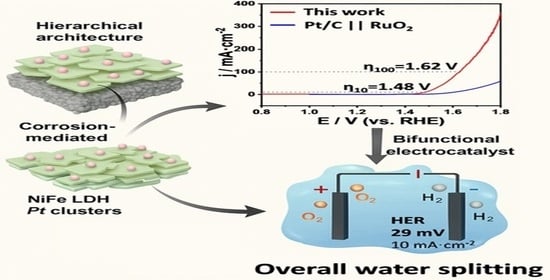
Durable Pt-Decorated NiFe-LDH for High-Current-Density Electrocatalytic Water Splitting Under Alkaline Conditions
Abstract
1. Introduction
2. Results and Discussion
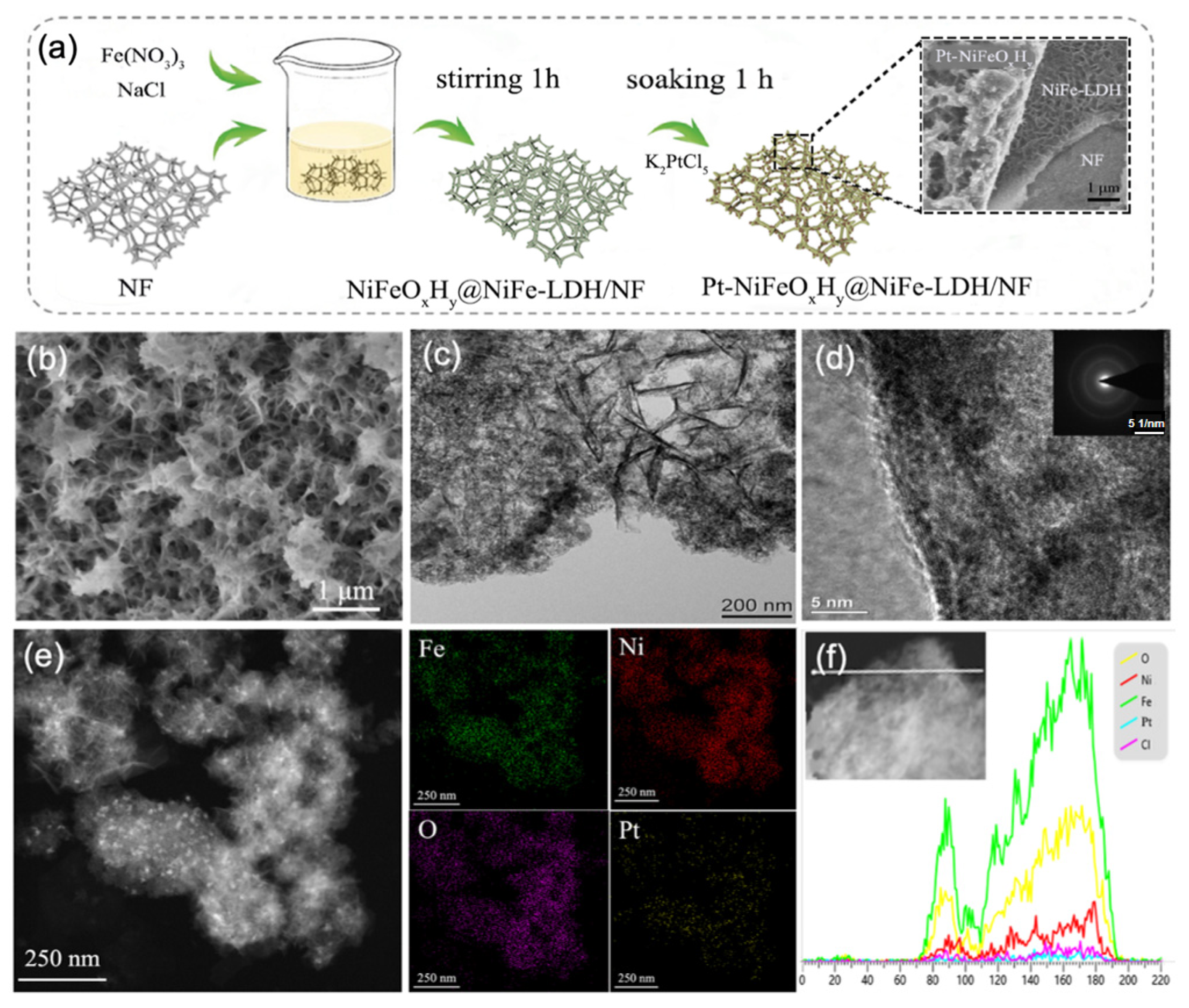
2.1. Synthesis and Characterization
2.2. Electrocatalyst Characterizations
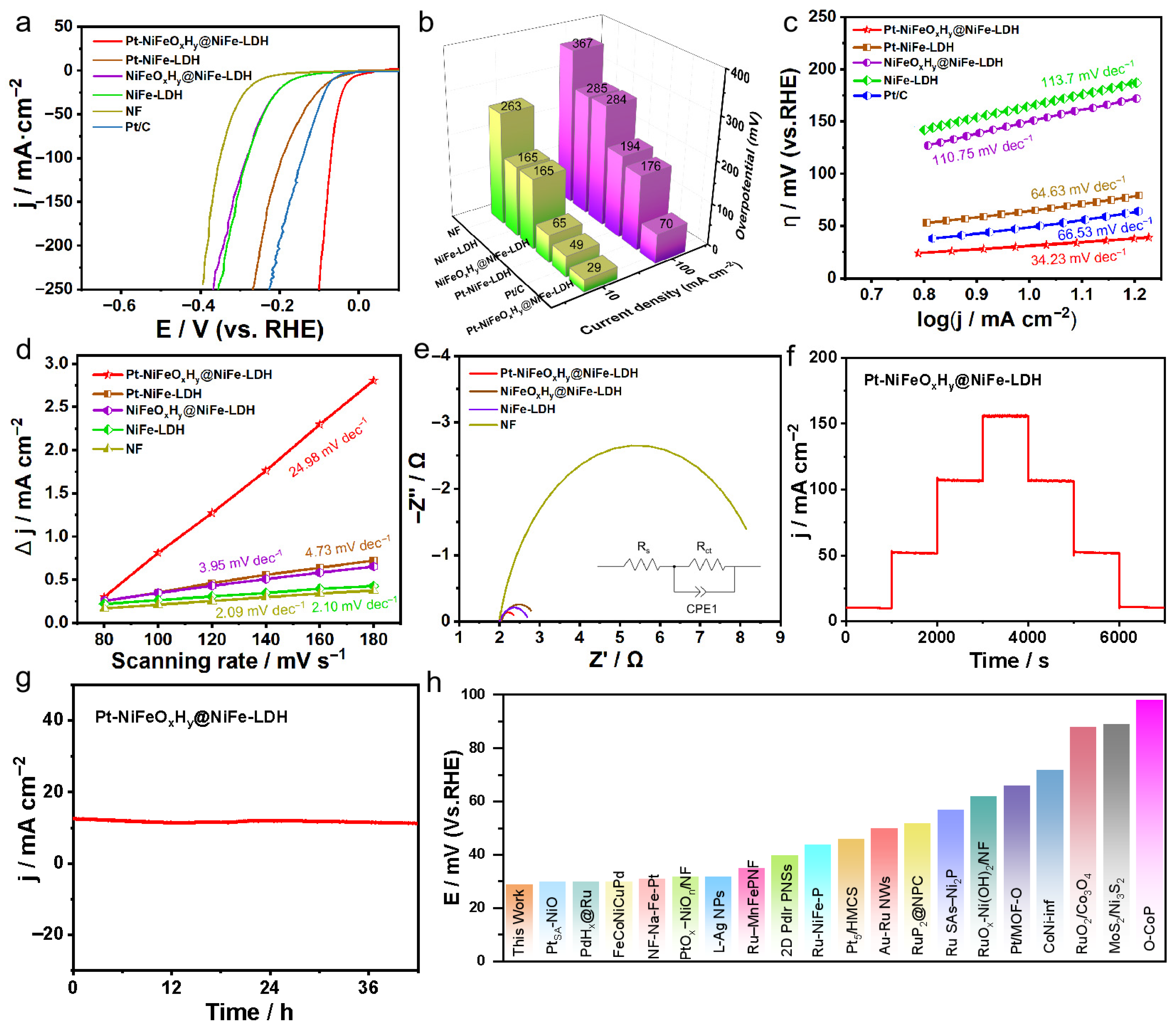
2.2.1. Hydrogen Evolution Reaction Performance
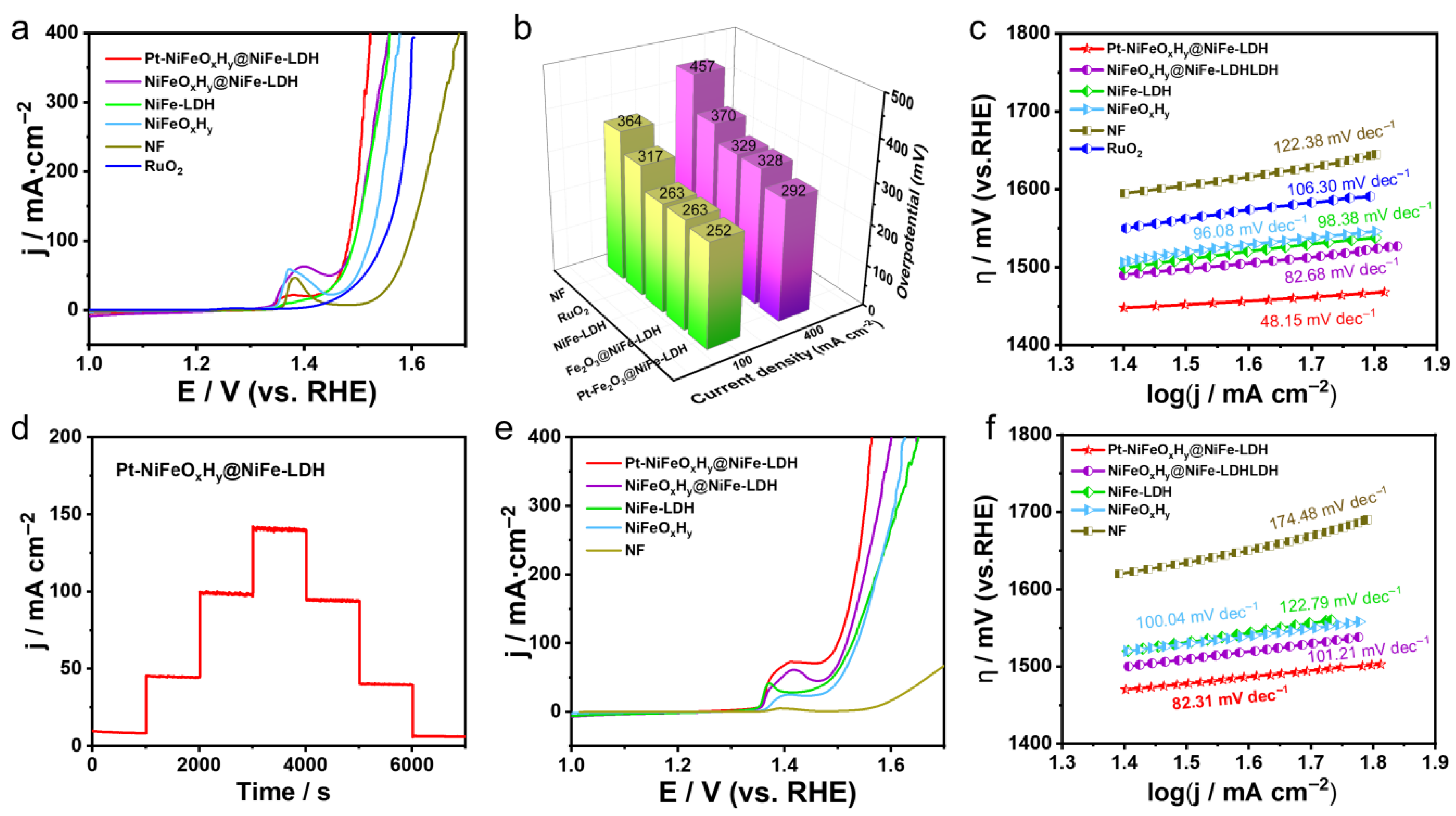
2.2.2. Oxygen Evolution Reaction Performance
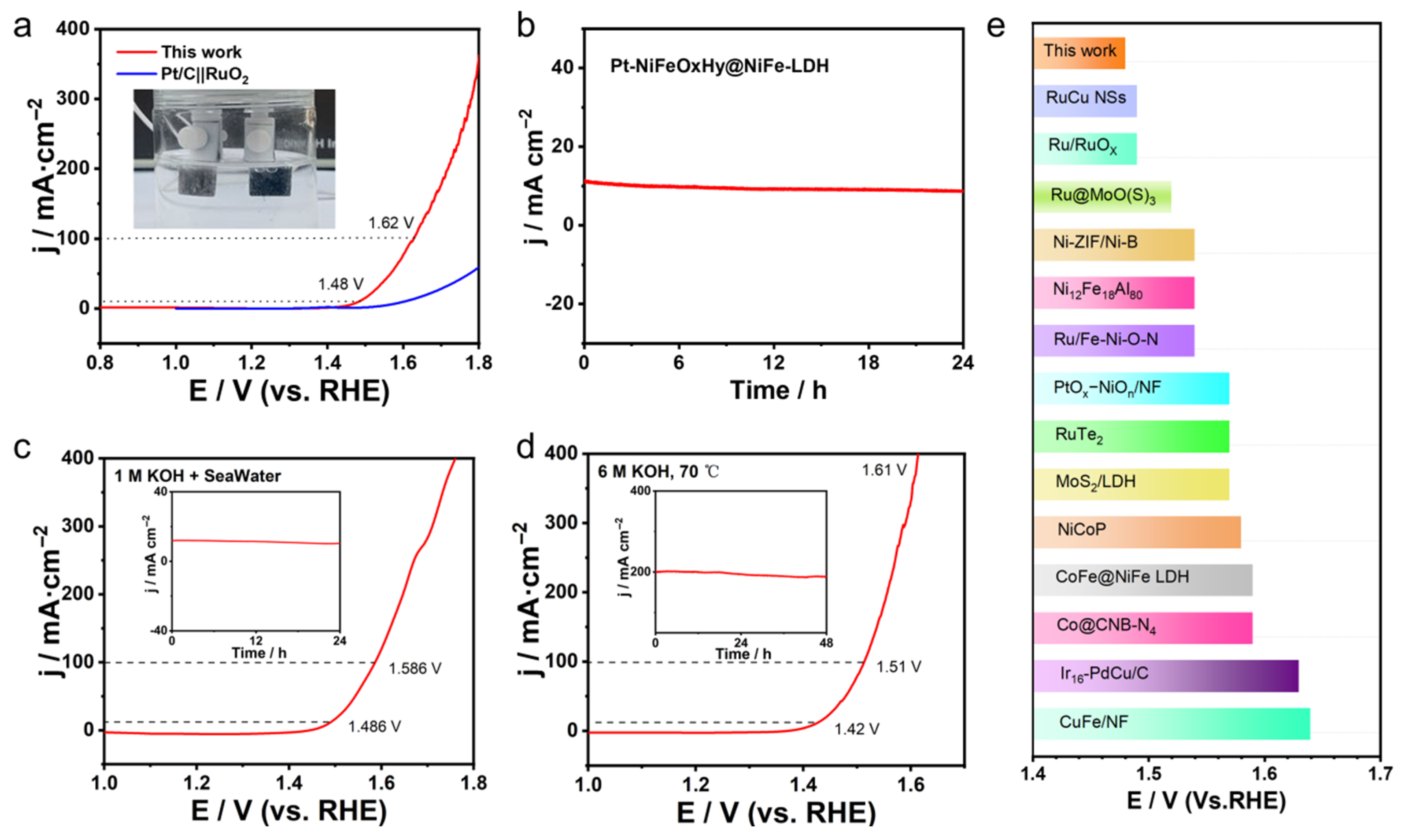
2.2.3. Overall Water-Splitting Performance
3. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Walter, M.G.; Warren, E.L.; McKone, J.R.; Boettcher, S.W.; Mi, Q.; Santori, E.A.; Lewis, N.S. Solar Water Splitting Cells. Chem. Rev. 2010, 110, 6446–6473. [Google Scholar] [CrossRef]
- Dresselhaus, M.S.; Thomas, I.L. Alternative Energy Technologies. Nature 2001, 414, 332–337. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Z.; Song, H.; Zhang, B.; Liu, J.; Shai, X.; Miao, L. Water Dissociation Kinetic-Oriented Design of Nickel Sulfides via Tailored Dual Sites for Efficient Alkaline Hydrogen Evolution. Adv. Funct. Mater. 2021, 31, 2008578. [Google Scholar] [CrossRef]
- Luo, J.; Im, J.-H.; Mayer, M.T.; Schreier, M.; Nazeeruddin, M.K.; Park, N.-G.; Tilley, S.D.; Fan, H.J.; Grätzel, M. Water Photolysis at 12.3% Efficiency via Perovskite Photovoltaics and Earth-Abundant Catalysts. Science 2014, 345, 1593–1596. [Google Scholar] [CrossRef] [PubMed]
