Nanosized Anisotropic Sm–Fe–N Particles with Metastable TbCu7-Type Structures Prepared by an Induction Thermal Plasma Process
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of the Raw Powder for ITP Process
2.2. Characterization
2.3. Numerical Calculation
- •
- The particles are spherical.
- •
- Bulk gas, metal vapors, and particles have the same temperature.
- •
- Heat generation due to vapor condensation on particles is negligible.
- •
- Metal vapor is considered an ideal gas owing to its high temperature.
- •
- •
- The electric charge of particles is negligible under the present conditions.
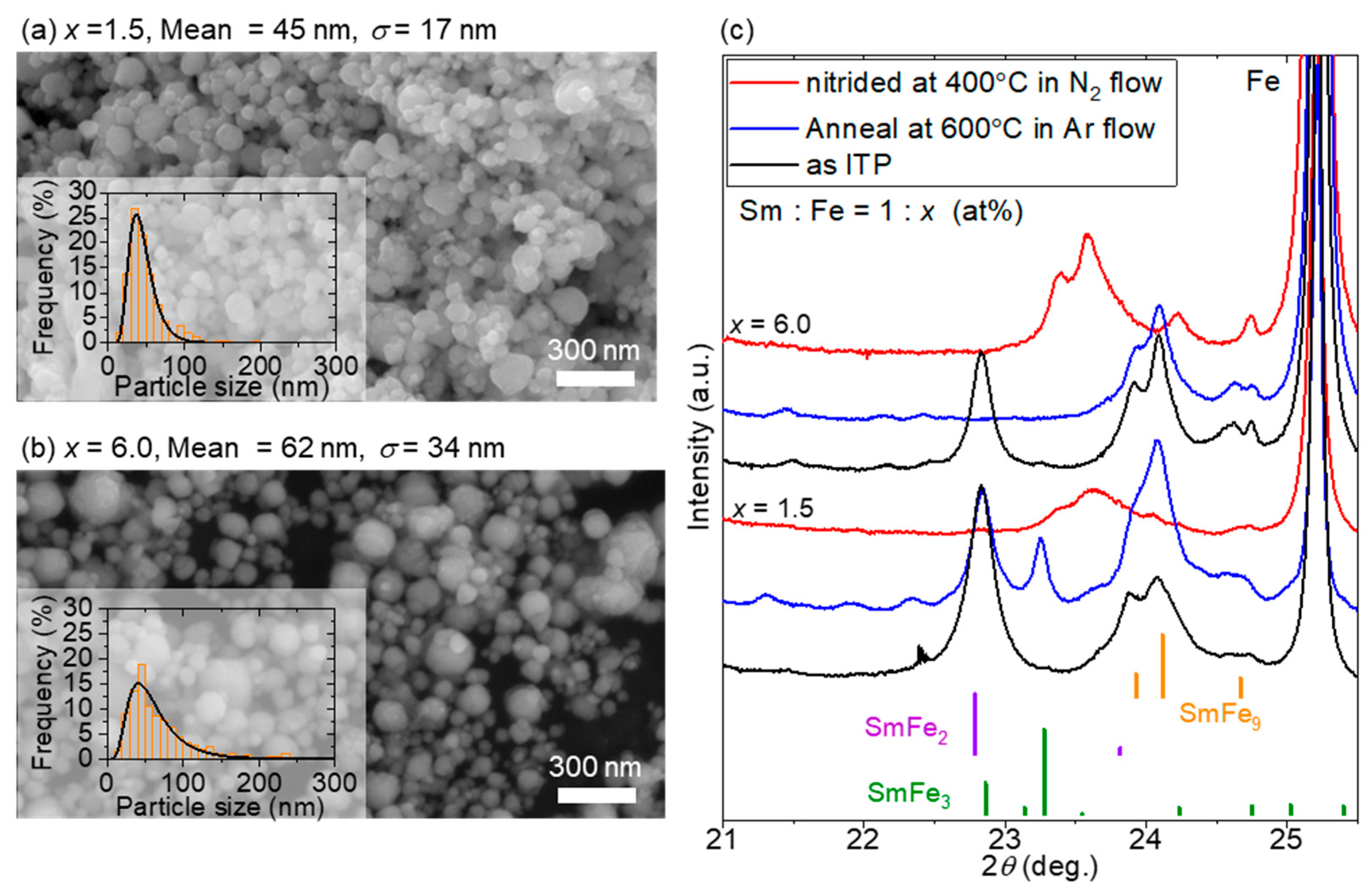
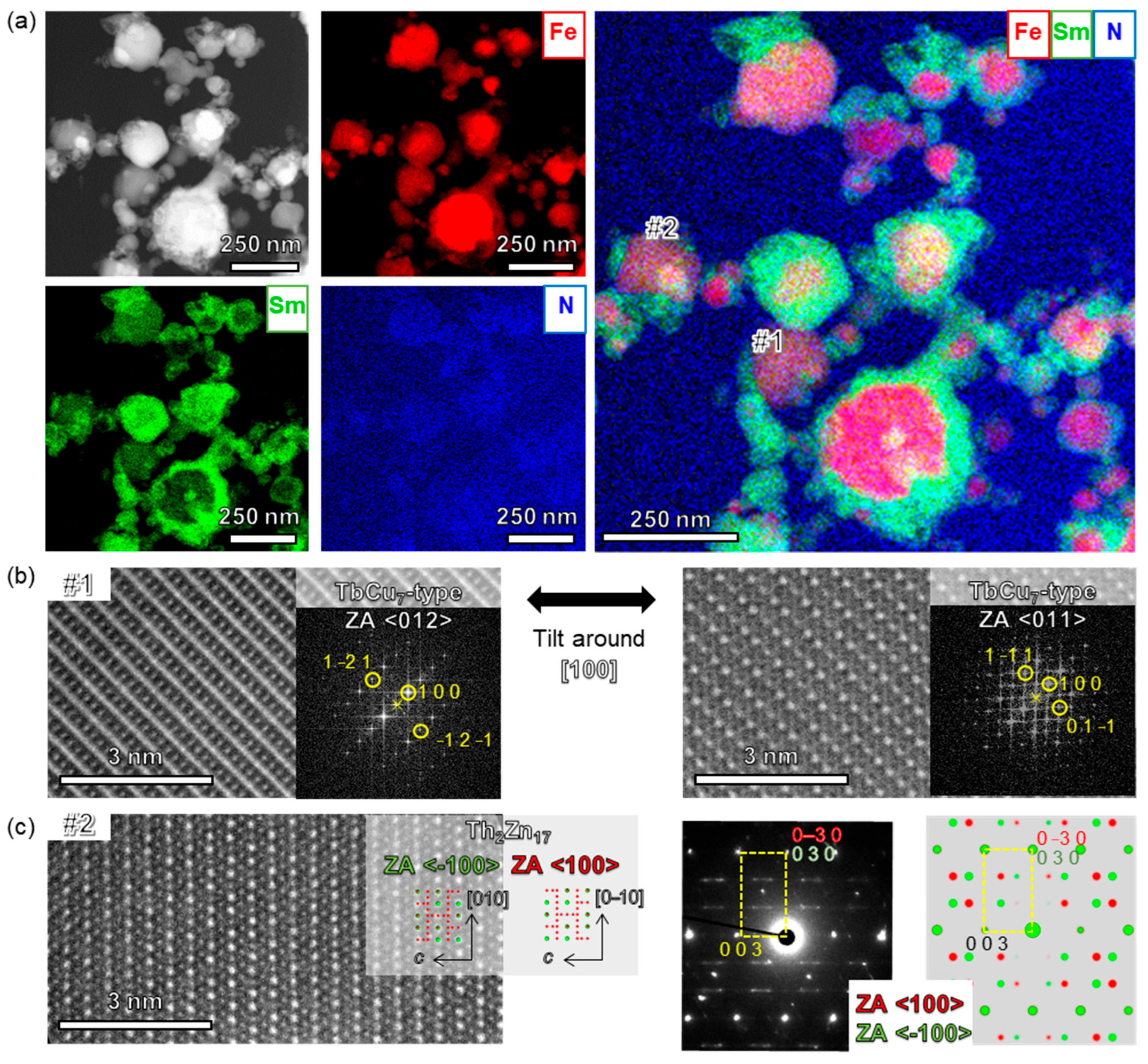
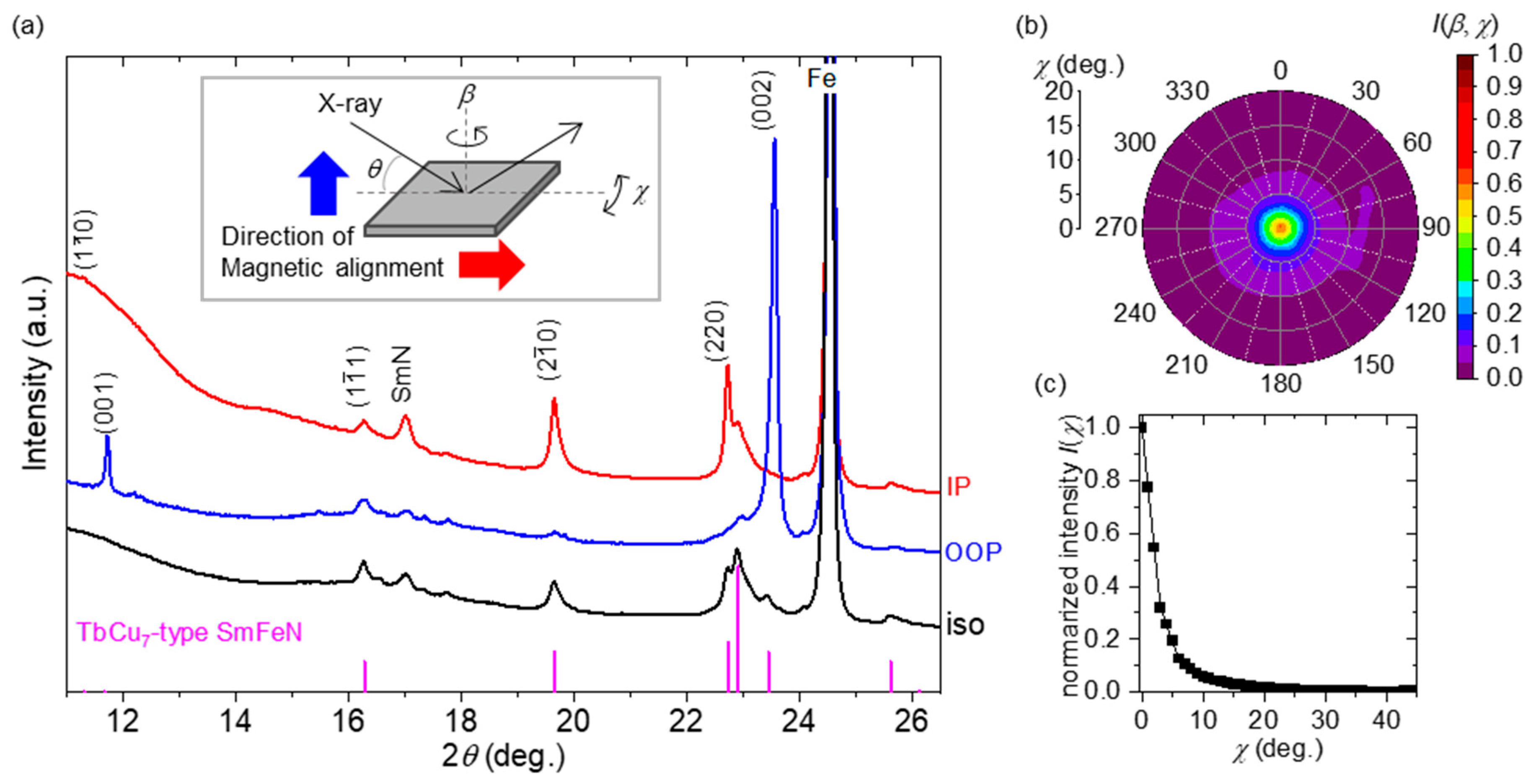
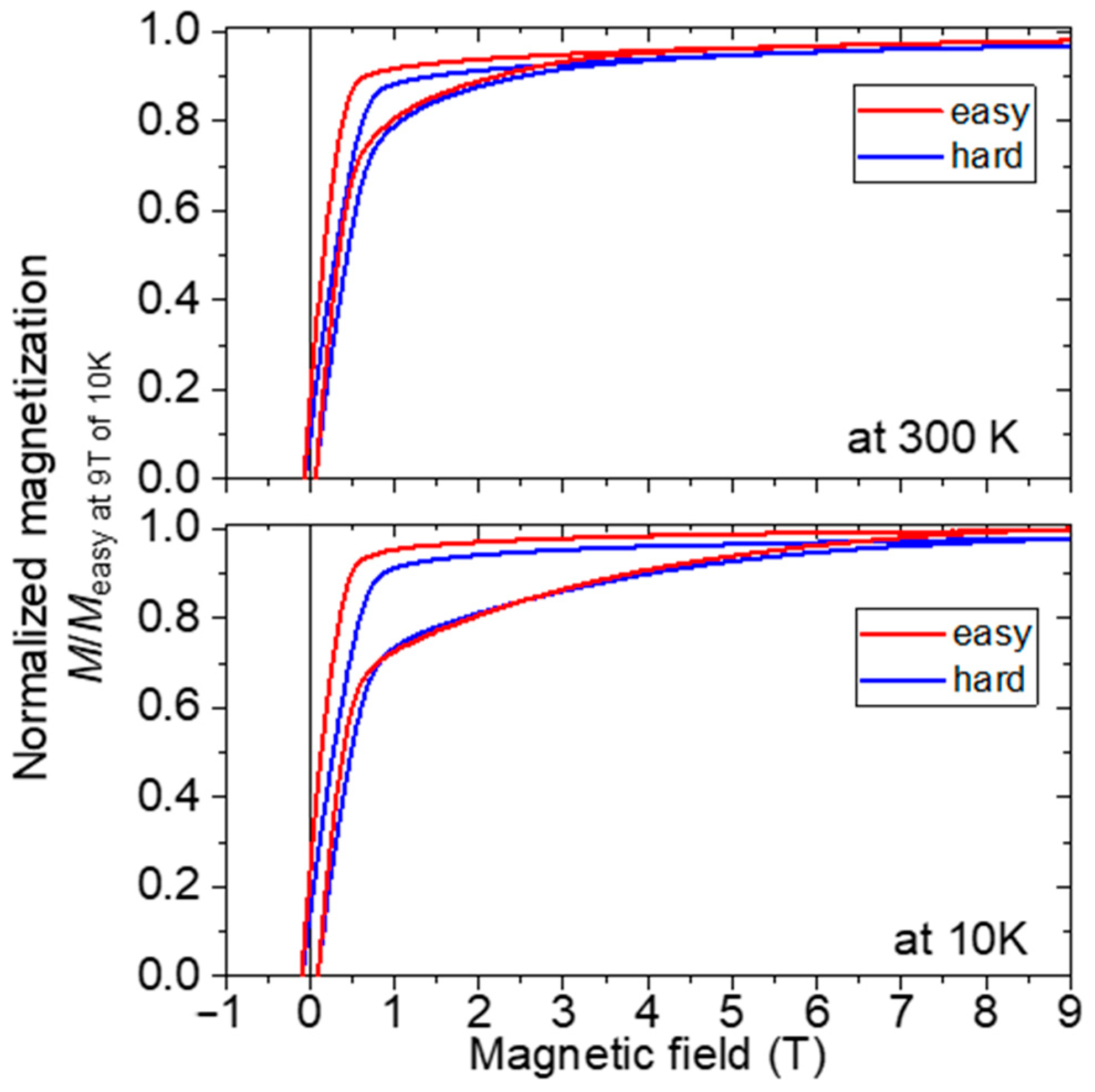
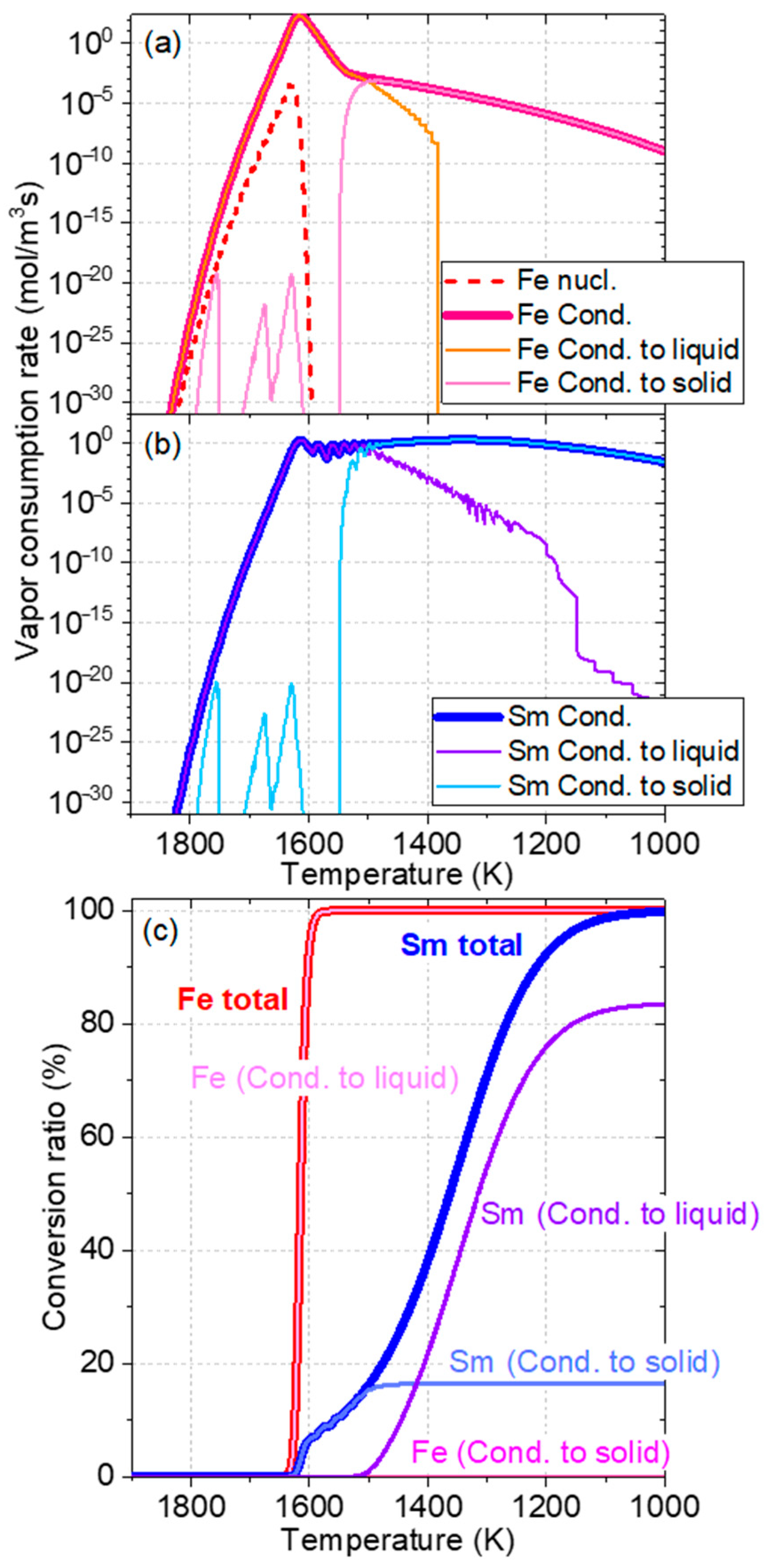
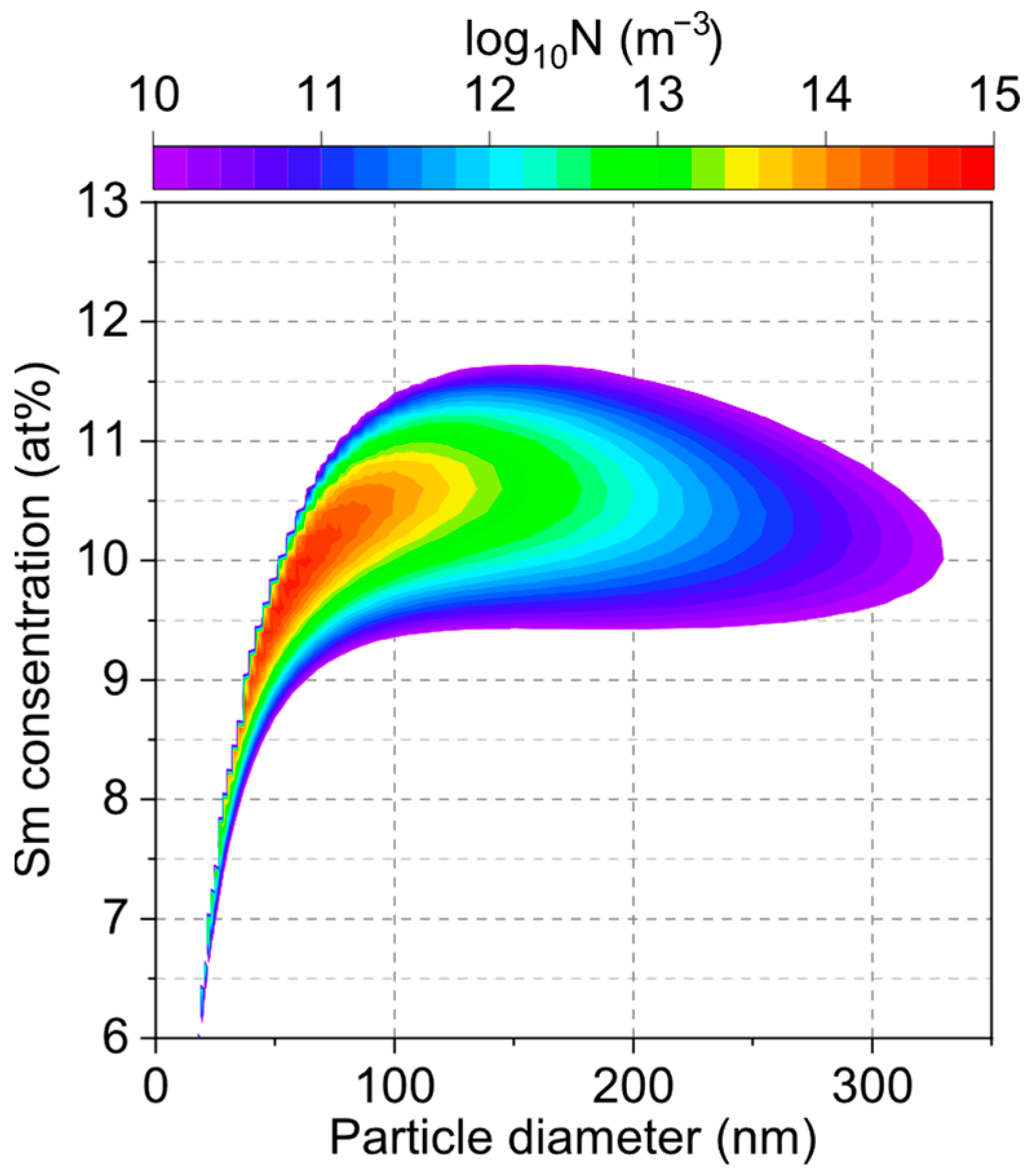
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sagawa, M.; Fujimura, S.; Togawa, N.; Yamamoto, H.; Matsuura, Y. New material for permanent magnets on a base of Nd and Fe (invited). J. Appl. Phys. 1984, 55, 2083–2087. [Google Scholar] [CrossRef]
- Croat, J.J. Permanent-Magnet Properties of Rapidly Quenched Rare Earth-Iron Alloys. IEEE Trans. Magn. 1982, 18, 1442–1447. [Google Scholar] [CrossRef]
- Löewe, K.; Brombacher, C.; Katter, M.; Gutfleisch, O. Temperature-dependent Dy diffusion processes in Nd–Fe–B permanent magnets. Acta Mater. 2015, 83, 248–255. [Google Scholar] [CrossRef]
- Zhou, T.; Chen, J.; Wang, Q.; Pan, W.; Huang, Q.; Liu, R.; Li, M.; Xie, G. Super-high coercivity NdFeB magnet fabricated with double Tb-rich/lean shells by double alloy method and grain boundary diffusion. J. Alloys Compd. 2023, 937, 168368. [Google Scholar] [CrossRef]
- Liu, L.; Sepehri-Amin, H.; Ohkubo, T.; Yano, M.; Kato, A.; Sakuma, N.; Shoji, T.; Hono, K. Coercivity enhancement of hot-deformed Nd-Fe-B magnets by the eutectic grain boundary diffusion process using Nd62Dy20Al18 alloy. Scr. Mater. 2017, 129, 44–47. [Google Scholar] [CrossRef]
