Hybrid Plasma Spray Synthesis of Spherical Si0.8Ge0.2 Alloy Nanoparticles for Lithium-Ion Battery Anodes
Abstract
1. Introduction
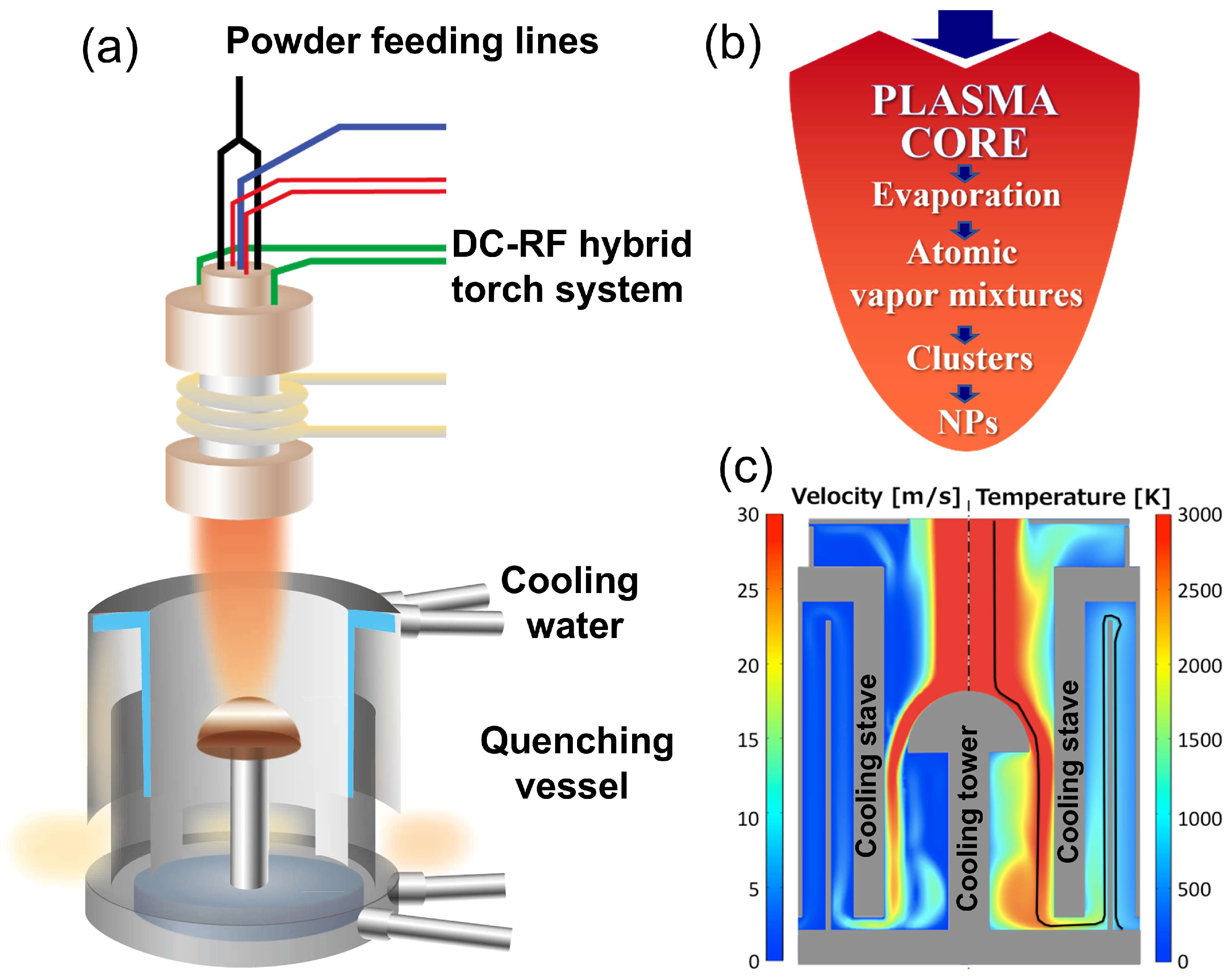
2. Methodology
3. Results and Discussion
3.1. Synthesis Mechanism and Structural Evolution of Si-Ge Nanospheres
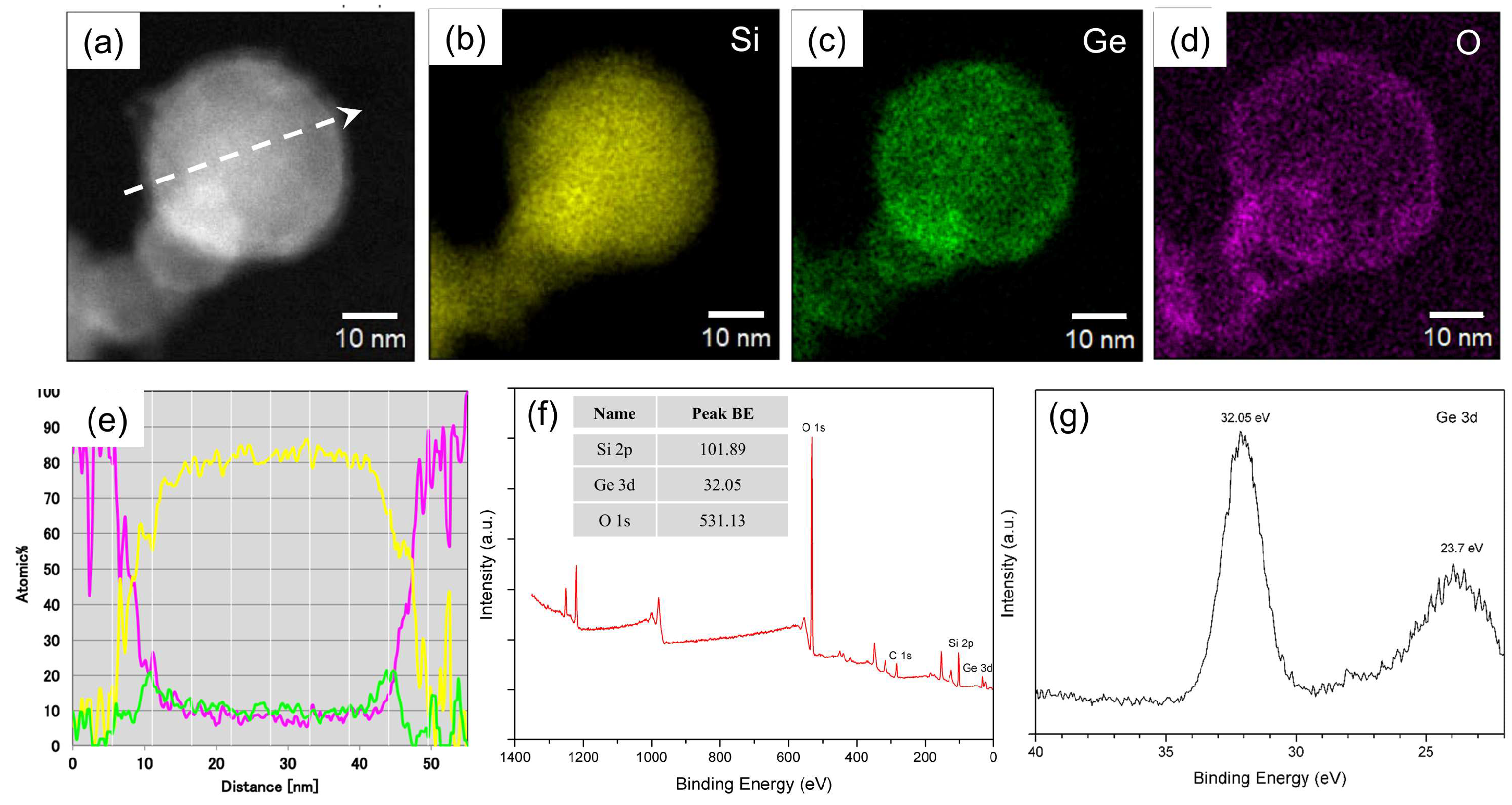
3.2. Morphological and Compositional Characterization of Si0.8Ge0.2 Nanospheres
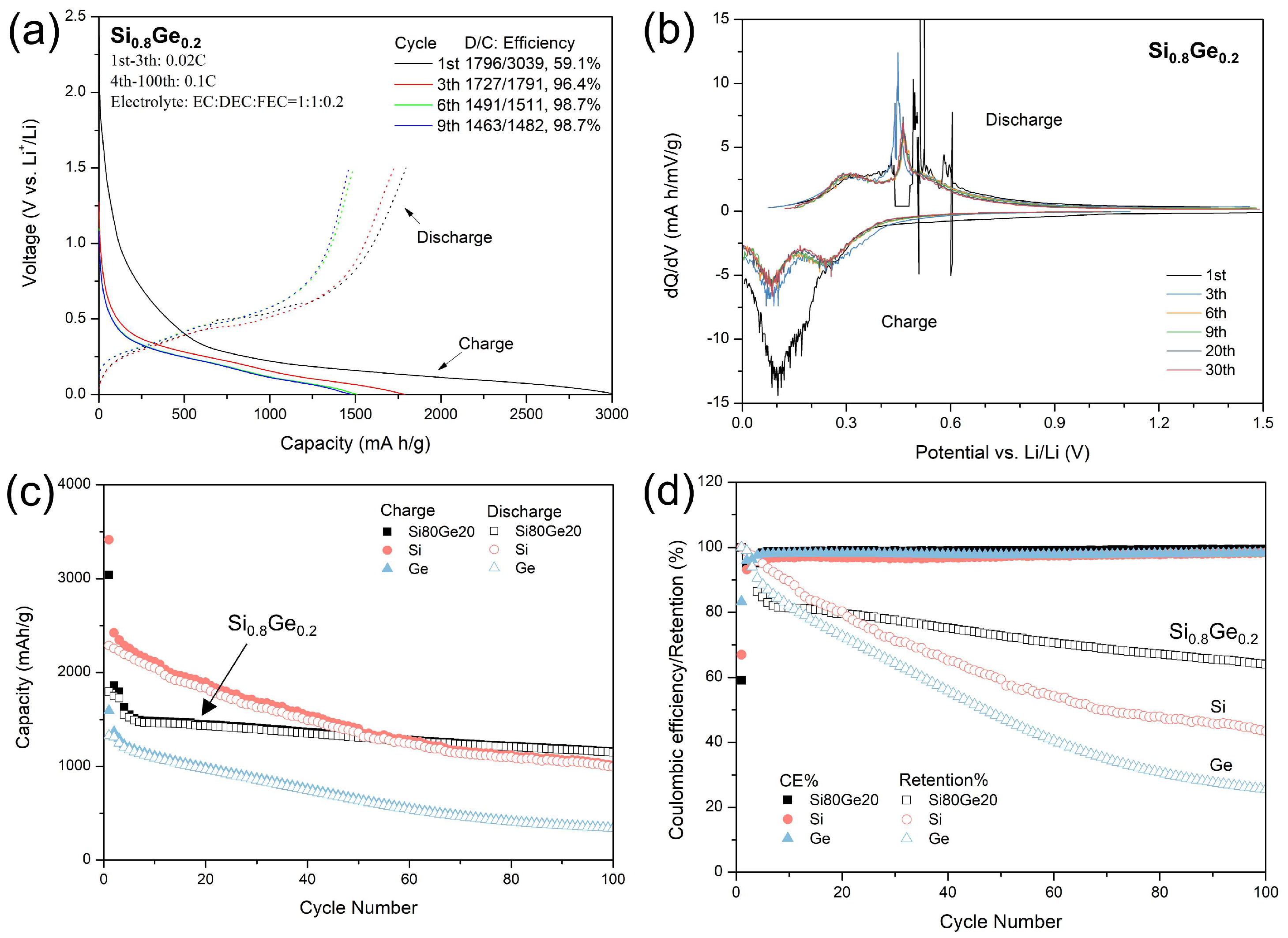
3.3. Electrochemical Performance as LIB Anodes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| FE-SEM | Field-emission scanning electron microscopy |
| EDS | Directory of open access journals |
| HRTEM | High-resolution transmission electron microscopy |
| STEM | Scanning transmission electron microscopy |
| HAADF | High-angle annular dark field |
| XRD | X-ray diffraction |
| XPS | X-ray photoelectron spectroscopy |
| NPs | Nanoparticles |
| PS-PVD | Plasma spraying physical vapor deposition |
| CE | Coulombic efficiency |
| MD | Molecular dynamics |
| DC-RF | Direct current–radio frequency |
References
- Obrovac, M.N.; Chevrier, V.L. Alloy Negative Electrodes for Li-Ion Batteries. Chem. Rev. 2014, 114, 11444–11502. [Google Scholar] [CrossRef] [PubMed]
- Zuo, X.; Zhu, J.; Müller-Buschbaum, P.; Cheng, Y.J. Silicon Based Lithium-Ion Battery Anodes: A Chronicle Perspective Review. Nano Energy 2017, 31, 113–143. [Google Scholar] [CrossRef]
- Keller, C.; Karuppiah, S.; Raaen, M.; Wang, J.; Perrenot, P.; Aldakov, D.; Reiss, P.; Haon, C.; Chenevier, P. Low-Cost Tin Compounds as Seeds for the Growth of Silicon Nanowire-Graphite Composites Used in High-Performance Lithium-Ion Battery Anodes. ACS Appl. Energy Mater. 2023, 6, 5249–5258. [Google Scholar] [CrossRef]
- Chan, C.K.; Zhang, X.F.; Cui, Y. High Capacity Li Ion Battery Anodes Using Ge Nanowires. Nano Lett. 2008, 8, 307–309. [Google Scholar] [CrossRef]
