Low-Cost Versatile Microfluidic Platform for Bioorthogonal Click-Mediated Nanoassembly of Hybrid Nanosystems
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Nanoparticle Synthesis
2.2.1. Synthesis of Azide-Functionalized Small Unilamellar Liposomes (Lip-N3)
2.2.2. Synthesis of Azide-Functionalized Mesoporous Silica Nanoparticles (MSN-N3)
2.2.3. Synthesis of Polymeric, DBCO-Functionalized Catalase Nanocapsules (DBCO-CatNCs)
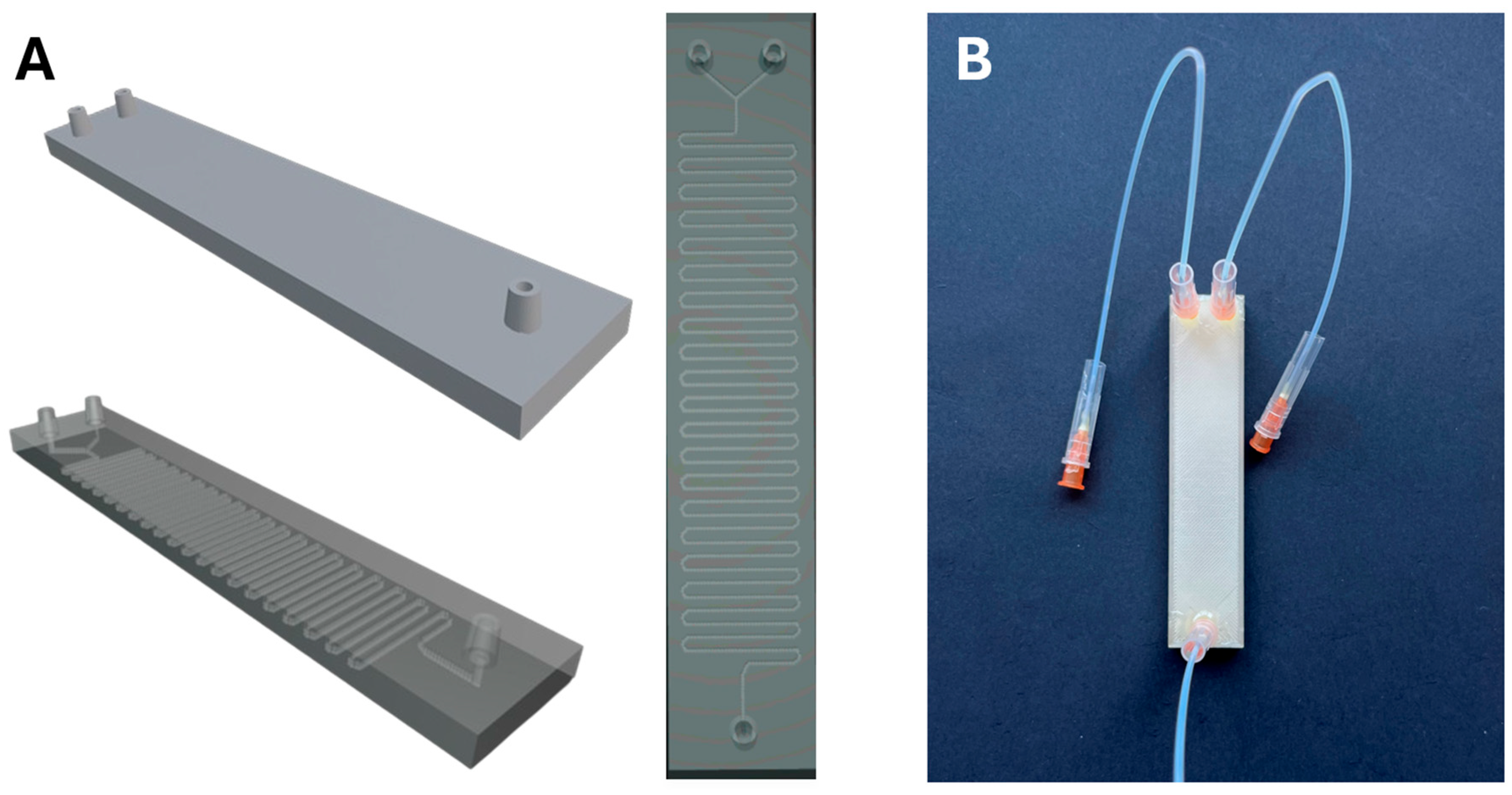
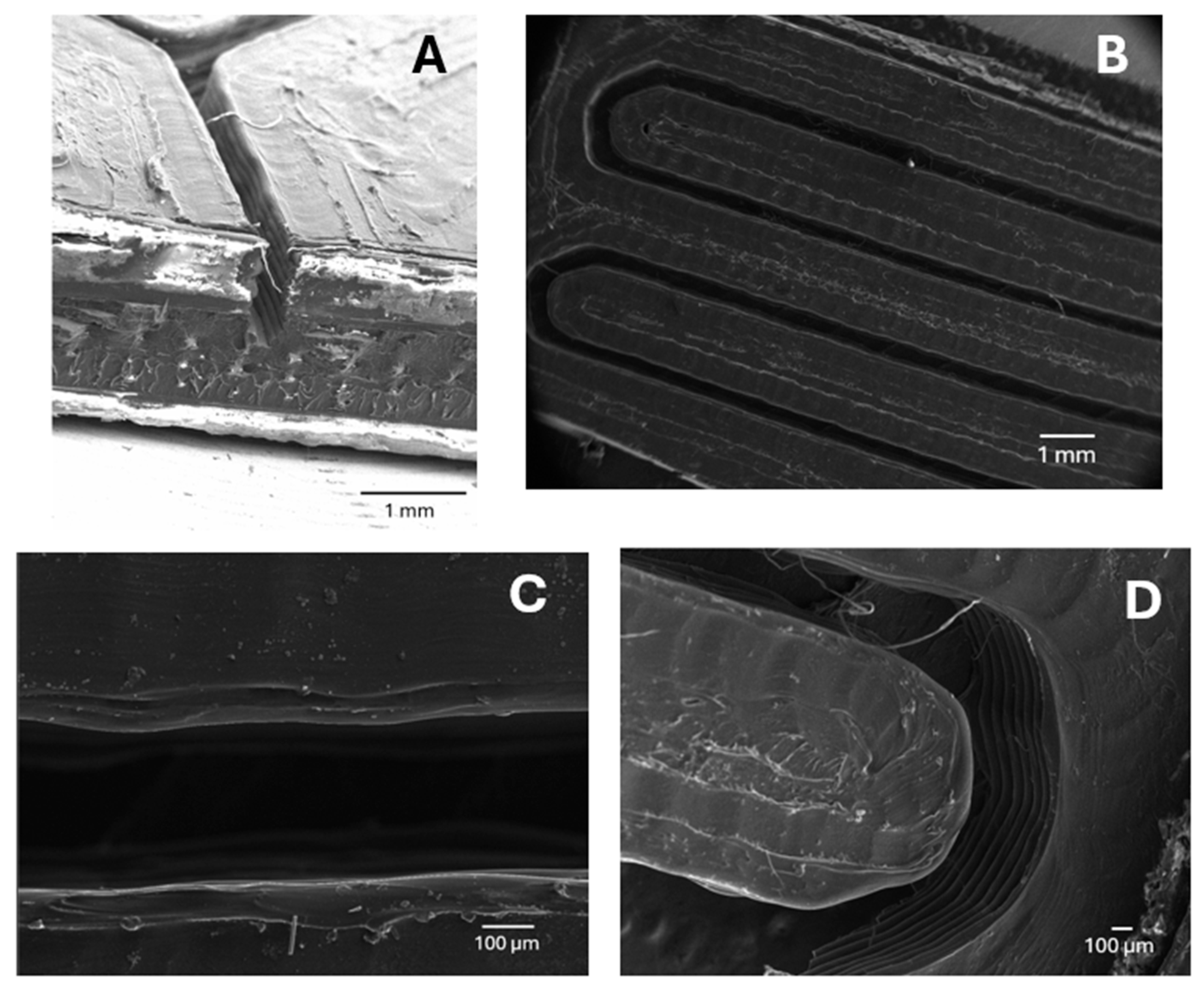
2.3. Microreactor Fabrication
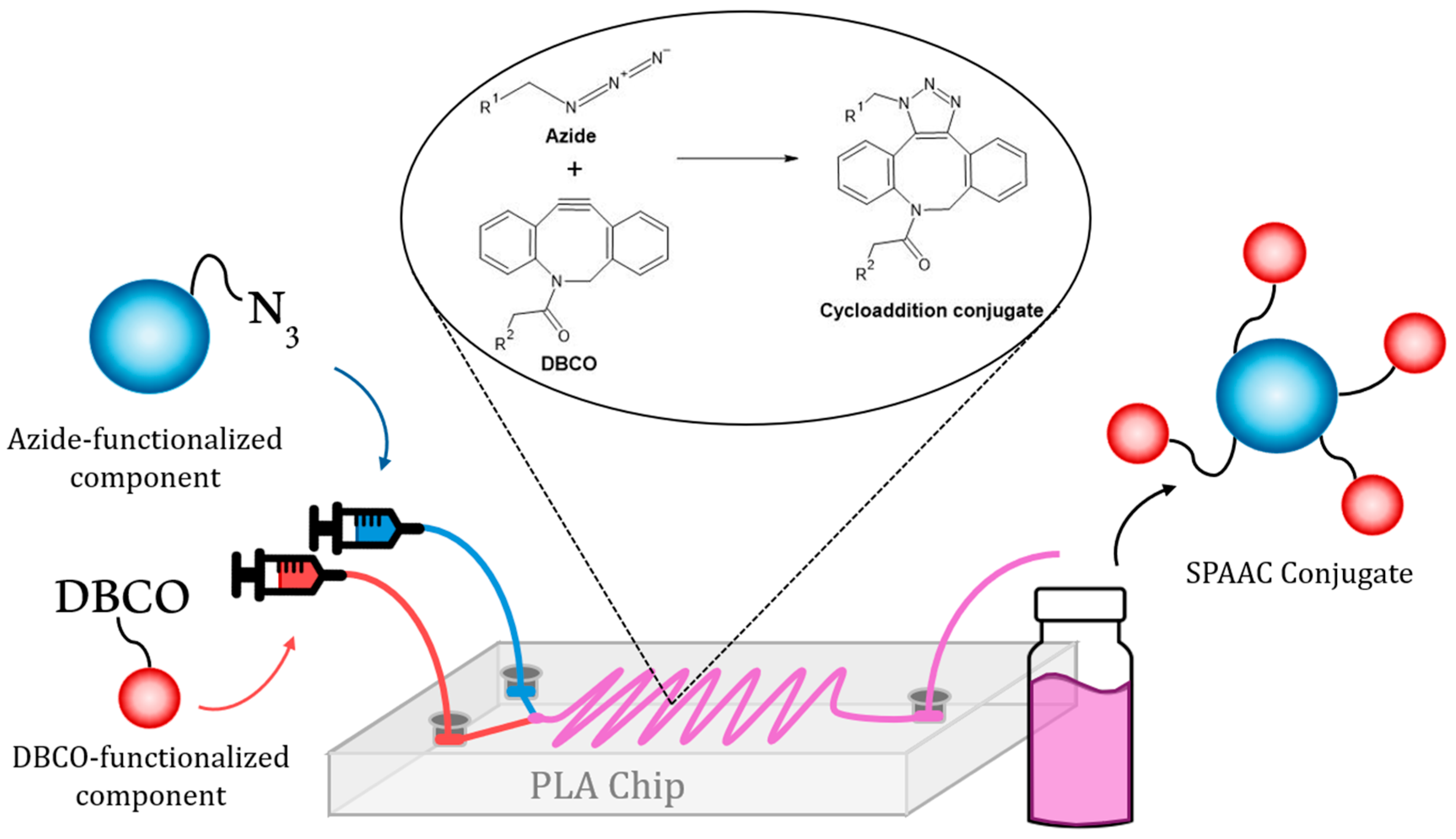
2.4. Microfluidic Functionalization of Nanomaterials with Organic Molecules
2.4.1. Functionalization of Lip-N3 with DBCO-TAMRA [(Lip-N3)-(DBCO-TAMRA)]
2.4.2. Functionalization of MSN-N3 with DBCO-TAMRA [(MSN-N3)-(DBCO-TAMRA)]
2.5. Assembly of Hybrid Nanosystems [(MSN-N3)-(DBCO-CatNC)]
2.5.1. Enzymatic Activity Assay
2.5.2. Colloidal Stability Assay
3. Results and Discussion
3.1. Synthesis of Azide-Functionalized Small Unilamellar Liposomes (Lip-N3) and Azide Modified Mesoporous Silica Nanoparticles (MSN-N3)
3.2. Microfluidic Device Characterization
3.3. Microfluidic Functionalization of Nanomaterials with Organic Molecules
3.3.1. Functionalization of Lip-N3 with DBCO-TAMRA [(Lip-N3)-(DBCO-TAMRA)]
3.3.2. Functionalization of MSN-N3 with DBCO-TAMRA-[(MSN-N3)-(DBCO-TAMRA)]
3.3.3. Comparative Evaluation of Microreactor Efficiency
3.4. Assembly of Hybrid Nanosystems
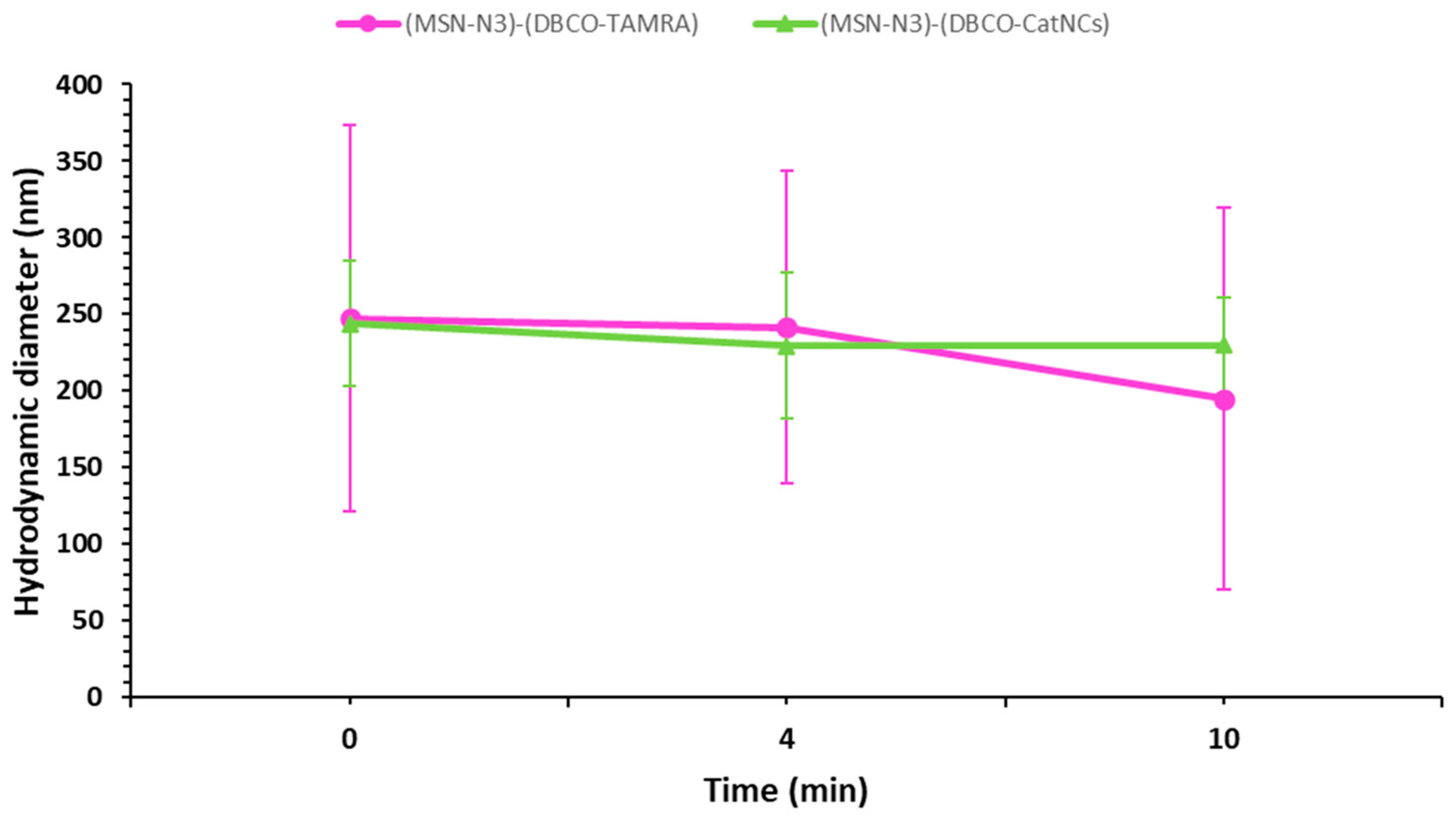
3.5. Colloidal Stability of the Nanosystems
3.6. Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| NP | Nanoparticle |
| FDM | Fusion deposition modeling |
| MCM-41 | Mobil Composition of Matter No. 41 |
| MSN | Mesoporous silica nanoparticle |
| MSN-NH2 | Aminated mesoporous silica nanoparticles |
| MSN-N3 | Azide-functionalized MSNs |
| Lip-N3 | Azide-functionalized liposomes |
| NC | Nanocapsule |
| CatNC | Catalase NC |