- Subbaraman, R.; Tripkovic, D.; Strmcnik, D.; Chang, K.-C.; Uchimura, M.; Paulikas, A.P.; Stamenkovic, V.; Markovic, N.M. Enhancing Hydrogen Evolution Activity in Water Splitting by Tailoring Li+-Ni(OH)2-Pt Interfaces. Science 2011, 334, 1256–1260. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Y.; Zheng, Y.; Jaroniec, M.; Qiao, S.Z. Design of Electrocatalysts for Oxygen- and Hydrogen-Involving Energy Conversion Reactions. Chem. Soc. Rev. 2015, 44, 2060–2086. [Google Scholar] [CrossRef]
- Zhu, Y.; Kuo, T.-R.; Li, Y.-H.; Qi, M.-Y.; Chen, G.; Wang, J.; Xu, Y.-J.; Chen, H.M. Emerging Dynamic Structure of Electrocatalysts Unveiled by in Situ X-Ray Diffraction/Absorption Spectroscopy. Energy Environ. Sci. 2021, 14, 1928–1958. [Google Scholar] [CrossRef]
- Roger, I.; Shipman, M.A.; Symes, M.D. Earth-Abundant Catalysts for Electrochemical and Photoelectrochemical Water Splitting. Nat. Rev. Chem. 2017, 1, 0003. [Google Scholar] [CrossRef]
- Panda, C.; Menezes, P.W.; Yao, S.; Schmidt, J.; Walter, C.; Hausmann, J.N.; Driess, M. Boosting Electrocatalytic Hydrogen Evolution Activity with a NiPt3@NiS Heteronanostructure Evolved from a Molecular Nickel-Platinum Precursor. J. Am. Chem. Soc. 2019, 141, 13306–13310. [Google Scholar] [CrossRef]
- Cherevko, S.; Geiger, S.; Kasian, O.; Kulyk, N.; Grote, J.-P.; Savan, A.; Shrestha, B.R.; Merzlikin, S.; Breitbach, B.; Ludwig, A.; et al. Oxygen and Hydrogen Evolution Reactions on Ru, RuO2, Ir, and IrO2 Thin Film Electrodes in Acidic and Alkaline Electrolytes: A Comparative Study on Activity and Stability. Catal. Today 2016, 262, 170–180. [Google Scholar] [CrossRef]