- Chan, C.K.; Peng, H.; Liu, G.; McIlwrath, K.; Zhang, X.F.; Huggins, R.A.; Cui, Y. High-Performance Lithium Battery Anodes Using Silicon Nanowires. Nat. Nanotechnol. 2008, 3, 31–35. [Google Scholar] [CrossRef]
- Gao, S.; Hong, S.; Park, S.; Jung, H.Y.; Liang, W.; Lee, Y.; Ahn, C.W.; Byun, J.Y.; Seo, J.; Hahm, M.G.; et al. Catalyst-Free Synthesis of Sub-5 Nm Silicon Nanowire Arrays with Massive Lattice Contraction and Wide Bandgap. Nat. Commun. 2022, 13, 3467. [Google Scholar] [CrossRef]
- Luo, J.; Arnot, D.J.; King, S.T.; Kingan, A.; Nicoll, A.; Tong, X.; Bock, D.C.; Takeuchi, E.S.; Marschilok, A.C.; Yan, S.; et al. Two-Dimensional Siloxene Nanosheets: Impact of Morphology and Purity on Electrochemistry. ACS Appl. Mater. Interfaces 2023, 15, 24306–24318. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Y.; Ma, H.; Wang, Z.; Xu, X.; Huang, X. Solid Silicon Nanosheet Sandwiched by Self-Assembled Honeycomb Silicon Nanosheets Enabling Long Life at High Current Density for a Lithium-Ion Battery Anode. ACS Appl. Mater. Interfaces 2023, 15, 15409–15419. [Google Scholar] [CrossRef]
- Park, S.W.; Ha, J.H.; Cho, B.W.; Choi, H.J. Designing of High Capacity Si Nanosheets Anode Electrodes for Lithium Batteries. Surf. Coat. Technol. 2021, 421, 127358. [Google Scholar] [CrossRef]
- Wang, J.H.; Chung, C.H.; Chi, P.W.; Paul, T.; Chandan, P.; Yeh, K.W.; Chang, C.C.; Prakoso, S.P.; Chiu, Y.C.; Wu, M.K. Si@C Core–Shell Nanostructure-Based Anode for Li-Ion Transport. ACS Appl. Nano Mater. 2023, 6, 12578–12587. [Google Scholar] [CrossRef]
- Gao, Y.; Song, S.; He, F.; Kong, X.; Xiao, Z.; Cui, X.; Cao, L.; Zhang, Y.; Liu, Z.; Yang, P. Controllable Synthesis of Hollow Dodecahedral Si@C Core–Shell Structures for Ultrastable Lithium-Ion Batteries. Small 2024, 20, 2406489. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Huang, H.; Chen, X.; Gao, T.; Li, J.; Yao, Y.; Xu, Z.; Zheng, M.; Liu, Z. Facile Synthesis of Graphite-SiOx/C Core–Shell Composite Anode for High Stable Lithium-Ion Batteries. Energy Fuels 2024, 38, 23140–23149. [Google Scholar] [CrossRef]
