Tetrabromocobalt Phthalocyanine-Functionalized Carbon Nanotubes as a High-Performance Anode for Lithium-Ion Batteries
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
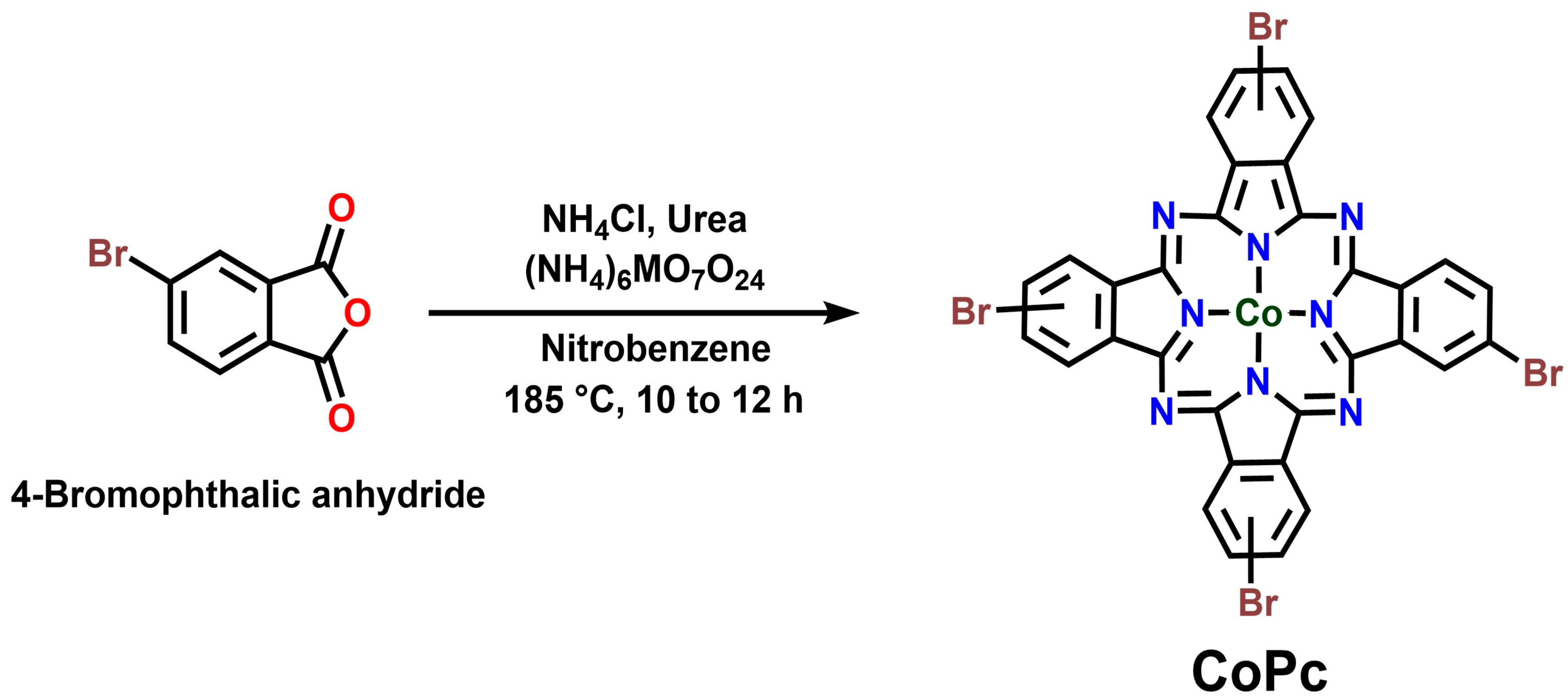
2.2. Synthesis of CoPc
2.3. Preparation of CoPc-CNT Hybrids
2.4. Electrochemical Measurements
3. Results and Discussion
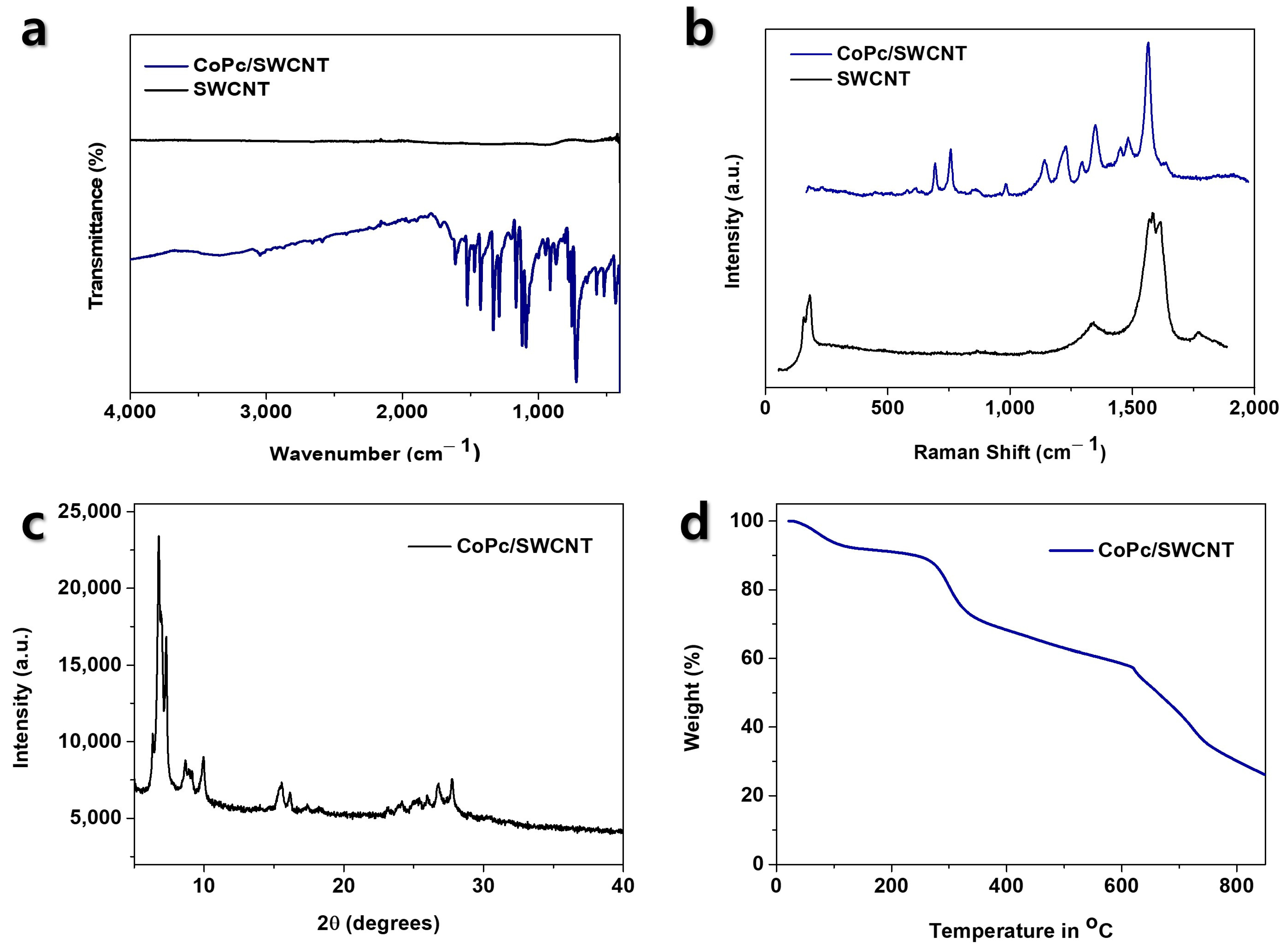
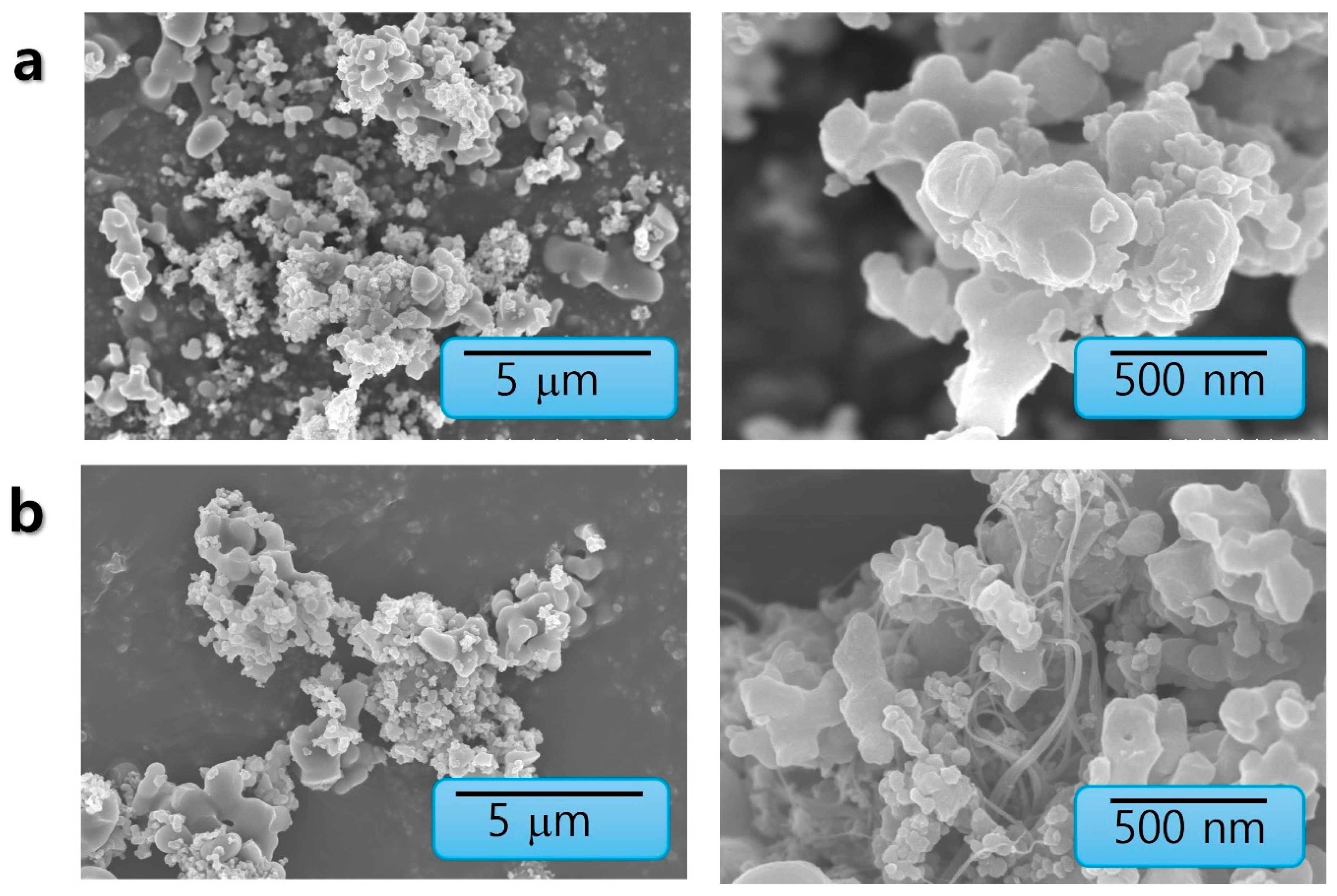
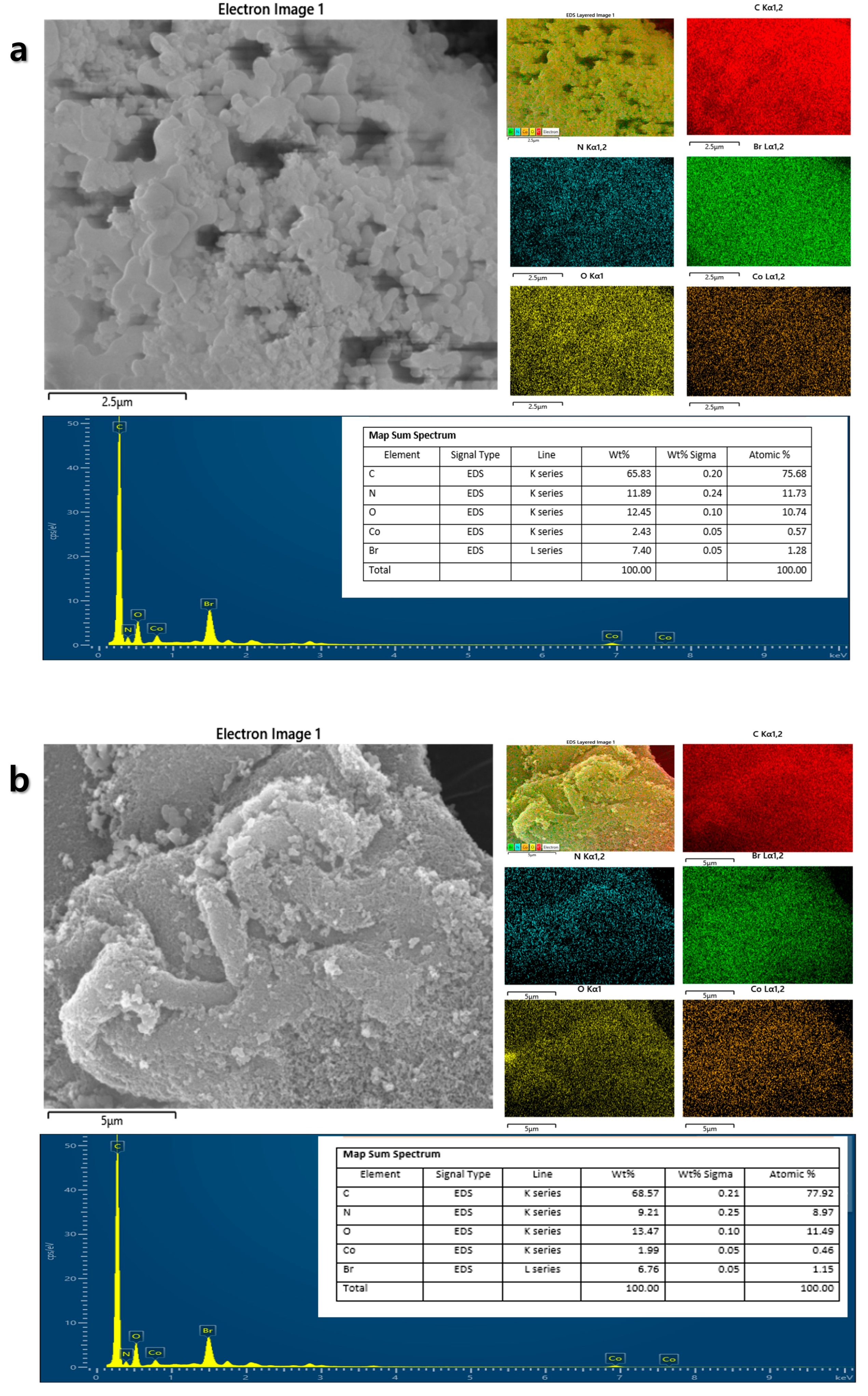
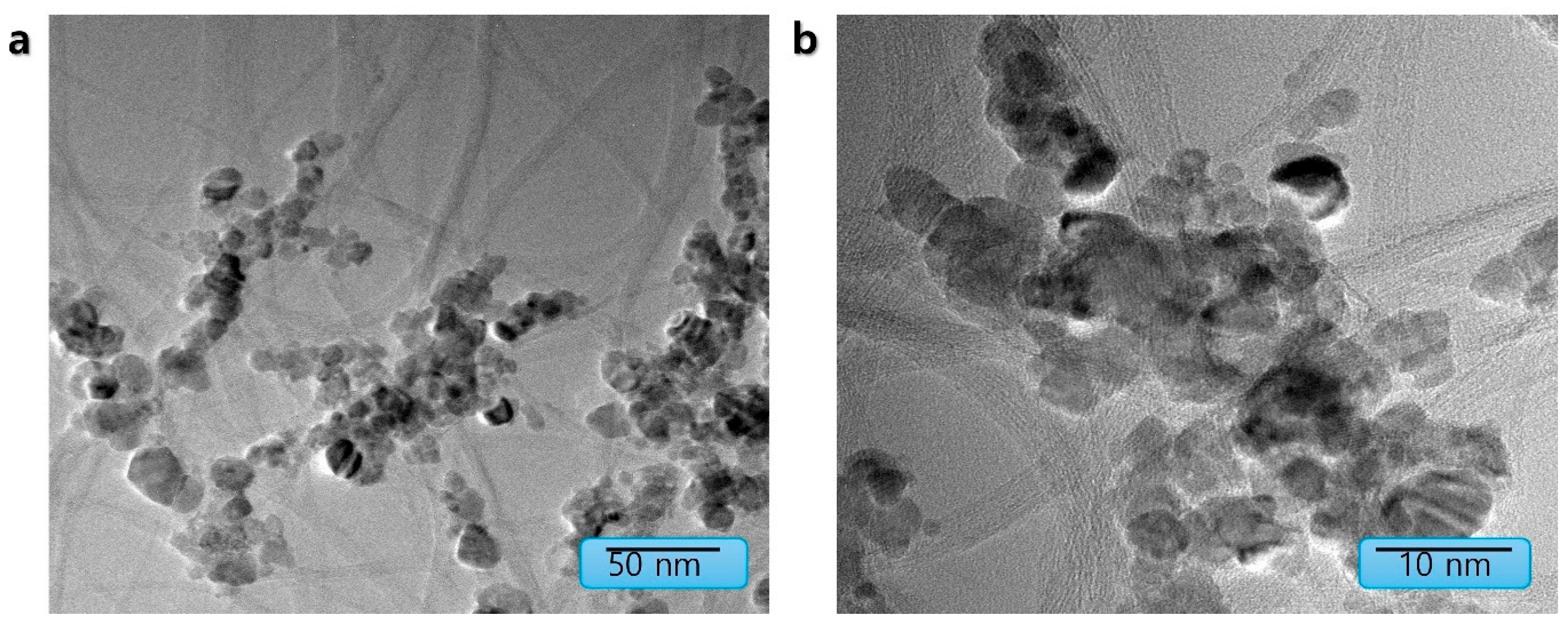
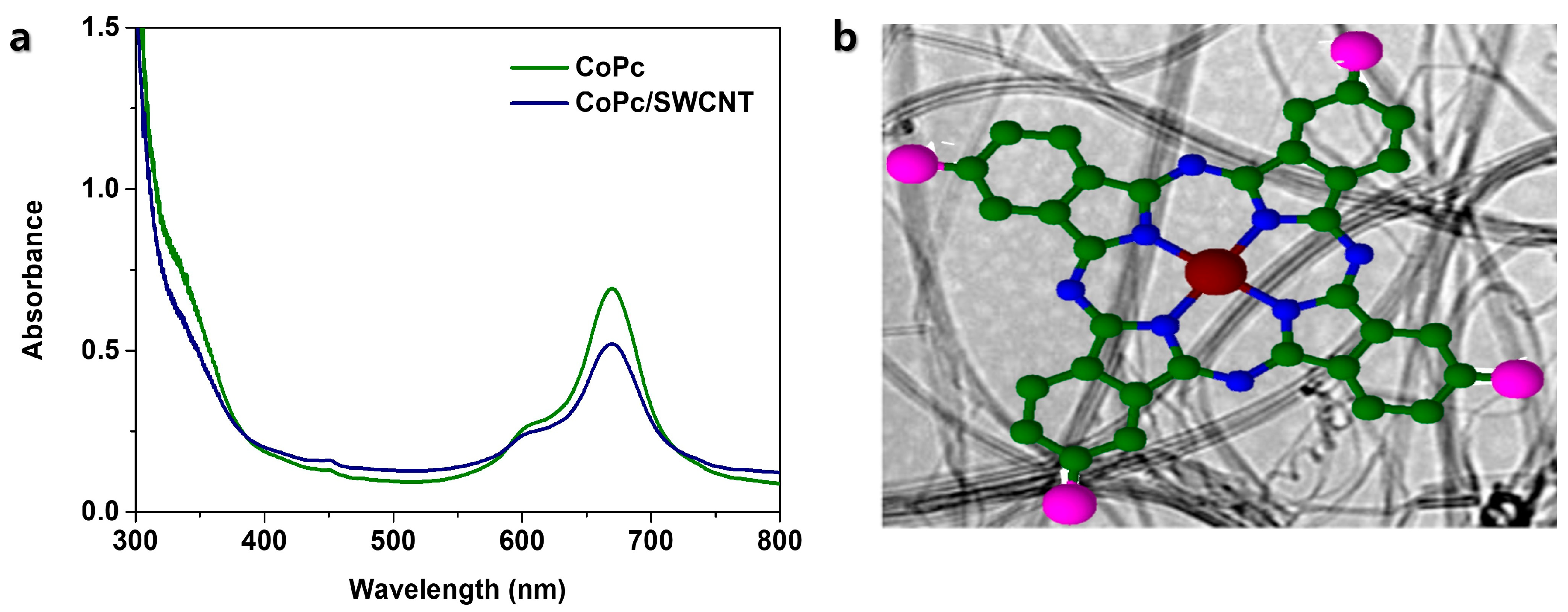
3.1. Material Synthesis and Structural Characterization
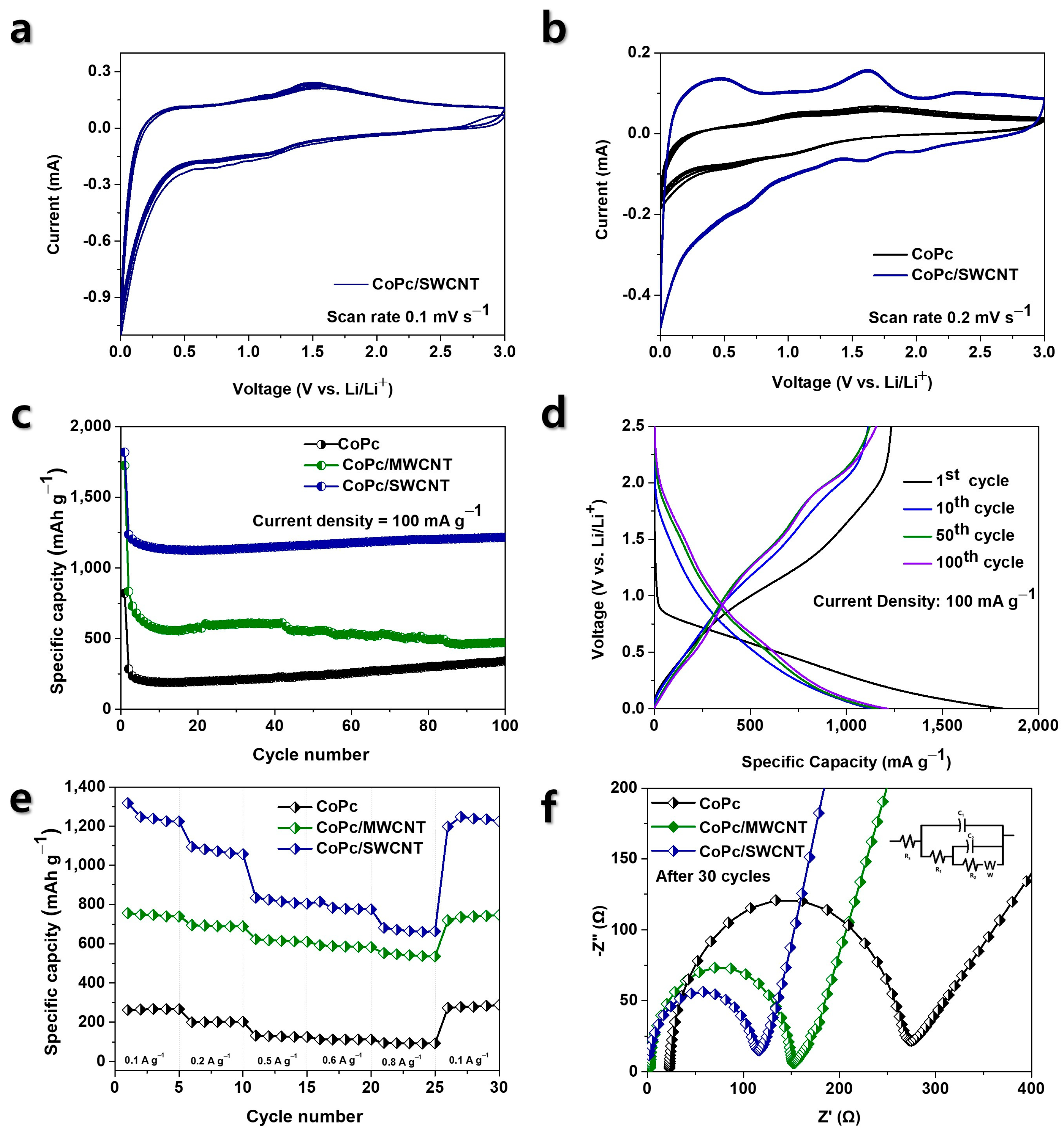
3.2. Electrochemical Performance as Li-Ion Battery Anodes
4. Conclusions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bahalkani, G.M.; Tayyab, J.S.H.S.; Tahreen, A.R.; Zahid, U.; Samad, A.; Rubab, K. Revolutionizing Energy Systems with Advanced Materials for High-Performance Batteries, Renewable Energy Integration, Smart Grids and Electric Vehicle Technologies. Glob. Sci. Acad. Res. J. Multidiscip. Stud. 2024, 3, 102–112. [Google Scholar] [CrossRef]
- Abro, G.E.M.; Zulkifli, S.A.B.; Kumar, K.; El Ouanjli, N.; Asirvadam, V.S.; Mossa, M.A. Comprehensive review of recent advancements in battery technology, propulsion, power interfaces, and vehicle network systems for intelligent autonomous and connected electric vehicles. Energies 2023, 16, 2925. [Google Scholar] [CrossRef]