| SPAAC | Strain-promoted azide-alkyne cycloaddition |
| TEOS | tetraethyl orthosilicate |
| APTES | 3-aminopropyltriethoxysilane |
| DIC | N,N′-diisopropylcarbodiimide |
| NHS | N-hydroxysuccinimide |
| DBCO | dibenzocyclooctyne |
| DBCO-TAMRA | DBCO-5-carboxytetramethylrhodamine |
| CatNC | Catalase nanocapsules |
| DBCO-CatNC | DBCO-functionalized CatNC |
| 6-AHA | 6-azidohexanoic acid |
| DBCO-NHS | Dibenzocyclooctyne-NHS ester |
| TMEDA | N,N,N′,N′-tetramethylethylenediamine |
| AA | Acrylamide |
| AM | aminoethyl methacrylamide |
| MBA | N,N′-methylenebisacrylamide |
| APS | Ammonium persulfate |
| PEG | polyethylene glycol |
| PBS | Phosphate-buffered saline |
| EtOH | Ethanol |
| DMF | N,N-dimethylformamide |
| DMSO | Dymtethyl sulfoxide |
| NH4NO3 | Ammonium nitrate |
| Na2CO3 | Sodium carbonate |
| CHCl3 | Chloroform |
| NH4OH | Ammonium hydroxide |
| DSPE-N3 | 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[azide(polyethylene glycol)-2000] |
| DSPC | 1,2-distearoyl-sn-glycero-3-phosphocholine |
| Chol | Cholesterol |
| DLS | Dynamic light scattering |
| ELS | Electrophoretic light scattering |
| MWCO | Molecular weight cut-off |
| rpm | Revolutions per minute |
| g | Relative centrifugal force |
| TEM | Transmission electron microscopy |
| SEM | Scanning electron microscopy |
| SEC | Size exclusion chromatography |
| UV/Vis | Ultraviolet-visible spectroscopy |
| PLA | Polylactic acid |
| kDa | Kilodalton |
| mL | Mililiter |
| µL | Microliter |
| µmol | Micromol |
| mV | Milivolt |
| µm | Micrometer |
| U/µg | Specific enzymatic activity (Absorbance units/microgram of material) |
| kCts. | Kilocounts (Scattering intensity in DLS analysis) |
References
- Wicki, A.; Witzigmann, D.; Balasubramanian, V.; Huwyler, J. Nanomedicine in Cancer Therapy: Challenges, Opportunities, and Clinical Applications. J. Control. Release 2015, 200, 138–157. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Jana, A.K.; Maiti, M.; Dhamija, I. Carbodiimide-Mediated Immobilization of Serratiopeptidase on Amino-, Carboxyl-Functionalized Magnetic Nanoparticles and Characterization for Target Delivery. J. Nanopart. Res. 2014, 16, 2233. [Google Scholar] [CrossRef]
