
- Goel, A.; Jhurani, S.; Aggarwal, B.B. Multi-targeted therapy by curcumin: How spicy is it? Mol. Nutr. Food Res. 2008, 52, 1010–1030. [Google Scholar] [CrossRef]
- Dhawale, K.; Sathiyanarayanan, A.; Bothiraja, C. Formulation development and evaluation of curcumin-loaded microemulsions to enhance the bioavailability and therapeutic efficacy. Nanotechnol. Percept. 2024, 20, 4829–4858. [Google Scholar]
- Anand, P.; Kunnumakkara, A.B.; Newman, R.A.; Aggarwal, B.B. Bioavailability of curcumin: Problems and promises. Mol. Pharm. 2007, 4, 807–818. [Google Scholar] [CrossRef] [PubMed]
- Lao, C.D.; Ruffin, M.T., IV; Normolle, D.; Heath, D.D.; Murray, S.I.; Bailey, J.M.; Boggs, M.E.; Crowell, J.; Rock, C.L.; Brenner, D.E. Dose escalation of a curcuminoid formulation. BMC Complement. Altern. Med. 2006, 6, 10. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, T.-Y.; Wu, Y.-C.; Gong, P.-X.; Li, H.-J. Foxtail millet prolamin as an effective encapsulant deliver curcumin by fabricating caseinate stabilized composite nanoparticles. Food Chem. 2022, 367, 130764. [Google Scholar] [CrossRef]
- Caro-León, F.J.; Silva-Campa, E.; Navarro-López, R.A.; Fernández-Quiroz, D.; Figueroa-León, V.A.; Trujillo-Ramirez, M.A.; López-Martínez, L.M.; Aguilar, M.R.; Álvarez-Bajo, O. Production and Characterization of Nanoparticulate Polyelectrolyte Complexes of Chitosan–Catechol, Ulvan, and Hyaluronic Acid. ACS Omega 2025, 10, 3474–3485. [Google Scholar] [CrossRef]
- Jha, A.; Goswami, P.; Kumar, M.; Bharti, K.; Manjit, M.; Satpute, A.P.; Gupta, A.; Moorkoth, S.; Koch, B.; Mishra, B. Cetuximab Functionalized Chitosan/Hyaluronic Acid-Based Nanoparticles Loaded with Cabazitaxel Enhances Anti-Tumor Efficacy in DMBA-Induced Breast Cancer Model in Rats through Spatial Targeting. Chem. Phys. Impact 2024, 9, 100750. [Google Scholar] [CrossRef]
- Thai, H.; Nguyen, C.T.; Thach, L.T.; Tran, M.T.; Mai, H.D.; Nguyen, T.T.T.; Le, G.D.; Can, M.V.; Tran, L.D.; Bach, G.L.; et al. Characterization of Chitosan/Alginate/Lovastatin Nanoparticles and Investigation of Their Toxic Effects In Vitro and In Vivo. Sci. Rep. 2020, 10, 909. [Google Scholar] [CrossRef]
- Tsai, Y.-C.; Miyajima, H.; Chou, M.-Y.; Fujita, S. Natural polysaccharide-based nanoparticles enhance intracellular delivery and cytotoxicity of Antrodia camphorata in breast cancer cells. Int. J. Mol. Sci. 2025, 26, 8420. [Google Scholar] [CrossRef]
- Boscariol, R.; Paulino, T.H.; Oliveira, J.M., Jr.; Balcão, V.M.; Vila, M.M.D.C. Characterization of commercially available turmeric for use in pharmaceutical products and food supplements. J. Braz. Chem. Soc. 2022, 33, 1392–1401. [Google Scholar] [CrossRef]
- Mishra, B.; Yadav, A.S.; Malhotra, D.; Mitra, T.; Sinsinwar, S.; Radharani, N.N.V.; Sahoo, S.R.; Patnaik, S.; Kundu, G.C. Chitosan nanoparticle-mediated delivery of curcumin suppresses tumor growth in breast cancer. Nanomaterials 2024, 14, 1294. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Guo, T.; Nan, J.; Yang, L.; Liao, G.; Park, H.J.; Li, J. Hyaluronic-acid-coated chitosan nanoparticles for insulin oral delivery: Fabrication, characterization, and hypoglycemic ability. Macromol. Biosci. 2022, 22, e2100493. [Google Scholar] [CrossRef]
- Yeerong, K.; Chantawannakul, P.; Anuchapreeda, S.; Juntrapirom, S.; Kanjanakawinkul, W.; Müllertz, A.; Rades, T.; Chaiyana, W. Chitosan alginate nanoparticles of protein hydrolysate from Acheta domesticus with enhanced stability for skin delivery. Pharmaceutics 2024, 16, 724. [Google Scholar] [CrossRef]
