Iron Redox Cycling in Persulfate Activation: Strategic Enhancements, Mechanistic Insights, and Environmental Applications—A Review
Abstract
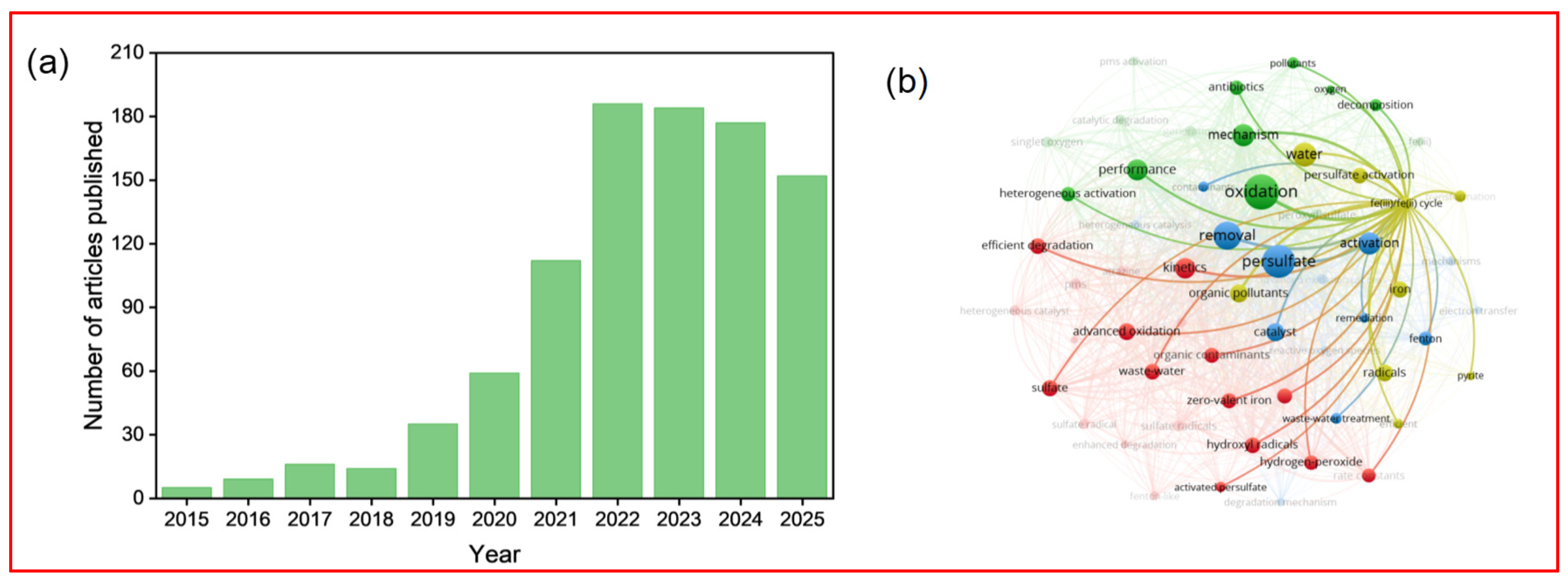
1. Introduction
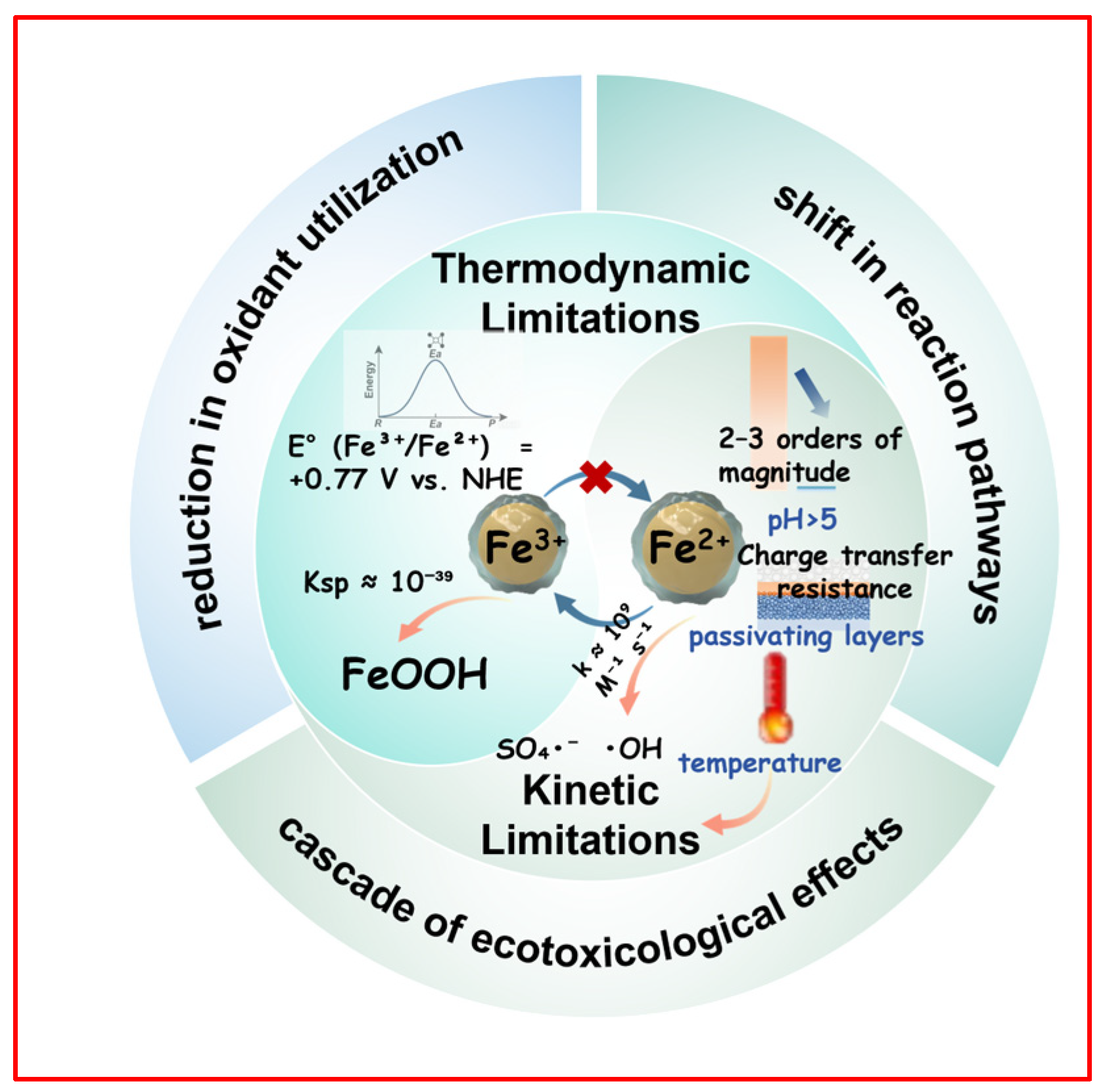
2. The Root Causes and Impacts of Low Iron Cycling Efficiency
2.1. Thermodynamic and Kinetic Limitations
2.2. Challenges of Fe3+ Precipitation
3. Iron Cycle Enhancement Strategies and Their Mechanisms
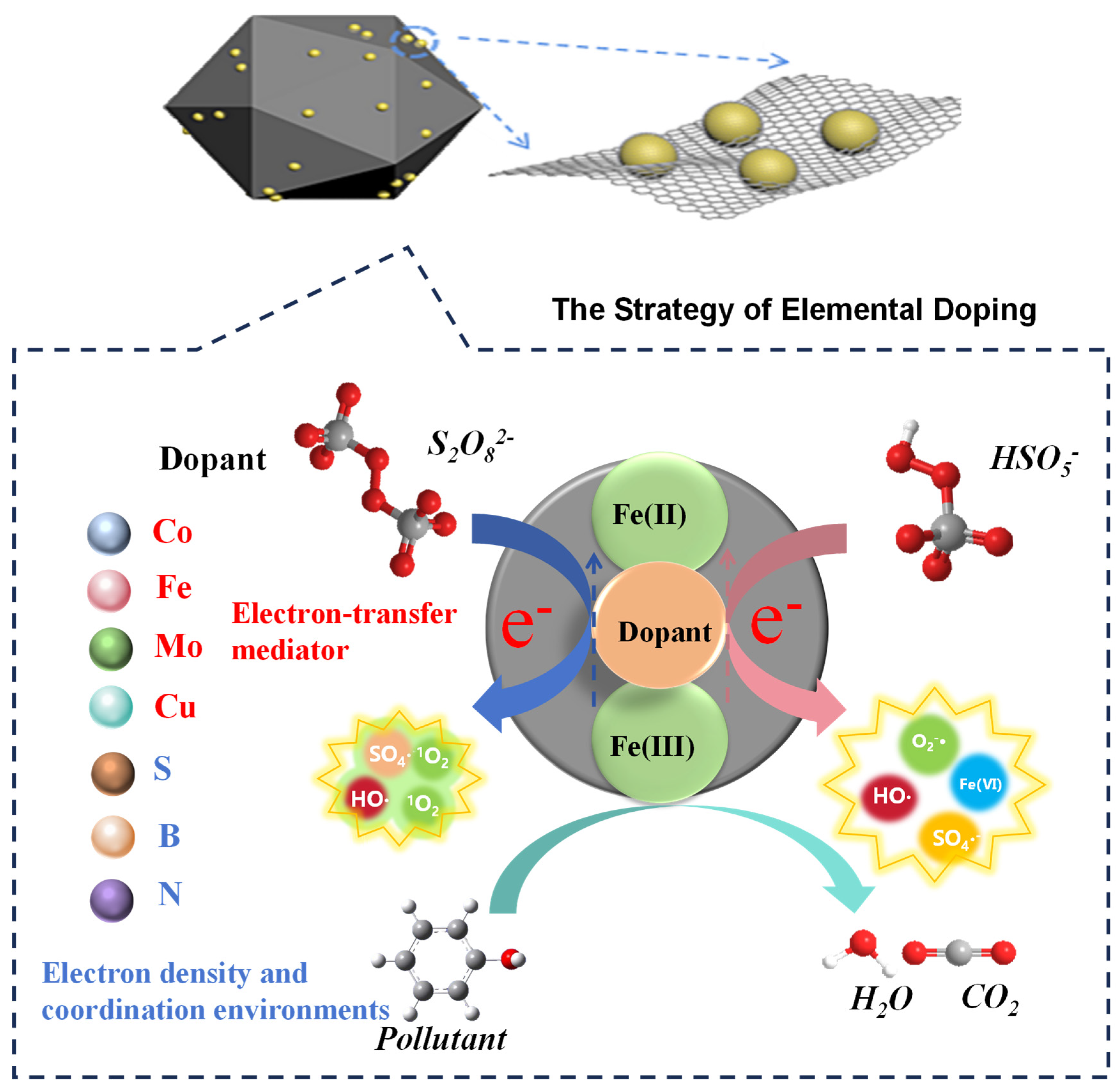
3.1. Catalyst Design and Modification Strategies for Enhanced Iron Cycling
3.1.1. Electronic Structure Modulation via Elemental Doping
3.1.2. Heterointerface Engineering for Enhanced Electron Transfer
3.1.3. Defect Engineering for Regulating Electron Transfer and Reaction Pathways
3.1.4. Morphological and Structural Engineering for Enhanced Mass and Electron Transport
| Strategy | Primary Mechanism & Electronic Modulation | Key Structural Feature | Dominant Enhancement in Iron Cycling | Typical Performance Outcome | References |
|---|---|---|---|---|---|
| Elemental Doping | Introduces foreign elements to modify the electronic structure and create electron-transfer mediators | Atomic-level dispersion of dopants; formation of active sites | Couples Fe3+/Fe2+ with a faster redox pair; lowers activation energy for Fe3+ reduction. | 26-fold increase in BPA degradation rate; >96% activity retention after 5 cycles. | [48,50] |
| Heterointerface Engineering | Constructs hybrid interfaces to provide continuous electron-transfer pathways and synergistic redox relays. | Core–shell structures; bimetallic junctions; carbon-supported composites. | Accelerates interfacial electron transfer from support or second metal to Fe3+. | 6-fold higher PDS activation; 96% degradation with minimal leaching (<10 μg/L). | [55,57] |
| Defect Engineering | Creates vacancies (Oᵥ, Sᵥ) that act as electron reservoirs and modulate surface adsorption/activation. | Oxygen/sulfur vacancies; under-coordinated metal sites; asymmetric coordination. | Serves as localized electron donor for Fe3+ reduction; stabilizes low-valence states. | 62.8% reduction in metal leaching; sustained activity over 192 h. | [22,67] |
| Morphological Engineering | Controls physical structure to shorten mass/electron transport distances and increase active site exposure. | Hollow structures; ordered pores; 2D nanosheets; hierarchical porosity. | Improves diffusion of reactants to sites and electrons to Fe centers. | 5-fold higher PMS activation rate; 99.8% degradation in 60 min. | [73,74] |
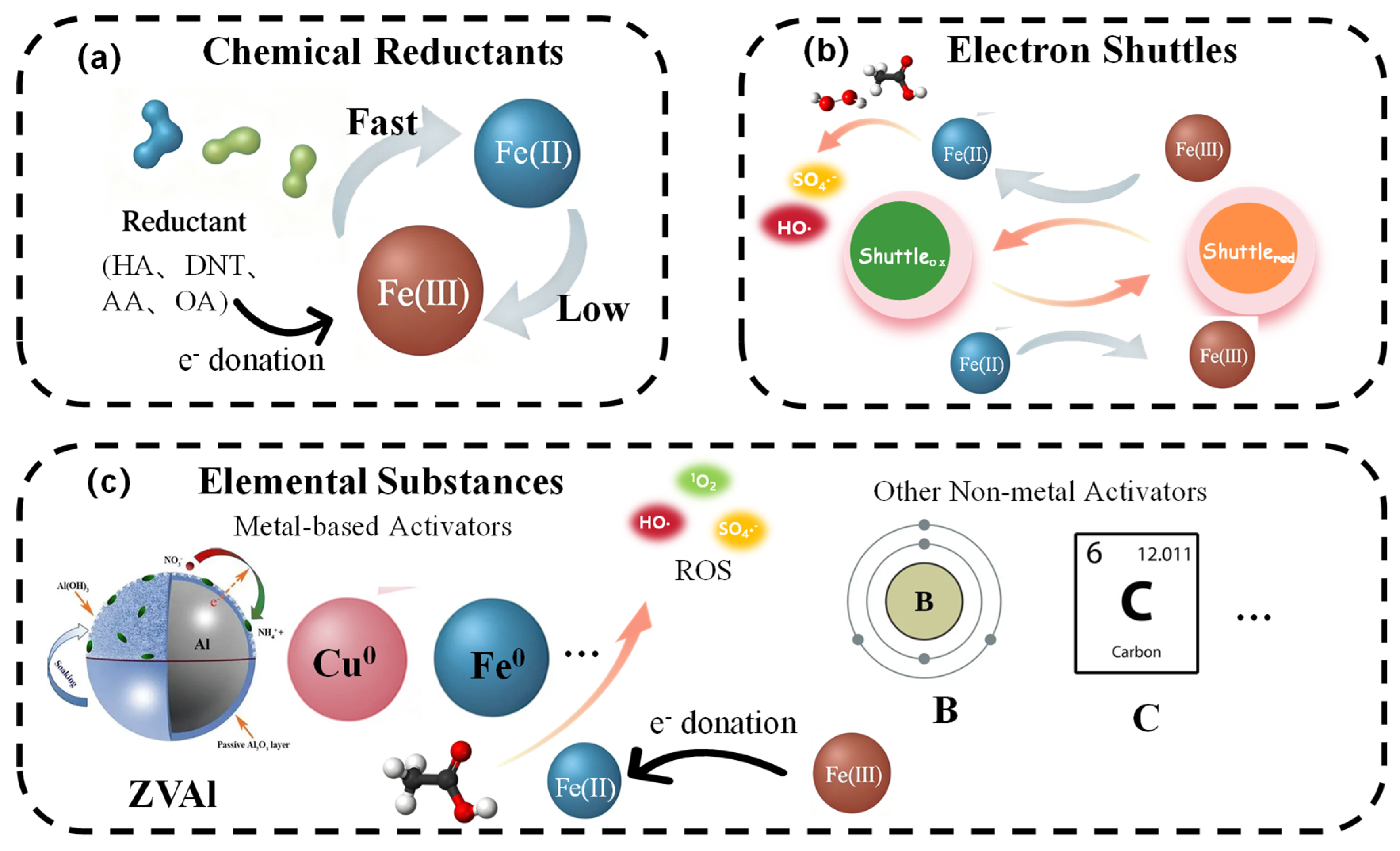
3.2. Reaction System Optimization Strategies
3.2.1. Introduction of Chemical Reductants
3.2.2. Electron Shuttles for Mediated Redox Cycling
3.2.3. Elemental Substances as Reductive Modulators
| Strategy | Core Mechanism & Action Scale | Primary Role in Iron Cycling | Key Advantage | Inherent Limitation/Risk | References |
|---|---|---|---|---|---|
| Chemical Reductants | Homogeneous molecular-scale reduction. Provides exogenous electrons via direct donation (e.g., HA, DTN) or complexation-mediated transfer (e.g., AA, Cys). | Overcomes kinetic barrier of Fe(III) reduction. Acts as a soluble electron source to rapidly regenerate Fe(II). | Immediate efficacy; Broad availability; Can shift reaction pathways (e.g., from Fe(IV) to radicals). | Consumable; Requires continuous dosing; Potential formation of harmful byproducts; Increases operational cost. | [12,78] |
| Electron Shuttles | Reversible molecular-mediated transfer. Establishes a catalytic cycle where mediators (e.g., quinones, humics) oscillate between redox states. | Decouples Fe(III) reduction from oxidant activation. Enables continuous electron transfer without mediator consumption. | Catalytic (non-consumable); High selectivity; Mitigates anion interference; Operates over a wide pH range. | Susceptible to radical attack; Performance pH-dependent for natural mediators; Cost barriers for synthetic analogs. | [81,83] |
| Elemental Substances | Heterogeneous interface & bulk electron donation. Metallic elements (e.g., ZVAl, Cu0) donate electrons; Carbon matrices act as conductive mediators. | Provides a sustained electron flux. Serves as a bulk electron reservoir or creates conductive networks for electron transfer. | Sustained release of electrons; Multifunctionality (e.g., adsorption, pH buffering); ZVAl offers a wide pH applicability. | Potential metal leaching (e.g., Al3+); Passivation of metal particles over time; Slower initial kinetics compared to molecular reductants. | [85,88] |
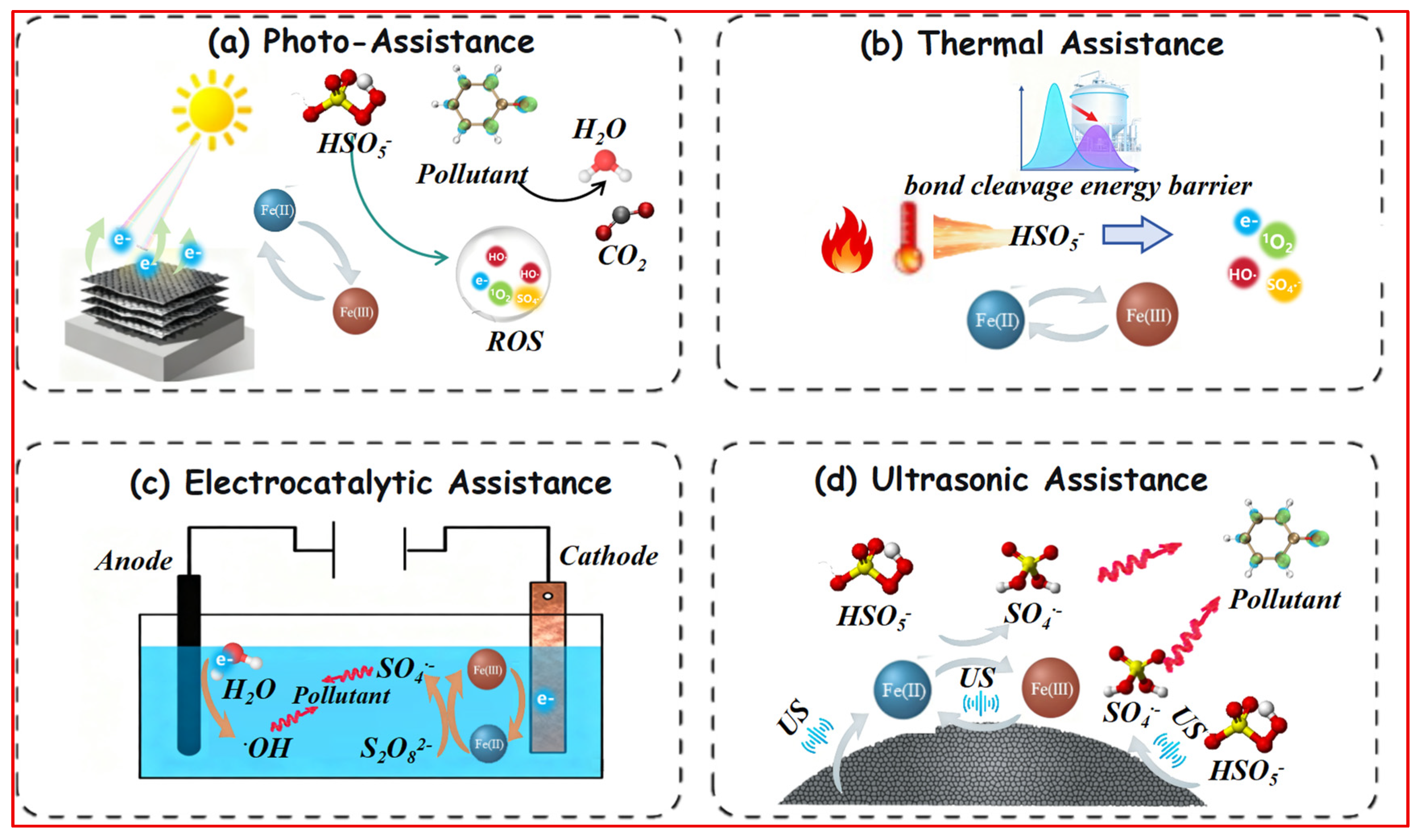
3.3. External Energy/Field Assistance Strategies
3.3.1. Photo-Assistance for Renewable Electron Injection
3.3.2. Thermal Assistance for Overcoming Activation Energy Barriers
3.3.3. Electrocatalytic Assistance for Controlled Iron Redox Cycling
3.3.4. Ultrasonic Assistance for Enhanced Interfacial Processes
| Strategy | Fundamental Principle & Action Scale | Primary Role in Iron Cycling | Unique Advantage | Practical Consideration & Limitation | References |
|---|---|---|---|---|---|
| Photo-Assistance | Photon-electron conversion. Utilizes photonic energy to generate electron-hole pairs for Fe3+ photoreduction and radical generation. | Provides renewable electrons; Expands reactive species diversity (e.g., via LMCT). | Solar energy utilization; Enables multi-pathway activation; High tunability via material design. | Dependent on light penetration; Catalyst requires photoactivity; Possible light shielding in complex matrices. | [90,95] |
| Thermal Assistance | Thermal energy input. Overcomes activation energy barriers for O–O bond cleavage and accelerates reaction kinetics. | Lowers activation energy for both persulfate decomposition and Fe3+ reduction; Enables autothermal cycles. | Universal applicability; No need for specialized catalysts; Effective for recalcitrant compounds. | High energy consumption; Limited control over reaction pathways; Potential for byproduct formation. | [100,101] |
| Electrocatalytic Assistance | Precise electron delivery. Applies potential to directionally drive electrons to Fe3+ at the cathode interface. | Sustains controlled Fe2+ regeneration via direct cathodic reduction; Decouples oxidation and reduction sites. | Precise redox control; Tunable reaction mechanisms; Minimal chemical consumption. | Electrode cost and fouling; Mass transfer limitations; System complexity and scalability. | [105,109] |