- Vajhadin, F.; Mazloum-Ardakani, M.; Raeisi, S.; Hemati, M.; Ebadi, A.; Haghiralsadat, F.; Tofighi, D. Glutaraldehyde Crosslinked Doxorubicin Promotes Drug Delivery Efficiency Using Cobalt Ferrite Nanoparticles. Colloids Surf. B Biointerfaces 2022, 220, 112870. [Google Scholar] [CrossRef] [PubMed]
- Braun, A.C.; Gutmann, M.; Lühmann, T.; Meinel, L. Bioorthogonal Strategies for Site-Directed Decoration of Biomaterials with Therapeutic Proteins. J. Control. Release 2018, 273, 68–85. [Google Scholar] [CrossRef] [PubMed]
- Sletten, E.M.; Bertozzi, C.R. Bioorthogonal Chemistry: Fishing for Selectivity in a Sea of Functionality. Angew. Chem.-Int. Ed. 2009, 48, 6974–6998. [Google Scholar] [CrossRef]
- Algar, W.R.; Prasuhn, D.E.; Stewart, M.H.; Jennings, T.L.; Blanco-Canosa, J.B.; Dawson, P.E.; Medintz, I.L. The Controlled Display of Biomolecules on Nanoparticles: A Challenge Suited to Bioorthogonal Chemistry. Bioconjug. Chem. 2011, 22, 825–858. [Google Scholar] [CrossRef]
- Shi, H.; Nie, K.; Dong, B.; Long, M.; Xu, H.; Liu, Z. Recent Progress of Microfluidic Reactors for Biomedical Applications. Chem. Eng. J. 2019, 361, 635–650. [Google Scholar] [CrossRef]
- Niculescu, A.-G.; Mihaiescu, D.E.; Grumezescu, A.M. A Review of Microfluidic Experimental Designs for Nanoparticle Synthesis. Int. J. Mol. Sci. 2022, 23, 8293. [Google Scholar] [CrossRef]
- Gimondi, S.; Ferreira, H.; Reis, R.L.; Neves, N.M. Microfluidic Devices: A Tool for Nanoparticle Synthesis and Performance Evaluation. ACS Nano 2023, 17, 14205–14228. [Google Scholar] [CrossRef]
- Scott, S.; Ali, Z. Fabrication Methods for Microfluidic Devices: An Overview. Micromachines 2021, 12, 319. [Google Scholar] [CrossRef]