- Khan, F.N.U.; Rasul, M.G.; Sayem, A.S.M.; Mandal, N. Maximizing energy density of lithium-ion batteries for electric vehicles: A critical review. Energy Rep. 2023, 9, 11–21. [Google Scholar] [CrossRef]
- Zhao, L.; Ding, B.; Qin, X.Y.; Wang, Z.; Lv, W.; He, Y.B.; Yang, Q.H.; Kang, F. Revisiting the roles of natural graphite in ongoing lithium-ion batteries. Adv. Mater. 2022, 34, 2106704. [Google Scholar] [CrossRef]
- Goriparti, S.; Miele, E.; De Angelis, F.; Di Fabrizio, E.; Zaccaria, R.P.; Capiglia, C. Review on recent progress of nanostructured anode materials for Li-ion batteries. J. Power Sources 2014, 257, 421–443. [Google Scholar] [CrossRef]
- Gopinadh, S.V.; Phanendra, P.V.; V, A.; John, B.; TD, M. Progress, challenges, and perspectives on alloy-based anode materials for lithium ion battery: A mini-review. Energy Fuels 2024, 38, 17253–17277. [Google Scholar] [CrossRef]
- Puttaningaiah, K.P.C.H.; Park, C.; Bae, J.S.; Park, S.H.; Hur, J. Harnessing benzimidazole moiety and annealing in ruthenium tetra-substituted benzimidazole phthalocyanine/carbon nanotube composite for enhanced Li-ion battery anodes. J. Alloys Compd. 2025, 1022, 179890. [Google Scholar]