- Xu, X.D.; Sasaki, T.T.; Li, J.N.; Dong, Z.J.; Sepehri-Amin, H.; Kim, T.H.; Ohkubo, T.; Schrefl, T.; Hono, K. Microstructure of a Dy-free Nd-Fe-B sintered magnet with 2 T coercivity. Acta Mater. 2018, 156, 146–157. [Google Scholar] [CrossRef]
- Hioki, K. High performance hot-deformed Nd-Fe-B magnets (Review). Sci. Technol. Adv. Mater. 2021, 22, 72–84. [Google Scholar] [CrossRef]
- Akiya, T.; Liu, J.; Sepehri-Amin, H.; Ohkubo, T.; Hioki, K.; Hattori, A.; Hono, K. High-coercivity hot-deformed Nd-Fe-B permanent magnets processed by Nd-Cu eutectic diffusion under expansion constraint. Scr. Mater. 2014, 81, 48–51. [Google Scholar] [CrossRef]
- Lee, S.; Sato, A.; Tamaoka, T.; Yubuta, K.; Auchi, M.; Sasaki, T.; Ohkubo, T.; Hono, K.; Murakami, Y. Extraction of phase information approximating the demagnetization field within a thin-foiled magnet using electron holography observation. Microscopy 2022, 72, 343–352. [Google Scholar] [CrossRef]
- Coey, J.M.D.; Lawler, J.F.; Sun, H.; Allan, J.E.M. Nitrogenation of R2Fe17 compounds: R=rare earth. J. Appl. Phys. 1991, 69, 3007–3010. [Google Scholar] [CrossRef]
- Iriyama, T.; Kobayashi, K.; Imaoka, N.; Fukuda, T.; Kato, H.; Nakagawa, Y. Effect of nitrogen content on magnetic properties of Sm2Fe17Nx(0<x<6). Magn. IEEE Trans. 1992, 28, 2326–2331. [Google Scholar] [CrossRef]
- Katter, M.; Wecker, J.; Schultz, L. Structural and hard magnetic properties of rapidly solidified Sm–Fe–N. J. Appl. Phys. 1991, 70, 3188–3196. [Google Scholar] [CrossRef]
- Sakurada, S.; Tsutai, A.; Hirai, T.; Yanagida, Y.; Sahashi, M.; Abe, S.; Kaneko, T. Structural and magnetic properties of rapidly quenched (R,Zr)(Fe,Co)10Nx (R=Nd,Sm). J. Appl. Phys. 1996, 79, 4611–4613. [Google Scholar] [CrossRef]
- Hagiwara, M.; Sanada, N.; Sakurada, S. Effect of Y substitution on the structural and magnetic properties of Sm(Fe0.8Co0.2)11.4Ti0.6. J. Magn. Magn. Mater. 2018, 465, 554–558. [Google Scholar] [CrossRef]
- Hirayama, Y.; Takahashi, Y.K.; Hirosawa, S.; Hono, K. Intrinsic hard magnetic properties of Sm(Fe1−xCox)12 compound with the ThMn12 structure. Scr. Mater. 2017, 138, 62–65. [Google Scholar] [CrossRef]