- Li, W.; Sun, X.; Yu, Y. Si-, Ge-, Sn-Based Anode Materials for Lithium-Ion Batteries: From Structure Design to Electrochemical Performance. Small Methods 2017, 1, 1600037. [Google Scholar] [CrossRef]
- Li, X.; Gu, M.; Hu, S.; Kennard, R.; Yan, P.; Chen, X.; Wang, C.; Sailor, M.J.; Zhang, J.G.; Liu, J. Mesoporous Silicon Sponge as an Anti-Pulverization Structure for High-Performance Lithium-Ion Battery Anodes. Nat. Commun. 2014, 5, 4105. [Google Scholar] [CrossRef] [PubMed]
- Avila Cardenas, A.; Herlin-Boime, N.; Monconduit, L.; Haon, C. Si–Ge Alloys as Promising Anodes for Sulfide-Based Solid-State Batteries: Role of the Powder Morphology on Performance. J. Energy Storage 2025, 132, 117912. [Google Scholar] [CrossRef]
- Du, Y.; Yang, Z.; Bai, L.; Ding, F.; Jin, H.; Yang, Y.; Lam, T.D.; Hou, G.; Yuan, F. Si/FeSi2 Nanoparticles Prepared by Thermal Plasma with Stress-Releasing Effect for Li-Ion Storage. ChemNanoMat 2021, 7, 467–475. [Google Scholar] [CrossRef]
- Gross, S.J.; Hsieh, M.T.; Mumm, D.R.; Valdevit, L.; Mohraz, A. Alleviating Expansion-Induced Mechanical Degradation in Lithium-Ion Battery Silicon Anodes via Morphological Design. Extrem. Mech. Lett. 2022, 54, 101746. [Google Scholar] [CrossRef]
- McDowell, M.T.; Lee, S.W.; Harris, J.T.; Korgel, B.A.; Wang, C.; Nix, W.D.; Cui, Y. In Situ TEM of Two-Phase Lithiation of Amorphous Silicon Nanospheres. Nano Lett. 2013, 13, 758–764. [Google Scholar] [CrossRef]
- Kambara, M. Powders: Plasma Spray PVD for High-Throughput Production. Encycl. Plasma Technol. 2017, 2, 1176–1190. [Google Scholar] [CrossRef]
- Ohta, R.; Fukada, K.; Tashiro, T.; Dougakiuchi, M.; Kambara, M. Effect of PS-PVD Production Throughput on Si Nanoparticles for Negative Electrode of Lithium Ion Batteries. J. Phys. D Appl. Phys. 2018, 51, 105501. [Google Scholar] [CrossRef]