- Zhang, H.; Zhou, W.; Lu, X.F.; Chen, T.; Lou, X.W. Implanting Isolated Ru Atoms into Edge-Rich Carbon Matrix for Efficient Electrocatalytic Hydrogen Evolution. Adv. Energy Mater. 2020, 10, 2000882. [Google Scholar] [CrossRef]
- Li, D.; Chen, X.; Lv, Y.; Zhang, G.; Huang, Y.; Liu, W.; Li, Y.; Chen, R.; Nuckolls, C.; Ni, H. An Effective Hybrid Electrocatalyst for the Alkaline HER: Highly Dispersed Pt Sites Immobilized by a Functionalized NiRu-Hydroxide. Appl. Catal. B Environ. 2020, 269, 118824. [Google Scholar] [CrossRef]
- Wang, P.; Qin, R.; Ji, P.; Pu, Z.; Zhu, J.; Lin, C.; Zhao, Y.; Tang, H.; Li, W.; Mu, S. Synergistic Coupling of Ni Nanoparticles with Ni3C Nanosheets for Highly Efficient Overall Water Splitting. Small 2020, 16, 2001642. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, Y.; Chen, T.; Zhang, J.; Zhang, J.; Lou, X.W. Unveiling the Activity Origin of Electrocatalytic Oxygen Evolution over Isolated Ni Atoms Supported on a N-Doped Carbon Matrix. Adv. Mater. 2019, 31, 1904548. [Google Scholar] [CrossRef]
- Zhang, H.; Zhou, W.; Dong, J.; Lu, X.F.; Lou, X.W. Intramolecular Electronic Coupling in Porous Iron Cobalt (Oxy)Phosphide Nanoboxes Enhances the Electrocatalytic Activity for Oxygen Evolution. Energy Environ. Sci. 2019, 12, 3348–3355. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, L.; Tan, Q.; Wang, J.; Liu, J.; Wan, H.; Miao, L.; Jiang, J. Simultaneous Interfacial Chemistry and Inner Helmholtz Plane Regulation for Superior Alkaline Hydrogen Evolution. Energy Environ. Sci. 2020, 13, 3007–3013. [Google Scholar] [CrossRef]