- Tozman, P.; Takahashi, Y.K.; Sepehri-Amin, H.; Ogawa, D.; Hirosawa, S.; Hono, K. The effect of Zr substitution on saturation magnetization in (Sm1-xZrx)(Fe0.8Co0.2)12 compound with the ThMn12 structure. Acta Mater. 2019, 178, 114–121. [Google Scholar] [CrossRef]
- Ohashi, K.; Tawara, Y.; Osugi, R.; Shimao, M. Magnetic properties of Fe-rich rare-earth intermetallic compounds with a ThMn12 structure. J. Appl. Phys. 1988, 64, 5714–5716. [Google Scholar] [CrossRef]
- Verhoef, R.; de Boer, F.R.; Zhi-dong, Z.; Buschow, K.H.J. Moment reduction in RFe12-xTx compounds (R=Gd, Y and T=Ti, Cr, V, Mo, W). J. Magn. Magn. Mater. 1988, 75, 319–322. [Google Scholar] [CrossRef]
- Miyake, T.; Terakura, K.; Harashima, Y.; Kino, H.; Ishibashi, S. First-Principles Study of Magnetocrystalline Anisotropy and Magnetization in NdFe12, NdFe11Ti, and NdFe11TiN. J. Phys. Soc. Jpn. 2014, 83, 043702. [Google Scholar] [CrossRef]
- Tozman, P.; Sepehri-Amin, H.; Tang, X.; Ohkubo, T.; Hono, K. Development of Co-lean (Sm,Y)(Fe,Co,Ti)12 compounds with large saturation magnetization. Appl. Phys. Express 2022, 15, 045505. [Google Scholar] [CrossRef]
- Tozman, P.; Sepehri-Amin, H.; Ohkubo, T.; Hono, K. Intrinsic magnetic properties of (Sm,Gd)Fe12-based compounds with minimized addition of Ti. J. Alloys Compd. 2021, 855, 157491. [Google Scholar] [CrossRef]
- Tozman, P.; Sepehri-Amin, H.; Takahashi, Y.K.; Hirosawa, S.; Hono, K. Intrinsic magnetic properties of Sm(Fe1-xCox)11Ti and Zr-substituted Sm1-yZry(Fe0.8Co0.2)11.5Ti0.5 compounds with ThMn12 structure toward the development of permanent magnets. Acta Mater. 2018, 153, 354–363. [Google Scholar] [CrossRef]
- Körner, W.; Krugel, G.; Urban, D.F.; Elsässer, C. Screening of rare-earth-lean intermetallic 1-11 and 1-11-X compounds of YNi9In2-type for hard-magnetic applications. Scr. Mater. 2018, 154, 295–299. [Google Scholar] [CrossRef]
- Fukazawa, T.; Akai, H.; Harashima, Y.; Miyake, T. First-principles study of spin-wave dispersion in Sm(Fe1−xCox)12. J. Magn. Magn. Mater. 2019, 469, 296–301. [Google Scholar] [CrossRef]