- Salentijn, G.I.; Oomen, P.E.; Grajewski, M.; Verpoorte, E. Fused Deposition Modeling 3D Printing for (Bio)Analytical Device Fabrication: Procedures, Materials, and Applications. Anal. Chem. 2017, 89, 7053–7061. [Google Scholar] [CrossRef]
- Wasti, S.; Adhikari, S. Use of Biomaterials for 3D Printing by Fused Deposition Modeling Technique: A Review. Front. Chem. 2020, 8, 315. [Google Scholar] [CrossRef] [PubMed]
- Bressan, L.P.; Robles-Najar, J.; Adamo, C.B.; Quero, R.F.; Costa, B.M.C.; de Jesus, D.P.; da Silva, J.A.F. 3D-Printed Microfluidic Device for the Synthesis of Silver and Gold Nanoparticles. Microchem. J. 2019, 146, 1083–1089. [Google Scholar] [CrossRef]
- Mastella, P.; Luin, S. Microfluidic Assembly of Poly(Glutamic Acid) Nanogels Through SPAAC Click Chemistry. Pharmaceutics 2025, 17, 1150. [Google Scholar] [CrossRef] [PubMed]
- Shepherd, S.J.; Issadore, D.; Mitchell, M.J. Microfluidic Formulation of Nanoparticles for Biomedical Applications. Biomaterials 2021, 274, 120826. [Google Scholar] [CrossRef]
- Saikia, A.; Newar, R.; Das, S.; Singh, A.; Deuri, D.J.; Baruah, A. Scopes and Challenges of Microfluidic Technology for Nanoparticle Synthesis, Photocatalysis and Sensor Applications: A Comprehensive Review. Chem. Eng. Res. Des. 2023, 193, 516–539. [Google Scholar] [CrossRef]
- Clay, N.E.; Whittenberg, J.J.; Leong, J.; Kumar, V.; Chen, J.; Choi, I.; Liamas, E.; Schieferstein, J.M.; Jeong, J.H.; Kim, D.H.; et al. Chemical and Mechanical Modulation of Polymeric Micelle Assembly. Nanoscale 2017, 9, 5194–5204. [Google Scholar] [CrossRef]
- Idiago-López, J.; Moreno-Antolín, E.; de la Fuente, J.M.; Fratila, R.M. Nanoparticles and Bioorthogonal Chemistry Joining Forces for Improved Biomedical Applications. Nanoscale Adv. 2021, 3, 1261–1292. [Google Scholar] [CrossRef]
- Gharib, G.; Bütün, İ.; Muganlı, Z.; Kozalak, G.; Namlı, İ.; Sarraf, S.S.; Ahmadi, V.E.; Toyran, E.; van Wijnen, A.J.; Koşar, A. Biomedical Applications of Microfluidic Devices: A Review. Biosensors 2022, 12, 1023. [Google Scholar] [CrossRef]