- Gaddimath, S.; Ramegowda, S.D.; Chandrakala, K.B.; Puttaningaiah, K.P.C.H.; Hur, J. Synergistic effect of nickel (II) phthalocyanine as a promising electrode material for high-performance lithium-ion batteries. J. Electroanal. Chem. 2025, 979, 118912. [Google Scholar] [CrossRef]
- Puttaningaiah, K.P.C.H.; Gaddimath, S.; Park, S.H.; Hur, J. Unveiling the effect of annealing on copper phthalocyanine/single-walled carbon nanotubes composite for bifunctional applications. J. Ind. Eng. Chem. 2025, 151, 186–199. [Google Scholar] [CrossRef]
- Channabasavana Hundi Puttaningaiah, K.P.; D Ramegowda, S.; Hur, J. Polymer Tetrabenzimidazole Aluminum Phthalocyanine Complex with Carbon Nanotubes: A Promising Approach for Boosting Lithium-Ion Battery Anode Performance. ACS Appl. Energy Mater. 2024, 7, 6793–6806. [Google Scholar] [CrossRef]
- Yang, S.; Yu, Y.; Gao, X.; Zhang, Z.; Wang, F. Recent advances in electrocatalysis with phthalocyanines. Chem. Soc. Rev. 2021, 50, 12985–13011. [Google Scholar] [CrossRef]
- CP, K.P.; Aralekallu, S.; Sajjan, V.A.; Palanna, M.; Kumar, S.; Sannegowda, L.K. Non-precious cobalt phthalocyanine-embedded iron ore electrocatalysts for hydrogen evolution reactions. Sustain. Energy Fuels 2021, 5, 1448–1457. [Google Scholar] [CrossRef]