- Harashima, Y.; Fukazawa, T.; Kino, H.; Miyake, T. Effect of R-site substitution and the pressure on stability of RFe12: A first-principles study. J. Appl. Phys. 2018, 124, 163902. [Google Scholar] [CrossRef]
- Skelland, C.; Ostler, T.; Westmoreland, S.C.; Evans, R.F.L.; Chantrell, R.W.; Yano, M.; Shoji, T.; Manabe, A.; Kato, A.; Ito, M.; et al. Probability Distribution of Substituted Titanium in RT (R. = Nd and Sm; T = Fe and Co) Structures. IEEE Trans. Magn. 2018, 54, 2103405. [Google Scholar] [CrossRef]
- Dilipan, A.R.; Ogawa, D.; Sepehri-Amin, H.; Tozman, P.; Hiroto, T.; Hono, K.; Takahashi, Y.K. Excellent intrinsic magnetic properties in the TbCu7-type Sm-Fe-N compound. Acta Mater. 2024, 274, 119996. [Google Scholar] [CrossRef]
- Kurokawa, N.; Matsuura, M.; Sakurada, S.; Sugimoto, S. Enhancement of magnetic properties and microstructural changes in TbCu7-type Sm-Fe-Co-Nb-B melt-spun ribbons. J. Magn. Magn. Mater. 2022, 556, 169414. [Google Scholar] [CrossRef]
- Kurokawa, N.; Matsuura, M.; Sakurada, S.; Sugimoto, S. Effects of Nb and B addition on intrinsic magnetic properties and phase of TbCu7-type Sm-Fe-Co-Nb-B alloys. In Proceedings of the 2023 IEEE International Magnetic Conference-Short Papers (INTERMAG Short Papers), Aobayama, Japan, 15–19 May 2023; pp. 1–2. [Google Scholar]
- Christodoulou, C.N.; Takeshita, T. Sm2Fe17-nitride-based permanent magnets produced by the hydrogenation-decomposition-desorption-recombination (HDDR) process. J. Alloys Compd. 1993, 196, 155–159. [Google Scholar] [CrossRef]
- Zhao, X.-g.; Zhang, Z.-d.; Liu, W.; Xiao, Q.-f.; Sun, X.K. Structural and magnetic properties of Sm–Fe–N magnets prepared by hydrogenation and nitrogenation processes. J. Magn. Magn. Mater. 1995, 148, 419–425. [Google Scholar] [CrossRef]
- Müller, K.H.; Cao, L.; Dempsey, N.M.; Wendhausen, P.A.P. Sm2Fe17 interstitial magnets (invited). J. Appl. Phys. 1996, 79, 5045–5050. [Google Scholar] [CrossRef]
- Sun, J.-B.; Cui, C.-X.; Zhang, Y.; Wang, R.; Li, L.; Yang, W.; Liu, Y.-L. Structural and nitrogenation of Sm2Fe16Ti1 alloy prepared by HDDR process. Mater. Chem. Phys. 2006, 97, 116–120. [Google Scholar] [CrossRef]