- Ohta, R.; Tanaka, T.; Takeuchi, A.; Dougakiuchi, M.; Fukuda, K.; Kambara, M. Feasibility of Silicon Nanoparticles Produced by Fast-Rate Plasma Spray PVD for High Density Lithium-Ion Storage. J. Phys. D Appl. Phys. 2021, 54, 494002. [Google Scholar] [CrossRef]
- Kambara, M.; Kitayama, A.; Homma, K.; Hideshima, T.; Kaga, M.; Sheem, K.-Y.; Ishida, S.; Yoshida, T. Nano-Composite Si Particle Formation by Plasma Spraying for Negative Electrode of Li Ion Batteries. J. Appl. Phys. 2014, 115, 143302. [Google Scholar] [CrossRef]
- Hu, Z.; Zhang, S.; Zhang, C.; Cui, G. High Performance Germanium-Based Anode Materials. Coord. Chem. Rev. 2016, 326, 34–85. [Google Scholar] [CrossRef]
- Duveau, D.; Fraisse, B.; Cunin, F.; Monconduit, L. Synergistic Effects of Ge and Si on the Performances and Mechanism of the GexSi1−x Electrodes for Li Ion Batteries. Chem. Mater. 2015, 27, 3226–3233. [Google Scholar] [CrossRef]
- Stokes, K.; Geaney, H.; Flynn, G.; Sheehan, M.; Kennedy, T.; Ryan, K.M. Direct Synthesis of Alloyed Si1–XGex Nanowires for Performance-Tunable Lithium Ion Battery Anodes. ACS Nano 2017, 11, 10088–10096. [Google Scholar] [CrossRef]
- Tersoff, J. Modeling Solid-State Chemistry: Interatomic Potentials for Multicomponent Systems. Phys. Rev. B 1989, 39, 5566–5568. [Google Scholar] [CrossRef]
- Scheerschmidt, K.; Kuhlmann, V. Relaxation of Semiconductor Nanostructures Using Molecular Dynamics with Analytic Bond Order Potentials. Z. Fuer Met./Mater. Res. Adv. Tech. 2007, 98, 1081–1085. [Google Scholar] [CrossRef]
- Deng, Q.; Liu, Q. Field-Programmable Gate Array Acceleration of the Tersoff Potential in LAMMPS. Eng. Rep. 2025, 7, e12694. [Google Scholar] [CrossRef]
- Yang, X.; Wu, M.; Jian, M.; Zhu, S.; Jiang, J.; Yang, L. Feasibility of Molecular Dynamics Simulation for Process Parameter Guidance of Silicon Nitride Thin Films by PECVD. Appl. Surf. Sci. 2024, 654, 159401. [Google Scholar] [CrossRef]