- Ameer, S.F.; Mohamed, M.Y.; Elzubair, Q.A.; Sharif, E.A.M.; Ibrahim, W.N. Curcumin as a novel therapeutic candidate for cancer: Can this natural compound revolutionize cancer treatment? Front. Oncol. 2024, 14, 1438040. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, B.B.; Kumar, A.; Bharti, A.C. Anticancer potential of curcumin: Preclinical and clinical studies. Anticancer Res. 2003, 23, 363–398. [Google Scholar]
- Kurien, B.T.; Singh, A.; Matsumoto, H.; Scofield, R.H. Improving the solubility and pharmacological efficacy of curcumin by heat treatment. Assay Drug Dev. Technol. 2007, 5, 567–576. [Google Scholar] [CrossRef]
- Hasan, M.; Ben Messaoud, G.; Michaux, F.; Tamayol, A.; Kahn, C.J.F.; Belhaj, N.; Linder, M.; Arab-Tehrany, E. Chitosan-coated liposomes encapsulating curcumin: Study of lipid–polysaccharide interactions and nanovesicle behavior. RSC Adv. 2016, 6, 45290–45304. [Google Scholar] [CrossRef]
- Chen, Q.; Qi, Y.; Jiang, Y.; Quan, W.; Luo, H.; Wu, K.; Li, S.; Ouyang, Q. Progress in research of chitosan chemical modification technologies and their applications. Mar. Drugs 2022, 20, 536. [Google Scholar] [CrossRef]
- Ebrahimi, H.A.; Esmaeli, S.; Khezri, S.; Salimi, A. Curcumin-loaded chitosan nanoparticle preparation and its protective effect on celecoxib-induced toxicity in rat isolated cardiomyocytes and mitochondria. Drug Res. 2023, 73, 125–136. [Google Scholar] [CrossRef]
- Sorasitthiyanukarn, F.N.; Muangnoi, C.; Rojsitthisak, P.; Rojsitthisak, P. Chitosan-alginate nanoparticles as effective oral carriers to improve the stability, bioavailability, and cytotoxicity of curcumin diethyl disuccinate. Carbohydr. Polym. 2021, 256, 117426. [Google Scholar] [CrossRef] [PubMed]
- Hong, W.; Gao, Y.; Lou, B.; Ying, S.; Wu, W.; Ji, X.; Yu, N.; Jiao, Y.; Wang, H.; Zhou, X.; et al. Curcumin-loaded hybrid nanoparticles: Microchannel-based preparation and antitumor activity in a mouse model. Int. J. Nanomed. 2021, 16, 4147–4159. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zou, L.-Q.; Niu, J.; Liu, W.; Peng, S.-F.; Liu, C.-M. The stability, sustained release and cellular antioxidant activity of curcumin nanoliposomes. Molecules 2015, 20, 14293–14311. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.N.; Hong, S.J.; Yu, M.H.; Shin, G.H.; Kim, J.T. Enhancement of storage stability and masking effect of curcumin by turmeric extract-loaded nanoemulsion and water-soluble chitosan coating. Pharmaceutics 2022, 14, 1547. [Google Scholar] [CrossRef]
- Arulmoorthy, M.P.; Srinivasan, M. Preparation, characterization and antimicrobial activity of curcumin loaded chitosan and alginate biocomposite edible films impregnated with silver nanoparticle. Sch. Acad. J. Biosci. 2015, 3, 712–719. [Google Scholar]
- Kumari, M.; Sharma, N.; Manchanda, R.; Gupta, N.; Syed, A.; Bahkali, A.H.; Nimesh, S. PGMD. PGMD/curcumin nanoparticles for the treatment of breast cancer. Sci. Rep. 2021, 11, 3824. [Google Scholar] [CrossRef] [PubMed]
- Asadi, S.; Madrakian, T.; Ahmadi, M.; Aguirre, M.Á.; Afkhami, A.; Uroomiye, S.S.; Ghaffari, F.; Ranjbar, A. Aerosol assisted synthesis of a pH responsive curcumin anticancer drug nanocarrier using chitosan and alginate natural polymers. Sci. Rep. 2023, 13, 19389. [Google Scholar] [CrossRef]
- Das, R.K.; Kasoju, N.; Bora, U. Encapsulation of curcumin in alginate-chitosan-pluronic composite nanoparticles for delivery to cancer cells. Nanomedicine 2010, 6, 153–160. [Google Scholar] [CrossRef]