- Okada, S.; Takagi, K. Novel synthesis of single-crystalline TbCu7-type Sm–Fe powder by low-temperature reduction-diffusion process using molten salt. J. Rare Earths 2022, 40, 1126–1133. [Google Scholar] [CrossRef]
- Sato, S.; Nishikawa, K.; Node, E.; Okada, S. Development of TbCu7-type Sm-Fe-N anisotropic magnet powder and its sintered magnets. J. Alloys Compd. 2022, 929, 167280. [Google Scholar] [CrossRef]
- Tada, S.; Tomimoto, T.; Kume, M. High-Coercivity Anisotoropic SmFeN Magnetic Materials. In Proceedings of the REPM’12 22th International Workshop on Rare-Earth Permanent Magnets and Their Applications, Nagasaki, Japan, 2–5 September 2012; pp. 48–53. [Google Scholar]
- Bilodeau, J.-F.; Proulx, P. A Mathematical Model for Ultrafine Iron Powder Growth in a Thermal Plasma. Aerosol Sci. Technol. 1996, 24, 175–189. [Google Scholar] [CrossRef]
- Désilets, M.; Bilodeau, J.F.; Proulx, P. Modelling of the reactive synthesis of ultra-fine powders in a thermal plasma reactor. J. Phys. D Appl. Phys. 1997, 30, 1951. [Google Scholar] [CrossRef]
- Watanabe, T.; Tonoike, N.; Honda, T.; Kanzawa, A. Flow, Temperature and Concentration Fields in Reactive Plasmas in an Inductively Coupled RF Discharge—Characteristics in Argon-Oxygen and Argon-Nitrogen Thermal Plasmas&mdash. J. Chem. Eng. Jpn. 1991, 24, 25–32. [Google Scholar] [CrossRef]
- Watanabe, T.; Okumiya, H. Formation mechanism of silicide nanoparticles by induction thermal plasmas. Sci. Technol. Adv. Mater. 2004, 5, 639. [Google Scholar] [CrossRef]
- Shigeta, M.; Watanabe, T. Numerical investigation of cooling effect on platinum nanoparticle formation in inductively coupled thermal plasmas. J. Appl. Phys. 2008, 103, 074903. [Google Scholar] [CrossRef]
- Pfender, E.; Fincke, J.; Spores, R. Entrainment of cold gas into thermal plasma jets. Plasma Chem. Plasma Process. 1991, 11, 529–543. [Google Scholar] [CrossRef]
- Mostaghimi, J.; Boulos, M.I. Thermal Plasma Sources: How Well are They Adopted to Process Needs? Plasma Chem. Plasma Process. 2015, 35, 421–436. [Google Scholar] [CrossRef]