- Abs Da Cruz, C.; Katcho, N.A.; Mingo, N.; Veiga, R.G.A. Thermal Conductivity of Nanocrystalline SiGe Alloys Using Molecular Dynamics Simulations. J. Appl. Phys. 2013, 114. [Google Scholar] [CrossRef]
- Erhart, P.; Albe, K. Analytical Potential for Atomistic Simulations of Silicon, Carbon, and Silicon Carbide. Phys. Rev. B 2005, 71, 035211. [Google Scholar] [CrossRef]
- Schelling, P.K. Phase Behavior and Kinetics of a New Bond-Order Potential for Silicon. Comput. Mater. Sci. 2008, 44, 274–279. [Google Scholar] [CrossRef]
- Kumagai, T.; Izumi, S.; Hara, S.; Sakai, S. Development of Bond-Order Potentials That Can Reproduce the Elastic Constants and Melting Point of Silicon for Classical Molecular Dynamics Simulation. Comput. Mater. Sci. 2007, 39, 457–464. [Google Scholar] [CrossRef]
- Wedekind, J.; Reguera, D. What Is the Best Definition of a Liquid Cluster at the Molecular Scale? J. Chem. Phys. 2007, 127, 154516. [Google Scholar] [CrossRef]
- Stukowski, A. Visualization and Analysis of Atomistic Simulation Data with OVITO--the Open Visualization Tool. Model. Simul. Mat. Sci. Eng. 2009, 18, 15012. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Y. Understanding the Crystallization Mechanism of Nonspherical Nanoclusters: A Dynamic Density Functional Study. Chem. Phys. Lett. 2023, 824, 140566. [Google Scholar] [CrossRef]
- Wang, W.B.; Kambara, M. A Molecular Dynamics Simulation of Inhomogeneous Silicon–Germanium Nucleation from Supersaturated Vapor Mixtures. AIP Adv. 2021, 11, 085119. [Google Scholar] [CrossRef]
- Wang, W.B.; Ohta, R.; Kambara, M. Study on Liquid-like SiGe Cluster Growth during Co-Condensation from Supersaturated Vapor Mixtures by Molecular Dynamics Simulation. Phys. Chem. Chem. Phys. 2022, 24, 7442–7450. [Google Scholar] [CrossRef]