| Ultrasonic Assistance | Cavitation-induced phenomena. Uses acoustic cavitation for surface cleaning, enhanced mass transfer, and sonochemistry. | Prevents passivation via surface renewal; Enhances mass transfer of reactants to active sites. | Operates under any pH; Mitigates catalyst fouling; Effective in viscous or particulate-laden systems. | High energy intensity; Limited reactor design scalability; Potential for equipment erosion. | [114,121] |
4. Application and Performance Evaluation in Environmental Remediation
4.1. Mechanism-Tailored Strategies for Different Contaminant Classes
4.2. Adaptability to Complex Environmental Matrices
4.3. Pollutant Degradation Kinetics and Mineralization Efficiency
4.4. Catalyst Stability and Recyclability in Iron-Enhanced Persulfate Systems
4.5. Environmental Risk and Cost-Effectiveness Analysis of Iron-Cycle Enhanced Systems
4.6. Comparative Analysis with Conventional AOPs
5. Challenges and Future Perspectives
5.1. Current Research Challenges
5.2. Future Research Directions
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Devendrapandi, G.; Liu, X.; Balu, R.; Ayyamperumal, R.; Arasu, M.V.; Lavanya, M.; Reddy, V.R.M.; Kim, W.K.; Karthika, P.C. Innovative remediation strategies for persistent organic pollutants in soil and water: A comprehensive review. Environ. Res. 2024, 249, 118404. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Cao, H.; Pan, W.; Wang, C.; Liang, Y. The role of dissolved organic matter during Per- and Polyfluorinated Substance (PFAS) adsorption, degradation, and plant uptake: A review. J. Hazard. Mater. 2022, 436, 129139. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Liu, X.; Sun, K.; Lin, C.; Ma, J.; He, M.; Ouyang, W. Persulfate-based advanced oxidation processes (AOPs) for organic-contaminated soil remediation: A review. Chem. Eng. J. 2019, 372, 836–851. [Google Scholar] [CrossRef]
- Cheng, M.; Zeng, G.; Huang, D.; Lai, C.; Xu, P.; Zhang, C.; Liu, Y. Hydroxyl radicals based advanced oxidation processes (AOPs) for remediation of soils contaminated with organic compounds: A review. Chem. Eng. J. 2016, 284, 582–598. [Google Scholar] [CrossRef]
- Kanakaraju, D.; Glass, B.D.; Oelgemoeller, M. Advanced oxidation process-mediated removal of pharmaceuticals from water: A review. J. Environ. Manag. 2018, 219, 189–207. [Google Scholar] [CrossRef]
- Poza-Nogueiras, V.; Rosales, E.; Pazos, M.; Angeles Sanroman, M. Current advances and trends in electro-Fenton process using heterogeneous catalysts—A review. Chemosphere 2018, 201, 399–416. [Google Scholar] [CrossRef]
- Giannakis, S.; Lin, K.-Y.A.; Ghanbari, F. A review of the recent advances on the treatment of industrial wastewaters by Sulfate Radical-based Advanced Oxidation Processes (SR-AOPs). Chem. Eng. J. 2021, 406, 127083. [Google Scholar] [CrossRef]
- Lee, J.; von Gunten, U.; Kim, J.-H. Persulfate-Based Advanced Oxidation: Critical Assessment of Opportunities and Roadblocks. Environ. Sci. Technol. 2020, 54, 3064–3081. [Google Scholar] [CrossRef]
- Matafonova, G.; Batoev, V. Recent advances in application of UV light-emitting diodes for degrading organic pollutants in water through advanced oxidation processes: A review. Water Res. 2018, 132, 177–189. [Google Scholar] [CrossRef]
- Zhao, G.; Zou, J.; Chen, X.; Liu, L.; Wang, Y.; Zhou, S.; Long, X.; Yu, J.; Jiao, F. Iron-based catalysts for persulfate-based advanced oxidation process: Microstructure, property and tailoring. Chem. Eng. J. 2021, 421, 127845. [Google Scholar] [CrossRef]
- Dong, H.; Xu, Q.; Lian, L.; Li, Y.; Wang, S.; Li, C.; Guan, X. Degradation of Organic Contaminants in the Fe(II)/Peroxymonosulfate Process under Acidic Conditions: The Overlooked Rapid Oxidation Stage. Environ. Sci. Technol. 2021, 55, 15390–15399. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wan, Y.; Li, Y.; Yao, G.; Lai, B. Surface Fe(III)/Fe(II) cycle promoted the degradation of atrazine by peroxymonosulfate activation in the presence of hydroxylamine. Appl. Catal. B Environ. 2019, 256, 117782. [Google Scholar]
- Liu, Z.; Pan, S.; Xu, F.; Wang, Z.; Zhao, C.; Xu, X.; Gao, B.; Li, Q. Revealing the fundamental role of MoO2 in promoting efficient and stable activation of persulfate by iron carbon based catalysts: Efficient Fe2+/Fe3+cycling to generate reactive species. Water Res. 2022, 225, 119142. [Google Scholar] [CrossRef] [PubMed]
- Sheng, B.; Yang, F.; Wang, Y.; Wang, Z.; Li, Q.; Guo, Y.; Lou, X.; Liu, J. Pivotal roles of MoS2 in boosting catalytic degradation of aqueous organic pollutants by Fe(II)/PMS. Chem. Eng. J. 2019, 375, 121989. [Google Scholar] [CrossRef]
- Wang, Z.; Jiang, J.; Pang, S.; Zhou, Y.; Guan, C.; Gao, Y.; Li, J.; Yang, Y.; Qu, W.; Jiang, C. Is Sulfate Radical Really Generated from Peroxydisulfate Activated by Iron(II) for Environmental Decontamination? Environ. Sci. Technol. 2018, 52, 11276–11284. [Google Scholar] [CrossRef]
- Ahmed, H. Heterogeneous catalysts in advanced oxidation processes: A comprehensive review on antibiotic removal from wastewater. Sep. Purif. Technol. 2025, 374, 133670. [Google Scholar] [CrossRef]
- Hu, H.; Liu, J.; Zheng, X.; Zhao, K.; Lin, Y.; Xu, X.; Long, H.; Zhang, Y.; Wang, X.; Chen, D.; et al. Recent advances in iron-based catalyst-driven persulfate activation for organic pollutant degradation. J. Water Process Eng. 2025, 71, 107423. [Google Scholar] [CrossRef]
- Wang, H.; Guo, W.; Yin, R.; Du, J.; Wu, Q.; Luo, H.; Liu, B.; Sseguya, F.; Ren, N. Biochar-induced Fe(III) reduction for persulfate activation in sulfamethoxazole degradation: Insight into the electron transfer, radical oxidation and degradation pathways. Chem. Eng. J. 2019, 362, 561–569. [Google Scholar] [CrossRef]
- Xiong, Y.; Zhou, T.; Bao, J.; Du, J.; Faheem, M.; Luo, L. Degradation mechanism of Bisphenol S via hydrogen peroxide/persulfate activated by sulfidated nanoscale zero valent iron. Environ. Sci. Pollut. Res. 2023, 30, 83545–83557. [Google Scholar] [CrossRef]
- Gao, Y.; Duan, X.; Li, B.; Jia, Q.; Li, Y.; Fan, X.; Zhang, F.; Zhang, G.; Wang, S.; Peng, W. Fe containing template derived atomic Fe-N-C to boost Fenton-like reaction and charge migration analysis on highly active Fe-N4 sites. J. Mater. Chem. A 2021, 9, 14793–14805. [Google Scholar] [CrossRef]