- Shigeta, M. Progress of computational plasma fluid mechanics. Jpn. J. Appl. Phys. 2023, 62, SL0801. [Google Scholar] [CrossRef]
- Girshick, S.L.; Chiu, C.P.; Muno, R.; Wu, C.Y.; Yang, L.; Singh, S.K.; McMurry, P.H. Thermal plasma synthesis of ultrafine iron particles. J. Aerosol Sci. 1993, 24, 367–382. [Google Scholar] [CrossRef]
- Watanabe, T.; Nezu, A.; Abe, Y.; Ishii, Y.; Adachi, K. Formation mechanism of electrically conductive nanoparticles by induction thermal plasmas. Thin Solid Film. 2003, 435, 27–32. [Google Scholar] [CrossRef]
- Wang, X.H.; Li, J.G.; Kamiyama, H.; Katada, M.; Ohashi, N.; Moriyoshi, Y.; Ishigaki, T. Pyrogenic Iron(III)-Doped TiO2 Nanopowders Synthesized in RF Thermal Plasma: Phase Formation, Defect Structure, Band Gap, and Magnetic Properties. J. Am. Chem. Soc. 2005, 127, 10982–10990. [Google Scholar] [CrossRef]
- Kong, P.C.; Lau, Y.C. Plasma synthesis of ceramic powders. Pure Appl. Chem. 1990, 62, 1809–1816. [Google Scholar] [CrossRef]
- Shigeta, M. Modeling and simulation of a turbulent-like thermal plasma jet for nanopowder production. IEEJ Trans. Electr. Electron. Eng. 2019, 14, 16–28. [Google Scholar] [CrossRef]
- Shigeta, M. Simulating Turbulent Thermal Plasma Flows for Nanopowder Fabrication. Plasma Chem. Plasma Process. 2020, 40, 775–794. [Google Scholar] [CrossRef]
- Shigeta, M.; Watanabe, T. Multi-component co-condensation model of Ti-based boride/silicide nanoparticle growth in induction thermal plasmas. Thin Solid Films 2007, 515, 4217–4227. [Google Scholar] [CrossRef]
- Shigeta, M.; Watanabe, T. Growth mechanism of silicon-based functional nanoparticles fabricated by inductively coupled thermal plasmas. J. Phys. D Appl. Phys. 2007, 40, 2407. [Google Scholar] [CrossRef]
- Kambara, M.; Kitayama, A.; Homma, K.; Hideshima, T.; Kaga, M.; Sheem, K.-Y.; Ishida, S.; Yoshida, T. Nano-composite Si particle formation by plasma spraying for negative electrode of Li ion batteries. J. Appl. Phys. 2014, 115, 143302. [Google Scholar] [CrossRef]