- Kunwar, A.; Barik, A.; Mishra, B.; Rathinasamy, K.; Pandey, R.; Priyadarsini, K.I. Quantitative cellular uptake, localization and cytotoxicity of curcumin in normal and tumor cells. Biochim. Biophys. Acta 2008, 1780, 673–679. [Google Scholar] [CrossRef] [PubMed]
- Singer, S.J. Some early history of membrane molecular biology. Annu. Rev. Physiol. 2004, 66, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Ipar, V.S.; Dsouza, A.; Devarajan, P.V. Enhancing curcumin oral bioavailability through nanoformulations. Eur. J. Drug Metab. Pharmacokinet. 2019, 44, 459–480. [Google Scholar] [CrossRef]
- Hu, S.; Xu, Y.; Meng, L.; Huang, L.; Sun, H. Curcumin inhibits proliferation and promotes apoptosis of breast cancer cells. Exp. Ther. Med. 2018, 16, 1266–1272. [Google Scholar] [CrossRef]
- Aibani, N.; Rai, R.; Patel, P.; Cuddihy, G.; Wasan, E.K. Chitosan nanoparticles at the biological interface: Implications for drug delivery. Pharmaceutics 2021, 13, 1686. [Google Scholar] [CrossRef]
- Wang, F.; Gómez-Sintes, R.; Boya, P. Lysosomal membrane permeabilization and cell death. Traffic 2018, 19, 918–931. [Google Scholar] [CrossRef]
- Fong, D.; Grégoire-Gélinas, P.; Cheng, A.P.; Mezheritsky, T.; Lavertu, M.; Sato, S.; Hoemann, C.D. Lysosomal rupture induced by structurally distinct chitosans either promotes a Type 1 IFN response or activates the inflammasome in macrophages. Biomaterials 2017, 129, 127–138. [Google Scholar] [CrossRef]
- Rainey, N.E.; Moustapha, A.; Petit, P.X. Curcumin, a multifaceted hormetic agent, mediates an intricate crosstalk between mitochondrial turnover, autophagy, and apoptosis. Oxid. Med. Cell. Longev. 2020, 2020, 3656419. [Google Scholar] [CrossRef] [PubMed]
- Gabr, S.A.; Elsaed, W.M.; Eladl, M.A.; El-Sherbiny, M.; Ebrahim, H.A.; Asseri, S.M.; Eltahir, Y.A.M.; Elsherbiny, N.; Eldesoqui, M. Curcumin modulates oxidative stress, fibrosis, and apoptosis in drug-resistant cancer cell lines. Life 2022, 12, 1427. [Google Scholar] [CrossRef] [PubMed]
- Elmore, S. Apoptosis: A review of programmed cell death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef] [PubMed]

| Abbreviation | CUR Amount Loaded (g) | CUR Concentration (w/w%) | Description |
|---|---|---|---|
| UL-NPs | 0 | 0% | CUR unloaded-NPs (blank control) |
| CUR0.7-NPs | 0.7 | 2.6% | NPs loaded with 0.7 g CUR (2.6% w/w) |
| CUR1.4-NPs | 1.4 | 5.2% | NPs loaded with 1.4 g CUR (5.2% w/w) |
| CUR2.1-NPs | 2.1 | 7.8% | NPs loaded with 2.1 g CUR (7.8% w/w) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsai, Y.-C.; Miyajima, H.; Chou, M.-Y.; Fujita, S. Curcumin-Loaded Polysaccharide Nanoparticles Enhance Aqueous Dispersibility and In Vitro Cytotoxicity in Breast Cancer Cell Lines. Nanomaterials 2025, 15, 1747. https://doi.org/10.3390/nano15221747
Tsai Y-C, Miyajima H, Chou M-Y, Fujita S. Curcumin-Loaded Polysaccharide Nanoparticles Enhance Aqueous Dispersibility and In Vitro Cytotoxicity in Breast Cancer Cell Lines. Nanomaterials. 2025; 15(22):1747. https://doi.org/10.3390/nano15221747
Chicago/Turabian StyleTsai, Yu-Chen, Hiroki Miyajima, Ming-Yang Chou, and Satoshi Fujita. 2025. "Curcumin-Loaded Polysaccharide Nanoparticles Enhance Aqueous Dispersibility and In Vitro Cytotoxicity in Breast Cancer Cell Lines" Nanomaterials 15, no. 22: 1747. https://doi.org/10.3390/nano15221747
APA StyleTsai, Y.-C., Miyajima, H., Chou, M.-Y., & Fujita, S. (2025). Curcumin-Loaded Polysaccharide Nanoparticles Enhance Aqueous Dispersibility and In Vitro Cytotoxicity in Breast Cancer Cell Lines. Nanomaterials, 15(22), 1747. https://doi.org/10.3390/nano15221747