- Channabasavana Hundi Puttaningaiah, K.P. Design and optimization of polyaniline/SWCNT anodes for improved lithium-ion storage. Polymers 2025, 17, 478. [Google Scholar] [CrossRef]
- Cao, Y.; Wang, M.; Wang, H.; Han, C.; Pan, F.; Sun, J. Covalent organic framework for rechargeable batteries: Mechanisms and properties of ionic conduction. Adv. Energy Mater. 2022, 12, 2200057. [Google Scholar] [CrossRef]
- Serrano, M.C.; Gutiérrez, M.C.; del Monte, F. Role of polymers in the design of 3D carbon nanotube-based scaffolds for biomedical applications. Prog. Polym. Sci. 2014, 39, 1448–1471. [Google Scholar] [CrossRef]
- Kandhola, G.; Park, S.; Lim, J.W.; Chivers, C.; Song, Y.H.; Chung, J.H.; Kim, J.; Kim, J.W. Nanomaterial-based scaffolds for tissue engineering applications: A review on graphene, carbon nanotubes and nanocellulose. Tissue Eng. Regen. Med. 2023, 20, 411–433. [Google Scholar] [CrossRef] [PubMed]
- Pineda, L.B. Non-Covalent Interactions of Metal Phthalocyanines with Carbon Nanotubes. Ph.D. Thesis, University of Groningen, Groningen, The Netherlands, 2023. [Google Scholar]
- Liu, D.; Shi, L.; Dai, Q.; Lin, X.; Mehmood, R.; Gu, Z.; Dai, L. Functionalization of carbon nanotubes for multifunctional applications. Trends Chem. 2024, 6, 186–210. [Google Scholar] [CrossRef]
- Akbulut, H.; Nalci, D.; Guler, A.; Duman, S.; Guler, M.O. Carbon-silicon composite anode electrodes modified with MWCNT for high energy battery applications. Appl. Surf. Sci. 2018, 446, 222–229. [Google Scholar] [CrossRef]