- Kodama, N.; Tanaka, Y.; Kita, K.; Uesugi, Y.; Ishijima, T.; Watanabe, S.; Nakamura, K. A method for large-scale synthesis of Al-doped TiO2 nanopowder using pulse-modulated induction thermal plasmas with time-controlled feedstock feeding. J. Phys. D Appl. Phys. 2014, 47, 195304. [Google Scholar] [CrossRef]
- Tanaka, Y.; Tsuke, T.; Guo, W.; Uesugi, Y.; Ishijima, T.; Watanabe, S.; Nakamura, K. A large amount synthesis of nanopowder using modulated induction thermal plasmas synchronized with intermittent feeding of raw materials. J. Phys. Conf. Ser. 2012, 406, 012001. [Google Scholar] [CrossRef]
- Shigeta, M.; Murphy, A.B. Thermal plasmas for nanofabrication. J. Phys. D Appl. Phys. 2011, 44, 174025. [Google Scholar] [CrossRef]
- Colombo, V.; Ghedini, E.; Gherardi, M.; Sanibondi, P.; Shigeta, M. A two-dimensional nodal model with turbulent effects for the synthesis of Si nano-particles by inductively coupled thermal plasmas. Plasma Sources Sci. Technol. 2012, 21, 025001. [Google Scholar] [CrossRef]
- Hirayama, Y.; Shigeta, M.; Liu, Z.; Yodoshi, N.; Hosokawa, A.; Takagi, K. Anisotropic Nd-Fe ultrafine particles with stable and metastable phases prepared by induction thermal plasma. J. Alloys Compd. 2021, 873, 159724. [Google Scholar] [CrossRef]
- Shigeta, M.; Watanabe, T. Two-Directional Nodal Model for Co-Condensation Growth of Multicomponent Nanoparticles in Thermal Plasma Processing. J. Therm. Spray Technol. 2009, 18, 1022. [Google Scholar] [CrossRef]
- Van Ende, M.-A.; Jung, I.-H. Critical thermodynamic evaluation and optimization of the Fe–B, Fe–Nd, B–Nd and Nd–Fe–B systems. J. Alloys Compd. 2013, 548, 133–154. [Google Scholar] [CrossRef]
- Guisbiers, G.; Kazan, M.; Van Overschelde, O.; Wautelet, M.; Pereira, S. Mechanical and Thermal Properties of Metallic and Semiconductive Nanostructures. J. Phys. Chem. C 2008, 112, 4097–4103. [Google Scholar] [CrossRef]
- Shigeta, M.; Tanaka, M. Visualization of electromagnetic-thermal-fluid phenomena in arc welding. Jpn. J. Appl. Phys. 2020, 59, SA0805. [Google Scholar] [CrossRef]