- Xu, J.; Chen, J.; Zhong, Y.; Cao, L.; Zhang, X.; Wang, Z.; Chen, J.; Lin, S.; Xu, Q.; Chen, Y.; et al. Ultrathin MoS2 nanosheet-wrapped Fe3O4 nanocrystals synergistically activate peroxymonosulfate for enhanced removal of organic pollutants. Colloids Surf. A-Physicochem. Eng. Asp. 2023, 671, 131599. [Google Scholar] [CrossRef]
- Wang, X.; Wang, W.; Peng, J.; Wei, P.; Yan, G.; Cao, Z. Boosting effect of oxygen vacancy on peroxymonosulfate activation over CoWO4 for sulfapyridine removal. Sep. Purif. Technol. 2025, 371, 133285. [Google Scholar] [CrossRef]
- Meng, X.-R.; Chen, Q.; Wu, M.-F.; Wu, Q.; Wang, S.-Q.; Jin, L.-P.; Zhou, F.; Ma, R.-L.; Zou, J.-P. Nano-flowers FeS/MoS2 composites as a peroxymonosulfate activator for efficient p-chlorophenol degradation. Chin. J. Struct. Chem. 2025, 44, 100543. [Google Scholar] [CrossRef]
- Liang, J.; Guo, M.; Xue, Y.; Gu, J.-N.; Li, J.; Shi, F.; Guo, X.; Min, X.; Jia, J.; Li, K.; et al. Constructing magnetically separable manganese-based spinel ferrite from spent ternary lithium-ion batteries for efficient degradation of bisphenol A peroxymonosulfate activation. Chem. Eng. J. 2022, 435, 135000. [Google Scholar] [CrossRef]
- Liang, J.; Xue, Y.; Gu, J.-n.; Li, J.; Shi, F.; Guo, X.; Guo, M.; Min, X.; Li, K.; Sun, T.; et al. Sustainably recycling spent lithium-ion batteries to prepare magnetically separable cobalt ferrite for catalytic degradation of bisphenol A peroxymonosulfate activation. J. Hazard. Mater. 2022, 427, 127910. [Google Scholar] [CrossRef]
- Kang, H.; Lee, D.; Lee, K.; Kim, H.; Lee, H.; Kim, M.; Lee, C. Nonradical activation of peroxymonosulfate by hematite for oxidation of organic compounds: A novel mechanism involving high-valent iron species. Chem. Eng. J. 2021, 426, 130743. [Google Scholar] [CrossRef]
- Luo, L.; Wang, Z.; Guo, Q.; Wei, X.; Hu, J.; Luo, Y.; Jiang, J. Evidence for the involvement of Fe(IV) in water treatment by Fe(III)-activated sulfite. Environ. Chem. Lett. 2022, 20, 91–99. [Google Scholar] [CrossRef]
- Wang, Z.; Qiu, W.; Pang, S.; Zhou, Y.; Gan, Y.; Guan, C.; Jiang, J. Further understanding the involvement of Fe(IV) in peroxydisulfate and peroxymonosulfate activation by Fe(II) for oxidative water treatment. Chem. Eng. J. 2019, 371, 842–847. [Google Scholar] [CrossRef]
- Li, S.; Tang, J.; Yu, C.; Liu, Q.; Wang, L. Efficient degradation of anthracene in soil by carbon-coated nZVI activated persulfate. J. Hazard. Mater. 2022, 431, 128581. [Google Scholar] [CrossRef]
- Yu, M.; Mao, X.; He, X.; Zheng, M.; Zhang, X.; Su, J.; Xi, B. Efficient degradation of sulfamethazine in a silicified microscale zero-valent iron activated persulfate process. Appl. Catal. B Environ. Energy 2022, 312, 121418. [Google Scholar] [CrossRef]
- He, D.; Cheng, Y.; Zeng, Y.; Luo, H.; Luo, K.; Li, J.; Pan, X.; Barcelo, D.; Crittenden, J.C. Synergistic activation of peroxymonosulfate and persulfate by ferrous ion and molybdenum disulfide for pollutant degradation: Theoretical and experimental studies. Chemosphere 2020, 240, 124979. [Google Scholar] [CrossRef]
- Yu, S.; Gu, X.; Lu, S.; Xue, Y.; Zhang, X.; Xu, M.; Qiu, Z.; Sui, Q. Degradation of phenanthrene in aqueous solution by a persulfate/percarbonate system activated with CA chelated-Fe(II). Chem. Eng. J. 2018, 333, 122–131. [Google Scholar] [CrossRef]
- Chen, Y.; Vu, H.C.; Miller, C.J.; Garg, S.; Pan, D.; Waite, T.D. Comparative Experimental and Computational Studies of Hydroxyl and Sulfate Radical-Mediated Degradation of Simple and Complex Organic Substrates. Environ. Sci. Technol. 2022, 56, 8819–8832. [Google Scholar] [CrossRef] [PubMed]
- Epold, I.; Trapido, M.; Dulova, N. Degradation of levofloxacin in aqueous solutions by Fenton, ferrous ion-activated persulfate and combined Fenton/persulfate systems. Chem. Eng. J. 2015, 279, 452–462. [Google Scholar] [CrossRef]
- Brienza, M.; Ahmed, M.; Escande, A.; Plantard, G.; Scrano, L.; Chiron, S.; Bufo, S.; Goetz, V. Relevance of a photo-Fenton like technology based on peroxymonosulphate for 17β-estradiol removal from wastewater. Chem. Eng. J. 2014, 257, 191–199. [Google Scholar] [CrossRef]
- Zhang, S.; Wei, Y.; Metz, J.; He, S.; Alvarez, P.; Long, M. Persistent free radicals in biochar enhance superoxide-mediated Fe(III)/Fe (II) cycling and the efficacy of CaO2 Fenton-like treatment. J. Hazard. Mater. 2022, 421, 126805. [Google Scholar] [CrossRef]
- Huang, Y.; Yu, X.; Gan, H.; Jiang, L.; Gong, H. Degradation and chlorination mechanism of fumaric acid based on SO4•−: An experimental and theoretical study. Environ. Sci. Pollut. Res. 2021, 28, 48471–48480. [Google Scholar] [CrossRef]
- Wang, S.; Wu, J.; Lu, X.; Xu, W.; Gong, Q.; Ding, J.; Dan, B.; Xie, P. Removal of acetaminophen in the Fe2+/persulfate system: Kinetic model and degradation pathways. Chem. Eng. J. 2019, 358, 1091–1100. [Google Scholar] [CrossRef]
- Xie, Y.; Guan, D.; Deng, Y.; Sato, Y.; Luo, Y.; Chen, G. Factors hindering the degradation of pharmaceuticals from human urine in an iron-activated persulfate system. J. Environ. Sci. 2024, 135, 130–148. [Google Scholar] [CrossRef]
- Xue, J.; Chen, M.; Wang, M. Potassium Hydrogen Phthalate Abatement by Activated Persulfate in ZVI-PS Systems. Water Air Soil Pollut. 2020, 231, 235. [Google Scholar] [CrossRef]
- Xue, W.; Li, J.; Chen, X.; Liu, H.; Wen, S.; Shi, X.; Guo, J.; Gao, Y.; Xu, J.; Xu, Y. Recent advances in sulfidized nanoscale zero-valent iron materials for environmental remediation and challenges. Environ. Sci. Pollut. Res. 2023, 30, 101933–101962. [Google Scholar] [CrossRef]
- Yi, Y.; Tu, G.; Tsang, P.; Fang, Z. Insight into the influence of pyrolysis temperature on Fenton-like catalytic performance of magnetic biochar. Chem. Eng. J. 2020, 380, 122518. [Google Scholar] [CrossRef]
- Zou, X.; Zhou, T.; Mao, J.; Wu, X. Synergistic degradation of antibiotic sulfadiazine in a heterogeneous ultrasound-enhanced Fe0/persulfate Fenton-like system. Chem. Eng. J. 2014, 257, 36–44. [Google Scholar] [CrossRef]
- Zou, J.; Ma, J.; Zhang, J. Comment on Electrolytic Manipulation of Persulfate Reactivity by Iron Electrodes for TCE Degradation in Groundwater. Environ. Sci. Technol. 2014, 48, 4630–4631. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Liu, Q.; Mai, Z.; Wang, M.; Zhao, H.; Xu, K. Strategies for improving performance of iron-based catalysts in activating heterogeneous Fenton-like oxidation in pollutants degradation: From the perspective of materials structure design. Process Saf. Environ. Prot. 2024, 190, 794–820. [Google Scholar] [CrossRef]