- Prabhu, C.K.; Nemakal, M.; Aralekallu, S.; Mohammed, I.; Palanna, M.; Sajjan, V.A.; Akshitha, D.; Sannegowda, L.K. A comparative study of carboxylic acid and benzimidazole phthalocyanines and their surface modification for dopamine sensing. J. Electroanal. Chem. 2019, 847, 113262. [Google Scholar] [CrossRef]
- CP, K.P.; Naveen, K.R.; Aralekallu, S.; Sannegowda, L.K. Novel polymeric cobalt tetrabenzimidazole phthalocyanine for nanomolar detection of hydrogen peroxide. RSC Sustain. 2023, 1, 128–138. [Google Scholar]
- Guan, J.G.; Wang, W.; Gong, R.Z.; Yuan, R.Z.; Gan, L.H.; Tam, K.C. One-Step Synthesis of Cobalt− Phthalocyanine/Iron Nanocomposite Particles with High Magnetic Susceptibility. Langmuir 2002, 18, 4198–4204. [Google Scholar] [CrossRef]
- Puttaningaiah, K.P.C.H.; Hur, J. Recent advances in phthalocyanine-based hybrid composites for electrochemical biosensors. Micromachines 2024, 15, 1061. [Google Scholar] [CrossRef]
- Seoudi, R.; El-Bahy, G.S.; El Sayed, Z.A. Ultraviolet and visible spectroscopic studies of phthalocyanine and its complexes thin films. Opt. Mater. 2006, 29, 304–312. [Google Scholar] [CrossRef]