- Takaki, S.; Makita, K.; Okamoto, A. Examination of Crystal Orientation in Green Compact and Sintered Nd-Fe-B Magnets by X-ray Diffraction. J. Jpn. Soc. Powder Powder Metall. 2002, 50, 45–49. [Google Scholar] [CrossRef][Green Version]
- Park, K.; Hirayama, Y.; Shigeta, M.; Liu, Z.; Kobashi, M.; Takagi, K. Anisotropic Sm-Co nanopowder prepared by induction thermal plasma. J. Alloys Compd. 2021, 882, 160633. [Google Scholar] [CrossRef]
- Shigeta, M.; Watanabe, T. Effect of Saturation Pressure Difference on Metal–Silicide Nanopowder Formation in Thermal Plasma Fabrication. Nanomaterials 2016, 6, 43. [Google Scholar] [CrossRef]
- Shigeta, M.; Hirayama, Y.; Ghedini, E. Computational Study of Quenching Effects on Growth Processes and Size Distributions of Silicon Nanoparticles at a Thermal Plasma Tail. Nanomaterials 2021, 11, 1370. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hirayama, Y.; Wang, J.; Shigeta, M.; Tsurumi, S.; Sugimoto, M.; Liu, Z.; Takagi, K.; Ozaki, K. Nanosized Anisotropic Sm–Fe–N Particles with Metastable TbCu7-Type Structures Prepared by an Induction Thermal Plasma Process. Nanomaterials 2025, 15, 1045. https://doi.org/10.3390/nano15131045
Hirayama Y, Wang J, Shigeta M, Tsurumi S, Sugimoto M, Liu Z, Takagi K, Ozaki K. Nanosized Anisotropic Sm–Fe–N Particles with Metastable TbCu7-Type Structures Prepared by an Induction Thermal Plasma Process. Nanomaterials. 2025; 15(13):1045. https://doi.org/10.3390/nano15131045
Chicago/Turabian StyleHirayama, Yusuke, Jian Wang, Masaya Shigeta, Shunsuke Tsurumi, Makoto Sugimoto, Zheng Liu, Kenta Takagi, and Kimihiro Ozaki. 2025. "Nanosized Anisotropic Sm–Fe–N Particles with Metastable TbCu7-Type Structures Prepared by an Induction Thermal Plasma Process" Nanomaterials 15, no. 13: 1045. https://doi.org/10.3390/nano15131045
APA StyleHirayama, Y., Wang, J., Shigeta, M., Tsurumi, S., Sugimoto, M., Liu, Z., Takagi, K., & Ozaki, K. (2025). Nanosized Anisotropic Sm–Fe–N Particles with Metastable TbCu7-Type Structures Prepared by an Induction Thermal Plasma Process. Nanomaterials, 15(13), 1045. https://doi.org/10.3390/nano15131045