- Wang, K.; Li, H.; Yu, Y.; Bai, Y.; Zhang, R. A mini review on construction strategies of novel metal-based catalysts with dual reaction centers (DRCs) employed in advanced oxidation processes (AOPs). J. Taiwan Inst. Chem. Eng. 2024, 164, 105678. [Google Scholar] [CrossRef]
- Wang, K.; Yang, Y.; Zhang, T.; Liang, Y.; Wang, Q. Degradation of methylene blue with magnetic Co-doped Fe3O4@FeOOH nanocomposites as heterogeneous catalysts of peroxymonosulfate. RSC Adv. 2019, 9, 17664–17673. [Google Scholar] [CrossRef]
- Li, T.; Pan, J.; Wang, X.; Fan, Z.; Shi, T.; Wang, L.; Gao, B. Insights into the fundamental role of Mo doping in facilitating the activation of peroxydisulfate by iron-based catalysts: Accelerating the generation of sulfate radicals. Chem. Eng. J. 2023, 477, 147000. [Google Scholar] [CrossRef]
- Liu, T.; Li, C.; Chen, X.; Chen, Y.; Cui, K.; Wang, D.; Wei, Q. Peroxymonosulfate Activation by Fe@N Co-Doped Biochar for the Degradation of Sulfamethoxazole: The Key Role of Pyrrolic N. Int. J. Mol. Sci. 2024, 25, 10528. [Google Scholar] [CrossRef]
- Duan, Y.; Liu, Y.; Wang, Y.; Wang, H.; Yin, W.; Xu, G. Recyclable Fe/S co-doped nanocarbon derived from metal-organic framework as a peroxymonosulfate activator for efficient removal of 2,4-dichlorophenol. Environ. Sci. Pollut. Res. 2023, 30, 6906–6918. [Google Scholar] [CrossRef]
- Jiang, L.; Liu, X.; Yang, M.; Jiang, R.; Gao, J.; Dai, Q. Catalytic degradation of bisphenol S via peroxymonosulfate activation by bimetallic Fe, Co-embedded N-enriched wheat straw biochar: Influencing factors, performance and mechanism. J. Water Process Eng. 2025, 74, 107864. [Google Scholar] [CrossRef]
- Li, X.; Wu, L.; Zhang, A.; Wu, S.; Lin, Y.; Yang, C. Cobalt doping amount determines dominant reactive species in peroxymonosulfate activation via porous carbon catalysts co-doped by cobalt and nitrogen. J. Environ. Sci. 2024, 138, 212–226. [Google Scholar] [CrossRef] [PubMed]
- Xiong, S.; Deng, Y.; Gong, D.; Tang, R.; Zheng, J.; Li, L.; Zhou, Z.; Su, L.; Liao, C.; Yang, L. Magnetically modified in-situ N-doped Enteromorpha prolifera derived biochar for peroxydisulfate activation: Electron transfer induced singlet oxygen non-radical pathway. Chemosphere 2021, 284, 131404. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Wang, Q.; Li, C.; Cui, M.; Chen, Y.; Liu, R.; Cui, K.; Wu, K.; Nie, X.; Wang, S. Synthesizing and characterizing Fe3O4 embedded in N-doped carbon nanotubes-bridged biochar as a persulfate activator for sulfamethoxazole degradation. J. Clean. Prod. 2022, 353, 131669. [Google Scholar] [CrossRef]
- Ma, H.; Zhang, X.; Feng, G.; Ren, B.; Pan, Z.; Shi, Y.; Xu, R.; Wang, P.; Liu, Y.; Wang, G.; et al. Carbon nanotube membrane armed with confined iron for peroxymonosulfate activation towards efficient tetracycline removal. Sep. Purif. Technol. 2023, 312, 123319. [Google Scholar] [CrossRef]
- He, D.; Chen, P.; Zheng, P.; Yang, M.; Liu, L.; Yuan, S. Iron-nitrogen co-doped biochar (FeN@BC) as particle electrode for three-dimensional (3D) electro-peroxydisulfate process for tetracycline degradation. AIP Adv. 2024, 14, 035245. [Google Scholar] [CrossRef]
- Yang, B.; Tong, J.; Tong, J.; Peng, H.; Xiang, Y.; Ruan, M.; Chen, Z.; Yang, Z.; Xiong, W. Microwave synthesis of Fe-Cu diatomic active center MOF: Synergistic cyclic catalysis of persulfate for degrading norfloxacin. Environ. Sci.-Nano 2023, 10, 2778–2789. [Google Scholar] [CrossRef]
- Xiong, W.; Hu, F.; Liu, Y.; Nie, G.; Xiao, L. Core-shell FeMn@NG derived from cellulose supported Prussian blue analogs for peroxymonosulfate activation: Non-radical mechanism and ultra-low metal leaching. J. Environ. Chem. Eng. 2022, 10, 108523. [Google Scholar] [CrossRef]
- Lan, H.; Zhou, J.; Hou, Z.; An, X.; Liu, H.; Qu, J. Defect modulation of MOF-derived ZnFe2O4/CNTs microcages for persulfate activation: Enhanced nonradical catalytic oxidation. Chem. Eng. J. 2022, 431, 133369. [Google Scholar] [CrossRef]
- Shang, Y.; Chen, C.; Zhang, P.; Yue, Q.; Li, Y.; Gao, B.; Xu, X. Removal of sulfamethoxazole from water via activation of persulfate by Fe<sub>3</sub>C@NCNTs including mechanism of radical and nonradical process. Chem. Eng. J. 2019, 375, 122004. [Google Scholar]
- Li, Q.; Wang, L.; Zhang, L.; Xie, H. Rapid degradation of tetrabromobisphenol A under the UV/TiO2/KPS systems in alkaline aqueous solutions. Res. Chem. Intermed. 2019, 45, 757–768. [Google Scholar] [CrossRef]
- Zhong, C.; Wang, J.; Liu, Y.; Zhang, C.; Li, Z.; Liu, Z.; Zhao, C.; Yang, L.; Xu, X. Modulation of sulfur vacancies in MoS2/Fe3P enhancing non-radical pathway of the Fenton-like reaction for complex real water. Appl. Catal. B Environ. Energy 2025, 370, 125193. [Google Scholar] [CrossRef]
- He, J.; Yang, Y.; Hong, P.; Li, Y.; Xie, C.; Wu, Z.; Li, M.; Kong, L. Oxygen Vacancies of Mn/CeOx-H induced non-radical activation of peroxymonosulfate through electron mediation for bisphenol A degradation. J. Environ. Chem. Eng. 2023, 11, 111078. [Google Scholar] [CrossRef]
- Zhou, Z.; Tan, Y.; Yan, T.; Wu, X.; He, G.; Li, H.; Zhou, J.; Tong, H.; Yu, L.; Zeng, J. CoFe2O4-x/expanded graphite composite with abundant oxygen vacancies as a high-performance peroxymonosulfate activator for antibiotic degradation. Colloids Surf. A-Physicochem. Eng. Asp. 2025, 718, 136808. [Google Scholar] [CrossRef]
- Meng, B.; Fu, H.; Liu, S.-S.; Zhao, C.; Wang, F.; Li, S.; Guo, Y.; Meng, B.; Wang, J.-F.; Wang, C.-C. Polymerization deposition route governed by the coordination environment of interface asymmetric oxygen vacancy for efficient BPA removal via a Fenton-like reaction. Sep. Purif. Technol. 2025, 366, 132824. [Google Scholar] [CrossRef]
- Liang, X.; Wang, D.; Zhao, Z.; Li, T.; Gao, Y.; Hu, C. Coordination Number Dependent Catalytic Activity of Single-Atom Cobalt Catalysts for Fenton-Like Reaction. Adv. Funct. Mater. 2022, 32, 2203001. [Google Scholar] [CrossRef]
- Zhao, X.; Tong, J.; Bai, S.; Qian, J. Dual-metal-catalyzed Fenton-like reaction on CdxZn1-xS @biochar: Mechanistic insights into sulfide-metal interactions for water purification. Appl. Catal. B Environ. Energy 2025, 379, 125654. [Google Scholar] [CrossRef]
- Wang, Y.; Li, H.; Zong, Y.; Zhou, Z.; Ye, G.; Lei, Z.; Wu, D. Defect-Engineered Inert Interfaces on Iron-Rich Clay Minerals Boost Exclusive Electron Transfer Pathway in Fenton-Like Reactions. Adv. Funct. Mater. 2025, e12238. [Google Scholar] [CrossRef]