- Yenilmez, H.Y.; Sevim, A.M.; Bayır, Z.A. Synthesis and photophysics of new metallo phthalocyanine complexes with thiazole groups and their fluorescence quenching studies with benzoquinone. Synth. Met. 2013, 176, 11–17. [Google Scholar] [CrossRef]
- Adeniyi, O.; Nwahara, N.; Mwanza, D.; Nyokong, T.; Mashazi, P. Nanohybrid electrocatalyst based on cobalt phthalocyanine-carbon nanotube-reduced graphene oxide for ultrasensitive detection of glucose in human saliva. Sens. Actuators B Chem. 2021, 348, 130723. [Google Scholar] [CrossRef]
- Liang, F.; Zhang, J.; Hu, Z.; Ma, C.; Ni, W.; Zhang, Y.; Zhang, S. Intrinsic defect-rich graphene coupled cobalt phthalocyanine for robust electrochemical reduction of carbon dioxide. ACS Appl. Mater. Interfaces 2021, 13, 25523–25532. [Google Scholar] [CrossRef]
- Prabhu, C.K.; Naveen, K.R.; Hur, J. Cobalt–benzimidazole swapped metal–organic macrocycle with reduced graphene oxide as a hybrid electrocatalyst for highly efficient oxygen evolution reaction. RSC Appl. Interfaces 2024, 1, 301–312. [Google Scholar] [CrossRef]
- Fu, L.; Qu, Q.; Holze, R.; Kondratiev, V.V.; Wu, Y. Composites of metal oxides and intrinsically conducting polymers as supercapacitor electrode materials: The best of both worlds? J. Mater. Chem. A 2019, 7, 14937–14970. [Google Scholar] [CrossRef]