- Hou, Y.; Lohe, M.R.; Zhang, J.; Liu, S.; Zhuang, X.; Feng, X. Vertically Oriented Cobalt Selenide/NiFe Layered-Double-Hydroxide Nanosheets Supported on Exfoliated Graphene Foil: An Efficient 3D Electrode for Overall Water Splitting. Energy Environ. Sci. 2016, 9, 478–483. [Google Scholar] [CrossRef]
- Tang, C.; Cheng, N.; Pu, Z.; Xing, W.; Sun, X. NiSe Nanowire Film Supported on Nickel Foam: An Efficient and Stable 3D Bifunctional Electrode for Full Water Splitting. Angew. Chem. Int. Ed. 2015, 54, 9351–9355. [Google Scholar] [CrossRef]
- Liu, J.; Zheng, Y.; Jiao, Y.; Wang, Z.; Lu, Z.; Vasileff, A.; Qiao, S.-Z. NiO as a Bifunctional Promoter for RuO2 toward Superior Overall Water Splitting. Small 2018, 14, 1704073. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Sheng, W.; Zhuang, Z.; Fang, Q.; Gu, S.; Jiang, J.; Yan, Y. Efficient Water Oxidation Using Nanostructured α-Nickel-Hydroxide as an Electrocatalyst. J. Am. Chem. Soc. 2014, 136, 7077–7084. [Google Scholar] [CrossRef]
- Lu, F.; Zhou, M.; Zhou, Y.; Zeng, X. First-Row Transition Metal Based Catalysts for the Oxygen Evolution Reaction under Alkaline Conditions: Basic Principles and Recent Advances. Small 2017, 13, 1701931. [Google Scholar] [CrossRef]
- McCrory, C.C.L.; Jung, S.; Ferrer, I.M.; Chatman, S.M.; Peters, J.C.; Jaramillo, T.F. Benchmarking Hydrogen Evolving Reaction and Oxygen Evolving Reaction Electrocatalysts for Solar Water Splitting Devices. J. Am. Chem. Soc. 2015, 137, 4347–4357. [Google Scholar] [CrossRef]