- Lai, L.; Zhou, H.; Hong, Y.; Luo, M.; Shi, Y.; Zhang, H.; Xiong, Z.; Yao, G.; Lai, B. Activation of peroxymonosulfate by FeVO3-x for the degradation of carbamazepine: Vanadium mediated electron shuttle and oxygen vacancy modulated interface chemistry. Chin. Chem. Lett. 2024, 35, 108580. [Google Scholar] [CrossRef]
- Fang, H.; Zhu, G.; Hu, Y.; Zhou, C.; Xu, M.; You, M.; Zhao, J.; Xue, S.; Zhang, J.; Liu, G. Flower-like C/CoO/Co3O4 heterostructures with abundant oxygen vacancies for effective peroxymonosulfate activation and tetracycline degradation. J. Solid State Chem. 2025, 350, 125469. [Google Scholar] [CrossRef]
- Ma, C.; Wang, J.; Wang, F.; Zhu, Y.; Li, Y.; Fan, X.; Zhang, F.; Zhang, G.; Peng, W. Facile synthesis of iron oxide supported on porous nitrogen doped carbon for catalytic oxidation. Sci. Total Environ. 2021, 785, 147296. [Google Scholar] [CrossRef]
- Sun, Y.; Ma, C.; Wu, D.; Liu, X.; Li, N.; Fan, X.; Li, Y.; Zhang, G.; Zhang, F.; Peng, W. Coating CoFe2O4 shell on Fe particles to increase the utilization efficiencies of Fe and peroxymonosulfate for low-cost Fenton-like reactions. Water Res. 2023, 244, 120542. [Google Scholar] [CrossRef]
- Wang, W.; Liu, Y.; Yue, Y.; Wang, H.; Cheng, G.; Gao, C.; Chen, C.; Ai, Y.; Chen, Z.; Wang, X. The Confined Interlayer Growth of Ultrathin Two-Dimensional Fe3O4 Nanosheets with Enriched Oxygen Vacancies for Peroxymonosulfate Activation. ACS Catal. 2021, 11, 11256–11265. [Google Scholar] [CrossRef]
- Song, Y.; Wang, H.; Shi, Z.; Cui, J.; Wu, D. Selective etching induced Fe3O4 cubes with orderly aligned porous structure for the effective lithium-ion storage and bisphenol A degradation. Sep. Purif. Technol. 2024, 334, 126054. [Google Scholar] [CrossRef]
- Ge, X.; Liu, J.; Song, X.; Wang, G.; Zhang, H.; Zhang, Y.; Zhao, H. Hierarchical iron containing γ-MnO2 hollow microspheres: A facile one-step synthesis and effective removal of As(III) via oxidation and adsorption. Chem. Eng. J. 2016, 301, 139–148. [Google Scholar] [CrossRef]
- Peng, T.; Zhang, H.; Xia, S.; Zhou, S.; Shi, Z.; Li, G.; Deng, L. MoS2 Nanosheets Anchored onto MIL-100(Fe)-Derived FeS2 as a Peroxymonosulfate Activator for Efficient Sulfamethoxazole Degradation: Insights into the Mechanism. ACS ES&T Water 2023, 3, 213–226. [Google Scholar]
- Wen, L.; Li, X.; Na, Y.; Chen, H.; Liu, M.; Yang, S.; Ding, D.; Wang, G.; Liu, Y.; Chen, Y.; et al. Surface reconstructed Fe@C1000 for enhanced Fenton-like catalysis: Sustainable ciprofloxacin degradation and toxicity reduction. Environ. Pollut. 2024, 345, 123534. [Google Scholar] [CrossRef]
- Lai, L.; Zhou, P.; Zhou, H.; Sun, M.; Yuan, Y.; Liu, Y.; Yao, G.; Lai, B. Heterogeneous Fe(III)/Fe(II) circulation in FeVO4 by coupling with dithionite towards long-lasting peroxymonosulfate activation: Pivotal role of vanadium as electron shuttles. Appl. Catal. B Environ. 2021, 297, 120470. [Google Scholar] [CrossRef]
- Li, Y.; Dong, H.; Xiao, J.; Li, L.; Hou, Y.; Chu, D.; Hou, X.; Xiang, S.; Dong, Q. Ascorbic acid-assisted iron silicate composite activated peroxydisulfate for enhanced degradation of aqueous contaminants: Accelerated Fe(III)/Fe(II) cycle and the interaction between iron and silicate. Chem. Eng. J. 2023, 455, 140733. [Google Scholar] [CrossRef]
- Yuan, Y.; Chen, S.; Yao, B.; Chen, A.; Pei, L.; Luo, S.; Zhou, Y. Fe3+-cysteine enhanced persulfate fenton-like process for quinclorac degradation: A wide pH tolerance and reaction mechanism. Environ. Res. 2023, 224, 115447. [Google Scholar] [CrossRef]
- Huang, M.; Zhu, C.; Zhu, F.; Fang, G.; Zhou, D. Mechanism of significant enhancement of VO2-Fenton-like reactions by oxalic acid for diethyl phthalate degradation. Sep. Purif. Technol. 2021, 279, 119671. [Google Scholar] [CrossRef]
- Li, Z.-Y.; Liu, Y.-L.; He, P.-N.; Zhang, X.; Wang, L.; Gu, H.-T.; Zhang, H.-C.; Ma, J. Further understanding the role of hydroxylamine in transformation of reactive species in Fe(II)/peroxydisulfate system. Chem. Eng. J. 2021, 418, 129464. [Google Scholar] [CrossRef]
- Li, X.; Ma, J.; Gao, Y.; Liu, X.; Wei, Y.; Liang, Z. Enhanced atrazine degradation in the Fe(III)/peroxymonosulfate system via accelerating Fe(II) regeneration by benzoquinone. Chem. Eng. J. 2022, 427, 131995. [Google Scholar] [CrossRef]
- Wu, J.; Cagnetta, G.; Wang, B.; Cui, Y.; Deng, S.; Wang, Y.; Huang, J.; Yu, G. Efficient degradation of carbamazepine by organo-montmorillonite supported nCoFe2O4-activated peroxymonosulfate process. Chem. Eng. J. 2019, 368, 824–836. [Google Scholar] [CrossRef]
- Ren, T.; Yang, S.; Jiang, Y.; Sun, X.; Zhang, Y. Enhancing surface corrosion of zero-valent aluminum (ZVAl) and electron transfer process for the degradation of trichloroethylene with the presence of persulfate. Chem. Eng. J. 2018, 348, 350–360. [Google Scholar] [CrossRef]
- Wan, Z.; Hu, X.; Li, C.; Zhang, J.; Wang, Q.; Fang, L.; Zhang, L.; Guo, Q.; Sun, D. Simultaneous oxidation and absorption of nitric oxide and sulfur dioxide by peroxymonosulfate activated by bimetallic metal-organic frameworks. J. Environ. Chem. Eng. 2023, 11, 109417. [Google Scholar] [CrossRef]
- Qiu, X.; Zhao, Y.; Jia, Z.; Li, C.; Jin, R.; Mutabazi, E. Fe and Zn co-doped carbon nanoparticles as peroxymonosulfate activator for efficient 2,4-dichorophenol degradation. Environ. Res. 2024, 240, 117313. [Google Scholar] [CrossRef]
- Wang, J.; Xie, T.; Liu, X.; Wu, D.; Li, Y.; Wang, Z.; Fan, X.; Zhang, F.; Peng, W. Enhanced redox cycle of Fe3+/Fe2+ on Fe@NC by boron: Fast electron transfer and long-term stability for Fenton-like reaction. J. Hazard. Mater. 2023, 445, 130605. [Google Scholar] [CrossRef]
- Xu, K.; Zhu, Z.; Hu, C.; Zheng, J.; Peng, H.; Liu, B. Superior degradation of organic contaminants by the UV/CoFe2O4/PI system: Kinetics, pathways, mechanisms and DFT calculation. Sep. Purif. Technol. 2024, 336, 126197. [Google Scholar] [CrossRef]
- Wang, X.; Li, J.; Chen, K.; Li, J.; Jia, Y.; Mei, Q.; Wang, Q. Facile synthesis of oxygen vacancies enriched ZnFe2O4 for effective photocatalytic peroxodisulfate activation. Sep. Purif. Technol. 2022, 303, 122205. [Google Scholar] [CrossRef]
- Chen, Y.; Zhao, D.; Sun, T.; Cai, C.; Dong, Y. The preparation of MoS2/8-FeOOH and degradation of RhB under visible light. J. Environ. Chem. Eng. 2023, 11, 110353. [Google Scholar] [CrossRef]