- Dehsheikh, H.G.; Boroujerdnia, M. Synthesis and investigation of thermal conductivity carbon nanotubes: MWCNT and SWCNT. Int. J. Bio-Inorg. Hybr. Nanomater. 2016, 5, 83–94. [Google Scholar]
- Islam, M.H.; Afroj, S.; Uddin, M.A.; Andreeva, D.V.; Novoselov, K.S.; Karim, N. Graphene and CNT-based smart fiber-reinforced composites: A review. Adv. Funct. Mater. 2022, 32, 2205723. [Google Scholar] [CrossRef]
| Materials | Initial Discharge Capacity (mAh g−1) | 1 st Discharge Capacity. (mAh g−1) | Capacity Retention After 100 Cycles (%) |
|---|---|---|---|
| CoPc | 821 | 290 | 39.82 |
| CoPc/MWCNT | 1729 | 834 | 28.22 |
| CoPc/SWCNT | 1823 | 1240 | 66.70 |
| Cycle Number | Coulombic Efficiency (%) | ||
|---|---|---|---|
| CoPc | CoPc/MWCNT | CoPc/SWCNT | |
| 1st | 50.93 | 65.93 | 70.21 |
| 2nd | 73.46 | 84.16 | 97.64 |
| 3rd | 82.67 | 89.64 | 98.56 |
| 4th | 86.58 | 92.17 | 99.00 |
| 5th | 88.53 | 93.86 | 99.24 |
| 6th | 89.05 | 95.08 | 99.34 |
| 7th | 90.15 | 95.89 | 99.45 |
| 8th | 91.25 | 96.81 | 99.53 |
| 9th | 91.56 | 97.27 | 99.57 |
| 10th | 91.89 | 97.76 | 99.60 |
| Rate Capacity [mAh g−1] | 0.1 A g−1 | 0.2 A g−1 | 0.5 A g−1 | 0.6 A g−1 | 0.8 A g−1 | 0.1 A g−1 | Regained Capacity (%) |
|---|---|---|---|---|---|---|---|
| CoPc | 269 | 201 | 123 | 107 | 89 | 270 | 100.3 |
| CoPc/MWCNT | 746 | 687 | 620 | 589 | 542 | 743 | 99.5 |
| CoPc/SWCNT | 1237 | 1068 | 816 | 775 | 666 | 1230 | 99.4 |