- Hu, C.; Zhang, L.; Gong, J. Recent Progress Made in the Mechanism Comprehension and Design of Electrocatalysts for Alkaline Water Splitting. Energy Environ. Sci. 2019, 12, 2620–2645. [Google Scholar] [CrossRef]
- Strmcnik, D.; Lopes, P.P.; Genorio, B.; Stamenkovic, V.R.; Markovic, N.M. Design principles for hydrogen evolution reaction catalyst materials. Nano Energy 2016, 29, 29–36. [Google Scholar] [CrossRef]
- Seh, Z.W.; Kibsgaard, J.; Dickens, C.F.; Chorkendorff, I.; Nørskov, J.K.; Jaramillo, T.F. Combining theory and experiment in electrocatalysis: Insights into materials design. Science 2017, 355, eaad4998. [Google Scholar] [CrossRef]
- Jiao, Y.; Zheng, Y.; Davey, K.; Qiao, S.Z. Activity origin and catalyst design principles for electrocatalytic hydrogen evolution on heteroatom-doped graphene. Nat. Energy 2016, 1, 16130. [Google Scholar] [CrossRef]
- Duan, J.; Chen, S.; Jaroniec, M.; Qiao, S.Z. Heteroatom-doped graphene-based materials for energy-relevant electrocatalytic processes. ACS Catal. 2015, 5, 5207–5234. [Google Scholar] [CrossRef]
- Liang, C.; Zou, P.; Nairan, A.; Zhang, Y.; Liu, J.; Liu, K.; Hu, S.; Kang, F.; Fan, H.J.; Yang, C. Exceptional Performance of Hierarchical Ni-Fe oxyhydroxide@NiFe Alloy Nanowire Array Electrocatalysts for Large Current Density Water Splitting. Energy Environ. Sci. 2020, 13, 86–95. [Google Scholar] [CrossRef]