- Bendre, A.; Bhat, M.P.; Lee, K.H.; Altalhi, T.; Alruqi, M.A.; Kurkuri, M. Recent Developments in Microfluidic Technology for Synthesis and Toxicity-Efficiency Studies of Biomedical Nanomaterials. Mater. Today Adv. 2022, 13, 100205. [Google Scholar] [CrossRef]
- Schneid, A.d.C.; Silveira, C.P.; Galdino, F.E.; Ferreira, L.F.; Bouchmella, K.; Cardoso, M.B. Colloidal Stability and Redispersibility of Mesoporous Silica Nanoparticles in Biological Media. Langmuir 2020, 36, 11442–11449. [Google Scholar] [CrossRef]
- Chen, Y.; Su, Y.C.; Roffler, S.R. Polyethylene Glycol Immunogenicity in Nanomedicine. Nat. Rev. Bioeng. 2025, 3, 742–760. [Google Scholar] [CrossRef]
- Mehta, P.; Shende, P. Evasion of Opsonization of Macromolecules Using Novel Surface-Modification and Biological-Camouflage-Mediated Techniques for next-Generation Drug Delivery. Cell Biochem. Funct. 2023, 41, 1031–1043. [Google Scholar] [CrossRef]
- Zhou, Q.; Xiang, J.; Qiu, N.; Wang, Y.; Piao, Y.; Shao, S.; Tang, J.; Zhou, Z.; Shen, Y. Tumor Abnormality-Oriented Nanomedicine Design. Chem. Rev. 2023, 123, 10920–10989. [Google Scholar] [CrossRef] [PubMed]
- Allen, C.; Dos Santos, N.; Gallagher, R.; Chiu, G.N.C.; Shu, Y.; Li, W.M.; Johnstone, S.A.; Janoff, A.S.; Mayer, L.D.; Webb, M.S.; et al. Controlling the Physical Behavior and Biological Performance of Liposome Formulations through Use of Surface Grafted Poly(Ethylene Glycol). Biosci. Rep. 2002, 22, 225–250. [Google Scholar] [CrossRef]
- Parra-Nieto, J.; Arroyo-Nogales, A.; Marcos-Fernández, D.; Jimenez-Falcao, S.; Arribas, C.; Megias, D.; Gonzalez-Murillo, Á.; Ramirez, M.; Baeza, A. Dual-Pore Protocells with Multitasking Capacities for Simultaneous Delivery of Therapeutic Enzymes and Drugs in Macrophage Depletion Therapy. Biomater. Sci. 2024, 12, 5372–5385. [Google Scholar] [CrossRef]