- He, Y.; Qian, J.; Wang, P.; Xie, T.; Dionysiou, D.D.; Lu, B.; Tang, S. Synergized selenium-vacancy heterogeneous interface and carbon nanotubes for insight into efficient oxidation of pollutants via photocatalytic peroxymonosulfate activation. Appl. Catal. B Environ. Energy 2023, 330, 122620. [Google Scholar] [CrossRef]
- Yuan, Y.; Wang, W.; Nie, M.; Yan, C.; Wang, P.; Ding, M. Visible light-mediated activation of periodate for bisphenol A degradation in the presence of Fe3+and gallic acid at neutral pH. Chem. Eng. J. 2024, 479, 147541. [Google Scholar] [CrossRef]
- An, L.; Kong, X.; Jiang, M.; Li, W.; Lv, Q.; Hou, X.; Liu, C.; Su, P.; Ma, J.; Yang, T. Photo-assisted natural chalcopyrite activated peracetic acid for efficient micropollutant degradation. Water Res. 2024, 257, 121699. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Lin, H.; Chen, X.; Pan, Y.; Li, X.; Zhuang, Z.; Chen, H.; Wang, X.; Luo, M.; Zheng, K.; et al. Fe, Mn-Prussian blue analogue@MXene composites for efficient photocatalytic peroxydisulfate-activated degradation of tetracycline hydrochloride and photoelectrochemical desalination. Chem. Eng. J. 2023, 476, 146682. [Google Scholar] [CrossRef]
- Liu, L.; Zhan, R.; Zhang, M.; Li, J.; Wang, Z.; Mi, H.; Zhang, Y. Insights into the performance, mechanism, and ecotoxicity of levofloxacin degradation in CoFe2O4 catalytic peroxymonosulfate process. J. Environ. Chem. Eng. 2022, 10, 107435. [Google Scholar] [CrossRef]
- Wang, Y.; Ding, L.; Liu, C.; Lu, Y.; Wu, Q.; Wang, C.; Hu, Q. 0D/2D/2D ZnFe2O4/Bi2O2CO3/BiOBr double Z-scheme heterojunctions for the removal of tetracycline antibiotics by permonosulfate activation: Photocatalytic and non-photocatalytic mechanisms, radical and non-radical pathways. Sep. Purif. Technol. 2022, 283, 120164. [Google Scholar] [CrossRef]
- Li, X.; Zhu, W.; Sun, S.-P. Peracetic acid-based UVA photo-Fenton reaction: Dominant role of high-valent iron species toward efficient selective degradation of emerging micropollutants. J. Hazard. Mater. 2023, 454, 131448. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, F.; Zhou, B.; Chen, H.; Yuan, R.; Zhang, Y.; Geng, H.; Liu, Y.; Wang, H. Photo-assisted Fe2+ modified molybdenum disulfide activated potassium persulfate to degrade sulfadiazine: Insights into the degradation pathway and mechanism from density functional theory. Chem. Eng. J. 2022, 435, 134904. [Google Scholar] [CrossRef]
- Shen, L.; Xu, Z.; Xiao, Z.; Zeng, Y.; Chen, K.; Lin, C.; Chen, W.; Chen, P.; Lv, W.; Liu, G. Insight into the degradation of difloxacin by heat/persulfate system: Mechanism, pathway and application. Sep. Purif. Technol. 2025, 362, 131661. [Google Scholar] [CrossRef]
- Yang, J.; Zeng, Z.; Huang, Z.; Cui, Y. Acceleration of Persulfate Activation by MIL-101(Fe) with Vacuum Thermal Activation: Effect of FeII/FeIII Mixed-Valence Center. Catalysts 2019, 9, 906. [Google Scholar] [CrossRef]
- Wang, B.W.; Gao, C.C.; Wang, W.S.; Zhao, H.B.; Zheng, Y.; Zheng, C.G. Effect of heating rate on chemical looping combustion of coal with Fe2O3 oxygen carrier. In Proceedings of the Asia-Pacific Power and Energy Engineering Conference (APPEEC), Wuhan, China, 25–28 March 2011. [Google Scholar]
- Chen, X.; Hu, X.; Gao, Y. Removal of NO in simulated flue with aqueous solution of peroxymonosulfate activated by high temperature and Fe(II). Chem. Eng. J. 2019, 359, 419–427. [Google Scholar] [CrossRef]
- Rodriguez-Chueca, J.; Giannakis, S.; Marjanovic, M.; Kohantorabi, M.; Gholami, M.R.; Grandjean, D.; de Alencastro, L.F.; Pulgarin, C. Solar-assisted bacterial disinfection and removal of contaminants of emerging concern by Fe2+-activated HSO5- vs. S2O82- in drinking water. Appl. Catal. B Environ. 2019, 248, 62–72. [Google Scholar] [CrossRef]
- Lin, H.; Zhong, X.; Ciotonea, C.; Fan, X.; Mao, X.; Li, Y.; Deng, B.; Zhang, H.; Royer, S. Efficient degradation of clofibric acid by electro-enhanced peroxydisulfate activation with Fe-Cu/SBA-15 catalyst. Appl. Catal. B Environ. 2018, 230, 1–10. [Google Scholar] [CrossRef]
- Lin, H.; Li, Y.; Mao, X.; Zhang, H. Electro-enhanced goethite activation of peroxydisulfate for the decolorization of Orange II at neutral pH: Efficiency, stability and mechanism. J. Taiwan Inst. Chem. Eng. 2016, 65, 390–398. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, Y. Zero valent iron-electro-Fenton-peroxymonosulfate (ZVI-E-Fenton-PMS) process for industrial wastewater treatment. RSC Adv. 2023, 13, 15063–15076. [Google Scholar] [CrossRef]
- Chen, X.; Han, Y.; Gao, P.; Li, H. New insight into the mechanism of electro-assisted pyrite minerals activation of peroxymonosulfate: Synergistic effects, activation sites and electron transfer. Sep. Purif. Technol. 2021, 274, 118817. [Google Scholar] [CrossRef]
- Bai, Y.; Sun, X.; Dang, Y.; Yu, S.; Zhu, J.-J.; Zhou, Y. A self-circulating electro-fenton-like process over Fe3O4-CaO2 cathode for highly efficient degradation of levofloxacin. Chemosphere 2023, 313, 137520. [Google Scholar] [CrossRef]
- Zeng, H.; Zhao, X.; Zhao, F.; Park, Y.; Sillanpaa, M. Accelerated Fe3+/Fe2+ Cycle using Atomic H* on Pd/Al2O3: A Novel Mechanism for an Electrochemical System with Particle Electrode for Iron Sludge Reduction in the Fe2+/Peroxydisulfate Oxidation Process. Chem. Eng. J. 2020, 382, 122972. [Google Scholar] [CrossRef]
- Yan, S.; Zhang, X.; Zhang, H. Persulfate activation by Fe(III) with bioelectricity at acidic and near-neutral pH regimes: Homogeneous versus heterogeneous mechanism. J. Hazard. Mater. 2019, 374, 92–100. [Google Scholar] [CrossRef]
- Jiang, X.; Huang, Y.; Xu, W.; Liu, Z.; Song, L.; Ruan, B.; Lin, Z.; Zhang, H.; Wang, H.; Yeung, K.L. Synergistic enhancement of sulfamethoxazole degradation via nitrogen-sulfur co-doped iron-based biochar in a 3D electro-Fenton system. Sep. Purif. Technol. 2025, 375, 133881. [Google Scholar] [CrossRef]
- Long, X.; Huang, R.; Li, Y.; Wang, J.; Zhang, M.; Zhang, I.Y. Understanding the electro-cocatalytic peroxymonosulfate-based systems with BDD versus DSA anodes: Radical versus nonradical dominated degradation mechanisms. Sep. Purif. Technol. 2023, 309, 123120. [Google Scholar] [CrossRef]
- Pang, Y.; Ruan, Y.; Feng, Y.; Diao, Z.; Shih, K.; Hou, L.A.; Chen, D.; Kong, L. Ultrasound assisted zero valent iron corrosion for peroxymonosulfate activation for Rhodamine-B degradation. Chemosphere 2019, 228, 412–417. [Google Scholar] [CrossRef]
- Bao, X.; Zhang, Y.; Zhang, M.; Luo, G.; Deng, Y.; He, Y.; Zhang, Y. Ultrasound irritation enhanced activation of peroxymonosulfate with Fe0 for humic acid removal: Less formation of Fe-HA complex. Desalin. Water Treat. 2021, 237, 108–120. [Google Scholar] [CrossRef]