- Zou, X.; Liu, Y.; Li, G.-D.; Wu, Y.; Liu, D.-P.; Li, W.; Li, H.-W.; Wang, D.; Zhang, Y.; Zou, X. Ultrafast Formation of Amorphous Bimetallic Hydroxide Films on 3D Conductive Sulfide Nanoarrays for Large-Current-Density Oxygen Evolution Electrocatalysis. Adv. Mater. 2017, 29, 1700404. [Google Scholar] [CrossRef]
- Yu, L.; Zhou, H.; Sun, J.; Qin, F.; Yu, F.; Bao, J.; Yu, Y.; Chen, S.; Ren, Z. Cu Nanowires Shelled with NiFe Layered Double Hydroxide Nanosheets as Bifunctional Electrocatalysts for Overall Water Splitting. Energy Environ. Sci. 2017, 10, 1820–1827. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, J.; Xi, L.; Yu, Y.; Chen, N.; Sun, S.; Wang, W.; Lange, K.M.; Zhang, B. Single-Atom Au/NiFe Layered Double Hydroxide Electrocatalyst: Probing the Origin of Activity for Oxygen Evolution Reaction. J. Am. Chem. Soc. 2018, 140, 3876–3879. [Google Scholar] [CrossRef]
- Lim, T.; Jung, G.Y.; Kim, J.H.; Park, S.O.; Park, J.; Kim, Y.-T.; Kang, S.J.; Jeong, H.Y.; Kwak, S.K.; Joo, S.H. Atomically Dispersed Pt-N4 Sites as Efficient and Selective Electrocatalysts for the Chlorine Evolution Reaction. Nat. Commun. 2020, 11, 412. [Google Scholar] [CrossRef]
- Liu, D.; Li, X.; Chen, S.; Yan, H.; Wang, C.; Wu, C.; Haleem, Y.A.; Duan, S.; Lu, J.; Ge, B.; et al. Atomically Dispersed Platinum Supported on Curved Carbon Supports for Efficient Electrocatalytic Hydrogen Evolution. Nat. Energy 2019, 4, 512–518. [Google Scholar] [CrossRef]
- Shen, Y.; Li, S.; Wang, C.; Xu, Y.; Chao, Y.; Shi, C.; Wen, M. Preparation and Electrocatalytic Property of Three-Dimensional Nano-Dendritic Platinum Oxide Film. Surf. Interface Anal. 2022, 54, 524–533. [Google Scholar] [CrossRef]