- Charifi, Z.; Bouarissa, N. The Effect of the Violation of Vegard’s Law on the Optical Bowing in Si1−XGex Alloys. Phys. Lett. A 1997, 234, 493–497. [Google Scholar] [CrossRef]
- Ge, G.; Li, G.; Wang, X.; Chen, X.; Fu, L.; Liu, X.; Mao, E.; Liu, J.; Yang, X.; Qian, C.; et al. Manipulating Oxidation of Silicon with Fresh Surface Enabling Stable Battery Anode. Nano Lett. 2021, 21, 3127–3133. [Google Scholar] [CrossRef]
- Ratynski, M.; Hamankiewicz, B.; Buchberger, D.A.; Czerwinski, A. Surface Oxidation of Nano-Silicon as a Method for Cycle Life Enhancement of Li-Ion Active Materials. Molecules 2020, 25, 4093. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.H.; Zhong, L.; Huang, S.; Mao, S.X.; Zhu, T.; Huang, J.Y. Size-Dependent Fracture of Silicon Nanoparticles during Lithiation. ACS Nano 2012, 6, 1522–1531. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Shao, Z.; Li, X. Advances in Metal and Metal Oxide Modified Silicon Anodes for Lithium-Ion Batteries: A Review. Russ. J. Phys. Chem. A 2025, 99, 1717–1729. [Google Scholar] [CrossRef]
- Galashev, A.Y.; Zaikov, Y.P. New Si–Cu and Si–Ni Anode Materials for Lithium-Ion Batteries. J. Appl. Electrochem. 2019, 49, 1027–1034. [Google Scholar] [CrossRef]
- Wu, J.; Dong, Q.; Zhang, Q.; Xu, Y.; Zeng, X.; Yuan, Y.; Lu, J. Fundamental Understanding of the Low Initial Coulombic Efficiency in SiOx Anode for Lithium-Ion Batteries: Mechanisms and Solutions. Adv. Mater. 2024, 36, 2405751. [Google Scholar] [CrossRef]
- Ham, S.Y.; Sebti, E.; Cronk, A.; Pennebaker, T.; Deysher, G.; Chen, Y.T.; Oh, J.A.S.; Lee, J.B.; Song, M.S.; Ridley, P.; et al. Overcoming Low Initial Coulombic Efficiencies of Si Anodes through Prelithiation in All-Solid-State Batteries. Nat. Commun. 2024, 15, 2991. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, C.; Huang, G.; Fu, S.; Fan, Y.; Chen, Y.; Tan, T.; Ma, X.; Meng, H.; Kim, Y.; et al. Strategies for Enhancing the Initial Coulombic Efficiency of Si-Based Anodes in Lithium-Ion Batteries. Carbon 2025, 245, 120746. [Google Scholar] [CrossRef]