- Chi, N.; Liu, J.; Feng, L.; Guo, Z.; Chen, Y.; Pan, T.; Zheng, H. FeS redox power motor for PDS continuous generation of active radicals on efficient degradation and removal of diclofenac: Role of ultrasonic. Chemosphere 2022, 300, 134574. [Google Scholar] [CrossRef] [PubMed]
- Xiang, W.; Chen, H.; Zhong, Z.; Zhang, C.; Lu, X.; Huang, M.; Zhou, T.; Yu, P.; Zhang, B. Efficient degradation of carbamazepine in a neutral sonochemical FeS/persulfate system based on the enhanced heterogeneous-homogeneous sulfur-iron cycle. Sep. Purif. Technol. 2022, 282, 120041. [Google Scholar] [CrossRef]
- Lv, Z.; You, H.; Leng, H.; Li, W.; Yang, S.; Li, Z.; Zhu, J.; Zhang, G. Revealing the catalytic behavior of different types ZVI (powder Fe0, sponge Fe1, foam Fe2) in US/Fex/PDS and its effect on the dewaterability of waste activated sludge. Chem. Eng. J. 2024, 500, 157010. [Google Scholar] [CrossRef]
- Liu, J.; Zhou, J.; Ding, Z.; Zhao, Z.; Xu, X.; Fang, Z. Ultrasound irritation enhanced heterogeneous activation of peroxymonosulfate with Fe3O4 for degradation of azo dye. Ultrason. Sonochem. 2017, 34, 953–959. [Google Scholar] [CrossRef]
- Wang, J.; Liang, M. Fe3O4@BPC nanocatalyst: Enhanced activation of peroxymonosulfate for effective degradation of norfloxacin. Mater. Lett. 2023, 352, 135223. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, J.; Wang, Q.; Zhang, G. Positive Effects of Ultrasound-Assisted Catalytic Activation of Peroxymonosulfate Combined with Fe-Niox Catalyst for the Orangeii Degradation In Acidic Solution. Fresenius Environ. Bull. 2020, 29, 7002–7013. [Google Scholar]
- Bazipour, F.; Jorfi, S.; Maleki, H.; Babaei, A. Catalytic Degradation of Acid Orange 7 Using CoFe2O4@Biochar Heterogeneous Catalytic Ozonation Process in Aqueous Solutions. Water Air Soil Pollut. 2024, 235, 811. [Google Scholar] [CrossRef]
- Hong, Q.; Liu, C.; Wang, Z.; Li, R.; Liang, X.; Wang, Y.; Zhang, Y.; Song, Z.; Xiao, Z.; Cui, T.; et al. Electron transfer enhancing Fe(II)/Fe(III) cycle by sulfur and biochar in magnetic FeS@biochar to active peroxymonosulfate for 2,4-dichlorophenoxyacetic acid degradation. Chem. Eng. J. 2021, 417, 129238. [Google Scholar] [CrossRef]
- Lee, Y.; Lo, S.; Chiueh, P.; Liou, Y.; Chen, M. Microwave-hydrothermal decomposition of perfluorooctanoic acid in water by iron-activated persulfate oxidation. Water Res. 2010, 44, 886–892. [Google Scholar] [CrossRef] [PubMed]
- Sühnholz, S.; Gawel, A.; Kopinke, F.; Mackenzie, K. Evidence of heterogeneous degradation of PFOA by activated persulfate-FeS as adsorber and activator. Chem. Eng. J. 2021, 423, 130102. [Google Scholar] [CrossRef]
- Tran, T.; Abrell, L.; Brusseau, M.; Chorover, J. Iron-activated persulfate oxidation degrades aqueous Perfluorooctanoic acid (PFOA) at ambient temperature. Chemosphere 2021, 281, 130824. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, J.; Guo, Z.; Zheng, X.; Guo, P.; Xu, J.; Lei, Y. The decomplexation of Cu-EDTA by electro-assisted heterogeneous activation of persulfate via acceleration of Fe(II)/Fe(III) redox cycle on Fe-MOF catalyst. Chem. Eng. J. 2022, 430, 133025. [Google Scholar] [CrossRef]
- Zhao, J.; Zhong, P.; Luo, W.; Zhang, S.; Xu, S.; Yu, Q.; Qiu, X. Insight into in-situ chemical oxidation by Fe(II)-containing minerals: The role of inherent Fe(II)-OH in Fe(II)-Al LDHs. Chem. Eng. J. 2022, 433, 133835. [Google Scholar] [CrossRef]
- Zhao, Y.; Huang, B.; An, H.; Dong, G.; Feng, J.; Wei, T.; Ren, Y.; Ma, J. Enhanced activation of peroxymonosulfate by Sr-doped LaFeO3 perovskite for Orange I degradation in the water. Sep. Purif. Technol. 2021, 256, 117838. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, S.-Y.; Yang, X.; Huang, X.-F.; Yang, Y.-H.; He, E.-K.; Wang, S.; Qiu, R.-L. Degradation of 2,2′,4,4′-tetrabromodiphenyl ether (BDE-47) by a nano zerovalent iron-activated persulfate process: The effect of metal ions. Chem. Eng. J. 2017, 317, 613–622. [Google Scholar] [CrossRef]
- Liu, B.; Song, W.; Wu, H.; Xu, Y.; Sun, Y.; Yu, Y.; Zheng, H.; Wan, S. Enhanced oxidative degradation of norfloxacin using peroxymonosulfate activated by oily sludge carbon-based nanoparticles CoFe2O4/OSC. Chem. Eng. J. 2020, 400, 125947. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, X.; Yan, Y.; Yan, J.; Pan, Y.; Zhang, Y.; Lai, B. Enhanced sulfamethoxazole degradation by peroxymonosulfate activation with sulfide-modified microscale zero-valent iron (S-mFe0): Performance, mechanisms, and the role of sulfur species. Chem. Eng. J. 2019, 376, 121302. [Google Scholar] [CrossRef]
- Pan, T.; Wang, Y.; Yang, X.; Huang, X.-F.; Qiu, R.-L. Gallic acid accelerated BDE47 degradation in PMS/Fe(III) system: Oxidation intermediates autocatalyzed redox cycling of iron. Chem. Eng. J. 2020, 384, 123248. [Google Scholar] [CrossRef]
- Nie, G.; Huang, J.; Hu, Y.; Ding, Y.; Han, X.; Tang, H. Heterogeneous catalytic activation of peroxymonosulfate for efficient degradation of organic pollutants by magnetic Cu/Fe3O4 submicron composites. Chin. J. Catal. 2017, 38, 227–239. [Google Scholar] [CrossRef]
- Feng, Y.; Lee, P.-H.; Wu, D.; Shih, K. Surface-bound sulfate radical-dominated degradation of 1,4-dioxane by alumina-supported palladium (Pd/Al2O3) catalyzed peroxymonosulfate. Water Res. 2017, 120, 12–21. [Google Scholar] [CrossRef]
- Lee, H.; Kim, H.-I.; Weon, S.; Choi, W.; Hwang, Y.S.; Seo, J.; Lee, C.; Kim, J.-H. Activation of Persulfates by Graphitized Nanodiamonds for Removal of Organic Compounds. Environ. Sci. Technol. 2016, 50, 10134–10142. [Google Scholar] [CrossRef]
- Zhuo, S.-N.; Ren, H.-Y.; Cao, G.-L.; Xie, G.-J.; Xing, D.-F.; Ren, N.-Q.; Liu, B.-F. Highly efficient activation of persulfate by encapsulated nano-Fe0 biochar for acetaminophen degradation: Rich electron environment and dominant effect of superoxide radical. Chem. Eng. J. 2022, 440, 135947. [Google Scholar] [CrossRef]
- Du, J.; Guo, W.; Che, D.; Ren, N. Weak magnetic field for enhanced oxidation of sulfamethoxazole by Fe0/H2O2 and Fe0/persulfate: Performance, mechanisms, and degradation pathways. Chem. Eng. J. 2018, 351, 532–539. [Google Scholar] [CrossRef]
- Li, Z.; Wang, F.; Zhang, Y.; Lai, Y.; Fang, Q.; Duan, Y. Activation of peroxymonosulfate by CuFe2O4-CoFe2O4 composite catalyst for efficient bisphenol a degradation: Synthesis, catalytic mechanism and products toxicity assessment. Chem. Eng. J. 2021, 423, 130093. [Google Scholar] [CrossRef]