- Wei, X.; Chen, C.; Fu, X.Z.; Wang, S. Oxygen vacancies-rich metal oxide for electrocatalytic nitrogen cycle. Adv. Energy Mater. 2024, 14, 2303027. [Google Scholar] [CrossRef]
- Zhan, Y.; Li, Y.; Yang, Z.; Wu, X.; Ge, M.; Zhou, X.; Hou, J.; Zheng, X.; Lai, Y.; Pang, R.; et al. Synthesis of a MoSx-O-PtOx Electrocatalyst with High Hydrogen Evolution Activity Using a Sacrificial Counter-Electrode. Adv. Sci. 2019, 6, 1801663. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, L.; Liu, H.; Jia, B.; Qu, X.; Qin, M. Durable Pt-Decorated NiFe-LDH for High-Current-Density Electrocatalytic Water Splitting Under Alkaline Conditions. Nanomaterials 2025, 15, 1683. https://doi.org/10.3390/nano15211683
Liu L, Liu H, Jia B, Qu X, Qin M. Durable Pt-Decorated NiFe-LDH for High-Current-Density Electrocatalytic Water Splitting Under Alkaline Conditions. Nanomaterials. 2025; 15(21):1683. https://doi.org/10.3390/nano15211683
Chicago/Turabian StyleLiu, Luan, Hongru Liu, Baorui Jia, Xuanhui Qu, and Mingli Qin. 2025. "Durable Pt-Decorated NiFe-LDH for High-Current-Density Electrocatalytic Water Splitting Under Alkaline Conditions" Nanomaterials 15, no. 21: 1683. https://doi.org/10.3390/nano15211683
APA StyleLiu, L., Liu, H., Jia, B., Qu, X., & Qin, M. (2025). Durable Pt-Decorated NiFe-LDH for High-Current-Density Electrocatalytic Water Splitting Under Alkaline Conditions. Nanomaterials, 15(21), 1683. https://doi.org/10.3390/nano15211683