| Electrode | Rs (Ω) | R1 (Ω) | R2 (Ω) | C1 (F) | C2 (F) | W (Ω) |
|---|---|---|---|---|---|---|
| CoPc | 22.82 | 237.4 | 0.01 | 7.297 × 10−8 | 66.96 | 0.001183 |
| CoPc/MWCNT | 2.578 | 108.5 | 146 | 1.517 × 10−7 | 5.922 × 10−8 | 0.004626 |
| CoPc/SWCNT | 0.0013 | 88.7 | 1850 | 1.41 × 10−7 | 1.657 × 10−6 | 0.001617 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Puttaningaiah, K.P.C.H. Tetrabromocobalt Phthalocyanine-Functionalized Carbon Nanotubes as a High-Performance Anode for Lithium-Ion Batteries. Nanomaterials 2025, 15, 1713. https://doi.org/10.3390/nano15221713
Puttaningaiah KPCH. Tetrabromocobalt Phthalocyanine-Functionalized Carbon Nanotubes as a High-Performance Anode for Lithium-Ion Batteries. Nanomaterials. 2025; 15(22):1713. https://doi.org/10.3390/nano15221713
Chicago/Turabian StylePuttaningaiah, Keshavananda Prabhu Channabasavana Hundi. 2025. "Tetrabromocobalt Phthalocyanine-Functionalized Carbon Nanotubes as a High-Performance Anode for Lithium-Ion Batteries" Nanomaterials 15, no. 22: 1713. https://doi.org/10.3390/nano15221713
APA StylePuttaningaiah, K. P. C. H. (2025). Tetrabromocobalt Phthalocyanine-Functionalized Carbon Nanotubes as a High-Performance Anode for Lithium-Ion Batteries. Nanomaterials, 15(22), 1713. https://doi.org/10.3390/nano15221713