- Antoniou, A.I.; Giofrè, S.; Seneci, P.; Passarella, D.; Pellegrino, S. Stimulus-Responsive Liposomes for Biomedical Applications. Drug Discov. Today 2021, 26, 1794–1824. [Google Scholar] [CrossRef] [PubMed]
- Andra, V.V.S.N.L.; Pammi, S.V.N.; Bhatraju, L.V.K.P.; Ruddaraju, L.K. A Comprehensive Review on Novel Liposomal Methodologies, Commercial Formulations, Clinical Trials and Patents. Bionanoscience 2022, 12, 274–291. [Google Scholar] [CrossRef] [PubMed]
- Lombardo, D.; Kiselev, M.A. Methods of Liposomes Preparation: Formation and Control Factors of Versatile Nanocarriers for Biomedical and Nanomedicine Application. Pharmaceutics 2022, 14, 543. [Google Scholar] [CrossRef]
- Gandek, T.B.; van der Koog, L.; Nagelkerke, A. A Comparison of Cellular Uptake Mechanisms, Delivery Efficacy, and Intracellular Fate between Liposomes and Extracellular Vesicles. Adv. Healthc. Mater. 2023, 12, 2300319. [Google Scholar] [CrossRef]
- Hu, X.L.; Gan, H.Q.; Meng, F.-D.; Han, H.H.; Shi, D.T.; Zhang, S.; Zou, L.; He, X.P.; James, T.D. Fluorescent Probes and Functional Materials for Biomedical Applications. Front. Chem. Sci. Eng. 2022, 16, 1425–1437. [Google Scholar] [CrossRef]
- Kukulka, M.; Pem, B.; Vazdar, K.; Cwiklik, L.; Vazdar, M. UV Absorption Spectra of TAMRA and TAMRA Labeled Peptides: A Combined Density Functional Theory and Classical Molecular Dynamics Study. J. Comput. Chem. 2025, 46, e70096. [Google Scholar] [CrossRef] [PubMed]
- Archipowa, N.; Wittmann, L.; Köckenberger, J.; Ertl, F.J.; Gleixner, J.; Keller, M.; Heinrich, M.R.; Kutta, R.J. Characterization of Fluorescent Dyes Frequently Used for Bioimaging: Photophysics and Photocatalytical Reactions with Proteins. J. Phys. Chem. B 2023, 127, 9532–9542. [Google Scholar] [CrossRef]