- Pu, M.; Ma, Y.; Wan, J.; Wang, Y.; Wang, J.; Brusseau, M.L. Activation performance and mechanism of a novel heterogeneous persulfate catalyst: Metal-organic frameworkMIL-53(Fe) with FeII/FeIII mixed-valence coordinatively unsaturated iron center. Catal. Sci. Technol. 2017, 7, 1129–1140. [Google Scholar] [CrossRef]
- Lu, J.; Zhou, Y.; Lei, J.; Ao, Z.; Zhou, Y. Fe3O4/graphene aerogels: A stable and efficient persulfate activator for the rapid degradation of malachite green. Chemosphere 2020, 251, 126402. [Google Scholar] [CrossRef]
- Zhao, Y.; Song, M.; Cao, Q.; Sun, P.; Chen, Y.; Meng, F. The superoxide radicals’ production persulfate activated with CuFe2O4@ Biochar composites to promote the redox pairs cycling for efficient degradation of o-nitrochlorobenzene in soil. J. Hazard. Mater. 2020, 400, 122887. [Google Scholar] [CrossRef]
- Qu, S.; Li, C.; Sun, X.; Wang, J.; Luo, H.; Wang, S.; Ta, J.; Li, D. Enhancement of peroxymonosulfate activation and utilization efficiency via iron oxychloride nanosheets in visible light. Sep. Purif. Technol. 2019, 224, 132–141. [Google Scholar] [CrossRef]
- Wang, S.; Xu, W.; Wu, J.; Gong, Q.; Xie, P. Improved sulfamethoxazole degradation by the addition of MoS2 into the Fe2+/peroxymonosulfate process. Sep. Purif. Technol. 2020, 235, 116170. [Google Scholar] [CrossRef]
- Luo, H.; Zhou, X.; Guo, X.; Fang, Z.; Chen, Q.; Zhou, J. WS2 as highly active co-catalyst for the regeneration of Fe(II) in the advanced oxidation processes. Chemosphere 2021, 262, 128067. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Feng, Y.; Yang, B.; Yang, Z.; Shih, K. Activation of peroxymonosulfate by molybdenum disulfide-mediated traces of Fe(III) for sulfadiazine degradation. Chemosphere 2021, 283, 131212. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Sun, X.; Dang, Y.; Zhu, J.-J.; Zhao, Y.; Xu, X.; Zhou, Y. A novel electrochemically enhanced homogeneous PMS-heterogeneous CoFe2O4 synergistic catalysis for the efficient removal of levofloxacin. J. Hazard. Mater. 2022, 424, 127651. [Google Scholar] [CrossRef]
- Jiang, F.; Li, Y.; Zhou, W.; Yang, Z.; Ning, Y.; Liu, D.; Tang, Z.; Yang, S.; Huang, H.; Wang, G. Enhanced degradation of monochlorobenzene in groundwater by ferrous iron/persulfate process with cysteine. Chem. Eng. J. 2020, 387, 124048. [Google Scholar] [CrossRef]
- Wang, C.; Liu, H.; Li, X.; Cao, Y.; Jia, K. Degradation of organic pollutants in natural magnetite-activated peroxymonosulfate system enhanced by hydroxylamine: Catalytic performance, reaction pathway, and enhancement mechanism. J. Water Process Eng. 2024, 66, 106000. [Google Scholar] [CrossRef]
- Li, R.; Lu, X.; Yan, B.; Li, N.; Chen, G.; Cheng, Z.; Hou, L.A.; Wang, S.; Duan, X. Sludge-derived biochar toward sustainable Peroxymonosulfate Activation: Regulation of active sites and synergistic production of reaction oxygen species. Chem. Eng. J. 2022, 440, 135897. [Google Scholar] [CrossRef]
- Tan, C.; Cheng, X.; Xu, T.; Chen, K.; Xiang, H.; Su, L. Crystalline boron significantly accelerates Fe(III)/PMS reaction as an electron donor: Iron recycling, reactive species generation, and acute toxicity evaluation. Chem. Eng. J. 2023, 452, 139154. [Google Scholar] [CrossRef]


| Intrinsic Nature | Specific Manifestation & Mechanism | Impact on Catalytic Performance & System Efficiency | References |
|---|---|---|---|
| Kinetic Limitations | The intrinsic electron transfer rate for the reduction of Fe3+ (especially hydrolyzed species, e.g., Fe(OH)2+) is prohibitively slow | Becomes the unequivocal rate-limiting step, causing a precipitous drop in radical flux and extending treatment half-lives by 1–2 orders of magnitude. | [12,15] |
| Kinetic Limitations | Fe2+ acts as a potent scavenger for SO4•−/•OH radicals (k ≈ 109 M−1s−1), competing with target pollutants. | Leads to significant oxidant waste (utilization efficiency drops by 70–80%) and rapidly depletes the dissolved Fe2+ pool. | [31,44] |
| Thermodynamic Limitations | Hydrolysis of Fe3+ forms insoluble (oxyhydr)oxides (e.g., FeOOH), representing the thermodynamically stable state in aqueous media, especially at neutral-alkaline pH. | Depletes bioavailable iron, terminating the homogeneous cycle. Traps iron in a solid phase, making reduction unfavorable. | [13,15] |
| Thermodynamic Limitations | Formation of insoluble Fe3+ salts (e.g., FePO4) sequesters Fe3+ via strong coordination bonds (Ksp ≈ 10−22), thermodynamically stabilizing the Fe3+ state and lowering the effective Fe3+/Fe2+ redox potential from +0.77 V to ~+ 0.36 V. | Thermodynamically suppresses the driving force for Fe2+ regeneration, exacerbating the kinetic bottleneck. | [39] |
| Interfacial & System-Level Constraints | The precipitation and deposition of iron oxides onto heterogeneous catalysts form a physical barrier, drastically increasing charge transfer resistance. | Blocks active sites and impedes electron transfer from the bulk to the surface, leading to rapid catalyst deactivation. | [41,42] |
| Interfacial & System-Level Constraints | Cycle stagnation shifts oxidation pathway, which exhibit different selectivity. | May lead to incomplete degradation and the generation of more toxic transformation products. | [27,35,36] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.; Du, F.; Shi, H.; Du, H.; Xiao, P. Iron Redox Cycling in Persulfate Activation: Strategic Enhancements, Mechanistic Insights, and Environmental Applications—A Review. Nanomaterials 2025, 15, 1712. https://doi.org/10.3390/nano15221712
Zhang Z, Du F, Shi H, Du H, Xiao P. Iron Redox Cycling in Persulfate Activation: Strategic Enhancements, Mechanistic Insights, and Environmental Applications—A Review. Nanomaterials. 2025; 15(22):1712. https://doi.org/10.3390/nano15221712
Chicago/Turabian StyleZhang, Zutao, Fengyang Du, Hongliang Shi, Huanzheng Du, and Peiyuan Xiao. 2025. "Iron Redox Cycling in Persulfate Activation: Strategic Enhancements, Mechanistic Insights, and Environmental Applications—A Review" Nanomaterials 15, no. 22: 1712. https://doi.org/10.3390/nano15221712
APA StyleZhang, Z., Du, F., Shi, H., Du, H., & Xiao, P. (2025). Iron Redox Cycling in Persulfate Activation: Strategic Enhancements, Mechanistic Insights, and Environmental Applications—A Review. Nanomaterials, 15(22), 1712. https://doi.org/10.3390/nano15221712