- Zhang, F.; Zhu, G.; Wang, K.; Qian, X.; Zhao, Y.; Luo, W.; Yang, J. Boosting the Initial Coulombic Efficiency in Silicon Anodes through Interfacial Incorporation of Metal Nanocrystals. J. Mater. Chem. A Mater. 2019, 7, 17426–17434. [Google Scholar] [CrossRef]
- Loaiza, L.C.; Salager, E.; Louvain, N.; Boulaoued, A.; Iadecola, A.; Johansson, P.; Stievano, L.; Seznec, V.; Monconduit, L. Understanding the Lithiation/Delithiation Mechanism of Si1−XGeX Alloys. J. Mater. Chem. A Mater. 2017, 5, 12462–12473. [Google Scholar] [CrossRef]
- Tersoff, J. Empirical Interatomic Potential for Silicon with Improved Elastic Properties. Phys. Rev. B 1988, 38, 9902. [Google Scholar] [CrossRef]
- Wang, X.; Ramírez-Hinestrosa, S.; Dobnikar, J.; Frenkel, D. The Lennard-Jones Potential: When (Not) to Use It. Phys. Chem. Chem. Phys. 2020, 22, 10624–10633. [Google Scholar] [CrossRef] [PubMed]
- Delhommelle, J.; Millié, P. Inadequacy of the Lorentz-Berthelot Combining Rules for Accurate Predictions of Equilibrium Properties by Molecular Simulation. Mol. Phys. 2001, 99, 619–625. [Google Scholar] [CrossRef]
- Stillinger, F.H. Rigorous Basis of the Frenkel-Band Theory of Association Equilibrium. J. Chem. Phys. 1963, 38, 1486–1494. [Google Scholar] [CrossRef]
- Zhang, H.; Li, J. High-Performance SiGe Anode Materials Obtained by Dealloying a Sr-Modified Al–Si–Ge Eutectic Precursor. RSC Adv. 2023, 13, 2672–2679. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, S.; Bian, X.; Feng, J.; An, Y.; Yuan, C. Morphology- and Porosity-Tunable Synthesis of 3D Nanoporous SiGe Alloy as a High-Performance Lithium-Ion Battery Anode. ACS Nano 2018, 12, 2900–2908. [Google Scholar] [CrossRef]