- Oude Blenke, E.; Klaasse, G.; Merten, H.; Plückthun, A.; Mastrobattista, E.; Martin, N.I. Liposome Functionalization with Copper-Free “Click Chemistry”. J. Control. Release 2015, 202, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.S.; Hurley, K.R.; Haynes, C.L. Critical Considerations in the Biomedical Use of Mesoporous Silica Nanoparticles. J. Phys. Chem. Lett. 2012, 3, 364–374. [Google Scholar] [CrossRef] [PubMed]
- Hao, N.; Nie, Y.; Zhang, J.X.J. Microfluidics for Silica Biomaterials Synthesis: Opportunities and Challenges. Biomater. Sci. 2019, 7, 2218. [Google Scholar] [CrossRef]
- Niculescu, V.C. Mesoporous Silica Nanoparticles for Bio-Applications. Front. Mater. 2020, 7, 486705. [Google Scholar] [CrossRef]
- Mukherjee, P.; Khademhosseini, A.; Seaberg, J.; Montazerian, H.; Hossen, M.N.; Bhattacharya, R. Hybrid Nanosystems for Biomedical Applications. ACS Nano 2021, 15, 2099–2142. [Google Scholar] [CrossRef]
- Cuoghi, S.; Caraffi, R.; Anderlini, A.; Baraldi, C.; Enzo, E.; Vandelli, M.A.; Tosi, G.; Ruozi, B.; Duskey, J.T.; Ottonelli, I. Challenges of Enzyme Therapy: Why Two Players Are Better than One. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2024, 16, e1979. [Google Scholar] [CrossRef]
- Simmchen, J.; Baeza, A.; Ruiz-Molina, D.; Vallet-Regí, M. Improving Catalase-Based Propelled Motor Endurance by Enzyme Encapsulation. Nanoscale 2014, 6, 8907–8913. [Google Scholar] [CrossRef]
- Parra-Nieto, J.; García del Cid, M.A.; Galeano, C.; de Carcer, I.A.; García-García, L.; Gonzalez-Murillo, Á.; Megias, D.; Ramirez, M.; Baeza, A. Multifunctional Nanoassemblies for Cytotoxic Drug and Therapeutic Enzymes Delivery in Neuroblastoma Therapy. Adv. Mater. Interfaces 2023, 10, 2201356. [Google Scholar] [CrossRef]
- Sun, H.; Jiang, C.; Wu, L.; Bai, X.; Zhai, S. Cytotoxicity-Related Bioeffects Induced by Nanoparticles: The Role of Surface Chemistry. Front. Bioeng. Biotechnol. 2019, 7, 414. [Google Scholar] [CrossRef] [PubMed]
- Khadanga, V.; Mishra, P.C. A Review on Toxicity Mechanism and Risk Factors of Nanoparticles in Respiratory Tract. Toxicology 2024, 504, 153781. [Google Scholar] [CrossRef] [PubMed]
- Fabozzi, A.; Della Sala, F.; di Gennaro, M.; Barretta, M.; Longobardo, G.; Solimando, N.; Pagliuca, M.; Borzacchiello, A. Design of Functional Nanoparticles by Microfluidic Platforms as Advanced Drug Delivery Systems for Cancer Therapy. Lab. Chip 2023, 23, 1389–1409. [Google Scholar] [CrossRef]


| Sample | Hydrodynamic Diameter (nm) | ζ-Potential (mV) |
|---|---|---|
| Lip-N3 | 82 ± 6 | −5 ± 1 |
| MSN-NH2 | 154 ± 10 | 18 ± 1 |
| MSN-N3 | 136 ± 3 | −18 ± 1 |
| CatNCs | 102 ± 47 | −21 ± 2 |
| DBCO-CatNCs | 50 ± 4 | −24 ± 1 |
| [(Lip-N3)-(DBCO-TAMRA)] | 70 ± 9 | −16 ± 5 |
| [(MSN-N3)-(DBCO-TAMRA)] | 129 ± 15 | −7 ± 1 |
| [(MSN-N3)-(DBCO-CatNCs)] | 267 ± 33 | −28.8 ± 0.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Larre, J.; García del Cid, M.A.; Benita-Donadios, D.; Vela-Cruz, Á.; Jiménez-Falcao, S.; Baeza, A. Low-Cost Versatile Microfluidic Platform for Bioorthogonal Click-Mediated Nanoassembly of Hybrid Nanosystems. Nanomaterials 2025, 15, 1663. https://doi.org/10.3390/nano15211663
González-Larre J, García del Cid MA, Benita-Donadios D, Vela-Cruz Á, Jiménez-Falcao S, Baeza A. Low-Cost Versatile Microfluidic Platform for Bioorthogonal Click-Mediated Nanoassembly of Hybrid Nanosystems. Nanomaterials. 2025; 15(21):1663. https://doi.org/10.3390/nano15211663
Chicago/Turabian StyleGonzález-Larre, Javier, María Amor García del Cid, Diana Benita-Donadios, Ángel Vela-Cruz, Sandra Jiménez-Falcao, and Alejandro Baeza. 2025. "Low-Cost Versatile Microfluidic Platform for Bioorthogonal Click-Mediated Nanoassembly of Hybrid Nanosystems" Nanomaterials 15, no. 21: 1663. https://doi.org/10.3390/nano15211663
APA StyleGonzález-Larre, J., García del Cid, M. A., Benita-Donadios, D., Vela-Cruz, Á., Jiménez-Falcao, S., & Baeza, A. (2025). Low-Cost Versatile Microfluidic Platform for Bioorthogonal Click-Mediated Nanoassembly of Hybrid Nanosystems. Nanomaterials, 15(21), 1663. https://doi.org/10.3390/nano15211663