- Adegoke, T.E.; Abdul Ahad, S.; Bangert, U.; Geaney, H.; Ryan, K.M. Solution Processable Si/Ge Heterostructure NWs Enabling Anode Mass Reduction for Practical Full-Cell Li-Ion Batteries. Nanoscale Adv. 2023, 5, 6514–6523. [Google Scholar] [CrossRef]
- Kim, H.; Son, Y.; Park, C.; Lee, M.J.; Hong, M.; Kim, J.; Lee, M.; Cho, J.; Choi, H.C. Germanium Silicon Alloy Anode Material Capable of Tunable Overpotential by Nanoscale Si Segregation. Nano Lett. 2015, 15, 4135–4142. [Google Scholar] [CrossRef]
- Meng, F.; Wang, F.; Yu, H.; Zhao, Z.; Lv, Y.; Ma, C.; Zhang, D.; Liu, X. Liquid Metal-Modified Nanoporous SiGe Alloy as an Anode for Li-Ion Batteries and Its Self-Healing Performance. ACS Appl. Energy Mater. 2021, 4, 14575–14581. [Google Scholar] [CrossRef]
- Abel, P.R.; Chockla, A.M.; Lin, Y.M.; Holmberg, V.C.; Harris, J.T.; Korgel, B.A.; Heller, A.; Mullins, C.B. Nanostructured Si(1-x)Gex for Tunable Thin Film Lithium-Ion Battery Anodes. ACS Nano 2013, 7, 2249–2257. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, W.-B.; Li, W.; Du, J.; Ohta, R.; Kambara, M. Hybrid Plasma Spray Synthesis of Spherical Si0.8Ge0.2 Alloy Nanoparticles for Lithium-Ion Battery Anodes. Nanomaterials 2025, 15, 1718. https://doi.org/10.3390/nano15221718
Wang W-B, Li W, Du J, Ohta R, Kambara M. Hybrid Plasma Spray Synthesis of Spherical Si0.8Ge0.2 Alloy Nanoparticles for Lithium-Ion Battery Anodes. Nanomaterials. 2025; 15(22):1718. https://doi.org/10.3390/nano15221718
Chicago/Turabian StyleWang, Wen-Bo, Wenfang Li, Jun Du, Ryoshi Ohta, and Makoto Kambara. 2025. "Hybrid Plasma Spray Synthesis of Spherical Si0.8Ge0.2 Alloy Nanoparticles for Lithium-Ion Battery Anodes" Nanomaterials 15, no. 22: 1718. https://doi.org/10.3390/nano15221718
APA StyleWang, W.-B., Li, W., Du, J., Ohta, R., & Kambara, M. (2025). Hybrid Plasma Spray Synthesis of Spherical Si0.8Ge0.2 Alloy Nanoparticles for Lithium-Ion Battery Anodes. Nanomaterials, 15(22), 1718. https://doi.org/10.3390/nano15221718






